Abstract
Background and purpose:
DNA fragmentation factor 40 (DFF40) as an apoptotic molecule can represent a novel approach to cancer treatment. Lycosin-I (LYC-I), a peptide derived from spider venom, was considered for the targeted delivery of DFF40 to cancer cells. This study attempted to produce soluble DFF40-LYC-I and evaluate its selective lethal effects on HeLa cells.
Experimental approach:
pTWINl vector was used to produce LYC-I and DFF40-LYC-I in E. coli BL21 (DE3) fused to inteins 1 and 2. IPTG concentration and incubation temperature were optimized to achieve the highest level of soluble product. To remove inteins 1 and 2 from the recombinant peptide or protein, pH shift and dithiothreitol were used for a 24-h incubation period at room temperature, respectively. MTT assay was performed to assess the biological effects of these bio-molecules on HeLa and HUVEC cell lines.
Findings/Results:
LYC-I and DFF40-LYC-I were detected in SDS-PAGE with bands of approximately 57 and 97 kDa, respectively. Furthermore, the 3 and 43 kDa bands showed the purified molecules. The IC50 value of DFF40-LYC-I and DFF40 was determined as 6.6 and 17.03 μg/mL for HeLa, respectively. LYC-I had no cytotoxic effects on both cell lines, even at high concentrations.
Conclusion and implications:
A new fusion protein with targeted cancer treatment potential was produced for the first time by LYC-I with a safe profile on normal cells. This fusion protein exhibited higher cytotoxic effects in cancer cells compared to normal cells. However, additional investigations are required to determine the apoptosis induction and evaluate selective toxicity against other cancer and normal cell lines.
Keywords: DFF40, DFF40-LYC-I, Lycosin-I, Targeted therapy.
INTRODUCTION
Chemotherapy is widely used to treat and alleviate the various types of cancer, although it is accompanied by harmful effects. Chemotherapy medications affect all proliferating cells, including healthy and cancer cells, resulting in bone marrow suppression and thrombocytopenia (1). Cancer cells with a slow growth pattern, on the other hand, respond less to chemotherapy medications since they have a stronger effect on proliferating cells (2). Additionally, rapid resistance to chemotherapy drugs is another issue proposed in treating cancer patients (3).
Molecules inducing apoptosis such as DNA fragmentation factor (DFF) are novel approaches in cancer treatment. DFF is a heterodimer protein, which is composed of two subunits including DFF40 and DFF45. DFF40 with a molecular weight of 40 kDa has 338 amino acids. This protein functions as an endonuclease to trigger double-stranded DNA molecule degradation at the 3'OH end (4). DFF45, another subunit, in fusion with DFF40, can block the endonuclease activity of DFF40. DFF40 is activated by caspases 3 and 7, which separate the subunit of DFF45. Therefore, DFF40 is known as caspase-activated DNase (CAD) (5).
Evidence has shown that the expression of DFF40 and DNA fragmentation activity decreased in glioblastoma cells, which confirms the role of DFF40 in increasing cell mortality (6). Also, a chimeric protein produced by binding gonadotropin-releasing hormone (GnRH) to DFF40 targeted colon adenocarcinoma cells. The cells treated by this fusion protein underwent apoptosis and G1 phase arrest, and as a result, their proliferation was reduced (7). Furthermore, in vivo investigations revealed that GnRH-DFF40 decreases tumor size in xenograft mice with colon adenocarcinoma (7). An immunotoxin containing DFF40 and granulocyte-macrophage colony-stimulating factor (GM-CSF) produced in E. coli has targeted acute myeloid leukemia cells. This protein induced apoptosis in the cells by concentration-and time-dependent selective effects, according to cytotoxicity assays (8). Another study investigated the response of the T-47D breast cancer cell line to doxorubicin when transfected with a recombinant vector containing the DFF40 gene and exhibited an increase in the mortality of cancer cells (9). Hence, DFF40 is assumed to be a strong toxic agent damaging cancer cells and increasing their mortality. Accordingly, the use of agents that can only transfer this apoptotic agent to cancer cells requires minimizing the influence on normal cells, improving targeted cytotoxicity, and reducing the dose of cytotoxic drugs in cancer cells. Therefore, lycosin-I (LYC-I), a peptide derived from spider venom was proposed as specifically targeted delivery of DFF-40 into cancer cells.
Spider venom contains a variety of peptides and proteins with therapeutic effects such as LYC-I which has antimicrobial properties (10). This 24-amino acid residue peptide is extracted from the venom of a spider called Lycosa singorensis (11). In vitro and in vivo studies have demonstrated the anti-cancer and apoptosis properties of LYC-I via increasing cyclin-dependent kinase inhibitor proteins in cancer cell lines such as HeLa, A549, HT1080, HCT116, HepG2, etc. (12,13). The ability of this peptide to bind to cancer cell membranes and enter plasma to induce mitochondrial-dependent apoptosis has also been demonstrated (12). Furthermore, LYC-1 showed a strong interaction with lipid membrane and formation of stable aggregates of peptide molecules on the bilayer, which led to its cell penetration. LYC-I, on the other hand, has no toxic effects on human normal cell lines (14). As a result, in addition to the specific cytotoxic effects, this peptide can act as a suitable carrier for the delivery of numerous cytotoxic agents to cancer cells (14). Few studies have investigated LYC-I role as a targeted transport of drugs in cancer cells. Tan et al. demonstrated the ability of LYC-I for the targeted delivery of gold nanoparticles to HeLa and SW480 cells, while it had no lethal effects on human umbilical vein endothelial cell (HUVEC) and HEK293 normal cells due to the inability to penetrate the normal cells (14).
Finally, the aim of this study was to produce the recombinant DFF40-LYC-I in soluble form. Furthermore, as the first stage of evaluation of the newly designed molecule, we assess its cytotoxic effects on the HeLa cell line, which serves as a model for cervical cancer cells.
