Abstract
Although research suggests that callous-unemotional (CU) traits are underpinned by deficits in social affiliation and reduced sensitivity to threat, there has been little investigation of the biophysiological regulatory mechanisms underlying these processes in infancy. The current study uses data from the Durham Child Health and Development Study (DCHD; n = 206) to examine whether and how the combination of infants’ behavioral reactivity and levels of respiratory sinus arrhythmia (RSA), an indicator of parasympathetic nervous system functioning, during the still-face episode of the still-face paradigm at 6 months differentiates risk for CU traits and oppositional defiant behaviors (ODD) at age 3 years, as well as whether these relations vary by children’s attachment security. Results indicate that reduced negative affect during the still-face episode at 6 months predicts higher CU traits (B = −0.28, β = −0.27, p = 0.003) and ODD (B = −0.35, β = −0.24, p = 0.007) at 3 years. Results also show that comparatively lower RSA, i.e. engaged parasympathetic system, predicts higher CU traits (B = −0.10, β = −0.34, p = 0.013), but not ODD. Tests of moderation suggest the combination of blunted negative affect but comparatively lower RSA levels during a social stressor constitutes risk for later CU traits for children who are also insecurely attached (simple slope = −0.70, t = 2.88, p = 0.006 at −1 SD). Findings contribute to our understanding of the complex and interactive risk processes which precede the development of CU traits.
Keywords: Regulation, Infancy, Callous-unemotional traits, Attachment, PNS Functioning, Respiratory Sinus Arrhythmia
Introduction
Callous-unemotional (CU) traits are characterized by shallow affect, reduced emotionality, lower guilt and empathy, and reduced prosociality. CU traits indicate an increased risk for patterns of more severe and persistent aggression and violence that differentiates them from other externalizing behavior problems (Frick et al., 2014a; Lynam et al., 2009). Impaired social affiliation and reduced biobehavioral sensitivity to environmental threat have been proposed as core deficits differentiating CU traits from other externalizing behavior problems (see STAR model; Waller & Wagner, 2019; Viding & McCrory, 2019). These deficits are thought to have cascading consequences that may exacerbate risk for maladaptive outcomes via disruptions to socioemotional and interpersonal processes across development (Waller & Wagner, 2019). However, despite this growing body of literature, very little is known about the neurophysiological mechanisms that may underpin these deficits in infancy.
Recent theoretical work suggests that parasympathetic nervous system (PNS) functioning may be a useful indicator of early risk for CU traits as it provides insight into neurophysiological systems underlying variation in social functioning (Wagner & Waller, 2020). Respiratory sinus arrhythmia (RSA) is an index of PNS functioning that supports the regulation of behavioral, emotional, and attentional responses to nuanced environmental and social challenge (Porges, 2007; Wagner & Waller, 2020). To date, research examining links between the PNS, including RSA, in infancy and later CU traits has focused primarily on differences in baseline or basal functioning. While resting state PNS functioning is informative of trait-level capacity for emotional and social regulation, examination of PNS activity in response to social threat may provide additional clarity into how the social engagement system facilitates (mal)adaptive responses to emotional and social cues (Hastings & Kahle, 2019). That is, individual variability in levels of RSA in the context social threat and stress provide insight into the interface between the prefrontal cortex (PFC) and the PNS during processes of self-regulation (Thayer et al., 2009). As such, examining links between RSA in these contexts and CU traits is an important next step given many of the core deficits linked with CU traits appear to stem from errors in processing and responding to environmental cues across an array of social contexts (Waller & Wagner, 2019).
In addition to deficits in affiliation, threat sensitivity, and their underlying neurophysiological correlates, there is substantial evidence for the influences of the early parent–child relationship on children’s externalizing behavior problems and CU traits (Wagner et al., 2019; Wagner et al., 2016; Waller et al., 2019). Over time, early caregiving experiences are internalized and translated into internal working models which inform children’s primary attachment relationships (Bretherton & Munholland, 2008). Insecure attachment is a known risk factor for CU traits (Pasalich et al., 2012; Rehder et al., 2020), and disorganized family representations have been shown to mediate links between early maladaptive caregiving and later CU traits but not conduct problems (Wagner et al., 2015). Moreover, it is well known that children’s attachment insecurity, which reflects a history of caregiving experiences across infancy, may exacerbate links between temperamental and regulatory risk and later psychopathology (Glenn, 2019; Wagner, et al., 2016a, 2016b). However, the extent to which children’s attachment security moderates the hypothesized links between biobehavioral regulation during social stress in infancy and later CU traits remains unexplored.
To address these gaps in the literature, the current study examines whether and how individual variability in PNS activity across the Face-to-Face Still-Face Paradigm at age 6-months (FFSFP; Tronick et al., 1978) differentiates risk for later CU traits and oppositional defiant behaviors (ODD), a sub category of externalizing behaviors distinguishable from CU traits by 3 years of age (Frick et al., 2014b). While ODD is characterized by a difficult temperament, disobedience, anger, and irritability, these children have notably better outcomes in regard to violence and criminal behavior as compared to children high on CU traits (Frick et al., 2014b). As such, understanding the underlying biological mechanisms that differentiate risk for CU traits and ODD would contribute to isolating core markers in early development. In addition, the current study tests whether children’s attachment security moderates these links. This research provides additional insight into whether and how infants’ parasympathetic regulation contributes to the theorized influences of threat sensitivity and social affiliation on CU traits in early childhood.
Threat Sensitivity, Affiliative Reward, and CU Traits
Early interactions with caregivers provide an opportunity for infants to engage in social learning, consolidate regulatory capabilities, and build a foundation for increasingly complex interactions (Beebe et al., 2010; Feldman et al., 1999; Sameroff, 2010; Tompkins et al., 2018). Reciprocal interactions between infants and caregivers, which are maintained by the rewarding nature of these affiliative experiences, promote bonding, provide a foundation for the transfer of regulatory responsibility to the developing child, and contribute to the formation of secure attachments (Nelson & Panksepp, 1998; Waller & Wagner, 2019). Deficits in affiliative reward, often manifest behaviorally as decreased frequency in attempts to engage in reciprocal social interactions, may disrupt critical caregiving processes, thus interfering with the development of socioemotional and interpersonal skills which otherwise might be protective against CU traits (Waller & Wagner, 2019).
There is accumulating behavioral evidence that deficits in social affiliation precede CU trait emergence, including reduced preferential orientation to the human face at 5-weeks (Bedford et al., 2014), less attention to the parent during parent-infant interactions at 6 months (Bedford et al., 2017), less imitation of the arbitrary actions of others (Wagner et al., 2020), and lower displays of affection toward adoptive parents in infancy (Waller et al., 2016). Using data from the same sample as the current study, Wagner and colleagues (2016a) have previously shown that reduced gaze towards the caregiver during the free play episode of the still-face paradigm is predictive of later CU traits (Wagner et al., 2016a). Research with older children also provides preliminary insight into the biological underpinnings of these associations. For example, children with CU traits show reduced neural responsivity to social stimuli that might otherwise promote affiliation including amygdala hyporeactivity to facial expressions of emotions (Lozier et al., 2014; Viding et al., 2012) and reduced reactivity while imagining others’ feelings (Sethi et al., 2018).
Impairments in responsivity to threat and fear learning in childhood have long been considered characteristic deficits of children with CU traits (Barker et al., 2011; Blair et al., 1997, 2013; Dadds & Salmon, 2003; Frick et al., 2014a, b; Goffin et al., 2018; Marsh, 2019; Patrick et al., 2009; Waller et al., 2019). Well established theoretical models suggest that neurobiologically-based deficits in fearful arousal contribute to impaired reinforcement learning (Blair, 2013) and may lead to a failure to inhibit maladaptive behavioral responses to the emotional displays of others (Dadds & Salmon, 2003). Supporting evidence includes research showing that preschool-aged children with CU traits demonstrate profound deficits in recognizing fearful faces (Kimonis et al., 2016), and longitudinal research links behavioral fearlessness with CU traits across early childhood (Goffin et al., 2018; Waller et al., 2017). A growing body of research also demonstrates that children and adolescents with CU traits show reduced neurophysiological (e.g., electrodermal, autonomic, neurological) responses to fear and threat stimuli (see: Blair, 2013; Marsh, 2019).
Taken together, there is strong evidence identifying deficits in social affiliation and threat sensitivity as core mechanisms underlying the emergence and stability of CU traits. Importantly, however, because the majority of this research has relied heavily on behavioral measures of affiliation and fear, the neurophysiological mechanisms underlying the links between CU traits and impaired social affiliation or fear response in early life remain underexplored.
PNS Functioning and the Parent-Infant Relationship
The majority of studies examining the relations between CU traits and ANS functioning have focused on heart rate (HR) or heart period. For example, consistent with phenotypic impairments in threat sensitivity and affiliation, high CU traits are often linked with lower resting HR (Dietrich et al., 2007) and reduced HR reactivity across emotionally evocative or stress-inducing contexts (Isen et al., 2010; Raine, 2002). However, measures of HR do not allow for sympathetic and parasympathetic influences to be disentangled, which limits the conclusions that can be drawn regarding underlying regulatory mechanisms. Moreover, it is difficult to draw direct comparisons with studies employing measures of RSA given HR is subject to modulation by some combination of parasympathetic, sympathetic, and other (e.g., respiration) influences (Cacioppo et al., 2007).
