Abstract
Turmeric has attracted a significant amount of interest in recent years due to its strong antimicrobial properties. The tissue culture of turmeric is preferred to obtain disease-free, highest number of plantlets with good uniform chemistry. However, there is a need to increase the speed of the whole process to meet the growing demand for planting materials and to save time and resources. Iron oxide nanoparticles (Fe3O4 NPs) showed positive effects on callus initiation time, proliferation rate, percent root response, shoot length, percent rooting, and number of roots per explant. Highest callus induction, i.e., 80%, was recorded in cultures that were grown in the presence of 15 mg/L of Fe3O4 NPs. Callus initiated earlier in culture tubes that received green synthesized iron nanoparticles in a concentration between 10–15 mg/L. Biofabricated nanoparticles were characterized for their size, physiochemical, and optical properties through UV–Vis spectroscopy, FTIR, XRD, and SEM. Curcuminoids profiling was performed by implementing LC-Ms that revealed increased quantities in plantlets grown in nano-supplemented media when compared to the control.
Keywords: nanotechnology, turmeric, curcuminoids, Fe3O nanoparticles, antimicrobial
1. Introduction
Turmeric is the common name of Curcuma longa L., which belongs to the family Zingiberaceae. Curcuma longa is a perennial herbaceous plant. This genus has 70 species of rhizomatous plants that are extensively grown throughout Asia, i.e., Pakistan, China, India, and other tropical areas [1]. Turmeric rhizomes have traditionally been utilized as antibacterial, antifungal, antiviral, insecticides, and for skin problems, wound healing, and as an anti-inflammatory agent [2]. The most valuable portion of turmeric is the rhizome, which is renowned for having antimicrobial properties. Curcuma longa extract is an oleoresin, made up of a substantial percentage of yellow-brown pigments and a light component of volatile oil [3]. Curcuminoids are most prevalently found in turmeric, which are naturally phenolic substances. Curcuminoids mainly consist of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Among these compounds, curcumin is the primary component of curcuminoids, which are accountable for the antimicrobial properties of turmeric. Curcumin is a naturally yellow-orange crystalline compound that is not soluble in water but is soluble in organic solvents like methanol, ethanol, and acetone. It was initially identified in 1910 as a minimal molecular weight compound with a melting point of 183 °C [4]. It has been demonstrated that turmeric extracts are highly effective at slowing the growth of various pathogenic bacteria. Ref. [5] stated that the phenolic chemicals called curcuminoids found in turmeric are what give it its antibacterial properties against B. subtilus, S. aureus, and E. coli. Turmeric oil demonstrated efficacious action against seven different fungi that cause agricultural products to decay when they are kept [6]. According to reports, curcumin has antifungal properties; for instance, it effectively combats human pathogenic fungi like Paracoccidioides brasiliensis and Candida spp. [7]. Curcumin has also shown high to average antifungal effects against plant pathogenic fungi including Erysiphe graminis, Puccinia recondita, Phytophthora infestans, and Rhizoctonia solani, which are causing powdery mildew in barley, wheat leaf rust, late blight of tomato, Botrytis blight in cucumber, and sheath blight of rice [8]. The extracts of Curcuma longa, C. zedoaria, C. amada, and C. aromatica successfully inhibited the growth of many fungi, i.e., Aspergillus niger, A. terreus, and Curvularia pallescens [9]. Due to their anti-inflammatory and therapeutic properties, curcuminoids, especially curcumin, have gained special importance.
Turmeric rarely flowers, and being a sterile triploid plant generally reproduced by vegetative propagation of rhizomes. It is reported that out of almost 10–25 lateral buds produced by each rhizome during one growing season, only 4–6 can develop plantlets in field conditions. Accumulation of curcuminoids in rhizomes is quite slow throughout the growing season [10]. In addition to genotypic differences, variation in field and processing conditions results in differences in the chemical composition of commercial turmeric rhizomes. Tissue culture can be an option to escape all these problems. Fast multiplication of turmeric by micropropagation is required to create a continuous source of evenly sized, high-quality, and disease-free plantlets, especially for commercial purposes. Variation in responses has been observed in plants, including turmeric, with variation in media used for micropropagation [11,12]. Several reports confirm the positive role of nanomaterials in micropropagation of plants [13].
Nanotechnology is a novel field with innovative uses in plant biotechnology and agriculture. Natural substances are found in plant extracts, including flavonoids, amino acids, saponins, vitamins, terpenes, phenolics, tannins, inositols, and resins [14]. Several plant sources with these chemicals have been explored to synthesize nanoparticles with reduced involvement of synthetic chemicals, hence reducing their toxicity. Green synthesized NPs increase crop yield, hasten the germination of plants and regeneration, and strengthen plant resistance to a range of biotic and abiotic diseases [15]. Fe3O4 NPs are one of the most significant oxides that have several uses in material engineering, biological remediation, medicine, cosmetics, and agriculture [16]. Fe3O4 NPs have prospective uses as agricultural fertilizer because of their capacity to produce chlorophyll, their redox activity, magnetic sensitivity, environmentally friendly nature, and ease of availability [17]. The industrial application of apple peel extract has been reported seldom, despite the fact that apples are among the main fruits used in the manufacturing of juices, sauces, and canned fruits. The apple peels are richer in polyphenolic and flavonoid compounds than pulp. Being a great source of antioxidants and reducing compounds, apple peels may be used as an attractive substitute for the green synthesis of metal nanoparticles. Thus far, a few studies have been reported on the biogenic synthesis of nanoparticles from fruit peels. They have synthesized a range of nanoparticles from single-extract mixtures of multiple fruit peels, including orange, pomegranate, apple, and banana [18,19]. Iron is essential for plant development and functioning, the synthesis of proteins, auxin control, metabolism of carbohydrates, and stress response [20]. Fe3O4 NPs have greatly increased the regeneration and growth of chickpea, sorghum, tomato, maize, strawberry, and rapeseed crops [21,22,23,24]. The best that we are aware of is that there has not been any research conducted regarding the effect of green synthesized Fe3O4 NPs in tissue culture investigations of Curcuma longa.
In this study, we present a unique green manufacturing process for apple peel extract-based Fe3O4 NPs. Furthermore, the effects of biosynthesized Fe3O4 NPs have been assessed for enhancing callogenesis, shoot regeneration patterns, root induction, and curcuminoid contents in turmeric plants. The objective of this enhancement was to increase the efficacy of micropropagation to save time and resources involved.
2. Materials and Methods
2.1. Green Synthesis of Fe3O4 Nanoparticles
Fresh apples (variety: Kala kulu) were purchased from a local market in Lahore. The apples were washed and peeled. Small pieces of apple peel were dried in sunlight for 5 days and then in a hot air oven at 60 °C for 48 h. Dried peels were powdered and sieved with a 2 mm mesh size.
