Abstract
Glucocorticoids exert pleiotropic effects on all tissues to regulate cellular and metabolic homeostasis. Synthetic forms are used therapeutically in a wide range of conditions for their anti-inflammatory benefits, at the cost of dose and duration-dependent side effects. Significant variability occurs between tissues, disease states, and individuals with regard to both the beneficial and deleterious effects. The glucocorticoid receptor (GR) is the site of action for these hormones and a vast body of work has been conducted understanding its function. Traditionally, it was thought that the anti-inflammatory benefits of glucocorticoids were mediated by transrepression of pro-inflammatory transcription factors, while the adverse metabolic effects resulted from direct transactivation. This canonical understanding of the GR function has been brought into question over the past 2 decades with advances in the resolution of scientific techniques, and the discovery of multiple isoforms of the receptor present in most tissues. Here we review the structure and function of the GR, the nature of the receptor isoforms, and the contribution of the receptor to glucocorticoid sensitivity, or resistance in health and disease.
Keywords: glucocorticoids, glucocorticoid receptor, glucocorticoid receptor isoforms, hypothalamic-pituitary-adrenal axis, glucocorticoid sensitivity
Graphical Abstract
Graphical Abstract.
Essential Points.
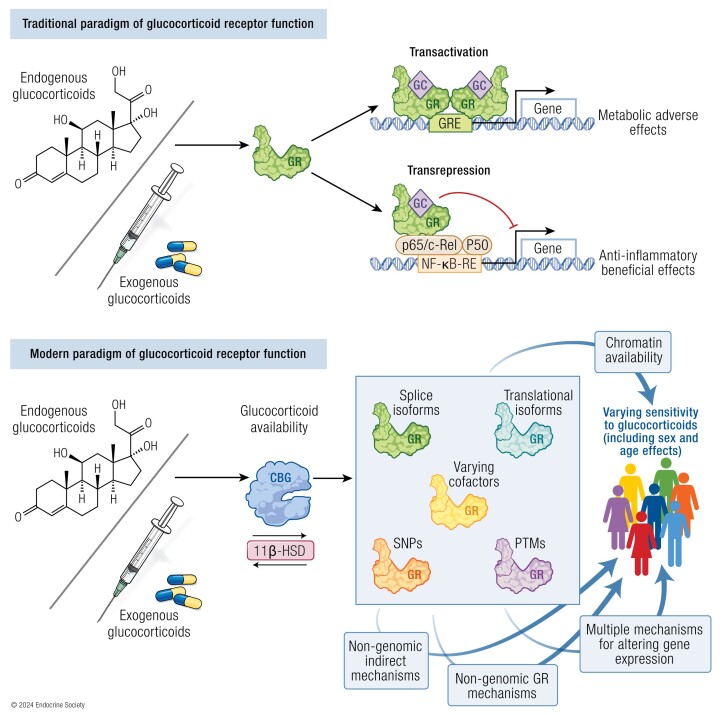
The glucocorticoid receptor, through expression of isoforms, interaction with cofactors, interaction with DNA and RNA, and interaction with other transcription factors, plays a key role in glucocorticoid sensitivity and tissue-selective action.
The glucocorticoid receptor isoforms diversify glucocorticoid function; a greater understanding of their functions and the role they play in disease is required.
The canonical understanding of glucocorticoid receptor function has been challenged by recent data and is much more complex and varied than previously understood.
Glucocorticoids are 21-carbon corticosteroid hormones derived from cholesterol in the adrenal gland, under control of the hypothalamic-pituitary-adrenal (HPA) axis (1). They exert pleiotropic effects on all tissues to regulate cellular and metabolic homeostasis, including, but not limited to, functions such as the response to stress, inflammation, metabolism, sodium and water balance, and reproductive function (2). In humans, cortisol is the main physiological glucocorticoid, and it acts via binding to the glucocorticoid receptor (GR), a member of the highly conserved nuclear receptor subfamily 3 group of intracellular hormone receptors (3). Ligand binding to GR results predominantly in transcriptional regulation, and in some conditions and tissues, glucocorticoids may regulate up to 20% of the genome (4). In recent years, researchers have identified a number of splice and transcription initiation site isoforms of the GR, which allow diversification of glucocorticoid action and, in part, mediate cellular sensitivity to glucocorticoids (3, 5).
Therapeutically, the anti-inflammatory and immunosuppressive actions of glucocorticoids are harnessed to treat a wide range of autoimmune and inflammatory conditions, with synthetic glucocorticoids forming critical components of organ transplantation and chemotherapeutic regimens in addition to hormone replacement in hypocortisolism (6). It was estimated that 1% of the UK population was on chronic glucocorticoid therapy in the 1990s (7); however, more recent data from Denmark suggests the population prevalence was around 3% between 1999 to 2014, increasing with age to up to almost 11% in those over 80 years of age (8). Their immunosuppressive benefits need to be balanced against an extensive, dose-dependent side-effect profile that includes diabetes mellitus, hypertension, dyslipidemia, weight gain, myopathy, and osteoporosis, culminating in excess mortality (9-13). Currently, we understand that sensitivity to glucocorticoids (to both beneficial and adverse effects) varies between individuals, yet clinically, we have no way to assess this objectively a priori or to measure glucocorticoid activity upon treatment commencement, and so we are limited to the retrospective review of clinical response, which may expose patients to the risk of harmful side effects or ineffective dosing. The desire to improve the efficacy of glucocorticoids while avoiding the side effects has led to research into the development of selective GR agonists (SEGRAs) which have yet to be successfully translated from preclinical studies (14). A greater understanding of interindividual differences in glucocorticoid sensitivity is expected to elucidate potential biomarkers for assessment/monitoring of patients who require glucocorticoid therapy, thereby improving the safety of treatment. Here we review the recent advances in understanding of the GR and its isoforms, and the role that they play in mediating glucocorticoid sensitivity.
Glucocorticoids and Hypothalamic-Pituitary-Adrenal Axis Function
Glucocorticoids are synthesized in the zona fasciculata, the middle of 3 layers of the adrenal cortex, through a series of enzymatic modifications of cholesterol and downstream steroidogenic precursors; in humans, cortisol is the primary glucocorticoid (Fig. 1) (15). This occurs under control of the HPA axis, comprising neurons of the hypothalamic paraventricular nucleus (PVN), corticotrope cells of the anterior pituitary, and the aforementioned adrenocortical cells (1). The HPA axis controls both basal cortisol secretion and the response to stress; its function is tightly regulated, including negative feedback loops, and in nonstressed conditions it displays circadian and ultradian rhythmicity.
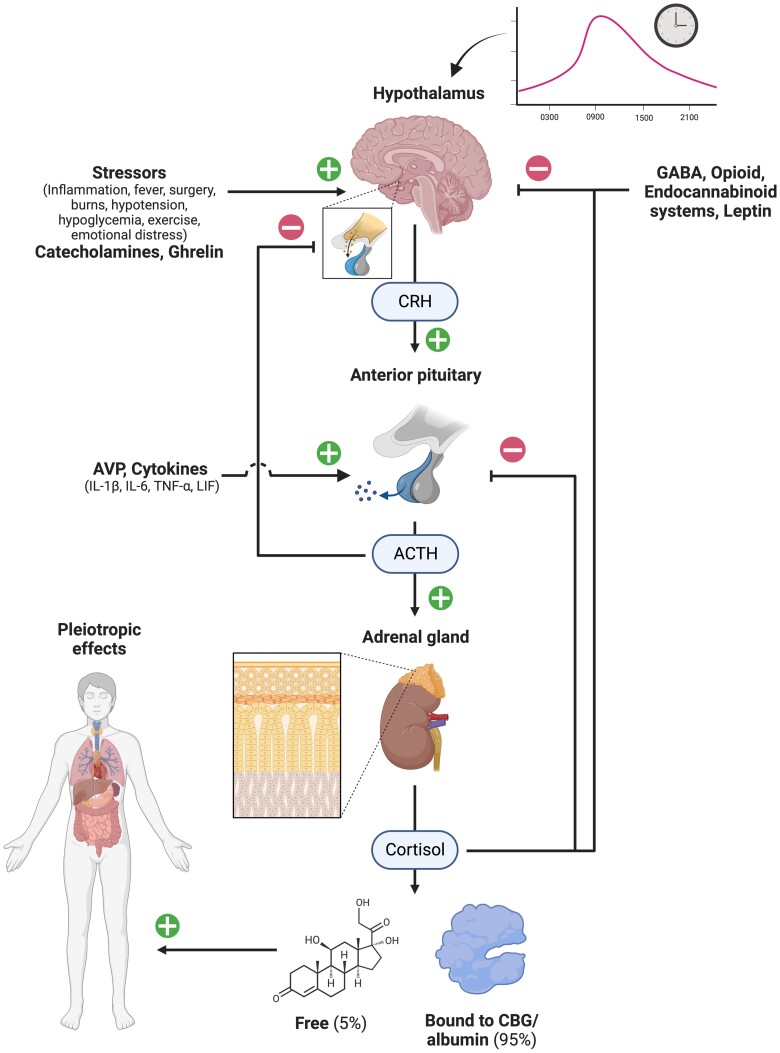
Figure 1.
Hypothalamic-pituitary-adrenal axis. The hypothalamus secretes CRH with a circadian rhythm, which acts on anterior pituitary corticotrope cells, stimulating secretion of ACTH (among other hormones). This induces cortisol secretion from the adrenal gland. Cortisol circulates predominantly bound to CBG and induces pleiotropic effects in almost all tissues. Several external inputs modulate this system and negative feedback loops constrain the secretion at each level, ultimately maintaining normal serum cortisol levels. (+) indicates stimulates; (−) indicates inhibits. Abbreviations: ACTH, adrenocorticotropic hormone; AVP, arginine vasopressin; CBG, corticosteroid-binding globulin; CRH, corticotropin-releasing hormone; GABA, gamma-aminobutyric acid; IL-1β, interleukin 1β; IL-6, interleukin 6; LIF, leukemia inhibitory factor; TNF-α, tumor necrosis factor α. Created with Biorender.com.
HPA Axis Regulation and Feedback
Corticotropin-releasing hormone (CRH) is a peptide hormone synthesized predominantly in parvocellular neurons of the PVN, and secreted into the hypophyseal portal vessels which traverse the pituitary stalk and deliver CRH to the anterior pituitary (16, 17). CRH binds to its cognate receptor on anterior pituitary corticotrope cells to stimulate production and secretion of pro-opiomelanocortin (POMC) (16, 18). Stimulation of POMC by CRH is potentiated by arginine vasopressin (AVP; also known as anti-diuretic hormone, ADH), another hypothalamic hormone, but can also be enhanced by other hormones, including angiotensin II, cholecystokinin, and atrial natriuretic peptide, among others (19, 20). POMC is a large hormone precursor that is cleaved in a tissue-specific fashion variably into α, β, and γ-melanocyte stimulating hormones, β-endorphin, β and γ-lipoproteins, and importantly for the HPA axis, adrenocorticotropic hormone (ACTH) (18, 21). ACTH binding to its receptor melanocortin receptor type 2 (MC2R) sets in motion acute, and subacute changes in the enzymatic activity in the zona fasciculata that yield an increase in production of cortisol. Once secreted, cortisol bioavailability is regulated by binding to circulating proteins: 80% to 90% to corticosteroid-binding globulin (CBG) and 5% to 15% to albumin (22). This acts as a reservoir for hydrophobic cortisol, ensuring it is delivered appropriately to tissues with maintenance of the ∼5% free cortisol fraction that is bioactive and can increase to 25% when CBG is absent or inactivated (23, 24). Furthermore, CBG can be cleaved by neutrophil elastase, resulting in a conformational change that reduces the binding affinity for cortisol, allowing targeted delivery of cortisol to sites of acute inflammation (25).
The HPA axis is regulated by negative feedback at multiple levels. Ultrashort feedback loops occur in the hypothalamus (CRH-mediated) (26) and pituitary (ACTH-mediated) through autocrine inhibition (27). Short feedback loops inhibiting CRH secretion occur in the hypothalamus, either through neural projections from the arcuate nucleus in response to CRH (1) or hormonal by ACTH. The latter has been demonstrated via in vitro studies, which has been confirmed to occur in vivo in patients with Addison disease or hypopituitarism (who have baseline elevated CRH), but not in normal subjects (in whom it is assumed long-feedback from cortisol masks this mechanism (28). As cortisol is secreted in response to ACTH, it acts via the GR in long-feedback loops to inhibit CRH and AVP mRNA transcription in the hypothalamus and POMC transcription and post-translational modification (into ACTH) in the anterior pituitary, thereby limiting exposure to glucocorticoids in response to stress (29, 30). This can also occur rapidly (< 20 minutes) through nongenomic, GR-mediated mechanisms, likely acting to prevent pre-formed ACTH release from corticotropes and blocking CRH stimulation, thus inhibiting ongoing pulsatile secretion of ACTH and cortisol (31). Supraphysiological doses of glucocorticoids can suppress HPA axis function through inducing constant, potent, negative feedback on the hypothalamus and pituitary. This is estimated to occur in 48% to 63% of those receiving long-term treatment (32, 33), but has also been demonstrated after short or recurrent courses of high-dose glucocorticoids (34-36). It is likely that interindividual differences in glucocorticoid sensitivity mediate the unpredictability in development of secondary adrenal insufficiency in this setting.
External inputs into HPA axis function come from multiple sources. Central catecholaminergic pathways in the medulla reciprocally stimulate CRH secretion in the hypothalamus, and inhibitory inputs arise from central gamma-aminobutyric acid (GABA), opioid, and endocannabinoid neuronal systems (16, 17). Ghrelin and leptin have contrasting effects on the HPA axis, the former stimulatory (predominantly acting via AVP), the latter inhibitory (37, 38). The HPA axis is one of the key effectors of the stress response and is tightly linked to the immune system, both regulating and being regulated by it: the immune-endocrine axis. Pro-inflammatory cytokines interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and leukemia inhibitory factor (LIF) all increase ACTH secretion either directly or via potentiating CRH stimulation (1, 39, 40).
Circadian/ultradian rhythms
CRH, ACTH, and cortisol, are secreted in a pulsatile manner with levels rising from the early hours of the morning, peaking shortly after waking, and falling throughout the day and evening (1, 41). Reported peak morning serum cortisol concentrations using liquid chromatography–tandem mass spectrometry (LC-MS/MS; the most accurate current methodology for measurement of steroid hormones) are 350 nmol/L (2.5-97.5th percentiles; 165-635) in males and 323 nmol/L (166-617) in females (42). In an older study which measured cortisol using reverse phase high performance liquid chromatography (HPLC), nadir concentrations were (mean ± SD) 50 ± 30 nmol/L (43), while stressed values in critically ill patients range from means/medians of 744 to 1350 nmol/L depending on the cause, severity, and duration of illness (44-46). Using microdialysis, free tissue concentrations of cortisol retain this pattern in ambulatory healthy volunteers, along with all measurable adrenal steroids and metabolites except for dehydroepiandrosterone sulfate (DHEA-S) (47). This study confirmed that a drop to nadir levels only occurs following sleep commencement. This circadian rhythm is driven by an increase in secretory pulse amplitude in the morning and a reduction in pulse frequency overnight (1, 48). The circadian pattern of secretion is controlled by light-dark and sleep-wake patterns (through the central clock mechanism of the hypothalamic suprachiasmatic nucleus [SCN]), food intake, and stressors (49, 50). Additionally, there is evidence of a native adrenal clock mechanism with daily rhythmic expression of steroidogenic acute regulatory protein (StAR) contributing to an intrinsic adrenal circadian rhythm (51). Furthermore, cortisol can act to reset other peripheral clock mechanisms by enhancing the expression of clock-related genes; however, there is no effect on the central clock as SCN neurons lack GR (50, 52). Overlaid on the circadian pattern of secretion is an ultradian rhythm with pulses of ACTH produced every 1 to 2 hours (50). This pulsatility enhances the ability of the HPA axis to respond to stress and loss of the circadian and derangement of ultradian rhythm are some of the first changes in endogenous Cushing syndrome (53).
Sex differences in HPA axis function
Sexual dimorphism occurs in HPA axis function between male and female subjects. This is mediated by interactions with the hypothalamic-pituitary-gonadal axis and its effector hormones (estrogens, progestogens, and androgens), either directly or indirectly. Other sex effects include the influence of chromosomal genetic factors, and biochemical and structural differences of the central nervous system arising from epigenetic programming during development that impact the integration of complex signals to the PVN (54, 55). These differences in HPA axis function are associated with sexual dimorphism in diseases impacted by HPA axis dysregulation, with women being disproportionately affected by autoimmune disease and major depressive disorders, while men more commonly suffer cardiovascular disease and infection (54). The most striking discrepancy is in the epidemiology of all causes of Cushing syndrome (endogenous cortisol excess), which are heavily biased toward female individuals (56).
In rodent studies, the sex differences in HPA axis function are consistent, demonstrating elevated basal and stress-induced corticosterone levels in female rodents (regardless of the stressor) with more frequent and greater amplitude of secretory pulses measured when assessing diurnal rhythmicity (55). In humans, sex differences are not as well defined, depending on the timing of assessment (basal vs stressed) and the nature of the stressor. No difference was found in serum cortisol levels between men and women when sampled every 15 minutes for 24 hours, despite an increase in the pulse frequency, amplitude, mean ACTH, and area under the curve (AUC) for ACTH in male subjects, suggesting there may be differences in either ACTH sensitivity or negative feedback regulation between males and females (48).
Differences between sexes alter with age, particularly in female individuals, which vary with impact of hypothalamic-pituitary-gonadal axis hormonal changes. Young female individuals have lower basal ACTH (57). Basal cortisol levels are elevated in the follicular compared to luteal phase of the menstrual cycle when measured both in serum (total) or saliva (free) (58, 59). In the extremis, HPA axis function becomes progressively more altered from normal physiology through the course of pregnancy, with eventual development of positive, placental CRH–driven feed-forward regulation and exceedingly high cortisol levels approaching parturition (60). As women age, CBG decreases compared to men, but this is not associated with changes in free cortisol (61). Relative to men, older data suggest lower mean 24-hour cortisol levels in premenopausal women, a difference that was lost following menopause, suggesting a relative greater increase in total serum cortisol as women age (62). This is associated with a delay in the onset and shortening of duration of the “quiescent phase” (cortisol persistently < 138 nmol/L) of the diurnal cycle which was more pronounced in aging women.
Following a standardized laboratory-induced psychological stress (Trier Social Stress Test), male subjects exhibit a greater ACTH response than female subjects (except those in the luteal phase) (63), and greater salivary free cortisol peak and during recovery period (64, 65). Stress involving a social rejection trigger may induce a greater cortisol response in female subjects (66), however this finding could not be replicated (67, 68). Physical triggers similarly demonstrate sexual dimorphism in HPA axis response with no difference in response to endurance exercise (69, 70), female participants generating greater cortisol levels following heat and cold exposure (71, 72), while males demonstrate greater salivary cortisol response to noxious stimuli (73). Finally, responses to pharmacological manipulation of the HPA axis differ, with female subjects demonstrating greater response of ACTH and cortisol to naloxone (65), ACTH (and more prolonged cortisol peak) to ovine CRH (74), ACTH and cortisol to combined human CRH and AVP (57, 75), cortisol following dexamethasone-suppressed CRH (76), and salivary free cortisol with ACTH1-24 stimulation (greater in luteal phase) (63). As aging occurs, the responsivity to pharmacological tests of HPA function tends to increase, and this occurs disproportionately so in females (76-78).
The underlying pathophysiological mechanisms of sexual dimorphism in HPA axis function are reviewed in depth elsewhere (55), where relevant data is available, sex differences will be covered in subsequent sections of this paper.
Physiological Effects and Therapeutic Use of Glucocorticoids
Glucocorticoids have pleiotropic effects on a wide range of tissues, and by acting predominantly through the GR, they regulate up to 20% of the genome (4). This expansive repertoire of transcriptional regulation accounts for the extensive and varied physiological regulation, beneficial therapeutic, and significant adverse effects.
In addition to responding to inflammatory stimuli, other stressors induce the HPA axis’ counterregulatory response to return the body to homeostasis. This has been demonstrated with fever, surgery, burns, hypotension, exercise, emotional stress, and hypoglycemia (79-83). The latter forms the basis for the insulin tolerance test as the gold standard for assessing adequate HPA axis function (84, 85). The effects on individual systems coalesce to prepare the body to deal with the stressor and prevent decompensation.
The name for glucocorticoids is derived from the hormones’ actions on glucose metabolism. The metabolic alterations in response to an elevation in glucocorticoid levels function to mobilize substrates for use in energy production. In the liver, glucocorticoids stimulate glycogen synthesis and gluconeogenesis through upregulation of synthetic enzymes, while peripherally cortisol inhibits uptake and utilization of glucose in muscle and fat and potentiates glucagon and catecholamine effects (86-88). The net effect is insulin resistance and mobilization of glucose into the circulation. Hyperglycemia is common with therapeutic treatment, occurs within hours of commencement, and predominantly affects postprandial glucose levels (89, 90). While stimulating lipolysis, glucocorticoids also promote adipocyte differentiation and adipogenesis, and long-term excess glucocorticoid exposure is associated with visceral and central adiposity (91, 92). Weight gain is reported in up to 70% of long-term glucocorticoid users, with a magnitude of 4% to 8% of body weight over 2 years on 5 to 10 mg prednisolone equivalent (93, 94). Furthermore, protein catabolism is stimulated in skeletal muscle, bones, and skin to provide amino acids for use in oxidative pathways (95, 96). With prolonged, excessive exposure to glucocorticoids, this culminates in clinical features such as dermal atrophy, sarcopenia, and osteoporosis (6, 97-99). Additionally, cortisol is necessary for maintenance of adequate circulating volume and blood pressure through interaction with the renin-angiotensin-aldosterone system to promote salt and water retention, increasing vascular sensitivity to catecholamines and inhibiting AVP (100-103). Glucocorticoids are essential for development in a range of tissues, but most importantly the lung, where they induce fetal lung maturation (104). GR-knockout mice die shortly after birth due to respiratory failure, and exogenous glucocorticoids are administered prenatally (maternally) to accelerate fetal lung development with impending premature delivery (105). Glucocorticoids are similarly important for neural function and in excess can cause a range of neurocognitive side effects which are dose- and duration-dependent but can occur within 1 week of commencement (106, 107). In normal physiological concentrations, glucocorticoids may contribute to neurodegenerative diseases through induction of neuronal cell death (108). Cortisol interacts with the other endocrine systems with induction of growth hormone transcription but antagonism of insulin-like growth factor 1 (IGF-1), and inhibition of thyroid-stimulating hormone (TSH) secretion, 5′-deiodinase, gonadotropin-releasing hormone (GnRH) pulsatility, and luteinizing (LH) and follicle-stimulating hormone (FSH) secretion (109, 110). Despite these wide-ranging impacts of glucocorticoids, therapeutically, they are predominantly used for their potent immunosuppressive effects.
Physiologically, the overarching function of glucocorticoids on the immune system is to enhance innate immunity and control inflammation, supporting efficient clearance of pathogens while limiting tissue damage and preventing an overwhelming systemic inflammatory response, thereby returning homeostasis (111). These contrasting pro- and anti-inflammatory effects are determined by the cell type, cellular activation state, stage of the inflammatory response, and dosage of glucocorticoids (1). Glucocorticoids are involved in enhancing the innate immune response through upregulation of some pattern-recognition receptors (toll-like receptor 2 [TLR2]; NOD-like receptors [NLR]P1, NLRP3, NLRC4) to enhance recognition of evolutionarily conserved pathogen/damage-associated molecular patterns (PAMP/DAMPs) (112, 113). This increases activation of transcription factors activator protein 1 (AP-1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and subsequent induction of pro-inflammatory cytokines in a synergistic fashion to aid with chemotaxis, and initiation of the immune and acute-phase response to an insult (111). The resultant secretion of IL-6, IL-1β, and TNF-α enhances glucocorticoid secretion, providing a feed-forward mechanism to limit inflammation (1, 114). Subsequent direct inhibition of AP-1 and NF-κB and upregulation of their inhibitors by glucocorticoids downregulates the inflammatory response (111). In the resolution phase of inflammation, glucocorticoids impair neutrophil recruitment and stimulate neutrophil and lymphocyte apoptosis with recruitment and activation of macrophages at the site of injury to promote clearance of cellular debris and tissue/wound healing (111). At therapeutic doses, the immunosuppressive effects of glucocorticoids predominate with inhibition of leukocyte migration into tissues, neutrophil egress from the bone marrow, induction of eosinophil apoptosis, reduction in circulating lymphocyte counts (T more than B cells) through apoptosis and sequestration, and a reduction in immunoglobulins (115-118). While these immunosuppressant actions of glucocorticoids are harnessed to effectively treat autoimmune and inflammatory disorders, they predispose to opportunistic infections in those on prolonged treatment. The relative risk may be as high as 1.6 compared to placebo, and this risk is increased further by concomitant use of other immunosuppressive or biological therapy (119). There is a particular susceptibility to invasive fungal and viral infections. The infection risk is also dependent on other factors such as the use of additional immunosuppressive agents, age, and comorbidities (120).