There is growing theoretical and empirical support for examining the relations between PNS functioning, specifically, and CU traits. The activity of the parasympathetic branch of the ANS regulates cardiac output to support engagement and disengagement across nuanced changes in the environment, regulating the distribution of behavioral, emotional, and attentional resources (Porges, 2001). CU traits are associated with functional differences in the same brain regions that influence cardiac functioning through the PNS (Holzman & Bridgett, 2017), links which have led researchers to argue that individual variability in PNS functioning might enhance our understanding of the etiology of CU traits (see Wagner & Waller, 2020 for review). Measures of baseline RSA provide insight into individual differences in the resources individuals may have to call upon in service of effective regulation, or the capacity to adaptively regulate in response to environmental challenge or threat (Porges, 1996; Wagner & Waller, 2020). A growing body of research links individual variability in resting PNS functioning and emotional and behavioral regulation (Porges, 2007), as well as various forms of psychopathology including CU traits (Beauchaine & Cicchetti, 2019). For example, low resting RSA has been linked with increased psychopathology including lower prosocial behavior and deficits in emotional regulation, social competence, and social regulatory capacities (Beauchaine et al., 2013). Mills-Koonce and colleagues (2015) found that lower baseline RSA at 15 months of age was related to children showing higher CU traits at age 7, and Wagner and colleagues (2015) found that lower baseline RSA across the first two years of life predicted higher CU traits at age 36 months. These findings from studies in infancy and early childhood seem to stand in contrast to studies of older children linking low resting HR (typically associated with higher RSA) with aggression, antisocial behaviors, and CU traits (Fanti et al., 2017; Portnoy et al., 2014; Raine et al., 1997; Sijtsema et al., 2010).
While measures of baseline PNS functioning index trait-level capacity to organize physiological resources in response to challenge or threat, examination of the functioning of the PNS via RSA during experiences of social stress or threat provides insight into individual variability in one’s capacity to adaptively respond to environmental social cues (Hastings & Kahle, 2019). Via projections made through efferent fibers originating with the cranial and spinal nerves, the PNS makes moment-to-moment adjustments in metabolic output in response to the shifting environmental demands presented under conditions that encompass all situations that do not constitute severe threat of harm (Porges, 1996). Because the prefrontal cortical areas that modulate the activity of the PNS are active during the exercise of volitional self-regulatory processes (Buhle et al., 2014), examining PNS activity during contexts of social interaction provides insight into how these experiences are perceived and the metabolic resources called upon to support the effective navigation of the experience. Thus, patterns of PNS functioning indexed via RSA provide insight into the substrates of behaviors underpinned by prefrontal cortex activation, influencing self-regulation, social communication, and attention (Porges, 2007; Thayer et al., 2009, 2012).
In the context of challenge or threat, lower levels of RSA indicate increased engagement of the PNS and may suggest the cue is perceived as requiring allocation of physiological resources in support of a behavioral response (Beauchaine, 2015; Berntson et al., 2008; Cacioppo et al., 2007). In these contexts, brief periods of comparatively lower RSA may be advantageous insofar as it allows individuals to engage a behavioral response, while a blunted response (e.g., comparatively higher RSA) may indicate a failure to appropriately engage (Beauchaine & Cicchetti, 2019). As such, exploring the parallel processes involved in behavioral and physiological responses to environmental challenges can provide critical insight into how specific experiences are perceived. The current study advances this literature by examining whether and how variability in patterns of behavioral response and PNS activity during social stress in infancy confer specific risk for later CU traits.
In addition to the large body of research examining neurophysiological risk mechanisms, longitudinal studies demonstrate that multiple aspects (e.g., harsh-intrusive behaviors, sensitivity and warmth) of the early parent–child relationship exert enduring influence on risk for CU traits (Wagner et al., 2019; Waller et al., 2013). Moreover, this body of research highlights the importance of the parent-infant relationship in particular. For example, Willoughby and colleagues (2013) found that harsh parenting in infancy but not toddlerhood predicted CU traits (Willoughby et al., 2013) and another study found that positive reinforcement from an adoptive mother at 18 months buffered the effects of heritable risk for CU traits (Hyde et al., 2016). Ongoing interactions with caregivers across infancy are translated into internalized representations of the parent-infant relationship (Bretherton, 1985; Bretherton & Munholland, 2008), and guide children in seeking and interpreting their social world across development. Indeed, a growing number of longitudinal studies demonstrate links between insecure attachment and later CU traits (Pasalich et al., 2012; Rehder et al., 2020), and one paper suggests that the ways in which children internalize early experiences with caregivers mediates links between early risky parenting and later CU traits (Wagner et al., 2015).
Research shows that infants and young children demonstrating PNS dysregulation (i.e., patterns of RSA functioning which are discordant from corresponding behavioral responses) are most susceptible to negative influences of unpredictable, harsh, or otherwise maladaptive environments in infancy, including stressful or volatile caregiving relationships (Beauchaine & Cicchetti, 2019; Belsky & Pluess, 2009; Ellis & Boyce, 2008; Wagner et al., 2018). We advance this literature by examining whether observed negative affect and RSA functioning during the still-face episode of the FFSFP combine to influence later ODD and CU traits, and whether these associations vary as a function of children’s attachment security.
The FFSFP is a parent-infant social challenge task which involves three distinct stages of structured interaction: a period of naturalistic interaction (face-to-face episode), 2-min sustaining an emotionless face during which the parent cannot respond to infant bids for engagement (still-face episode), and a return to normal interaction (reunion episode). The still-face episode violates expectations for social interactions and is typically experienced as threatening and distressing by infants aged 2- through 12-months, thus providing insight into self-regulatory strategies during times of heightened social stress (Adamson & Frick, 2003; Mesman et al., 2009; Tronick et al., 1998; Weinberg et al., 2008; Willoughby et al., 2011). Research suggests that infants who engage in more synchronous play with their caregivers demonstrate more advantageous RSA regulation during the still-face, indicating adaptive autonomic responses to social stress (Moore & Calkins, 2004; Moore et al., 2009). The current study leverages the FFSFP to test if RSA functioning during a social stressor in infancy differentiates risk for later CU traits from ODD, as well as whether these associations vary as a function of attachment quality.
Current Study
The current study addresses gaps in the literature and advances our understanding of precursors to CU traits in the following ways. First, we tested whether and how individual variability in behavioral responding during the still-face episode, RSA functioning during the still-face episode, and attachment insecurity predicted CU traits and ODD at 3 years. Consistent with extant theory and research including previous findings suggesting reduced negative affect during the still-face is a risk factor for CU traits, we hypothesized infants who demonstrate lower levels of negative affect and comparatively higher levels of RSA during the face-to-face and still-face episodes of the FFSFP would exhibit higher levels of CU traits at 3 years. Furthermore, given research suggesting PNS dysregulation (i.e., low baseline RSA or comparatively lower levels of RSA during challenge and stress) as a general risk factor for externalizing behavior problems, we predicted that later ODD would be associated with lower levels of RSA across this period. Second, we tested the extent to which the combined influence of reduced negative affect and variation in levels of RSA during stress differentiated risk for ODD and CU traits, as well as whether and how these associations varied as a function of children’s attachment security. Specifically, consistent with research identifying PNS dysregulation as a susceptibility factor, we predicted attachment insecurity would exacerbate the hypothesized relations between RSA functioning during a social stressor and later ODD and CU traits.
Methods
Participants
The Durham Child Health and Development Study (DCHD) is a prospective longitudinal study of 206 healthy, full-term children and their families. Participants were recruited when the children were 3 months old, and subsequently observed in 6-month increments through 36 months, and again once a year from preschool through second grade. Families were recruited from a largely urban community in accordance with stratified sampling to ensure variation in SES related developmental processes, with measures taken to ensure there was approximately equal representation across self-identified racial categories and income (56% African-American, 44% European-American; 53% below 200% of the poverty level). Demographic information was collected during the first visit at 3-months of age and updated at each subsequent visit.
The current study uses observational and parent-questionnaire data collected from the 6-month, 12-month, and 36-month time points. All ratings and observations occurred in a laboratory setting except for the observation of parent–child interactions during free play (contributing to relationship quality), which were conducted at the participants’ homes. At each visit, infants and their mothers participated in several joint and individual activities and mothers completed a standardized interview and demographic questionnaires. Families were compensated $50 for their participation at each timepoint and transportation was provided to families who required assistance getting to and from the laboratory. Parental consent was provided prior to data collection at every time point. All procedures and protocols were approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Measures
Face-to-Face Still-Face Paradigm.
The infants were observed in the FFSFP (Adamson & Frick, 2003; Tronick et al., 1978) during the 6-month lab visit to assess infants’ regulatory capacities while experiencing a stressful context. Mothers were given a set of standardized instructions for each episode of the FFSFP (i.e., FF face-to-face, SF still-face, reunion). During the FFSF, the mothers were instructed to talk and interact with their child for 2 min normally (FF episode), then to turn away from the child for 15 s. After returning to face the child, the mothers were instructed to maintain a fixed stare, refraining from facial movements or display of affect for 2 min (SF episode). After turning head away for another 15 s, the mothers were to again interact normally with the child for two-minutes. Negative child affect was demonstrated most often during the face-to-face episode and still-face episodes (Ekas et al., 2013; Mesman et al., 2009). The FFSFP was stopped if the infant was unable to be soothed at any point during the procedure. The episodes were recorded to ensure the behaviors of mothers and infants could be observed and coded for the entire interaction.
Infant affect was coded by a trained team of research assistants unaware of the hypotheses of the current study. In separate viewings of the tapes, research assistants coded infant facial affect in 1-s intervals. Affect was coded as positive, neutral, or negative. Coders were trained to reliability using a large pool of pre-existing video recordings of FFSFP interactions. To assess interobserver agreement in the current study, 15% of the interactions were selected randomly and coded by a second coder. Inter-observer agreement was determined if they coded the same behavior within one second of each other. Reliability was calculated using kappa to correct for chance agreement (K = 0.89). Affect used in the current analyses was computed as proportions of the total valid interaction time that an infant spent in positive and negative affective states.