MATERIALS AND METHODS
Protein design
In order to design the fusion protein containing DFF40 and LYC-I, the amino acid sequence of each part was extracted from the UniProt database with accession number O76075.1 and from the previous study (15). The three-dimensional structure of this fusion protein with the orientation of DFF-40 at N-and LYC-1 at C-terminal of the fusion protein was predicted by Modeller 9.24 (San Francisco, USA) by multiple homology modelling strategy using the crystal structure of caspase-activated DNase (CAD) as a template for DFF-40 (PDB code: 1V0D) and an alpha-helical synthetic peptide from L. erythrognatha spider venom for the LYC-I peptide (PDB code: 6CL3). Among 1000 constructed models by Modeller software, the model with the lowest value of discrete optimized protein energy (DOPE) was considered the best one for protein docking, and analyzed by ProSA, PROCHECK, and Verify3D servers. After confirming the suitable quality of the fusion protein, reverse-translate of the amino acid sequence was performed for coding sequence design.
Production of recombinant vectors
The recombinant pTWIN1-DFF40 plasmid was prepared according to the previous study (15). Subcloning steps were as follows: First, the plasmid was transformed to the CaCl2 competent E. coli TOP 10 bacteria (Pasteur Institute of Iran, Tehran, Iran) and cultured in 10 mL of Luria Bertanii (LB) containing 100 μg/mL ampicillin, overnight. After the plasmid extraction using GeneJET Plasmid Miniprep Kit (Thermo Scientific Inc., USA), this recombinant vector underwent a PCR with specific primers (forward: ATGCCATGGCGATGCTGC, reverse: GGAATTCCTGGCGTTTAC) in order to change the restriction sites at the 5' and 3' of the DFF40 gene. After the digestion of the PCR product by FastDigest™ NcoI and EcoRI restriction enzymes (Thermo Scientific Inc., USA), and gel extraction, the ligation procedure was performed to attach the mentioned digested fragment and pTWIN1-LYC1 vector (synthesized by Zist Eghtesad Mad Co., Tehran, Iran) treated with the same restriction endonucleases by T4 DNA ligase. The accuracy of the recombinant vector containing DFF40-LYC1 was assessed by double digestion with NcoI and EcoRI restriction enzymes as well as DNA sequencing (Gene Fanavaran Co., Iran). Finally, the recombinant vectors (pTWIN1-LYC1 and pTWIN 1-DFF40-L Y C1) were transformed to the E. coli BL21 (DE3) as the expression strain. The recombinant colonies of E. coli BL21 (DE3) were selected on LB-agar plates containing 100 μg/mL ampicillin.
Soluble expression of LYC-I and DFF40-LYCI molecules
After the overnight cultivation, fresh cultures were inoculated. When an optical density in 600 nm vawelength (OD600) reached to 0.6, the total expression of the DFF40-LYCI and LYC-I in fusion to inteins 1 and 2 of the pTWIN1 vector was induced by 1 mM i sopropyl β-d-1-thiogalactopyranoside (IPTG) at 37 °C for 4 h. Furthermore, in order to produce the mentioned proteins in a soluble form, the optimization of recombinant protein expression was performed at the various IPTG concentrations (0.1, 0.3, and 0.5 mM) in different incubation temperatures such as 7, 22, and 37 ° C for lycosin-I and 7, 15, and 37 °C for the fusion protein for 17 h. In each stage, the cells were harvested via centrifugation at 7000 g at 4 °C for 10 min. Finally, the evaluation of the protein expression was performed by 12% sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Purification of recombinant proteins
The intein-mediated purification with an affinity chitin-binding tag (IMPACT) purification system (New England Biolabs, Massachusetts, USA) was used to purify the recombinant peptides and proteins. Based on the cloning of the coding sequence of DFF40-LYC-I gene between the inteins 1 and 2 of pTWIN1 vector (Fig. S1 (663.5KB, tif) ), the self-cleavage activity of inteins was induced by lowering the pH level from 8.5 to 6.5 and dithiothreitol (DTT) for inteins 1 and 2, respectively.
In detail, the induction of intein cleavage from DFF40-LYC-I was performed as follows: first, wash buffer (B1: Tris-HCl 20 mM, NaCl 500 mM, and EDTA 1 mM, pH 8.5) was added to the expression pellets and mixed using a vortex mixer. Then, for cell lysis, sonication protocol including three 30-s cycles with 80 watts amplitude and 60-s intervals followed by three 15-s cycles with 80 watts amplitude and 60-s intervals. was performed (16). Next, the samples were centrifuged at 7000 rpm for 30 min in order to separate the soluble protein from protein inclusion bodies.
The supernatant was poured into a column containing chitin resin and incubated for 30 min. The sample was passed through the column and then, the column was washed by B1 buffer at least 5 times. After that, 5 mL of cleavage buffer (B2: Tris-HCl 20 mM, NaCl 500 mM, EDTA 1 mM, 0.3% Triton®-X100, and 0.2% Tween 20, pH 6.5) was added to the column and put at room temperature for 24 h. Finally, the column supernatant was discharged, and 5 ml of cleavage buffer (B3: Tris-HCl 20 mM, NaCl 500 mM, EDTA 1 mM, DTT 50 mM, 0.3% Triton-X100, and 0.2% Tween 20, pH 8.5) was added to the column and left at room temperature for 24 h. For LYC-I, there were some changes in the IMPACT protocol. B3 buffer was used prior to B2, and B2 was replaced with PBS pH 6.5 for inducing the auto cleavage ability of intein 1. On the next day, the purified column samples were collected and analyzed by SDS-PAGE containing glycine for the fusion protein and tricine for the LYC-I.
Preparation of samples for in vitro evaluation
In order to buffer exchange for DFF40-LYC-I, a dialysis membrane (cut-off 12 kDa) was used to remove the B3 buffer. In this stage, PBS was used as a physiologic buffer for 1-h intervals and finally, a 16-h incubation at the temperature of 4 °C followed by determining the protein concentration via Bradford methods and finally the sterilization with 0.22 pm filters.