Therapeutic uses of glucocorticoids
Since the discovery that cortisone could improve clinical features and markers of inflammation in patients with rheumatoid arthritis (RA) (121), glucocorticoids have been used widely to treat autoimmune and inflammatory diseases in oral, parenteral, inhaled, topical, ocular, and intraarticular formulations (6). They have additionally been used as essential components of chemotherapeutic regimens, for their direct cytotoxic effects in lymphoproliferative malignancies, for their antiemetic and orexigenic properties in other cancers, and their anti-inflammatory properties in edematous brain metastases (36, 122). Furthermore, glucocorticoids form part of the backbone of organ transplantation immunosuppression regimens and are utilized in the perioperative setting for nausea and vomiting prophylaxis or to reduce laryngeal edema after endotracheal intubation (123, 124).
Pig adrenal extract was first used to treat Addison disease by William Osler in 1896 and since the mid-20th century, glucocorticoids have been used as life-saving treatment for patients with both primary (also requiring mineralocorticoid replacement) and secondary adrenal insufficiency (125). Unlike in therapeutic treatment of inflammatory disease, treatment of adrenal insufficiency aims to mimic the physiological diurnal secretion of cortisol which is estimated to be in the region of 5 to 8 mg/m2 (body surface area) per day (126). Hydrocortisone twice or thrice daily is the preferred formulation due to the short half-life; however, longer-acting medications may be used to aid with compliance (126). These regimens do not truly mimic physiological secretion which begins prior to waking, leading to development of delayed-release hydrocortisone and the use of continuous subcutaneous hydrocortisone infusions that can more closely mimic the cortisol circadian rhythm (126, 127).
Over time, there has been development/discovery of a wide range of glucocorticoids with varying potencies, degrees of mineralocorticoid activity, and pharmacokinetic profiles. Hydrocortisone is the pharmacologic name for cortisol, and progressive alterations of the molecule have yielded a range of synthetic glucocorticoids, the common feature being a 17-carbon androstane structure of 3 hexane rings and 1 pentane ring, with a hydroxyl group at carbon-11 being critical for glucocorticoid activity (128). Cortisone acetate and prednisone are inactive precursors that require conversion by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) to the active hydrocortisone and prednisolone respectively (128). Table 1 lists common glucocorticoids used in clinical practice.
Table 1.
Common glucocorticoids used in clinical practice
| Medication | Equivalent dosage (mg) | Anti-inflammatory potency (relative) | HPA axis suppression potency (relative) | Mineralocorticoid potency (relative) | Duration of action (h) |
|---|---|---|---|---|---|
| Hydrocortisone | 20 | 1 | 1 | 1 | 8-12 |
| Cortisone acetate | 25 | 0.8 | 0.8 | 0.8 | 8-12 |
| Prednis(ol)one | 5 | 3 | 4 | 0.75 | 12-36 |
| Methylprednisolone | 4 | 6.2 | 4 | 0.5 | 12-36 |
| Dexamethasone | 0.75 | 26 | 17 | 0 | 36-72 |
| Betamethasone | 0.6 | 30 | — | 0 | 36-72 |
| Budesonide (inh) | 1.3 | — | 19.5 | — | 24 |
| Fluticasone p. (inh) | 0.6 | — | 34 | — | 24 |
Adverse effects from glucocorticoid treatment are predictable and dose- and duration-dependent (6), and they contribute significantly to morbidity and mortality in those being treated (9-13). In hormone replacement for adrenal insufficiency, traditional doses were supraphysiological based on earlier estimates of endogenous cortisol production (132). More recent studies have focused on replicating the 5 to 8 mg/m2 (body surface area) per day. Guidelines recommend hydrocortisone 15 to 25 mg per day with consideration of weight-based dosing for some patients (126). Weight-based dosing and administration of hydrocortisone in the fasted state has been shown to reduce cortisol area under the curve (AUC) variability and to provide levels closer to physiological replacement than fixed dosing (132). Despite this, cortisol peaks and troughs are respectively higher and lower than physiological secretion and could contribute to development of side effects and reduced quality of life. Increasing the frequency of dosing provides a more physiological profile (126). Furthermore, retrospective studies suggest that higher glucocorticoid doses, and particularly dexamethasone use, are associated with increased risk of adverse metabolic effects; the data for prednisolone are less certain (126, 127, 133). Monitoring of glucocorticoid replacement remains predominantly clinical, and many patients report ongoing symptoms related to glucocorticoid excess or deficiency and metabolic side effects of over-replacement despite treatment with recommended doses (134, 135). As a result of this inaccuracy in management, patients with Addison disease are at risk of premature mortality (136).
In therapeutic uses, dosage of glucocorticoids is disease specific and often protocol driven. For systemic, high-dose glucocorticoids to treat autoimmune/inflammatory diseases or in organ transplantation, regimens typically begin at a moderate-high doses with or without an initial very high-dose induction “pulse” (137, 138). Many regimens use a 1 mg/kg prednisolone equivalent starting dose and wean down over time, often months. Reduction in dosage is dependent on clinical or biochemical measures of underlying disease activity and/or emergence of side effects, with consideration of HPA axis suppression once approaching physiological dosing (138, 139). Addition of alternative immunosuppressants is often used to reduce steroid exposure. Alternatively, in oncology regimens, high doses of glucocorticoids are delivered intermittently to coincide with cytotoxic medications (36). For many of these uses, aside from the initial weight-based commencement dose, little consideration is made to interindividual sensitivity to glucocorticoids and there currently is no mechanism/biomarker to assess the extent of glucocorticoid activity, which is likely to result in many patients being unnecessarily exposed to excess glucocorticoids. The glucocorticoid receptor (GR) is a key regulator of glucocorticoid sensitivity and further understanding of how it does so may provide a promising avenue by which to assess an individual's sensitivity.
Glucocorticoid Receptor
The GR is a member of the highly conserved nuclear receptor subfamily 3, which also includes the estrogen, progesterone, androgen, and mineralocorticoid receptors (140). GR is encoded by a single gene: nuclear receptor subfamily 3, group C, member 1 (NR3C1), located on chromosome 5 (5q31) (141). All members of the family have a similar structure centering around the highly conserved central DNA-binding domain, and heterodimerization of the receptors and ligand promiscuity between the family allow for diverse and complex interactions between the hormones (140). Of note, cortisol, and many synthetic glucocorticoids, are natural agonists of the mineralocorticoid receptor (MR), binding with up to 100-fold the affinity of the hormone for GR; however, transcriptional regulation by cortisol via GR is much more pronounced than via MR (142). The MR is protected from excessive cortisol activation by 11β-HSD2 in many key tissues (see section on glucocorticoid activity); however, this is not the case in cardiac myocytes or macrophages, where the MR is key for cellular function and glucocorticoids remain the primary physiological ligand (143). Glucocorticoid function mediated by the MR has been reviewed elsewhere in depth (144-148).
Structure of the Glucocorticoid Receptor
The nuclear receptor subfamily 3 group C member 1 (NR3C1) gene consists of 9 exons, of which exons 2 to 9 encode for the GR protein (149). Exon 1 encodes the 5′ untranslated region (UTR) of which 13 variants have been identified, with differing upstream promoter regions, and these appear to be involved in regulation of protein isoform expression, with certain promoter usage related to specific isoforms (150-152).
Exon 1 includes promoters for 9 exon 1 variants, including 2 that can be alternatively spliced into a total of 13 exon 1 promoter variants (153-156). The presence of multiple GR untranslated exon 1 sequences arising from different promoters is seen across species and nuclear receptors (154, 155). In humans, these occur up to 31 kilobase pairs (kb) upstream of exon 2, have variable splice donor sites, and a common exon 2 splice acceptor site that includes an in-frame stop codon, 12 base pairs (bp) proximal to the initial exon 2 ATG, ensuring they are not translated into the GR protein (154). There is no difference in transcript stability between the variants tested, nor translational efficiency (157). Alternative promoter usage has been demonstrated to impact splice variant expression in a number of genes, modulated via transcription factor and cofactor recruitment, which could be important for the expression of different GR isoforms (158).
Full-length GR (GRα-Α) is a 777–amino acid, modular protein composed of 3 major functional domains (140). The N-terminal domain (NTD) encoded by exon 2 comprises the initial 421 amino acids and is the most variable region between nuclear receptors in the same species, and in the same receptor between species (3). The NTD contains the constitutive, ligand-independent transcriptional activation function 1 (AF1), which is essential for maximal transcriptional activation and is important for GR interaction with other transcription factors, chromatin re-modelers, and the basal transcriptional machinery (149). The central DNA-binding domain (DBD) contains 2 highly conserved zinc finger motifs which are critical for binding of GR to glucocorticoid response elements (GRE) in the DNA sequence (159). Additionally, this region also contains a nuclear localization (NLS1), nuclear retention, and nuclear export signal which mediate cellular transport of the receptor. The second zinc finger includes a specific 5–amino acid sequence (D-box) required for canonical receptor dimerization (149). More recent data demonstrate that mutations of this region do not abrogate dimerization completely as previously thought (160). Furthermore, the C-terminal end of the DBD comprises the hinge region, which provides flexibility for conformational change and allosteric interactions with transcription cofactors, the basal transcriptional machinery, and DNA itself (161). The hinge region also participates in bidirectional signaling between the components of the receptor in response to DNA binding (162). A final ligand-binding domain (LBD) forms the carboxy-terminal portion of the protein. The LBD forms a hydrophobic pocket necessary for glucocorticoid binding, in addition to mediating crucial interactions with coregulators, other transcription factors, and cytoplasmic chaperone proteins (163). Also included in the LBD are the ligand-dependent transcriptional activation function (AF2) and a second nuclear localization signal (NLS2) (149). Structural analysis of the LBD demonstrates that it may play a role in the ability of GR to dimerize in addition to impacting the conformation of these dimers (164).
Function of the Glucocorticoid Receptor
The canonical understanding of GR function begins with unliganded GR residing predominantly in the cytoplasm, bound to a multi-protein anchoring complex. This multi-protein complex includes chaperones (heat shock protein 90 [hsp90], hsp70), immunophilins (FK506-binding protein 5 [FKBP5], FKBP4), and tyrosine kinases (c-Src), which prevent GR from acting as a transcription factor while maintaining a conformation with a high affinity for glucocorticoid (165). Ligand binding to GR induces a conformational change in the receptor leading to exchange of FKBP5 for FKBP4 within the components of the multi-protein complex (166). This leads to dissociation of some components of the multi-protein complex and exposure of the NLS1 and NLS2, facilitating nuclear translocation of GR where it can modify target gene expression via a number of mechanisms (Fig. 2) (167). The affinity of the GR ligand alters the diffusion coefficient and nuclear residence time, with higher-affinity ligands inducing longer nuclear translocation (168, 169).
Figure 2.
Mechanisms of glucocorticoid receptor (GR) action. GR is maintained in the cytoplasm by an anchoring complex [1] which modifies the conformation of GR to allow high affinity for glucocorticoids [2]. Glucocorticoids cross the membrane where they can be activated/inactivated by 11β-ΗSD isoforms [3]. Binding of active glucocorticoid to GR leads to dissociation of the complex [4]. GR can have a series of nongenomic effects in the cytoplasm (see text for details), or predominantly enters the nucleus. GR binds to open chromatin (determined by pioneering transcription factors) [5] where it can: directly activate or repress transcription at canonical GREs, direction of regulation determined by cofactors, gene, other transcription factors [6]; repress transcription at nGREs [7]; interact with other transcription factors either as a monomer or dimer at shared binding sites to enhance/repress transcription [8, 9], whether this can occur without direct DNA binding (tethering) [8] has been brought into question; or compete for binding sites with other transcription factors [10]. Unliganded GR is bound throughout open chromatin [11] and dissociates in favor of glucocorticoid-bound dimers upon exposure. Created with Biorender.com.
Homodimers of GR can bind to the consensus sequence of GRE in the promoter, intron, or exon of target genes to induce transactivation (149). The GRE consensus sequence consists of an imperfect palindrome with 2 half-sites, each of which cooperatively bind 1 subunit of the GR homodimer, separated by a crucial 3-bp spacer which is necessary to allow for appropriate GR conformation (3). Chromatin immunoprecipitation (ChIP) and deep sequencing (ChIP-seq) studies have demonstrated that in the absence of glucocorticoid stimulation, there is widespread chromatin occupancy by GR monomers, which dissociate in favor of GR dimer binding to GRE in response to glucocorticoids (170, 171); GR occupation of GRE leads to the recruitment of cofactors and chromatin remodeling complexes to positively regulate transcription by RNA polymerase II (RNApolII). The DNA sequence of the GRE alters GR confirmation, activation site usage, and transcriptional activity with differences seen with as little as a 1-bp alteration (172). Binding to GRE, however, does not always lead to transactivation. Occupation of canonical GREs by GR has been shown to induce suppression of target genes, suggesting that other regulatory mechanisms (eg, recruitment of certain cofactors) can modulate GR-GRE activity (149). Furthermore, negative GRE (nGRE) with a more variable consensus sequence have been identified that repress transcription upon binding of 2 everted GR monomers (preventing interaction of the D-box motif and hence dimerization), involving recruitment of corepressors and histone deactylases (173, 174). In addition to direct DNA-binding mechanisms, GR is thought to interact with DNA-bound transcriptions factors (tethering), either amplifying (eg, signal transducer and activator of transcription 3; STAT3) or suppressing (eg, NF-κB) their function through means such as altering coactivator binding or hindrance of interaction with transcriptional machinery (2, 175-177). Recent data have brought this mechanism into question. Substitution of a cysteine residue in the first zinc finger for glycine completely abrogates GR DNA binding but leaves all other functions/domains and post-translational modification intact (178). This mutation demonstrates perinatal lethality (similar to GR knockout), and while tethering was demonstrable, no changes in associated gene activation/repression were detected in response to ligand when assessed by multiple means, suggesting that DNA binding by GR is necessary to alter gene expression, potentially through failure of interaction with key coactivators (eg, GRIP-1) (178). Furthermore, only a small proportion of GR-regulated genes are found at sites with concomitant NF-κB and AP-1 binding, and the majority of those that are contain a classic GRE, suggesting that tethering is not widespread at a genome level (170). Monomeric GR can even repress inflammatory gene transcription by binding to a cryptic GRE half-site within the AP-1 response element in the absence of AP-1 binding (179). A final genomic mechanism of transcriptional regulation by a composite of GRE binding and tethering to/interacting with transcription factors bound at an adjacent DNA response element has also been demonstrated (eg, STAT3 and 5, AP-1) (2, 175). At the same locus, differing interacting transcription factors can variably upregulate or downregulate the gene in response to glucocorticoids (170, 180). It may be that the formerly demonstrated DNA-independent tethering actually represents composite binding and requires direct DNA interaction with a GRE (175).
Some effects of GR have been demonstrated to occur rapidly upon ligand binding—within seconds to minutes—suggesting a nongenomic mechanism (3, 181). Glucocorticoids can activate the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway through phosphorylation within minutes of administration in a GR-dependent, mRNA-independent manner; this pathway is essential for NF-κB phosphorylation and activation (182). This pathway is also involved in GR-dependent upregulation of endothelial nitric oxide synthase activity in vascular endothelial cells treated for 10 minutes with dexamethasone, an effect resistant to transcriptional inhibition (182). Furthermore, GR interacts with p65 and IκBα in the cytoplasm and glucocorticoids can reduce p65 entry into the nucleus (183). Similarly, GR can prevent AP-1 activity by associating with c-Jun N-terminal kinase (JNK) via the LBD of GR, inhibiting phosphorylation and hence activation of JNK, subsequently competitively inhibiting active JNK from phosphorylating c-Jun in AP-1 (184). Glucocorticoid-mediated liberation from the cytoplasmic multi-protein complex can lead to GR translocation to the mitochondria and induction of apoptotic pathways without gene regulation, and this correlates with lymphocyte sensitivity to glucocorticoid-mediated apoptosis (185). Furthermore, membrane-bound GR has been demonstrated in a range of tissues, is upregulated in immune cells in response to lipopolysaccharide (LPS) stimulation, and rapidly activates pro-apoptotic, immune regulation, and metabolic pathways upon exposure to membrane-impermeable glucocorticoids (181, 186, 187). Unliganded GR is essential for T-cell receptor signaling at the membrane through LCK/FYN kinase activation, and glucocorticoid treatment causes dissociation of GR from the complex preventing this (188, 189). Finally, liberation of the other components of the anchor complex allows them to participate in signal transduction pathways such as c-Src's activation of pathways leading to annexin-1 phosphorylation and inhibition of arachidonic acid metabolism (190). At least a portion of the described nongenomic effects may even be independent of GR (181). Glucocorticoids have been shown to rapidly decrease via protein kinase A, or increase via protein kinase C, basal or stimulated intracellular calcium concentration in a cell type–specific manner which is not affected by the GR antagonist mifepristone (also known as RU-486) or cycloheximide (mRNA synthesis inhibitor) treatment (191, 192). It is thought these effects are mediated by nonspecific interactions between glucocorticoids and the cell membrane, which alter its physiochemical properties and the function of membrane-associated proteins, and may mediate some of the immunosuppressive benefits at supratherapeutic doses (eg, pulse methylprednisolone treatment) (181, 193).
Glucocorticoid Receptor Isoforms
While a significant body of work has developed an in-depth understanding of canonical GR function, a number of splice and translation initiation isoforms have recently been discovered which diversify GR function as detailed below and shown in Fig. 3. Both splice and translational isoforms are conserved across species, having been described in analysis of various tissues of mice, rats, guinea pigs, pigs, sheep, dogs, and zebrafish (5, 194-196). Other splice variants have been described in the slender African lungfish (197). An example of conservation of GR isoforms across species is derived from work conducted in the placenta, where 8 known GR isoforms are detectable in human (152, 198, 199), mouse (200), guinea pig (201), and sheep placentas (202). Data presented here relate to the human GR.
Figure 3.
Splice and translation initiation isoforms of the glucocorticoid receptor. NR3C1 gene is composed of 9 exons, exon 1 contains 11 untranslated promoter variants, while exons 2 to 9 encode the GR, which occurs as a number of splice, and translational initiation isoforms, see text for details. Abbreviations: AF-1, transcriptional activation function 1; DBD, DNA-binding domain; GR, glucocorticoid receptor; H, hinger region; LBD, ligand-binding domain; NTD, N-terminal domain. Created with Biorender.com.
Alternative splice site variants
Gene splicing is the process of removing noncoding introns and joining protein-encoding exons of pre-mRNA to form mature mRNA that can be translated (203). This process is mediated by a large ribonucleoprotein complex, the spliceosome, under the guidance of regulatory sequences within the pre-mRNA which recruit splicing factors to control splicing. Alternative splicing is a common feature of eukaryotic gene expression and can generate multiple mRNA isoforms from a single gene (203). Direction of alternative splicing can occur through variation in RNA-binding protein recruitment (eg, heterogenous nuclear ribonucleoproteins, serine-arginine repeat proteins), RNA-RNA base pair interactions and pre-mRNA secondary structure, and interactions between the spliceosome and histones/chromatin structure and RNApolII (204).
The full-length GRα mRNA results from splicing of exons 2 to 8 and the end of exon 8 to a donor site at the beginning of exon 9—9a; this results in the majority of exon 9 forming the 3′ UTR (149). Our understanding of GRα-A function is as above, or as described for its other translational isoforms in the subsequent section.
GRβ
GRβ is generated through alternative splicing of exon 8 to a distal splice acceptor site in exon 9—9β (205). The resulting protein is identical to GRα through to amino acid 727 (NTD, DBD, and part of LBD) with a further nonhomologous 15 amino acids. This results in a shorter protein lacking α-helix 12 of the LBD, which is essential for formation of the hydrophobic pocket, thereby preventing glucocorticoid binding, and which forms part of the AF2 domain, thereby altering cofactor interactions (206-208). Alternative splicing to GRβ can be directed by dehydroepiandrosterone (DHEA)-induced serine/arginine-rich splicing factor 9 (209). The only known ligand of GRβ is GR antagonist mifepristone (210). GRβ has been found to be a dominant negative regulator of GRα activity in a range of tissues. It is expressed predominantly in the nucleus and can form nontransactivating heterodimers with GRα, and competes for both coactivator and GRE binding, preventing glucocorticoid-mediated GRα activity (211-214). Nuclear localization of GRβ is not universal and cytoplasmic expression has been demonstrated in a number of cell types/lines (human monocytes, COS-1, HeLa, U2OS) (206, 210, 213). Furthermore, mifepristone has been variably shown to induce nuclear translocation of GRβ in a cell type–specific manner (210, 215). GRβ mRNA is detectable in almost all tissues and cells, but with expression at a fraction of GRα levels, and the protein has been detected in most tissues, but this has not been consistently demonstrated (213, 216). In patients with Cushing syndrome compared to healthy controls, the relative expression of GRβ mRNA was higher in hypercortisolism and declined after successful treatment. In parallel to this, binding affinity of dexamethasone was lower in peripheral blood mononuclear cells (PBMC) in patients with hypercortisolism than in healthy controls and increased after treatment; however, this difference was not seen in skin fibroblasts (217-220).
While traditionally thought of as acting solely as an inhibitor of GRα activity, GRβ has been demonstrated to have its own, unique transcriptional regulation repertoire, while also sharing some regulatory functions with GRα (eg, repression of IL-5 and IL-13 via histone deacetylase recruitment to their promoters) (214, 215, 221, 222). The mechanism behind this is not fully elucidated, however in vitro data has been confirmed to be active in vivo from a study of GR wild-type and knockout mice which demonstrated that the effect of GRβ on gene transcription in the liver varies, dependent on the presence or absence of GRα (222). Furthermore, despite lacking the α-helix 12 and presumed interruption of the AF2 domain, GRβ can still bind corepressors in the presence of mifepristone to a similar extent to GRα, meanwhile, coactivator binding on dexamethasone stimulation is abrogated (206). Further work is needed to elucidate the mechanisms of GRβ's non-GRα-dependent functions.
Pro-inflammatory cytokines can increase GRβ expression through a number of mechanisms, and it is thought that this contributes to glucocorticoid resistance seen in inflammatory illnesses such as sepsis (223, 224). The serine/arginine-rich proteins (SRps) are a highly conserved family of proteins involved in regulating mRNA splicing through preferentially binding to different splice sites and initiating spliceosome formation. SRp30c and SRp40 have been shown to favor GRβ splicing of GR mRNA in response to IL-8 or bombesin, while SRp20 expression favors GRα splicing (225, 226). The effect of these SRps in regulating GR mRNA splicing is supported by the presence of predicted binding sites for each isoform in their respective segments of GR exon 9 (167). Furthermore, micro-RNAs can alter splice isoform expression; micro-RNAs are small, noncoding RNA molecules that regulate gene expression post-transcriptionally by binding to mRNA and causing degradation of the transcript (227). In T cells, expression of microRNA (miR)-124 reduces both GRα mRNA and protein resulting in a relative increase in the proportion of GRβ mRNA (which was unchanged) (223). Additionally, miR-144 has been demonstrated to be targeted to the 3′ UTR of GRβ mRNA; mutation of miR-144 or blockade leads to downregulation of GRβ, and overexpression leads to increased expression of GRβ (228).
GRγ
GRγ was first discovered in cancer cell lines and upregulation in these tissues is associated with glucocorticoid resistance (229, 230). This isoform results from alternative splicing of the intron between exons 3 and 4, resulting in addition of a single arginine residue between the DNA-binding zinc finger motifs (229). This insertion resides close to the NLS1 domain and unliganded, GRγ shows a predominantly cytoplasmic distribution compared to GRα, with slower nuclear import in response to dexamethasone, which is associated with delayed onset of transactivation (231). Interestingly, there is a concentration of GRγ residing close to the cell membrane particularly concentrated around membrane ruffles, and potentially this isoform may be the putative membrane GR (231). GRγ typically accounts for around 3.8% to 8.7% of total GR mRNA (232, 233); however, as much as 40% of total GR has been seen in PBMC of patients with glucocorticoid-resistant multiple sclerosis (who also had reduced total and GRγ expression compared to glucocorticoid-sensitive controls) (234). Additionally, GRγ mRNA is upregulated in PBMC from patients with glucocorticoid-resistant immune thrombocytopenic purpura (235).
The GRγ gene regulatory profile and DNA-binding regions show up to 94% similarity to GRα in response to glucocorticoids (162, 236), but despite a higher affinity for GR binding sites than GRα, GRγ has been shown to have approximately 50% of the transcriptional activity (229, 230). In the unliganded state, transcriptional regulation compared to GRα is more distinct (231). These differences in GRγ-specific regulation of genes appear to be in part directed by the DNA sequence at the GR binding site (162, 172). Additionally, GRγ demonstrates an abundance of mitochondrial function–related gene pathways in transcriptome and protein interaction studies relative to GRα, which is associated with increased mitochondrial mass, basal (unliganded) respiration, and ATP production in HEK cells overexpressing each isoform (231).
