Abstract
Background and Objectives
Relapse and MRI activity usually decline with aging but are replaced by progression independent of relapse activity (PIRA) in patients with multiple sclerosis (PwMS). However, several older PwMS continue to experience clinical relapses, and the impact on their disease remains undetermined. We aimed to determine the impact of an index relapse on disease outcomes in patients older than 50 years and to identify risk factors of disadvantageous outcomes.
Methods
We performed a secondary analysis from 3 prospective cohorts in Germany. We evaluated all PwMS 50 years and older with a relapse ≤60 days before a baseline visit and at least 18 months of follow-up compared with a control cohort of PwMS without a relapse. Patients were stratified according to age (“50–54” vs “55–59” vs “60+”) or disease outcomes (“stable” vs “active” vs “progressive,” according to the Lublin criteria). We analyzed relapses, MRI activity, relapse-associated worsening, and PIRA. Regression analysis was performed to evaluate the association of specific baseline risk factors and treatment regimen changes with disease outcomes at month 18.
Results
A total of 681 patients were included in the “relapse cohort” (50+: 361; 55+: 220; 60+: 100). The “control cohort” comprised 232 patients (50+: 117; 55+: 71; 60+: 44). Baseline epidemiologic parameters were balanced among cohorts and subgroups. We observed increased abundance of inflammatory activity and relapse-independent disability progression in the “relapse” vs “control” cohort. In the “relapse” cohort, we identified 273 patients as “stable” (59.7%), 114 patients as “active” (24.9%), and 70 patients as “progressive” (15.3%) during follow-up. Cardiovascular risk factors (CVRFs) and older age at baseline were identified as risk factors of progressive, whereas disease-modifying treatment (DMT) administration at baseline favored stable disease. DMT during follow-up was associated with stable over active, but not over progressive disease.
Discussion
A relapse—suggesting underlying active disease—in PwMS older than 50 years was associated with continued disease activity and increased risk of PIRA. Presence of CVRF and absence of DMT at baseline appeared as risk factors of disadvantageous disease courses. An escalation of DMT switch was associated with stable over active but not progressive disease.
Introduction
The treatment goals in multiple sclerosis (MS) have changed dramatically throughout the past decade, and “no evidence of disease activity” appears achievable for most people with MS (PwMS).1
Although older data suggested a risk of becoming wheelchair dependent to be as high as 50% in 10 years,2 newer data suggest a drop below 30%.3,4 In line, the excess mortality of PwMS compared with the general population of previously roughly 7 years is expected to further decline.5,6
However, several aspects regarding the disease course under treatment and the impact of relapses on disability worsening remain unclear. In younger patients (with early disease), a relapse has a relevant impact on long-term disability.7 Although relapses may be a strong indicator of ongoing underlying disease activity in older PwMS, its impact on disease outcomes has not been evaluated systematically in this age group. Specifically, the association of relapses and subsequent progression independent of relapse activity (PIRA) remains unclear in older PwMS.
Furthermore, evidence on the effectiveness of disease-modifying treatment (DMT) is less profound in older PwMS. These patients are expected to profit less from DMT as inflammatory activity is thought to decrease with aging.8 However, older patients are more prone to experience adverse events from treatment because they are more likely to have comorbidities and to receive multiple drugs to treat those.9 Thus, treatment discontinuation is repeatedly discussed in older patients, yet studies to date failed to demonstrate safe and effective protocols for such decision.10,11
We thus performed a post hoc analysis of our large multicenter prospective cohort of PwMS to evaluate 3 key questions regarding PwMS older than 50 years: (1) Are relapses associated with active or progressive disease in PwMS older than 50 years? (2) What risk factors are present at relapse onset and are associated with active or progressive disease courses? (3) Are switches of the DMT in response to an index relapse associated with active or progressive disease courses in patients older than 50 years?
Methods
Patients
The study was conducted at 3 tertiary centers in Germany (Heinrich-Heine-University of Duesseldorf, University of Duisburg-Essen, Justus-Liebig-University Giessen). Local prospective clinical databases containing longitudinal data on all PwMS were screened for the period from January 1, 2016, to December 31, 2021. All patients fulfilled the 2017 revised McDonald criteria for relapsing MS.12 None of the patients fulfilled Lorscheider criteria for secondary progressive MS,13 and none of the patients was found to have primary progressive MS.
In a first step, we identified all patients older than 50 years. Among these, we separated the “whole cohort” into patients with a documented clinical relapse (“relapse cohort”) and patients without a relapse (“control cohort”). In the “relapse cohort,” we selected all patients with a documented visit no later than 2 months after the documented “index” relapse (time from symptom onset of a later confirmed relapse to visit) and defined this as “baseline” visit. Patients with at least 18 months of follow-up were included in further analyses. A timespan of ±15 days to a calculated visit was allowed. Among the “control cohort,” we used the last available epoch of 18 months to mitigate selection bias.
Prospectively collected baseline data comprised sex, age, disease duration since MS onset, number of previous DMTs, Expanded Disability Status Scale (EDSS) scores, smoker status, body mass index, and MRI data (presence of a baseline MRI not older than 2 months before a baseline visit; presence of new or enlarging T2-hyperintense MRI lesions [T2Ls]). In addition, data on further neurologic or autoimmune conditions were evaluated as present.
Prospectively collected follow-up data comprised EDSS scores, information on new relapses, and qualified MRI data (availability of a follow-up MRI scan obtained ±1 month before or after a follow-up visit; presence of new or enlarging T2L). All MRI scans included a 3D-fluid-attenuated inversion recovery sequence, which was used for lesion detection and quantification, and all MRI data from the cohort were either obtained at the centers themselves or were re-evaluated during clinical routine by a local neuroradiologist. Only MS-specific lesions were included in the analysis according to most recent recommendations14; nonspecific subcortical lesions were excluded.
Within all selected patients, we performed in-depth medical chart reviews for acquisition of data not stored in the longitudinal cohort databases including presence of diabetes mellitus and cardiovascular disease.
Furthermore, chart review was used to validate comorbidities. Such comorbidities comprised musculoskeletal disorders severely impairing mobility or muscle strength, other severe neurologic disorders than MS, severe psychiatric disorders, further autoimmune disorders, and active neoplastic disorders.
Patients with other autoimmune disorders and those with neoplastic disorders were excluded because these disorders interfere with DMT and often lead to an independent cognitive and physical deterioration, which may introduce severe bias into the evaluation of MS disease progression. Patients with severe psychiatric conditions were excluded because these disorders are known as independent contributors to disability worsening15 that could not be quantified here in detail. Patients with severe musculoskeletal or neurologic disorders were excluded given the impact of these diseases on the reliability of the EDSS score.
