Abstract
Obstructive sleep apnea (OSA), a common sleep-disordered breathing condition, is characterized by intermittent hypoxia (IH) and sleep fragmentation and has been implicated in the pathogenesis and severity of nonalcoholic fatty liver disease (NAFLD). Abnormal molecular changes mediated by IH, such as high expression of hypoxia-inducible factors, are reportedly involved in abnormal pathophysiological states, including insulin resistance, abnormal lipid metabolism, cell death, and inflammation, which mediate the development of NAFLD. However, the relationship between IH and NAFLD remains to be fully elucidated. In this review, we discuss the clinical correlation between OSA and NAFLD, focusing on the molecular mechanisms of IH in NAFLD progression. We meticulously summarize clinical studies evaluating the therapeutic efficacy of continuous positive airway pressure treatment for NAFLD in OSA. Additionally, we compile potential molecular biomarkers for the co-occurrence of OSA and NAFLD. Finally, we discuss the current research progress and challenges in the field of OSA and NAFLD and propose future directions and prospects.
Keywords: intermittent hypoxia, hypoxia-inducible factor 1 alpha, nonalcoholic fatty liver disease, oxidative stress, dyslipidemia, leptin resistance
Introduction
Obstructive sleep apnea (OSA) is common in the general population and is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, resulting in periods of intermittent hypoxia (IH), snoring, and sleep fragmentation (SF).1 OSA is increasingly recognized as a multisystem disorder because of its association with multiple adverse health outcomes, including cardiovascular and metabolic diseases.2 Nocturnal polysomnography (PSG) is the preferred method for diagnosing OSA. This test should report an apnea/hypopnea index (AHI), which is the sum of apneas (cessation of respiratory airflow for >10 s) and hypopneas (characterized by at least a 30% reduction in airflow for >10 s associated with oxygen desaturation or arousal on electroencephalogram) per hour of sleep.3 The severity of OSA is assessed using the AHI and described as mild (5–14.9), moderate (15–29.9), and severe (≥30).4 The prevalence of OSA, defined by an AHI of more than five events per hour, has increased significantly over the past few decades.5,6 In Lausanne, the prevalence of moderate-to-severe OSA (AHI ≥ 15/h) was 23.4% in women and 49.7% in men.5 Furthermore, a recent study showed that the prevalence of mild-to-severe sleep-disordered breathing (SDB) was 43.2%, moderate-to-severe SDB was 11.6%, and severe SDB (AHI ≥ 30/h) was 2.7% in 1810 participants with complete respiratory polygraphic data.7 Furthermore, the economic costs associated with sleep disorders (including OSA) are substantial.8
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver disease condition characterized by the accumulation of lipid droplets in >5% of hepatocytes.9 NAFLD has become an important global public health problem, with obesity being the major risk factor. The global prevalence of NAFLD in the general population is approximately 25%, reaching even 90% in the morbidly obese patient population and increasing with the prevalence of obesity.10,11 The first stage of NAFLD is characterized by simple steatosis, followed by further liver deterioration leading to overt steatohepatitis (nonalcoholic steatohepatitis [NASH]), which is characterized by steatosis, hepatocyte ballooning, and inflammatory infiltration. NASH may be followed by the development of fibrosis, liver cirrhosis, liver failure, and possibly hepatocellular carcinoma.12,13
The independent risk factors for OSA include advanced age, male sex, large neck circumference, abdominal obesity, being overweight or obese, and snoring.7,14 OSA, a metabolic-related disease, is a risk factor for many metabolic diseases through IH mediation in the general population, such as type 2 diabetes mellitus and cardiovascular and cerebrovascular diseases, particularly NAFLD.15–17 Increasing evidence links the severity of OSA with the occurrence and progression of NAFLD, particularly concerning the impact of moderate-to-severe OSA on the development of NAFLD. A recent review summarized the many related links between OSA and NAFLD, focusing on describing the evidence and some mechanisms linking OSA to NAFLD.18 However, based on some clinical studies, the therapeutic efficacy of continuous positive airway pressure (CPAP), a classic method for treating OSA, for NAFLD remains controversial, and there is currently a lack of comprehensive reviews summarizing this aspect. Therefore, this review focuses on introducing recent and comprehensive molecular mechanisms and screening indicators of NAFLD combined with OSA and summarizing clinical studies on the effectiveness of CPAP treatment for NAFLD.
Clinical Evidence Linking OSA to NAFLD
Clinical Evidence
Increasing clinical evidence has shown the association of OSA with the presence and severity of NAFLD, particularly in adults.17,19,20 An observational study involving 334 patients showed that the severity of OSA evaluated with AHI was associated with a decrease in transaminase levels, high-density lipoprotein levels, body mass index (BMI), homeostatic model assessment for insulin resistance (HOMA-IR) index, and fibrosis-4 index (FIB-4), demonstrating sufficient sensitivity and specificity for the degree of fibrosis.21 Similarly, AHI was reported as an independent predictor of significant fibrosis in NAFLD patients with OSA.22 Moreover, in patients with metabolic comorbidities, severe OSA was independently associated with increased liver stiffness, evaluated via liver stiffness measurement, which can help diagnose the stage of liver fibrosis.23 Furthermore, a multisite cross-sectional study of 1285 patients reported that the risk of hepatic steatosis, but not hepatic fibrosis, increased with OSA severity and sleep-related hypoxemia, adjusted for confounders, including central obesity.24 Additionally, a meta-regression analysis of nine studies (2272 participants) found that OSA was associated with steatosis, lobular inflammation, ballooning degeneration, and fibrosis. The severity of OSA was also associated with ALT levels but not AST levels.25 Moreover, another meta-analysis involving 18 cross-sectional studies (2183 participants) found that OSA was associated with the severity and prevalence of NAFLD, NASH, and fibrosis, independent of age, sex, BMI, and abdominal obesity.26
There are many common risk factors for OSA and NAFLD, including male sex, older age, and obesity.27 Additionally, OSA seems to affect the severity of NAFLD after adjusting for these common risk factors. OSA severity is directly correlated with NAFLD severity, and OSA may lead to a higher risk of NASH in obese patients, which remains statistically significant after adjusting for BMI and other related factors.17,28,29 In short, the presence and severity of OSA are closely related to the histological elements of NAFLD, including the steatosis degree, lobular inflammation, and fibrosis stage.
Some studies have shown that OSA is associated with the presence and severity of NAFLD in children. In one study, OSA affected 60% of 65 children with biopsy-proven NAFLD, and the presence and severity of OSA were associated with features of NASH and fibrosis, similar to those observed in nonobese children with NAFLD.30 Similarly, pediatric obese or nonobese adolescents with NAFLD who had OSA/hypoxia showed more severe fibrosis than their non-OSA/hypoxia counterparts.31,32 The hypoxia and hedgehog pathways were activated in pediatric NAFLD patients with OSA and associated with disease severity.33 Moreover, a previous meta-analysis also demonstrated that OSA is associated with NAFLD, as the OSA group had higher liver enzyme levels and progressive liver fibrosis than the control group in the pediatric population.34 In summary, OSA is associated with NAFLD progression not only in adults but also in children.
Effect of CPAP
CPAP helps people breathe during sleep by providing adjustable air pressure through a mask or nosepiece. Generally, OSA is associated with metabolic comorbidities, and CPAP treatment can improve many metabolic diseases, including diabetes35,36 and hypertension.37,38 Does CPAP improve NAFLD in OSA patients? There are several studies on whether CPAP improves the clinical features of NAFLD in patients with OSA and NAFLD (Table S1). However, few randomized controlled trials have succeeded in demonstrating a clinical effect of CPAP on noninvasive markers of NAFLD severity. In the largest such study of CPAP effects in NAFLD/OSA, Ng et al’s randomized clinical trial involving 120 patients, a 6-month CPAP intervention did not lead to improvements in hepatic steatosis and fibrosis among NAFLD patients with OSA.39 However, a main weakness of the study was that the “sham CPAP” group had a large reduction in respiratory event index. Therefore, it is difficult to conclude that the failure to demonstrate a significant difference between CPAP and sham CPAP is due to a lack of effect of CPAP versus a significant effect of subtherapeutic CPAP.
The results of studies on the efficacy of different CPAP treatments for NAFLD have been variable (Table S1). There may be several reasons for these discrepant findings. First, the studies employed different approaches for diagnosing NAFLD. Pathological and histological features are critical for NAFLD management, and the degree of steatosis and fibrosis may affect the treatment difficulty. Moreover, the parameters used to assess NAFLD severity vary. There is no doubt that the diagnostic approach and severity classification have a significant impact on treatment outcomes. Additionally, one of the limitations of some studies is the lack of clear division of OSA classification in terms of treatment, which may contribute to inconsistent treatment effects. Second, CPAP compliance was not well-controlled in some studies. High-quality, accurate use of CPAP is important, as is the duration of use each night. Moreover, the duration of the entire trial is also critical in determining the effect. In general, the longer the use, the better and more reliable the results. More importantly, confounding factors (eg, high BMI) need to be corrected to increase the reliability of the evidence.
In Conclusion, whether CPAP improves NAFLD in OSA patients remains inconclusive. These studies have not definitively demonstrated whether the relationship between OSA and NAFLD is causal or merely associative, and there is currently no clear evidence. It is possible that OSA and NAFLD mutually exacerbate each other and contribute to their development. Alternatively, they may represent different outcomes resulting from common features such as obesity and metabolic abnormalities. It is also possible that they indeed have unknown causal relationships. Given the current mixed results of the studies, future research is needed to improve compliance with CPAP use, extend follow-up periods, and increase the sample size to provide more convincing evidence of the effect of CPAP in NAFLD cases.