RSA.
During the 6-month laboratory visit, children were equipped with heartrate monitors used to collect vagal tone data. Researchers placed two disposable pediatric electrodes on the child’s chest connected to a preamplifier and transmitted to a vagal tone monitor for R-wave detection (VTM-1, Delta Biometrics, Inc., Bethesda, MD). Heart rate data was continuously collected for all procedures during the visit. IBI files were edited using MXEdit software to account for movement artifact (Delta Biometrics, Bethesda, MD). Measures of RSA were extracted using Porges’ (1985) method, applying an algorithm to the sequential IBI data using a moving 21-point polynomial to detrend periodicities in heart period slower than RSA. A band-pass filter extracts the variance of the IBIs within the frequency band of spontaneous respiration in children. This estimate of RSA is derived by calculating the natural logarithm of the variance, reported in units of ln(msec)2.
RSA was calculated every 15 s during each 2-min episodes (baseline, FF, SF, reunion) and an average of these epochs was used to index individual variability in PNS functioning during each episode. These durations are typical for studies of short duration tasks (Huffman et al., 1998). RSA from the face-to-face episode, which immediately preceded the still-face episode, was included as a covariate in all analyses in order to isolate the associations between levels of RSA during the still-face episode and study outcomes. Simulation studies have demonstrated that adjusting for autoregressive effects (e.g., examining RSA during the still-face episode controlling for RSA during the preceding free play episode) provides superior statistical estimation as compared to change scores or percent change from baseline scores (Zhang et al., 2014). As such, individual variability in PNS activity during the still-face episode was isolated by controlling for RSA levels in the face-to-face episode immediately prior. Comparatively lower levels of RSA during the still-face episode represent increased engagement of the PNS in response to social stress. Studies aiming to examine the RSA functioning across a specific context have adopted similar autoregressive approaches (see Miller et al., 2013; Gatzke-Kopp et al., 2015).
CU Traits and ODD.
Measures of ODD and CU behaviors were created using items from the Achenbach System of Empirically Based Assessment, (ASEBA; Achenbach & Rescorla, 2000, a.k.a Child Behavior Checklist) which was completed by the infants’ primary caregiver at the 36-month visit. The ASEBA is a standardized assessment that indexes behavioral and emotional problems using caregivers’ ratings of their child’s behavior over the 2 months prior using DSM-referenced scales (Achenbach & Rescorla, 2000). A scoring profile drawn from DSM scales for ODD comprised of defiant, disobedient, angry moods, stubborn, temper tantrum, and uncooperative. Items for “no guilt after misbehave,” “punish does not change behavior,” “unresponsive to affection,” “shows little affection,” and “too little fear” from the ASEBA measure early CU traits (Waller et al., 2015; Willoughby et al., 2011, 2013, 2014). The ODD behavior measure demonstrated adequate internal consistency at 36-months (α = 0.83). Internal consistencies for the CU behavior measure items were modest at 36-months (α = 0.65), but they were comparable to those reported by other studies using the ASEBA at these ages (Song et al., 2016; Waller et al., 2012, 2014). Centered scores for ODD and CU behaviors in early childhood were used to support interpretability of regression coefficients and interaction plots.
Attachment.
Attachment quality was assessed at the 12-month laboratory visit through the Strange Situation Paradigm (Ainsworth et al., 1978), where the child undergoes separations and reunions with their primary attachment figure. The procedure is designed to trigger specific attachment behaviors that are characteristic of children from 10- to 18-months. The child is placed in a series of “strange situations,” each of which is defined by increasing levels of stress and demands on the attachment system. There are eight episodes presented in a standard order with the least stressful occurring first. Classifications were made (e.g., insecure, secure) using behavior scales for proximity seeking, contact maintenance, resistance, and avoidance. The focal points for classification purposes occur during the reunion episodes, which reveals the child’s patterned strategy and its effectiveness in using the mother as a secure base. Attachment was coded by an experienced research assistant who successfully passed a centralized reliability exam (Main & Solomon, 1990). All analyses used a dichotomous variable to characterize children’s attachment security (0 = secure, 1 = insecure). Distributions of attachment insecurity were consistent with reports from other community samples (Fearon et al., 2010), but the current sample size limited the ability to test associations among sub classifications of insecurity (e.g., disorganized, resistant, and avoidant).
Additional Covariates.
Additional covariates were selected a priori based on their theoretical relations with the primary predictors and outcome to account for confounding contributions to the processes of interest. Demographic and income variables were included to control for the documented associations between these characteristics, biobehavioral markers, and children’s later ODD or CU traits. Specifically, information on children’s sex and race was collected upon entry into the study. Families’ poverty status was calculated as the federal poverty threshold for the appropriate family size using income data provided by parents at the 6-month time point (U.S. Bureau of the Census, 2009). Distress to limitations, a subscale of infant temperament, was assessed through the Infant Behavior Questionnaire (Goldsmith & Rothbart, 1991) which mothers completed at the 6-month lab visit. The questionnaire rates the frequency of temperament-related behaviors that may have occurred in a variety of everyday situations and that were observed over the 1–2 weeks prior. The current study includes a measure of distress to limitations at 6-months as a covariate to account for the influences of children’s difficult temperament on the parent–child relationship, as well as potential responder bias.
In addition, relationship quality at 6-months was assessed through observation of parent–child interactions which were later coded by trained and reliable research assistants who rated the interactions using a 5-point scale (Vernon-Feagans et al., 2013). Relationship quality assesses the degree to which the parent-infant relationship is characterized by shared experience, intimacy and coordination. This may be reflected by reciprocal play, communication, and shared enjoyment. Dyads receiving low scores may demonstrate interactions which are stifled, conflictual, or non-reciprocal. These interactions may also appear perfunctory or mechanical whereas dyads with high scores demonstrate shared emotion, engagement, interest, and acceptance. Reliability across coders was high (intraclass correlations > 0.80 coder pairs). The current study includes a measure of relationship quality derived from observations outside of the context of the FFSFP in order to isolate the relations between infants biobehavioral functioning during the FFSFP and later ODD and CU traits. That is, our aim was to control for variance due to the general quality of interactions between the parent and infant as to isolate the specific experience of the still-face episode. Finally, measures of negative affect and RSA during the face-to-face episode, which immediately preceded the still-face episode, were included in all analyses to isolate the associations between affect and RSA during the still-face and later CU traits and ODD.
Analytic Plan
The proposed hypotheses were addressed by estimating a series of saturated path models in Mplus 8.3 (Muthén & Muthén) using full information maximum likelihood (FIML) (Enders & Bandalos, 2001) which is a well-recognized method for analyzing missing data. CU traits and ODD were included as covarying outcomes in all models to isolate the associations between study predictors and outcomes at age 3 years. First, CU traits and ODD at 3 years were included as covarying dependent variables in a model testing the direct influences of observed negative affect during the still-face, RSA during the still-face, and children’s attachment security, over and above study covariates including infants’ RSA during the preceding episode (i.e., face-to-face). Next, a multiple group approach was used to test whether and how observed negative affect and RSA during the still-face episode combine to influence later ODD and CU traits, and whether these associations vary as a function of children’s attachment security. Significant main effect and interaction terms which varied across secure and insecure groups were formally tested using the MODEL TEST command in Mplus 8.3 which conducts a joint test of equality of the model parameters across groups using the Wald test with k-1 degrees of freedom (Muthén & Muthén, 2009). A multiple groups modeling approach was taken to compare insecurely versus securely attached to leverage the categorical nature of the variable and aid interpretation (e.g., parsimony). Model covariates included the child’s sex, child’s race, poverty status, distress-to-limitations, relationship quality, and children’s negative affect and RSA from the face-to-face episode which immediately preceded the still-face episode.
The nature of significant interactions was elucidated following the recommendations provided by Roisman and colleagues (Roisman et al., 2012). First, in order to estimate a snapshot of the association between the predictor and outcome at two specific reference points, significant interactions were probed at one standard deviation above and below the mean for the moderator variables (still-face RSA). Second, regions of significance (RoS) analyses, which identify the exact range of values of the moderator for which the independent and dependent variables are significantly associated, were used to determine at which levels of still-face RSA does reduced negative affect predict study outcomes, but also for which levels of negative affect observed associations were significant. Significant interactions were probed, and simple slopes were examined to identify regions of significance.
Results
Table 1 presents the bivariate correlations, means, and standard deviations for the model covariates and variables of interest. Negative affect during the still-face episode is negatively correlated with poverty level and positively correlated with negative affect during the face-to-face episode. RSA during the still-face episode is negatively correlated with race, and positively correlated with temperamental distress to limitations and RSA during the face-to-face episode. ODD is negatively associated with gender. CU traits are negatively correlated with poverty level. CU traits and ODD are positively correlated.
Table 1.