Cytotoxic activity evaluation
The evaluation of the cytotoxic effects of recombinant proteins including DFF40, produced according to our previous project (15), LYC-I and DFF40-LYC-I was performed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on HeLa cancer cells compared with HUVEC as normal cells. Briefly, cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 culture medium enriched with 10% (v/v) fetal bovine serum and antibiotics (100 IU/mL of penicillin and 100 μg/mL of streptomycin) and incubated in the incubator at 37 °C and 5% CO2.
In this test, 180 μL of RPMI medium cont aini ng cells (in the final concentration as 3 × 104 cells/mL) was added to each well of a 96-well plate and incubated at 37 °C for 24 h. On the next day, DFF40-LYC-I, DFF40, and LYC-I were added to the wells in the final volume of 20 μL to reach the final concentrations of 1.9, 3.8, 7.5, 15, and 30 μg/mL and incubated at 37 °C for a 48-h period. After adding 20 μL of MTT solution (5 mg/mL) to each well, the plate was incubated in a CO2 incubator for 3 h. Then, 150 μL of dimethyl sulfoxide was added to each well and slowly mixed to dissolve the formazan cry stals. In the end, the OD was measured at 570 nm using a microplate reader (Bio-Rad, USA). PBS-treated cells were used as the negative control, and the cell-free culture medium was used as the blank.
Statistical analysis
In the biological assay stage, the MTT test was repeated in triplicate during independent experiments with four repeated wells for each concentration of proteins or peptides. All data were expressed as mean ± SD. Data analysis was performed by SPSS 23 software. One-way ANOVA followe d by Tukey's post hoc test was used to di sti nguish the differences between or among groups. The significance was assumed as P < 0.05.
RESULTS
Three-dimensional structure analysis
Among 1000 predicted structures for the DFF40-LYC-I fusion protein using Modeller software one with the lowest DOPE score was selected as the best model (-26661.34180) (Fig. 1A). Furthermore, the ProSA web-server results showed a Z-score in the range of about 6.23, with suitable local model quality (Fig. 1B). VERIFY3D, on the other hand, revealed that more than 80% of the amino acids have scored > 0.2 in the 3D/1D profile. Finally, Ramachandran plot analysis exhibited that 91.2% of residues were in the “most favored” regain (Fig. 1C).
Fig. 1.
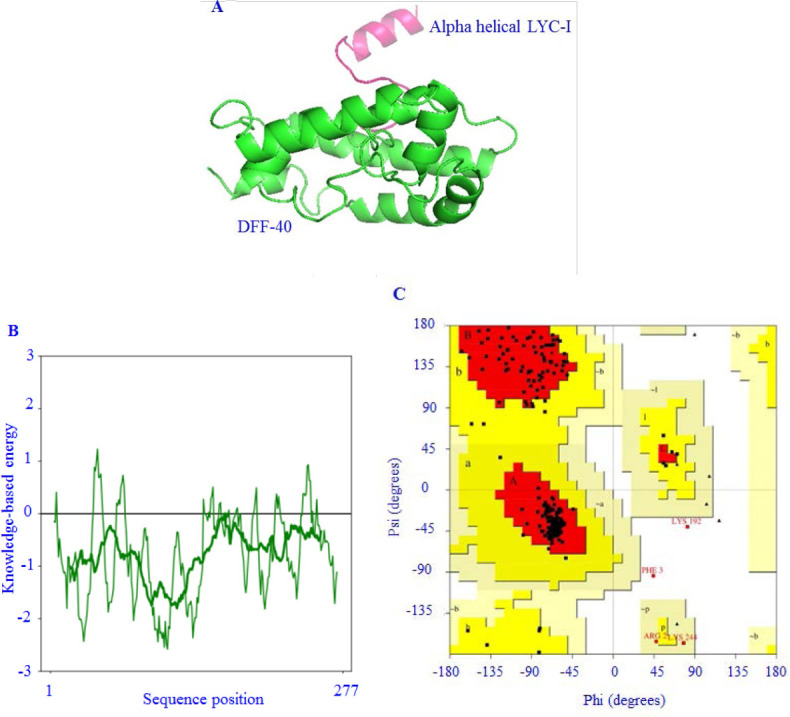
In silico design of DFF40-LYC-I fusion protein. (A) Predicted three-dimensional structure of fusion protein after the visualization by PyMol; (B) local energy distribution of the predicted protein data bank from protein structure analysis web server; (C) Rhamachandran plot analysis of predicted protein. DFF, DNA fragmentation factor; LYC-I, lycosin-I.
PCR and sub-cloning procedures
The 0.8% agarose gel electrophoresis confirmed the successfulness of PCR by relieving a band of about 1200 bps related to DFF40 with modified ends. Furthermore, the double digestion of recombinant pTWIN-DFF40-LYC-I by NcoI and EcoRI restriction endonucleases established the accuracy of the sub-cloning procedure by revealing a band at approximately 1200 bps (Fig. S2 (1MB, tif) ).
Expression and purification of LYC-I and DFF40-LYC-I
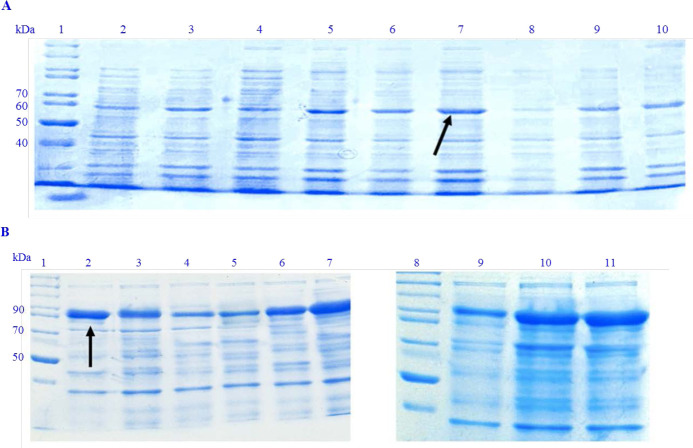
SDS-PAGE analysis confirmed the expression of LYC-I and DFF40-LYC-I with bands at 57 kDa and 97 kDa (fused to inteins 1 and 2), respectively (Figs. 2 and 3). On the other hand, the optimum conditions for the soluble expression of each peptide or protein was varied; in fact, DFF40-LYC-I showed the most soluble expression level in the least inducer concentration (0.1 mM IPTG).