Possible Mechanisms Linking IH to NAFLD
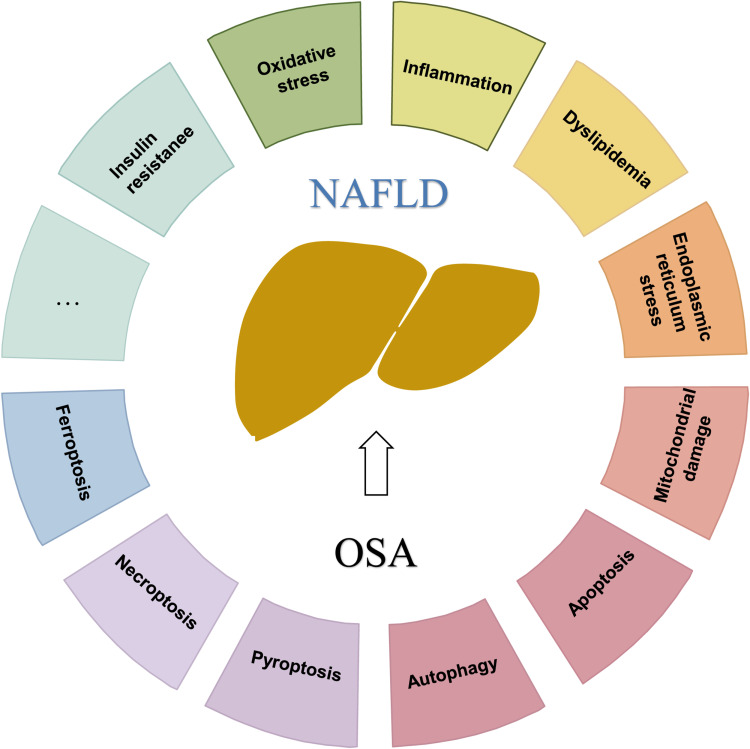
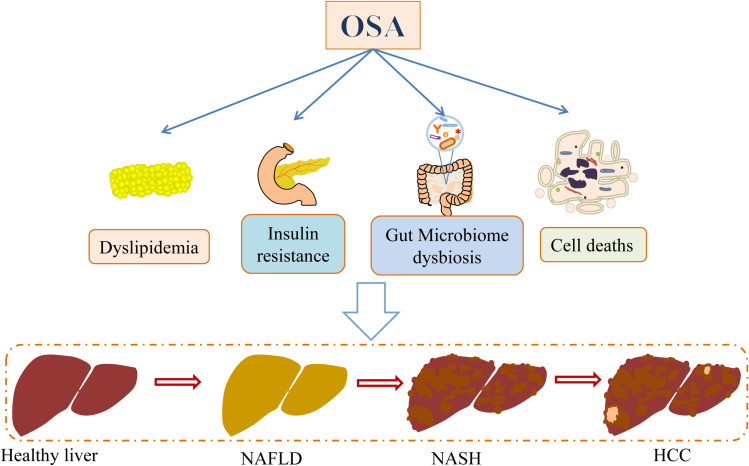
OSA may cause pathological changes in cells and the body through various mechanisms (Figure 1). As mentioned previously, IH and sleep disorders, which are characteristics of OSA, play important roles in OSA and NAFLD pathogenesis.17 IH induces tissue hypoxia, which increases the expression of hypoxia-inducible factor 1 alpha (HIF-1α) and downstream genes involved in oxidative stress, inflammation, lipogenesis, and overactivation of the sympathetic nervous system, thereby leading to proinflammatory cytokine overproduction, vascular endothelial dysfunction, pancreatic beta cell injury, metabolic dysregulation, and insulin resistance (IR). Oxidative stress, inflammation, IR, and lipid metabolism disorders are key factors in OSA and NAFLD physiopathology (Figure 2). Although IH is the main mediator of the increased risk of NAFLD induced by OSA, SF also plays an important role in NAFLD onset and development.
Figure 1.
Obstructive sleep apnea (OSA) may cause pathological changes in cells and the body through various mechanisms. The characteristics of OSA, such as intermittent hypoxia, sleep fragmentation, and poor sleep quality, may cause pathological changes in cells and the body through various mechanisms.
Figure 2.
Role of obstructive sleep apnea (OSA) in NAFLD development and progression. In general, OSA may lead to lipid metabolism impairment, insulin resistance, gut microbiota dysregulation, and various liver cell death mechanisms (such as apoptosis, pyroptosis, and ferroptosis), thereby participating in NAFLD occurrence and development.
IH and Oxidative Stress
NAFLD is strongly associated with oxidative stress.40,41 IH and subsequent reoxygenation lead to the production of reactive oxygen species (ROS), resulting in systemic oxidative stress in OSA patients.42,43 IH remodels hepatic mitochondrial structure and function throughincreasedhepatic DNA damage and ROS production, leading to an imbalance between oxidative stress and antioxidant defense. This effect was observed in mice directly exposed to 2 weeks of IH, simulated with 1-min cycles alternating 30s of hypoxia (5% FiO2) and 30s of normoxia (21% FiO2), which serves as a more appropriate simulation of OSA patients.44
The hypoxia signaling pathway likely plays a significant role in the contribution of IH to oxidative stress in NAFLD.45,46 HIFs, comprising hypoxia-dependent α-subunits (including HIF-1α, HIF-2α, and HIF-3α) and a common constitutively expressed β-subunit, serve as master regulators of oxygen homeostasis by activating the transcription of target genes. Although the regulation and function of HIF-1α and HIF-2α are well established, those of HIF-3α remain less understood.47,48 Extensive evidence supports the association between elevated HIF-1α levels and NAFLD development, emphasizing its role in regulating oxidative stress, immunity, and inflammation.49,50 Emerging evidence suggests that IH increases HIF-1α protein expression and transactivation, leading to various biological and pathophysiological changes in OSA.47,51,52 Although there are fewer clinical studies on HIF-1α levels in NAFLD patients with OSA, several studies have indicated that IH mediates HIF-1α to exacerbate inflammation and liver fibrosis in NAFLD mouse models.53,54 In IH mouse models simulated via intraperitoneal injection of sodium nitrite, HIF-1α protein levels increased, impacting the Treg/Th17 balance and accelerating the development of NASH and fibrosis. Interestingly, these conditions were ameliorated by HIF1α siRNA treatment.53 Another study highlights the key role of HIF-1α in liver fibrosis development, angiogenesis, and inflammatory factors, demonstrating improvement through HIF-1α intervention in rats with NASH and IH treated with sodium nitrite (NaNO2).54 The mechanism suggests that IH, as a systemic stimulus, induces HIF-1α, placing the body in a state of oxidative stress and inflammation, recognized for its role in the development of liver fibrosis in NAFLD.55
The endoplasmic reticulum (ER) stress pathway is another potential mechanism through which IH contributes to oxidative stress in NAFLD. IH activates the ER stress pathway, leading to dysregulation of hepatocyte lipid homeostasis and subsequent accumulation of toxic lipids. This triggers ER stress and activates the unfolded protein response (UPR), which is a physiological mechanism for maintaining protein folding homeostasis.49 However, excessive UPR activation can result in pathological conditions, including hepatocellular damage and apoptosis.56–58 Tauroursodeoxycholic acid (TUDCA), a bile acid derivative used for treating cholelithiasis and cholestatic liver diseases, plays a crucial role in enhancing protein folding to alleviate ER stress.59–61 A previous animal study demonstrated that IH induces ER stress, leading to elevated levels of tumor necrosis factor and interleukin-1β in hepatic tissues, ultimately leading to hepatic injury. However, TUDCA exhibited a protective effect on the liver against IH-induced injury and apoptosis by effectively inhibiting ER stress.62 Despite these findings, the specific mechanism underlying IH-induced UPR in NAFLD development remains unclear. Understanding how IH-induced UPR contributes to the pathogenesis of NAFLD could provide valuable insights into potential therapeutic targets. Further investigations on the intricate interplay among IH, ER stress, and NAFLD development could contribute to the development of novel treatment strategies for this complex liver condition.
IH and Dyslipidemia
NAFLD is characterized by the excessive accumulation of fat in the liver, with disturbances in hepatic and systemic lipid metabolism identified as key pathological mechanisms.63 Interestingly, there is increasing evidence demonstrating that OSA is a risk factor for various metabolic disorders due to sleep disturbance and IH, particularly affecting lipid metabolism. OSA and dyslipidemia exhibit a strong bidirectional relationship.2,64–67 OSA and IH appear to play pivotal roles in NAFLD development by enhancing adipose tissue lipolysis, leading to increased availability of free fatty acids (FFAs) in the serum, promoting hepatic biosynthesis, delaying postprandial lipid clearance, and reducing mitochondrial fat oxidation (FAO).68 Severe sleep apnea has been reported to increase FFA levels during sleep in patients with heart failure, a phenomenon prevented by supplemental oxygen.69 Plasma FFA levels were also found to be increased during sleep in OSA patients without heart failure, with CPAP attenuating lipolysis. Additionally, CPAP withdrawal in OSA patients leads to increased FFA levels, associated with increased sympathetic and adrenocortical activities.70 Animal models further support the role of IH in dyslipidemia and abnormal liver lipids. Mice, including lean mice, exposed to IH exhibited increased levels of serum cholesterol and other lipids. However, this effect was not observed in obese mice, potentially due to baseline hypercholesterolemia masking the effects of IH on lipid metabolism.71,72 IH appears to cause hepatic lipid biosynthesis by upregulating lipid biosynthesis genes, including sterol regulatory element-binding protein 1, stearoyl-coenzyme A desaturase 1, and fatty acid translocase CD36 (which facilitates FFA uptake in the liver) in mice.73–76 Moreover, IH may contribute to hyperlipidemia by affecting lipid clearance, reducing triglyceride (TG)-rich lipoprotein clearance, and inhibiting lipoprotein lipase activity in adipose tissues.77 These factors collectively contribute to increased serum lipid levels and metabolic burden on the liver, ultimately resulting in hepatic fat accumulation, dyslipidemia, and steatohepatitis.