Zero-order Bivariate Correlations Between Model Outcomes
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1. Sex (Male = 1) | - | |||||||||||
| 2. Race (EA = 0, AA = 1) | 0.08 | - | ||||||||||
| 3. Poverty Status | 0.01 | 0.19** | - | |||||||||
| 4. Distress to Limitations (6 m) | −0.03 | −0.23** | −0.20** | - | ||||||||
| 5. Relationship Quality (6 m) | −0.03 | 0.28** | 0.30** | −0.01 | - | |||||||
| 6. Negative Affect, FF (6 m) | 0.06 | 0.08 | −0.02 | −0.08 | 0.14 | - | ||||||
| 7. RSA, FF (6 m) | 0.01 | −0.21* | −0.08 | 0.15 | 0.15 | −0.23* | - | |||||
| 8. Negative Affect, SF (6 m) | 0.12 | −0.03 | −0.21** | 0.08 | −0.07 | 0.32** | 0.02 | - | ||||
| 9. RSA, SF (6 m) | 0.06 | −0.26** | −0.10 | 0.21* | 0.01 | −0.10 | 0.76** | −0.05 | - | |||
| 10. ODD (36 m) | 0.16* | 0.08 | −0.02 | 0.09 | −0.12 | −0.08 | −0.16 | −0.17 | −0.08 | - | ||
| 11. CU traits (36 m) | 0.12 | 0.04 | −0.20** | 0.06 | −0.17 | −0.10 | −0.13 | −0.13 | −0.19 | 0.66** | - | |
| 12. Insecure Attachment (12 m) | 0.13 | −0.04 | −0.01 | −0.03 | −0.04 | 0.06 | −0.08 | 0.04 | −0.03 | 0.13 | 0.10 | - |
| Sample Size | 206 | 206 | 206 | 179 | 135 | 165 | 112 | 159 | 108 | 178 | 178 | 148 |
| Mean | 0.51 | 0.43 | 0.49 | 3.56 | 4.62 | 0.06 | 3.57 | 0 | 0 | 0.51 | 0 | 0.39 |
| Standard Deviation | 0.5 | 0.5 | 0.5 | 0.88 | 1.28 | 0.14 | 0.97 | 0.28 | 1.03 | 0.43 | 0.31 | 0.49 |
FF face-to-face episode, SF still-face episode, AA African American, EA European American
p ≤ 0.05
p ≤ 0.01
Path Models.
Results from the main effects saturated path model (Fig. 1; Table 2) showed that reduced negative affect during the still-face episode significantly negatively predicted both ODD, B = −0.35, β = −0.24, p = 0.007, and CU-traits B = −0.28, β = −0.27, p = 0.003 at 3 years, and that RSA during the still-face episode significantly negatively predicted only CU traits B = −0.10, β = −0.34, p = 0.013. Children’s attachment security did not directly predict ODD or CU traits.
Fig. 1.
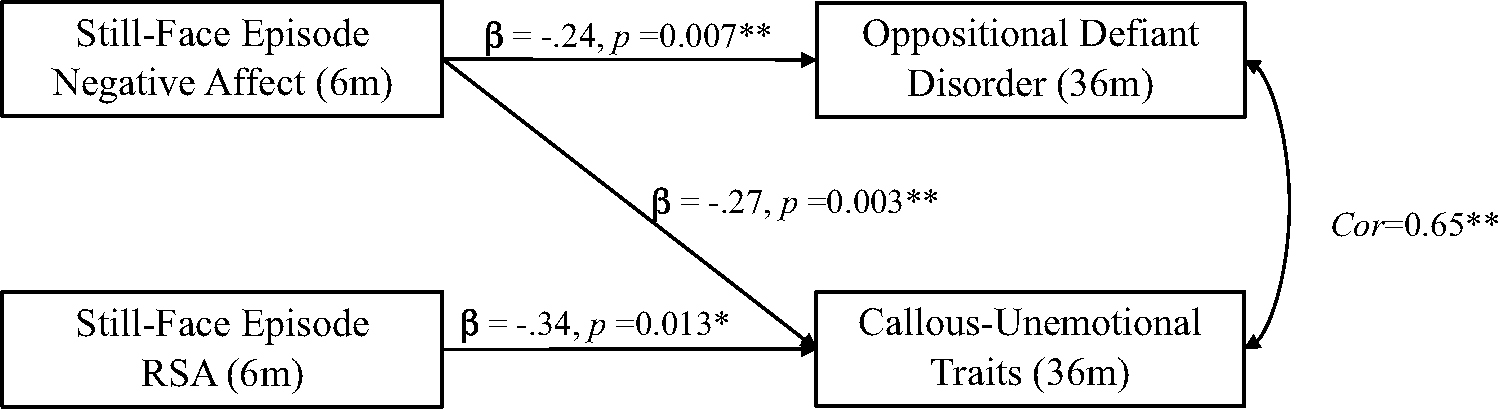
Direct Effects. Path model and standardized estimates of the longitudinal relations between negative affect and RSA during the SF episode at 6-months, and CU traits and ODD at 36-months. Exogenous covariates (gender, race, poverty line, distress to limitations, relationship quality, affect during FF episode, and RSA during FF episode at 6-months) are not included in this diagram but were allowed to covary.
Notes: p ≤ .05*, p ≤ .01**; FF face-to-face episode, SF still-face episode
Table 2.
Model 1: Unstandardized and standardized estimates for longitudinal linear pathway model
| Oppositional Defiant Disorder at 36 m on |
Callous-Unemotional Traits at 36 m on |
|||
|---|---|---|---|---|
| B(β) | CI | B(β) | CI | |
|
| ||||
| Sex (Male = 1) | 0.16(0.18)* | (0.03,0.28) | 0.09(0.15)* | (0.01,0.18) |
| Race (EA = 0, AA = 1) | 0.10(0.12) | (−0.03,0.24) | 0.06(0.09) | (−0.04,0.16) |
| Poverty Status | −0.04(−0.05) | (−0.17,0.09) | −0.15(−0.24)** | (−0.24,−0.05) |
| Distress to Limitations | 0.07(0.14) | (−0.01,0.15) | 0.04(0.10) | (−0.02,0.09) |
| Relationship Quality | −0.03(−0.10) | (−0.09,0.02) | −0.03(−0.1) | (−0.08,0.02) |
| Negative Affect, FF (6 m) | −0.11(−0.04) | (−0.55,0.33) | −0.05(−0.02) | (−0.36,0.26) |
| RSA, FF(6 m) | −0.04(−0.09) | (−0.17,0.09) | 0.05(0.15) | (−0.04,0.14) |
| Negative Affect, SF (6 m) | −0.35(−0.24)** | (−0.61,−0.10) | −0.28(−0.27)** | (−0.47,−0.09) |
| RSA, SF (6 m) | −0.02(−0.05) | (−0.14,0.10) | −0.10(−0.34)* | (−0.18,−0.02) |
| Insecure Attachment | 0.11(0.12) | (−0.04,0.26) | 0.07(0.10) | (−0.04,0.17) |
FF face-to-face episode, SF still-face episode, AA African American, EA European American
p ≤ 0.05
p ≤ 0.01
Next, multiple group models indicated that infants negative affect and RSA during the still-face interacted to predict CU traits for children who were insecurely attached at 12 months but not children who were securely attached. The interaction between infants’ negative affect and RSA during the still-face did not significantly predict ODD. In order to conduct formal tests of equality across groups, the paths regressing CU traits on the interaction term were assigned arbitrary labels and were compared using the MODEL TEST command in Mplus (Muthén & Muthén, 2009). Results confirm that negative affect and RSA interacted to predict CU traits for insecurely attached children but not securely attached children, b = 0.42, β = 0.42, p = 0.023, Wald’s X2(1) = 6.77, p = 0.009.1
Tests of moderation effects revealed a significant interaction between RSA and negative affect during the still-face in the prediction of CU traits, b = 0.41, β = 0.42, p = 0.023, but only for infants who were insecurely attached. Examination of simple slopes revealed that the combination of comparatively less negative affect and lower RSA during the still-face episode predicted higher CU traits for children who were insecurely attached, simple slope = −0.70, t = 2.88, p = 0.006 at −1 SD (Fig. 2; Table 3). Specifically, the RoS analysis indicated that negative affect at just above the centered mean or lower (lower threshold for RoS = 0.30) during the still-face predicted CU traits for individuals demonstrating levels just below the centered mean or lower (lower threshold for RoS = −0.09). The upper thresholds for RoS for both negative affect and RSA were outside of the range of observed data. All standardized and unstandardized parameter estimates and confidence intervals, including parameter estimates between the model covariates and variables of interest, are shown in Table 3.
Fig. 2.