Fig. 2.
Different conditions of total expression of recombinant proteins. (A) Recombinant LYC-I peptide in fusion to inteins 1 and 2. Lane 1, unstained protein ladder 26614 PageRuler™(Thermoscietific, USA); lane 2, total E. coli proteins induced by 0.1 mM IPTG at 7 °C; lane 3, total E. coli proteins induced by 0.3 mM IPTG at 7 °C; lane 4, total E. coli proteins induced by 0.5 mM IPTG at 7 °C; lane 5, total E. coli proteins induced by 0.1 mM IPTG at 22 °C; lane 6, total E. coli proteins induced by 0.3 mM IPTG at 22 °C; lane 7, total E. coli proteins induced by 0.5 mM IPTG at 22 °C; lane 8, total E. coli proteins induced by 0.1 mM IPTG at 37 °C; lane 9, total E. coli proteins induced by 0.3 mM IPTG at 37 °C; lane 10, total E. coli proteins induced by 0.5 mM IPTG at 37 °C; (B) recombinant DFF40-LYC-I in fusion to inteins 1 and 2. Lanes 1 and 8, unstained protein ladder 26614 PageRuler™; lane 2, total E. coli proteins induced by 0.1 mM IPTG at 22 °C; lane 3, total E. coli proteins induced by 0.3 mM IPTG at 22 °C; lane 4, total E. coli proteins induced by 0.5 mM IPTG at 22 °C; lane 5, total E. coli proteins induced by 0.1 mM IPTG at 7 °C; lane 6, total E. coli proteins induced by 0.3 mM IPTG at 7 °C; lane 7, total E. coli proteins induced by 0.5 mM IPTG at 7 °C; lane 9, total E. coli proteins induced by 0.1 mM IPTG at 37 °C; lane 10, total E. coli proteins induced by 0.3 mM IPTG at 37 °C; lane 11, total E. coli proteins induced by 0.5 mM IPTG at 37 °C. LYC-I, lycosin-I; E. coli, Escherichia coli; IPTG, isopropyl β-d-1-thiogalactopyranoside; DFF, DNA fragmentation factor.
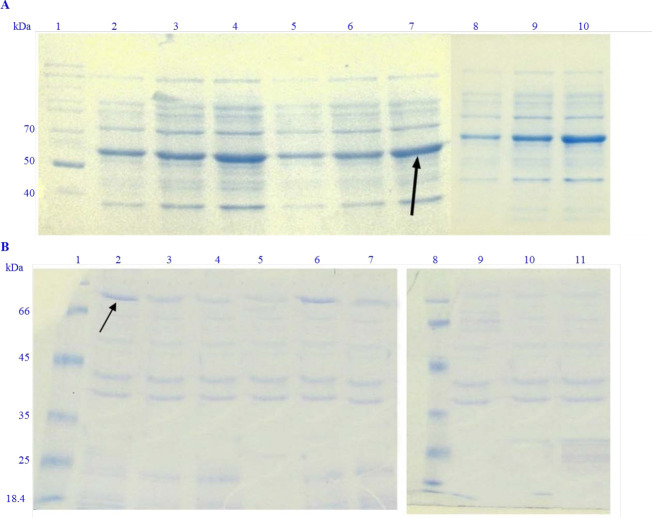
Fig. 3.
Different conditions of the soluble expression of recombinant proteins. (A). LYC-I peptide in fusion to inteins 1 and 2. Lane 1, unstained protein ladder 26614 PageRuler™; Lane 2, expression of E. coli protein solution induced by 0.1 mM IPTG at 7 °C; Lane 3, expression of E. coli protein solution induced by 0.3 mM IPTG at 7 °C; Lane 4, expression of E. coli protein solution induced by 0.5 mM IPTG at 7 °C; lane 5, expression of E. coli protein solution induced by 0.1 mM IPTG at 22 °C; lane 6, expression of E. coli protein solution induced by 0.3 mM IPTG at 22 °C; lane 7, expression of E. coli protein solution induced by 0.5 mM IPTG at 22 °C; lane 8, expression of E. coli protein solution induced by 0.1mM IPTG at 37 °C; lane 9, expression of E. coli protein solution induced by 0.3 mM IPTG at 37 °C; lane 10, expression of E. coli protein solution induced by 0.5 mM IPTG at 37 °C; (B) DFF40-LYC-I in fusion to inteins 1 and 2. Lanes 1 and 8, unstained protein ladder 26614 PageRuler™; lane 2, expression of E. coli proteins induced by 0.1 mM IPTG at 7 °C; lane 3, expression of E. coli proteins induced by 0.3 mM IPTG at 7 °C; lane 4, expression of E. coli proteins induced by 0.5 mM IPTG at 7 °C; lane 5, expression of E. coli proteins induced by 0.1 mM IPTG at 15 °C; lane 6, expression of E. coli proteins induced by 0.3 mM IPTG at 15 °C; lane 7, expression of E. coli proteins induced by 0.5 mM IPTG at 15 °C; lane 9 expression of E. coli proteins induced by 0.1 mM IPTG at 37 °C; lane 10, expression of E. coli proteins induced by 0.3 mM IPTG at 37 °C; lane 11, expression of E. coli proteins induced by 0.5 mM IPTG at 37 °C. LYC-I, lycosin-I; E. coli, Escherichia coli; IPTG, isopropyl β-d-1-thiogalactopyranoside; DFF, DNA fragmentation factor.
On the other hand, the best temperature for the fusion protein expression was determined as 22 °C. These data for the LYC-I fused to the inteins 1 and 2 were determined as 0.5 mM IPTG and incubated at 22 °C for 24 h.