The peel extract was prepared by mixing 10 g of apple peel powder in 100 mL of double distilled water and stirring at 75 °C for 2 h [25]. The solution was left for 10 to 15 min to cool and then filtered using Whatman filter paper. While placing the extract on a magnetic stirrer, 3.01 g of iron chloride (FeCl3) and 8.61 g of sodium acetate (C2H3NaO2) were added. After mixing for 2 h at 80 °C, the solution was cooled to 25 °C. The solution was centrifuged at 4000 rpm for 15 min, the upper liquid portion was discarded, and the remaining solid part was washed with distilled water two to three times and then kept in a hot air oven at 50 °C for 2 days to dry completely. The final product was ground into a fine powder and preserved at room temperature.
2.2. Characterization of Synthesized Nanoparticles
2.2.1. Ultraviolet Visible (UV–Vis) Spectroscopy
A UV–Vis spectrophotometer was used to determine the optical properties of Fe3O4 NPs. The nanoparticles detected by a UV–Vis spectrophotometer (Lambda 35, Perkin Elmer, USA) between 200 and 800 nm were identified as Fe3O4 NPs.
2.2.2. Fourier Transform Infrared Spectroscopy (FT-IR)
FTIR spectrum was obtained using an Agilent Technologies Cary 630 FTIR, (Fourier transform infrared spectrophotometer) Japan. On the basis of their chemical composition, synthesized nanoparticles were examined. The solution was characterized in the 500–4000 cm−1 range by using the KBr pellet method.
2.2.3. X-ray Diffraction (XRD) Analysis
The phase variety and the size of the grains of the created nanoparticles were determined using X-ray diffraction spectroscopy (XRD) (Philips PAN analytical, UK). CUK radiation was used to evaluate the produced nanoparticles at 30 kV of voltage and 20 MA of current, with a scan rate of 0.030/s. The prepared samples’ particle sizes were determined using Scherrer’s equation as follows:
where D is the size of the crystals, λ is the X-ray wavelength, θ is the Braggs angle in radians, β is full width at half the maximum of the peak, and k is a constant.
2.2.4. Scanning Electron Microscopy (SEM)
The morphological properties of synthesized apple peel extract-derived Fe3O4 NPs (JSM-6480 LV, UK) were examined using scanning electron microscopy (SEM). To make the sample conductor, a thin layer of platinum was coated on them. The sample was then examined in the SEM with an accelerating voltage of 20 KV.
2.3. Tissue Culture of Turmeric Plant
The turmeric rhizomes were purchased from a nursery in Lahore, Punjab, Pakistan. Clean rhizomes were then buried in wet sand to begin sprouting. The tips of newly sprouting shoots were removed and utilized as explants. To further clean the sprouting rhizomes, they were treated with a fungicide solution containing 1% bavistin for 30 min. The sprouting rhizome buds were removed until the explants’ length was 2–3 cm and the buds were still intact. The removed rhizome segments that enclosed the sprouting buds were subsequently treated for 30 min with a solution of 1% Cabrio, and then they were rinsed with double-distilled water. Then, they were surface sterilized in a laminar flow chamber with 0.2% mercuric chloride (HgCl2) for 10 min. The explants were treated with 70% ethanol. Finally, the explants were washed 4 to 5 times with double distilled water. All the other procedures, including final washing with sterile water to remove traces of chloride, cutting, and culturing, were carried out in a laminar flow chamber.
Sprouting rhizome bud explants were cultured in culture tubes using MS [25] culture medium supplemented with 1 mL/L of IAA (indole acetic acid), 8.88 mL/L of BAP (6 benzylaminopurine), and five different concentrations (1, 5, 10, 15, and 20 mg/L) of Fe3O4 NPs. The cultured tubes were incubated in vitro for four weeks, maintaining the standard culture temperature of 25 °C at 16/8 h photoperiod. The data were collected after 30 days of culturing. The whole experiment was run in triplicate.
2.4. Preparation of Turmeric Extract
Samples (control and grown in presence of 15 mg/L of Fe3O4 NPs) of tissue cultured plantlets of C. longa were extracted using an ultrasonic apparatus and ethanol/water (7:3) for 15 min. Each extract was filtered and then evaporated under vacuum in a rotary evaporator at 40 °C. The residue was diluted in a suitable volume of MeOH-water and filtered through a syringe filter (polytetrafluoroethylene, PTFE, 2.5 mm, 0.45 mm) before further analysis through LC-Ms.
2.5. Liquid Chromatography–Mass Spectroscopy (LC-MS)
The LC-Ms analysis was carried out by using an Agilent Jet Stream, (Agilent, Santa Clara, CA, USA) with a 996 photodiode assay (PDA) detector together with an automatic liquid chromatographic sampler and an auto injection system hyphenated to a Micromass Quattro Ultima tandem quadrupole mass spectrometer equipped with an electrospray ionization (ESI) source. The separation was accomplished by using a ZORBAX C-18 CA, USA column. The system produced a steady flow of 100 mL/min, and the mobile phase contained 0.1% formic acid and 70% acetonitrile. The injection’s volume was 0.5 µL. The temperature control mode was set at 30 °C. The electrospray interface (ESI) was used for the MS operation mode. A mode 22 multiple syringe pump was used to carry out the analyte infusion experiment. Quantification of the compounds was based on the peak area ratios, carried out by comparing the mean area response of three replicates of extracted samples.
2.6. Statistical Analysis
Statistical analyses were performed using the Excel software (version 2019) add-in DSAASTAT 1.101. The experiments were repeated twice, and mean data are presented. The experimental data were analyzed statistically by performing a one-way analysis of variance (ANOVA) and Tukey’s test (p = 0.05) to identify significant level differences between treatment means. Data visualization was performed using Excel software, and the standard error of the mean was presented as error bars in the graphical representations.
3. Results
3.1. Effect of Fe3O4 NPs on the Regeneration of Turmeric Plant through Tissue Culture
3.1.1. Effect of Fe3O4 NPs on Callus Induction
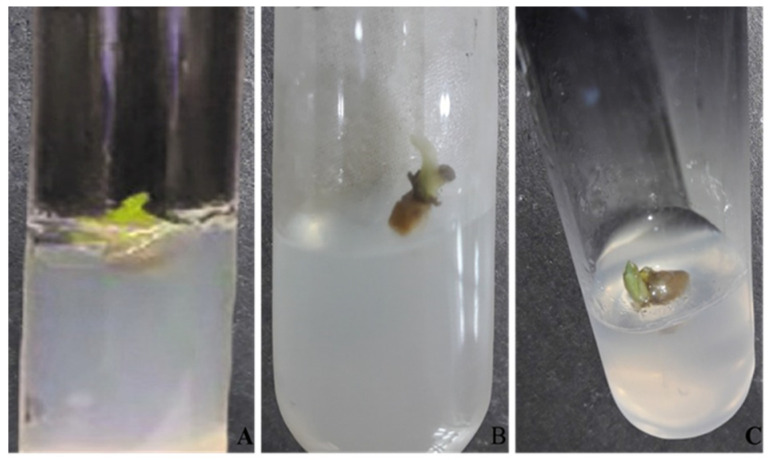
Green synthesized Fe3O4 NPs significantly enhanced rate of callus induction (Figure 1). Increase in concentration of nanoparticles from 1–15 mg/L linearly increased percentage of callus induction. However, further increase in Fe3O4 NPs to 20 mg/L declined this percentage. The highest Fe3O4 NPs concentration of 15 mg/L showed an 80% rate of callus induction that was 30% more than the control. At 10 mg/L of Fe3O4 NPs, the explant showed a satisfactory growth rate with a percentage of 70% in comparison to the control, which showed a 50% rate of callus induction. In the presence of nanoparticles, not only callus induction increased but also the time for callus initiation decreased significantly. Explant in the presence of Fe3O4 NPs in 15 mg/L was initiated after 28 days in comparison to the control, where initiation was recorded at the 42nd day of inoculation (Table 1).