To date, this isoform has only been described using measurement of RNA/cDNA (229, 230, 232, 233), or functional reporter assays in cell lines (229, 230). With only one additional amino acid, it would be impossible to separate this isoform from GRα using Western blot methodology, the most common approach to isoform protein measurement.
GR-A
GR-A was first described in a resistant multiple myeloma cell line and is formed through alternative splicing of the 3′ exon 4 splice donor site to the 5′ donor site in exon 8 (237). The absence of exons 5 to 7 yields a 65-kDa receptor lacking the amino portion of the LBD, including a nuclear localization signal and AF2 (237). The physiological activity of this isoform is unknown and to date has only otherwise been confirmed in the placenta (198). However, a 67-kDa isoform, which may represent GR-A, was the predominant isoform expressed in nasal mucosa of healthy volunteers and polyps from patients with chronic rhinosinusitis (although this study found conflicting findings using 2 different GR antibodies) (238) and was also detected in human dorsolateral prefrontal cortex at higher levels than GRα-A (239). No changes in GR-A expression were seen in hippocampal or prefrontal cortex of mice exposed to chronic unpredictable stress, unlike other isoforms.
GR-P
GR-P mRNA was first recognized in a glucocorticoid-resistant multiple myeloma cell line where its upregulation (in combination with downregulation of GRα) occurred at emergence of the resistant population followed by eventual downregulation over months whether or not there was ongoing exposure to dexamethasone (240). The isoform is formed by failure of splicing between exon 7 and 8, with inclusion of the initial segment of the intervening intron (237, 240). The resulting receptor lacks the carboxy-terminal half of the LBD, including the dimerization signal and AF2, resulting in a 74-kDa protein that fails to bind ligand (241).
Subsequently, GR-P has been shown to be present in normal PBMCs or lymphocytes where is constitutes 4% to 24% of total GR mRNA and is correlated with total GR per cell using dexamethasone binding assays (217, 242, 243). Additionally, this isoform has been demonstrated in human placenta (where it is increased in those undergoing induction of labor or cesarean section) (150, 198, 199, 244), postmortem hippocampus and prefrontal cortex samples (in the latter, mRNA expression was lower in suicide-completers compared to controls) (245), leukemic cells of glucocorticoid-naïve multiple myeloma, acute lymphoblastic/myeloid leukemia (ALL/AML), and non-Hodgkin lymphoma patients, and ovarian, hepatocellular, prostate, and ACTH-secreting small cell lung cancer cell lines (241, 242). GR-P was not detectable in paraganglioma samples (242). There is significant variability of the level of GR-P expressed in these malignancies; however, in most samples it is greater than in healthy lymphocytes. Additionally, GR-P mRNA is expressed to a greater extent in marrow mononuclear cells from patients with T-ALL compared to B-ALL (246). GR-P expression in these studies did not find any association with glucocorticoid resistance (242). DMS-79 cells (ACTH-secreting small cell lung cancer line), known to be resistant to glucocorticoids, showed no detectable dexamethasone binding with an abundance of GR-P protein detected (241). However, this glucocorticoid resistance is likely mediated by negligible expression of GRα, as transfection with GRα into the similar COR L24 cell line restored glucocorticoid-mediated transcriptional activation and repression (247).
Transfection studies have demonstrated that GR-P alone does not activate a known glucocorticoid-responsive reporter (mouse mammary tumor virus-chloramphenicol acetyltransferase; MMTV-CAT) (241). However, GR-P modulates GRα activity in a cell type–specific manner. In COS-1 (monkey kidney fibroblast; lacking endogenous GR) and HeLa (human cervical epithelial carcinoma; expressing endogenous GRα) and 2 multiple myeloma cell lines, GR-P increased dexamethasone-stimulated luciferase reporter activity in a dose-dependent manner when present with GRα (242). In contrast, in the CHO (Chinese hamster ovary) cell line, GR-P co-transfection reduced dexamethasone reporter stimulation by 50%. Furthermore, GR-P may undergo glucocorticoid-mediated negative feedback, with GR-P mRNA significantly decreased following glucocorticoid treatment in human placenta, with a greater extent using dexamethasone compared to hydrocortisone (150).
Other splice variants
While the splice variants discussed above have been described and some of their effects on function characterized, it is possible there are many more isoforms that could play a role in the modulation of GR function. In a study considering 97 healthy volunteers, 18 burn patients, and 35 patients with asthma, an additional 21 GR splice isoforms were identified in RNA extracted from buffy coat (248, 249). These arose through retention of varying lengths of introns B, D, G, and H, with many lacking the LBD and/or DBD. Only 2 variants have been characterized and whether they were expressed to a greater extent in health or disease was not reported. GR-S1 and GR-S1(-349A) were identified in a single subject, both retain intron H (between exon 8 and 9), with the latter also containing a single nucleotide deletion at position 349 that induces a frameshift with premature stop codon and truncated protein of 118 amino acids (249). These demonstrate 10% and < 1% of the transactivation potential of wild-type GR in a luciferase assay; however, the latter had greater than 10-fold the activity of wild-type at higher hydrocortisone concentrations. This augmented response is abrogated by removing the 3′ UTR, which is known to reduce the stability or interfere with the processing of mRNA. Another of these variants had previously been reported: GRΔ313 to 338 (250). This GR has a 78-bp, 26–amino acid deletion of exon 2, downstream of AF1. Unlike the larger study, this isoform was not detected in any immune cells but was expressed in lung, adipose tissue, thyroid, and salivary gland; no functional studies were undertaken (250).
Alternative translation initiation variants
Translation of mRNA into protein by the ribosomal complex begins at a start codon (AUG) which encodes for methionine. Scanning of mRNA by the 40S subunit of the ribosome stops upon recognition of AUG by the complementary tRNA, instigating formation of the remainder of the ribosome complex and initiation of protein translation (251). The base pair context (Kozak context) in which AUG is placed affects the ability of the 40S subunit to recognize the start codon and begin translation, allowing alternative translation initiation at downstream AUGs (251). The process of ribosomal leaky scanning involves translation initiation at multiple AUGs whereby the 40S subunit will not reliably begin translation at the proximal AUG with suboptimal Kozak context, but it will scan past and commence at a more optimal downstream AUG (252, 253). The frequency of recognition of a start codon can be altered by mRNA secondary structure downstream of the first AUG, availability of eukaryotic initiation factors, or queueing of ribosomes at downstream codons (251-253). Ribosomal shunting is the process of discontinuous scanning of mRNA that allows the 40S subunit to bypass sections of the 5′ UTR directly to downstream AUG codons (252). The mechanisms by which this occurs are not completely understood but may be dependent on upstream short open reading frames and the presence of complex stem-loop structures in mRNA (252-254).
The human GR gene includes 8 N-terminal AUG codons within exon 2. Mutational studies have shown that translation initiation at each of these codons is responsible for the production of N-terminal translational isoforms of GRα: GRα-A (94 kDa; full-length GR), GRα-B (91 kDa), GRα-C1-3 (82-84 kDa), and GRα-D1-3 (53-56 kDa) (194). The Kozak context for the first start codon is suboptimal, and placement within the optimal context leads to an absence of GRα-Β and significant reduction in GRα-C1-3, suggesting that these isoforms are generated through ribosomal leaky scanning (194, 255). However, insertion of scanning inhibitory structures in upstream mRNA at some locations but not others could inhibit expression of GRα-D1-3, in addition to GRα-C1-3, suggesting ribosomal shunting is responsible for translation initiation of both C (in part) and D isoforms (194). As all other GR splice variants described to date contain an intact exon 2, it is expected that alternatively spliced translation initiation isoforms exist (eg, GRβ-C3), resulting in 40 potential isoforms; however, only the GRα translational isoforms have been confirmed (3, 199).
Translation initiation isoforms of GRα have progressively shorter NTD, and truncation of this domain alters interaction with cofactors and the transcriptional machinery (3), but does not affect dexamethasone binding affinity, receptor half-life, or GRE binding capacity (at genes for H+/K+ ATPase subunit α, vitamin D receptor, NF-κB inhibitor α [IκBα], caspase 6, and granzyme A [GZMA]) when expressed alone in U2OS cells (256). While not tested, given that the NTD is involved in AP-1 interaction, it could be expected this function may be altered (177). As a result, these isoforms have been shown to have both shared and unique gene regulatory profiles upon stimulation with glucocorticoid (194, 256-259). The various translational isoforms of GRα contribute to tissue specificity of glucocorticoid activity, leading to an alteration in glucocorticoid response. This is evidenced by varying levels of isoforms expressed in different cells/tissues (194, 198, 257), contrasting effects of some isoforms on cellular function (eg, apoptosis) (258), or differential activation of the same function in different cells (257), and changes in isoform expression occurring during cellular (259) or organ (239) maturation, or in response to different disease states (198).
GRα-A
GRα-A is the canonical full-length GR (777 amino acids, 94 kDa) and much of the earlier described understanding of the function of GR is thought to be mediated by this isoform. Hence only features specific to this isoform are detailed here. As with other isoforms, expression of GRα-A changes through development. In the dorsolateral prefrontal cortex, neuronal GRα-A expression increases from birth through to adolescence where it peaks, before falling and remaining stable throughout life (239). GRα-A protein expression is stimulated in PBMCs by LPS and peptidoglycan but not lipoteichoic acid (260). GRα-A expression is reduced in PBMC of patients with chronic rhinosinusitis and asthma compared to healthy volunteers with no change in GRβ, leading to a reduced GRα-A:GRβ ratio, which is thought to contribute to glucocorticoid resistance in these diseases (261). GRα-A mRNA is elevated compared to controls in PBMC of patients with chronic adrenal insufficiency on glucocorticoid replacement, but not those with acute, induced hypocortisolism (metyrapone test) (217). Furthermore, GRα-A mRNA is reduced in the neutrophils, monocytes, skeletal muscle, and subcutaneous adipose tissues of critically ill patients in an intensive care unit at up to 4 weeks (262). Other GRα translational isoforms were not considered in these studies.
Interestingly, despite being the prototypical isoform, GRα-A expression in the placenta is low relative to other isoforms, making up less than 5% of nuclear receptor expression and even lower in the cytoplasm (198, 199). It thought that due to this, the interaction of GRα-A with other isoforms contributes to the sex-specific response of the fetus to excess glucocorticoid exposure: relative resistance in males (GRβ) and hypersensitivity in females (GRα-C, GRα-D) (263).
GRα-B
GRα-B results from translation initiation at the second AUG codon, producing a 751–amino acid sequence of 91 kDa, retaining all the AF1 domain. GRα-B is expressed in a tissue-specific manner, being highest in the rat liver and thymus, where it is more extensively expressed than GRα-A (194). Additionally, GRα-B is one of the most abundant isoforms in CD3+ T cells (257), and dendritic cells switch isoform expression following maturation to GRα-Α and GRα-Β rendering them sensitive to dexamethasone-induced apoptosis and resistant to glucocorticoid inhibition of antigen uptake (259). Most studies report similar magnitude of transcriptional regulation and apoptosis induction to GRα-A when transfected into cells in isolation using GRE, MMTV, and NF-κB reporters, demonstrated in COS1, U2OS, and Jurkat T-ALL cell lines (194, 256-258). One study found a 1.5- to 2-fold elevation of transcriptional activation compared to GRα-A despite a lower protein expression for equivalent vector transfection (255); this could be explained by the greater expression of an 83-kDa “degradation product” in the GRα-B transfected cells, as this is now known to correspond to the more active GRα-C isoform. Despite a similar magnitude of effect on transcription as GRα-A, GRα-B has been shown to regulate fewer genes and a proportion of distinct genes to other isoforms (194, 256).
GRα-C 1-3
The GRα-C isoforms 1 to 3 are 692–, 688–, and 680–amino acid proteins with molecular weight between 82 and 84 kDa, generated from further downstream start codons (still upstream of the AF1 domain—residues 187-244) (264). GRα-C1 and GRα-C2 isoforms have a similar GRE-driven luciferase reporter response to dexamethasone as GRα-A and GRα-Β in both COS1 and U2OS cells. GRα-C3 appears to be the most transcriptionally active GR isoform, with the greatest luciferase and MMTV reporter activity on dexamethasone stimulation across a range of receptor and dexamethasone levels (194, 264). In addition to being more effective at transactivation, GRα-C3 regulates the most total and distinct genes of any translational isoform (194) and displays a completely different transcriptome to wild-type GR in mouse embryonic fibroblasts (265). This renders GRα-C3-expressing cells more sensitive to glucocorticoids through multiple mechanisms. GR-null osteosarcoma cell lines (which are resistant to glucocorticoid-induced apoptosis) are rendered sensitive by stable-transfection with GRα-C3, and dexamethasone induces apoptosis to a greater extent than GRα-Α through enhanced inhibition of NF-κB and nGRE-mediated inhibition of anti-apoptotic genes Bcl-XL, survivin, and cIAP1 (258). This translates to earlier induction of apoptosis (12 hours vs 20 hours in other isoforms), and a greater extent of apoptosis 48 hours after stimulation (50% vs 30% in GRα-A), and co-expression of GRα-A and GRα-C3 increases cell death by 19% (256). GRα-C3-expressing Jurkat T-ALL cells showed similar rates of dexamethasone-induced apoptosis (50% at 48 hours) with nGRE-mediated inhibition of anti-apoptotic MYC and miR-17, and GRE-mediated expression of pro-apoptotic BIM, miR-15b (257). The underlying mechanism of GRα-C3 hyperactivity compared to other isoforms appears to be due to steric hindrance of residues 98 to 115 (particularly Asp101) which, when unmasked by the shorter N-terminal of GRα-C3, improves recruitment of specific coactivators to the AF1 domain when bound to DNA (particularly CBP/p300) (264).
GRα-C3 is expressed most abundantly in rat pancreas and colon (194), and it is not detected in human dorsolateral prefrontal cortex (239) or in either immature or activated dendritic cells (259). In the placenta, GRα-C3 is higher in female fetuses than male (198), increased in male preterm births compared to term, reduced in female preterm placentae (199), and increased with maternal alcohol intake (244). This isoform represents 10% to 20% of GR in T cells and can be upregulated in response to concanavalin A activation (257). While varying levels of GRα-C3 in tissue is expected to vary the cellular sensitivity to glucocorticoids, this isoform also appears to have both tissue- and gene-specific regulation of target genes. When comparing apoptosis stimulation in Jurkat T-ALL and U2 osteosarcoma cells, apoptosis-related genes AKAP13, ATG12, CDKN2D, GZMA, ING1, ITPR1, SATB1 were commonly regulated by GRα-C3 between cells, but most other modulated apoptosis pathway genes were cell type–specific (257). Furthermore, there appears to be promoter-specific regulation, with higher amounts of CBP/p300 and RNA polymerase II recruitment and acetylated histone H4 at the GZMA promoter when activated by GRα-C3 compared to other isoforms, a change that was not seen for IκBα, which was also regulated by this isoform (256).
GRα-D 1-3
GRα-D1-3 isoforms have the shortest NTD, consisting of sequences of 462, 447, and 441 amino acids, generating proteins of 53 to 56 kDa. Unlike the other translation initiation isoforms, the GRα-D isoforms reside primarily in the nucleus both liganded and unliganded (194, 256). Deletion of the residues 98 to 335 (present in GRα-A, -B, and -C, but not -D) of GRα-A leads to a GR with similar nuclear localization to the GRα-D isoforms, the thought is that changes in the conformation of the protein resulting from this region being absent exposes either the nuclear localization and/or nuclear retention domains (258). GRα-D isoforms have the lowest transactivation activity and regulate the fewest total and distinct genes in COS1 and U2OS cells, and increasing concentrations do not alter GRα-A activity (194, 256). In cell-free in vitro coimmunoprecipitation studies, GRα-D isoforms interact with NF-κB to a similar extent to GR wild-type (258). However, when expressed in GR-null osteosarcoma cells, GRα-D isoforms do not readily interact with the p65 subunit of NF-κB; these isoforms do not induce apoptosis and regulate the fewest apoptosis-related genes (256, 258), and either do not alter basal NF-κΒ reporter activity (258), or reduce it by 50%, associated with dexamethasone inhibition of cytokine (TNF-α, IL-8, granulocyte-macrophage colony stimulating factor [GM-CSF]) release in response to LPS (256). It is postulated that this reduction in NF-κB inhibition is due to the need for GR to be localized in the same compartment as the p65 subunit for optimal interaction of these proteins, the latter of which is predominantly expressed in the cytoplasm (258). Furthermore, Jurkat T-ALL cells transfected with GRα-D3 were also resistant to dexamethasone-induced apoptosis but did downregulate the pro-inflammatory genes inducible T-cell costimulatory, IL-8, TNF-α, TNF-related apoptosis-inducing ligand, TNF superfamily member 14, lymphotoxin-β, GM-CSF commonly to GRα-C3 (257). GRα-D3 appears to also regulate gene expression in a glucocorticoid-independent manner, having been shown to activate certain promoter regions by ChIP-seq, unlike other isoforms (256). Interestingly, a 50-kDa GR protein, likely one of the D isoforms, was upregulated in PBMCs isolated from human donors following incubation with LPS and peptidoglycan, suggesting a potential role in the innate immune response (260).
Like the other translation initiation isoforms, GRα-D isoforms display variation in tissue expression. In rodents GRα-D are expressed to the greatest extent in spleen and bladder, with negligible expression in bone (194, 256). GRα-D isoforms represent 5% of GR expressed in the CD3+ T cells and GRα-D is the predominant isoform expressed in immature dendritic cells (257, 259). In the human cortex, GRα-D is the most abundantly expressed in neonates, before falling through childhood and adolescence with some recovery in adults (239). In keeping with this, samples from neonates < 130 days old displayed strong GR immunohistochemistry staining in the nuclei of pyramidal neurons which progressed to greater soma staining in older tissue samples. Additionally, GRα-D1 is upregulated in the dorsolateral prefrontal and orbitofrontal cortex in bipolar disorder and schizophrenia (266, 267), and in nasal polyps of patients with chronic rhinosinusitis (compared to uncinate mucosa of healthy volunteers) (238). Sexual dimorphism is seen in the brain, with unstressed female mice expressing GRα-D isoforms to a greater extent than males in the hippocampus and prefrontal cortex, with chronic stress leading to a reduction in cytosolic expression in females and increased nuclear expression in males (268). GRα-D isoforms account for the majority of GR expressed in human placenta, with the D1 isoform being the only nuclear isoform detected in human umbilical endothelial cells (198, 199). Finally, gestational alcohol consumption was associated with a reduced frequency of GRα-D detection in placental extract cytoplasm lysate (244).
Exon 1 promoter variants
The GR exon 1 promoter variants demonstrate variable expression between tissues, and are associated with expression of certain GR isoforms, suggesting they may contribute to tissue specificity of glucocorticoid action (152, 155, 156, 269). Variant 1C3 and 1H are the most widely detectable between tissues, and 1D appears to be exclusively expressed in the hippocampus (155). Expression of these exon 1 promoter variants is altered in response to inflammation in human placenta and varies depending on sex of the fetus (150, 152). This is associated with altered protein expression of various GR isoforms depending on sex, with different patterns of change in males and females in response to maternal asthma during pregnancy (152). In multiple cell types, exon 1 variant 1A3 is associated with an increase in GRα-B relative to GRα-A protein expression (157), while 1B is correlated with GR-P, and 1C with GRα mRNA (243). In suicide-completers, increased methylation in the 1J and 1C promoters in prefrontal cortex was associated with reduced GR-P mRNA expression (245). Variants 1B and 1C are downregulated in response to glucocorticoid treatment of trophoblast cell lines in vitro (150), whereas the distal three 1A splice variants and 1I are upregulated by dexamethasone in T but not B-cell ALL lines (154, 156, 270). Using luciferase reporter assays, 1B and 1C (or a combination) demonstrate varying efficacy in driving luciferase activity dependent on cell type (269). Overall, the 1A variants appear to be more responsive to glucocorticoid-mediated regulation than 1B and 1C (157, 270). Transcription factor binding sites have been identified in a number of the promoter regions that may explain the differing tissue distribution of these variant transcripts, including: SP1, AP-1, AP-2, YY1, NGF-1A, NF-κB (p65), IRF 1/2, Nur77, COUP, c-Myb, c-Ets, and nGRE (the latter 3 appear to form a unit mediating glucocorticoid regulation of those exon 1 variant transcripts that are glucocorticoid-responsive) (152, 154, 180, 245, 269, 271). Taken together, these data suggest that the multiple exon 1 promoter variants allow diversification of GR transcriptional regulation between cellular environments and may contribute to splice variant control of downstream isoforms. More work is needed to understand how expression of these exon 1 promoter variants are controlled and how they affect downstream GR isoform protein expression.
Post-Translational Modification
Post-translational modification involves the covalent modification of GR, altering protein stability and interaction with other proteins and hence cellular localization and transcriptional activity (149).
Phosphorylation of GR is the most well-described post-translational modification, which occurs at a number of serine and tyrosine residues, predominantly in the AF1 domain, under the control of phosphorylating kinases (cyclin-dependent kinases, p38 mitogen-activated protein kinases [MAPKs], JNKs, extracellular signal-regulated kinases [ERKs], and glycogen synthase kinase 3β [GSK-3β]) and dephosphorylating phosphatases (protein phosphatase [PP]I1, PP2a, and PP5) (149). The effects of phosphorylation are both site and kinase dependent. Phosphorylation of Ser211 by p38 induces a conformational change in AF1 favoring coregulator recruitment and thereby enhancing transcriptional activity, yet phosphorylation at the same residue by ERK or JNK yields transcriptional activity that is relatively reduced (272). In general, phosphorylation by ERKs, JNKs, or GSK-3β blunts GR activity (149). Concomitant phosphorylation of Ser203 and Ser211 is required for maximal transcriptional activity of GR, whereas phosphorylation of Ser226 and Ser404 reduces transcriptional activity by promoting nuclear export of the receptor (149, 273). Abnormal phosphorylation status of GR has been linked with glucocorticoid resistance in a number of inflammatory diseases (274-276), and maternal asthma is linked to Ser226 phosphorylation in the placenta (198). MAPK phosphatase 1 (MKP1) is upregulated by GR and inhibits MAPK expression, and hence reduces inhibitory GR phosphorylation. Macrophage migration inhibitory factor (MIF) suppressed MKP1 and is thought to antagonize the immunosuppressive effects of glucocorticoids in RA, systemic lupus erythematosus (SLE), asthma, and inflammatory bowel disease (IBD) (277, 278).
Ubiquination involves the attachment of ubiquitin residues to a protein that signals it for proteasomal degradation. The main site for ubiquination of GR resides in the NTD (Lys419, present in all known isoforms) and leads to a reduction in transcriptional activity (due to GR turnover) and export from the nucleus (279-281). SUMOylation is the addition of small ubiquitin-related modifier-1 (SUMO-1) protein to lysine residues that, while related to ubiquitin, do not immediately signal GR for proteasomal degradation. SUMOylation has been demonstrated to increase or decrease transcriptional activity of GR depending on the promoter, modification residue, or tissue (149). Acetylation is a process significantly involved in GR activity in the form of histone (de-)acetylation in response to GR binding GRE; however, the receptor itself can be acetylated. Glucocorticoids induce acetylation in the hinge region of GR on binding (149) and clock gene transcription factors acetylate GR reducing its transcriptional activity at GREs (49). Furthermore, to transrepress NF-κΒ, GR must be deacetylated by histone deacetylase 2 (HDAC2) (49, 282). GR does not undergo S-palmitoylation in a highly conserved region of the LBD, unlike other nuclear receptors (such as the estrogen receptor [ER]), where this post-translational modification is necessary for receptor association with the cell membrane (283, 284). The GR does not appear to undergo palmitoylation at any site (285). Recently, N-homocysteinilation of GR in the LBD has been identified in the rat hypothalamus in a model of perinatal methyl-donor deficiency, associated with reduced GR-GR interaction, nuclear translocation, and GR-responsive Agouti-related protein expression (286). Finally, the impact of nitrosylation on GR function is controversial and the literature has demonstrated conflicting results, while oxidative states appear to reduce the activity of GR through reduction of ligand binding and reducing agents can rescue this (149).