HIFs, particularly HIF-2α, appear to play a crucial role in the hepatic lipid metabolism abnormalities induced by IH in NAFLD. Levels of CD36, a molecule involved in lipid transport, are significantly elevated in the livers of patients with OSA compared to controls, showing a positive correlation with key respiratory parameters such as AHI and oxygen desaturation index, which are well-known indicators of OSA severity.74 Another study observed high expressions of both HIF-2α and CD36 in the livers of NAFLD patients. Knockdown of CD36 in hypoxic liver cells notably reduced lipid accumulation, whereas knockdown of HIF-2α reduced both lipid accumulation and CD36 expression. Additionally, mice deficient in HIF-2α exhibited decreased CD36 levels and improved NASH characteristics.78 These findings strongly support the role of HIF-2α in hepatic lipid accumulation by regulating CD36 expression and function. Furthermore, hypoxia-induced overexpression of HIF-2α suppressed peroxisome proliferator-activated receptor (PPAR) in steatotic human hepatocytes (L02 cells) treated with FFAs and in a high-fat diet (HFD)-fed mouse model of NAFLD. This resulted in IR, suppression of fatty acid β-oxidation, or increased lipogenesis, exacerbating NAFLD progression. However, these effects were mitigated by HIF-2α silencing or PPARα activation.79 Interestingly, oxygen therapy, involving treatment of HFD-fed mice with 88% oxygen concentration and palmitic acid (PA)-treated primary hepatocytes with 100% oxygen concentration for 1 h twice daily, alleviated hepatic steatosis by inhibiting hepatic de novo lipogenesis. This was achieved by reducing HIF-2α expression (but not HIF-1α) and improving glucose metabolism and insulin sensitivity.80 These findings collectively suggest that IH exacerbates NAFLD progression by inducing HIF-2α expression.
The role of HIF-1α in lipid metabolism in NAFLD is also noteworthy. In a choline-deficient diet-induced NAFLD mouse model, livers deficient in HIF-1α exhibited more pronounced steatosis and increased TG accumulation compared to wild-type livers.81 However, despite its importance, HIF-1α is considered dispensable in NAFLD development. Both HIF-1α and HIF-2α are significantly activated in primary hepatocytes treated with cobalt chloride (CoCl2), a hypoxic inducer simulating HIF effects, or cultured in a 1% O2 environment. However, neither palmitate acid- nor hypoxia-induced hepatic steatosis improved with siHIF-1α or siHIF-2α treatments, respectively, suggesting the involvement of an HIF-independent pathway in hepatic steatosis.82 Additionally, IH exacerbates liver fibrosis in hepatocyte-specific deletion of HIF-1α (Hif1α-/-),83 implying that the role of IH in liver fibrosis may extend beyond its direct effect on HIF-1α. In conclusion, the intricate relationship among OSA, IH, and NAFLD involves dysregulated lipid metabolism and the pivotal role of HIFs, particularly HIF-2α. Further research is needed to unravel the complex interplay between IH, HIFs, and alternative pathways contributing to the pathogenesis of NAFLD.
IH and Insulin and/or Leptin Resistance
Leptin plays a crucial role in reducing food intake and increasing energy expenditure, whereas insulin is essential for metabolizing ingested nutrients, particularly glucose. However, insulin and/or leptin resistance, characterized by impaired insulin and/or leptin action, are strongly implicated in NAFLD development.84–88 Several factors contribute to the specific role of IR in the pathological mechanisms of NAFLD.89–91 First, IR increases the breakdown of peripheral adipose tissues, elevating FFA levels in the bloodstream, which then enter the liver. Second, IR leads to a compensatory increase in insulin synthesis and secretion by pancreatic β-cells, resulting in hyperinsulinemia and increased de novo fat synthesis in the liver. Finally, IR causes adipose tissue dysfunction and abnormal adipokine secretion, resulting in elevated IL-6 and TNF-α levels.
Interestingly, many epidemiological studies have indicated a strong association between OSA and insulin and/or leptin resistance.92–94 In rats, exposure to IH may contribute to the induction of a state of insulin and/or leptin resistance, potentially leading to increased food intake and weight gain.65 There are several plausible mechanisms through which IH leads to IR.93,95–97 First, hypoxia affects the phosphorylation of insulin receptor tyrosine kinase, reducing the effectiveness of insulin receptors. Additionally, hypoxia activates the sympathetic nervous system and peripheral chemoreceptors, resulting in increased adrenaline and glucocorticoid levels, which antagonize the biological effects of insulin. Moreover, hypoxia induces oxidative stress and upregulates the expression of inflammatory factors, contributing to IR. IH may mediate IR through inflammatory remodeling of adipose tissue independent of obesity.93,98 A possible molecular mechanism involves IH triggering the production of multiple inflammatory mediators through the activation of downstream NF-κB pathways, including TNF-α and C-C motif chemokine ligand 2, which play roles in the development and perpetuation of IR and chronic adipose tissue inflammation.99,100 Additionally, IH can promote TNF-α, IL-1β, and IL-6 production by activating the MAPK signaling pathway, leading to inflammation and cell apoptosis.101 Moreover, IH can result in abnormal expression and secretion of cytokines from cells, including hepatokines, adipokines, and myokines, further exacerbating IR.97 For example, selenoprotein P, a hepatokine associated with the development of IR, was found to be upregulated by IH via a microRNA-203-mediated mechanism.102,103 In summary, a close relationship among OSA, IR, and NAFLD is plausible.
Numerous studies have shown that OSA/IH is associated with the development of NAFLD through the induction of IR.104–106 In one study, the HOMA-IR index was independently associated with NAFLD in patients with OSA, even after adjusting for confounders.104,105 Another study indicated that AHI and HOMA-IR index were independent risk factors for elevated ALT levels in obese NAFLD patients with OSA.106 In conclusion, the induction of insulin and/or leptin resistance may serve as a critical link between OSA and NAFLD development. However, the specific molecular mechanisms remain unclear, and exploring the effects of IH on inflammatory molecules and pathways may offer valuable insights into understanding IR.
IH and Gut Microbiota
The balanced gut microbiota plays critical physiological roles in the host immune system, normal gut–liver circulation, and metabolism.107,108 Accumulating evidence suggests that gut dysfunction, particularly dysbiosis and alterations in gut metabolites, may contribute to NAFLD development and progression.109,110 Furthermore, gut microbial composition is associated with NAFLD severity and outcomes.111–114 Several studies have reported that both obese and nonobese NAFLD patients exhibit fecal dysbiosis and distinct microbial community compositions compared to controls, including bacteria, fungi, and viruses.111,115–119 It appears that gut microbiota dysbiosis significantly impacts NAFLD, irrespective of obesity.116,119 Furthermore, regulating the gut microbiome may represent a therapeutic target in NAFLD.120–124 Several plausible mechanisms explain how gut microbiota may influence NAFLD progression. Briefly, dysbiosis leads to intestinal inflammation and gut barrier dysfunction, resulting in changes in the translocation of live bacteria and microbial products (including lipopolysaccharides [LPSs], trimethylamine N-oxide, short-chain fatty acids [SCFAs], bile acids, and ethanol) due to increased intestinal permeability. This promotes liver injury and inflammation.110,125–129 SCFAs produced by the gut microbiome have important effects on energy metabolism, immunity, and gut function regulation.130,131 The types and amounts of SCFAs are altered in NAFLD.110 LPS activation of toll-like receptors (TLRs) and inflammasomes also contributes to the pathological mechanism.132 Fecal microbiota transplantation from healthy donors has been shown to reduce small intestinal permeability, highlighting the role of gut microbiota in NAFLD.133 Additionally, there are several clinical trials investigating drugs that regulate bile acid synthesis in NAFLD.134,135
There is increasing evidence indicating an association between OSA and altered gut microbiota. Although animal models have demonstrated that IH, SF, intermittent hypercapnia, or sleep deprivation (which OSA can cause) lead to dysbiosis,136–142 data on changes in gut microbiota in OSA patients are limited. Two pediatric studies have shown altered gut microbiota function and composition,143 decreased microbiota diversity, and increased inflammation.144 Another study revealed gut microbial dysbiosis in OSA patients, characterized by changes in gut function, reduction in SCFA-producing bacteria, and increased pathogen levels along with elevated IL-6 levels.145 Additionally, alterations in gut microbiota and glucometabolic changes have been observed in healthy young individuals with recurrent partial sleep deprivation.146 This suggests that OSA contributes to the development and progression of NAFLD by inducing gut microbiota dysbiosis, which in turn could promote intrahepatic and systemic inflammation.147,148 Moreover, OSA-associated changes in gut microbiota have been implicated in the development of type 2 diabetes mellitus and exacerbation of outcomes in coronavirus disease 2019 by promoting systemic inflammation.149,150 Interestingly, a study found that OSA with the Prevotella enterotype (Prevotella-dominated gut microbiomes) significantly disrupted arousal-related parameters or sleep stages.151 Notably, NASH microbiomes are often categorized into the Prevotella enterotype, whereas healthy microbiomes are less represented by Prevotella.152 This indicates a potential mechanism underlying the association between OSA and NAFLD. However, studies on the abundance of Prevotella in patients with OSA or NAFLD have yielded inconsistent results,109,112,145,152 possibly due to small sample sizes or variations among regions and ethnic groups. Therefore, further studies on the composition of microbial communities in OSA and NAFLD are warranted.
However, relatively little is known about how OSA induces gut microbiota involvement in NAFLD development and progression. An increase in LPSs due to impaired gut barrier function leads to the activation of TLRs, which are crucial in liver inflammation and fibrosis.148,153,154 One study highlighted the prominence of low-level endotoxemia and systemic inflammation in obese children with OSA.147 Moreover, another study monitoring sleep and related indices in 80 children with biopsy-proven NAFLD revealed that OSA (defined as AHI ≥ 1) is associated with increased endotoxemia, impaired gut barrier function, increased TLR-4-mediated hepatic susceptibility to endotoxemia, and expansion of the adiponectin-deficient hepatic progenitor cell pool.148 However, the study focused on children and had a relatively small sample size. It should be noted that the definition of OSA in children (AHI ≥ 1) slightly differs from that in adults. Several potential mechanisms underlie how gut microbiota links OSA to NAFLD. In short, IH- or SP-induced changes in the gut microbiome lead to increased intestinal permeability, gut barrier dysfunction, and an increase in harmful microbial metabolites, as discussed above. This, in turn, promotes the translocation of live bacteria and microbial products, ultimately contributing to liver inflammation and fibrosis.92,109,148 Additionally, alterations in the gut microbiota induced by IH or SF also contribute to other pathological changes, including IR and lipid metabolism, as previously discussed, further exacerbating the development and progression of NAFLD.
In summary, existing evidence does not conclusively establish whether the gut microbiota is truly involved in the association between OSA and NAFLD. This review provides a conceptual framework, but further clinical evidence and foundational research are necessary to confirm the role of gut microbiota in OSA-associated NAFLD. Importantly, compelling evidence demonstrating the efficacy of targeting the gut microbiota to mitigate NAFLD progression mediated by IH is required. Continued research into the intricate interplay among OSA, gut microbiota, and NAFLD will contribute to a more comprehensive understanding of their associations, transitioning from speculative theories to evidence-based conclusions.