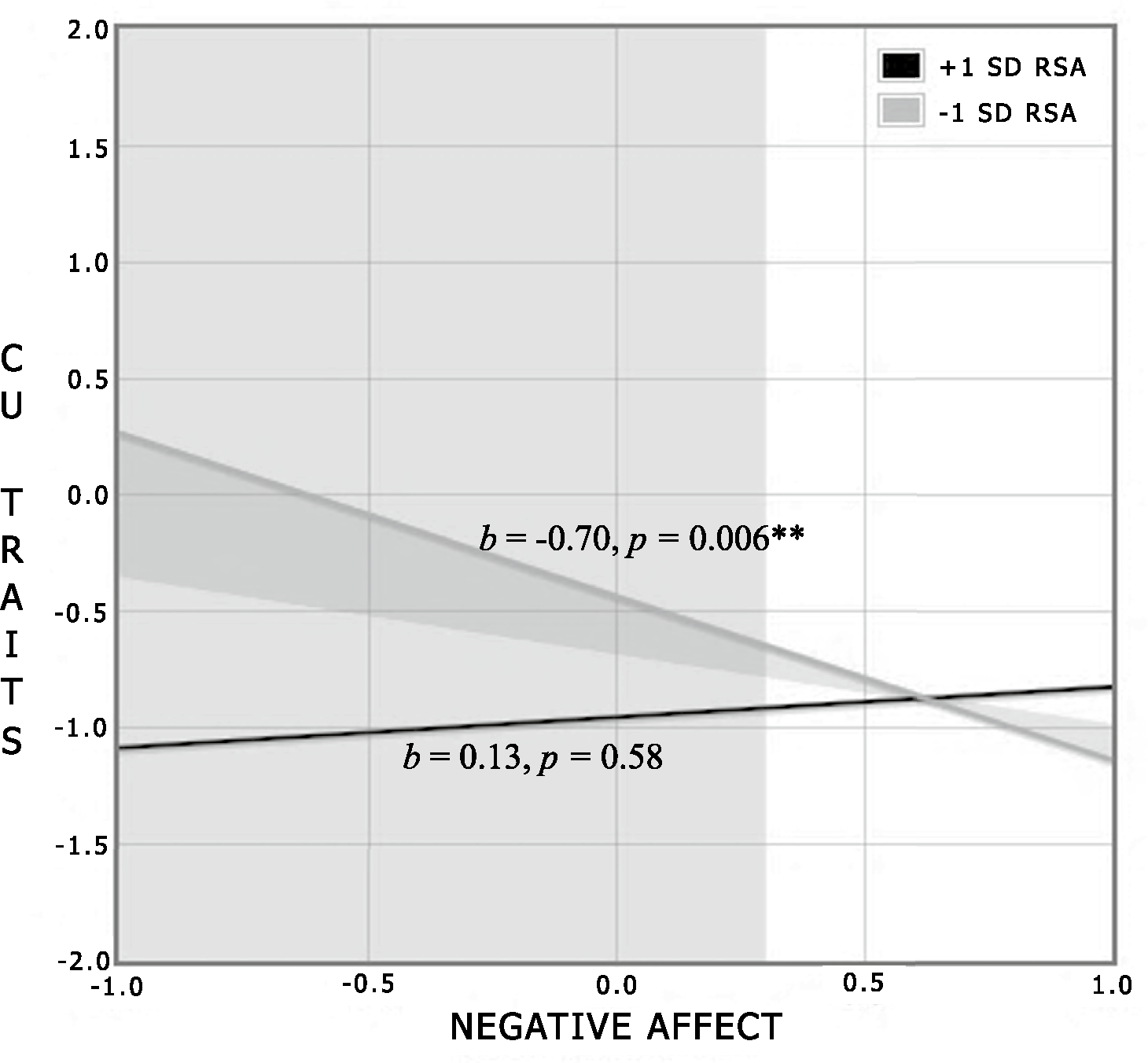
Interaction. Regions of significance and simple slope estimates for the interaction between negative affect and RSA during the still-face in the prediction of CU traits. The shaded areas represent the point at which negative affect predicts higher levels of CU traits for infants with comparatively lower RSA during the still-face episode.
Table 3.
Model 2: Unstandardized and standardized estimates for the prediction of CU traits and ODD
| Oppositional Defiant Disorder at 36 m on | Callous Unemotional Traits at 36 m on | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Secure Attachment (12 m) | Insecure Attachment (12 m) | Secure Attachment (12 m) | Insecure Attachment (12 m) | |||||
| B(β) | CI | B(β) | CI | B(β) | CI | B(β) | CI | |
| Sex (Male = 1) | 0.07(0.09) | (−0.11,0.26) | 0.20(0.21) | (−0.06,0.45) | 0.06(0.11) | (−0.06,0.18) | 0.23(0.32)* | (0.05,0.41) |
| Race (EA = 0, AA=1) | 0.05(0.05) | (−0.14,0.23) | 0.27(0.29) | (−0.07,0.62) | 0.02(0.04) | (−0.11,0.16) | −0.00(−0.00) | (−0.26,0.26) |
| Poverty Status | −0.01(−0.02) | (−0.2,0.17) | −0.05(−0.05) | (−0.37,0.28) | −0.13(−0.23)* | (−0.27,0.01) | −0.21(−0.30) | (−0.46,0.04) |
| Distress to Limitations | 0.02(0.05) | (−0.08,0.12) | 0.20(0.35) | (0.03,0.37) | 0.02(0.07) | (−0.06,0.10) | 0.12(0.29) | (−0.002,0.25) |
| Relationship Quality | 0.01(0.03) | (−0.07,0.09) | −0.12(−0.33) | (−0.26,0.02) | 0.02(0.10) | (−0.06,0.10) | −0.08(−0.27) | (−0.18,0.03) |
| Negative Affect, FF (6 m) | 0.33(0.111) | (−0.99,1.65) | −0.10(−0.03) | (−0.88,0.69) | −0.31 (−0.15) | (−1.36,0.74) | 0.29(0.13) | (−0.22,0.79) |
| RSA, FF(6 m) | −0.08(−0.22) | (−0.29,0.13) | 0.06(0.12) | (−0.26,0.39) | −0.00(−0.01) | (−0.16,0.16) | 0.19(0.49)* | (0.02,0.37) |
| Negative Affect, SF (6 m) | −0.14(−0.10) | (−0.55,0.27) | −0.50(−0.31) | (−1.13,0.13) | −0.07(−0.31) | (−0.22,0.08) | −0.28(−0.23) | (−0.59,0.03) |
| RSA, SF (6 m) | 0.05(0.14) | (−0.14,0.23) | −0.17(−0.38) | (−0.42,0.09) | −0.02(−0.02) | (−0.41,0.37) | −0.26(−0.77)** | (−0.41,−0.11) |
| Interaction (Neg Affect, SF x RSA, SF) | −0.28(−0.20) | (−0.67,0.11) | −0.01(−O.Ol) | (−0.57,0.54) | −0.18(−0.18) | (−0.45,0.09) | 0.42(0.42)* | (0.06,0.77) |
FF face-to-face episode, SF still-face episode, AA African American, EA European American
p ≤ 0.05
p ≤ 0.01
Discussion
The current study is the first to examine links between PNS activity via RSA during a social stressor in infancy and later CU traits. Previous research from this sample shows that reduced negative affect during the still-face episode at 6 months is a risk factor for later CU traits (Wagner et al., 2016a), a pattern of behavioral response that, when considered in the context of existing literature with older children and adolescents, suggest reduced or blunted neurophysiological reactivity. Prior to the current investigation, this reduced behavioral response to the still-face episode was taken to indicate reduced sensitivity to threat. However, contrary to our hypotheses, the current findings show that comparatively lower RSA (e.g., more engaged) during the still-face episode is a specific risk factor for CU traits for infants who are insecurely attached. This illustrates that, while affective reactivity may be subdued, infants at risk for CU traits display a parasympathetic response to this social stressor in infancy. These findings are contrary to the notion that CU traits are preceded by failures to engage with or process social threat. Moreover, multiple group moderation analyses suggest that the risk posed by the combination of reduced behavioral response and comparatively lower RSA during the still-face episode is specific to those children who were insecurely attached.
In one of the first studies to examine links between biological indicators of engagement with threat in infancy and later CU traits, Mills-Koonce and colleagues (Mills-Koonce et al., 2015) found that increased cortisol levels at 15 months in response to being exposed to a scary mask was predictive of later CU traits, but these relations were not present when the task was employed at 6 months. That is, for children who later develop CU traits, the fear task did not elicit a physiological response at 6 months, though there was a prominent fear response at 15 months. The authors suggest that, when coupled with research with older children, the relations between reduced or blunted biobehavioral engagement and CU traits may be emergent during the first 2 years of life. However, the current findings indicate that the patterns of comparatively higher physiological activity in response to social stressors linked with later CU traits may be present earlier in infancy. Moreover, the current findings may also imply that research examining risk associated with neurophysiological regulation in infancy benefits from employing tasks which are ecologically-valid with regard to this specific developmental period. That is, exposure to a scary mask is a nonsocial experience that typically elicits fear in late infancy and early toddlerhood (Goldsmith & Rothbart, 1991) and may not be as appropriate for studying individual differences in fear processing at 6 months.
In addition to providing evidence which extends downward the associations between neurophysiological responses to threat and fear and later CU traits from toddlerhood into infancy, the current study benefits from the use of a behavioral paradigm that leverages the parent-infant relationship as a source of social stress, a relationship that is core to early social experiences (Greenberg et al., 1993). Our findings in the current study suggest that children who are high on CU traits may show less affective social engagement in infancy for reasons other than errors in the perceived saliency or deficits in affiliative motivation. Future research should seek to test these research questions across both social and nonsocial fear-eliciting tasks at different developmental stages throughout infancy and early childhood. Moreover, testing whether associations in the prefrontal and limbic brain structures linked with both CU traits and PNS functioning are present in infancy would be an important next step.
Patterns of adaptation and maladaptation across development are influenced by dynamic and collaborative interplay between the child and their caregiving experiences (Cox & Paley, 1997; Magnusson & Cairns, 1996). Several studies, including those using genetically-informed designs (Hyde et al., 2016; Waller et al., 2016), suggest that parental harshness, insensitivity, and low warmth are risk factors for CU traits (Wagner et al., 2015, 2019; Waller et al., 2013). How these early caregiving experiences are internalized influences the formation of attachment security and may be important for understanding the development of CU traits (Pasalich et al., 2012; Rehder et al., 2020; Wagner et al., 2015).
The patterns of biobehavioral regulation observed in insecurely attached children often reflect strategies that, while adaptive in the short term, are based on experiences of rejection, fear, and uncertainty and may confer risk for a range of psychopathology (Miller et al., 2013). The current study found that the combination of reduced affective reactivity and comparatively lower RSA (e.g., engaged PNS) was a specific risk factor for later CU traits, but only for children who formed insecure attachments. This pattern of functioning has been observed previously in insecure-avoidant attached children and may indicate experiences of rejecting or inconsistent parenting behaviors (Pasalich et al., 2012; Rehder et al., 2020). That is, a history of maladaptive caregiving experiences may result in learned responses characterized by reduced external behavioral bids (e.g., negative affect) for soothing and regulatory support from the parent, despite the dysregulating aspects of the experience (Donovan, 1998; Moore, 2009). Future research should investigate whether and how these developmental processes inform the emergence and stability of CU traits.