Figure 1.
Comparison of callus induction on MS media supplemented with different concentrations of Fe3O4 NPs after 28 days of inoculation. (A) Simple MS media (control); (B) MS media supplemented with 10 mg/L; (C) MS media supplemented with 15 mg/L.
Table 1.
Effect of different concentrations of Fe3O4 NPs on callus induction.
| Treatment | Callus Initiation (Day) | Callus Induction (%) | Proliferation Rate |
|---|---|---|---|
| No Fe3O4 NPs (Control) | 42 | 50 | ++ |
| Fe3O4 NPs (1 mg/L) | 42 | 52 | ++ |
| Fe3O4 NPs (5 mg/L) | 39 | 55 | ++ |
| Fe3O4 NPs (10 mg/L) | 33 | 70 | +++ |
| Fe3O4 NPs (15 mg/L) | 28 | 80 | ++++ |
| Fe3O4 NPs (20 mg/L) | 41 | 40 | + |
++++: Excellent; +++: Good; ++: Satisfactory; +: Poor.
3.1.2. Effect of Fe3O4 NPs on In Vitro Shoot Growth of Turmeric
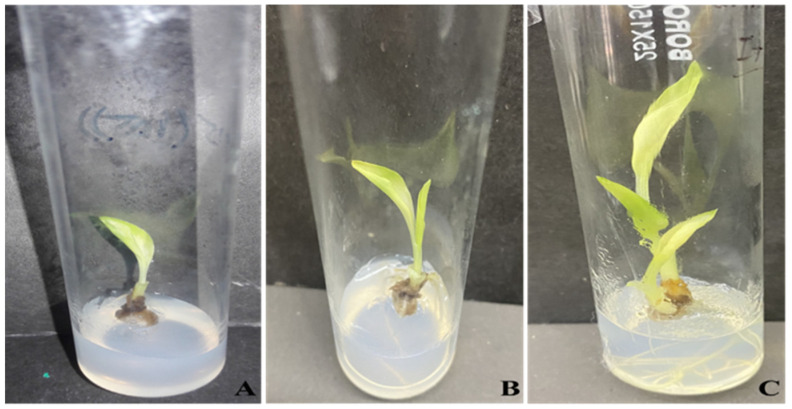
Table 2 shows that among all the concentrations of Fe3O4 NPs, the MS media supplemented with 15 mg/L showed greater effects on shoot growth by showing the highest percentage of 70% of shoot growth response, an average of 2.5 shoots per explant, 9 cm as the highest shoot height, 4 leaves per plantlet, and 100 mg of fresh weight. Noticeably, the lowest response rate (42%), least number of shoots, and minimum dry weight were recorded when the explants were cultured in MS media without nanoparticles. In general, the MS media supplemented with 15 mg/L of Fe3O4 NPs showed the most efficient shoot regeneration (Figure 2).
Table 2.
Effect of Fe3O4 NPs on in vitro shoot growth of turmeric.
| Treatments | Percent Response (%) | Shoot Height (cm) | No. of Leaves per Plantlet | Shoot Fresh Weight (mg) |
|---|---|---|---|---|
| No Fe3O4 NPs (Control) | 42.3 ± 0.8 b | 3.4 ± 0.4 b | 1.3 ± 0.3 b | 51.3 ± 0.6 b |
| Fe3O4 NPs (1 mg/L) | 44.0 ± 1.0 b | 3.7 ± 0.2 b | 1.3 ± 0.3 b | 52.6 ± 0.7 b |
| Fe3O4 NPs (5 mg/L) | 46.0 ± 0.5 b | 4.5 ± 0.5 bc | 2.0 ± 0.5 b | 57.1 ± 0.8 c |
| Fe3O4 NPs (10 mg/L) | 60.0 ± 1.1 c | 6.2 ± 0.7 c | 3.0 ± 1.0 c | 74.1 ± 1.0 d |
| Fe3O4 NPs (15 mg/L) | 70.0 ± 0.5 d | 9.0 ± 0.7 d | 5.6 ± 0.8 d | 100.3 ± 0.1 e |
| Fe3O4 NPs (20 mg/L) | 33.2 ± 0.5 a | 2.1 ± 0.4 a | 1.1 ± 0.4 a | 40.2 ± 0.7 a |
Data represent mean ± standard error. Different letters denote statistically significant differences between treatments as evaluated by the ANOVA and Tukey’s multiple range test at the p = 0.05 level.
Figure 2.
Effects of Fe3O4 NPs on in vitro growth of turmeric. (A) Simple MS media (control); (B) MS media supplemented with 10 mg/L; (C) MS media supplemented with 15 mg/L.
3.1.3. Effect of Fe3O4 NPs on In Vitro Root Induction
The explant when cultured in MS media supplemented with 1, 5, 10, 15, and 20 mg/L of Fe3O4 NPs showed an increase rate of root induction. The highest percentage of root induction of 75%, highest number of roots of six roots per plantlet, and highest fresh weight of 51 mg per plantlet were recorded at 15 mg/L concentration as shown in Table 3, while minimal root induction of 45% was shown in control media without nanoparticles. In comparison to the control, the percentage of rooting was 30 and 17% higher in 15 and 10 mg/L of Fe3O4 NPs.
Table 3.
Effect of Fe3O4 NPs on root induction of turmeric.
| Treatments | Percent Rooting (%) | No of Roots | Root Fresh Weight (mg) |
|---|---|---|---|
| No Fe3O4 NPs (Control) | 45.3 ± 1.8 a | 1.3 ± 0.3 a | 27.0 ± 1.7 a |
| Fe3O4 NPs (1 mg/L) | 47.6 ± 0.3 a | 1.6 ± 0.3 a | 29.2 ± 0.5 a |
| Fe3O4 NPs (5 mg/L) | 49.3 ± 0.8 a | 2.3 ± 0.6 ab | 30.2 ± 0.6 a |
| Fe3O4 NPs (10 mg/L) | 62.0 ± 2.1 b | 4.0 ± 0.5 b | 38.2 ± 1.9 b |
| Fe3O4 NPs (15 mg/L) | 75.0 ± 2.1 c | 7.0 ± 0.5 c | 51.1 ± 2.2 c |
| Fe3O4 NPs (20 mg/L) | 43.3 ± 0.9 a | 1.1 ± 0.2 a | 25.0 ± 2.1 a |
Data represent mean ± standard error. Different letters denote statistically significant differences between treatments as evaluated by the ANOVA and Tukey’s multiple range test at the p = 0.05 level.