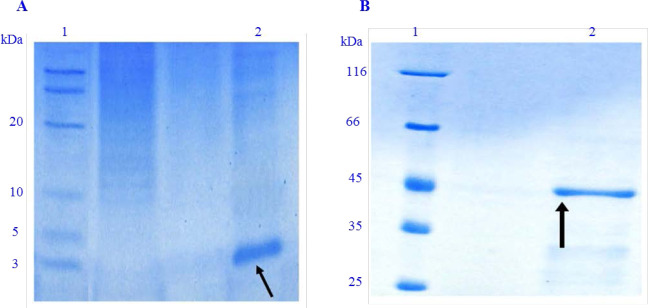
After inducing the cleavage of inteins 1 and 2 from the recombinant proteins, the appearance of bands at approximately 3 KDa for native LYC-I (Fig. 4A) and 43 KDa for the chimeric protein (DFF40-LYC-I) (Fig. 4B) on Tricine and glycin SDS-PAGE proved successful purification by the IMPACT system.
Fig. 4.
Purification of peptide and protein using the IMPACT system. (A). Purified LYC-I. Lane 1, low range unstained protein ladder 26635 (Thermoscientif, USA); lane 2, elution of LYC-I removed from the column; (B) purified DFF40-LYC-I. Lane 1, unstained protein ladder 26612 (Thermoscientif, USA); lane 2, elution of DFF40-LYC-I removed from the column. IMPACT, intein mediated purification with an affinity chitin-binding tag; LYC-I, lycosin-I; DFF, DNA fragmentation factor.
Finally, according to the Bradford method, the final yield of the purified LYC-I and the fusion protein under the optimized conditions was calculated as 477.5 and 973 pg/L of bacterial culture medium, respectively.
Biological assay
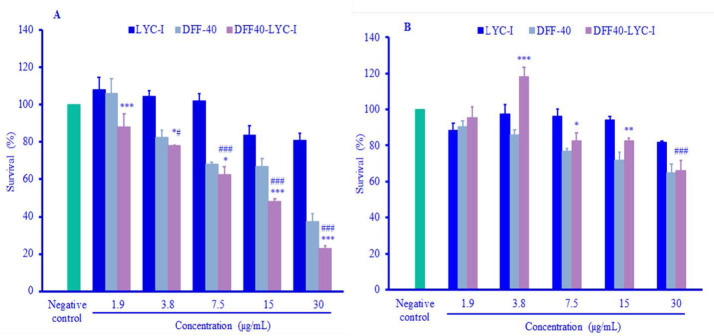
DFF40 and DFF40-LYC-I showed cytotoxic effects on both HeLa and HUVEC cell lines in a concentration-dependent manner. For HeLa cells, there were significant differences in the cytotoxicity between DFF40 and the fusion protein in the same concentrations (Fig. 5A). In other words, the cytotoxicity of the fusion protein was more than the native DFF40. For the LYC-I, on the other hand, there were no significant toxic effects even in the highest concentration (P = 0.92) (Fig. 5A). This result exhibits the cytotoxicity of fusion protein due to the toxic moiety of DFF40.
Fig. 5.
Cytotoxicity assays of DFF40, LYC-I and DFF40-LYC-I against different cell lines after 48 h of incubation. (A) HeLa cell line; (B) HUVEC cell line. Data were presented as mean ± SD (n=3). *P . 0.05, **P . 0.01, and ***P . 0.001 represent significant differences than DFF40 in the same concentrations. #P . 0.05 and ###P . 0.001 represent significant differences versus negative control. LYC-I, lycosin-I; DFF, DNA fragmentation factor.
Regarding the HUVEC, there were significant differences between the cytotoxicity of DFF40 and the fusion protein in an opposite manner (Fig. 5B).
The toxic effect of DFF40 was more than the DFF40-LYC-I in the concentrations of 3.8, 7.5, and 15 μg/mL (Fig. 5B). The fusion protein showed significant toxicity at concentrations of 3.8, 7.5, 15, and 30 μg/mL in HeLa cell line in comparison with negative control (Fig. 5A). Whereas the fusion protein showed significant cytotoxicity only at 30 μg/mL in HUVEC cell line compared to the negative control (Fig. 5B).
Similar to the HeLa cell line, it was shown no significant toxicity of the LYC-I in the normal cells (Fig. 5B).
Finally, based on the concentrations vs. survival percent graphs, the calculated IC 50 of each surveyed compound against two cell lines was reported as follows: HeLa cells demonstrated the IC50 values of about 9.4 and 6.6 μg/mL for DFF40 and DFF40-LYC-I, respectively. Although the IC50 values for the normal cell line were not calculable in three recombinant peptides and proteins in surveyed concentrations.
DISCUSSION
The present study aimed to produce a new and specific fusion protein of DFF40 and LYC-I in Escherichia coli that can be potentially used for cancer treatment. The purification of this bio-molecule was performed using the IMPACT system and finally, its cytotoxic effects were assessed on cancer cells. According to the Bradford assay, after the optimization at the temperatures of 15 and 22 °C and the IPTG concentrations of 0.1 and 0.5 mM, the yield of the purified proteins of DFF40-LYC-I and LYC-I were 973 and 477.5 μg/L of bacterial culture medium, respectively. Biological studies on the HeLa cell line showed that the LYC-I is a safe carrier peptide while DFF40-LYC-I has more cytotoxicity and probably cell penetration than the native apoptotic protein at 48-h incubation, significantly.
In the optimization procedure used in the present study, the temperature and incubation time after the induction, as the most important factors that affect the protein expression were assessed. In general, lowering the temperature after induction reduces the formation of inclusion bodies by reducing the expression of synthesized proteins (17). Lowering the induction temperature has been proposed as the most efficient way to increase the soluble expression of some proteins.