Results indicate that an engaged PNS in response to a social stressor, despite a reduced affective response, is predictive of later CU traits, but only for children who also experience early risk environments. Rather, findings are consistent with the Adaptive Calibration Model (ACM; Del Giudice et al., 2011) which proposes that patterns of regulation are optimally calibrated to match environmental pressures, and that repeated exposure to environmental stress may influence the recalibration of physiological systems across development (Gunnar & Quevedo, 2007; McEwen, 1998). That is, it is possible that the patterns of blunted physiological response reported later in life may develop as a consequence of alterations in hormone and ANS functioning triggered by repeated exposure to chronic, severe stress, abuse, and trauma (i.e., secondary variant; Glenn, 2019; Thomson et al., 2020). Indeed, there is substantial evidence in both animal and human literatures that the responsivity and regulation of stress physiology is shaped by early social experiences (see Gunnar & Donzella, 2002; Van Goozen et al., 2008). Moreover, consistent with differential susceptibility and biological sensitivity to context models (Belsky & Pluess, 2009; Ellis & Boyce, 2008), PNS engagement may represent a plasticity or susceptibility factor which promotes positive outcomes or confers risk for negative outcomes depending on the quality of the environment (e.g., parent-infant relationship; Dunbar et al., 2021). Each of these theoretical frameworks suggests a potential point for intervention, as suboptimal caregiving environments are often associated with the development of conduct problems (Elizur et al., 2017; Pasalich et al., 2012; Wagner et al., 2015).
Future research should consider the possibility that emergence of fearlessness associated with later CU traits may be, in part, consequence of a complex developmental progression characterized by (1) hyperreactivity and dysregulation in response to learned social and nonsocial fear stimuli in infancy; (2) extinction of a behavioral fear response driven by short-term adaptive responses to risky environmental contexts in infancy (e.g., rejecting, harsh parenting); (3) generalization of the fearless phenotype from social to nonsocial stimuli; and (4) eventual recalibration from hyper- to hypo-reactive physiological responses. The current study contributes one small piece to this complex, developmental puzzle by exploring the implications of differences in neurophysiological regulation across an ecologically valid context in infancy for later CU traits and ODD.
To our knowledge, this is the first study to examine links between PNS functioning during social stress in infancy and later CU traits and ODD. Our use of the FFSFP to achieve these goals is a strength given its long history of eliciting infants’ behavioral responses to disrupted interactions with caregivers, thus providing an assessment of specific affective and social processes which serve as the basis for emotional and social development across infancy and childhood. Our findings are also strengthened by the longitudinal prospective design of the sample and its demographic diversity, which allows for greater generalizability than is possible with convenience or clinically-based samples. The current findings should also be interpreted in the context of the following limitations. First, our examination of the influences of early attachment security in the current required that we use data from the FFSFP and the assessment of attachment from two different time points. That is, children’s attachment security, which was assessed at 12 months, is treated as a moderator of preceding assessments. However, despite the fact that the FFSFP and attachment assessments were not conducted concurrently, existing literature supports the use of attachment as a moderator in the current study given literature suggesting that the formation of internal working models or schemas underlying the formation of attachment relationships are informed by a history of experiences with caregivers starting at birth (Bretherton & Munholland, 1999). Second, although rates of insecurity in the current sample are consistent with other community samples (Fearon et al., 2010), future research should employ larger samples so that specific insecure attachment classifications (e.g., resistant, avoidant, disorganized) can be examined, something that was not possible given the size of the current sample.
One remaining empirical question is whether the relation between narrow measures of PNS activity and regulation in infancy and later CU traits varies across the type of social (e.g., interacting with parents versus strangers) or nonsocial (e.g., fear versus other emotions) stressors. It is likely that the interplay between the type of task and age have important implications for our understanding of the associations between early neurophysiological regulation and risk for CU traits. The current study provides preliminary foundation for future research, and contributes to an ongoing effort to extend the study of neurophysiological precursors in infancy to ODD and CU traits in childhood, a line of inquiry which has the potential to aid in the development of early and targeted intervention.
Funding
Manuscript preparation was supported by the National Institute of Mental Health (R03MH123762 awarded to NJW) and institutional support from Boston University (NJW). This study was funded by the National Science foundation through Children’s Research Initiative Grant (BCS-0126475).
Footnotes
Conflict of Interest The authors report no conflict of interest.
Tests of a continuous 3-way interaction between infants negative affect during the still-face, RSA during the still-face, and children’s attachment security significantly predicted CU traits, b = 0.483, p = 0.002, but not ODD, b = 0.296, p = 0.249, but multiple group models are presented to leverage the dichotomous nature of children’s attachment security and to aid interpretability.
Compliance with Ethical Standards
Ethics Approval This study was approved by the appropriate institutional research ethics committees and was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Consent to Participate Informed consent was obtained from all individual participants.
Consent for Publication All necessary consents have been obtained.
References
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA preschool forms and profiles (Vol. 30). Burlington, VT: University of Vermont, Research center for children, youth [Google Scholar]
- Adamson LB, & Frick JE (2003). The still face: A history of a shared experimental paradigm. Infancy, 4(4), 451–473. 10.1207/S15327078IN0404_01 [DOI] [Google Scholar]
- Ainsworth MDS, Blehar MC, Waters E, & Wall SN (1978). Patterns of attachment: A psychological study of the strange situation. In Patterns of Attachment: A Psychological Study of the Strange Situation. Lawrence Erlbaum. 10.4324/9780203758045 [DOI] [Google Scholar]
- Barker ED, Oliver BR, Viding E, Salekin RT, & Maughan B (2011). The impact of prenatal maternal risk, fearless temperament and early parenting on adolescent callous-unemotional traits: A 14-year longitudinal investigation. Journal of Child Psychology and Psychiatry, 52(8), 878–888. 10.1111/j.1469-7610.2011.02397.x [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3(509), 43–47. 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, & Cicchetti D (2019). Emotion dysregulation and emerging psychopathology: A transdiagnostic, transdisciplinary perspective. Development and Psychopathology, 31(3), 799–804. 10.1017/S0954579419000671 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp LM, Neuhaus E, Chipman J, Reid MJ, & Webster-Stratton C (2013). Sympathetic- and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. Journal of Consulting and Clinical Psychology, 81(3), 481–493. 10.1037/a0032302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford R, Pickles A, Sharp H, Wright N, & Hill J (2014). Reduced Face Preference in Infancy: A Developmental Precursor to Callous-Unemotional Traits? Biological Psychiatry, 78(2), 144–150. 10.1016/j.biopsych.2014.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford R, Wagner NJ, Rehder PD, Propper CB, Willoughby MT, & Mills-Koonce WR (2017). The role of infants’ mother-directed gaze, maternal sensitivity, and emotion recognition in childhood callous unemotional behaviours. European Child and Adolescent Psychiatry, 26(8), 947–956. 10.1007/s00787-017-0967-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe B, Jaffe J, Markese S, Buck K, Chen H, Cohen P, Bahrick L, Andrews H, & Feldstein S (2010). The origins of 12-month attachment: A microanalysis of 4-month mother-infant interaction. Attachment and Human Development, 12(1–2), 3–141. 10.1080/14616730903338985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, & Pluess M (2009). Beyond Diathesis Stress: Differential Susceptibility to Environmental Influences. Psychological Bulletin, 135(6), 885–908. 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, & Cacioppo JT (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45(4), 643–652. 10.1111/j.1469-8986.2008.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (2013). The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience, 14(11), 786–799. 10.1038/nrn3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Jones L, Clark F, & Smith M (1997). The psychopathic individual: A lack of responsiveness to distress cues? Journal of Abnormal Child Psychology, 40, 449–458. 10.1111/j.1469-8986.1997.tb02131.x [DOI] [PubMed] [Google Scholar]
- Bretherton I (1985). Attachment theory: Retrospect and prospect. Monographs of the Society for Research. Child Development, 50, 3–35. 10.2307/3333824 [DOI] [Google Scholar]
- Bretherton I, & Munholland KA (1999). Internal working models in attachment relationships: a construct revisited [W:] Handbook of attachment. Theory, Research and Clinical Application, 90–101. [Google Scholar]
- Bretherton I, & Munholland KA (2008). Internal working models in attachment relationships: Elaborating a central construct in attachment theory.