IH and Cell Death
It is well established that hepatocyte injury and death contribute to NAFLD pathology. Although apoptosis and necrosis have been shown to be the dominant drivers of progression and fibrosis,155,156 more recently, other types of cell death, including necroptosis, pyroptosis (inflammatory programmed cell death), and ferroptosis,155–159 have also been recognized to play important roles in NAFLD progression. However, autophagy, which maintains cellular homeostasis, plays a dual role in NAFLD. Autophagy plays a protective role in hepatocytes, whereas defects in autophagy in the endothelial cells of the liver in NAFLD patients promote liver inflammation and fibrosis.160,161 In contrast, autophagy is detrimental to hepatic stellate cells, thereby promoting liver fibrosis.162
Increasing evidence suggests that OSA promotes hepatocyte injury and death via IH, contributing to NAFLD development.30,148,163–167 In children with NAFLD and OSA, the duration of hemoglobin desaturation (<90%) was associated with increased levels of circulating markers of hepatocyte apoptosis and fibrogenesis.30,148 In NAFLD animal models, IH can exacerbate hepatocyte apoptosis to ameliorate liver inflammation and injury via ER and oxidative stress, which can be reversed by ammonium pyrrolidine dithiocarbonate (a scavenger of ROS), Lactobacillus rhamnosus GG culture supernatant (improving lipid metabolism by increasing circulating adiponectin levels), or 4-phenylbutyric acid.164,165,168. Additionally, OSA is implicated in necroptosis in NAFLD, a programmed cell death mechanism triggering inflammation by receptor-interacting protein kinase-3 (RIPK3) and mixed-lineage kinase domain-like pseudokinase (MLKL). IH appears to exacerbate oxidative stress and inflammation by promoting PA-induced necroptosis in liver LO2 cells and high-fat choline-deficient mice. Liver injury is significantly reduced by blocking the function of RIPK3 to induce necroptosis or TBHQ (an activator of Nrf2 to reduce oxidative stress).167 Moreover, Nrf2 dysregulation is associated with hepatic ferroptosis, inducing CIH-induced liver injury in rats.169 Furthermore, melatonin can enhance autophagy to prevent IH-exacerbated hepatocellular damage by activating SIRT1-mediated autophagy signaling in an HFD-fed mouse model.170 However, CIH can increase hepatic steatosis and ER stress by promoting the dysregulated activity of hepatic autophagy, implying the involvement of IH in NAFLD development by mediating autophagy dysregulation.171 HIF-1α may be a driver of cell death in NAFLD patients with OSA. One study found that CoCl2 exacerbated FFA-induced TG levels and higher cell death rates by promoting inflammatory signals, including NLRP3 inflammasome/caspase-1 activation,163 which contributes not only to pyroptosis but also to apoptosis, necroptosis, and ferroptosis.172 In conclusion, OSA promotes various types of cell death in NAFLD progression via IH; however, considering the crosstalk and/or overlap between different cell death pathways, pan-cell death, such as PANoptosis,173 but not a single cell death, is likely to play a role in NAFLD. Future research should explore the role of PANoptosis in NAFLD.
Possible Markers
The diagnosis of OSA through PSG and the definitive diagnosis of NAFLD via liver biopsy both present significant challenges. PSG, although considered the gold standard for OSA diagnosis, is a complex and often expensive procedure that requires overnight monitoring in a sleep laboratory. On the other hand, liver biopsy, the gold standard for diagnosing NAFLD, involves an invasive procedure with potential risks and discomfort for the patient. Given these challenges, there is a pressing need for simpler and more accessible diagnostic tools for both OSA and NAFLD, enabling quicker and less invasive diagnosis. This would facilitate earlier intervention and lead to better patient outcomes.
Table 1 provides a comprehensive overview of the current state of research on markers for OSA and NAFLD. These markers span various categories, including polysomnographic parameters, genomics, inflammatory proteins, proteomics, metabolism-related markers, and liver imaging indices. Among these markers, PSG parameters serve as indicators of OSA severity and may be pivotal in assessing the severity of NAFLD. For instance, mean SaO2% has been linked to predicting obese and OSA patients who are at a higher risk of advanced fibrosis,174–176 whereas AHI is significantly correlated with lobular inflammation and fibrosis.22,106,177,178 This also suggests that significant abnormalities in PSG parameters in OSA patients warrant attention to the health of their liver.
Table 1.
Possible Screening Markers for OSA and NAFLD Patients
| Type | Name | References | Possible Significance |
|---|---|---|---|
| PSG parameters | LaSO2 | [104,105,174,177–179] | Mean SaO2% may predict patients with obesity and OSA and those at a higher risk of developing advanced fibrosis; AHI was significantly correlated with lobular inflammation and fibrosis; the others may be predictors of NAFLD among OSA patients. |
| Mean SaO2% | [174–176] | ||
| TS90 | [104,177] | ||
| ODI | [104,174,180] | ||
| AHI | [22,106,177,178] | ||
| Snoring index | [181] | ||
| Genomics | The allele Gly972Arg | [182] | Associated with an increased risk of OSA and NAFLD development. |
| Polymorphism of IRS1, CRP, IL-6, and LEPR | [183] | ||
| Proteomics | LOX | [184] | May serve as a biomarker for liver fibrosis in OSA. |
| Metabonomics | TG | [105] | May predict NAFLD development. |
| HOMA-IR | [104–106] | ||
| Leptin, adiponectin | [185] | ||
| Inflammatory markers | MIF, IL-6 | [185] | Associated with an increased risk of OSA and NAFLD. |
| Hs-CRP | [106,185] | ||
| TNF-α | [185] | ||
| Others | BMI | [105,178,186] | BMI may predict the development of NAFLD and OSA; FLI and HSI may serve as good screening tools for detecting NAFLD in OSA patients. |
| FLI | [27,187,188] | ||
| HSI | [187] |
Abbreviations: PSG, polysomnographic; LaSO2, lowest oxygen saturation; Mean SaO2%, mean percentage oxyhemoglobin; TS90, total sleep time spent with oxygen saturation of <90%; ODI, oxygen desaturation index; AHI, apnea/hypopnea index; IRS1, insulin receptor substrate 1; CRP, C-reactive protein; IL-6, interleukin-6; LEPR, leptin receptor; LOX, lysyl oxidase; TG, triglyceride; HOMA-IR, homeostatic model assessment for insulin resistance; MIF, macrophage migration inhibitory factor; Hs-CRP, highly sensitive C-reactive protein; TNF-α, tumor necrosis factor alpha; BMI, body mass index; FLI, fatty liver index; HIS, hepatic steatosis index.
Some metabolic and inflammatory markers, such as TGs, TNF-α, and IL-6, may also have diagnostic value for OSA and NAFLD;105,185 however, it is important to note their lack of specificity. Nevertheless, certain biomarkers may hold particular significance for guiding the diagnosis of OSA and NAFLD. For example, a study involving 35 patients with severe obesity who underwent liver biopsy, PSG, and serum lysyl oxidase (LOX) testing found that AHI was elevated in patients with fibrosis. LOX, an enzyme involved in cross-linking collagen levels, also showed elevated serum levels in patients with evidence of hepatic fibrosis. Moreover, patients with severe OSA exhibited higher baseline LOX levels compared to healthy controls. These findings suggest that LOX serves as a biomarker of liver fibrosis in patients with severe obesity, OSA, and NAFLD.184 Additionally, a study involving 431 newly diagnosed OSA patients found that the fatty liver index and hepatic steatosis index values were significantly elevated in the NAFLD group, indicating their potential use as screening tools for NAFLD in adults with obstructive sleep apnea/hypopnea syndrome.187 However, the clinical significance of these markers requires further investigation. It is necessary to expand sample sizes and adopt more rigorous research designs to explore the diagnostic value of these markers for OSA and NAFLD.
In conclusion, although each marker may have its strengths and limitations, combining multiple markers into predictive models could enhance diagnostic accuracy and provide valuable insights into disease progression. In the future, simpler and more effective diagnostic methods are needed to diagnose and treat OSA or NAFLD earlier. Additionally, identifying individuals at high risk of developing OSA-related complications, including NAFLD, is crucial such that treatment efforts can be appropriately focused on them.
Discussion and Outlook
HIFs play a critical role in the pathological state induced by OSA, and controlling disease progression may involve intervening in the excessive activation of downstream pathways regulated by HIFs. However, the HIF signaling pathway also plays a crucial role in various molecular and signaling pathways. Effectively targeting HIFs or their downstream key molecules in NAFLD patients with OSA while minimizing potential side effects poses a significant challenge and remains a key focus for future research.
The proportion of OSA patients with NAFLD or vice versa is not clearly defined; hence, large-scale epidemiological studies are necessary to elucidate this relationship. Moreover, NAFLD and OSA may exacerbate each other, forming a vicious cycle. Therefore, based on existing evidence, clinicians must remain vigilant regarding the coexistence of these conditions and raise awareness about the potential complications of OSA. Timely screening for OSA is crucial for diagnosis, especially considering that many snorers may not exhibit severe symptoms and thus overlook the chronic metabolic risks associated with OSA. The efficacy of CPAP in treating OSA combined with NAFLD remains uncertain. Therefore, further exploration of appropriate inclusion criteria, outcome indicators, observation time, and standardized CPAP treatment protocols is necessary to determine the exact effect of CPAP. Additionally, combining CPAP with other weight loss methods, such as exercise, may enhance its effectiveness in improving OSA and its comorbidities, including NAFLD. Clinicians should recognize the close relationship between OSA and metabolic diseases and provide management education, including guidance on increased exercise and weight loss, particularly for individuals with NAFLD and those who are obese. However, further studies are needed to investigate the causal relationship between OSA and NAFLD and elucidate their underlying mechanisms to develop more effective treatment strategies.
Conclusion
Although confounding factors, such as obesity, have introduced significant complexity into the study of OSA and metabolic diseases, increasing evidence indicates that OSA is independently involved in the development of NAFLD. OSA mediates systemic or local inflammation and oxidative stress, primarily through IH and SF, leading to disturbances in glucose and lipid metabolism, dysregulation of the gut microbiome, various modes of cell death, and ultimately, the development of NAFLD. It is important to note the substantial interplay between the risk factors for NAFLD and OSA, making it challenging to discern cause and effect. Clarifying the relationship between NAFLD and OSA represents a long and arduous journey.