3.2. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis of Turmeric
For the determination of curcuminoids in turmeric, a liquid chromatography–mass spectrometry (LC-MS) technique was performed and evaluated as per the guidelines for standard laboratory procedures. A full scan in positive ion modes (range of scan from 200 m/z to 500 m/z) was used to determine the analytes. To identify curcumin full scan mass spectra (precursor ion is 369 [M + H+]), demethoxycurcumin (precursor ion is 339 [M + H+]), bisdemethoxycurcumin (precursor ion is 309 [M + H+]), and dihydrocurcumin (precursor ion is 371 [M + H+]), 60 V of voltage was used. Collision energies of 15 eV were optimum for the primary production of curcumin at 177 m/z. The compounds were identified using positive ionization mode by observing the precursor–product mixtures in MRM mode. After optimization, the mass transition for curcumin was m/z 369–m/z 177 and had excellent symmetry and significant intensity. The retention time of curcumin was 17.91 min (Table 4).
Table 4.
Detection of curcuminoids and their metabolites.
| Compound | MW | RT | Formula | m/z | Concentration mg/g | |
|---|---|---|---|---|---|---|
| With NPs | Without NPs | |||||
| Curcumin | 368 | 17.91 | C12H20O6 | 369, 285, 177 | 13.73 | 10.35 |
| Demethoxycurcumin | 338 | 14.10 | C20H18O5 | 339, 177, 147 | 8.05 | 6.49 |
| Bisdemethoxycurcumin | 308 | 10.75 | C19H16O4 | 309, 225, 147 | 2.16 | 1.13 |
| Dihydrocurcumin | 370 | 10.50 | C12H22O6 | 371, 137, 177 | 0.02 | 0.01 |
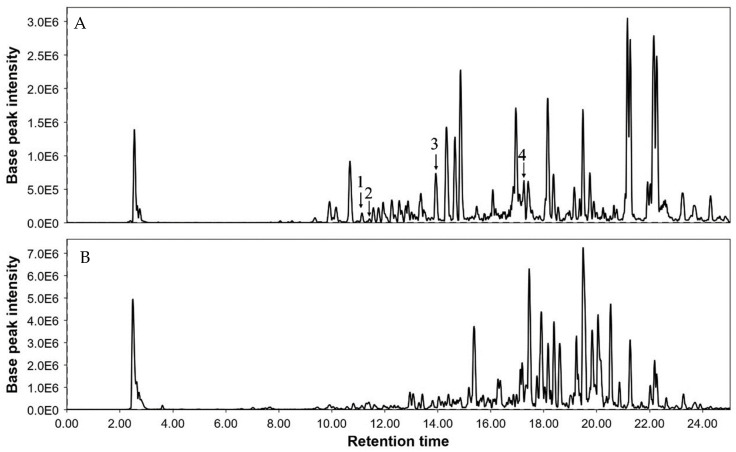
The curcuminoids combination revealed relatively lower quantities of dihydrocurcumin. Figure 3 shows a representation of chromatograms of turmeric plant extracts, both of control and grown in the presence of Fe3O4 NPs. The quantities of curcumin, demethoxycurcumin, bisdemethoxycurcumin, and dihydrocurcumin in the extract of plants grown in media supplemented with Fe3O4 NPs were found to be 13.73, 8.05, 2.16, and 0.02 mg/g, respectively. The content of curcumin in both extracts was greater than that of the other three compounds. While in the extract of the control plants all the contents of curcumin, demethoxycurcumin, bisdemethoxycurcumin, and dihydrocurcumin were in lower quantity than in plants grown in the presence of Fe3O4 NPs.
Figure 3.
Representative chromatograms of turmeric extract of plants grown in presence of Fe3O4 NPs (A) and control plants (B). Where 1 = dihydrocurcumin, 2 = bisdemethoxycurcumin, 3 = demethoxycurcumin, and 4 = curcumin.
3.3. Characterization of Green Synthesized Fe3O4 NPs
3.3.1. UV–Vis Spectroscopy
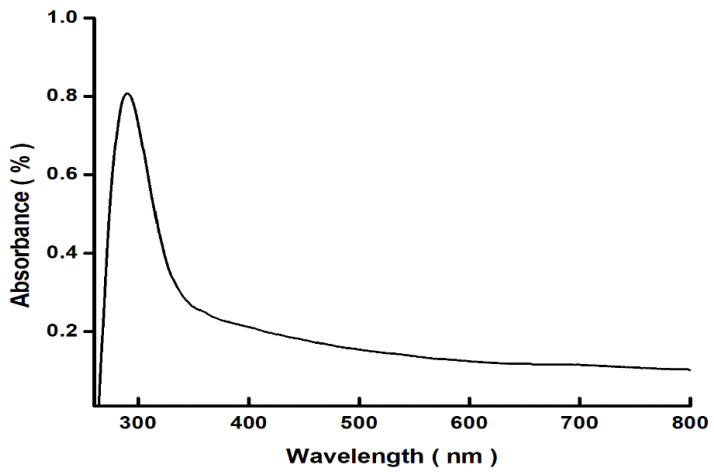
The ultraviolet–visible (UV) absorption spectrum of the green synthesized Fe3O4 NPs is shown in Figure 4 as the wavelength range of 250–800 nm. The major absorption bands of Fe3O4 NPs were identified at 290 nm. Upon exposure to light, Fe3O4 NPs absorb light, which may result in the excitation of electrons from the valence to the conduction band.
Figure 4.
A graphic illustration of the UV–Vis spectrophotometric study displaying the absorbance spectra of green Fe3O4 NPs that were synthesized using apple peel extract.
3.3.2. Fourier Transformed Infrared Spectroscopy (FTIR)
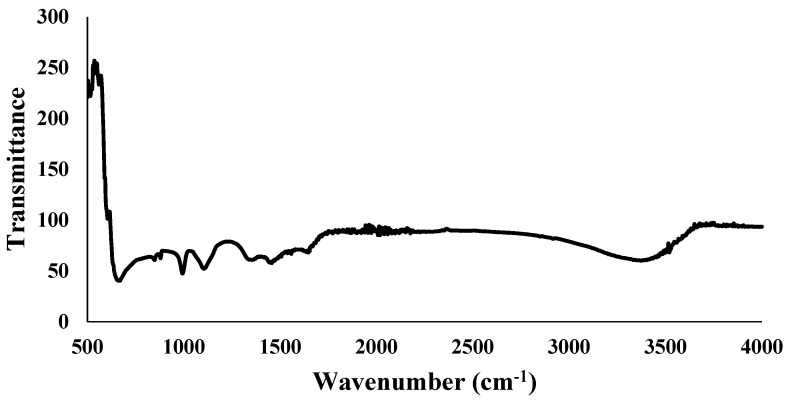
Fourier transformed infrared spectroscopy revealed detailed data about the profile of infrared spectra absorption on the Fe3O4 NPs sample. FTIR analysis for the wavelength range of 500–4000 cm−1 was used for the characterization of Fe3O4 NPs. The data plot displays the infrared light wavelength as distinct absorption peaks at specific wavenumbers that are caused by the vibration of particular functional groups. The stretching vibration of Fe-O functional groups takes place at the absorption of wavenumbers between 525 cm−1 to 571 cm−1 (Figure 5). The presence of the H-O-H bending vibration can be noted in the wavenumber range of 1327–1665 cm−1, whereas the OH group vibrates stretching between the wavenumber intervals from 3229 cm−1 to 3519 cm−1.