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, & Ochsner KN (2014). Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG, & Berntson GG (2007). Handbook of Psychophysiology. Cambridge University Press. 10.1017/s0033291707001201 [DOI] [Google Scholar]
- Cox MJ, & Paley B (1997). Families as systems. Annual Review of Psychology, 48(1), 243–267. [DOI] [PubMed] [Google Scholar]
- Dadds MR, & Salmon K (2003). Punishment insensitivity and parenting: Temperament and learning as interacting risks for antisocial behavior. Clinical Child and Family Psychology Review, 6(2), 69–86. 10.1023/A:1023762009877 [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews, 35(7), 1562–1592. 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Riese H, Sondeijker FEPL, Greaves-Lord K, Van Roon AM, Ormel J, Neeleman JAN, & Rosmalen JGM (2007). Externalizing and Internalizing Problems in Relation to Autonomic Function: A Population-Based Study in Preadolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 46(3), 378–386. 10.1097/CHI.0b013e31802b91ea [DOI] [PubMed] [Google Scholar]
- Donovan WL (1998). Conflict and depression predict maternal sensitivity to infant cries. Infant Behavior and Development, 21(3), 505–517. 10.1016/S0163-6383(98)90023-6 [DOI] [Google Scholar]
- Dunbar AS, Zeytinoglu S, & Leerkes EM (2021). When is Parental Suppression of Black Children’s Negative Emotions Adaptive? The Role of Preparation for Racial Bias and Children’s Resting Cardiac Vagal Tone. Research on child and adolescent psychopathology, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekas NV, Haltigan JD, & Messinger DS (2013). The dynamic still-face effect: Do infants decrease bidding over time when parents are not responsive? Developmental Psychology, 49(6), 1027–1035. 10.1037/a0029330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizur Y, Somech LY, & Vinokur AD (2017). Effects of Parent Training on Callous-Unemotional Traits, Effortful Control, and Conduct Problems: Mediation by Parenting. Journal of Abnormal Child Psychology, 45(1), 15–26. 10.1007/s10802-016-0163-7 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, & Boyce WT (2008). Biological sensitivity to context. Current Directions in Psychological Science, 17(3), 183–187. 10.1111/j.1467-8721.2008.00571.x [DOI] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8, 430–457. [PubMed] [Google Scholar]
- Fanti KA, Kyranides MN, Georgiou G, Petridou M, Colins OF, Tuvblad C, & Andershed H (2017). Callous-unemotional, impulsive-irresponsible, and grandiose-manipulative traits: Distinct associations with heart rate, skin conductance, and startle responses to violent and erotic scenes. Psychophysiology, 54(5), 663–672. 10.1111/psyp.12837 [DOI] [PubMed] [Google Scholar]
- Fearon RP, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Lapsley A-M, & Roisman GI (2010). The Significance of Insecure Attachment and Disorganization in the Development of Children ‘ s Externalizing Behavior : A Meta-Analytic Study Anne-Marie Lapsley and Glenn I. Roisman Published by : Wiley on behalf of the Society for Research in Child Deve. Child Development, 81(2), 435–456. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Leckman JF, Kuint J, & Eidelman AI (1999). The nature of the mother’s tie to her infant: Maternal bonding under conditions of proximity, separation, and potential loss. Journal of Child Psychology and Psychiatry and Allied Disciplines, 40(6), 929–939. 10.1017/S0021963099004308 [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, & Kahn RE (2014a). Annual research review: A developmental psychopathology approach to understanding callous-unemotional traits in children and adolescents with serious conduct problems. Journal of Child Psychology and Psychiatry and Allied Disciplines, 55(6), 532–548. 10.1111/jcpp.12152 [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, & Kahn RE (2014b). Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A Comprehensive Review. Psychological Bulletin, 140(1), 1–57. 10.1037/a0033076 [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Greenberg M, & Bierman K (2015). Children’s parasympathetic reactivity to specific emotions moderates response to intervention for early-onset aggression. Journal of Clinical Child & Adolescent Psychology, 44(2), 291–304. [DOI] [PubMed] [Google Scholar]
- Glenn AL (2019). Early life predictors of callous-unemotional and psychopathic traits. Infant Mental Health Journal, 40(1), 39–53. 10.1002/imhj.21757 [DOI] [PubMed] [Google Scholar]
- Goffin KC, Boldt LJ, Kim S, & Kochanska G (2018). A Unique Path to Callous-Unemotional Traits for Children who are Temperamentally Fearless and Unconcerned about Transgressions: A Longitudinal Study of Typically Developing Children from age 2 to 12. Journal of Abnormal Child Psychology, 46(4), 769–780. 10.1007/s10802-017-0317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, & Rothbart MK (1991). Contemporary instruments for assessing early temperament by questionnaire and in the laboratory. In In Angleitner A, & Strelau J (Eds.), Explorations in temperament: International perspectives on theory and measurement (pp. 249–272). [Google Scholar]
- Greenberg MT, Speltz ML, & Deklyen M (1993). The role of attachment in the early development of disruptive behavior problems. Development and Psychopathology, 5, 191. [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1–2), 199–220. 10.1016/S0306-4530(01)00045-2 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Quevedo KM (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Hastings PD, & Kahle S (2019). Get bent into shape: The nonlinear, multi-system, contextually-embedded psychophysiology of emotional development. In Handbook of Emotional Development (pp. 27–55). [Google Scholar]
- Holzman JB, & Bridgett DJ (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neuroscience and Biobehavioral Reviews, 74, 233–255. 10.1016/j.neubiorev.2016.12.032 [DOI] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, Del Carmen R, Pedersen FA, Doussard-Roosevelt JA, & Porges SW (1998). Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development, 69(3), 624–635. 10.1111/j.1467-8624.1998.tb06233.x [DOI] [PubMed] [Google Scholar]
- Hyde LW, Waller R, Trentacosta CJ, Shaw DS, Neiderhiser JM, Ganiban JM, Reiss D, & Leve LD (2016). Heritable and nonheritable pathways to early callous-unemotional behaviors. American Journal of Psychiatry, 173(9), 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isen J, Raine A, Baker LA, Dawson M, Bezdjian S, & Lozano DI (2010). Sex-specific association between psychopathic traits and electrodermal reactivity in children. Journal of Abnormal Psychology, 119(1), 216–225. 10.1037/a0017777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonis ER, Fanti KA, Anastassiou-Hadjicharalambous X, Mertan B, Goulter N, & Katsimicha E (2016). Can callous-unemotional traits be reliably measured in preschoolers? Journal of Abnormal Child Psychology, 44(4), 625–638. [DOI] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, Van Meter JW, & Marsh AA (2014). Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry, 71(6), 627–636. 10.1001/jamapsychiatry.2013.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynam DR, Charnigo R, Moffitt TE, Raine A, Loeber R, & Stouthamer-Loeber M (2009). The stability of psychopathy across adolescence. Development and Psychopathology, 21, 1133–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson D, & Cairns RB (1996). Developmental science: Toward a unified framework. In Cairns RB, Elder GHJ, & Costello EJ (Eds.), Cambridge studies in social and emotional development. Developmental science. (pp. 7–30). Cambridge University Press. 10.1017/CBO9780511571114.003 [DOI] [Google Scholar]
- Main M, & Solomon J (1990). Procedures for identifying infants as disorganized/disoriented during the Ainsworth Strange Situation. Attachment in the Preschool Years: Theory, Research, and Intervention, 1, 121–160. [Google Scholar]
- Marsh AA (2019). The Caring Continuum: Evolved Hormonal and Proximal Mechanisms Explain Prosocial and Antisocial Extremes. Annual Review of Psychology, 70(1), 347–371. 10.1146/annurev-psych-010418-103010 [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 804, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- Mesman J, van IJzendoorn MH, & Bakermans-Kranenburg MJ (2009). The many faces of the Still-Face Paradigm: A review and meta-analysis. Developmental Review, 29(2), 120–162. 10.1016/j.dr.2009.02.001 [DOI] [Google Scholar]
- Miller JG, Chocol C, Nuselovici JN, Utendale WT, Simard M, Hastings PD, Chochol C, Nuselovici JN, Utendale WT, Simard M, & Hastings PD (2013). Children’s dynamic RSA change during anger and its relations with parenting, temperament, and control of aggression. Biological Psychology, 92(2), 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Koonce WR, Wagner NJ, Willoughby MT, Stifter CA, Blair C, & Granger DA (2015). Greater fear reactivity and psychophysiological hyperactivity among infants with later conduct problems and callous-unemotional traits. Journal of Child Psychology and Psychiatry and Allied Disciplines, 56(2), 147–154. 10.1111/jcpp.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA (2009). Infants’ and mothers’ vagal reactivity in response to anger. Child Psychology and Psychiatry, 50(11), 1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA, & Calkins SD (2004). Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Developmental Psychology, 40, 1068–1080. [DOI] [PubMed] [Google Scholar]
- Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mills-Koonce WR, & Cox MJ (2009). Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development, 80(1), 209–223. 10.1111/j.1467-8624.2008.01255.x [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2009). Statistical analysis with latent variables. In Wiley; (Vol. 123, Issue 6). Wiley New York, NY. http://www.ncbi.nlm.nih.gov/pubmed/22210541 [Google Scholar]
- Nelson EE, & Panksepp J (1998). Brain substrates of infant-mother attachment: Contributions of opioids, oxytocin, and norepinephrine. Neuroscience and Biobehavioral Reviews, 22(3), 437–452. 10.1016/S0149-7634(97)00052-3 [DOI] [PubMed] [Google Scholar]
- Pasalich DS, Dadds MR, Hawes DJ, & Brennan J (2012). Attachment and callous-unemotional traits in children with early-onset conduct problems. Journal of Child Psychology and Psychiatry and Allied Disciplines, 53(8), 838–845. 10.1111/j.1469-7610.2012.02544.x [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Fowles DC, & Krueger RF (2009). Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology, 21(3), 913–938. 10.1017/S0954579409000492 [DOI] [PubMed] [Google Scholar]
- Porges SW (1985). Method and apparatus for evaluating rhythmic oscillations in a periodic physiological response system (Patent No. 4,510,944).