Funding Statement
This work was supported by the Hubei Provincial Natural Science Foundation of China (No. 2024AFB050), the National Key Research and Development Program (No. 2021YFC2500702), the National Natural Science Foundation of China (Nos. 82270104 and 82201268), the Scientific Research Project of the Hubei Provincial Commission of Health (No. WJ2023Z010), the Young Doctors’ Innovation and Development Program (No. HXQNJJ-2023-010), the Moderate and Severe Asthma Diagnosis and Treatment Scientific Research Project (No. Z001), and the Fundamental Research Funds for the Central Universities (No. 2042023kf0044).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Levy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015. doi: 10.1038/nrdp.2015.15 [DOI] [PubMed] [Google Scholar]
- 2.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi: 10.1016/j.jacc.2013.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malhotra RK, Kirsch DB, Kristo DA, et al. Polysomnography for obstructive sleep apnea should include arousal-based scoring: an American academy of sleep medicine position statement. J Clin Sleep Med. 2018;14(7):1245–1247. doi: 10.5664/jcsm.7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323(14):1389–1400. doi: 10.1001/jama.2020.3514 [DOI] [PubMed] [Google Scholar]
- 5.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wachinou AP, Houehanou C, Ade S, et al. Prevalence of sleep-disordered breathing in an African general population: the Benin Society and Sleep (BeSAS) study. Lancet Respir Med. 2022;10(9):831–839. doi: 10.1016/S2213-2600(22)00046-7 [DOI] [PubMed] [Google Scholar]
- 8.Streatfeild J, Smith J, Mansfield D, Pezzullo L, Hillman D. The social and economic cost of sleep disorders. Sleep. 2021;44(11). doi: 10.1093/sleep/zsab132 [DOI] [PubMed] [Google Scholar]
- 9.Scorletti E, Carr RM. A new perspective on NAFLD: focusing on lipid droplets. J Hepatol. 2021. doi: 10.1016/j.jhep.2021.11.009 [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 11.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 12.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–1523. doi: 10.1126/science.1204265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. [DOI] [PubMed] [Google Scholar]
- 14.Lyons MM, Bhatt NY, Pack AI, Magalang UJ. Global burden of sleep-disordered breathing and its implications. Respirology. 2020;25(7):690–702. doi: 10.1111/resp.13838 [DOI] [PubMed] [Google Scholar]
- 15.Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. [DOI] [PubMed] [Google Scholar]
- 16.Hirotsu C, Haba-Rubio J, Togeiro SM, et al. Obstructive sleep apnoea as a risk factor for incident metabolic syndrome: a joined Episono and HypnoLaus prospective cohorts study. Eur Respir J. 2018;52(5). doi: 10.1183/13993003.01150-2018 [DOI] [PubMed] [Google Scholar]
- 17.Aron-Wisnewsky J, Minville C, Tordjman J, et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56(1):225–233. doi: 10.1016/j.jhep.2011.04.022 [DOI] [PubMed] [Google Scholar]
- 18.Tang H, Lv F, Zhang P, Liu J, Mao J. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease. Front Endocrinol. 2023;14:1254459. doi: 10.3389/fendo.2023.1254459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benotti P, Wood GC, Argyropoulos G, et al. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity. 2016;24(4):871–877. doi: 10.1002/oby.21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minville C, Hilleret M-N, Tamisier R, et al. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest. 2014;145(3):525–533. doi: 10.1378/chest.13-0938 [DOI] [PubMed] [Google Scholar]
- 21.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104–1112. doi: 10.1016/j.cgh.2009.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283–291. doi: 10.1007/s12072-015-9615-3 [DOI] [PubMed] [Google Scholar]
- 23.Trzepizur W, Boursier J, Le Vaillant M, et al. Increased liver stiffness in patients with severe sleep apnoea and metabolic comorbidities. Eur Respir J. 2018;51(6). doi: 10.1183/13993003.00601-2018 [DOI] [PubMed] [Google Scholar]
- 24.Trzepizur W, Boursier J, Mansour Y, et al. Association between severity of obstructive sleep apnea and blood markers of liver injury. Clin Gastroenterol Hepatol. 2016;14(11):1657–1661. doi: 10.1016/j.cgh.2016.04.037 [DOI] [PubMed] [Google Scholar]
- 25.Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2018;22(3):841–851. doi: 10.1007/s11325-018-1625-7 [DOI] [PubMed] [Google Scholar]
- 26.Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14(5):417–431. doi: 10.1111/obr.12020 [DOI] [PubMed] [Google Scholar]
- 27.Chung GE, Cho EJ, Yoo JJ, et al. Nonalcoholic fatty liver disease is associated with the development of obstructive sleep apnea. Sci Rep. 2021;11(1):13473. doi: 10.1038/s41598-021-92703-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanne F, Gagnadoux F, Chazouilleres O, et al. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41(6):1290–1296. doi: 10.1002/hep.20725 [DOI] [PubMed] [Google Scholar]
- 29.Fu Y, Zhang N, Tang W, et al. Chronic intermittent hypoxia contributes to non-alcoholic steatohepatitis progression in patients with obesity. Hepatol Internat. 2022;16(4):824–834. doi: 10.1007/s12072-022-10347-2 [DOI] [PubMed] [Google Scholar]
- 30.Nobili V, Cutrera R, Liccardo D, et al. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med. 2014;189(1):66–76. doi: 10.1164/rccm.201307-1339OC [DOI] [PubMed] [Google Scholar]
- 31.Sundaram SS, Halbower A, Pan Z, et al. Nocturnal hypoxia-induced oxidative stress promotes progression of pediatric non-alcoholic fatty liver disease. J Hepatol. 2016;65(3):560–569. doi: 10.1016/j.jhep.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundaram SS, Sokol RJ, Capocelli KE, et al. Obstructive sleep apnea and hypoxemia are associated with advanced liver histology in pediatric nonalcoholic fatty liver disease. J Pediatr. 2014;164(4):699–706e1. doi: 10.1016/j.jpeds.2013.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundaram SS, Swiderska-Syn M, Sokol RJ, et al. Nocturnal hypoxia activation of the hedgehog signaling pathway affects pediatric nonalcoholic fatty liver disease severity. Hepatol Commun. 2019;3(7):883–893. doi: 10.1002/hep4.1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen LD, Chen MX, Chen GP, et al. Association between obstructive sleep apnea and non-alcoholic fatty liver disease in pediatric patients: a meta-analysis. Pediatr Obes. 2021;16(3):e12718. doi: 10.1111/ijpo.12718 [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Ceron E, Barquiel B, Bezos AM, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. a randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476–485. doi: 10.1164/rccm.201510-1942OC [DOI] [PubMed] [Google Scholar]
- 36.Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. a randomized controlled trial. Am J Respir Crit Care Med. 2015;192(1):96–105. doi: 10.1164/rccm.201408-1564OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro-Grattoni AL, Torres G, Martinez-Alonso M, et al. Blood pressure response to CPAP treatment in subjects with obstructive sleep apnoea: the predictive value of 24-h ambulatory blood pressure monitoring. Eur Respir J. 2017;50(4). doi: 10.1183/13993003.00651-2017 [DOI] [PubMed] [Google Scholar]
- 38.Sapina-Beltran E, Torres G, Benitez I, et al. Differential blood pressure response to continuous positive airway pressure treatment according to the circadian pattern in hypertensive patients with obstructive sleep apnoea. Eur Respir J. 2019;54(1). doi: 10.1183/13993003.00098-2019 [DOI] [PubMed] [Google Scholar]
- 39.Ng SSS, Wong VWS, Wong GLH, et al. Continuous positive airway pressure does not improve nonalcoholic fatty liver disease in patients with obstructive sleep apnea. a randomized clinical trial. Am J Respir Crit Care Med. 2021;203(4):493–501. doi: 10.1164/rccm.202005-1868OC [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025 [DOI] [PubMed] [Google Scholar]
- 41.Greatorex S, Kaur S, Xirouchaki CE, et al. Mitochondrial- and NOX4-dependent antioxidant defence mitigates progression to non-alcoholic steatohepatitis in obesity. J Clin Invest. 2023. doi: 10.1172/JCI162533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanek A, Brozyna-Tkaczyk K, Myslinski W. Oxidative stress markers among obstructive sleep apnea patients. Oxid Med Cell Longev. 2021;2021:9681595. doi: 10.1155/2021/9681595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy P, Naughton MT, Tamisier R, Cowie MR, Bradley TD. Sleep apnoea and heart failure. Eur Respir J. 2022;59(5). doi: 10.1183/13993003.01640-2021 [DOI] [PubMed] [Google Scholar]
- 44.Gaucher J, Vial G, Montellier E, et al. Intermittent hypoxia rewires the liver transcriptome and fires up fatty acids usage for mitochondrial respiration. Front Med. 2022;9:829979. doi: 10.3389/fmed.2022.829979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim LJ, Pham LV, Polotsky VY. Sleep apnea, hypoxia inducible factor, and fatty liver: more questions than answers? Am J Respir Cell Mol Biol. 2021;65(4):337–338. doi: 10.1165/rcmb.2021-0204ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holzner LMW, Murray AJ. Hypoxia-inducible factors as key players in the pathogenesis of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Front Med. 2021;8:753268. doi: 10.3389/fmed.2021.753268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang P, Yao Q, Lu L, Li Y, Chen PJ, Duan C. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014;6(6):1110–1121. doi: 10.1016/j.celrep.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 48.Ravenna L, Salvatori L, Russo MA. HIF3alpha: the little we know. FEBS J. 2016;283(6):993–1003. doi: 10.1111/febs.13572 [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol. 2018;15(1):21–32. doi: 10.1038/s41574-018-0096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, de Carvalho Ribeiro M, Iracheta-Vellve A, et al. Macrophage-specific hypoxia-inducible factor-1alpha contributes to impaired autophagic flux in nonalcoholic steatohepatitis. Hepatology. 2019;69(2):545–563. doi: 10.1002/hep.30215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92(3):967–1003. doi: 10.1152/physrev.00030.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabryelska A, Szmyd B, Szemraj J, Stawski R, Sochal M, Białasiewicz P. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J Clin Sleep Med. 2020;16(10):1761–1768. doi: 10.5664/jcsm.8682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J, Li W, Zhu W, et al. Chronic intermittent hypoxia promotes the development of experimental non-alcoholic steatohepatitis by modulating Treg/Th17 differentiation. Acta Biochim Biophys Sin. 2018;50(12):1200–1210. doi: 10.1093/abbs/gmy131 [DOI] [PubMed] [Google Scholar]