For this study, injectable treatments (glatiramer acetate, beta-interferons), teriflunomide, and dimethyl fumarate were termed “platform treatments,” and fingolimod, cladribine, alemtuzumab, ocrelizumab, and natalizumab were termed “highly active treatments.” The resulting data set underwent anonymized statistical analysis.
Stratification and Outcome Measures
The presence of 6 months confirmed disability worsening (CDW) was assessed by EDSS. Similar to clinical trials in MS,16 an increase of 1.0 was deemed relevant in patients with a baseline EDSS of 1.0–5.5, whereas an increase of 0.5 was considered relevant in patients with a baseline EDSS of ≥6.0. This definition was identical for relapse-associated worsening (RAW) and PIRA. RAW itself was defined as worsening of disability after a relapse within the past ≤90 days, whereas PIRA was defined as worsening in the absence of a relapse. CDW required confirmation in a relapse-free interval.
Treatment outcomes among the “relapse” and “control” cohorts were evaluated using survival analysis. Next, patients were stratified according to age (“50–54” vs “55–59” vs “60+”) to evaluate disease outcomes after a relapse among different age strata and performed survival analysis as described below. We refrained from the use of age as a continuous variable because it was neither normally distributed nor appeared as a linear covariate in our regression models.
Afterward, we stratified the patients according to their disease outcome at month 18 from the baseline visit. According to the proposed classification of PwMS published by Lublin et al.,17 we categorized patients' disease courses into “stable,” “active,” or “progressive.”
“Stable” comprised patients without relapses and disability worsening and with not more than 1 new or enlarging T2L within follow-up scans given their low risk of future disability accrual (“minimal evidence of disease activity” [MEDA]).18
“Active” comprised all patients with ongoing signs of inflammation including clinical relapses and/or more than 1 new or enlarging T2L during follow-up scans. RAW was accepted in those patients.
“Progressive” consisted of all patients exhibiting PIRA during follow-up irrespective of relapses or new or enlarging T2L. An increase of disability later than 90 days after a relapse was accepted as PIRA (patients fulfilling criteria for active and progressive during follow-up were categorized as progressive).
For determination of risk factors present already at baseline favoring one of the abovementioned outcomes, we included age group; sex; DMT type at baseline; and the cardiovascular risk factors (CVRFs) smoking, obesity, arterial hypertension, diabetes mellitus, and manifest cardiovascular disease; disease duration; and new MRI lesions at baseline as covariates. For further analysis, we included a 3-step risk score (“0–1 risk factors” vs “2–3 risk factors” vs “4–5 risk factors”) and again evaluated risk factors at baseline upon their contribution to a distinct disease outcome.
Ultimately, we investigated the association of treatment intervention after the index relapse and disease outcomes. We evaluated all patients with a DMT switch within 6 months from baseline and those patients without DMT switch. Patients who had a drug switch after month 6 were excluded from this analysis given the assumed therapeutic lag of a new DMT on disability progression. The treatment switches were defined according to the abovementioned classification of DMT (“none → none”; “none → platform”; “none → highly active”; “platform → platform”; “platform → highly active”; “highly active → highly active”).
Statistical Analysis
Baseline epidemiologic characteristics were evaluated using descriptive statistics. Comparisons among patient subgroups were made using the χ2 test or Fisher exact test for categorical variables (depending on the estimated cell probability) or the Mann-Whitney rank sum test or the Kruskal-Wallis test for continuous variables.
Univariate survival analyses after stratification according to age groups were conducted using the Kaplan-Meier method and a log-rank test.
Risk factors at baseline and the impact of DMT changes in the cohort stratified according to disease outcome were performed using multinomial logistic regression models including all defined covariates in a single step. Significance levels were derived from a likelihood-ratio test, a p value <0.05 was considered significant. Statistical analysis was performed using SPSS 29 (IBM Corp., Armonk, NY).
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the local ethical boards, and patients gave informed consent upon cohort enrollment (institutional review board of the Heinrich-Heine-University Dusseldorf, Germany [5951R]; institutional review board of the University Duisburg-Essen [20-9510-BO]; institutional review board of the Justus-Liebig-University Giessen [53/23]).
Data Availability
All authors were given unrestricted access to data published herein during manuscript preparation. Anonymized data will be shared with qualified investigators upon reasonable request.
Results
Patients
We identified 1,199 patients older than 50 years. In total, 811 patients had at least 1 clinical relapse, whereas 388 patients had no relapse. In total, 1,007 relapses were observed in the cohort and this crudely translates to an annualized relapse rate of 0.14 from 2016 to 2021. The median follow-up of the cohort was 23 months (interquartile range [IQR] 19–31).
Of the identified 811 patients in the “relapse cohort,” 73 were excluded because of lack of follow-up and 57 were excluded because of comorbidities (Figure 1A). Finally, 681 patients were analyzed in the “relapse cohort” (visit availability 2,710/2,724, 99.5%). The median age at first relapse was 54 years (IQR 52–57).
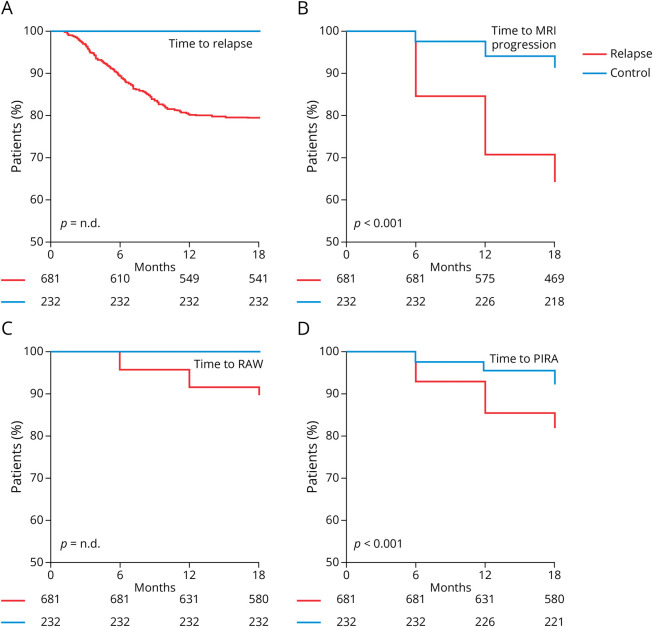
Figure 1. Comparison of Outcome Parameters Between the “Relapse” and “Control” Cohorts.
(A) Kaplan-Meier plot indicating the proportion of relapse-free patients over time among “relapse” (red line) and “control” (blue line) patients. (B) Proportion of patients without new or enlarging T2-hyperintense MRI lesions over time. (C) Proportion of patients without relapse-associated worsening (RAW) over time. (D) Proportion of patients without progression independent of relapse activity (PIRA) over time. Numbers at risk are indicated below the x-axis. Significance levels were calculated using a log-rank test where appropriate.