Feghhi-Najafabadi et al. investigated the expression of a mutated form of Interleukin-6 receptor in E. coli BL21 (DE3) and reported that the expressed protein was inactive at 37 °C and only those expressed at 15 °C were in soluble and active form (18). The previous study attempted to produce another fusion protein containing DFF40 and showed that the optimum temperature for the soluble expression is 7 °C (13). However, the current project determined the temperature of 22 °C as the most suitable expression condition for the LYC-I. Therefore, it was assumed that the presence of LYC-I in the fusion of the DFF40 leads to the increase in the solubility of the protein at higher temperature in comparison to the only DFF40. The production of DFF40-LYC-I was negligible at 7 °C, and increased temperature to 15 °C resulted in the emergence of the related band in SDS-PAGE. However, a very small increase in temperature from this level, leads to the decreased expression of soluble protein.
Beyond the incubation temperature, the addition of low amounts of inducers (e.g. IPTG) results in low expression induction but in soluble form, regardless of the reduction in the protein expression costs (19). Actually, the addition of higher amounts of these expensive inducers causes the waste of money and may have toxic effects on cell growth (20). Based on the IMPACT system, in order to obtain soluble recombinant protein, the temperature of 15 °C and IPTG with the final concentration of 0.4 mM are advised. However, the conditions must be optimized for several recombinant proteins One study was performed to produce a fusion protein by the same expression system, IPTG was used in the final concentration of 0.1 mM for DFF40-iRGD in soluble form (15). Again, for the recombinant production of LYC-I, 0.5 mM IPTG could successfully induce its expression unlike the fusion protein which is produced in active form in lower inducer concentration.
Recent studies have used IMPACT system for the purification of recombinant proteins because of its facility and cost-effectiveness than other purification systems based on peptide tags (18,21,22). Amrollahi-Nia et al. used the IMPACT system with a pH shift and the addition of Triton-X100 and Tween 20 in order to enhance the cleavage ability of intein 1 fused to DFF40 and DFF40-iRGD (15). In Sun's study, the human brain natriuretic hormone was expressed using the Ssp DnaB intein, and after refolding, the protein was purified by the chitin column. This purification was performed with high efficiency by changing the pH to 7 at 25 °C for 16 h (23). In another study, the IMPACT system was used to express and purify the PorA protein, an outer membrane protein, in the outer layer of the Neisseria meningitidis membrane. This protein was fused to intein-1 and the purified protein was obtained by changing the temperature and time. This purification was performed at pH 7 and a temperature of 4 °C within 5 days (24).
Because of the potential anti-microbial effects of LYC-I on host cells (E. coli), the N-and C-terminal cloning strategy was used in order to cover both C-and N-amino acid sequences of LYC-I and minimize its cell lysis ability. DTT is the advice of IMPACT system to induce the self-cleavage ability of intein 2 fused to the C-end of LYC-I and the fusion protein (25). In two cases, based on the last amino acid in the C-terminal, incubation was achieved for 24 h at room temperature. In another study, uricase was produced in the pTXB1 vector and purified using the IMPACT system with the addition of DTT at 4 °C for 40 h (26). The other example in this regard is the purification of BR2 derived from buforin IIb as an anti-microbial peptide with an arginine at its carboxyl end cleaved by keeping the chitin column at room temperature for 40 h (22). So, the production of two bio-molecules in this study was performed only in 48 h at room temperature with a final yield of 93%.
DFF40 has been used in several studies to induce apoptosis in cancer cells (9, 28). Bagheri et al. treated T-47D cells containing the DFF40 encoding vector, with doxorubicin and examined their apoptosis (9). The results indicated that the presence of this protein increased the apoptosis effects of chemotherapeutic agents, and thus it is effective in reducing the resistance of cancer cells to apoptosis caused by chemotherapy agents (9). Furthermore, the transfection of T-47D cancer cells with a eukaryotic vector, pIRES2-EGFP-DFF40, to increase the expression of DFF40 evaluated the effect of this protein on the rate of cell death caused by chemotherapy with sulfonamide medicines such as acetazolamide, sulfabenzamide, sulfathiazole, and sulfastamide. The results indicated that the treatment of tumor cells with acetazolamide increases DFF40-induced death (9).
In addition to using the DFF40 base gene therapy strategies discussed before (27), the DFF40 protein has also been used in various studies using various targeting agents to induce apoptosis in cancer cells (9,27). Ben-Yehudah et al. investigated the production of GnRH-DFF40 fusion protein and its effects on DNA fragmentation. The results indicated DNA fragmentation in the COLO205 cell line treated with 5 μg/mL of the fusion protein (7). In another study, Mathew et al. synthesized GM-CSF protein fused to DFF40 and investigated its effects on tumor cells. In the MTT assay, this fusion protein significantly killed THP-1 cells at a concentration of 200 nM indicating a high potency of this protein in inducing cell apoptosis (8). Also, the cells treated with 200 nM GM-CSF-DFF40 were assessed by Annexin V-fluorescein-5-isothiocyanate staining and showed 30-35% apoptosis in this cell line (8). Moreover, Amrollahi-Nia's study investigated the effects of DFF40-iRGD on breast cancer cell lines and revealed the IC50 value of about 0.61 μg/mL at 72-h incubation time and the apoptosis induction of about 76% after 24-h treatment on MDA-MB-231 cell line (15).
The current results showed that LYC-I had no cytotoxic effects on normal and cancer cells. On the contrary, this peptide has been shown to induce apoptosis in cancer cells such as HeLa, A549, HT1080, HCT116, HepG2, etc. by increasing cyclin-dependent kinas inhibitor proteins (14). The peptide also is able to interact with cancer cell membranes and enter plasma to induce mitochondrial apoptosis (12). On the other hand, it has been shown that LYC-I has no adverse effect on normal cells such as HEK293 and erythrocytes (14). Similar to the current study results, when this peptide was used at the concentration of 0.8 μM against HeLa cells, no inhibition was observed in cell proliferation (14). Similarly, the present study exhibited that there were no significant cytotoxic effects even at 1 μM final concentration. However, when this peptide was used as the targeting moiety in the current study, the cytotoxicity of the fusion protein increased significantly in HeLa cells compared to the native DFF40. On the other hand, LYC-I showed no significant cytotoxicity against these cells in the same molar ratios. Therefore, more cytotoxic effects of the fusion protein than DFF40 and also the negligible toxicity of LYC-I on HeLa cells indicated the more penetration ability of the fusion protein due to the presence of the targeting peptide with penetration ability to cancer cells.