- 54.Wu W, Li W, Wei J, et al. Chronic intermittent hypoxia accelerates liver fibrosis in rats with combined hypoxia and nonalcoholic steatohepatitis via angiogenesis rather than endoplasmic reticulum stress. Acta Biochim Biophys Sin. 2019;51(2):159–167. doi: 10.1093/abbs/gmy169 [DOI] [PubMed] [Google Scholar]
- 55.da Rosa DP, Forgiarini LF, Baronio D, Feijo CA, Martinez D, Marroni NP. Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Mediators Inflamm. 2012;2012:879419. doi: 10.1155/2012/879419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paschetta E, Belci P, Alisi A, et al. OSAS-related inflammatory mechanisms of liver injury in nonalcoholic fatty liver disease. Mediators Inflamm. 2015;2015:815721. doi: 10.1155/2015/815721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song MJ, Malhi H. The unfolded protein response and hepatic lipid metabolism in non alcoholic fatty liver disease. Pharmacol Ther. 2019;203:107401. doi: 10.1016/j.pharmthera.2019.107401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henkel AS. Unfolded protein response sensors in hepatic lipid metabolism and nonalcoholic fatty liver disease. Semin Liver Dis. 2018;38(4):320–332. doi: 10.1055/s-0038-1670677 [DOI] [PubMed] [Google Scholar]
- 59.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cha BH, Kim JS, Ahn JC, et al. The role of tauroursodeoxycholic acid on adipogenesis of human adipose-derived stem cells by modulation of ER stress. Biomaterials. 2014;35(9):2851–2858. doi: 10.1016/j.biomaterials.2013.12.067 [DOI] [PubMed] [Google Scholar]
- 61.Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59(8):1899–1905. doi: 10.2337/db10-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hou Y, Yang H, Cui Z, Tai X, Chu Y, Guo X. Tauroursodeoxycholic acid attenuates endoplasmic reticulum stress and protects the liver from chronic intermittent hypoxia induced injury. Exp Ther Med. 2017;14(3):2461–2468. doi: 10.3892/etm.2017.4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luukkonen PK, Qadri S, Ahlholm N, et al. Distinct contributions of metabolic dysfunction and genetic risk factors in the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2022;76(3):526–535. doi: 10.1016/j.jhep.2021.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonsignore MR, Borel AL, Machan E, Grunstein R. Sleep apnoea and metabolic dysfunction. Eur Respir Rev. 2013;22(129):353–364. doi: 10.1183/09059180.00003413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciriello J, Moreau JM, Caverson MM, Moranis R. Leptin: a potential link between obstructive sleep apnea and obesity. Front Physiol. 2021;12:767318. doi: 10.3389/fphys.2021.767318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kono M, Tatsumi K, Saibara T, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131(5):1387–1392. doi: 10.1378/chest.06-1807 [DOI] [PubMed] [Google Scholar]
- 67.Wei Z, Chen Y, Upender RP. Sleep disturbance and metabolic dysfunction: the roles of adipokines. Int J Mol Sci. 2022;23(3). doi: 10.3390/ijms23031706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barros D, Garcia-Rio F. Obstructive sleep apnea and dyslipidemia: from animal models to clinical evidence. Sleep. 2019;42(3). doi: 10.1093/sleep/zsy236 [DOI] [PubMed] [Google Scholar]
- 69.Jun JC, Drager LF, Najjar SS, et al. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep. 2011;34(9):1207–1213. doi: 10.5665/SLEEP.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chopra S, Rathore A, Younas H, et al. Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab. 2017;102(9):3172–3181. doi: 10.1210/jc.2017-00619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J, Thorne LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97(7):698–706. doi: 10.1161/01.RES.0000183879.60089.a9 [DOI] [PubMed] [Google Scholar]
- 72.Li J, Grigoryev DN, Ye SQ, et al. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol. 2005;99(5):1643–1648. doi: 10.1152/japplphysiol.00522.2005 [DOI] [PubMed] [Google Scholar]
- 73.Li J, Nanayakkara A, Jun J, Savransky V, Polotsky VY. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol Genomics. 2007;31(2):273–280. doi: 10.1152/physiolgenomics.00082.2007 [DOI] [PubMed] [Google Scholar]
- 74.Rey E, Del Pozo-Maroto E, Maranon P, et al. Intrahepatic expression of fatty acid translocase CD36 is increased in obstructive sleep apnea. Front Med. 2020;7:450. doi: 10.3389/fmed.2020.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savransky V, Jun J, Li J, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103(10):1173–1180. doi: 10.1161/CIRCRESAHA.108.178533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savransky V, Nanayakkara A, Li J, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175(12):1290–1297. doi: 10.1164/rccm.200612-1771OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drager LF, Li J, Shin MK, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33(6):783–790. doi: 10.1093/eurheartj/ehr097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rey E, Melendez-Rodriguez F, Maranon P, et al. Hypoxia-inducible factor 2alpha drives hepatosteatosis through the fatty acid translocase CD36. Liver Int. 2020;40(10):2553–2567. doi: 10.1111/liv.14519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J, Chen J, Fu H, et al. Hypoxia exacerbates nonalcoholic fatty liver disease via the HIF-2alpha/PPARalpha pathway. Am J Physiol Endocrinol Metab. 2019;317(4):E710–E722. doi: 10.1152/ajpendo.00052.2019 [DOI] [PubMed] [Google Scholar]
- 80.Yu L, Wang H, Han X, et al. Oxygen therapy alleviates hepatic steatosis by inhibiting hypoxia-inducible factor-2alpha. J Endocrinol. 2020;246(1):57–67. doi: 10.1530/JOE-19-0555 [DOI] [PubMed] [Google Scholar]
- 81.Arai T, Tanaka M, Goda N. HIF-1-dependent lipin1 induction prevents excessive lipid accumulation in choline-deficient diet-induced fatty liver. Sci Rep. 2018;8(1):14230. doi: 10.1038/s41598-018-32586-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X, Huang C, Li X, et al. HFD and HFD-provoked hepatic hypoxia act as reciprocal causation for NAFLD via HIF-independent signaling. BMC Gastroenterol. 2020;20(1):366. doi: 10.1186/s12876-020-01515-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mesarwi OA, Moya EA, Zhen X, et al. Hepatocyte HIF-1 and Intermittent Hypoxia Independently Impact Liver Fibrosis in Murine Nonalcoholic Fatty Liver Disease. Am J Respir Cell Mol Biol. 2021;65(4):390–402. doi: 10.1165/rcmb.2020-0492OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Du X, Di malta C, Fang Z, et al. Nuciferine protects against high-fat diet-induced hepatic steatosis and insulin resistance via activating TFEB-mediated autophagy-lysosomal pathway. Acta Pharm Sin B. 2022;12(6):2869–2886. doi: 10.1016/j.apsb.2021.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lanaspa MA, Kuwabara M, Andres-Hernando A, et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci U S A. 2018;115(12):3138–3143. doi: 10.1073/pnas.1713837115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Polyzos SA, Kountouras J, Mantzoros CS. Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism. 2015;64(1):60–78. doi: 10.1016/j.metabol.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 87.Shearer AM, Wang Y, Fletcher EK, et al. PAR2 promotes impaired glucose uptake and insulin resistance in NAFLD through GLUT2 and Akt interference. Hepatology. 2022. doi: 10.1002/hep.32589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li RY, Qin Q, Yang HC, et al. TREM2 in the pathogenesis of AD: a lipid metabolism regulator and potential metabolic therapeutic target. Mol Neurodegener. 2022;17(1):40. doi: 10.1186/s13024-022-00542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al-Mansoori L, Al-Jaber H, Prince MS, Elrayess MA. Role of inflammatory cytokines, growth factors and adipokines in adipogenesis and insulin resistance. Inflammation. 2022;45(1):31–44. doi: 10.1007/s10753-021-01559-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guerra S, Mocciaro G, Gastaldelli A. Adipose tissue insulin resistance and lipidome alterations as the characterizing factors of non-alcoholic steatohepatitis. Eur J Clin Invest. 2022;52(3):e13695. doi: 10.1111/eci.13695 [DOI] [PubMed] [Google Scholar]
- 91.Parikh MP, Gupta NM, McCullough AJ. Obstructive Sleep Apnea and the Liver. Clin Liver Dis. 2019;23(2):363–382. doi: 10.1016/j.cld.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gileles-Hillel A, Kheirandish-Gozal L, Gozal D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat Rev Endocrinol. 2016;12(5):290–298. doi: 10.1038/nrendo.2016.22 [DOI] [PubMed] [Google Scholar]
- 93.Murphy AM, Thomas A, Crinion SJ, et al. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J. 2017;49(4). doi: 10.1183/13993003.01731-2016 [DOI] [PubMed] [Google Scholar]
- 94.Koh HE, van Vliet S, Cao C, et al. Effect of obstructive sleep apnea on glucose metabolism. Eur J Endocrinol. 2022;186(4):457–467. doi: 10.1530/EJE-21-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li M, Li X, Lu Y. Obstructive sleep apnea syndrome and metabolic diseases. Endocrinology. 2018;159(7):2670–2675. doi: 10.1210/en.2018-00248 [DOI] [PubMed] [Google Scholar]
- 96.Ota H, Fujita Y, Yamauchi M, Muro S, Kimura H, Takasawa S. Relationship between intermittent hypoxia and type 2 diabetes in sleep apnea syndrome. Int J Mol Sci. 2019;20(19). doi: 10.3390/ijms20194756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uchiyama T, Ota H, Ohbayashi C, Takasawa S. Effects of intermittent hypoxia on cytokine expression involved in insulin resistance. Int J Mol Sci. 2021;22(23). doi: 10.3390/ijms222312898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Poulain L, Thomas A, Rieusset J, et al. Visceral white fat remodelling contributes to intermittent hypoxia-induced atherogenesis. Eur Respir J. 2014;43(2):513–522. doi: 10.1183/09031936.00019913 [DOI] [PubMed] [Google Scholar]