Among the 388 patients from the “control cohort,” 133 were excluded because of insufficient follow-up and 23 were excluded because of comorbidities. Thus, 232 patients (visit availability 661/696, 95%) were analyzed in the “control cohort” (eFigure 1). The epidemiologic characteristics at baseline are given in Table 1.
Table 1.
Baseline Epidemiologic Characteristics of the “Relapse” and “Control” Cohorts
| Whole cohort (n = 913) | Relapse cohort (n = 681) | Control cohort (n = 232) | p Value | |
| Age, y, median (IQR) | 54 (52–57) | 54 (52–57) | 54 (52–57) | 0.125a |
| Male patients, n (%) | 301 (33.0) | 230 (33.8) | 71 (30.6) | 0.419b |
| Disease duration since diagnosis, y, median (IQR) | 15 (11–20) | 15 (11–20) | 15 (11–20) | 0.269a |
| Patients with of late-onset MS, n (%) | 66 (7.2) | 46 (6.8) | 20 (8.6) | 0.378b |
| No. of previous DMTs, median (IQR) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.080a |
| Baseline EDSS, median (IQR) | 3 (2.5–3.5) | 3 (2.5–3.5) | 3 (2.5–3.5) | 0.130a |
| Baseline MRI available, n (%) | 850 (93.1) | 643 (94.4) | 207 (89.2) | 0.006b |
| New or enlarging T2-lesions in baseline MRI, n (%) | 630 (69.0) | 596 (92.7) | 19 (8.2) | <0.001b |
| DMT at baseline, n (%) | ||||
| None | 144 (15.8) | 122 (17.9) | 22 (9.5) | <0.001b |
| Platform treatment | 562 (61.6) | 425 (62.4) | 137 (59.1) | |
| Active treatment | 207 (22.7) | 134 (19.7) | 73 (31.5) | |
| Time on current DMT at baseline, mo, median (IQR) | 34 (27–41) | 34 (27–40) | 33 (27–40) | 0.768a |
| Patients with risk factors | ||||
| Active smoker | 160 (17.5) | 123 (18.1) | 37 (15.9) | 0.465b |
| Hypertension | 184 (20.2) | 141 (20.7) | 43 (18.5) | 0.477b |
| Obesity | 296 (32.4) | 222 (32.6) | 74 (31.9) | 0.843b |
| Cardiovascular disease | 105 (11.5) | 85 (12.5) | 20 (8.6) | 0.111b |
| Type-2 diabetes | 96 (10.5) | 78 (11.5) | 18 (7.8) | 0.113b |
Abbreviations: DMT = disease-modifying treatment; EDSS = Expanded Disability Status Scale; IQR = interquartile range; MS = multiple sclerosis.
Platform treatments comprised injectable substances (glatiramer acetate, beta-interferons), teriflunomide, and dimethyl fumarate. Active treatments comprised natalizumab, ocrelizumab, fingolimod, cladribine, and alemtuzumab.
Statistical significance was calculated using Kruskal-Wallis test for continuous variables (a) and χ2 test or Fisher exact test for categorical variables (b).
Among the “relapse cohort,” more patients were treatment naïve (17.9% vs 9.5%; p < 0.001) and fewer patients received highly active treatment (19.7% vs 31.5%; p < 0.001). None of the treatment-naïve patients received DMT within the past 2 years, yet 14 of 22 “control” patients (64%) and 71 of 122 “relapse” patients (58%) had a history of earlier platform DMT administration (p = 0.8143).
CVRFs were more frequent in the “relapse cohort,” however, not reaching statistical significance (12.5% vs 8.6%; p = 0.111).
Association of Relapses in PwMS Older Than 50 Years With Active and Progressive Disease
Within follow-up, 140 patients from the “relapse cohort” experienced at least 1 further clinical relapse (21%). In total, we observed 180 relapses during follow-up, 31 of those patients did not receive DMT at baseline (22%), whereas 90 received platform treatment (64%) and 19 received a highly active DMT (14%).
As per definition, no relapse occurred in the “control cohort” (Figure 1A). Patients from the “relapse cohort” were also substantially more prone to develop new T2L (mean estimators for the time to event 533.2 [control] vs 466.6 [relapse] days; p < 0.001; Figure 1B). Of note, the availability of MRI scans was lower in the “control” cohort vs the “relapse” cohort (87.4% vs 94.5%; p < 0.001; eTable 1). However, even after imputation of missing scans as “positive” among the “control” cohort, the difference toward the “relapse cohort” remained significant (p < 0.001).
In terms of disability worsening, we observed that 70 of 681 (10.3%) patients had RAW in the “relapse cohort” (Figure 1C). We also found that the abundance of PIRA was significantly higher in the “relapse cohort” (18.2% vs 7.7%; mean estimators 535.6 [control] vs 508.3 [relapse] days; p < 0.001; Figure 1D).
Among patients with disability worsening from both cohorts, PIRA was less abundant in patients with minimal baseline disability (EDSS 0–1.5: 37.5% of patients with CDW) compared with patients with moderate or high disability at baseline (EDSS 2–3.5: 67.8% of patients with CDW; EDSS ≥4: 73.6% of patients with CDW; p = 0.025). The median increase of the EDSS was 1.0 points (IQR 1.0–1.0) among patients experiencing RAW and 1.0 points (IQR 1.0–1.5) among patients experiencing PIRA.
Thus, our findings show that clinical relapses are associated with both subsequent active and progressive disease in PwMS older than 50 years.
Association of Age With the Risk of Active or Progressive Disease in the “Relapse Cohort”
Patients were stratified according to their age (age 50–54 vs age 55–59 vs age 60+ years), and disease courses during follow-up were compared using the Kaplan-Meier method. Baseline characteristics among strata were well balanced apart from those baseline parameters that increase with age per se (disease duration, proportion of patients with late-onset MS [LOMS; defined as onset of MS symptoms beyond age 50 years]). In addition, diabetes (50–54: 8.0% vs 55–59: 12.3% vs 60+: 22.0%; p < 0.001) and cardiovascular diseases (50–54: 9.7% vs 55–59: 10.9% vs 60+: 26.0%; p < 0.001) were more abundant among PwMS of age 60+ years (eTable 2). MRI scan frequencies remained balanced after stratification (50–54: 94.6%; 55–59: 94.7%; 60+: 93.3%; p = 0.675; for details see eTable 1). Time from index relapse to the baseline visit and time to follow-up visits were comparable among groups (eFigure 2).
We evaluated the time to relapse among age strata and found a slight prolongation of time to first-relapse among patients older than 60 years (mean estimators 472.5 [50–54] vs 473.3 [55–59] vs 492.5 [60+] days; p = 0.415; Figure 2A). Overall, 71 of 180 (39.4%) relapses occurred within month 6 and 87 of 180 (48.3%) relapses occurred within month 12 of follow-up from the index relapse; only 20 of 180 relapses were observed between months 12 and 18 (11.1%).