- Porges SW (1996). Physiological regulation in high-risk infants: A model for assessment and potential intervention. Development & Psychopathology, 8, 29–42. [Google Scholar]
- Porges SW (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42(2), 123–146. [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The Polyvagal Perspective. Biological Psychology, 74, 116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy J, Raine A, Chen FR, Pardini DA, Loeber R, & Jennings JR (2014). Heart rate and antisocial behavior: The mediating role of impulsive sensation seeking. Criminology: An Interdisciplinary Journal, 52(2), 292–311. 10.1111/1745-9125.12038 [DOI] [Google Scholar]
- Raine A (2002). Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology, 30(4), 311–326. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, & Mednick SA (1997). Low resting heart rate at age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius child health project. Journal of the American Academy of Child and Adolescent Psychiatry, 36(10), 1457–1464. 10.1097/00004583-199710000-00029 [DOI] [PubMed] [Google Scholar]
- Rehder PD, Mills-Koonce WR, Wagner NJ, Zvara B, Willoughby MT (2020). Attachment quality assessed from children’s family drawings links to child conduct problems and callous-unemotional behaviors Attachment & Human Development 6734 10.1080/14616734.2020.1714676 [DOI] [PMC free article] [PubMed]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, & Haydon KC (2012). Distinguishing differential susceptibility from diathesis-stress: recommendations for evaluating interaction effects. Development and Psychopathology, 24(2), 389–409. 10.1017/s0954579412000065 [DOI] [PubMed] [Google Scholar]
- Sameroff AJ (2010). A unified theory of development: A dialectic integration of nature and nurture. Child Development, 81(1), 6–22. 10.1111/j.1467-8624.2009.01378.x [DOI] [PubMed] [Google Scholar]
- Sethi A, O’Nions E, McCrory EJ, Bird G, & Viding E (2018). An fMRI investigation of empathic processing in boys with conduct problems and varying levels of callous-unemotional traits. NeuroImage: Clinical, 18(February), 298–304. 10.1016/j.nicl.2018.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijtsema JJ, Veenstra R, Lindenberg S, van Roon AM, Verhulst FC, Ormel J, & Riese H (2010). Mediation of Sensation Seeking and Behavioral Inhibition on the Relationship Between Heart Rate and Antisocial Behavior: The TRAILS Study. Journal of the American Academy of Child & Adolescent Psychiatry, 49(5), 493–502. 10.1016/j.jaac.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Song J-H, Waller R, Hyde LW, & Olson SL (2016). Early callous-unemotional behavior, theory-of-mind, and fearful/inhibited temperament predict externalizing problems in middle and late childhood. Journal of Abnormal Child Psychology, 44(6), 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ III., & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, & Johnsen B (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine, 37(2), 141–153. [DOI] [PubMed] [Google Scholar]
- Thomson ND, Gillespie SM, & Centifanti LCM (2020). Callous-unemotional traits and fearlessness: A cardiovascular psychophysiological perspective in two adolescent samples using virtual reality. Development and Psychopathology, 32(3), 803–815. [DOI] [PubMed] [Google Scholar]
- Tompkins V, Benigno JP, Kiger Lee B, & Wright BM (2018). The relation between parents’ mental state talk and children’s social understanding: A meta-analysis. Social Development, 27(2), 223–246. 10.1111/sode.12280 [DOI] [Google Scholar]
- Tronick EZ, Als H, Adamson LB, Wise S, & Brazelton TB (1978). The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child Psychiatry, 17, 1–13. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Bruschweiler-Stern N, Harrison AM, Lyons-Ruth K, Morgan AC, & Nahum JP (1998). Dyadically expanded states of consciousness and the process of therapeutic change. Infant Mental Health Journal, 19, 290–299. [DOI] [Google Scholar]
- U.S. Census Bureau (2009). Estimates And Projections – Household Income. Retrieved from https://www.census.gov/library/publications/2008/compendia/statab/128ed/population.html
- Van Goozen SHM, Fairchild G, & Harold GT (2008). The role of neurobiological deficits in childhood antisocial behavior. Current Directions in Psychological Science, 17(3), 224–228. 10.1111/j.1467-8721.2008.00579.x [DOI] [Google Scholar]
- Vernon-Feagans L, Cox MJ, Investigators LPIFLP, Willoughby MT, Burchinal MR, Garrett-Peters P, Mills-Koonce WR, Conger RD, & Bauer PJ (2013). The Family Life Project: An epidemiological and developmental study of young children living in poor rural communities. Monographs of the Society for Research in Child Development, 78(5), serial-310. [DOI] [PubMed] [Google Scholar]
- Viding E, & McCrory EJ (2019). Towards understanding atypical social affiliation in psychopathy. The Lancet Psychiatry, 6(5), 437–444. 10.1016/S2215-0366(19)30049-5 [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, & McCrory EJ (2012). Amygdala response to preattentive masked fear in children with conduct problems: The role of callous-unemotional traits. American Journal of Psychiatry, 169(10), 1109–1116. [DOI] [PubMed] [Google Scholar]
- Wagner NJ, Hastings PD, & Rubin KH (2018). Callous-Unemotional Traits and Autonomic Functioning in Toddlerhood Interact to Predict Externalizing Behaviors in Preschool. Journal of Abnormal Child Psychology, 46(7), 1439–1450. 10.1007/s10802-017-0374-6 [DOI] [PubMed] [Google Scholar]
- Wagner NJ, Mills-Koonce WR, Propper CB, Willoughby MT, Rehder PD, Moore GA, & Cox MJ (2016a). Associations between Infant Behaviors during the Face-To-Face Still-Face Paradigm and Oppositional Defiant and Callous-Unemotional Behaviors in Early Childhood. Journal of Abnormal Child Psychology, 44(8), 1439–1453. 10.1007/s10802-016-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner NJ, Mills-Koonce WR, Willoughby MT, Cox MJ, Vernon-Feagans L, Blair C, Burchinal MR, Crnic KA, Crouter A, Garrett-Peters P, Greenberg MT, Frank JL, Stifter CA, Werner E, & Lanza S (2019). Parenting and Cortisol in Infancy Interactively Predict Conduct Problems and Callous-Unemotional Behaviors in Childhood. Child Development, 90(1), 279–297. 10.1111/cdev.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner NJ, Mills-Koonce WR, Willoughby MT, Zvara B, & Cox MJ (2015). Parenting and Children’s Representations of Family Predict Disruptive and Callous-Unemotional Behaviors. Developmental Psychology, 51(7), 935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner NJ, Propper CB, Gueron-Sela N, & Mills-Koonce WR (2016b). Dimensions of maternal parenting and infants’ autonomic functioning interactively predict early internalizing behavior problems. Journal of Abnormal Child Psychology, 44(3), 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner NJ, & Waller R (2020). Leveraging parasympathetic nervous system activity to study risk for psychopathology: The special case of callous-unemotional traits. Neuroscience and Biobehavioral Reviews. [DOI] [PubMed] [Google Scholar]
- Wagner NJ, Waller R, Flom M, Ronfard S, Fenstermacher S, & Saudino KJ (2020). Less imitation of arbitrary actions is a specific developmental precursor to callous–unemotional traits in early childhood. Journal of Child Psychology and Psychiatry and Allied Disciplines, 61(7), 818–825. 10.1111/jcpp.13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Gardner F, & Hyde LW (2013). What are the associations between parenting, callous–unemotional traits, and antisocial behavior in youth? A systematic review of evidence. Clinical Psychology Review, 33(4), 593–608. [DOI] [PubMed] [Google Scholar]
- Waller R, Gardner F, Hyde LW, Shaw DS, Dishion TJ, & Wilson MN (2012). Do harsh and positive parenting predict parent reports of deceitful-callous behavior in early childhood? Journal of Child Psychology and Psychiatry, 53, 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Gardner F, Viding E, Shaw DS, Dishion TJ, Wilson MN, & Hyde LW (2014). Bidirectional associations between parental warmth, callous unemotional behavior, and behavior problems in high-risk preschoolers. Journal of Abnormal Child Psychology, 42(8), 1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Hyde LW, Grabell A, Alves M, & Olson SL (2015). Differential associations of early callous-unemotional, oppositional, and ADHD behaviors: multiple domains within early-starting conduct problems? Journal of Child Psychology and Psychiatry. 10.1111/jcpp.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Shaw DS, & Hyde LW (2017). Observed fearlessness and positive parenting interact to predict childhood callous-unemotional behaviors among low-income boys. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58(3), 282–291. 10.1111/jcpp.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Trentacosta CJ, Shaw DS, Neiderhiser JM, Ganiban JM, Reiss D, Leve LD, & Hyde LW (2016). Heritable temperament pathways to early callous-unemotional behaviour. British Journal of Psychiatry, 209(6), 475–482. 10.1192/bjp.bp.116.181503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, & Wagner NJ (2019). The Sensitivity to Threat and Affiliative Reward (STAR) model and the development of callous-unemotional traits. Neuroscience and Biobehavioral Reviews, 107(October), 656–671. 10.1016/j.neubiorev.2019.10.005 [DOI] [PubMed] [Google Scholar]
- Waller R, Wagner NJ, Flom M, Ganiban JM, & Saudino KJ (2019). Fearlessness and low social affiliation as unique developmental precursors of callous-unemotional behaviors in preschoolers. Psychological Medicine. 10.1017/S003329171900374X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg KM, Beeghly M, Olson KL, & Tronick EZ (2008). A Still-face Paradigm for Young Children: 2½ Year-olds’ Reactions to Maternal Unavailability during the Still-face. The Journal of Developmental Processes, 3(1), 4–22. https://pubmed.ncbi.nlm.nih.gov/22384309 [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Mills-Koonce WR, Gottfredson NC, & Wagner NJ (2014). Measuring callous unemotional behaviors in early childhood: Factor structure and the prediction of stable aggression in middle childhood. Journal of Psychopathology and Behavioral Assessment, 36(1), 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Mills-Koonce WR, Propper CB, & Waschbusch DA (2013). Observed parenting behaviors interact with a polymorphism of the brain-derived neurotrophic factor gene to predict the emergence of oppositional defiant and callous-unemotional behaviors at age 3 years. Development and Psychopathology, 25, 903–917. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Waschbusch DA, Moore GA, & Propper CB (2011). Using the ASEBA to screen for callous unemotional traits in early childhood: Factor structure, temporal stability, and utility. Journal of Psychopathology and Behavioral Assessment, 33(1), 19–30. 10.1007/s10862-010-9195-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Paul J, Nantha-Aree M, Buckley N, Shahzad U, Cheng J, Debeer J, Winemaker M, Wismer D, Punthakee D, Avram V, & Thabane L (2014). Empirical comparison of four baseline covariate adjustment methods in analysis of continuous outcomes in randomized controlled trials. Clinical Epidemiology, 6(1), 227–235. 10.2147/CLEP.S56554 [DOI] [PMC free article] [PubMed] [Google Scholar]