Figure 5.
Fourier transform infrared (FTIR) spectra of green synthesized Fe3O4 NPs using apple peel extract.
3.3.3. X-ray Diffraction (XRD)
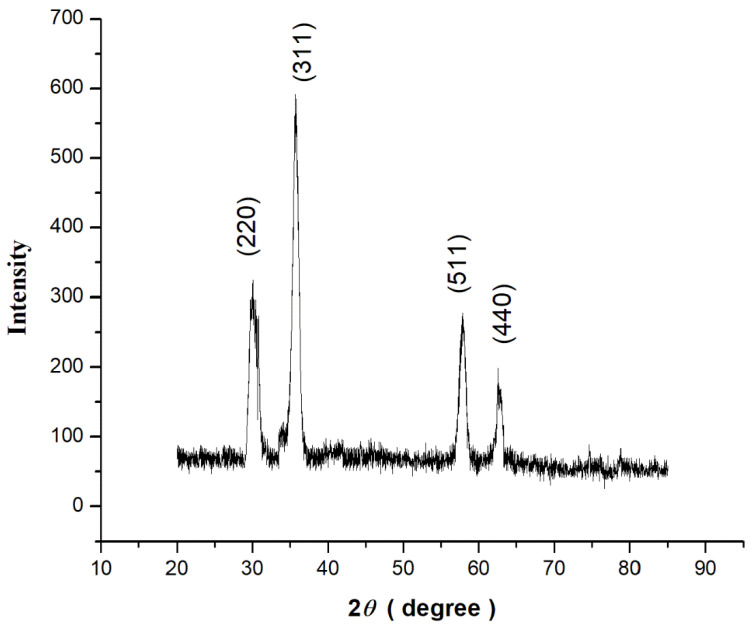
The crystalline structure and phase composition of Fe3O4 NPs synthesized using apple peel extract were revealed by X-ray diffraction (XRD) analysis. The XRD pattern exhibits unique diffraction peaks at certain angles, which correspond to the Fe3O4 NPs’ crystal planes. The locations and strengths of these points allow the crystal structure to be identified. The creation of magnetite Fe3O4 NPs is suggested by the existence of distinctive peaks at 30.1° 35.6°, 57.2°, and 62.6° [23]. The relative strengths of the peaks in diffraction reveal the amount of various crystal phases inside Fe3O4 NPs. To determine the phase composition, the peak intensity ratios may be compared to standardized reference data. The XRD pattern shows that the magnetite phase predominates in the Fe3O4 NPs synthesized with apple peel extract.
The lack of further diffraction peaks, or the presence of just faint, minor peaks, confirms the Fe3O4 NPs phase purity. Any unusual peaks might indicate the presence of contaminants or subsequent phases. The XRD pattern of Fe3O4 NPs shows relatively high phase purity, validating the effective production of magnetite Fe3O4 NPs as shown in Figure 6.
Figure 6.
X-ray diffraction (XRD) pattern (before extension of the peaks) of green synthesized Fe3O4 NPs using apple peel extract.
3.3.4. Scanning Electron Microscopy (SEM)
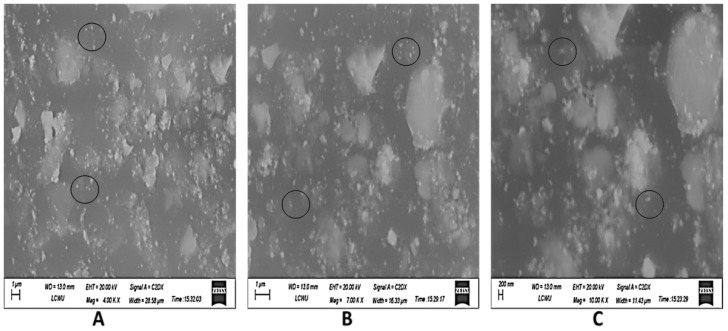
Fe3O4 NPs made from the apple peel extract were examined using SEM (scanning electron microscopy), which revealed information on the shape, size distribution, and surface properties of the nanoparticles. An explanation of how to read Fe3O4 NPs generated from extracts of apple peel from SEM pictures is given below (Figure 7). The size of the nanoparticles ranged from those that are smaller (10–20 nanometers) to those that are bigger (100–200 nanometers).
Figure 7.
Scanning electron microscopy (SEM) image of green synthesized Fe3O4 NPs. (A) = 4×, (B) = 7×, and (C) = 10×.
4. Discussion
In recent times, nanoparticles with the ability to improve plant physiological systems have been synthesized for their application in agriculture and biotechnology. They have been presented as mineral substances, especially important micronutrients needed for plant defense, as stimuli of resistance, and as agents that reduce the occurrence of plant pathogens [26]. In the present study, Fe3O4 NPs were green synthesized as iron plays an important function in many plant physiological systems, i.e., redox reactions and the formation of chlorophyll. Iron is an essential component for the metabolism and development of plants, and its insufficient amount may cause a prevalent nutritional disease in several plants, leading to reduced production and output [27]. These characteristics show the potential use of nanoparticles in tissue culture regeneration of plants to enhance their growth.
In the current study, the Fe3O4 NPs showed a great effect on callus induction and plant growth. In comparison to the control, callus induction was 30% higher in those that were grown in 15 mg/L of synthesized nanoparticles. Being small in size, nanoparticles are easily taken up by the plants through various reported mechanisms. The transportation within plants can be symplastic or apoplastic [28]. It is known that rapidly growing cells within the explant show extensive multiplication, and therefore there is a need for active synthesis of DNA. This great prerequisite for nucleic acid synthesis supports augmented manufacturing of nucleoside triphosphate (NTP), an initial substrate for synthesis of nucleic acids. This increased NTP synthesis increases pH level within cells, and Fe3O4 NPs are chemically stable in alkaline pH, hence available for rapid plant growth. It is also known that because of their large surface area, nanoparticles can attach to organic chemicals or to carrier proteins present in the cell membranes that help them in migration [29]. Once within a cell, these nanoparticles may have an impact on genetic regulation [30], thus affecting physiological and biochemical processes within a cell. This may lead to enhanced callus induction, growth, and disease resistance. A similar study conducted by [31] confirmed the positive role of iron nanoparticles on plantlet regeneration of Panax vietnamensis. However, they reported adverse effects on growth when nanoparticles were added in a concentration of 11.2 mg/L, whereas in the current study, a concentration of 15 mg/L was found to support both callus induction and growth in turmeric. Beside differences in plants, this may be due to differences in synthesis of nanoparticles used. Ref. [32] used chemically synthesized iron nanoparticles [33], whereas the present investigation was carried out with green synthesized iron nanoparticles. This also confirms that green synthesis protocols can reduce toxicity of metal and metal oxide nanoparticles. Further increase in concentration of Fe3O4 NPs to 20 mg/L incurred adverse effects on micropropagated plantlets. Ref. [34] reported that iron oxide nanoparticles at high concentrations can decrease cell viability. High iron content can trigger the formation of reactive oxygen species (ROS), resulting in damage to plant cells. According to [35], iron can transmit electrons to oxygen to form H2O2, thus extraordinary reactive OH radicals accumulate, causing degradation of cell wall polysaccharides [36]. The positive effects of treatment with Fe3O4 NPs on K accumulation could be due to iron-dependent activation of NADPH oxidases, since the activity of these enzymes is essential for controlling intracellular K+ homeostasis via ROS-gated ion channels [26].