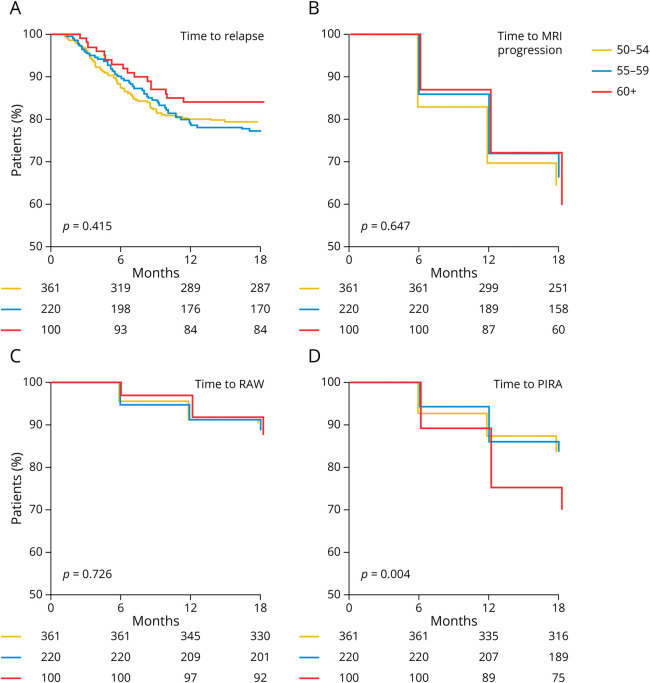
Figure 2. Analysis of Disease Outcomes in the “Relapse” Cohort Stratified by Age Groups.
(A) Kaplan-Meier plot indicating the proportion of relapse-free patients over time among “relapse” (red line) and “control” (blue line) patients. Numbers at risk are indicated below the x-axis. (B) Proportion of patients without new or enlarging T2-hyperintense MRI lesions over time. (C, D) Proportion of patients without relapse-associated worsening (RAW; C) over time and without progression independent of relapse activity (PIRA; D). Significance levels were calculated using a log-rank test.
New or enlarging T2L remained equally abundant during follow-up (month 6: 17.6% of scans; month 12: 18.7%; month 18: 12.3%; mean estimators: 461.7 vs 471.5 vs 473.8 days; p = 0.647; Figure 2B). Proportions of RAW appeared equal among age strata (mean estimators 525.1 vs 524.0 vs 528.8 days; p = 0.726; Figure 2C). However, PwMS older than age 60 years were more susceptible to development of PIRA (mean estimators 513.0 vs 512.3 vs 482.9 days; p = 0.004; Figure 2D).
PwMS older than age 60 years hence appeared more prone to worsening of disability after a relapse than the younger age groups examined here.
Stratification of Outcomes and Risk Factors for Development of Active or Progressive Disease
The “relapse cohort” was stratified according to their outcome at month 18 from baseline as described in the methods chapter into stable, active, and progressive disease (Figure 3A). In total, 393 patients (57.7%) were stable, 164 were active (24.1%), and 124 were progressive (18.2%). None of the patients experienced both RAW and PIRA during follow-up.
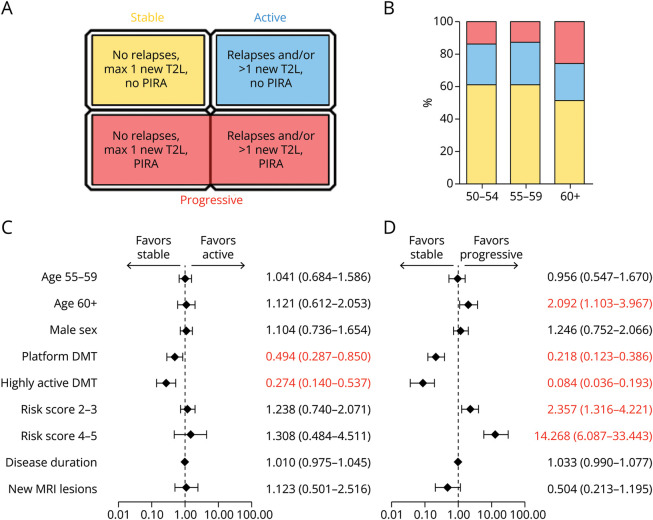
Figure 3. Analysis of Risk Factors of Active or Progressive Disease Courses as Present at Baseline.
(A) Stratification criteria for patient outcome subgroups used in this study. (B) Distribution of patient outcome groups among the 3 age strata. Color scheme from (A) applies as legend for outcome groups. (C, D) We here conducted a multinomial regression model using the outcome type at month 18 as dependent variable. Results are shown as forest plot indicating the odds ratio ±95% CI on a logarithmic scale. Reference categories were “age 50–54” years for age strata, “no DMT at baseline” for DMT, and “0–1” for cardiovascular risk factors. Odds ratios are listed next to the respective covariate. Red numbers indicate covariates selected in the model (p < 0.05). (C) The results for the comparison of active vs stable disease. (D) The results for the comparison of progressive disease vs stable disease. The complete regression model is given in eTable 4. DMT = disease-modifying treatment; PIRA = progression independent of relapse activity; RAW = relapse-associated worsening; T2L = T2-hyperintense MRI lesion.
Most baseline epidemiologic characteristics remained balanced after stratification. DMT administration at baseline, however, differed significantly (proportion of treatment-naïve patients 11.2%/22.0%/33.9% [stable/active/progressive]; proportion of patients with platform DMT 63.45/64.0%/57.3%; proportion of patients with highly active DMT 25.4%/14.0%/8.9%; p < 0.001). Furthermore, CVRFs were more abundant among progressive patients (patients with ≤2 CVRF 17.8%/20.1%/45.2% [stable/active/progressive]; p < 0.001; eTable 3). Frequencies of MRI scans (eTable 1) and time spans between visits (eFigure 2) were equally distributed among groups.
Among 393 stable patients, we found no evidence of disease activity in 337 of 393 patients (86%), whereas in 56 of 393 patients, we observed a solitary new or enlarging T2L suggesting MEDA (14%).
A total of 164 patients developed active disease. One hundred twenty of 164 (73.2%) patients experienced clinical relapses and >1 experienced new or enlarging T2L in the cranial MRI. Twenty (12.2%) patients had a clinical relapse without new MRI lesions and 24 (14.6%) patients developed multiple new or enlarging T2L in absence of a clinical relapse.