Glucocorticoid Sensitivity
It is known from extensive experience that interindividual differences in sensitivity to both the beneficial and unwanted effects of exogenous glucocorticoids exist (287). This has been demonstrated in healthy individuals based on HPA axis response to dexamethasone suppression or in vitro response of PBMCs to glucocorticoids (288, 289). Furthermore, sensitivity has diurnal and seasonal variation, and is modified by sex and age (76, 290, 291). Clinically, variations in glucocorticoid sensitivity can manifest in an individual being sensitive to the side effects of glucocorticoids, preventing dose escalation necessary for disease control, or presenting as resistance of the disease process to glucocorticoid treatment (275). Much rarer is primary generalized glucocorticoid resistance (Chrousos syndrome) due (predominantly) to inherited (or sporadic) mutations in the GR, rendering individuals partially resistant to glucocorticoid action in target tissues, resulting in compensatory hypercortisolemia without clinical features of Cushing syndrome (292). The clinical features of this condition range from asymptomatic to severe, and result from elevation in adrenal androgens (virilization, precocious puberty, hyperandrogenism, oligo-/amenorrhea, infertility) and mineralocorticoids (hypertension, hypokalemia, alkalosis which may in part relate to excess cortisol activating the MR), driven by ACTH secretion which is resistant to cortisol negative feedback (293). Conversely, a glucocorticoid hypersensitivity syndrome has been described due to an activating point mutation in NR3C1 (D401H in the distal NTD), associated clinically with peripheral manifestations of glucocorticoid excess but elevated cortisol and ACTH (suggestive of tissue selectivity of the mutation, potentially endowing resistance to negative feedback in the hypothalamus/pituitary) (294). Transfection studies demonstrate increased transcriptional activation of the mutant receptor with an additive effect to wild-type GR, but otherwise identical function. Furthermore, transient glucocorticoid hypersensitivity has been reported in a child with a suppressed HPA axis and no GR abnormality identified, with a postulated post-receptor defect as the cause (295).
Glucocorticoid resistance has been described in a wide variety of autoimmune, inflammatory, and malignant disease processes leading to treatment failure. It is estimated to occur in up to 30% of patients (275). This can be primary glucocorticoid resistance, where there is no initial response to therapy, or can be secondary/treatment-emergent resistance, that is, a loss of disease control despite initial response. Primary glucocorticoid resistance has been described in a number of inflammatory diseases including asthma (296, 297), RA (298), IBD (299-301), SLE (302, 303), and multiple sclerosis (304). In asthma, glucocorticoid resistance correlates with severity of disease (305) and smoking (306). Bronchoalveolar lavage fluid of glucocorticoid-resistant patients contains increased inflammatory cells compared to sensitive patients (275), and circulating PBMCs demonstrate in vitro resistance to glucocorticoids, suggesting disease induction of a generalized resistance (307). A similar finding occurs in RA and IBD: glucocorticoid resistance correlates with severe disease and harvested PBMCs are glucocorticoid resistant in vitro (298, 308). The development of resistance to glucocorticoids during treatment occurs more commonly in lymphoproliferative malignancies, particularly at relapse in ALL (309, 310), but has been also demonstrated in other conditions (300, 301). Furthermore, states of glucocorticoid resistance occur in severe systemic inflammatory responses such as acute respiratory distress syndrome (ARDS) and sepsis, generating the concept of critical illness-related corticosteroid insufficiency (CIRCA) (311). An intensely pro-inflammatory insult induces tissue glucocorticoid resistance, at least in part through upregulation of GRβ without adequate compensation from the HPA axis, resulting in what has been termed relative adrenal insufficiency, uncontrolled inflammation, and eventual multi-organ failure (223, 311, 312). This is exacerbated by the decline in CBG, and hence cortisol availability to tissue, that occurs in septic shock, with low plasma concentrations independently associated with mortality (313).
Despite use of glucocorticoids to treat inflammatory diseases for over 70 years, to date there is no accurate, accepted method to measure or predict glucocorticoid sensitivity in patients requiring treatment. This is reviewed by Quax et al, but in summary, the majority of studies assess either GR number, GR binding capacity/affinity, GRβ expression, or in vitro functional bioassays in PBMCs or other circulating leukocytes (287). The results are conflicting and while the functional assays aim to account for all components of glucocorticoid function (eg, GR, coactivators, transcription factor interactions etc), none of the tests directly assess the glucocorticoid response of the underlying diseased tissue (eg, synovium in RA) which may account for the unreliability. The following is a discussion of the main described molecular mechanisms which can alter glucocorticoid sensitivity.
Mechanisms of Glucocorticoid Sensitivity/Resistance and Cellular Specificity
Glucocorticoid Availability
At a pre-receptor level, glucocorticoid metabolism can contribute to altered glucocorticoid sensitivity. The 11β-hydroxysteroid dehydrogenase (11β-HSD) system consists of 2 distinct enzymes that reversibly catalyze the interconversion of the active glucocorticoid cortisol to the inactive cortisone (11β-HSD2) and vice versa (11β-HSD1) (314). 11β-HSD1 has bidirectional activity enzymatic function and intracellular co-localization with hexose-6-phosphate dehydrogenase, which generates an abundance of the reduced form of cofactor nicotinamide adenine dinucleotide phosphate (NADPH), which is necessary for oxoreductase activity (315). At a physiological level, 11β-HSD2 colocalizes with the MR in epithelial cells of the nephron distal tubule and collecting duct, vascular beds, and subpopulations of hypothalamic neurons to protect the MR from activation by cortisol, which has a similar binding affinity to aldosterone (and circulates at a 2 to 3 log-fold increased concentration in the circulation) (143, 316). Upregulation of 11β-HSD1 occurs in response to both inflammation and glucocorticoids in a tissue-specific, synergistic way, with reciprocal inhibition of 11β-HSD2 (314, 317, 318). This leads to 11β-HSD1 potentiating glucocorticoid activity. Conversely, transfection of 11β-HSD2 into cell lines renders them glucocorticoid resistant (319). Furthermore, a relative increase in 11β-HSD2 expression has been demonstrated in a range of inflammatory diseases and tumors/malignancies associated with glucocorticoid resistance (314, 319, 320). Expression of 11β-HSD2 in corticotrope adenomas likely contributes to the pathophysiology by inhibiting negative feedback from hypercortisolism (321). Polymorphisms in the genes encoding these enzymes have been associated with a reduction (11β-HSD1) or increase (11β-HSD2) in glucocorticoid action (322, 323).
In addition to metabolism by 11β-HSD2, glucocorticoid bioavailability may be reduced by P-glycoprotein (multidrug-resistance gene; MDR1) (275). P-glycoprotein is a drug efflux pump responsible for transport of drugs, including glucocorticoids, out of cells. High levels of MDR1 have been demonstrated in lymphocytes from patients with IBD, SLE, and RA who demonstrate glucocorticoid resistance (324-326).
Finally, the response of GR to glucocorticoids is further altered by the affinity and rhythm of the ligand. Higher-affinity glucocorticoids induce a stronger transcriptional output from MMTV reporter assays. Continuous hormone stimulation leads to peak GR occupancy and transcription of the reporter construct after approximately 1 hour of treatment, whereas GR occupancy closely follows hormone exposure in pulsatile stimulation (327, 328). In keeping with this, GR nuclear residence time is shorter at the end of the circadian cycle with shorter transcriptional regulation (329).
Glucocorticoid Receptor Isoform Expression
As the GR mediates the majority of the effects of glucocorticoids, it plays a key role in determining cellular, tissue, and organismal sensitivity to glucocorticoids. Even within the same type of tissue, GR expression can vary dependent on localization; GR mRNA expression in abdominal adipocytes was shown to be greater in females and lower in males, in contrast to gluteal adipocytes which were comparable between sexes (330). The majority of studies published on the role GR plays in sensitivity to glucocorticoids have not considered GR isoforms, which may account for some of the conflicting data (also partly attributable to varying measurement techniques for GR: mRNA, immunohistochemistry, dexamethasone binding assay, flow cytometry). As described above, the individual splice and translational isoforms of GR display differing effects on transcriptional regulation and downstream cellular processes. As yet, there is limited understanding of the complex interplay between these isoforms in vivo. However, with the available evidence, it appears that variability in GR isoform expression contributes to cellular, tissue, and organismal glucocorticoid sensitivity. This is rationalized on the basis of a number of arguments:
-
1.
Variation in GR isoform function
As detailed earlier, the individual GR splice and translation initiation isoforms demonstrate variation in their ability to regulate both activation and repression of gene transcription, and in keeping with the different mechanisms of action, the alteration in efficacy of these functions is not always linked. In addition to the strength of transcriptional regulation, the isoforms display different breadths of gene expression control, with both shared and unique gene regulation between isoforms.
-
1.
Cell type/tissue–specific GR isoform expression
The variation seen in the relative expression of individual native GR isoforms between tissues, and the demonstration of tissue-specific differences in function when expressed in isolation suggests that the relative expression of GR isoforms contributes, at least in part, to the variation in tissue sensitivity to glucocorticoids. How this tissue-selective expression of isoforms is controlled is not yet elucidated completely.
-
1.
Change in GR isoform expression alters cellular sensitivity to glucocorticoids
Through both experimental manipulation and observation of natural phenomenon, it has been demonstrated that changing the balance of GR isoforms alters cellular sensitivity to glucocorticoids. In GR-transfected COS1 and U2OS cells with a stable level of GRα-A, increasing concentrations of GRα-C3 increases transactivation by glucocorticoids (194). Immature dendritic cells are resistant to glucocorticoid-mediated apoptosis yet sensitive to glucocorticoid-mediated inhibition of antigen uptake, and they have been demonstrated to express predominantly GRα-D isoforms (259). With LPS-induced maturation, dendritic cells demonstrated a switch to predominant expression of GRα-A and GRα-B isoforms, bestowing sensitivity of apoptotic mechanisms and resistance to inhibition of antigen uptake when treated with dexamethasone. A recent study demonstrated loss/reduction in GR protein expression is associated with dexamethasone resistance in relapsed T-ALL; however, only GRα-A was assessed with no consideration given to the other isoforms (309). The limitations of GRα mRNA measurement as a surrogate for GR activity has recently been highlighted in neural tissue and cells, with a lack of correlation to target gene expression (331) and an increase GRα-A protein expression following LPS stimulation without a change in mRNA levels (260). This may be in part related to alterations in isoform expression and highlights the need for measurement of GR protein, rather than mRNA, expression.
-
1.
Association between GR isoform expression and phenotypic traits
When arguments 1 to 3 are taken together, they suggest that variations in GR isoform expression contribute to cellular and tissue sensitivity to glucocorticoids. If the relative balance of isoforms is shifted in a specific tissue, such as one involved in a disease process of interest, that could render the disease process more sensitive or resistant to treatment with glucocorticoids, which has been demonstrated as discussed below.
Female fetuses are hyperresponsive to excess cortisol exposure and in keeping with this, the placenta of female offspring have a greater expression of GRα-C3 than males, who are relatively resistant to the effects of excess cortisol and express higher levels of GRβ (198, 263). Furthermore, in male fetuses, GRβ expression is correlated with cord blood cortisol, suggesting that either isoform expression is directly responding to glucocorticoid exposure or greater cortisol levels are generated to compensate for the relative resistance (198).
Elevated GRβ expression (or a reduction in GRα:GRβ ratio) in particular has been widely reported to confer glucocorticoid resistance to disease states, and has been demonstrated in vitro and in vivo (261, 307, 310, 332, 333). A case report of a patient with generalized glucocorticoid resistance but without a detectable GR mutation, and chronic lymphocytic leukemia (CLL), was found to have significantly elevated GRβ and reduced GRα expression in normal B lymphocytes which corresponded to a resistance to glucocorticoid-induced apoptosis in her leukemic lymphocytes despite a retention of sensitivity to other apoptosis inducers (334). This finding of a reduced GRα:GRβ ratio is a repeated finding in leukemic cells, either in T-cell ALL (which is less sensitive to glucocorticoids than B-cell lineage cancers) (310, 335), or in overt glucocorticoid resistance disease (310), although some studies dispute this (336). Furthermore, a reduction in GRα:GRβ ratio has been demonstrated in circulating PBMC of patients with nonhematological conditions, suggesting that systemic inflammation resulting from a localized disease process can affect whole body glucocorticoid sensitivity (261, 337).
In primary blast cells from children with ALL and poor prednisolone clinical response treated ex vivo with dexamethasone, GR-γ was upregulated at 4 hours with maintenance of an almost 2-fold elevation out to 10 hours compared to good clinical responders in whom GR-γ returned to baseline by 10 hours (233). GR-γ has 50% of the transcriptional activity of GRα and as each transcript can only be spliced to one isoform, this suggests reduced glucocorticoid-induced transcriptional activity by the more abundant, less effective isoform contributes to the poor prednisolone response in these patients. In further support of this, GR-γ mRNA expression was found to be correlated with in vitro glucocorticoid resistance to apoptosis in childhood ALL cells (336).
Glucocorticoid Receptor Single Nucleotide Polymorphisms
Single nucleotide polymorphisms (SNPs) in GR have been shown to both increase and reduce transactivation potential of GR, both at baseline and in the presence of glucocorticoids (338). Furthermore, SNPs can confer dose-dependent changes to glucocorticoid sensitivity, with T1463C showing reduced activity compared to GRα and A2297G at baseline and lower concentrations of hydrocortisone, methylprednisolone, and dexamethasone, but significantly more transactivation than both other forms at higher doses of these glucocorticoids (338). Differential effects can also be seen depending on the ligand (339). More than 3000 SNPs have been reported, the majority are nonsynonymous, and while more are described in exons (most commonly affecting the NTD), by population frequency, they more commonly occur in introns (248, 340). While the majority of polymorphisms have not been characterized, the resultant changes in GR function as a result of some SNPs can translate into alterations in glucocorticoid sensitivity for the individual, with clinical features described for a number of more common SNPs.
The ER22/23EK polymorphism occurs in the transactivation domain (exon 2), resulting from a change of 2 adjacent codons from glutamic acid-arginine to glutamic acid-lysine residues (341). PBMCs from SNP carriers, and cell transfection studies, demonstrate decreased GRE-mediated transactivation compared to wild-type GR but no change in transrepression of NF-κB activity (342). The mechanism by which this occurs may be alteration of transcriptional cofactor interactions (while not occurring in the core AF1 region, this mutation may alter protein conformation, and hence interactions), or a relative increase in GRα-Α over GRα-B due to changed mRNA secondary structure (the latter has narrower transactivation but similar transrepressive activity) (255, 340, 342, 343). Additionally, carriers of this SNP show impaired cortisol suppression by dexamethasone (344) associated with lower fasting insulin and low-density lipoprotein cholesterol levels (344, 345), increased insulin sensitivity (344), lower C-reactive protein (CRP; a marker of systemic inflammation) (345), higher lean body mass (males only) (346), taller stature (346, 347), lower risk of dementia (348), and improved longevity (344, 345). These clinical findings are in keeping with resistance to the transactivation effects of GR, while no reliable association has been found with autoimmune or inflammatory diseases (aside from a single study in multiple sclerosis) in keeping with preserved transrepression activity of the GR harboring this SNP (341). In fact, ER22/23EK carriers may be at risk of immunosuppression secondary to higher circulating cortisol levels inducing NF-κB inhibition, as evidenced by higher rates of Staphylococcus aureus nasal carriage (349).
Another well-described SNP in GR is the N363S mutant (point mutation in codon 363 of exon leading to substitution of an asparagine to serine residue) (341). In vitro studies have been conflicting regarding enhanced transactivation ability compared to wild-type receptor with positive (342) and negative (350-352) studies; transrepression of NF-κB has been shown to be no different to wild-type GR (342, 351). Despite this, wild-type GR and N363S variants regulate gene expression differently, with unique genes up/downregulated at baseline and in response to dexamethasone, including apparent dysregulation of genes involved in insulin signaling and pro-inflammatory mediators (351, 353). These differences translate clinically to increased sensitivity to glucocorticoids, with SNP carriers shown to have a greater reduction in serum cortisol and greater increase in serum insulin in response to 0.25 mg dexamethasone (350). This is associated with increased body mass index (350, 354-357), waist-hip ratio (358), total and low-density lipoprotein cholesterol and triglycerides (359, 360), and risk of coronary artery disease (360). In a large population cohort study from the Netherlands, N363S carrier status increased the risk of metabolic syndrome in those ≤ 47 years of age, particularly in men (361). Furthermore, in female children with congenital adrenal hyperplasia, concomitant N363S heterozygosity was associated with milder genital virilization at birth, presumed due to reduced ACTH-stimulated androgen production in utero (362). Similar to the ER22/23EK variant, with no difference in NF-κB inhibition, there is no association seen with the incidence or severity of autoimmune/inflammatory diseases (341).
The BclI polymorphism results from a change in intron 2 that is associated with increased sensitivity to glucocorticoids in response to dexamethasone suppression through an unknown mechanism (341). This has been associated with higher body mass index, waist-to-hip ratio, lower lean muscle mass, higher insulin levels, and hypertension. In contrast to the N363S SNP, carriers of BclI are enriched in those with less severe Graves ophthalmopathy, and those with Crohn disease but not ulcerative colitis (341). Furthermore, SNP carriers with Crohn disease had improved response to glucocorticoid therapy and were less likely to require additional courses of treatment. In keeping with increased sensitivity to glucocorticoids, BclI carriers treated with glucocorticoids have an increased risk of squamous cell carcinoma than noncarriers, a known risk with immunosuppression (341).
An A to G substitution in the 3′ UTR of exon 9β, known as the 9β polymorphism, appears to increase the stability of GRβ mRNA, resulting in increased expression of this splice isoform (341). This results in a reduction in glucocorticoid-mediated transrepression without altering transactivation. As the immunosuppressive effects of GR are predominantly mediated by transrepression, it is unsurprising that this SNP is associated with a reduced risk of Staphylococcus aureus nasal carriage, elevated IL-6, and C-reactive protein, steroid dependence in childhood nephrotic syndrome (363), and increase risk of RA and coronary artery disease (340).
A GR variant with 3 nonsynonymous SNPs (A214G, T962C, A2297G), dubbed GR NS-1, which induces amino acid changes at each of the affected positions, demonstrated double the transactivation potential of wild-type GR in a luciferase reporter assay; however, is suppressed by higher doses of hydrocortisone. This hyperactivity appears to be driven by the most distal SNP when GR constructs with the individual SNPs were tested (364).
Experimental studies of point mutations in the DNA-binding region of GR just distal to the D-box (A465T, GRdim), in which dimerization is attenuated but not absent, have been demonstrated to impair the immunosuppressive effects of glucocorticoids in mice across a range of conditions (30). Using ChIP-Seq in GRdim mouse liver and macrophages, and U2OS cells transfected with an equivalent mutation in human GRα, GR monomers demonstrate differential DNA-binding regions and gene regulation with occupation of GRE half-sites by mutant GR, some which were also activated by GRα (171, 236). A point mutation described in this region (R477H) in humans manifests generalized glucocorticoid resistance and similarly impairs ability of GR to interact with canonical GRE (presumed in part through impaired dimerization) but maintains ligand affinity, coactivator binding and the ability to transrepress NF-κB (365-367).
While little is known about the majority of GR SNPs reported, it is clear from the more commonly studied variants, that glucocorticoid sensitivity can be altered by genomic changes that affect GR function or isoform expression in a dose, ligand, and tissue-specific manner.
Glucocorticoid Receptor Cofactors, Transcription Factors, and Heterocomplex
While the expression of GR isoforms and presence of SNPs clearly alters glucocorticoid sensitivity, other components required for GR function contribute to this complex milieu.
Transcriptional regulation by GR and other nuclear hormone receptors relies on interaction with cofactors which complex upon DNA binding (368). Transcriptional cofactors are multi-protein complexes which are involved in molecular signaling through post-translational modification and enzymatic functions to regulate gene transcription by RNApolII (368, 369). Coactivators such as the steroid receptor coactivator family (SRC-1 and SRC-3) interact with the AF1 and AF2 regions of GR to promote transcriptional activity through histone acetyltransferase activity, thereby increasing gene accessibility to the basal transcriptional machinery (369, 370). Other coactivators have been shown to positively regulate transcriptional activity through remodeling of higher-order chromatin structures, another mechanism by which gene accessibility can be increased (370). Similar to transactivation, transrepression via GR binding to nGRE is dependent on the presence of corepressors such as silencing mediator for retinoid or thyroid-hormone receptors (SMRT) and nuclear receptor co-repressor 1 (NCoR) (173). SMRT and NCoR recruit histone deacetylases to complex with themselves and GR at the nGRE, thereby inhibiting transcription through chromatin remodeling, and hence a reduction in accessibility of the target gene to RNApolII (368, 371). In keeping with this, reduced expression and activity of histone deacetylase 2 (HDAC2) confers glucocorticoid resistance in chronic obstructive pulmonary disease and severe asthma (372). Alterations to the availability of coactivators/corepressors have been shown to affect both the amount of gene transcription in response to ligand binding to nuclear receptors, the potency of ligand (373), and the equilibrium between coactivators and corepressors alters cellular sensitivity to glucocorticoids (374). Additionally, the environment of the cell/host can alter cofactor interactions, such as occurs with the reduction in GR:p300 interactions induced by TNF-α, leading to glucocorticoid resistance in mice and a range of cell lines (375). Conversely, GR sequesters p300 at GR-responsive genes leading to downregulation of transcription at non-GR-bound loci (376). Furthermore, variations in cofactor availability between tissues contributes to tissue specificity of hormone action, for example SRC-1 expression is greater in endometrial than breast tissue and at least in part contributes to the opposing action of tamoxifen (selective estrogen receptor modulator) binding to the ER at these sites (377). Even within the same tissue, different but related cell types recruit different cofactors, as is the case with glial cells and SRCs (378). Finally, recruitment by GR of coactivators and corepressors is ligand-specific, and recruitment of cofactors correlates strongly with transactivation/transrepression efficacy (379). This ligand-selective cofactor recruitment occurs as a result of differing conformation alterations in the LBD that are induced dependent on the ligand (163, 380, 381).
While the availability of coactivators/repressors impacts DNA availability following GR binding, the preexisting chromatin landscape also impacts GR activity. ChIP-seq studies have demonstrated that majority of GR binding induced by dexamethasone occurs at preexisting loci of accessible chromatin between a pair of sites enriched with histone modifications that are associated with transcriptional activation (382-384). This appears to remain true when considering STAT3 tethering/interaction (175). H3K9 acetylation (HK39ac) is preferentially found at the transcriptional start site of genes and is associated with transcriptional activation; however, in one study, this was inversely correlated with GR binding (384). Two enzymes catalyze the majority of H3K9ac (GCN5 and PCAF), and knockdown of these enzymes increased the number of genome-wide GR binding sites suggesting a role in modulating GR activity (384). Histone variant H2A.Z is enriched at GR binding loci and may direct GR:GRE binding when chromatin is accessible (382). Furthermore, cell type–specific differences in GR and other nuclear receptor binding preferentially occur at enhancer sites distal to promoter regions (384, 385), or at open chromatin locations with weaker response element motifs (386), and binding site methylation contributes to inhibition of binding in a cell type–specific manner (385, 386). Conversely, shared nuclear receptor binding sites between cell types tend to occur at higher-affinity response elements that require ligand-mediated chromatin remodeling to access DNA (382, 385, 386). That the pattern of chromatin availability differs between cell types in the unliganded state suggests this contributes to GRE occupancy following glucocorticoid activation. The concept of pioneering transcription factors arises from these data—tissue-specific transcription factors that are required for initial chromatin opening and remodeling and determine the available repertoire of open chromatin, and hence GR regulatory opportunity (387).
Glucocorticoid-activated GR interacts with other transcription factors to modulate their activity through tethering or composite binding as detailed earlier. Changes to the activity of these transcription factors can alter sensitivity to glucocorticoids. Knockout of the closely related MR in keratinocytes reduces GR breadth of genomic binding, and speed and magnitude of gene expression in response to dexamethasone, without altering pattern of differential gene expression (388). LIF is a cytokine of the IL-6 family that acts via the Janus kinase (JAK)/STAT signal transduction pathway leading to STAT3 transcriptional activation. Co-treatment of corticotrope cell lines with a combination of LIF and dexamethasone alters STAT3 and GR recruitment to promoter regions (compared to either ligand independently) and results in synergistic transcriptional regulation with a diversification in gene sets regulated (389). Additionally, in vivo treatment of mice with the combination of LIF and dexamethasone leads to tissue-specific upregulation of pro-inflammatory “Cell Defense” genes (Gene Ontology), in some tissues to a similar or greater extent than LPS treatment (389). The stimulatory/inhibitory effects of GR on other transcription factors can be reciprocal and GR inhibition has been described for NF-κB and AP-1 (390), and upregulation of AP-1 by TNF-α has been shown to contribute to glucocorticoid resistance in patients with asthma (391, 392). While traditionally thought to inhibit NF-κB function, glucocorticoids and medroxyprogesterone acetate act via GR to synergistically upregulate cytokine, chemokine (C-C motif) ligand 20 (CCL20) with inflammatory stimuli in a manner dependent on functional NF-κB transactivation (393). The effect of GR on inflammation is also signal-dependent based on transcription factor interactions: pro-inflammatory genes induced by LPS binding to TLR4 that are downregulated by dexamethasone are glucocorticoid resistant when induced by polyinosinic:polycytidylic acid, which signals through TLR3 and does not upregulate gene expression via NF-κB (394). Furthermore, GR can work synergistically with other nuclear receptors to inhibit NF-κB gene transcription (394, 395).