Growth of turmeric plantlets was also found to be increased in the presence of Fe3O4 NPs in concentrations between 10–20 mg/L. The increased growth indicates that iron, as a micronutrient, has supported growth-related physiological processes [14,19]. It is also reported that the effects of nanoparticles in plants depend on concentrations, species of plants, and exposed timing [37]. Similar findings were presented by [22] in the chickpea plant, which showed an increase in shoot growth. Therefore, it is reasonable to believe that Fe3O4 NPs affected plant metabolism, and they may also cling to plant roots and alter the morphology and physiology of turmeric plantlets [26].
An increase in callus induction can indirectly be correlated to the antimicrobial potential of iron nanoparticles [38,39,40]. Microbial contamination at different stages of tissue culture is a main reason for low propagation rates. Ref. [41] explained the role of nanoparticles as elicitors in tissue culture. It is well documented that metal and metal oxide nanoparticles can activate defense in plant cells by activating enzymes such as superoxide dismutase, catalase, peroxidases, and polyphenol oxidases [42].
Plant growth regulators (PGR), including auxins, cytokinins, gibberellins, abscisic acid, and ethylene, have key roles in plant growth and development. The role of auxin is most important in initiation of the apical meristem. Cytokinin is involved in germination, meristematic functions, and leaf senescence, whereas abscisic acid controls germination. Ref. [43] in a study explained the role of biosynthesized AgNPs on hormones and hence on regenerating rice calli. They reported downregulation of PGR genes in nanoparticle-treated cells. The lowest expression was recorded in cells treated with 10 mg/L of nanoparticles that showed a 20% increase in callus initiation and induction in rice. In this investigation, beside callus induction, callus initiation was also improved with various doses of Fe3O4 NPs. Moreover, 15 mg/L nano-supplemented media showed callus initiation 14 days earlier than the control.
The green synthesis of Fe3O4 NPs was validated with a UV–Vis absorption band at 236 nm and stretching vibrations of Fe-O at the absorption of wave numbers between 525 and 571 cm−1. These optical characteristics were found to be closely similar to the previous investigations as of [44,45]. XRD revealed diffraction peaks corresponding to the cubic phase of metallic iron, resulting in a highly crystalline structure. The size of the synthesized nanoparticles was as small as 10–20 nm, making them easier to uptake and transport by the cells. However, larger particles of 100 nm and more were also present. It is known that small-sized nanoparticles can easily cling to the cell membranes and create small pores that then help relatively larger nanoparticles to travel in a system.
Plants are a significant source of potentially valuable active components, including novel antimicrobial agents [46]. Curcuminoids are vital compounds of turmeric. Synthesized Fe3O4 NPs not only supported micropropagation of turmeric but also increased curcuminoids in the whole plant. The findings are consistent with some other workers [47,48,49] reporting an enhanced production of curcuminoids after application of ZnO nanoparticles on Curcuma longa. Hence the study confirms that the green synthesized Fe3O4 NPs, when used in concentrations of 15 mg/L, can positively affect micropropagation as well as curcuminoids synthesis in turmeric [50].
5. Conclusions
This study reveals that biofabricated Fe3O4 NPs can be a more biologically compatible, efficient, eco-friendly, and reasonable method that may be employed effectively in investigations using plant tissue culture. Fe3O4 NPs 15 mg/L showed 80% increase in callus induction, 70% enhancement in shoot growth, and 75% increase in root induction in the turmeric plantlets. The treatment also supported 0.3 times more production of curcuminoids. Fast callus initiation can help us save time and valuable resources and thus can enhance the efficacy of micropropagation of turmeric.
Author Contributions
Conceptualization, T.A.; Methodology, A.A. and Z.A.S.; Formal analysis, H.R. and W.A.; Investigation, M.I.; Writing—review & editing, Z.-e.-H.A.; Project administration, H.R., W.A., A.A., G.L. and Z.A.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Humaira Rizwana Researchers Supporting Project number (RSPD2024R1048), King Saud University, Saudi Arabia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Brijesh H., Ajjappala B. Micropropagation strategies in medicinally important turmeric (Curcuma sp.): Current research and future challenges. [(accessed on 26 June 2024)];J. Appl. Biol. Biotechnol. 2023 11:1–8. doi: 10.7324/JABB.2023.65814. Available online: http://www.jabonline.in. [DOI] [Google Scholar]
- 2.Kumar A., Singh A.K., Kaushik M.S., Mishra S.K., Raj P., Singh P.K., Panday K.D. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: A review. 3 Biotech. 2017;7:357. doi: 10.1007/s13205-017-0971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yixuan L., Quaria M.A., Sivasamy S., Jianzohng S., Daochen Z. Curcumin production and bioavailability: A comprehensive review of curcumin extraction, synthesis, biotransformation and delivery systems. Ind. Crops Prod. 2021;172:114050. doi: 10.1016/j.indcrop.2021.114050. [DOI] [Google Scholar]
- 4.Sinchana N.S., Kattimani K.N., Prabhuling G., Sudesh K., Jagadeesha N. Standardization of tissue culture protocol for turmeric (Curcuma longa L.) Cv. Salem. Int. J. Chem. Stud. 2020;8:2721–2726. doi: 10.22271/chemi.2020.v8.i1ao.8681. [DOI] [Google Scholar]
- 5.Chitra R. Comparative studies on growth and Yield of Conventional and Tissue culture plants of Turmeric (Curcuma longa) var. CO2. J. Hortic. Sci. 2019;14:162–165. doi: 10.24154/jhs.v14i2.802. [DOI] [Google Scholar]