Finally, 124 patients were identified as progressive (18.2% of the cohort). Figure 3B shows the proportions of outcome groups among the age strata (50–54: 59.8%/24.1%/16.1% [stable/active/progressive]; 50–59: 58.6%/25.0%/16.4%; 60+: 48.0%/22.0%/30.0%; p = 0.025).
Disease duration at baseline was not significantly different among age strata (median 15 vs 15 vs 17 years for stable, active, and progressive patients, respectively; p = 0.155). Slight but significant differences remained for EDSS at baseline (median 3.0 vs 3.0 vs 3.5 for stable, active, and progressive patients, respectively; p > 0.019).
A multinomial regression analysis containing the epidemiologic parameters available at baseline as covariates and the disease outcome as dependent variable was conducted to identify risk factors at baseline.
Compared with treatment-naïve patients at baseline, administration of both platform and highly active DMT favored stable over active (platform: odds ratio [OR] 0.494, 95% CI 0.287–0.850; p = 0.011; highly active: OR 0.274, 95% CI 0.140–0.537; p < 0.001) and progressive disease (platform: OR 0.218, 95% CI 0.123–0.386; p < 0.001; highly active: OR 0.084, 95% CI 0.036–0.193; p < 0.001; Figure 3, C and D, for full regression model, see eTable 4). A baseline age older than 60 years remained in favor of progressive disease (OR 2.092, 95% CI 1.103–3.967; p = 0.024). The presence of 2–3 (OR 2.357, 95% CI 1.316–4.221; p = 0.004) or 4–5 (OR 14.268, 95% CI 6.087–33.443, p < 0.001) CVRFs (ref. 0–1 CVRF) greatly enhanced the risk of progressive disease, whereas no such association was detectable in terms of active disease. A regression model depicting the contribution of a single CVRF is presented in eFigure 3 and eTable 5.
These data indicate that a higher baseline age and presence of (multiple) CVRF are associated with progressive, but not active disease. Presence of DMT at baseline favored stable disease in either comparison.
Association of DMT Switches After the Index Relapse With Disease Outcomes
Of the 681 patients from the “relapse cohort,” 237 (34.8%) started (or switched) DMT until month 6. Of those, 194 out of 237 patients (28.4% of all patients; 81.5% of all switchers/starters) underwent treatment change until month 3. In total, 209 of 237 patients underwent treatment escalation (88.2%), whereas 26 of 237 patients had a “lateral switch” from 1 platform treatment to another or 1 highly active treatment to another (11.8%).
Additional 43 patients underwent DMT change beyond month 6. In total, 280 patients had a change of their DMT (41.1%). None of the patients de-escalated or stopped their baseline DMT.
Multinomial regression demonstrated that, after adjustment for the further baseline parameters, remaining without DMT was significantly associated with active (OR 2.327, 95% CI 1.002–5.404) and progressive (OR 8.749, 95% CI 3.563–21.488) disease in treatment-naïve patients. Treatment escalation from platform to highly active treatment (OR 0.403, 95% CI 0.236–0.689) or lateral switches from 1 highly active treatment to another (OR 0.404, 95% CI 0.239–0.684) favored stable over active disease, yet were not selected in the regression analysis for stable vs progressive disease (escalation: OR 0.694, 95% CI 0.337–1.428; lateral switch: OR 0.494, 95% CI 0.229–1.063). None of the other DMT switches was associated significantly with a specific disease outcome. Again, presence of CVRF favored progressive over stable disease (2–3 risk factors: OR 2.811, 95% CI 1.517–5.211; p = 0.001; 4–5 risk factors: OR 9.811, 95% CI 3.986–24.148; p < 0.001) (Table 2). Thus, initiation or escalation of DMT in response to a relapse was associated with stable over active, but not over progressive disease.
Table 2.
Association of DMT Switches and Disease Outcomes
| Regression coefficient | Odds ratio | Lower 95% CI | Upper 95% CI | p Value | |
| Active | |||||
| Age group 55–59 years (50–54 = ref.) | 0.037 | 1.037 | 0.681 | 1.580 | 0.864 |
| Age group 60+ years (50–54 = ref.) | 0.131 | 1.140 | 0.625 | 2.082 | 0.669 |
| Male sex (female = ref.) | 0.126 | 1.134 | 0.758 | 1.695 | 0.541 |
| Risk score 2–3 (0–1 = ref.) | 0.289 | 1.335 | 0.797 | 2.236 | 0.272 |
| Risk score 4–5 (0–1 = ref.) | 0.214 | 1.238 | 0.407 | 3.773 | 0.707 |
| DMT sequence “none → none” (“platform → platform” = ref.) | 0.845 | 2.327 | 1.002 | 5.404 | 0.049 |
| DMT sequence “none → platform” (“platform → platform” = ref.) | 0.727 | 2.069 | 0.724 | 5.913 | 0.175 |
| DMT sequence “none → active” (“platform → platform” = ref.) | −0.294 | 0.745 | 0.296 | 1.875 | 0.532 |
| DMT sequence “platform → active” (“platform → platform” = ref.) | −0.908 | 0.403 | 0.236 | 0.689 | 0.001 |
| DMT sequence “active → active” (“platform → platform” = ref.) | −0.905 | 0.404 | 0.239 | 0.684 | 0.001 |
| Disease duration since MS diagnosis (continuous variable, increase per year) | 0.005 | 1.005 | 0.971 | 1.041 | 0.756 |
| Progressive | |||||
| Age group 55–59 years (50–54 = ref.) | −0.005 | 0.995 | 0.547 | 1.808 | 0.987 |
| Age group 60+ years (50–54 = ref.) | 0.420 | 1.521 | 0.741 | 3.125 | 0.253 |
| Male sex (female = ref.) | 0.260 | 1.296 | 0.748 | 2.248 | 0.355 |
| Risk score 2–3 (0–1 = ref.) | 1.034 | 2.811 | 1.517 | 5.211 | 0.001 |
| Risk score 4–5 (0–1 = ref.) | 2.283 | 9.811 | 3.986 | 24.148 | <0.001 |
| DMT sequence “none → none” (“platform → platform” = ref.) | 2.169 | 8.749 | 3.563 | 21.488 | <0.001 |
| DMT sequence “none → platform” (“platform → platform” = ref.) | 0.864 | 2.373 | 0.601 | 9.369 | 0.218 |
| DMT sequence “none → active” (“platform → platform” = ref.) | 0.609 | 1.839 | 0.631 | 5.358 | 0.264 |
| DMT sequence “platform → active” (“platform → platform” = ref.) | −0.366 | 0.694 | 0.337 | 1.428 | 0.320 |
| DMT sequence “active → active” (“platform → platform” = ref.) | −0.706 | 0.494 | 0.229 | 1.063 | 0.071 |
| Disease duration since MS diagnosis (continuous variable, increase per year) | 0.039 | 1.044 | 0.992 | 1.099 | 0.169 |
Abbreviations: DMT = disease-modifying treatment; MS = multiple sclerosis.