GR function has also been demonstrated to be altered by RNA binding. Noncoding RNAs (ncRNA) are regulatory molecules, which are not translated into protein, affect cellular function through modifying mRNA transcription, translation, and stability and interacting with and influencing the function, abundance, and subcellular localization of proteins (396, 397). The gene growth arrest–specific 5 (Gas5) includes a noncoding RNA which accumulates in response to nutrient depletion and can bind to ligand-activated GR at the DBD, acting as a “decoy” GRE to prevent GR nuclear translocation and binding to DNA, inhibiting transcriptional activity (398, 399). More recently, it has been demonstrated that GR binds as a monomer to a diverse range of hairpin RNA motifs through interaction with the DBD and the hinge region, resulting in a unique GR conformation and competitive inhibition of double-stranded DNA binding (400). These data demonstrate a structure-specific nature of binding, rather than sequence-specific, voiding the need for a GRE-like motif in the RNA, and has been confirmed using RNA-immunoprecipitation (401). Mutagenesis of a GR which disproportionately impairs GR-RNA binding demonstrated dexamethasone-induced upregulation of a set of genes co-occurring at GR-ChIP sites relative to wild-type GR in U2OS cells, confirming in vivo RNA-mediated inhibition of GR transactivation (401). A subset of genes was identified as upregulated with the mutant GR in the absence of dexamethasone, and not responsive to glucocorticoid stimulation. Enrichment analysis maps these genes to other unrelated transcription factor signaling concentrated in 3 small regions on chromosomes 3, 7, and 8, suggesting chromatin remodeling at these sites in the absence of GR-RNA interaction (401). In addition to impacting GR function, GR can bind a large array of mRNA transcripts and enhance degradation, suggesting a further mechanism by which GR can alter the cellular transcriptome following exposure to glucocorticoids (402). A greater understanding of the impact of RNA on GR function is required, but the available data suggest GR-RNA binding could have significant, likely cell type–specific effects on glucocorticoid sensitivity.
Alterations to the function of the multi-protein heterocomplex that anchors the GR in the cytoplasm prior to ligand binding are another mechanism through which glucocorticoid sensitivity can vary. Chaperone proteins hsp90 and hsp70 in the heterocomplex are not only essential for maintaining GR in the cytoplasm but also necessary to open the hydrophobic ligand-binding pocket and facilitate glucocorticoid binding (hsp90) and subsequently traffic GR to the nucleus (hsp70) (403). Glucocorticoid resistance has been associated with aberrant hsp90 expression, a reduction in the hsp90:GR ratio, and a significant reduction in hsp70 mRNA, while others have found an increase in the hsp90:GR ratio and no difference in hsp90/70 expression in glucocorticoid-resistant tissues (234, 404-408). Two other key components of the chaperone complex are the immunophilins FKBP5 and FKBP4 (409). The former is bound to the GR-hsp90 complex in the unliganded state and reduces hormone affinity, thus assisting with anchoring the complex in the cytoplasm (159, 166, 409). Upon glucocorticoid binding, FKBP5 dissociates, FKBP4 binds and increases hormone-binding affinity and signals nuclear translocation of GR (161, 166, 409). FKBP5 is stimulated by glucocorticoids providing a short negative feedback loop (410-412), and the balance between FKBP5:4 alters GR activity (166, 411, 413). New world primates are glucocorticoid resistant which is reduced by FK506 treatment, demonstrate elevated FKBP5 expression, and cell extracts from a variety of species can reduce GR binding of high affinity ligands (414). Furthermore, overexpression of FKBP5 has been shown to induce glucocorticoid resistance in a mouse asthma model, and knockdown increases glucocorticoid sensitivity of multiple cell types (410, 413). FKBP5 mRNA is increased in neutrophils and monocytes of critically ill patients during the first week of admission to the intensive care unit associated with a reduction in GILZ expression in the former cell type (262).
Taken together, the complex interplay of GR interactions with chaperone proteins, transcriptional coactivators and corepressors, and other transcription factors modulate GR activity, contribute to cell/tissue specificity of glucocorticoid action, and have been demonstrated to alter glucocorticoid sensitivity and contribute to glucocorticoid resistance in disease states.
Selective GR Agonists/Modulators
After the precedent set by the development and progression to widespread clinical use of selective estrogen receptor modulators (SERMs), which display estrogen receptor agonist or antagonist activity dependent on the tissue (415), much effort has been made to develop selective GR agonists or modulators (SEGRAMs). Many molecules have been designed and investigated, with very few progressing to clinical trials, and none yet assessed in a phase III trial or marketed to date.
The goal of separating the beneficial anti-inflammatory effects from the adverse metabolic effects arose from the traditional understanding that the former were due to GR-mediated transrepression, and the latter transactivation. However, this dichotomous view of GR function is inaccurate in its simplicity, and as outlined in this paper, many components of the GR signaling pathway are involved in determining the downstream effects. Systematic reviews in asthma and RA have considered a total of 15 phase 1 and 2 trials for 4 agents, the majority dose-finding phase I studies (416, 417). AD5423 was developed for respiratory use and demonstrated improved sputum eosinophilia and lung function in allergic asthma, and while being found to have limited adverse effects compared to budesonide, demonstrated no benefits to lung function in a population with chronic obstructive pulmonary disease (418, 419). AD7594 was similarly investigated for use in asthma and demonstrated improvements in spirometry, symptoms, and rescue inhaler use compared to placebo, with no difference in serum cortisol or other markers of systemic glucocorticoid activity; it has not been compared to glucocorticoids (420). GW80086X improved lung function compared to placebo in some studies, with no evidence of reduced urinary free cortisol, but it is no longer under development (416). Fosdagrocorat was developed in an oral formulation to treat RA and was found to yield nonsignificant improvements in glucose metabolism at equipotent anti-inflammatory doses to prednisolone; its development has been discontinued (421, 422). Mapracorat (ZK245186) is a nonsteroidal SEGRAM with demonstrated preclinical in vivo anti-inflammatory actions and lower adverse effects compared to glucocorticoids; it progressed to clinical trials completed between 2013 to 2020 which have not been reported (14, 423). Recorilant is unique among SEGRAMs in its development as a competitive antagonist of GR for treatment of endogenous hypercortisolism; a dose-finding phase 2 study demonstrated improvement in clinical features of Cushing syndrome in both low- and high-dose arms (424).
Other mechanisms of achieving the desired effect of glucocorticoids while improving their safety have been attempted with variable success. The importance of 11β-HSD1 in local tissue cortisol generation has been targeted, with a range of molecules unable to provide sufficient benefit through enzymatic inhibition secondary to invariable compensation by the HPA axis to restore tissue cortisol exposure (14). A number of phytochemicals and their metabolites from plants used in traditional medicines or proven/purported to have beneficial health effects have been tested for GR activity due to the structural similarity to glucocorticoids. Compound A is a stable analogue of a molecule from the Salsola tuberculatiformis Botschantzev shrub which has demonstrated in animal studies potent anti-inflammatory effects with limited metabolic adverse effects (425, 426); however, more recent data suggest that it alters cofactor recruitment rather than activating GR, its binding to which is also in question (14). A more detailed discussion is outside of the scope of this review and is covered in depth elsewhere (14, 426); however, to the best of our knowledge, no SEGRAM investigation has considered GR isoforms and the implications on the molecule's function.
Future Directions
Our understanding of GR function has progressed in keeping with advances in molecular bioscience techniques over the past decades. As a key variation from canonical GR, expression of the described GR isoforms appears to have a significant role in enhancing, attenuating, or diversifying GR function in a tissue-specific manner under the influence of the complex interplay of other components of GR signaling. Knowledge of their individual physiological roles is limited and the majority of evidence available comes from cell culture experiments with isolated isoform expression or overexpression. Further work is needed to elucidate both the functions of the individual isoforms (gene regulation, cofactor interactions, nongenomic effects), and how these interact to orchestrate a coordinated response to glucocorticoids. There is an importance to understanding this milieu across all tissues, and at organismal level, in both physiological and pathological environments, and the implications of differences between the two on our current model for glucocorticoid activity in both health and disease. Very little is known about sexual dimorphism in the expression and function of the GR isoforms. Further elucidation is required, which could be expected to complicate interpretation of this model. As a key regulator of glucocorticoid sensitivity, future attempts to quantify sensitivity to glucocorticoids should take into account isoform expression and might elucidate some of the factors contributing to prior conflicting results. Progress in this field may have translational implications: if our understanding of isoform function improves, and the regulation of isoform expression is elucidated, it may provide an avenue to develop treatments that could either be used as an adjunct to glucocorticoids to enhance the beneficial and minimize the harmful effects, or to provide an avenue for effective, isoform-specific SEGRA medications.
Conclusion
The traditional paradigm of GR signaling has been replaced by a significantly more complex system, which coalesces to contribute to varied glucocorticoid sensitivity between cell types, tissues, organisms, and between individuals of the same species. The identification of GR isoforms and elucidation of their function, along with a number of advances in our understanding of GR molecular mechanisms, lays a solid foundation on which further knowledge can be built toward truly understanding the diverse functions of glucocorticoids. Consequently, this understanding will allow the development of tailored, safer treatment regimens and newer agents that maximize advantageous therapeutic benefits while minimizing patient harm.
Abbreviations
- 11β-HSD
11β-hydroxysteroid dehydrogenase
- ACTH
adrenocorticotropic hormone
- AF1
activation function 1
- ALL
acute lymphoblastic leukemia
- AP-1
activator protein 1
- AVP
arginine vasopressin
- bp
base pair
- CBG
corticosteroid-binding globulin
- ChIP
chromatin immunoprecipitation
- ChIP-seq
chromatin immunoprecipitation sequencing
- CRH
corticotropin-releasing hormone
- DBD
DNA-binding domain
- ERK
extracellular signal-regulated kinase
- FKBP5
FK506-binding protein 5
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- HPA
hypothalamic-pituitary-adrenal
- hsp70/90
heat shock protein 70/90
- IL-
interleukin
- JNK
c-Jun N-terminal kinase
- LBD
ligand-binding domain
- LIF
leukemia inhibitory factor
- LPS
lipopolysaccharide
- MAPK
p38 mitogen-activated protein kinase
- miR
microRNA
- MMTV
mouse mammary tumor virus
- MR
mineralocorticoid receptor
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells (nuclear factor kappa-B)
- nGRE
negative glucocorticoid response element
- NLS1
nuclear localization signal 1
- NR3C1
nuclear receptor subfamily 3 group C member 1 (GR gene)
- NTD
N-terminal domain
- PBMC
peripheral blood mononuclear cell
- POMC
pro-opiomelanocortin
- PVN
paraventricular nucleus
- RA
rheumatoid arthritis
- RNApolII
RNA polymerase II
- SEGRA(M)
selective glucocorticoid receptor agonist (or modulator)
- SNP
single nucleotide polymorphism
- SRC
steroid receptor coactivator
- SRp
serine/arginine-rich protein
- StAR
steroidogenic acute regulatory protein
- TNF
tumor necrosis factor
- UTR
untranslated region
Contributor Information
Jack Lockett, Mater Research Institute, The University of Queensland, Translational Research Institute, Woolloongabba, QLD 4101, Australia; Faculty of Medicine, The University of Queensland, Herston, QLD 4006, Australia; Department of Diabetes and Endocrinology, Princess Alexandra Hospital, Metro South Health, Woolloongabba, QLD 4102, Australia.
Warrick J Inder, Faculty of Medicine, The University of Queensland, Herston, QLD 4006, Australia; Department of Diabetes and Endocrinology, Princess Alexandra Hospital, Metro South Health, Woolloongabba, QLD 4102, Australia.
Vicki L Clifton, Mater Research Institute, The University of Queensland, Translational Research Institute, Woolloongabba, QLD 4101, Australia.
Funding
J.L. receives scholarships from The University of Queensland and Mater Research Institute for his research higher degree. W.J.I., J.L., and V.L.C. are supported by a Research Support Scheme Project Grant from Metro South Health for this and ongoing work. V.L.C. is funded by a Senior Research Fellowship (APP1136100) from National Health and Medical Research Council and an Amplify Fellowship from The University of Queensland.
Disclosures
All authors have no disclosures related to this work.
References
- 1. Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351‐1362. [DOI] [PubMed] [Google Scholar]
- 2. Ramamoorthy S, Cidlowski JA. Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin North Am. 2016;42(1):15‐31, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cain DW, Cidlowski JA. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29(4):545‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galon J, Franchimont D, Hiroi N, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16(1):61‐71. [DOI] [PubMed] [Google Scholar]
- 5. Bhaumik S, Lockett J, Cuffe J, Clifton VL. Glucocorticoids and their receptor isoforms: roles in female reproduction, pregnancy, and foetal development. Biology (Basel). 2023;12(8):1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93(2):105‐111. [DOI] [PubMed] [Google Scholar]
- 8. Laugesen K, Jørgensen JOL, Petersen I, Sørensen HT. Fifteen-year nationwide trends in systemic glucocorticoid drug use in Denmark. Eur J Endocrinol. 2019;181(3):267‐273. [DOI] [PubMed] [Google Scholar]
- 9. Oh TK, Song IA. Trends in long-term glucocorticoid use and risk of 5-year mortality: a historical cohort study in South Korea. Endocrine. 2020;69(3):634‐641. [DOI] [PubMed] [Google Scholar]
- 10. Mebrahtu TF, Morgan AW, Keeley A, Baxter PD, Stewart PM, Pujades-Rodriguez M. Dose dependency of iatrogenic glucocorticoid excess and adrenal insufficiency and mortality: a cohort study in England. J Clin Endocrinol Metab. 2019;104(9):3757‐3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H, Ryu J, Nam E, et al. Increased mortality in patients with corticosteroid-dependent asthma: a nationwide population-based study. Eur Respir J. 2019;54(5):1900804. [DOI] [PubMed] [Google Scholar]
- 12. Movahedi M, Costello R, Lunt M, Pye SR, Sergeant JC, Dixon WG. Oral glucocorticoid therapy and all-cause and cause-specific mortality in patients with rheumatoid arthritis: a retrospective cohort study. Eur J Epidemiol. 2016;31(10):1045‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis JD, Scott FI, Brensinger CM, et al. Increased mortality rates with prolonged corticosteroid therapy when compared with antitumor necrosis factor-α-directed therapy for inflammatory bowel disease. Am J Gastroenterol. 2018;113(3):405‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pofi R, Caratti G, Ray DW, Tomlinson JW. Treating the side effects of exogenous glucocorticoids; can we separate the good from the bad? Endocr Rev. 2023;44(6):975‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord. 2007;8(4):289‐300. [DOI] [PubMed] [Google Scholar]
- 16. Tsigos C, Chrousos GP. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am. 1994;23(3):451‐466. [PubMed] [Google Scholar]
- 17. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374‐381. [DOI] [PubMed] [Google Scholar]
- 18. Lundblad JR, Roberts JL. Regulation of proopiomelanocortin gene expression in pituitary. Endocr Rev. 1988;9(1):135‐158. [DOI] [PubMed] [Google Scholar]
- 19. Vale W, Vaughan J, Smith M, Yamamoto G, Rivier J, Rivier C. Effects of synthetic ovine corticotropin-releasing factor, glucocorticoids, catecholamines, neurohypophysial peptides, and other substances on cultured corticotropic cells. Endocrinology. 1983;113(3):1121‐1131. [DOI] [PubMed] [Google Scholar]
- 20. Rotondo F, Butz H, Syro LV, et al. Arginine vasopressin (AVP): a review of its historical perspectives, current research and multifunctional role in the hypothalamo-hypophysial system. Pituitary. 2016;19(4):345‐355. [DOI] [PubMed] [Google Scholar]
- 21. Fridmanis D, Roga A, Klovins J. ACTH receptor (MC2R) specificity: what do we know about underlying molecular mechanisms? Front Endocrinol (Lausanne). 2017;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Breuner CW, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J Endocrinol. 2002;175(1):99‐112. [DOI] [PubMed] [Google Scholar]
- 23. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin Chim Acta. 2005;359(1-2):189‐194. [DOI] [PubMed] [Google Scholar]
- 24. Bae YJ, Kratzsch J. Corticosteroid-binding globulin: modulating mechanisms of bioavailability of cortisol and its clinical implications. Best Pract Res Clin Endocrinol Metab. 2015;29(5):761‐772. [DOI] [PubMed] [Google Scholar]
- 25. Meyer EJ, Nenke MA, Lewis JG, Torpy DJ. Corticosteroid-binding globulin: acute and chronic inflammation. Expert Rev Endocrinol Metab. 2017;12(4):241‐251. [DOI] [PubMed] [Google Scholar]
- 26. Silverman AJ, Hou-Yu A, Chen WP. Corticotropin-releasing factor synapses within the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 1989;49(3):291‐299. [DOI] [PubMed] [Google Scholar]
- 27. Morris DG, Kola B, Borboli N, et al. Identification of adrenocorticotropin receptor messenger ribonucleic acid in the human pituitary and its loss of expression in pituitary adenomas. J Clin Endocrinol Metab. 2003;88(12):6080‐6087. [DOI] [PubMed] [Google Scholar]
- 28. Suda T, Tomori N, Yajima F, et al. A short negative feedback mechanism regulating corticotropin-releasing hormone release. J Clin Endocrinol Metab. 1987;64(5):909‐913. [DOI] [PubMed] [Google Scholar]
- 29. Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1‐24. [DOI] [PubMed] [Google Scholar]
- 30. Langgartner D, Koenen M, Kupfer S, et al. Intact GR dimerization is critical for restraining plasma ACTH levels during chronic psychosocial stress. Neurobiol Stress. 2023;24:100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell GM, Henley DE, Leendertz J, et al. Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. J Neurosci. 2010;30(17):6106‐6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sacre K, Dehoux M, Chauveheid MP, et al. Pituitary-adrenal function after prolonged glucocorticoid therapy for systemic inflammatory disorders: an observational study. J Clin Endocrinol Metab. 2013;98(8):3199‐3205. [DOI] [PubMed] [Google Scholar]
- 33. Schlaghecke R, Kornely E, Santen RT, Ridderskamp P. The effect of long-term glucocorticoid therapy on pituitary-adrenal responses to exogenous corticotropin-releasing hormone. N Engl J Med. 1992;326(4):226‐230. [DOI] [PubMed] [Google Scholar]
- 34. Livanou T, Ferriman D, James VH. Recovery of hypothalamo-pituitary-adrenal function after corticosteroid therapy. Lancet. 1967;2(7521):856‐859. [DOI] [PubMed] [Google Scholar]
- 35. Henzen C, Suter A, Lerch E, Urbinelli R, Schorno XH, Briner VA. Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet. 2000;355(9203):542‐545. [DOI] [PubMed] [Google Scholar]
- 36. Han HS, Park JC, Park SY, et al. A prospective multicenter study evaluating secondary adrenal suppression after antiemetic dexamethasone therapy in cancer patients receiving chemotherapy: a Korean south west oncology group study. Oncologist. 2015;20(12):1432‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Korbonits M, Kaltsas G, Perry LA, et al. The growth hormone secretagogue hexarelin stimulates the hypothalamo-pituitary-adrenal axis via arginine vasopressin. J Clin Endocrinol Metab. 1999;84(7):2489‐2495. [DOI] [PubMed] [Google Scholar]
- 38. Malendowicz LK, Rucinski M, Belloni AS, Ziolkowska A, Nussdorfer GG. Leptin and the regulation of the hypothalamic-pituitary-adrenal axis. Int Rev Cytol. 2007;263:63‐102. [DOI] [PubMed] [Google Scholar]
- 39. Ray DW, Ren SG, Melmed S. Leukemia inhibitory factor (LIF) stimulates proopiomelanocortin (POMC) expression in a corticotroph cell line. Role of STAT pathway. J Clin Invest. 1996;97(8):1852‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bateman A, Singh A, Kral T, Solomon S. The immune-hypothalamic-pituitary-adrenal axis. Endocr Rev. 1989;10(1):92‐112. [DOI] [PubMed] [Google Scholar]
- 41. Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33(1):14‐22. [DOI] [PubMed] [Google Scholar]
- 42. Kunz S, Wang X, Ferrari U, et al. Age- and sex-adjusted reference intervals for steroid hormones measured by liquid chromatography-tandem mass spectrometry using a widely available kit. Endocr Connect. 2024;13(1):e230225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nomura S, Fujitaka M, Sakura N, Ueda K. Circadian rhythms in plasma cortisone and cortisol and the cortisone/cortisol ratio. Clin Chim Acta. 1997;266(2):83‐91. [DOI] [PubMed] [Google Scholar]
- 44. Vermes I, Beishuizen A, Hampsink RM, Haanen C. Dissociation of plasma adrenocorticotropin and cortisol levels in critically ill patients: possible role of endothelin and atrial natriuretic hormone. J Clin Endocrinol Metab. 1995;80(4):1238‐1242. [DOI] [PubMed] [Google Scholar]
- 45. Annane D, Sébille V, Troché G, Raphaël JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283(8):1038‐1045. [DOI] [PubMed] [Google Scholar]
- 46. Widmer IE, Puder JJ, König C, et al. Cortisol response in relation to the severity of stress and illness. J Clin Endocrinol Metab. 2005;90(8):4579‐4586. [DOI] [PubMed] [Google Scholar]
- 47. Upton TJ, Zavala E, Methlie P, et al. High-resolution daily profiles of tissue adrenal steroids by portable automated collection. Sci Transl Med. 2023;15(701):eadg8464. [DOI] [PubMed] [Google Scholar]
- 48. Horrocks PM, Jones AF, Ratcliffe WA, et al. Patterns of ACTH and cortisol pulsatility over twenty-four hours in normal males and females. Clin Endocrinol (Oxf). 1990;32(1):127‐134. [DOI] [PubMed] [Google Scholar]
- 49. Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab. 2010;21(5):277‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicolaides NC, Charmandari E, Kino T, Chrousos GP. Stress-related and circadian secretion and target tissue actions of glucocorticoids: impact on health. Front Endocrinol (Lausanne). 2017;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Son GH, Chung S, Choe HK, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008;105(52):20970‐20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344‐2347. [DOI] [PubMed] [Google Scholar]
- 53. Johnston DG, Alberti KG, Nattrass M, Barnes AJ, Bloom SR, Joplin GF. Hormonal and metabolic rhythms in Cushing's syndrome. Metabolism. 1980;29(11):1046‐1052. [DOI] [PubMed] [Google Scholar]
- 54. Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113‐132. [DOI] [PubMed] [Google Scholar]
- 55. Goel N, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Compr Physiol. 2014;4(3):1121‐1155. [DOI] [PubMed] [Google Scholar]
- 56. Lyraki R, Schedl A. The sexually dimorphic adrenal cortex: implications for adrenal disease. Int J Mol Sci. 2021;22(9):4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Born J, Ditschuneit I, Schreiber M, Dodt C, Fehm HL. Effects of age and gender on pituitary-adrenocortical responsiveness in humans. Eur J Endocrinol. 1995;132(6):705‐711. [DOI] [PubMed] [Google Scholar]
- 58. Klusmann H, Schulze L, Engel S, et al. HPA axis activity across the menstrual cycle—a systematic review and meta-analysis of longitudinal studies. Front Neuroendocrinol. 2022;66:100998. [DOI] [PubMed] [Google Scholar]
- 59. Hamidovic A, Karapetyan K, Serdarevic F, Choi SH, Eisenlohr-Moul T, Pinna G. Higher circulating cortisol in the follicular vs. luteal phase of the menstrual cycle: a meta-analysis. Front Endocrinol. 2020;11:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lockett J, Inder W, McIntyre DH, Barrett HL, Kumar S, Clifton VL. Adrenal Disease in Pregnancy. In: Petraglia F, Di Tommaso M, Mecacci F, eds. Hormones and Pregnancy: Basic Science and Clinical Implications. 1st ed. Cambridge University Press; 2022:164. [Google Scholar]