- 99.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746 [DOI] [PubMed] [Google Scholar]
- 100.Uchiyama T, Itaya-Hironaka A, Yamauchi A, et al. Intermittent hypoxia up-regulates CCL2, RETN, and TNFalpha mRNAs in adipocytes via down-regulation of miR-452. Int J Mol Sci. 2019;20(8). doi: 10.3390/ijms20081960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y, Hai B, Niu X, et al. Chronic intermittent hypoxia disturbs insulin secretion and causes pancreatic injury via the MAPK signaling pathway. Biochem Cell Biol. 2017;95(3):415–420. doi: 10.1139/bcb-2016-0167 [DOI] [PubMed] [Google Scholar]
- 102.Mita Y, Nakayama K, Inari S, et al. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat Commun. 2017;8(1):1658. doi: 10.1038/s41467-017-01863-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uchiyama T, Ota H, Itaya-Hironaka A, et al. Up-regulation of selenoprotein P and Hip/PAP mRNAs in hepatocytes by intermittent hypoxia via down-regulation of miR-203. Biochem Biophys Rep. 2017;11:130–137. doi: 10.1016/j.bbrep.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding H, Huang JF, Xie HS, et al. The association between glycometabolism and nonalcoholic fatty liver disease in patients with obstructive sleep apnea. Sleep Breath. 2019;23(1):373–378. doi: 10.1007/s11325-018-1744-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qi JC, Huang JC, Lin QC, et al. Relationship between obstructive sleep apnea and nonalcoholic fatty liver disease in nonobese adults. Sleep Breath. 2016;20(2):529–535. doi: 10.1007/s11325-015-1232-9 [DOI] [PubMed] [Google Scholar]
- 106.Zhang L, Zhang X, Meng H, Li Y, Han T, Wang C. Obstructive sleep apnea and liver injury in severely obese patients with nonalcoholic fatty liver disease. Sleep Breath. 2020;24(4):1515–1521. doi: 10.1007/s11325-020-02018-z [DOI] [PubMed] [Google Scholar]
- 107.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han H, Jiang Y, Wang M, et al. Intestinal dysbiosis in nonalcoholic fatty liver disease (NAFLD): focusing on the gut-liver axis. Crit Rev Food Sci Nutr. 2021:1–18. doi: 10.1080/10408398.2021.1966738 [DOI] [PubMed] [Google Scholar]
- 109.Aron-Wisnewsky J, Vigliotti C, Witjes J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17(5):279–297. doi: 10.1038/s41575-020-0269-9 [DOI] [PubMed] [Google Scholar]
- 110.Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–425. doi: 10.1038/nrgastro.2016.85 [DOI] [PubMed] [Google Scholar]
- 111.Lang S, Demir M, Martin A, et al. Intestinal virome signature associated with severity of nonalcoholic fatty liver disease. Gastroenterology. 2020;159(5):1839–1852. doi: 10.1053/j.gastro.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–775. doi: 10.1002/hep.28356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bajaj JS, Vargas HE, Reddy KR, et al. Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17(4):756–765e3. doi: 10.1016/j.cgh.2018.07.022 [DOI] [PubMed] [Google Scholar]
- 114.Ponziani FR, Bhoori S, Castelli C, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69(1):107–120. doi: 10.1002/hep.30036 [DOI] [PubMed] [Google Scholar]
- 115.Demir M, Lang S, Hartmann P, et al. The fecal mycobiome in non-alcoholic fatty liver disease. J Hepatol. 2022;76(4):788–799. doi: 10.1016/j.jhep.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee G, You HJ, Bajaj JS, et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. 2020;11(1):4982. doi: 10.1038/s41467-020-18754-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Del Chierico F, Nobili V, Vernocchi P, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65(2):451–464. doi: 10.1002/hep.28572 [DOI] [PubMed] [Google Scholar]
- 118.Tsai MC, Liu YY, Lin CC, et al. Gut microbiota dysbiosis in patients with biopsy-proven nonalcoholic fatty liver disease: a cross-sectional study in Taiwan. Nutrients. 2020;12(3). doi: 10.3390/nu12030820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen F, Esmaili S, Rogers GB, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71(4):1213–1227. doi: 10.1002/hep.30908 [DOI] [PubMed] [Google Scholar]
- 120.Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault NA. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. 2019;110(1):139–149. doi: 10.1093/ajcn/nqz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hadi A, Mohammadi H, Miraghajani M, Ghaedi E. Efficacy of synbiotic supplementation in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis of clinical trials: synbiotic supplementation and NAFLD. Crit Rev Food Sci Nutr. 2019;59(15):2494–2505. doi: 10.1080/10408398.2018.1458021 [DOI] [PubMed] [Google Scholar]
- 122.Khan A, Ding Z, Ishaq M, et al. Understanding the effects of gut microbiota dysbiosis on nonalcoholic fatty liver disease and the possible probiotics role: recent updates. Int J Biol Sci. 2021;17(3):818–833. doi: 10.7150/ijbs.56214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee NY, Yoon SJ, Han DH, et al. Lactobacillus and Pediococcus ameliorate progression of non-alcoholic fatty liver disease through modulation of the gut microbiome. Gut Microbes. 2020;11(4):882–899. doi: 10.1080/19490976.2020.1712984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scorletti E, Afolabi PR, Miles EA, et al. Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology. 2020;158(6):1597–1610e7. doi: 10.1053/j.gastro.2020.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–577. doi: 10.1016/j.jhep.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 126.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146(6):1513–1524. doi: 10.1053/j.gastro.2014.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Agus A, Clement K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70(6):1174–1182. doi: 10.1136/gutjnl-2020-323071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68(2):359–370. doi: 10.1136/gutjnl-2018-316307 [DOI] [PubMed] [Google Scholar]
- 129.Lang S, Schnabl B. Microbiota and fatty liver disease-the known, the unknown, and the future. Cell Host Microbe. 2020;28(2):233–244. doi: 10.1016/j.chom.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Freitas PL, Barros MVC, Froes RBL, Franca LM, Paes AMA. Prebiotic effects of plant-derived (poly)phenols on host metabolism: is there a role for short-chain fatty acids? Crit Rev Food Sci Nutr. 2022;1–9. doi: 10.1080/10408398.2022.2100315 [DOI] [PubMed] [Google Scholar]
- 131.Wang Z, Liu J, Li F, et al. The gut-lung axis in severe acute Pancreatitis-associated lung injury: the protection by the gut microbiota through short-chain fatty acids. Pharmacol Res. 2022;182:106321. doi: 10.1016/j.phrs.2022.106321 [DOI] [PubMed] [Google Scholar]
- 132.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Craven L, Rahman A, Nair Parvathy S, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am J Gastroenterol. 2020;115(7):1055–1065. doi: 10.14309/ajg.0000000000000661 [DOI] [PubMed] [Google Scholar]
- 134.Harrison SA, Rinella ME, Abdelmalek MF, et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, Phase 2 trial. Lancet. 2018;391(10126):1174–1185. doi: 10.1016/S0140-6736(18)30474-4 [DOI] [PubMed] [Google Scholar]
- 135.Loomba R, Ling L, Dinh DM, et al. The commensal microbe veillonella as a marker for response to an FGF19 analog in NASH. Hepatology. 2021;73(1):126–143. doi: 10.1002/hep.31523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moreno-Indias I, Torres M, Montserrat JM, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015;45(4):1055–1065. doi: 10.1183/09031936.00184314 [DOI] [PubMed] [Google Scholar]
- 137.Khalyfa A, Ericsson A, Qiao Z, Almendros I, Farre R, Gozal D. Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: effects of physical activity. EBioMedicine. 2021;64:103208. doi: 10.1016/j.ebiom.2021.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lucking EF, O’Connor KM, Strain CR, et al. Chronic intermittent hypoxia disrupts cardiorespiratory homeostasis and gut microbiota composition in adult male Guinea-pigs. EBioMedicine. 2018;38:191–205. doi: 10.1016/j.ebiom.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y, Luo H, Niu Y, et al. Chronic intermittent hypoxia induces gut microbial dysbiosis and infers metabolic dysfunction in mice. Sleep Med. 2022;91:84–92. doi: 10.1016/j.sleep.2022.02.003 [DOI] [PubMed] [Google Scholar]
- 140.Tripathi A, Melnik AV, Xue J, et al. Intermittent hypoxia and hypercapnia, a hallmark of obstructive sleep apnea, alters the gut microbiome and metabolome. mSystems. 2018;3(3). doi: 10.1128/mSystems.00020-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y, Xie B, Chen X, Zhang J, Yuan S. A key role of gut microbiota-vagus nerve/spleen axis in sleep deprivation-mediated aggravation of systemic inflammation after LPS administration. Life Sci. 2021;265:118736. doi: 10.1016/j.lfs.2020.118736 [DOI] [PubMed] [Google Scholar]
- 142.Triplett J, Ellis D, Braddock A, et al. Temporal and region-specific effects of sleep fragmentation on gut microbiota and intestinal morphology in Sprague Dawley rats. Gut Microbes. 2020;11(4):706–720. doi: 10.1080/19490976.2019.1701352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu J, Lu Y, Cai X, Chen Y, Shen Z, Lyv Q. Gut microbiota dysbiosis in 4- to 6-year-old children with obstructive sleep apnea-hypopnea syndrome. Pediatr Pulmonol. 2022. doi: 10.1002/ppul.25967 [DOI] [PubMed] [Google Scholar]
- 144.Valentini F, Evangelisti M, Arpinelli M, et al. Gut microbiota composition in children with obstructive sleep apnoea syndrome: a pilot study. Sleep Med. 2020;76:140–147. doi: 10.1016/j.sleep.2020.10.017 [DOI] [PubMed] [Google Scholar]