CONCLUSION
In this study, the soluble DFF40-LYC-I fusion protein was produced by the bacterial expression system. After purifying, its efficacy was determined on a cancer cell line. In the HeLa cell line, the effect of DFF40-LYC-I was significantly different from the normal cell line of HUVECs, and LYC-I showed no significant difference between the two cell lines. However, it is essential to show the penetration efficacy of the fusion protein in comparison to the native DFF40 without any targeting moiety. Furthermore, the induction of apoptosis must be determined in the fusion protein in comparison to native DFF40. The evaluation of cytotoxicity against other cancer cell lines and its safety on other normal cells also must be achieved. After the evaluation of the effectiveness of this new chimeric protein during in vitro studies, preclinical tests are essential before clinical trials.
Conflict of interest statement
All authors declared no conflict of interest in this study.
Authors' contributions
F. Shafiee designed the study, conducted the experiments, analyzed the data and revised the manuscript. Z. Shafiee-Ardestani performed the experiments and wrote the manuscript draft.
SUPPLEMENTARY MATERIAL
Schem of subcloning of LYC-I and DFF40-LYC-I into pTWIN-1 vector between inteins 1 and 2. LYC-I, lycosin-I; DFF, DNA fragmentation factor. CBD, Chitin binding domain.
Agarose gel electrophoresis of the DFF40-LYC-I coding sequence. Digestion of the recombinant pTWIN-1 containing DFF40-LYC-I fragment by NcoI and XhoI restriction endonucleases. Lane 1, DNA marker (Thermoscientific, USA); lanes 2 to 6, digested recombinant vectors. LYC-I, lycosin-I; DFF, DNA fragmentation factor.
Acknowledgments
This paper was extracted from the Pharm. D thesis submitted by Z. Shafiee-Ardestani which was financially supported by the Research Deputy of Isfahan University of Medical Sciences, with Grant No. 399247. The authors would like to appreciate the valuable technical assistance of laboratory experts in molecular biotechnology and cell culture laboratories.
REFERENCES
- 1.Horvath L, Boyer M, Clarke S, Beale P, Beith J, Underhill C, et al. Carboplatin and vinorelbine in untreated locally advanced and metastatic non-small cell lung cancer. Lung Can. 2001;32(2):173–178. doi: 10.1016/s0169-5002(00)00218-x. DOI: 10.1016/S0169-5002(00)00218-X. [DOI] [PubMed] [Google Scholar]
- 2.Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VHC, Groom AC, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. 2003;82(3):199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. DOI: 10.1023/B :BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 3.Gatti L, Zunino F. 1st. Totowa, New Jersey: Humana; 2005. Overview of tumor cell chemoresistance mechanisms. In: Blumenthal RD, editor. Chemosensitivity: Volume II: in vivo models, imaging, and molecular regulators; pp. 127–148. DOI: 10.1385/1592598897. [DOI] [PubMed] [Google Scholar]
- 4.Widlak P. The DFF40/CAD endonuclease and its role in apoptosis. Acta Biochim Pol. 2000;47(4):1037–1044. DOI: 10.18388/abp.2000_3957. [PubMed] [Google Scholar]
- 5.Gu J, Dong RP, Zhang C, McLaughlin DF, Wu MX, Schlossman SF. Functional interaction of DFF35 and DFF45 with caspase-activated DNA fragmentation nuclease DFF40. J Biol Chem. 1999;274(30):20759–20762. doi: 10.1074/jbc.274.30.20759. DOI: 10.1074/jbc.274.30.20759. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Osuna M, Martinez-Escardo L, Granados-Colomina C, Martinez-Soler F, Pascual-Guiral S, Iglesias-Guimarais V, et al. An intrinsic DFF40/CAD endonuclease deficiency impairs oligonucleosomal DNA hydrolysis during caspase-dependent cell death: a common trait in human glioblastoma cells. Neuro Oncol. 2016;18(7):950–961. doi: 10.1093/neuonc/nov315. DOI: 10.1093/neuonc/nov315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Yehudah A, Aqeilan R, Robashkevich D, Lorberboum-Galski H. Using apoptosis for targeted cancer therapy by a new gonadotropin releasing hormone-DNA fragmentation factor 40 chimeric protein. Clin Cancer Res. 2003;9(3):1179–1190. PMID: 12631624. [PubMed] [Google Scholar]
- 8.Mathew M, Zaineb KC, Verma RS. GM-CSF-DFF40: a novel humanized immunotoxin induces apoptosis in acute myeloid leukemia cells. Apoptosis. 2013;18(7):882–895. doi: 10.1007/s10495-013-0840-8. DOI: 10.1007/s10495-013-0840-8. [DOI] [PubMed] [Google Scholar]
- 9.Bagheri F, Safarian S, Eslaminejad MB, Sheibani N. Sensitization of breast cancer cells to doxorubicin via stable cell line generation and overexpression of DFF40. Biochem Cell Biol. 2015;93(6):604–610. doi: 10.1139/bcb-2015-0007. DOI: 10.1139/bcb-2015-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saez NJ, Senff S, Jensen JE, Er SY, Herzig V, Rash LD, et al. Spider-venom peptides as therapeutics. Toxins (Basel) 2010;2(12):2851–2871. doi: 10.3390/toxins2122851. DOI: 10.3390/toxins2122851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan L, Bai L, Wang L, He L, Li G, Du W, et al. Antifungal activity of spider venom-derived peptide lycosin-I against Candida tropicalis. Microbiol Res. 2018;216:120–128. doi: 10.1016/j.micres.2018.08.012. DOI: 10.1016/j.micres.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Deng M, Xiang J, Ma H, Hu W, Zhao Y, et al. A novel spider peptide toxin suppresses tumor growth through dual signaling pathways. Curr Mol Med. 2012;12(10):1350–1360. doi: 10.2174/156652412803833643. DOI: 10.2174/156652412803833643. [DOI] [PubMed] [Google Scholar]