- 61. Stolk RP, Lamberts SW, de Jong FH, Pols HA, Grobbee DE. Gender differences in the associations between cortisol and insulin in healthy subjects. J Endocrinol. 1996;149(2):313‐318. [DOI] [PubMed] [Google Scholar]
- 62. Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81(7):2468‐2473. [DOI] [PubMed] [Google Scholar]
- 63. Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154‐162. [DOI] [PubMed] [Google Scholar]
- 64. Liu JJW, Ein N, Peck K, Huang V, Pruessner JC, Vickers K. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): a meta-analysis. Psychoneuroendocrinology. 2017;82:26‐37. [DOI] [PubMed] [Google Scholar]
- 65. Uhart M, Chong RY, Oswald L, Lin P-I, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31(5):642‐652. [DOI] [PubMed] [Google Scholar]
- 66. Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52(4):318‐327. [DOI] [PubMed] [Google Scholar]
- 67. Blackhart GC, Eckel LA, Tice DM. Salivary cortisol in response to acute social rejection and acceptance by peers. Biol Psychol. 2007;75(3):267‐276. [DOI] [PubMed] [Google Scholar]
- 68. Linnen A-M, Ellenbogen MA, Cardoso C, Joober R. Intranasal oxytocin and salivary cortisol concentrations during social rejection in university students. Stress. 2012;15(4):393‐402. [DOI] [PubMed] [Google Scholar]
- 69. Kirschbaum C, Wüst S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J Clin Endocrinol Metab. 1992;75(6):1526‐1530. [DOI] [PubMed] [Google Scholar]
- 70. Friedmann B, Kindermann W. Energy metabolism and regulatory hormones in women and men during endurance exercise. Eur J Appl Physiol. 1989;59(1-2):1‐9. [DOI] [PubMed] [Google Scholar]
- 71. Gerra G, Volpi R, Delsignore R, et al. Sex-related responses of beta-endorphin, ACTH, GH and PRL to cold exposure in humans. Acta Endocrinol (Copenh). 1992;126(1):24‐28. [DOI] [PubMed] [Google Scholar]
- 72. Jezová D, Kvetnanský R, Vigas M. Sex differences in endocrine response to hyperthermia in sauna. Acta Physiol Scand. 1994;150(3):293‐298. [DOI] [PubMed] [Google Scholar]
- 73. Zimmer C, Basler H-D, Vedder H, Lautenbacher S. Sex differences in cortisol response to noxious stress. Clin J Pain. 2003;19(4):233‐239. [DOI] [PubMed] [Google Scholar]
- 74. Gallucci WT, Baum A, Laue L, et al. Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health Psychol. 1993;12(5):420‐425. [DOI] [PubMed] [Google Scholar]
- 75. Vicennati V, Ceroni L, Genghini S, Patton L, Pagotto U, Pasquali R. Sex difference in the relationship between the hypothalamic-pituitary-adrenal axis and sex hormones in obesity. Obesity (Silver Spring). 2006;14(2):235‐243. [DOI] [PubMed] [Google Scholar]
- 76. Heuser IJ, Gotthardt U, Schweiger U, et al. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol Aging. 1994;15(2):227‐231. [DOI] [PubMed] [Google Scholar]
- 77. Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30(1):80‐91. [DOI] [PubMed] [Google Scholar]
- 78. Greenspan SL, Rowe JW, Maitland LA, McAloon-Dyke M, Elahi D. The pituitary-adrenal glucocorticoid response is altered by gender and disease. J Gerontol. 1993;48(3):M72‐M77. [DOI] [PubMed] [Google Scholar]
- 79. Udelsman R, Norton JA, Jelenich SE, et al. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab. 1987;64(5):986‐994. [DOI] [PubMed] [Google Scholar]
- 80. Vaughan GM, Becker RA, Allen JP, Goodwin CW Jr, Pruitt B Jr, Mason A Jr. Cortisol and corticotrophin in burned patients. J Trauma. 1982;22(4):263‐273. [DOI] [PubMed] [Google Scholar]
- 81. Fish HR, Chernow B, O'Brian JT. Endocrine and neurophysiologic responses of the pituitary to insulin-induced hypoglycemia: a review. Metabolism. 1986;35(8):763‐780. [DOI] [PubMed] [Google Scholar]
- 82. Luger A, Deuster PA, Kyle SB, et al. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise. Physiologic adaptations to physical training. N Engl J Med. 1987;316(21):1309‐1315. [DOI] [PubMed] [Google Scholar]
- 83. Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15(4):321‐350. [DOI] [PubMed] [Google Scholar]
- 84. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888‐3921. [DOI] [PubMed] [Google Scholar]
- 85. Plumpton FS, Besser GM. The adrenocortical response to surgery and insulin-induced hypoglycemia in corticosteroid-treated and normal subjects. Br J Surg. 1968;55(11):857. [PubMed] [Google Scholar]
- 86. Watts LM, Manchem VP, Leedom TA, et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes. 2005;54(6):1846‐1853. [DOI] [PubMed] [Google Scholar]
- 87. Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol. 2005;40(3):129‐154. [DOI] [PubMed] [Google Scholar]
- 88. Olefsky JM. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975;56(6):1499‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schneiter P, Tappy L. Kinetics of dexamethasone-induced alterations of glucose metabolism in healthy humans. Am J Physiol. 1998;275(5):E806‐E813. [DOI] [PubMed] [Google Scholar]
- 90. Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab. 2011;96(6):1789‐1796. [DOI] [PubMed] [Google Scholar]
- 91. Hauner H, Entenmann G, Wabitsch M, et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest. 1989;84(5):1663‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rebuffé-Scrive M, Krotkiewski M, Elfverson J, Björntorp P. Muscle and adipose tissue morphology and metabolism in Cushing's syndrome. J Clin Endocrinol Metab. 1988;67(6):1122‐1128. [DOI] [PubMed] [Google Scholar]
- 93. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102(2):274‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420‐426. [DOI] [PubMed] [Google Scholar]
- 95. Kyrou I, Tsigos C. Stress mechanisms and metabolic complications. Horm Metab Res. 2007;39(6):430‐438. [DOI] [PubMed] [Google Scholar]
- 96. Canalis E. Clinical review 83: mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996;81(10):3441‐3447. [DOI] [PubMed] [Google Scholar]
- 97. van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13(10):777‐787. [DOI] [PubMed] [Google Scholar]
- 98. Bowyer SL, LaMothe MP, Hollister JR. Steroid myopathy: incidence and detection in a population with asthma. J Allergy Clin Immunol. 1985;76(2 Pt 1):234‐242. [DOI] [PubMed] [Google Scholar]
- 99. Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78(1):71‐100. [PMC free article] [PubMed] [Google Scholar]
- 100. Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing's syndrome. J Clin Endocrinol Metab. 1986;62(2):275‐279. [DOI] [PubMed] [Google Scholar]
- 101. Marver D. Evidence of corticosteroid action along the nephron. Am J Physiol. 1984;246(2 Pt 2):F111‐F123. [DOI] [PubMed] [Google Scholar]
- 102. Quinkler M, Stewart PM. Hypertension and the cortisol-cortisone shuttle. J Clin Endocrinol Metab. 2003;88(6):2384‐2392. [DOI] [PubMed] [Google Scholar]
- 103. Baylis C, Brenner BM. Mechanism of the glucocorticoid-induced increase in glomerular filtration rate. Am J Physiol. 1978;234(2):F166‐F170. [DOI] [PubMed] [Google Scholar]
- 104. Ballard PL, Ertsey R, Gonzales LW, Gonzales J. Transcriptional regulation of human pulmonary surfactant proteins SP-B and SP-C by glucocorticoids. Am J Respir Cell Mol Biol. 1996;14(6):599‐607. [DOI] [PubMed] [Google Scholar]
- 105. Grier DG, Halliday HL. Effects of glucocorticoids on fetal and neonatal lung development. Treat Respir Med. 2004;3(5):295‐306. [DOI] [PubMed] [Google Scholar]
- 106. McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66(4):1121‐1188. [DOI] [PubMed] [Google Scholar]
- 107. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361‐1367. [DOI] [PubMed] [Google Scholar]
- 108. Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69‐73. [DOI] [PubMed] [Google Scholar]
- 109. Geraghty AC, Kaufer D. Glucocorticoid regulation of reproduction. Adv Exp Med Biol. 2015;872:253‐278. [DOI] [PubMed] [Google Scholar]
- 110. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865‐871. [DOI] [PubMed] [Google Scholar]
- 111. Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24(3):109‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chinenov Y, Rogatsky I. Glucocorticoids and the innate immune system: crosstalk with the toll-like receptor signaling network. Mol Cell Endocrinol. 2007;275(1-2):30‐42. [DOI] [PubMed] [Google Scholar]
- 113. Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286(44):38703‐38713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Besedovsky HO, del Rey A, Klusman I, Furukawa H, Monge Arditi G, Kabiersch A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J Steroid Biochem Mol Biol. 1991;40(4-6):613‐618. [DOI] [PubMed] [Google Scholar]
- 115. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Galati A, Brown ES, Bove R, Vaidya A, Gelfand J. Glucocorticoids for therapeutic immunosuppression: clinical pearls for the practicing neurologist. J Neurol Sci. 2021;430:120004. [DOI] [PubMed] [Google Scholar]
- 117. Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84(3):304‐315. [DOI] [PubMed] [Google Scholar]
- 118. Settipane GA, Pudupakkam RK, McGowan JH. Corticosteroid effect on immunoglobulins. J Allergy Clin Immunol. 1978;62(3):162‐166. [DOI] [PubMed] [Google Scholar]
- 119. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954‐963. [DOI] [PubMed] [Google Scholar]
- 120. Sakuma Y, Katoh T, Owada K, et al. Initial functional status predicts infections during steroid therapy for renal diseases. Clin Nephrol. 2005;63(2):68‐73. [DOI] [PubMed] [Google Scholar]
- 121. Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocortical hormone in arthritis: preliminary report. Ann Rheum Dis. 1949;8(2):97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mantilla EC Jr, Abramowitz J, Dan TU, Pan E. Prolonged steroid dependence in adult patients with glioma. Anticancer Res. 2020;40(4):2059‐2064. [DOI] [PubMed] [Google Scholar]
- 123. Lim MA, Kohli J, Bloom RD. Immunosuppression for kidney transplantation: where are we now and where are we going? Transplant Rev (Orlando). 2017;31(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 124. DREAMS Trial Collaborators and West Midlands Research Collaborative . Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS trial). BMJ. 2017;357:j1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Swyer GI. Addison's disease. Br Med J. 1979;2(6181):25‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Isidori AM, Arnaldi G, Boscaro M, et al. Towards the tailoring of glucocorticoid replacement in adrenal insufficiency: the Italian Society of Endocrinology Expert Opinion. J Endocrinol Invest. 2020;43(5):683‐696. [DOI] [PubMed] [Google Scholar]
- 128. Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63(6):655‐670. [DOI] [PubMed] [Google Scholar]
- 129. Wilson AM, Lipworth BJ. Short-term dose-response relationships for the relative systemic effects of oral prednisolone and inhaled fluticasone in asthmatic adults. Br J Clin Pharmacol. 1999;48(4):579‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jennings BH, Andersson KE, Johansson SA. Assessment of systemic effects of inhaled glucocorticosteroids: comparison of the effects of inhaled budesonide and oral prednisolone on adrenal function and markers of bone turnover. Eur J Clin Pharmacol. 1991;40(1):77‐82. [DOI] [PubMed] [Google Scholar]
- 131. Newell-Price JDC, Auchus RJ. The adrenal cortex. In: Melmed S, Auchus RJ, Goldfine AB, Koenig RJ, Rosen CJ, eds. Williams Textbook of Endocrinology. 14th ed. Elsevier; 2020:497. [Google Scholar]
- 132. Mah PM, Jenkins RC, Rostami-Hodjegan A, et al. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2004;61(3):367‐375. [DOI] [PubMed] [Google Scholar]
- 133. Filipsson H, Monson JP, Koltowska-Häggström M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91(10):3954‐3961. [DOI] [PubMed] [Google Scholar]
- 134. Løvås K, Loge JH, Husebye ES. Subjective health status in Norwegian patients with Addison's disease. Clin Endocrinol (Oxf). 2002;56(5):581‐588. [DOI] [PubMed] [Google Scholar]
- 135. Giordano R, Marzotti S, Balbo M, et al. Metabolic and cardiovascular profile in patients with Addison's disease under conventional glucocorticoid replacement. J Endocrinol Invest. 2009;32(11):917‐923. [DOI] [PubMed] [Google Scholar]
- 136. Bergthorsdottir R, Leonsson-Zachrisson M, Odén A, Johannsson G. Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab. 2006;91(12):4849‐4853. [DOI] [PubMed] [Google Scholar]
- 137. Hellmich B, Águeda AF, Monti S, Luqmani R. Treatment of giant cell arteritis and takayasu arteritis-current and future. Curr Rheumatol Rep. 2020;22(12):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ruiz-Irastorza G, Ugarte A, Saint-Pastou Terrier C, et al. Repeated pulses of methyl-prednisolone with reduced doses of prednisone improve the outcome of class III, IV and V lupus nephritis: an observational comparative study of the Lupus-Cruces and lupus-Bordeaux cohorts. Autoimmun Rev. 2017;16(8):826‐832. [DOI] [PubMed] [Google Scholar]
- 139. Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):590‐599; quiz 600. [DOI] [PubMed] [Google Scholar]
- 140. Lu NZ, Wardell SE, Burnstein KL, et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58(4):782‐797. [DOI] [PubMed] [Google Scholar]
- 141. Hollenberg SM, Weinberger C, Ong ES, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318(6047):635‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rupprecht R, Reul JM, van Steensel B, et al. Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur J Pharmacol. 1993;247(2):145‐154. [DOI] [PubMed] [Google Scholar]
- 143. Fuller PJ, Yao Y-Z, Yang J, Young MJ. Structural determinants of activation of the mineralocorticoid receptor: an evolutionary perspective. J Hum Hypertens. 2021;35(2):110‐116. [DOI] [PubMed] [Google Scholar]
- 144. Rogerson FM, Brennan FE, Fuller PJ. Mineralocorticoid receptor binding, structure and function. Mol Cell Endocrinol. 2004;217(1-2):203‐212. [DOI] [PubMed] [Google Scholar]
- 145. Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4(3):965‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Fuller PJ, Yang J, Young MJ. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: coregulators as mediators of mineralocorticoid receptor signalling diversity. J Endocrinol. 2017;234(1):T23‐T34. [DOI] [PubMed] [Google Scholar]
- 147. Yang H, Narayan S, Schmidt MV. From ligands to behavioral outcomes: understanding the role of mineralocorticoid receptors in brain function. Stress. 2023;26(1):2204366. [DOI] [PubMed] [Google Scholar]
- 148. Yang J, Chen Y, Li X, Xu D. New insights into the roles of glucocorticoid signaling dysregulation in pathological cardiac hypertrophy. Heart Fail Rev. 2022;27(4):1431‐1441. [DOI] [PubMed] [Google Scholar]
- 149. Vandevyver S, Dejager L, Libert C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr Rev. 2014;35(4):671‐693. [DOI] [PubMed] [Google Scholar]
- 150. Johnson RF, Rennie N, Murphy V, Zakar T, Clifton V, Smith R. Expression of glucocorticoid receptor messenger ribonucleic acid transcripts in the human placenta at term. J Clin Endocrinol Metab. 2008;93(12):4887‐4893. [DOI] [PubMed] [Google Scholar]
- 151. Bockmühl Y, Murgatroyd CA, Kuczynska A, Adcock IM, Almeida OFX, Spengler D. Differential regulation and function of 5′-untranslated GR-exon 1 transcripts. Mol Endocrinol. 2011;25(7):1100‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Saif Z, Meakin AS, Clifton VL. A preferential switch between placental GR exon 1 promoter variants in the presence of maternal asthma or inflammation upregulates GRα D isoforms. Placenta. 2021;108:64‐72. [DOI] [PubMed] [Google Scholar]
- 153. Zong J, Ashraf J, Thompson EB. The promoter and first, untranslated exon of the human glucocorticoid receptor gene are GC rich but lack consensus glucocorticoid receptor element sites. Mol Cell Biol. 1990;10(10):5580‐5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Breslin MB, Geng CD, Vedeckis WV. Multiple promoters exist in the human GR gene, one of which is activated by glucocorticoids. Mol Endocrinol. 2001;15(8):1381‐1395. [DOI] [PubMed] [Google Scholar]
- 155. Turner JD, Muller CP. Structure of the glucocorticoid receptor (NR3C1) gene 5′ untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol. 2005;35(2):283‐292. [DOI] [PubMed] [Google Scholar]
- 156. Presul E, Schmidt S, Kofler R, Helmberg A. Identification, tissue expression, and glucocorticoid responsiveness of alternative first exons of the human glucocorticoid receptor. J Mol Endocrinol. 2007;38(1-2):79‐90. [DOI] [PubMed] [Google Scholar]
- 157. Pedersen KB, Geng C-D, Vedeckis WV. Three mechanisms are involved in glucocorticoid receptor autoregulation in a human T-lymphoblast cell line. Biochemistry. 2004;43(34):10851‐10858. [DOI] [PubMed] [Google Scholar]
- 158. Turner JD, Alt SR, Cao L, et al. Transcriptional control of the glucocorticoid receptor: CpG islands, epigenetics and more. Biochem Pharmacol. 2010;80(12):1860‐1868. [DOI] [PubMed] [Google Scholar]
- 159. Davies TH, Ning Y-M, Sánchez ER. Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry. 2005;44(6):2030‐2038. [DOI] [PubMed] [Google Scholar]
- 160. Presman DM, Ogara MF, Stortz M, et al. Live cell imaging unveils multiple domain requirements for in vivo dimerization of the glucocorticoid receptor. PLoS Biol. 2014;12(3):e1001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Davies TH, Ning Y-M, Sánchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277(7):4597‐4600. [DOI] [PubMed] [Google Scholar]
- 162. Thomas-Chollier M, Watson LC, Cooper SB, et al. A naturally occuring insertion of a single amino acid rewires transcriptional regulation by glucocorticoid receptor isoforms. Proce Natl Acad Sci U S A. 2013;110(44):17826‐17831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kauppi B, Jakob C, Färnegårdh M, et al. The three-dimensional structures of antagonistic and agonistic forms of the glucocorticoid receptor ligand-binding domain: RU-486 induces a transconformation that leads to active antagonism. J Biol Chem. 2003;278(25):22748‐22754. [DOI] [PubMed] [Google Scholar]
- 164. Jiménez-Panizo A, Alegre-Martí A, Tettey TT, et al. The multivalency of the glucocorticoid receptor ligand-binding domain explains its manifold physiological activities. Nucleic Acids Res. 2022;50(22):13063‐13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Smith DF, Toft DO. Steroid receptors and their associated proteins. Mol Endocrinol. 1993;7(1):4‐11. [DOI] [PubMed] [Google Scholar]
- 166. Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280(6):4609‐4616. [DOI] [PubMed] [Google Scholar]
- 167. Ramos-Ramírez P, Tliba O. Glucocorticoid receptor β (GRβ): beyond its dominant-negative function. Int J Mol Sci. 2021;22(7):3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Schaaf MJM, Cidlowski JA. Molecular determinants of glucocorticoid receptor mobility in living cells: the importance of ligand affinity. Mol Cell Biol. 2003;23(6):1922‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Groeneweg FL, van Royen ME, Fenz S, et al. Quantitation of glucocorticoid receptor DNA-binding dynamics by single-molecule microscopy and FRAP. PLoS One. 2014;9(3):e90532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Uhlenhaut NH, Barish GD, Yu RT, et al. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Mol Cell. 2013;49(1):158‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Lim H-W, Uhlenhaut NH, Rauch A, et al. Genomic redistribution of GR monomers and dimers mediates transcriptional response to exogenous glucocorticoid in vivo. Genome Res. 2015;25(6):836‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, Yamamoto KR. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324(5925):407‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Surjit M, Ganti KP, Mukherji A, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell. 2011;145(2):224‐241. [DOI] [PubMed] [Google Scholar]
- 174. Hudson WH, Youn C, Ortlund EA. The structural basis of direct glucocorticoid-mediated transrepression. Nat Struct Mol Biol. 2013;20(1):53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Langlais D, Couture C, Balsalobre A, Drouin J. The Stat3/GR interaction code: predictive value of direct/indirect DNA recruitment for transcription outcome. Mol Cell. 2012;47(1):38‐49. [DOI] [PubMed] [Google Scholar]
- 176. Tuckermann JP, Reichardt HM, Arribas R, Richter KH, Schütz G, Angel P. The DNA binding-independent function of the glucocorticoid receptor mediates repression of Ap-1–dependent genes in skin. J Cell Biol. 1999;147(7):1365‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Heck S, Kullmann M, Gast A, et al. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 1994;13(17):4087‐4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Escoter-Torres L, Greulich F, Quagliarini F, Wierer M, Uhlenhaut NH. Anti-inflammatory functions of the glucocorticoid receptor require DNA binding. Nucleic Acids Res. 2020;48(15):8393‐8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Weikum ER, de Vera IMS, Nwachukwu JC, et al. Tethering not required: the glucocorticoid receptor binds directly to activator protein-1 recognition motifs to repress inflammatory genes. Nucleic Acids Res. 2017;45(14):8596‐8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Geng C-D, Schwartz JR, Vedeckis WV. A conserved molecular mechanism is responsible for the auto-up-regulation of glucocorticoid receptor gene promoters. Mol Endocrinol. 2008;22(12):2624‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O. Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci. 2019;40(1):38‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hafezi-Moghadam A, Simoncini T, Yang Z, et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8(5):473‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Widén C, Gustafsson J-A, Wikström A-C. Cytosolic glucocorticoid receptor interaction with nuclear factor-kappa B proteins in rat liver cells. Biochem J. 2003;373(Pt 1):211‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bruna A, Nicolàs M, Muñoz A, Kyriakis JM, Caelles C. Glucocorticoid receptor-JNK interaction mediates inhibition of the JNK pathway by glucocorticoids. EMBO J. 2003;22(22):6035‐6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Boldizsar F, Talaber G, Szabo M, et al. Emerging pathways of non-genomic glucocorticoid (GC) signalling in T cells. Immunobiology. 2010;215(7):521‐526. [DOI] [PubMed] [Google Scholar]
- 186. Vernocchi S, Battello N, Schmitz S, et al. Membrane glucocorticoid receptor activation induces proteomic changes aligning with classical glucocorticoid effects. Mol Cell Proteomics. 2013;12(7):1764‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bartholome B, Spies CM, Gaber T, et al. Membrane glucocorticoid receptors (mGCR) are expressed in normal human peripheral blood mononuclear cells and up-regulated after in vitro stimulation and in patients with rheumatoid arthritis. FASEB J. 2004;18(1):70‐80. [DOI] [PubMed] [Google Scholar]
- 188. Löwenberg M, Verhaar AP, Bilderbeek J, et al. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO Rep. 2006;7(10):1023‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Löwenberg M, Verhaar AP, van den Brink GR, Hommes DW. Glucocorticoid signaling: a nongenomic mechanism for T-cell immunosuppression. Trends Mol Med. 2007;13(4):158‐163. [DOI] [PubMed] [Google Scholar]
- 190. Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130(2):289‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Urbach V, Verriere V, Grumbach Y, Bousquet J, Harvey BJ. Rapid anti-secretory effects of glucocorticoids in human airway epithelium. Steroids. 2006;71(4):323‐328. [DOI] [PubMed] [Google Scholar]
- 192. Steiner A, Vogt E, Locher R, Vetter W. Stimulation of the phosphoinositide signalling system as a possible mechanism for glucocorticoid action in blood pressure control. J Hypertens Suppl. 1988;6(4):S366‐S368. [DOI] [PubMed] [Google Scholar]
- 193. Buttgereit F, Scheffold A. Rapid glucocorticoid effects on immune cells. Steroids. 2002;67(6):529‐534. [DOI] [PubMed] [Google Scholar]
- 194. Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18(3):331‐342. [DOI] [PubMed] [Google Scholar]
- 195. Schaaf MJM, Champagne D, van Laanen IH, et al. Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish. Endocrinology. 2008;149(4):1591‐1599. [DOI] [PubMed] [Google Scholar]
- 196. Kállai BM, Csöndes J, Kiss G, Bodrogi L, Rónai Z, Mészáros T. Restrained expression of canine glucocorticoid receptor splice variants α and P prognosticates fatal disease outcome in SIRS. Sci Rep. 2021;11(1):24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Katsu Y, Oana S, Lin X, Hyodo S, Bianchetti L, Baker ME. Cloning of nine glucocorticoid receptor isoforms from the slender African lungfish (Protopterus dolloi). PLoS One. 2022;17(8):e0272219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Saif Z, Hodyl NA, Hobbs E, et al. The human placenta expresses multiple glucocorticoid receptor isoforms that are altered by fetal sex, growth restriction and maternal asthma. Placenta. 2014;35(4):260‐268. [DOI] [PubMed] [Google Scholar]