A multinomial logistic regression model was developed using the disease outcome as dependent variable (active vs stable [ref.] and progressive vs stable [ref.]) and the listed factors with the indicated reference categories.
Discussion
In this study, we performed a secondary analysis of a large 3-center cohort of PwMS comprising 681 PwMS older than 50 years, who experienced at least 1 index clinical relapse, and 232 patients without a relapse.
With respect to our initial 3 key questions, we found that (1) clinical relapses are associated with subsequent active or progressive disease in PwMS beyond the age of 50 years. In terms of risk factors present at relapse (key question 2), we found that older age and presence of (multiple) CVRF profoundly increased the risk of progressive disease. Presence of a DMT at baseline favored stable over active and progressive disease. In addition, a DMT switch in response to an index relapse was associated with stable over active, but not over progressive disease (key question 3).
Clinical relapses are easy to detect among clinical cohorts and thus were used here as “index” event, yet are not an isolated event but reflect ongoing chronic inflammation. However, their abundance usually decreases with longer disease duration and older age while PIRA becomes the predominant driver of disability accrual.8,19,20
Nonetheless, our data indicate that—at least in this selected German tertiary center cohort—clinical relapses remain a common phenomenon in PwMS beyond age 50 years and underline that treatment strategies are urgently required for these patients.
Whereas early presence of PIRA has been shown to negatively affect disease outcomes,21 our data indicate that—in older patients—this interaction appears bidirectional as clinical relapses increased the frequency of progressive disease during follow-up.22
The fraction of patients with an active course was similar among all age strata, whereas progressive disease became more abundant among older patients. Disease duration and baseline EDSS showed only minor differences among age groups, although the proportion of patients with LOMS increased over age strata and LOMS appeared as independent risk factor of increased disability progression in a previous study.23
We evaluated potential risk factors of active or progressive disease present at the index relapse and found cardiovascular disease was associated with both active and progressive disease. The association of CVRF factors to development and worsening of MS is well known.24-26 Some studies clearly associated these risk factors with increased relapse rates and disability worsening in patients with MS.25 In addition, accelerated brain volume loss is also a major consequence of cardiovascular disease.27,28 Brain volume loss has been determined as a pivotal correlate to PIRA and subsequently progressive MS.29 By contrast, DMT at baseline was associated with stable disease and this effect was stronger for highly active than for platform substances.
Evidence guiding DMT administration in older patients remains sparse. Patients older than 55 years are usually excluded from clinical trials of approved DMT.30 Furthermore, current DMTs predominantly address inflammatory disease activity, and given the assumed decline of this in the elderly, drug cessation in long-term stable and relapse-free PwMS around age 55 years has been repeatedly proposed.31,32 Currently, older patients are less likely to receive DMT per se, and the presence of comorbidities has been identified to further reduce the probability of treatment.33 Unfortunately, comorbidities can further disadvantageously affect DMT safety or complicate drug surveillance.7,32,33 In this study, we described clinical courses after DMT modification. Compared with continuous platform DMT, treatment switch toward highly active treatment was associated with stable over active disease. These findings support treatment escalation in older patients, similar to recommendations for the general MS population as published elsewhere.34,35
Notably, induction of highly active DMT in previously naïve patients was not significantly favoring stable over active disease. Besides insufficient sample size, another potential explanation could be that—in treatment-naïve patients—highly active DMT took too long to exert a protective effect. In line, a recent study determined a therapeutic lag from 12 to 30 weeks in terms of relapse reduction and a lag from 30 to 70 weeks in terms of disability progression.36
In progressive patients, treatment induction in previously untreated patients or escalation from platform to highly active treatment did not favor stable disease anymore. Reasons for this remain unclear, yet various possible explanations exist: (1) The delay from initiation of highly active DMT to full effect can exceed 6 months and thus DMT may simply have been initiated too late to prevent progression during the observational period36; (2) compartmentalization of chronic inflammation within the CNS as a consequence of ongoing disease activity, which may result in a reduced effect of DMT37 and favored a state of “smoldering” MS with a progressive phenotype.38
Finally, pathomechanisms in established progressive MS differ from relapsing disease and involve mitochondrial dysfunction and release of reactive oxygen species and iron accumulation.39,40 Consistent with this, we noted that experiencing a relapse heightened the likelihood of disease progression, whereas managing relapses did not affect the progressive component. Given that current DMTs do not address many of the pathogenetic aspects of progressive MS, it appears reasonable that initiation of DMT in patients remains ineffective once this cascade toward neurodegeneration and thus PIRA commenced. However, a relapse might accelerate this cascade.
Previous data on platform and highly active DMT have repeatedly shown decreased relapse severity in patients receiving DMT compared with placebo.41,42 Thus, it appears likely that (even platform) DMT at baseline also protects from the hypothetical “smoldering” MS mentioned above as well.
Our study has some general limitations related to its design. Besides being observational and hence strongly limited in terms of evaluation of treatment effects, some information potentially relevant to analysis such as socioeconomic background were not available. Others had to be obtained or at least validated by chart reviews. Consequently, all analyses are exploratory by design. Furthermore, our patient cohort is likely to have undergone a selection bias resulting from recruitment exclusively at tertiary centers and this is eventually reflected in the high proportion of patients with a clinical relapse (67.6%). This has likely resulted in an over-representation of active and progressive patients in our sample.
Among the strengths of our study are that data availability throughout the cohort was high, including availability of >92% of MRI scans, and patients underwent standardized examination throughout. Moreover, the data set was complete regarding information on DMT administration. Also, the frequency of risk factors at baseline was representative of the German general population.18
Taken together, our findings indicate a substantial risk of active or progressive disease courses of MS for older patients after a clinical relapse. It remains unclear whether this disease stadium can be halted or even reverted by therapeutic intervention, and if so, how long a “therapeutic window” lasts.
The underlying phenomenon of (undetected) ongoing disease activity yet remains addressed incompletely. The recently published DISCOMS study did not show noninferiority of drug cessation against continuation in patients with previously stable MS older than 55 years.10,11 Whether risk stratification of PwMS with clinically inapparent yet ongoing disease activity will benefit from introduction of biomarkers such as neurofilament light chains remains to be elucidated. Its evaluation in older PwMS, however, appears warranted in the light of the data shown here.