- 145.C-y K, Liu -Q-Q, H-z S, et al. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci. 2019;133(7):905–917. doi: 10.1042/CS20180891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Benedict C, Vogel H, Jonas W, et al. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metab. 2016;5(12):1175–1186. doi: 10.1016/j.molmet.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kheirandish-Gozal L, Peris E, Wang Y, et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J Clin Endocrinol Metab. 2014;99(2):656–663. doi: 10.1210/jc.2013-3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nobili V, Alisi A, Cutrera R, et al. Altered gut-liver axis and hepatic adiponectin expression in OSAS: novel mediators of liver injury in paediatric non-alcoholic fatty liver. Thorax. 2015;70(8):769–781. doi: 10.1136/thoraxjnl-2015-206782 [DOI] [PubMed] [Google Scholar]
- 149.Mashaqi S, Kallamadi R, Matta A, et al. Obstructive sleep apnea as a risk factor for COVID-19 severity-the gut microbiome as a common player mediating systemic inflammation via gut barrier dysfunction. Cells. 2022;11(9). doi: 10.3390/cells11091569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tang SS, Liang CH, Liu YL, et al. Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome. World J Gastroenterol. 2022;28(21):2320–2333. doi: 10.3748/wjg.v28.i21.2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ko CY, Fan JM, Hu AK, et al. Disruption of sleep architecture in Prevotella enterotype of patients with obstructive sleep apnea-hypopnea syndrome. Brain Behav. 2019;9(5):e01287. doi: 10.1002/brb3.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–609. doi: 10.1002/hep.26093 [DOI] [PubMed] [Google Scholar]
- 153.Baumann A, Burger K, Brandt A, et al. GW9662, a peroxisome proliferator-activated receptor gamma antagonist, attenuates the development of non-alcoholic fatty liver disease. Metabolism. 2022;133:155233. doi: 10.1016/j.metabol.2022.155233 [DOI] [PubMed] [Google Scholar]
- 154.Imajo K, Fujita K, Yoneda M, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16(1):44–54. doi: 10.1016/j.cmet.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 155.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15(12):738–752. doi: 10.1038/s41575-018-0065-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147(4):765–783e4. doi: 10.1053/j.gastro.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022;29(3):467–480. doi: 10.1038/s41418-022-00941-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Beier JI, Banales JM. Pyroptosis: an inflammatory link between NAFLD and NASH with potential therapeutic implications. J Hepatol. 2018;68(4):643–645. doi: 10.1016/j.jhep.2018.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koh EH, Yoon JE, Ko MS, et al. Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis. Gut. 2021;70(10):1954–1964. doi: 10.1136/gutjnl-2020-322509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hammoutene A, Biquard L, Lasselin J, et al. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J Hepatol. 2020;72(3):528–538. doi: 10.1016/j.jhep.2019.10.028 [DOI] [PubMed] [Google Scholar]
- 161.Wu X, Poulsen KL, Sanz-Garcia C, et al. MLKL-dependent signaling regulates autophagic flux in a murine model of non-alcohol-associated fatty liver and steatohepatitis. J Hepatol. 2020;73(3):616–627. doi: 10.1016/j.jhep.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Allaire M, Rautou PE, Codogno P, Lotersztajn S. Autophagy in liver diseases: time for translation? J Hepatol. 2019;70(5):985–998. doi: 10.1016/j.jhep.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 163.Hernandez A, Geng Y, Sepulveda R, et al. Chemical hypoxia induces pro-inflammatory signals in fat-laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles. Biochim Biophys Acta Mol Basis Dis. 2020;1866(6):165753. doi: 10.1016/j.bbadis.2020.165753 [DOI] [PubMed] [Google Scholar]
- 164.Hwang SY, Yu SJ, Lee JH, Kim HY, Kim YJ. Reduction of oxidative stress attenuates lipoapoptosis exacerbated by hypoxia in human hepatocytes. Int J Mol Sci. 2015;16(2):3323–3334. doi: 10.3390/ijms16023323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu Q, Liu Y, Li F, et al. Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice. J Nutr Biochem. 2020;75:108256. doi: 10.1016/j.jnutbio.2019.108256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tian JL, Zhang Y, Chen BY. Sleep apnea hypopnea syndrome and liver injury. Chin Med J. 2010;123(1):89–94. [PubMed] [Google Scholar]
- 167.Zhang H, Zhou L, Zhou Y, et al. Intermittent hypoxia aggravates non-alcoholic fatty liver disease via RIPK3-dependent necroptosis-modulated Nrf2/NFkappaB signaling pathway. Life Sci. 2021;285:119963. doi: 10.1016/j.lfs.2021.119963 [DOI] [PubMed] [Google Scholar]
- 168.Xin L, Fan W, Tingting D, Zuoming S, Qiang Z. 4-phenylbutyric acid attenuates endoplasmic reticulum stress-mediated apoptosis and protects the hepatocytes from intermittent hypoxia-induced injury. Sleep Breath. 2019;23(2):711–717. doi: 10.1007/s11325-018-1739-y [DOI] [PubMed] [Google Scholar]
- 169.Chen LD, Wu RH, Huang YZ, et al. The role of ferroptosis in chronic intermittent hypoxia-induced liver injury in rats. Sleep Breath. 2020;24(4):1767–1773. doi: 10.1007/s11325-020-02091-4 [DOI] [PubMed] [Google Scholar]
- 170.Ren J, Jin M, You ZX, et al. Melatonin prevents chronic intermittent hypoxia-induced injury by inducing sirtuin 1-mediated autophagy in steatotic liver of mice. Sleep Breath. 2019;23(3):825–836. doi: 10.1007/s11325-018-1741-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang D, Si D, Li G, Ding Z, Yang X, Gao C. Dysregulated autophagic activity induced in response to chronic intermittent hypoxia contributes to the pathogenesis of NAFLD. Front Physiol. 2022;13:941706. doi: 10.3389/fphys.2022.941706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–2127. doi: 10.1038/s41423-021-00740-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Christgen S, Tweedell RE, Kanneganti TD. Programming inflammatory cell death for therapy. Pharmacol Ther. 2022;232:108010. doi: 10.1016/j.pharmthera.2021.108010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cakmak E, Duksal F, Altinkaya E, et al. Association between the severity of nocturnal hypoxia in obstructive sleep apnea and non-alcoholic fatty liver damage. Hepat Mon. 2015;15(11):e32655. doi: 10.5812/hepatmon.32655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bettini S, Serra R, Fabris R, et al. Association of obstructive sleep apnea with non-alcoholic fatty liver disease in patients with obesity: an observational study. Eat Weight Disord. 2022;27(1):335–343. doi: 10.1007/s40519-021-01182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin QC, Chen LD, Chen GP, et al. Association between nocturnal hypoxia and liver injury in the setting of nonalcoholic fatty liver disease. Sleep Breath. 2015;19(1):273–280. doi: 10.1007/s11325-014-1008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Turkay C, Ozol D, Kasapoglu B, Kirbas I, Yildirim Z, Yigitoglu R. Influence of obstructive sleep apnea on fatty liver disease: role of chronic intermittent hypoxia. Respir Care. 2012;57(2):244–249. doi: 10.4187/respcare.01184 [DOI] [PubMed] [Google Scholar]
- 178.Schwenger KJP, Ghorbani Y, Li C, et al. Obstructive sleep apnea and non-alcoholic fatty liver disease in obese patients undergoing bariatric surgery. Obes Surg. 2020;30(7):2572–2578. doi: 10.1007/s11695-020-04514-3 [DOI] [PubMed] [Google Scholar]
- 179.Mishra P, Nugent C, Afendy A, et al. Apnoeic-hypopnoeic episodes during obstructive sleep apnoea are associated with histological nonalcoholic steatohepatitis. Liver Int. 2008;28(8):1080–1086. doi: 10.1111/j.1478-3231.2008.01822.x [DOI] [PubMed] [Google Scholar]
- 180.Maalej S, Jedidi S, Hannachi H, et al. Prevalence et facteurs de risque de steatose hepatique chez les adultes tunisiens atteints de syndrome d’apnees hypopnees obstructives du sommeil [Prevalence and risk factors for fatty liver in Tunisian adults with obstructive sleep apnea]. Rev Mal Respir. 2020;37(1):8–14. French. doi: 10.1016/j.rmr.2019.11.643 [DOI] [PubMed] [Google Scholar]
- 181.Bahr K, Simon P, Leggewie B, Gouveris H, Schattenberg J. The snoring index identifies risk of non-alcoholic fatty liver disease in patients with obstructive sleep apnea syndrome. Biology. 2021;11(1). doi: 10.3390/biology11010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bhatt SP, Guleria R. Association of IRS1 (Gly972Arg) and IRS2 (Gly1057Asp) genes polymorphisms with OSA and NAFLD in Asian Indians. PLoS One. 2021;16(8):e0245408. doi: 10.1371/journal.pone.0245408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bhatt SP, Guleria R, Vikram NK, Vivekanandhan S, Singh Y, Gupta AK. Association of inflammatory genes in obstructive sleep apnea and non alcoholic fatty liver disease in Asian Indians residing in north India. PLoS One. 2018;13(7):e0199599. doi: 10.1371/journal.pone.0199599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mesarwi OA, Shin M-K, Drager LF, et al. Lysyl oxidase as a serum biomarker of liver fibrosis in patients with severe obesity and obstructive sleep apnea. Sleep. 2015;38(10):1583–1591. doi: 10.5665/sleep.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bhatt SP, Guleria R, Vikram NK, Gupta AK. Non-alcoholic fatty liver disease is an independent risk factor for inflammation in obstructive sleep apnea syndrome in obese Asian Indians. Sleep Breath. 2019;23(1):171–178. doi: 10.1007/s11325-018-1678-7 [DOI] [PubMed] [Google Scholar]
- 186.Lu YB, Weng YC, Huang YN, et al. Novel screening model of obstructive sleep apnea for snorers with suspected NAFLD undergoing liver sonography. BMC Pulm Med. 2021;21(1):387. doi: 10.1186/s12890-021-01759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen LD, Huang JF, Chen QS, et al. Validation of fatty liver index and hepatic steatosis index for screening of non-alcoholic fatty liver disease in adults with obstructive sleep apnea hypopnea syndrome. Chin Med J. 2019;132(22):2670–2676. doi: 10.1097/CM9.0000000000000503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen X, Lin X, Chen LD, et al. Obstructive sleep apnea is associated with fatty liver index, the index of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2016;28(6):650–655. doi: 10.1097/MEG.0000000000000598 [DOI] [PubMed] [Google Scholar]