- 199. Saif Z, Hodyl NA, Stark MJ, et al. Expression of eight glucocorticoid receptor isoforms in the human preterm placenta vary with fetal sex and birthweight. Placenta. 2015;36(7):723‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Cuffe JSM, Saif Z, Perkins AV, Moritz KM, Clifton VL. Dexamethasone and sex regulate placental glucocorticoid receptor isoforms in mice. J Endocrinol. 2017;234(2):89‐100. [DOI] [PubMed] [Google Scholar]
- 201. Saif Z, Dyson RM, Palliser HK, Wright IMR, Lu N, Clifton VL. Identification of eight different isoforms of the glucocorticoid receptor in Guinea pig placenta: relationship to preterm delivery, sex and betamethasone exposure. PLoS One. 2016;11(2):e0148226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Clifton VL, McDonald M, Morrison JL, et al. Placental glucocorticoid receptor isoforms in a sheep model of maternal allergic asthma. Placenta. 2019;83:33‐36. [DOI] [PubMed] [Google Scholar]
- 203. Matera AG, Wang Z. A day in the life of the spliceosome. Nat Rev Mol Cell Biol. 2014;15(2):108‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lee Y, Rio DC. Mechanisms and regulation of alternative Pre-mRNA splicing. Annu Rev Biochem. 2015;84:291‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996;271(16):9550‐9559. [DOI] [PubMed] [Google Scholar]
- 206. Min J, Perera L, Krahn JM, et al. Probing dominant negative behavior of glucocorticoid receptor β through a hybrid structural and biochemical approach. Mol Cell Biol. 2018;38(8):e00453-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95(6):2435‐2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Bledsoe RK, Montana VG, Stanley TB, et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110(1):93‐105. [DOI] [PubMed] [Google Scholar]
- 209. Buoso E, Galasso M, Ronfani M, et al. Role of spliceosome proteins in the regulation of glucocorticoid receptor isoforms by cortisol and dehydroepiandrosterone. Pharmacol Res. 2017;120:180‐187. [DOI] [PubMed] [Google Scholar]
- 210. Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27(6):2266‐2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Oakley RH, Webster JC, Sar M, Parker CR Jr, Cidlowski JA. Expression and subcellular distribution of the beta-isoform of the human glucocorticoid receptor. Endocrinology. 1997;138(11):5028‐5038. [DOI] [PubMed] [Google Scholar]
- 212. Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor beta isoform. Specificity and mechanisms of action. J Biol Chem. 1999;274(39):27857‐27866. [DOI] [PubMed] [Google Scholar]
- 213. de Castro M, Elliot S, Kino T, et al. The non-ligand binding beta-isoform of the human glucocorticoid receptor (hGR beta): tissue levels, mechanism of action, and potential physiologic role. Mol Med. 1996;2(5):597‐607. [PMC free article] [PubMed] [Google Scholar]
- 214. Charmandari E, Chrousos GP, Ichijo T, et al. The human glucocorticoid receptor (hGR) beta isoform suppresses the transcriptional activity of hGRalpha by interfering with formation of active coactivator complexes. Mol Endocrinol. 2005;19(1):52‐64. [DOI] [PubMed] [Google Scholar]
- 215. Kino T, Manoli I, Kelkar S, Wang Y, Su YA, Chrousos GP. Glucocorticoid receptor (GR) beta has intrinsic, GRalpha-independent transcriptional activity. Biochem Biophys Res Commun. 2009;381(4):671‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Pujols L, Mullol J, Roca-Ferrer J, et al. Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. Am J Physiol Cell Physiol. 2002;283(4):C1324‐C1331. [DOI] [PubMed] [Google Scholar]
- 217. Hagendorf A, Koper JW, de Jong FH, Brinkmann AO, Lamberts SW, Feelders RA. Expression of the human glucocorticoid receptor splice variants alpha, beta, and P in peripheral blood mononuclear leukocytes in healthy controls and in patients with hyper- and hypocortisolism. J Clin Endocrinol Metab. 2005;90(11):6237‐6243. [DOI] [PubMed] [Google Scholar]
- 218. Huizenga NA, De Herder WW, Koper JW, et al. Decreased ligand affinity rather than glucocorticoid receptor down-regulation in patients with endogenous Cushing's syndrome. Eur J Endocrinol. 2000;142(5):472‐476. [DOI] [PubMed] [Google Scholar]
- 219. Invitti C, Redaelli G, Baldi G, Cavagnini F. Glucocorticoid receptors in anorexia nervosa and Cushing's disease. Biol Psychiatry. 1999;45(11):1467‐1471. [DOI] [PubMed] [Google Scholar]
- 220. Nawata H, Higuchi K, Higashizima M, Kato K, Ibayashi H. Glucocorticoid receptors in cultured skin fibroblasts of normal and adrenocorticoid disorders. Endocrinol Jpn. 1984;31(2):109‐116. [DOI] [PubMed] [Google Scholar]
- 221. Kelly A, Bowen H, Jee Y-K, et al. The glucocorticoid receptor beta isoform can mediate transcriptional repression by recruiting histone deacetylases. J Allergy Clin Immunol. 2008;121(1):203‐208.e1. [DOI] [PubMed] [Google Scholar]
- 222. He B, Cruz-Topete D, Oakley RH, Xiao X, Cidlowski JA. Human glucocorticoid receptor β regulates gluconeogenesis and inflammation in mouse liver. Mol Cell Biol. 2015;36(5):714‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Ledderose C, Möhnle P, Limbeck E, et al. Corticosteroid resistance in sepsis is influenced by microRNA-124–induced downregulation of glucocorticoid receptor-α. Crit Care Med. 2012;40(10):2745‐2753. [DOI] [PubMed] [Google Scholar]
- 224. Guerrero J, Gatica HA, Rodríguez M, Estay R, Goecke IA. Septic serum induces glucocorticoid resistance and modifies the expression of glucocorticoid isoforms receptors: a prospective cohort study and in vitro experimental assay. Crit Care. 2013;17(3):R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Jain A, Wordinger RJ, Yorio T, Clark AF. Spliceosome protein (SRp) regulation of glucocorticoid receptor isoforms and glucocorticoid response in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012;53(2):857‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zhu J, Gong JY, Goodman OB Jr, Cartegni L, Nanus DM, Shen R. Bombesin attenuates pre-mRNA splicing of glucocorticoid receptor by regulating the expression of serine-arginine protein p30c (SRp30c) in prostate cancer cells. Biochim Biophys Acta. 2007;1773(7):1087‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. McBeth L, Nwaneri AC, Grabnar M, Demeter J, Nestor-Kalinoski A, Hinds TD Jr. Glucocorticoid receptor beta increases migration of human bladder cancer cells. Oncotarget. 2016;7(19):27313‐27324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kasai Y. Two naturallô occurring isoforms and their expression of a glucocorticoid receptor gene from an androgen dependent mouse tumor. FEBS Lett. 1990;274(1-2):99‐102. [DOI] [PubMed] [Google Scholar]
- 230. Ray DW, Davis JR, White A, Clark AJ. Glucocorticoid receptor structure and function in glucocorticoid-resistant small cell lung carcinoma cells. Cancer Res. 1996;56(14):3276‐3280. [PubMed] [Google Scholar]
- 231. Morgan DJ, Poolman TM, Williamson AJ, et al. Glucocorticoid receptor isoforms direct distinct mitochondrial programs to regulate ATP production. Sci Rep. 2016;6:26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Rivers C, Levy A, Hancock J, Lightman S, Norman M. Insertion of an amino acid in the DNA-binding domain of the glucocorticoid receptor as a result of alternative splicing. J Clin Endocrinol Metab. 1999;84(11):4283‐4286. [DOI] [PubMed] [Google Scholar]
- 233. Beger C, Gerdes K, Lauten M, et al. Expression and structural analysis of glucocorticoid receptor isoform gamma in human leukaemia cells using an isoform-specific real-time polymerase chain reaction approach. Br J Haematol. 2003;122(2):245‐252. [DOI] [PubMed] [Google Scholar]
- 234. Matysiak M, Makosa B, Walczak A, Selmaj K. Patients with multiple sclerosis resisted to glucocorticoid therapy: abnormal expression of heat-shock protein 90 in glucocorticoid receptor complex. Mult Scler. 2008;14(7):919‐926. [DOI] [PubMed] [Google Scholar]
- 235. Liang Y, Song MM, Liu SY, Ma LL. Relationship between expression of glucocorticoid receptor isoforms and glucocorticoid resistance in immune thrombocytopenia. Hematology. 2016;21(7):440‐446. [DOI] [PubMed] [Google Scholar]
- 236. Schiller BJ, Chodankar R, Watson LC, Stallcup MR, Yamamoto KR. Glucocorticoid receptor binds half sites as a monomer and regulates specific target genes. Genome Biol. 2014;15(7):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Moalli PA, Pillay S, Krett NL, Rosen ST. Alternatively spliced glucocorticoid receptor messenger RNAs in glucocorticoid-resistant human multiple myeloma cells. Cancer Res. 1993;53(17):3877‐3879. [PubMed] [Google Scholar]
- 238. Shao S, Wang Y, Zhao Y, et al. Identification of multiple isoforms of glucocorticoid receptor in nasal polyps of patients with chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2022;51(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Sinclair D, Webster MJ, Wong J, Weickert CS. Dynamic molecular and anatomical changes in the glucocorticoid receptor in human cortical development. Mol Psychiatry. 2011;16(5):504‐515. [DOI] [PubMed] [Google Scholar]
- 240. Moalli PA, Pillay S, Weiner D, Leikin R, Rosen ST. A mechanism of resistance to glucocorticoids in multiple myeloma: transient expression of a truncated glucocorticoid receptor mRNA. Blood. 1992;79(1):213‐222. [PubMed] [Google Scholar]
- 241. Gaitan D, DeBold CR, Turney MK, Zhou P, Orth DN, Kovacs WJ. Glucocorticoid receptor structure and function in an adrenocorticotropin-secreting small cell lung cancer. Mol Endocrinol. 1995;9(9):1193‐1201. [DOI] [PubMed] [Google Scholar]
- 242. de Lange P, Segeren CM, Koper JW, et al. Expression in hematological malignancies of a glucocorticoid receptor splice variant that augments glucocorticoid receptor-mediated effects in transfected cells. Cancer Res. 2010;61(10):3937‐3941. [PubMed] [Google Scholar]
- 243. Russcher H, Dalm VASH, de Jong FH, et al. Associations between promoter usage and alternative splicing of the glucocorticoid receptor gene. J Mol Endocrinol. 2007;38(1-2):91‐98. [DOI] [PubMed] [Google Scholar]
- 244. Young SL, Saif Z, Meakin AS, et al. Alterations to placental glucocorticoid receptor expression with alcohol consumption. Reprod Sci. 2021;28(5):1390‐1402. [DOI] [PubMed] [Google Scholar]
- 245. Rizavi HS, Khan OS, Zhang H, Bhaumik R, Grayson DR, Pandey GN. Methylation and expression of glucocorticoid receptor exon-1 variants and FKBP5 in teenage suicide-completers. Transl Psychiatry. 2023;13(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Sun X, Fang M, Guan Y, et al. Changes of glucocorticoid receptor isoforms expression in acute lymphoblastic leukemia correlate with glucocorticoid resistance. Pharmazie. 2015;70(5):316‐321. [PubMed] [Google Scholar]
- 247. Ray DW, Littlewood AC, Clark AJ, Davis JR, White A. Human small cell lung cancer cell lines expressing the proopiomelanocortin gene have aberrant glucocorticoid receptor function. J Clin Invest. 1994;93(4):1625‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Leventhal SM, Lim D, Green TL, Cantrell AE, Cho K, Greenhalgh DG. Uncovering a multitude of human glucocorticoid receptor variants: an expansive survey of a single gene. BMC Genet. 2019;20(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Baker AC, Green TL, Chew VW, et al. Enhanced steroid response of a human glucocorticoid receptor splice variant. Shock. 2012;38(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 250. Turner JD, Schote AB, Keipes M, Muller CP. A new transcript splice variant of the human glucocorticoid receptor: identification and tissue distribution of hGR Delta 313-338, an alternative exon 2 transactivation domain isoform. Ann N Y Acad Sci. 2007;1095:334‐341. [DOI] [PubMed] [Google Scholar]
- 251. Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene. 1999;234(2):187‐208. [DOI] [PubMed] [Google Scholar]
- 252. Sriram A, Bohlen J, Teleman AA. Translation acrobatics: how cancer cells exploit alternate modes of translational initiation. EMBO Rep. 2018;19(10):e45947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Haimov O, Sinvani H, Dikstein R. Cap-dependent, scanning-free translation initiation mechanisms. Biochim Biophys Acta. 2015;1849(11):1313‐1318. [DOI] [PubMed] [Google Scholar]
- 254. Pooggin MM, Fütterer J, Skryabin KG, Hohn T. A short open reading frame terminating in front of a stable hairpin is the conserved feature in pregenomic RNA leaders of plant pararetroviruses. J Gen Virol. 1999;80(Pt 8):2217‐2228. [DOI] [PubMed] [Google Scholar]
- 255. Yudt MR, Cidlowski JA. Molecular identification and characterization of A and B forms of the glucocorticoid receptor. Mol Endocrinol. 2001;15(7):1093‐1103. [DOI] [PubMed] [Google Scholar]
- 256. Lu NZ, Collins JB, Grissom SF, Cidlowski JA. Selective regulation of bone cell apoptosis by translational isoforms of the glucocorticoid receptor. Mol Cell Biol. 2007;27(20):7143‐7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Wu I, Shin SC, Cao Y, et al. Selective glucocorticoid receptor translational isoforms reveal glucocorticoid-induced apoptotic transcriptomes. Cell Death Dis. 2013;4(1):e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Gross KL, Oakley RH, Scoltock AB, Jewell CM, Cidlowski JA. Glucocorticoid receptor alpha isoform-selective regulation of antiapoptotic genes in osteosarcoma cells: a new mechanism for glucocorticoid resistance. Mol Endocrinol. 2011;25(7):1087‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Cao Y, Bender IK, Konstantinidis AK, et al. Glucocorticoid receptor translational isoforms underlie maturational stage-specific glucocorticoid sensitivities of dendritic cells in mice and humans. Blood. 2013;121(9):1553‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Greenhalgh DG, Green TL, Lim D, Cho K. Bacterial pathogen-associated molecular patterns upregulate human glucocorticoid receptor expression in peripheral blood mononuclear cells. Shock. 2022;58(5):393‐399. [DOI] [PubMed] [Google Scholar]
- 261. Taylor RJ, Schlosser RJ, Soler ZM, Mattos JL, Mulligan JK. Glucocorticoid receptor isoform expression in peripheral blood mononuclear leukocytes of patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(8):913‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Téblick A, Van Dyck L, Van Aerde N, et al. Impact of duration of critical illness and level of systemic glucocorticoid availability on tissue-specific glucocorticoid receptor expression and actions: a prospective, observational, cross-sectional human and two translational mouse studies. EBioMedicine. 2022;80:104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Clifton VL, Cuffe J, Moritz KM, et al. Review: the role of multiple placental glucocorticoid receptor isoforms in adapting to the maternal environment and regulating fetal growth. Placenta. 2017;54:24‐29. [DOI] [PubMed] [Google Scholar]
- 264. Bender IK, Cao Y, Lu NZ. Determinants of the heightened activity of glucocorticoid receptor translational isoforms. Mol Endocrinol. 2013;27(9):1577‐1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Oakley RH, Ramamoorthy S, Foley JF, Busada JT, Lu NZ, Cidlowski JA. Glucocorticoid receptor isoform-specific regulation of development, circadian rhythm, and inflammation in mice. FASEB J. 2018;32(10):5258‐5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Sinclair D, Tsai SY, Woon HG, Weickert CS. Abnormal glucocorticoid receptor mRNA and protein isoform expression in the prefrontal cortex in psychiatric illness. Neuropsychopharmacology. 2011;36(13):2698‐2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Sinclair D, Webster MJ, Fullerton JM, Weickert CS. Glucocorticoid receptor mRNA and protein isoform alterations in the orbitofrontal cortex in schizophrenia and bipolar disorder. BMC Psychiatry. 2012;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Aleksic M, Brkic Z, Petrovic Z, Francija E, Lukic I, Adzic M. Sex-specific contribution of glucocorticoid receptor alpha isoforms to anxiety and depressive-like behavior in mice. J Neurosci Res. 2022;100(5):1239‐1253. [DOI] [PubMed] [Google Scholar]
- 269. Nunez BS, Vedeckis WV. Characterization of promoter 1B in the human glucocorticoid receptor gene. Mol Cell Endocrinol. 2002;189(1-2):191‐199. [DOI] [PubMed] [Google Scholar]
- 270. Pedersen KB, Vedeckis WV. Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry. 2003;42(37):10978‐10990. [DOI] [PubMed] [Google Scholar]
- 271. Geng C-D, Vedeckis WV. Steroid-responsive sequences in the human glucocorticoid receptor gene 1A promoter. Mol Endocrinol. 2004;18(4):912‐924. [DOI] [PubMed] [Google Scholar]
- 272. Garza AMS, Khan SH, Kumar R. Site-specific phosphorylation induces functionally active conformation in the intrinsically disordered N-terminal activation function (AF1) domain of the glucocorticoid receptor. Mol Cell Biol. 2010;30(1):220‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Blind RD, Garabedian MJ. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J Steroid Biochem Mol Biol. 2008;109(1-2):150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Ramamoorthy S, Cidlowski JA. Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr Dev. 2013;24:41‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905‐1917. [DOI] [PubMed] [Google Scholar]
- 276. Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol. 2007;21(10):2311‐2319. [DOI] [PubMed] [Google Scholar]
- 277. Ayoub S, Hickey MJ, Morand EF. Mechanisms of disease: macrophage migration inhibitory factor in SLE, RA and atherosclerosis. Nat Clin Pract Rheumatol. 2008;4(2):98‐105. [DOI] [PubMed] [Google Scholar]
- 278. Rossi AG, Haslett C, Hirani N, et al. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). potential role in asthma. J Clin Invest. 1998;101(12):2869‐2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Wallace AD, Cidlowski JA. Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. J Biol Chem. 2001;276(46):42714‐42721. [DOI] [PubMed] [Google Scholar]
- 280. Wallace AD, Cao Y, Chandramouleeswaran S, Cidlowski JA. Lysine 419 targets human glucocorticoid receptor for proteasomal degradation. Steroids. 2010;75(12):1016‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Kino T, Liou S-H, Charmandari E, Chrousos GP. Glucocorticoid receptor mutants demonstrate increased motility inside the nucleus of living cells: time of fluorescence recovery after photobleaching (FRAP) is an integrated measure of receptor function. Mol Med. 2004;10(7-12):80‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203(1):7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Deng Q, Waxse B, Riquelme D, Zhang J, Aguilera G. Helix 8 of the ligand binding domain of the glucocorticoid receptor (GR) is essential for ligand binding. Mol Cell Endocrinol. 2015;408:23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Samarasinghe RA, Di Maio R, Volonte D, et al. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation. Proc Natl Acad Sci U S A. 2011;108(40):16657‐16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Nicolaides NC, Kino T, Roberts ML, et al. The role of S-palmitoylation of the human glucocorticoid receptor (hGR) in mediating the nongenomic glucocorticoid actions. J Mol Biochem. 2017;6(1):3‐12. [PMC free article] [PubMed] [Google Scholar]
- 286. Michel A, Kokten T, Saber-Cherif L, et al. Folate and cobalamin deficiencies during pregnancy disrupt the glucocorticoid response in hypothalamus through N-homocysteinilation of the glucocorticoid receptor. Int J Mol Sci. 2023;24(12):9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Quax RA, Manenschijn L, Koper JW, et al. Glucocorticoid sensitivity in health and disease. Nat Rev Endocrinol. 2013;9(11):670‐686. [DOI] [PubMed] [Google Scholar]
- 288. Chriguer RS, Elias LLK, da Silva IM Jr, Vieira JGH, Moreira AC, de Castro M. Glucocorticoid sensitivity in young healthy individuals: in vitro and in vivo studies. J Clin Endocrinol Metab. 2005;90(11):5978‐5984. [DOI] [PubMed] [Google Scholar]
- 289. Huizenga NATM, Koper JW, de Lange P, et al. Interperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitary-adrenal axis to a low dose of dexamethasone in elderly individuals. J Clin Endocrinol Metab. 1998;83(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 290. Blackhurst G, McElroy PK, Fraser R, Swan RL, Connell JM. Seasonal variation in glucocorticoid receptor binding characteristics in human mononuclear leucocytes. Clin Endocrinol (Oxf). 2001;55(5):683‐688. [DOI] [PubMed] [Google Scholar]
- 291. Cardinal J, Pretorius CJ, Ungerer JPJ. Biological and diurnal variation in glucocorticoid sensitivity detected with a sensitive in vitro dexamethasone suppression of cytokine production assay. J Clin Endocrinol Metab. 2010;95(8):3657‐3663. [DOI] [PubMed] [Google Scholar]
- 292. Charmandari E, Kino T, Ichijo T, Chrousos GP. Generalized glucocorticoid resistance: clinical aspects, molecular mechanisms, and implications of a rare genetic disorder. J Clin Endocrinol Metab. 2008;93(5):1563‐1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Charmandari E, Kino T. Chrousos syndrome: a seminal report, a phylogenetic enigma and the clinical implications of glucocorticoid signalling changes. Eur J Clin Invest. 2010;40(10):932‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Charmandari E, Ichijo T, Jubiz W, et al. A novel point mutation in the amino terminal domain of the human glucocorticoid receptor (hGR) gene enhancing hGR-mediated gene expression. J Clin Endocrinol Metab. 2008;93(12):4963‐4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Nicolaides NC, Lamprokostopoulou A, Polyzos A, et al. Transient generalized glucocorticoid hypersensitivity. Eur J Clin Invest. 2015;45(12):1306‐1315. [DOI] [PubMed] [Google Scholar]
- 296. Barnes PJ, Greening AP, Crompton GK. Glucocorticoid resistance in asthma. Am J Respir Crit Care Med. 1995;152(6 Pt 2):S125‐S140. [DOI] [PubMed] [Google Scholar]
- 297. Sher ER, Leung DY, Surs W, et al. Steroid-resistant asthma. Cellular mechanisms contributing to inadequate response to glucocorticoid therapy. J Clin Invest. 1994;93(1):33‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Chikanza IC, Kozaci DL. Corticosteroid resistance in rheumatoid arthritis: molecular and cellular perspectives. Rheumatology (Oxford). 2004;43(11):1337‐1345. [DOI] [PubMed] [Google Scholar]
- 299. Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121(2):255‐260. [DOI] [PubMed] [Google Scholar]
- 300. Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35(3):360‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Ho G-T, Chiam P, Drummond H, Loane J, Arnott IDR, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24(2):319‐330. [DOI] [PubMed] [Google Scholar]
- 302. Seki M, Ushiyama C, Seta N, et al. Apoptosis of lymphocytes induced by glucocorticoids and relationship to therapeutic efficacy in patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41(5):823‐830. [DOI] [PubMed] [Google Scholar]
- 303. Du J, Li M, Zhang D, et al. Flow cytometry analysis of glucocorticoid receptor expression and binding in steroid-sensitive and steroid-resistant patients with systemic lupus erythematosus. Arthritis Res Ther. 2009;11(4):R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. DeRijk RH, Eskandari F, Sternberg EM. Corticosteroid resistance in a subpopulation of multiple sclerosis patients as measured by ex vivo dexamethasone inhibition of LPS induced IL-6 production. J Neuroimmunol. 2004;151(1-2):180‐188. [DOI] [PubMed] [Google Scholar]
- 305. Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J. 2004;24(5):822‐833. [DOI] [PubMed] [Google Scholar]
- 307. Hew M, Bhavsar P, Torrego A, et al. Relative corticosteroid insensitivity of peripheral blood mononuclear cells in severe asthma. Am J Respir Crit Care Med. 2006;174(2):134‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol. 2003;178(3):339‐346. [DOI] [PubMed] [Google Scholar]
- 309. Wandler AM, Huang BJ, Craig JW, et al. Loss of glucocorticoid receptor expression mediates in vivo dexamethasone resistance in T-cell acute lymphoblastic leukemia. Leukemia. 2020;34(8):2025‐2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Koga Y, Matsuzaki A, Suminoe A, Hattori H, Kanemitsu S, Hara T. Differential mRNA expression of glucocorticoid receptor alpha and beta is associated with glucocorticoid sensitivity of acute lymphoblastic leukemia in children. Pediatr Blood Cancer. 2005;45(2):121‐127. [DOI] [PubMed] [Google Scholar]
- 311. Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36(6):1937‐1949. [DOI] [PubMed] [Google Scholar]
- 312. Vassiliou AG, Stamogiannos G, Jahaj E, et al. Longitudinal evaluation of glucocorticoid receptor alpha/beta expression and signalling, adrenocortical function and cytokines in critically ill steroid-free patients. Mol Cell Endocrinol. 2020;501:110656. [DOI] [PubMed] [Google Scholar]
- 313. Meyer EJ, Nenke MA, Davies ML, et al. Corticosteroid-Binding globulin deficiency independently predicts mortality in septic shock. J Clin Endocrinol Metab. 2022;107(6):1636‐1646. [DOI] [PubMed] [Google Scholar]
- 314. Chapman K, Holmes M, Seckl J. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93(3):1139‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Jang C, Obeyesekere VR, Dilley RJ, Alford FP, Inder WJ. 11Beta hydroxysteroid dehydrogenase type 1 is expressed and is biologically active in human skeletal muscle. Clin Endocrinol (Oxf). 2006;65(6):800‐805. [DOI] [PubMed] [Google Scholar]