Glossary
- CDW
confirmed disability worsening
- CVRF
cardiovascular risk factor
- DMT
disease-modifying treatment
- EDSS
Expanded Disability Status Scale
- IQR
interquartile range
- LOMS
late-onset MS
- MEDA
minimal evidence of disease activity
- MS
multiple sclerosis
- OR
odds ratio
- PIRA
progression independent of relapse activity
- PwMS
people with MS
- RAW
relapse-associated worsening
- T2L
T2-hyperintense MRI lesion
Appendix. Authors
| Name | Location | Contribution |
| Steffen Pfeuffer, MD | Department of Neurology, University Hospital Giessen, Justus-Liebig-University Giessen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Stephanie Wolff, MD | Department of Neurology, University Hospital Giessen, Justus-Liebig-University Giessen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Derya Aslan, MD | Department of Neurology, University Hospital Essen, University Duisburg-Essen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Leoni Rolfes, MD | Department of Neurology, Medical Faculty, Heinrich Heine University Düsseldorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Melanie Korsen, MD | Department of Neurology, Medical Faculty, Heinrich Heine University Düsseldorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Marc Pawlitzki, MD | Department of Neurology, Medical Faculty, Heinrich Heine University Düsseldorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Philipp Albrecht, MD | Department of Neurology, Medical Faculty, Heinrich Department of Neurology, Maria-Hilf-Clinic, Mönchengladbach, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Joachim Havla, MD | Institute of Clinical Neuroimmunology, LMU Hospital, Ludwig-Maximilians University Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Hagen B. Huttner, MD, PhD | Department of Neurology, University Hospital Giessen, Justus-Liebig-University Giessen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Christoph Kleinschnitz, MD | Department of Neurology, University Hospital Essen, University Duisburg-Essen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Sven G. Meuth, MD, PhD | Department of Neurology, Medical Faculty, Heinrich Heine University Düsseldorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Refik Pul, MD | Department of Neurology, University Hospital Essen, University Duisburg-Essen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Tobias Ruck, MD | Department of Neurology, Medical Faculty, Heinrich Heine University Düsseldorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
No targeted funding reported.
Disclosure
S. Pfeuffer has received travel grants from Roche Pharma, Sanofi-Aventis, and Merck, and lecturing honoraria from Merck, Novartis, Sanofi-Aventis, Mylan Healthcare, Roche, Hexal, Alexion, and Biogen, and research support from Diamed, Merck, Biogen, Novartis, and the German Multiple Sclerosis Society North-Rhine-Westphalia. S. Wolff has received honoraria from Mylan and research grants from Novartis. D. Aslan has received speaker honoraria from Merck Serono and Sanofi-Aventis. L. Rolfes has received travel reimbursements from Merck Serono and Sanofi-Aventis. M. Korsen has received honoraria for lecturing, consulting, and travel expenses from Biogen, Hexal, Merck Healthcare Germany, and Novartis, and research support from Novartis. M. Pawlitzki has received research funding from Novartis and speaker honoraria from ArgenX and Merckand has received research funding from the German Multiple Sclerosis Society North Rhine-Westphalia (DMSG) and the program “Innovative Medizinische Forschung” (IMF) of the Medical Faculty of the University of Muenster. P. Albrecht has received honoraria for lecturing and consulting as well as research support from Allergan/Abbvie, Celgene/Bristol-Myers Squibb, Biogen, Ipsen, Janssen Cilag, Merck, Merz, Novartis, and Roche and honoraria for lecturing and consulting from Bayer, Lilly, Sanofi, and Teva. J. Havla has received grants for OCT research from Friedrich-Baur-Stiftung and Merck, personal fees and nonfinancial support from Celgene, Janssen, Bayer, Merck, Alexion, Novartis, Roche, and Biogen, and nonfinancial support from the Guthy-Jackson Charitable Foundation (all outside the submitted work), and is partially funded by the German Federal Ministry of Education and Research (DIFUTURE, Grant Numbers 01ZZ1603[A-D] and 01ZZ1804[A-H]). H.B. Huttner has received honoraria, travel grants, and/or unrestricted research grants from Boehringer Ingelheim, Bayer AG, Daiichi Sankyo, Novartis, Medtronic, Alexion, CSL Behring, UCB Pharma, and AstraZeneca. C. Kleinschnitz has received honoraria for lecturing and consulting as well as financial research support from Ablynx, Almirall, Amgen, Bayer Vital, Bristol-Mayers Squibb, Biotronik, Boehringer Ingelheim, Biogen Idec, CSL Behring, Daiichi-Sankyo, Desitin, Eisai, Ever Pharma, Sanofi-Aventis, Merck Serono, Mylan, MedDay, Novartis, Pfizer, Roche, Siemens, Stago, and Teva. S.G. Meuth has received speaker honoraria and travel expenses from Almirall, Amicus Therapeutics Germany, Bayer Healthcare, Biogen Idec, Celgene, Diamed, Sanofi-Aventis, MedDay, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Chugai Pharma, QuintilesIMS, and Teva. , and has received research support from the German Ministry for Education and Research (BMBF), Bundesinstitut für Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology, Alexion, Almirall, Amicus Therapeutics Germany, Biogen Idec, Diamed, Fresenius Medical Care, Sanofi-Aventis, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. R. Pul has received honoraria for lecturing and consulting from Alexion, Bayer Healthcare, Biogen Idec, Bristol-Mayers Squibb, MedDay, Merck Serono, Mylan, Novartis, Roche, and Sanofi-Aventis, and has received research funding from HERZ Burgdorf, Merck Serono, and Novartis. T. Ruck has received grants from the German Ministry of Education, Science, Research and Technology, grants and personal fees from Sanofi-Aventis and Alexion, personal fees from Biogen Idec, Roche, and Teva and, personal fees and nonfinancial support from Merck Serono, outside the submitted work. Go to Neurology.org/N for full disclosures.
References
- 1.Bevan CJ, Cree BA. Disease activity free status: a new end point for a new era in multiple sclerosis clinical research? JAMA Neurol. 2014;71(3):269-270. doi: 10.1001/jamaneurol.2013.5486 [DOI] [PubMed] [Google Scholar]
- 2.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438. doi: 10.1056/NEJM200011163432001 [DOI] [PubMed] [Google Scholar]
- 3.Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175-187. doi: 10.1001/jama.2018.20588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Salter A, Wallstrom E, Cutter G, Stuve O. Evolution of clinical trials in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419826547. doi: 10.1177/1756286419826547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lunde HMB, Assmus J, Myhr KM, Bo L, Grytten N. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry. 2017;88(8):621-625. doi: 10.1136/jnnp-2016-315238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanai SA, Saini V, Benedict RH, et al. Aging and multiple sclerosis. Mult Scler. 2016;22(6):717-725. doi: 10.1177/1352458516634871 [DOI] [PubMed] [Google Scholar]
- 7.Fuh-Ngwa V, Charlesworth JC, Zhou Y, et al. The association between disability progression, relapses, and treatment in early relapse onset MS: an observational, multi-centre, longitudinal cohort study. Sci Rep. 2023;13(1):11584. doi: 10.1038/s41598-023-38415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlke F, Arnold DL, Aarden P, et al. Characterisation of MS phenotypes across the age span using a novel data set integrating 34 clinical trials (NO.MS cohort): age is a key contributor to presentation. Mult Scler. 2021;27(13):2062-2076. doi: 10.1177/1352458520988637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweitzer F, Laurent S, Fink GR, et al. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Curr Opin Neurol. 2019;32(3):305-312. doi: 10.1097/WCO.0000000000000701 [DOI] [PubMed] [Google Scholar]
- 10.Corboy JR, Fox RJ, Kister I, et al. Risk of new disease activity in patients with multiple sclerosis who continue or discontinue disease-modifying therapies (DISCOMS): a multicentre, randomised, single-blind, phase 4, non-inferiority trial. Lancet Neurol. 2023;22(7):568-577. doi: 10.1016/S1474-4422(23)00154-0 [DOI] [PubMed] [Google Scholar]
- 11.Coerver EME, Fung WH, Beukelaar JD, et al. Patient-reported outcomes in discontinuation of first-line disease-modifying therapy in stable multiple sclerosis (DOT-MS): results of a multicenter randomized controlled trial. Presented at: MSMilan; October 11-13, 2023; Milan, Italy. POSTER 791.