- 6.Ramkumar Kollana Y., Raj S.D., Suman P., Vani P.R., Sreeramulu S.H. Studies on nutritional evaluation and secondary metabolites of some selected curcuma species collected from Eastern Ghats of Udayagiri Hills, Gajapathi District, Odisha. Eur. J. Biomed. Pharm. Sci. 2020;7:276–281. [Google Scholar]
- 7.Panda D., Behera A.K., Padhan B., Nayak J.K. Chemical profiling of selected plants of Zingiberaceae used in ethnomedicine of Koraput, India. J. Stress Physiol. Biochem. 2020;16:50–60. [Google Scholar]
- 8.Ibanez M.D., Blazquez M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants. 2021;10:44. doi: 10.3390/plants10010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchent M.J., Molina P., Montecinos M., Guzman L., Balada C., Fassio C., Castro M. In Vitro Propagation of Easter Island Curcuma longa from Rhizome Explants Using Temporary Immersion System. Agronomy. 2021;11:2121. doi: 10.3390/agronomy11112121. [DOI] [Google Scholar]
- 10.Shashikant Rajak K.K., Koshore C., Parasad B.C., Trivedi M.P. Callus induction in rhizome of Curcuma caesia: A medicinal plant. J. Pharmacogn. Phytochem. 2019;8:1989–1993. [Google Scholar]
- 11.Flores S., Retana-Cordero M., Fisher P.R., Freyer R., Gomez C. Effect of Photoperiod, Propagative Material, and Production Period on Greenhouse-grown Ginger and Turmeric Plants. Hortiscience. 2021;56:1476–1485. doi: 10.21273/HORTSCI16025-21. [DOI] [Google Scholar]
- 12.Upendri HF L., Seran T.H. In vitro propagation of turmeric (Curcuma longa L.) through direct somatic embryogenesis with reference to types of explants and plant growth regulators: A review. Cienc. Agron. 2021;38 [Google Scholar]
- 13.Singh Y., Kumar U., Panigrahi S., Balyan P., Mehla S., Sihag P., Sagwal V., Singh K.P., White J.C., Dhankher O.P. Nanoparticles as novel elicitors in plant tissue culture applications: Current status and future outlook. Plant Physiol. Biochem. 2023;203:108004. doi: 10.1016/j.plaphy.2023.108004. [DOI] [PubMed] [Google Scholar]
- 14.Abbasi B.A., Iqbal J., Zahra S.A., Shahbaz A., Kanwal S., Rabbani A., Mahmood T. Bioinspired synthesis and activity characterization of iron oxide nanoparticles made using Rhamnus Triquetra leaf extract. Mater. Res. Express. 2020;6:1250e7. doi: 10.1088/2053-1591/ab664d. [DOI] [Google Scholar]
- 15.Pacheco I., Buzea C. Nanoparticle uptake by plants: Beneficial or detrimental? Phytotoxicity Nanoparticles. 2018:1–61. doi: 10.1007/978-3-319-76708-6_1. [DOI] [Google Scholar]
- 16.Asoufi H.M., Al-Antary T.M., Awwad A.M. Green route for synthesis hematite (α-Fe2O3) nanoparticles: Toxicity effect on the green peach aphid, Myzus persicae (Sulzer) Environ. Nanotechnol. Monit. Manag. 2018;9:107–111. [Google Scholar]
- 17.Abusalem M., Awwad A., Ayad J., Abu Rayyan A. Green synthesis of α-Fe2O3 nanoparticles using pistachio leaf extract influenced seed germination and seedling growth of tomatos. JJEES. 2019;10:161–166. [Google Scholar]
- 18.Niluxsshun M.C.D., Masilamani K., Mathiventhan U. Green Synthesis of Silver Nanoparticles from the Extracts of Fruit Peel of Citrus tangerina, Citrus sinensis, and Citrus limon for Antibacterial Activities. Bioinorg. Chem. Appl. 2021;2021:6695734. doi: 10.1155/2021/6695734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Priya Ashique S., Afzal O., Khalid M., Ahmad M.F., Upadhyay A., Kumar S., Garg A., Ramzan M., Hussain A., Altamimi M.A., et al. Biogenic nanoparticles from waste fruit peels: Synthesis, applications, challenges and future perspectives. Int. J. Pharm. 2023;643:123223. doi: 10.1016/j.ijpharm.2023.123223. [DOI] [PubMed] [Google Scholar]
- 20.Al-Amri N., Tombuloglu H., Slimani Y., Akhtar S., Barghouthi M., Almessiere M., Alshammari T., Baykal A., Sabit H., Ercan I., et al. Size effect of iron (III) oxide nanomaterials on the growth, and their uptake and translocation in common wheat (Triticum aestivum L.) Ecotoxicol. Environ. Saf. 2020;194:110377. doi: 10.1016/j.ecoenv.2020.110377. [DOI] [PubMed] [Google Scholar]
- 21.Shirsat S., Suthindhiran K. Iron oxide nanoparticles as iron micronutrient fertilizer—Opportunities and limitations. J. Plant Nutr. Soil Sci. 2023 doi: 10.1002/jpln.202300203. [DOI] [Google Scholar]
- 22.Abou Seeda M.A., El-Sayed A.A., Yassen A.A., Abou El-Nour EA A., Zaghloul S.M., Mervat G.M. Nickel, iron and their diverse role in plants: A review, approaches and future prospective. Middle East J. Appl. Sci. 2020;10:196–219. [Google Scholar]
- 23.Maswada H., Djanaguiraman M., Prasad P. Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J. Agron. Crop Sci. 2018;204:577–587. doi: 10.1111/jac.12280. [DOI] [Google Scholar]
- 24.Irum S., Jabeen N., Ahmad K.S., Shafique S., Khan T.F., Gul H., Anwaar S., Shah N.I., Mehmood A., Hussain S.Z. Biogenic iron oxide nanoparticles enhance callogenesis and regeneration pattern of recalcitrant Cicer arietinum L. PLoS ONE. 2020;15:e0242829. doi: 10.1371/journal.pone.0242829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmansah C., Kutman M.K., Muftuler F.B.Z. Preparation of iron oxide nanoparticles by banana peel extract and its usage in NDT. Measurement. 2022;204:112081. doi: 10.1016/j.measurement.2022.112081. [DOI] [Google Scholar]
- 26.Murashige T., Skoog F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962;15 doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 27.Feng Y., Kreslavski V.D., Shmarev A.N., Ivanov A.A., Zharmukhamedov S.K., Kosobryukhov A., Yu M., Allakherdiev S.I., Shabala S. Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants. Plants. 2022;11:1894. doi: 10.3390/plants11141894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Xi H., Wang P., Yin H. Nanopartcles in Plants: Uptake, Transpoty and Physiological Activity in Leaf and Root. Materials. 2023;16:3097. doi: 10.3390/ma16083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali I., Khan A., Ali A., Ullah Z., Dai D.Q., Khan N., Khan A., Al-Tawaha A.R., Sher H. Iron and zinc micronutrients and soil inoculation of Trichoderma harzianum enhance wheat grain quality and yield. Front. Plant Sci. 2022;13:960948. doi: 10.3389/fpls.2022.960948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tombuloglu H., Slimani Y., Tombuloglu G., Almessiere M., Baykal A. Uptake and translocation of magnetite (Fe3O4) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.) Chemosphere. 2019;226:110–122. doi: 10.1016/j.chemosphere.2019.03.075. [DOI] [PubMed] [Google Scholar]