- 13.Shen H, Xie Y, Ye S, He K, Yi L, Cui R. Spider peptide toxin lycosin-I induces apoptosis and inhibits migration of prostate cancer cells. Exp Biol Med (Maywood) 2018;243(8):725–735. doi: 10.1177/1535370218772802. DOI: 10.1177/1535370218772802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan H, Huang Y, Xu J, Chen B, Zhang P, Ye Z, et al. Spider toxin peptide lycosin-I functionalized gold nanoparticles for in vivo tumor targeting and therapy. Theranostics. 2017;7(12):3168–3178. doi: 10.7150/thno.19780. DOI: 10.7150/thno.19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amrollahi-Nia R, Akbari V, Shafiee F. DFF40-iRGD, a novel chimeric protein with efficient cytotoxic and apoptotic effects against triple-negative breast cancer cells. Biotechnol Lett. 2021;43(10):1967–1976. doi: 10.1007/s10529-021-03178-y. DOI: 10.1007/s10529-021-03178-y. [DOI] [PubMed] [Google Scholar]
- 16.Shafiee F, Rabbani M, Behdani M, Jahanian-Najafabadi A. Expression and purification of truncated diphtheria toxin, DT386, in Escherichia coli: an attempt for production of a new vaccine against diphtheria. Res Pharm Sci. 2016;11(5):428–434. doi: 10.4103/1735-5362.192496. DOI: 10.4103/1735-5362.192496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasina JA, Baneyx F. Expression of aggregation-prone recombinant proteins at low temperatures: a comparative study of the Escherichia coli cspA and tac promoter systems. Protein Expr Purif. 1997;9(2):211–218. doi: 10.1006/prep.1996.0678. DOI: 10.1006/prep.1996.0678. [DOI] [PubMed] [Google Scholar]
- 18.Feghhi-najafabadi S, Shafiee F. Recombinant production of a mutant form of soluble IL-6 receptor with inhibitory effects against interleukin-6. Iran J Biotechnol. 2022;20(1):98–105. doi: 10.30498/ijb.2021.278685.3021. e3021. DOI: 10.30498/ijb.2021.278685.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pourhadi M, Jamalzade F, Jahanian-Najafabadi A, Shafiee F. Expression, purification, and cytotoxic evaluation of IL24-BR2 fusion protein. Res Pharm Sci. 2019;14(4):320–328. doi: 10.4103/1735-5362.263556. DOI: 10.4103/1735-5362.263556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramírez OT, Zamora R, Espinosa G, Merino E, Bolívar F, Quintero R. Kinetic study of penicillin acylase production by recombinant E. coli in batch cultures. Proccess Biochem. 1994;29(3):197–206. DOI: 10.1016/0032-9592(94)85004-6. [Google Scholar]
- 21.Adelnia R, Shafiee F. Recombinant production and one-step purification of IL-1Ra in Escherichia coli and evaluation of its IL-1 antagonizing efficacy. Iran J Immunol. 2021;18(2):141–149. doi: 10.22034/iji.2021.89103.1929. DOI: 10.22034/iji.2021.89103.1929. [DOI] [PubMed] [Google Scholar]
- 22.Shafiee F, Minaiyan G, Moazen F, Jahanian-Najafabadi A. Recombinant production and intein-mediated purification of an antimicrobial peptide, BR2. Int J Pept Res Ther. 2017;23:501–507. DOI: 10.1007/s10989-017-9583-7. [Google Scholar]
- 23.Sun Z, Chen J, Yao H, Liu L, Wang J, Zhang J, et al. Use of Ssp dnaB derived mini-intein as a fusion partner for production of recombinant human brain natriuretic peptide in Escherichia coli. Protein Expr Purif. 2005;43(1):26–32. doi: 10.1016/j.pep.2005.05.005. DOI: 10.1016/j.pep.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Humphries HE, Christodoulides M, Heckels JE. Expression of the class 1 outer-membrane protein of Neisseria meningitidis in Escherichia coli and purification using a self-cleavable affinity tag. Protein Expr Purif. 2002;26(2):243–248. doi: 10.1016/s1046-5928(02)00534-x. [DOI] [PubMed] [Google Scholar]
- 25.Aranko AS, Iwai H. The inducible inter-mediated self-cleaving tag (IIST) system: a novel purification and amidation system for peptides and proteins. Molecules. 2021;26(19):1–13. doi: 10.3390/molecules26195948. 5948. DOI: 10.3390/molecules26195948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alishah K, Asad S, Khajeh K, Akbari N. Utilizing intein-mediated protein cleaving for purification of uricase, a multimeric enzyme. Enzyme Microb Technol. 2016;93(94):92–98. doi: 10.1016/j.enzmictec.2016.08.001. DOI: 10.1016/j.enzmictec.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Minaiyan G, Shafiee F, Akbari V. Survivin Promoter-driven DFF40 gene expression sensitizes melanoma cancer cells to chemotherapy. Int J Toxicol. 2021;40(4):380–387. doi: 10.1177/10915818211014170. DOI: 10.1177/10915818211014170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schem of subcloning of LYC-I and DFF40-LYC-I into pTWIN-1 vector between inteins 1 and 2. LYC-I, lycosin-I; DFF, DNA fragmentation factor. CBD, Chitin binding domain.
Agarose gel electrophoresis of the DFF40-LYC-I coding sequence. Digestion of the recombinant pTWIN-1 containing DFF40-LYC-I fragment by NcoI and XhoI restriction endonucleases. Lane 1, DNA marker (Thermoscientific, USA); lanes 2 to 6, digested recombinant vectors. LYC-I, lycosin-I; DFF, DNA fragmentation factor.