- 12.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 13.Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139(pt 9):2395-2405. doi: 10.1093/brain/aww173 [DOI] [PubMed] [Google Scholar]
- 14.Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016;15(3):292-303. doi: 10.1016/S1474-4422(15)00393-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKay KA, Tremlett H, Fisk JD, et al. Psychiatric comorbidity is associated with disability progression in multiple sclerosis. Neurology. 2018;90(15):e1316-e1323. doi: 10.1212/WNL.0000000000005302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. doi: 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 17.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286. doi: 10.1212/WNL.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prosperini L, Mancinelli C, Haggiag S, et al. Minimal evidence of disease activity (MEDA) in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2020;91(3):271-277. doi: 10.1136/jnnp-2019-322348 [DOI] [PubMed] [Google Scholar]
- 19.Lublin FD, Haring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145(9):3147-3161. doi: 10.1093/brain/awac016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132-1140. doi: 10.1001/jamaneurol.2020.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tur C, Carbonell-Mirabent P, Cobo-Calvo A, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. 2023;80(2):151-160. doi: 10.1001/jamaneurol.2022.4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch-Henriksen N, Thygesen LC, Sorensen PS, Magyari M. Worsening of disability caused by relapses in multiple sclerosis: a different approach. Mult Scler Relat Disord. 2019;32:1-8. doi: 10.1016/j.msard.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 23.D'Amico E, Patti F, Zanghi A, Chisari CG, Lo Fermo S, Zappia M. Late-onset and young-onset relapsing-remitting multiple sclerosis: evidence from a retrospective long-term follow-up study. Eur J Neurol. 2018;25(12):1425-1431. doi: 10.1111/ene.13745 [DOI] [PubMed] [Google Scholar]
- 24.Harding KE, Wardle M, Carruthers R, et al. Socioeconomic status and disability progression in multiple sclerosis: a multinational study. Neurology. 2019;92(13):e1497-e1506. doi: 10.1212/WNL.0000000000007190 [DOI] [PubMed] [Google Scholar]
- 25.Petruzzo M, Reia A, Maniscalco GT, et al. The Framingham cardiovascular risk score and 5-year progression of multiple sclerosis. Eur J Neurol. 2021;28(3):893-900. doi: 10.1111/ene.14608 [DOI] [PubMed] [Google Scholar]
- 26.Rosso M, Chitnis T. Association between cigarette smoking and multiple sclerosis: a review. JAMA Neurol. 2020;77(2):245-253. doi: 10.1001/jamaneurol.2019.4271 [DOI] [PubMed] [Google Scholar]
- 27.Kappus N, Weinstock-Guttman B, Hagemeier J, et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(2):181-187. doi: 10.1136/jnnp-2014-310051 [DOI] [PubMed] [Google Scholar]
- 28.Jakimovski D, Weinstock-Guttman B, Gandhi S, et al. Dietary and lifestyle factors in multiple sclerosis progression: results from a 5-year longitudinal MRI study. J Neurol. 2019;266(4):866-875. doi: 10.1007/s00415-019-09208-0 [DOI] [PubMed] [Google Scholar]
- 29.Cagol A, Schaedelin S, Barakovic M, et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. 2022;79(7):682-692. doi: 10.1001/jamaneurol.2022.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaughn CB, Jakimovski D, Kavak KS, et al. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol. 2019;15(6):329-342. doi: 10.1038/s41582-019-0183-3 [DOI] [PubMed] [Google Scholar]
- 31.Signori A, Schiavetti I, Gallo F, Sormani MP. Subgroups of multiple sclerosis patients with larger treatment benefits: a meta-analysis of randomized trials. Eur J Neurol. 2015;22(6):960-966. doi: 10.1111/ene.12690 [DOI] [PubMed] [Google Scholar]
- 32.Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577. doi: 10.3389/fneur.2017.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Tremlett H, Leung S, et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology. 2016;86(14):1287-1295. doi: 10.1212/WNL.0000000000002543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiendl H, Gold R, Berger T, et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord. 2021;14:17562864211039648. doi: 10.1177/17562864211039648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777-788. doi: 10.1212/WNL.0000000000005347 [DOI] [PubMed] [Google Scholar]
- 36.Roos I, Leray E, Frascoli F, et al. Delay from treatment start to full effect of immunotherapies for multiple sclerosis. Brain. 2020;143(9):2742-2756. doi: 10.1093/brain/awaa231 [DOI] [PubMed] [Google Scholar]
- 37.Faissner S, Plemel JR, Gold R, Yong VW. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov. 2019;18(12):905-922. doi: 10.1038/s41573-019-0035-2 [DOI] [PubMed] [Google Scholar]
- 38.Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the ‘real MS’. Ther Adv Neurol Disord. 2022;15:17562864211066751. doi: 10.1177/17562864211066751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43(4):655-661. doi: 10.1212/wnl.43.4.655 [DOI] [PubMed] [Google Scholar]
- 40.De Stefano N, Sormani MP, Giovannoni G, et al. Analysis of frequency and severity of relapses in multiple sclerosis patients treated with cladribine tablets or placebo: the CLARITY and CLARITY Extension studies. Mult Scler. 2022;28(1):111-120. doi: 10.1177/13524585211010294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647-656. doi: 10.1038/nrneurol.2012.168 [DOI] [PubMed] [Google Scholar]
- 42.Correale J, Gaitan MI, Ysrraelit MC, Fiol MP. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain. 2017;140(3):527-546. doi: 10.1093/brain/aww258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors were given unrestricted access to data published herein during manuscript preparation. Anonymized data will be shared with qualified investigators upon reasonable request.