- 31.Kokina I., Mickeviča I., Jahundoviča I., Ogurcovs A., Krasovska M., Jermaļonoka M., Mihailova I., Tamanis E., Gerbreders V. Plant Explants Grown on Medium Supplemented with Fe3O4 Nanoparticles Have a Significant Increase in Embryogenesis. J. Nanomater. 2017;2017:4587147. doi: 10.1155/2017/4587147. [DOI] [Google Scholar]
- 32.Gebre S.H. Bio-inspired synthesis of metal and metal oxide nanoparticles: The key role of phytochemicals. J. Clust. Sci. 2022;33:1–40. doi: 10.1007/s10876-022-02276-9. [DOI] [Google Scholar]
- 33.Nhut D.T., Duc H.H., Hoang N.H., Ngan H.T.M., Diem L.T., Tung H.T., Khai H.D., Mai N.T.N., Cuong D.M., Luan V.Q., et al. Efficient transgenic plantlet regeneration from hairy roots via somatic embryogenesis and hardening plantlets of Panax vietnamensis by iron nanoparticles-supplied culture. Plant Cell Tissue Organ Cult. (PCTOC) 2022;151:335–345. doi: 10.1007/s11240-022-02355-9. [DOI] [Google Scholar]
- 34.Hernández-Hernández A.A., Aguirre-Álvarez G., Cariño-Cortés R., Mendoza-Huizar L.H., Jiménez-Alvarado R. Iron oxide nanoparticles: Synthesis, functionalization, and applications in diagnosis and treatment of cancer. Chem. Pap. 2020;74:3809–3824. doi: 10.1007/s11696-020-01229-8. [DOI] [Google Scholar]
- 35.Marcus N., Lee J., Liman R.A.D., Ruallo J.M.S., Villaflores O.B., Ger T.R., Hsiao C.D. Potential cytotoxicity of Iron Oxide Nanaoparticles: A Review. Molecules. 2020;25:3159. doi: 10.3390/molecules25143159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezealigo U.S., Ezealigo B.N., Aisida S.O., Ezema F.I. Iron Oxide Nanaoparticles in Biological systems: Antbacterial and toxicology Perspective. JCIS Open. 2021;4:100027. doi: 10.1016/j.jciso.2021.100027. [DOI] [Google Scholar]
- 37.Madubuonu N., Aisida S.O., Ali A., Ahmad I., ZTing-Kai Botha S., Mazza M., Ezema F.I. Biosynthesis of iron oxide nanoparticles via a composite of Psidium guavaja-Moringa oleifera and their antibacterial and photocatalytic study. J. Photochem. Photobiol. B Biol. 2019;199:111601. doi: 10.1016/j.jphotobiol.2019.111601. [DOI] [PubMed] [Google Scholar]
- 38.Begum S., Sahoo T., Swain S., Nayak A., Das S.P.S., Rath S.K., Rath C.C. Fabrication of iron nanoparticles using different bioactive precursors, their characterization and bioactivity evaluation. Sustain. Chem. Environ. 2024;6:100100. doi: 10.1016/j.scenv.2024.100100. [DOI] [Google Scholar]
- 39.Jamzad M., Kamari B.M. Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis L. leaves and evaluation of the antimicrobial activity. J. Nanostruct. Chem. 2020;10:193–201. doi: 10.1007/s40097-020-00341-1. [DOI] [Google Scholar]
- 40.Madubuonu N., Aisida S.O., Ahmad I., Botha S., Zhao T.K., Maaza M. Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Appl. Phys. A Mater. Sci. Process. 2020;126:72. doi: 10.1007/s00339-019-3249-6. [DOI] [Google Scholar]
- 41.Parveen S., Wani A.H., Shah M.A., Devi H.S., Bhat M.Y., Koka J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018;115:287–292. doi: 10.1016/j.micpath.2017.12.068. [DOI] [PubMed] [Google Scholar]
- 42.Jadczak P., Kulpa D., Bihun M., Przewodowski W. Positive effect of AgNPs and AuNPs in in vitro cultures of Lavandula angustifolia Mill. Plant Cell Tissue Organ Cult. (PCTOC) 2019;139:191–197. doi: 10.1007/s11240-019-01656-w. [DOI] [Google Scholar]
- 43.Desai A.S., Singh A., Edis Z., Bloukh S.H., Shah P., Panday B., Agrawal N., Bhagat N. An In Vitro and In Vivo Study of the Efficacy and Toxicity of Plant-Extract-Derived Silver Nanoparticles. J. Funct. Biomater. 2022;13:54. doi: 10.3390/jfb13020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manickavasagam M., Pavan G., Vasudevan V. A comprehensive study of the hormetic influence of biosynthesized AgNPs on regenerating rice calli of indica cv. IR64. Sci. Rep. 2019;9:8821. doi: 10.1038/s41598-019-45214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhosale S.R., Bhosale R.R., Kamble G.S., Shukla S.S., Gadale S.R., Dhavale R.P., Anbhule P.V. Enriching the antimicrobial efficacy of iron oxide with bioderived mesoporous carbon. Org. Chem. Commun. 2024;161:112111. doi: 10.1016/j.inoche.2024.112111. [DOI] [Google Scholar]
- 46.Eldeeb B.A., El-Raheem WM A., Elbeltagi S. Green synthesis of biocompatible Fe3O4 magnetic nanoparticles using Citrus Sinensis peels extract for their biological activities and magnetic-hyperthermia applications. Sci. Rep. 2023;13:19000. doi: 10.1038/s41598-023-46287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira M.I., Magro M.Y., Ming L.C., da Silva M.B., Rodrigues L.F.O.S., Prado D.Z., Bonaiuto E., Baratella D., Roger J.D.A., Lima G.P.P., et al. Sustainable production of high purity curcuminoids from Curcuma longa by magnetic nanoparticles: A case study in Brazil. J. Clean. Prod. 2017;154:233–241. doi: 10.1016/j.jclepro.2017.03.218. [DOI] [Google Scholar]
- 48.Ajanaku C.O., Ademosun O.T., Atohengbe P.O., Ajayi S.O., Obafemi Y.D., Owolabi O.D., Akinduti P.A., Ajanaku P.A. Functional bioactive compounds in ginger, turmeric, and garlic. Front. Nutr. 2022;9:1012023. doi: 10.3389/fnut.2022.1012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khattab S., Alkuwayti M.A., Yap Y., Meligy A.M.A., Ismail M.B., Sherif F.E. Foliar Spraying of ZnO Nanoparticals on Curcuma longa Had Increased Growth, Yield, Expression of Curcuminoid Synthesis Genes, and Curcuminoid Accumulation. Horticulturae. 2023;9:355. doi: 10.3390/horticulturae9030355. [DOI] [Google Scholar]
- 50.Aldayel M.F. Enhancement of the Bioactive Compound Content and Antibacterial Activities in Curcuma longa Using Zinc Oxide Nanoparticles. Molecules. 2023;28:4935. doi: 10.3390/molecules28134935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.