- 316. Leckie C, Chapman KE, Edwards CR, Seckl JR. LLC-PK1 cells model 11 beta-hydroxysteroid dehydrogenase type 2 regulation of glucocorticoid access to renal mineralocorticoid receptors. Endocrinology. 1995;136(12):5561‐5569. [DOI] [PubMed] [Google Scholar]
- 317. Kaur K, Hardy R, Ahasan MM, et al. Synergistic induction of local glucocorticoid generation by inflammatory cytokines and glucocorticoids: implications for inflammation associated bone loss. Ann Rheum Dis. 2010;69(6):1185‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Sun K, Myatt L. Enhancement of glucocorticoid-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by proinflammatory cytokines in cultured human amnion fibroblasts. Endocrinology. 2003;144(12):5568‐5577. [DOI] [PubMed] [Google Scholar]
- 319. Rabbitt EH, Lavery GG, Walker EA, Cooper MS, Stewart PM, Hewison M. Prereceptor regulation of glucocorticoid action by 11beta-hydroxysteroid dehydrogenase: a novel determinant of cell proliferation. FASEB J. 2002;16(1):36‐44. [DOI] [PubMed] [Google Scholar]
- 320. Sai S, Nakagawa Y, Yamaguchi R, et al. Expression of 11beta-hydroxysteroid dehydrogenase 2 contributes to glucocorticoid resistance in lymphoblastic leukemia cells. Leuk Res. 2011;35(12):1644‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Korbonits M, Bujalska I, Shimojo M, et al. Expression of 11 beta-hydroxysteroid dehydrogenase isoenzymes in the human pituitary: induction of the type 2 enzyme in corticotropinomas and other pituitary tumors. J Clin Endocrinol Metab. 2001;86(6):2728‐2733. [DOI] [PubMed] [Google Scholar]
- 322. Feldman K, Szappanos A, Butz H, et al. The rs4844880 polymorphism in the promoter region of the HSD11B1 gene associates with bone mineral density in healthy and postmenopausal osteoporotic women. Steroids. 2012;77(13):1345‐1351. [DOI] [PubMed] [Google Scholar]
- 323. Melander O, Orho-Melander M, Bengtsson K, et al. Association between a variant in the 11 beta-hydroxysteroid dehydrogenase type 2 gene and primary hypertension. J Hum Hypertens. 2000;14(12):819‐823. [DOI] [PubMed] [Google Scholar]
- 324. Farrell RJ, Murphy A, Long A, et al. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology. 2000;118(2):279‐288. [DOI] [PubMed] [Google Scholar]
- 325. Tsujimura S, Saito K, Nawata M, Nakayamada S, Tanaka Y. Overcoming drug resistance induced by P-glycoprotein on lymphocytes in patients with refractory rheumatoid arthritis. Ann Rheum Dis. 2008;67(3):380‐388. [DOI] [PubMed] [Google Scholar]
- 326. Tsujimura S, Saito K, Nakayamada S, Nakano K, Tanaka Y. Clinical relevance of the expression of P-glycoprotein on peripheral blood lymphocytes to steroid resistance in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52(6):1676‐1683. [DOI] [PubMed] [Google Scholar]
- 327. Stavreva DA, Wiench M, John S, et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11(9):1093‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Nenseth HZ, Dezitter X, Tesikova M, et al. Distinctly different dynamics and kinetics of two steroid receptors at the same response elements in living cells. PLoS One. 2014;9(8):e105204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Stavreva DA, Garcia DA, Fettweis G, et al. Transcriptional bursting and co-bursting regulation by steroid hormone release pattern and transcription factor mobility. Mol Cell. 2019;75(6):1161‐1177.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Brönnegård M, Arner P, Hellström L, Akner G, Gustafsson JA. Glucocorticoid receptor messenger ribonucleic acid in different regions of human adipose tissue. Endocrinology. 1990;127(4):1689‐1696. [DOI] [PubMed] [Google Scholar]
- 331. Koorneef LL, Viho EMG, Wahl LF, Meijer OC. Do corticosteroid receptor mRNA levels predict the expression of their target genes? J Endocr Soc. 2022;7(2):bvac188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Goleva E, Li L-B, Eves PT, Strand MJ, Martin RJ, Leung DYM. Increased glucocorticoid receptor beta alters steroid response in glucocorticoid-insensitive asthma. Am J Respir Crit Care Med. 2006;173(6):607‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol. 2000;105(5):943‐950. [DOI] [PubMed] [Google Scholar]
- 334. Shahidi H, Vottero A, Stratakis CA, et al. Imbalanced expression of the glucocorticoid receptor isoforms in cultured lymphocytes from a patient with systemic glucocorticoid resistance and chronic lymphocytic leukemia. Biochem Biophys Res Commun. 1999;254(3):559‐565. [DOI] [PubMed] [Google Scholar]
- 335. Longui CA, Vottero A, Adamson PC, et al. Low glucocorticoid receptor alpha/beta ratio in T-cell lymphoblastic leukemia. Horm Metab Res. 2000;32(10):401‐406. [DOI] [PubMed] [Google Scholar]
- 336. Haarman EG, Kaspers GJL, Pieters R, Rottier MMA, Veerman AJP. Glucocorticoid receptor alpha, beta and gamma expression vs in vitro glucocorticod resistance in childhood leukemia. Leukemia. 2004;18(3):530‐537. [DOI] [PubMed] [Google Scholar]
- 337. Goleva E, Jackson LP, Gleason M, Leung DYM. Usefulness of PBMCs to predict clinical response to corticosteroids in asthmatic patients. J Allergy Clin Immunol. 2012;129(3):687‐693.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Lasker MV, Leventhal SM, Lim D, et al. Hyperactive human glucocorticoid receptor isoforms and their implications for the stress response. Shock. 2015;43(3):228‐232. [DOI] [PubMed] [Google Scholar]
- 339. Green TL, Tung KL, Lim D, Leventhal SM, Cho K, Greenhalgh DG. A novel human glucocorticoid receptor SNP results in increased transactivation potential. Biochem Biophys Rep. 2017;9:140‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Koper JW, van Rossum EFC, van den Akker ELT. Glucocorticoid receptor polymorphisms and haplotypes and their expression in health and disease. Steroids. 2014;92:62‐73. [DOI] [PubMed] [Google Scholar]
- 341. Manenschijn L, van den Akker EL, Lamberts SWJ, van Rossum EFC. Clinical features associated with glucocorticoid receptor polymorphisms. An overview. Ann N Y Acad Sci. 2009;1179:179‐198. [DOI] [PubMed] [Google Scholar]
- 342. Russcher H, Smit P, van den Akker ELT, et al. Two polymorphisms in the glucocorticoid receptor gene directly affect glucocorticoid-regulated gene expression. J Clin Endocrinol Metab. 2005;90(10):5804‐5810. [DOI] [PubMed] [Google Scholar]
- 343. Russcher H, van Rossum EFC, de Jong FH, Brinkmann AO, Lamberts SWJ, Koper JW. Increased expression of the glucocorticoid receptor-A translational isoform as a result of the ER22/23EK polymorphism. Mol Endocrinol. 2005;19(7):1687‐1696. [DOI] [PubMed] [Google Scholar]
- 344. van Rossum EFC, Koper JW, Huizenga NATM, et al. A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels. Diabetes. 2002;51(10):3128‐3134. [DOI] [PubMed] [Google Scholar]
- 345. van Rossum EFC, Feelders RA, van den Beld AW, et al. Association of the ER22/23EK polymorphism in the glucocorticoid receptor gene with survival and C-reactive protein levels in elderly men. Am J Med. 2004;117(3):158‐162. [DOI] [PubMed] [Google Scholar]
- 346. van Rossum EFC, Voorhoeve PG, te Velde SJ, et al. The ER22/23EK polymorphism in the glucocorticoid receptor gene is associated with a beneficial body composition and muscle strength in young adults. J Clin Endocrinol Metab. 2004;89(8):4004‐4009. [DOI] [PubMed] [Google Scholar]
- 347. Finken MJJ, Meulenbelt I, Dekker FW, et al. The 23 K variant of the R23K polymorphism in the glucocorticoid receptor gene protects against postnatal growth failure and insulin resistance after preterm birth. J Clin Endocrinol Metab. 2007;92(12):4777‐4782. [DOI] [PubMed] [Google Scholar]
- 348. van Rossum EFC, de Jong FJ, Koper JW, et al. Glucocorticoid receptor variant and risk of dementia and white matter lesions. Neurobiol Aging. 2008;29(5):716‐723. [DOI] [PubMed] [Google Scholar]
- 349. van den Akker ELT, Nouwen JL, Melles DC, et al. Staphylococcus aureus nasal carriage is associated with glucocorticoid receptor gene polymorphisms. J Infect Dis. 2006; 194(6):814‐818. [DOI] [PubMed] [Google Scholar]
- 350. Huizenga NATM, Koper JW, de Lange P, et al. A polymorphism in the glucocorticoid receptor gene may be associated with an increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab. 1998;83(1):144‐151. [DOI] [PubMed] [Google Scholar]
- 351. Jewell CM, Cidlowski JA. Molecular evidence for a link between the N363S glucocorticoid receptor polymorphism and altered gene expression. J Clin Endocrinol Metab. 2007;92(8):3268‐3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. de Lange P, Koper JW, Huizenga NA, et al. Differential hormone-dependent transcriptional activation and -repression by naturally occurring human glucocorticoid receptor variants. Mol Endocrinol. 1997;11(8):1156‐1164. [DOI] [PubMed] [Google Scholar]
- 353. Jewell CM, Katen KS, Barber LM, Cannon C, Garantziotis S, Cidlowski JA. Healthy glucocorticoid receptor N363S carriers dysregulate gene expression associated with metabolic syndrome. Am J Physiol Endocrinol Metab. 2016;311(4):E741‐E748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Di Blasio AM, van Rossum EF, Maestrini S, et al. The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol (Oxf). 2003;59(1):68‐74. [DOI] [PubMed] [Google Scholar]
- 355. Lin RC, Wang XL, Dalziel B, Caterson ID, Morris BJ. Association of obesity, but not diabetes or hypertension, with glucocorticoid receptor N363S variant. Obes Res. 2003;11(6):802‐808. [DOI] [PubMed] [Google Scholar]
- 356. Lin RC, Wang WY, Morris BJ. High penetrance, overweight, and glucocorticoid receptor variant: case-control study. BMJ. 1999;319(7221):1337‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Roussel R, Reis AF, Dubois-Laforgue D, Bellanné-Chantelot C, Timsit J, Velho G. The N363S polymorphism in the glucocorticoid receptor gene is associated with overweight in subjects with type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2003;59(2):237‐241. [DOI] [PubMed] [Google Scholar]
- 358. Dobson MG, Redfern CP, Unwin N, Weaver JU. The N363S polymorphism of the glucocorticoid receptor: potential contribution to central obesity in men and lack of association with other risk factors for coronary heart disease and diabetes mellitus. J Clin Endocrinol Metab. 2001;86(5):2270‐2274. [DOI] [PubMed] [Google Scholar]
- 359. Kuningas M, Mooijaart SP, Slagboom PE, Westendorp RG, van Heemst D. Genetic variants in the glucocorticoid receptor gene (NR3C1) and cardiovascular disease risk. The Leiden 85-plus study. Biogerontology. 2006;7(4):231‐238. [DOI] [PubMed] [Google Scholar]
- 360. Lin RCY, Wang XL, Morris BJ. Association of coronary artery disease with glucocorticoid receptor N363S variant. Hypertension. 2003;41(3):404‐407. [DOI] [PubMed] [Google Scholar]
- 361. Wester VL, Koper JW, van den Akker EL, Franco OH, Stolk RP, van Rossum EF. Glucocorticoid receptor haplotype and metabolic syndrome: the lifelines cohort study. Eur J Endocrinol. 2016;175(6):645‐651. [DOI] [PubMed] [Google Scholar]
- 362. Luczay A, Török D, Ferenczi A, Majnik J, Sólyom J, Fekete G. Potential advantage of N363S glucocorticoid receptor polymorphism in 21-hydroxylase deficiency. Eur J Endocrinol. 2006;154(6):859‐864. [DOI] [PubMed] [Google Scholar]
- 363. Teeninga N, Kist-van Holthe JE, van den Akker EL, et al. Genetic and in vivo determinants of glucocorticoid sensitivity in relation to clinical outcome of childhood nephrotic syndrome. Kidney Int. 2014;85(6):1444‐1453. [DOI] [PubMed] [Google Scholar]
- 364. Tung K, Baker AC, Amini A, et al. Novel hyperactive glucocorticoid receptor isoform identified within a human population. Shock. 2011;36(4):339‐344. [DOI] [PubMed] [Google Scholar]
- 365. Ruiz M, Lind U, Gåfvels M, et al. Characterization of two novel mutations in the glucocorticoid receptor gene in patients with primary cortisol resistance. Clin Endocrinol (Oxf). 2001;55(3):363‐371. [DOI] [PubMed] [Google Scholar]
- 366. Charmandari E, Kino T, Ichijo T, Zachman K, Alatsatianos A, Chrousos GP. Functional characterization of the natural human glucocorticoid receptor (hGR) mutants hGRalphaR477H and hGRalphaG679S associated with generalized glucocorticoid resistance. J Clin Endocrinol Metab. 2006;91(4):1535‐1543. [DOI] [PubMed] [Google Scholar]
- 367. Ruiz M, Hedman E, Gåfvels M, et al. Further characterization of human glucocorticoid receptor mutants, R477H and G679S, associated with primary generalized glucocorticoid resistance. Scand J Clin Lab Invest. 2013;73(3):203‐207. [DOI] [PubMed] [Google Scholar]
- 368. Lonard DM, O'Malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27(5):691‐700. [DOI] [PubMed] [Google Scholar]
- 369. Lonard DM, O'Malley BW. Expanding functional diversity of the coactivators. Trends Biochem Sci. 2005;30(3):126‐132. [DOI] [PubMed] [Google Scholar]
- 370. McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20(3):321‐344. [DOI] [PubMed] [Google Scholar]
- 371. Nagy L, Kao HY, Chakravarti D, et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89(3):373‐380. [DOI] [PubMed] [Google Scholar]
- 372. Barnes PJ. Histone deacetylase-2 and airway disease. Ther Adv Respir Dis. 2009;3(5):235‐243. [DOI] [PubMed] [Google Scholar]
- 373. Simons SS Jr, Kumar R. Variable steroid receptor responses: intrinsically disordered AF1 is the key. Mol Cell Endocrinol. 2013;376(1-2):81‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Wang Q, Blackford JA Jr, Song L-N, Huang Y, Cho S, Simons SS Jr. Equilibrium interactions of corepressors and coactivators with agonist and antagonist complexes of glucocorticoid receptors. Mol Endocrinol. 2004;18(6):1376‐1395. [DOI] [PubMed] [Google Scholar]
- 375. Dendoncker K, Timmermans S, Vandewalle J, et al. TNF-α inhibits glucocorticoid receptor-induced gene expression by reshaping the GR nuclear cofactor profile. Proc Natl Acad Sci U S A. 2019;116(26):12942‐12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Portuguez AS, Grbesa I, Tal M, et al. Ep300 sequestration to functionally distinct glucocorticoid receptor binding loci underlie rapid gene activation and repression. Nucleic Acids Res. 2022;50(12):6702‐6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295(5564):2465‐2468. [DOI] [PubMed] [Google Scholar]
- 378. Grenier J, Trousson A, Chauchereau A, Cartaud J, Schumacher M, Massaad C. Differential recruitment of p160 coactivators by glucocorticoid receptor between Schwann cells and astrocytes. Mol Endocrinol. 2006;20(2):254‐267. [DOI] [PubMed] [Google Scholar]
- 379. Ronacher K, Hadley K, Avenant C, et al. Ligand-selective transactivation and transrepression via the glucocorticoid receptor: role of cofactor interaction. Mol Cell Endocrinol. 2009;299(2):219‐231. [DOI] [PubMed] [Google Scholar]
- 380. Kroe RR, Baker MA, Brown MP, et al. Agonist versus antagonist induce distinct thermodynamic modes of co-factor binding to the glucocorticoid receptor. Biophys Chem. 2007;128(2-3):156‐164. [DOI] [PubMed] [Google Scholar]
- 381. Stojceski F, Buetti-Dinh A, Stoddart MJ, Danani A, Della Bella E, Grasso G. Influence of dexamethasone on the interaction between glucocorticoid receptor and SOX9: a molecular dynamics study. J Mol Graph Model. 2023;125:108587. [DOI] [PubMed] [Google Scholar]
- 382. John S, Sabo PJ, Johnson TA, et al. Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell. 2008;29(5):611‐624. [DOI] [PubMed] [Google Scholar]
- 383. John S, Sabo PJ, Thurman RE, et al. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet. 2011;43(3):264‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Love MI, Huska MR, Jurk M, et al. Role of the chromatin landscape and sequence in determining cell type-specific genomic glucocorticoid receptor binding and gene regulation. Nucleic Acids Res. 2017;45(4):1805‐1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Wiench M, John S, Baek S, et al. DNA methylation status predicts cell type-specific enhancer activity. EMBO J. 2011;30(15):3028‐3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Gertz J, Savic D, Varley KE, et al. Distinct properties of cell-type-specific and shared transcription factor binding sites. Mol Cell. 2013;52(1):25‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Mayran A, Drouin J. Pioneer transcription factors shape the epigenetic landscape. J Biol Chem. 2018;293(36):13795‐13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Carceller-Zazo E, Sevilla LM, Pons-Alonso O, et al. The mineralocorticoid receptor modulates timing and location of genomic binding by glucocorticoid receptor in response to synthetic glucocorticoids in keratinocytes. FASEB J. 2023;37(1):e22709. [DOI] [PubMed] [Google Scholar]
- 389. Langlais D, Couture C, Balsalobre A, Drouin J. Regulatory network analyses reveal genome-wide potentiation of LIF signaling by glucocorticoids and define an innate cell defense response. PLoS Genet. 2008;4(10):e1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Kassel O, Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol. 2007;275(1-2):13‐29. [DOI] [PubMed] [Google Scholar]
- 391. Palumbo ML, Prochnik A, Wald MR, Genaro AM. Chronic stress and glucocorticoid receptor resistance in asthma. Clin Ther. 2020;42(6):993‐1006. [DOI] [PubMed] [Google Scholar]
- 392. Loke T-K, Mallett KH, Ratoff J, et al. Systemic glucocorticoid reduces bronchial mucosal activation of activator protein 1 components in glucocorticoid-sensitive but not glucocorticoid-resistant asthmatic patients. J Allergy Clin Immunol. 2006;118(2):368‐375. [DOI] [PubMed] [Google Scholar]
- 393. Moliki JM, Nhundu TJ, Maritz L, Avenant C, Hapgood JP. Glucocorticoids and medroxyprogesterone acetate synergize with inflammatory stimuli to selectively upregulate CCL20 transcription. Mol Cell Endocrinol. 2023;563:111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Ogawa S, Lozach J, Benner C, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122(5):707‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Ratman D, Vanden Berghe W, Dejager L, et al. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol Cell Endocrinol. 2013;380(1-2):41‐54. [DOI] [PubMed] [Google Scholar]
- 396. Mattick JS. The functional genomics of noncoding RNA. Science. 2005;309(5740):1527‐1528. [DOI] [PubMed] [Google Scholar]
- 397. Barrandon C, Spiluttini B, Bensaude O. Non-coding RNAs regulating the transcriptional machinery. Biol Cell. 2008;100(2):83‐95. [DOI] [PubMed] [Google Scholar]
- 398. Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3(107):ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Hudson WH, Pickard MR, de Vera IMS, et al. Conserved sequence-specific lincRNA-steroid receptor interactions drive transcriptional repression and direct cell fate. Nat Commun. 2014;5:5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Parsonnet NV, Lammer NC, Holmes ZE, Batey RT, Wuttke DS. The glucocorticoid receptor DNA-binding domain recognizes RNA hairpin structures with high affinity. Nucleic Acids Res. 2019;47(15):8180‐8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Lammer NC, Ashraf HM, Ugay DA, et al. RNA binding by the glucocorticoid receptor attenuates dexamethasone-induced gene activation. Sci Rep. 2023;13(1):9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Ishmael FT, Fang X, Houser KR, et al. The human glucocorticoid receptor as an RNA-binding protein: global analysis of glucocorticoid receptor-associated transcripts and identification of a target RNA motif. J Immunol. 2011;186(2):1189‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306‐360. [DOI] [PubMed] [Google Scholar]
- 404. Kojika S, Sugita K, Inukai T, et al. Mechanisms of glucocorticoid resistance in human leukemic cells: implication of abnormal 90 and 70 kDa heat shock proteins. Leukemia. 1996;10(6):994‐999. [PubMed] [Google Scholar]
- 405. Qian X, Zhu Y, Xu W, Lin Y. Glucocorticoid receptor and heat shock protein 90 in peripheral blood mononuclear cells from asthmatics. Chin Med J (Engl). 2001;114(10):1051‐1054. [PubMed] [Google Scholar]
- 406. Tissing WJE, Meijerink JPP, den Boer ML, Brinkhof B, Pieters R. mRNA expression levels of (co)chaperone molecules of the glucocorticoid receptor are not involved in glucocorticoid resistance in pediatric ALL. Leukemia. 2005;19(5):727‐733. [DOI] [PubMed] [Google Scholar]
- 407. Lauten M, Beger C, Gerdes K, et al. Expression of heat-shock protein 90 in glucocorticoid-sensitive and -resistant childhood acute lymphoblastic leukaemia. Leukemia. 2003;17(8):1551‐1556. [DOI] [PubMed] [Google Scholar]
- 408. Ouyang J, Jiang T, Tan M, Cui Y, Li X. Abnormal expression and distribution of heat shock protein 90: potential etiologic immunoendocrine mechanism of glucocorticoid resistance in idiopathic nephrotic syndrome. Clin Vaccine Immunol. 2006;13(4):496‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Gross KL, Lu NZ, Cidlowski JA. Molecular mechanisms regulating glucocorticoid sensitivity and resistance. Mol Cell Endocrinol. 2009;300(1-2):7‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Kästle M, Kistler B, Lamla T, et al. FKBP51 modulates steroid sensitivity and NFκB signalling: a novel anti-inflammatory drug target. Eur J Immunol. 2018;48(11):1904‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Chun E, Lee H-S, Bang B-R, et al. Dexamethasone-induced FKBP51 expression in peripheral blood mononuclear cells could play a role in predicting the response of asthmatics to treatment with corticosteroids. J Clin Immunol. 2011;31(1):122‐127. [DOI] [PubMed] [Google Scholar]
- 412. Kelly MM, King EM, Rider CF, et al. Corticosteroid-induced gene expression in allergen-challenged asthmatic subjects taking inhaled budesonide. Br J Pharmacol. 2012;165(6):1737‐1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Hinds TD, Stechschulte LA, Elkhairi F, Sanchez ER. Analysis of FK506, timcodar (VX-853) and FKBP51 and FKBP52 chaperones in control of glucocorticoid receptor activity and phosphorylation. Pharmacol Res Perspect. 2014;2(6):e00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Scammell JG. The animal and cell models that uncovered FKBP51 as a regulator of glucocorticoid receptor function. J Cell Biochem. 2023:1‐4. [DOI] [PubMed] [Google Scholar]
- 415. Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618‐629. [DOI] [PubMed] [Google Scholar]
- 416. Rogliani P, Ritondo BL, Puxeddu E, Pane G, Cazzola M, Calzetta L. Experimental glucocorticoid receptor agonists for the treatment of asthma: a systematic review. J Exp Pharmacol. 2020;12:233‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Safy M, de Hair MJH, Jacobs JWG, Buttgereit F, Kraan MC, van Laar JM. Efficacy and safety of selective glucocorticoid receptor modulators in comparison to glucocorticoids in arthritis, a systematic review. PLoS One. 2017;12(12):e0188810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Gauvreau GM, Boulet L-P, Leigh R, et al. A nonsteroidal glucocorticoid receptor agonist inhibits allergen-induced late asthmatic responses. Am J Respir Crit Care Med. 2015;191(2):161‐167. [DOI] [PubMed] [Google Scholar]
- 419. Kuna P, Aurivillius M, Jorup C, Prothon S, Taib Z, Edsbäcker S. Efficacy and tolerability of an inhaled selective glucocorticoid receptor modulator—AZD5423—in chronic obstructive pulmonary disease patients: phase II study results. Basic Clin Pharmacol Toxicol. 2017;121(4):279‐289. [DOI] [PubMed] [Google Scholar]
- 420. Brown MN, Fuhr R, Beier J, et al. Efficacy and safety of AZD7594, an inhaled non-steroidal selective glucocorticoid receptor modulator, in patients with asthma: a phase 2a randomized, double blind, placebo-controlled crossover trial. Respir Res. 2019;20(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Buttgereit F, Strand V, Lee EB, et al. Fosdagrocorat (PF-04171327) versus prednisone or placebo in rheumatoid arthritis: a randomised, double-blind, multicentre, phase IIb study. RMD Open. 2019;5(1):e000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Stock T, Fleishaker D, Wang X, Mukherjee A, Mebus C. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a phase 2 randomized study. Int J Rheum Dis. 2017;20(8):960‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Schäcke H, Zollner TM, Döcke WD, et al. Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. Br J Pharmacol. 2009;158(4):1088‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Pivonello R, Bancos I, Feelders RA, et al. Relacorilant, a selective glucocorticoid receptor modulator, induces clinical improvements in patients with cushing syndrome: results from a prospective, open-label phase 2 study. Front Endocrinol (Lausanne). 2021;12:662865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Louw A, Swart P. Salsola tuberculatiformis botschantzev and an aziridine precursor analog mediate the in vivo increase in free corticosterone and decrease in corticosteroid-binding globulin in female wistar rats. Endocrinology. 1999;140(5):2044‐2053. [DOI] [PubMed] [Google Scholar]
- 426. Lesovaya EA, Chudakova D, Baida G, et al. The long winding road to the safer glucocorticoid receptor (GR) targeting therapies. Oncotarget. 2022;13:408‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]