Abstract
Background and Objectives
Clinical trials in neurodegenerative diseases often encounter selective enrollment and under-representation of certain patient populations. This delays drug development and substantially limits the generalizability of clinical trial results. To inform recruitment and retention strategies, and to better understand the generalizability of clinical trial populations, we investigated which factors drive participation.
Methods
We reviewed the literature systematically to identify barriers to and facilitators of trial participation in 4 major neurodegenerative disease areas: Alzheimer disease, Parkinson disease, amyotrophic lateral sclerosis, and Huntington disease. Inclusion criteria included original research articles published in a peer-reviewed journal and evaluating barriers to and/or facilitators of participation in a clinical trial with a drug therapy (either symptomatic or disease-modifying). The Critical Appraisal Skills Program checklist for qualitative studies was used to assess and ensure the quality of the studies. Qualitative thematic analyses were employed to identify key enablers of trial participation. Subsequently, we pooled quantitative data of each enabler using meta-analytical models.
Results
Overall, we identified 36 studies, enrolling a cumulative sample size of 5,269 patients, caregivers, and health care professionals. In total, the thematic analysis resulted in 31 unique enablers of trial participation; the key factors were patient-related (own health benefit and altruism), study-related (treatment and study burden), and health care professional-related (information availability and patient–physician relationship). When meta-analyzed across studies, responders reported that the reason to participate was mainly driven by (1) the relationship with clinical staff (70% of the respondents; 95% CI 53%–83%), (2) the availability of study information (67%, 95% CI 38%–87%), and (3) the use or absence of a placebo or sham-control arm (53% 95% CI 32%–72%). There was, however, significant heterogeneity between studies (all p < 0.001).
Discussion
We have provided a comprehensive list of reasons why patients participate in clinical trials for neurodegenerative diseases. These results may help to increase participation rates, better inform patients, and facilitate patient-centric approaches, thereby potentially reducing selection mechanisms and improving generalizability of trial results.
Introduction
Randomized clinical trials have been the gold standard for evaluating new disease-modifying therapies but present a particular challenge in neurodegenerative diseases.1,2 Besides our limited understanding of the underlying pathophysiologic mechanisms, and the clinical heterogeneity between patients,1 enrollment involves some unique challenges because of the debilitating nature of the diseases: trials require more time to complete and the costs incurred are higher than other therapeutic indications.3 These challenges are further exacerbated by barriers that hinder trial participation.
Participation rates in clinical trials have been estimated to be as low as 2%–8% of all patients living with a certain condition.4 Significantly, participation rates among ethnic minorities, older patients, and late-stage disease patients have been estimated to be even lower.5-8 Among patients who are aware of the option of participation and who are willing to participate, only a minor fraction is eligible,9,10 and even fewer patients will ultimately complete the study.11 This not only jeopardizes the generalizability of clinical trial results but also forms a major obstacle to feasibly executing a clinical trial and to developing a reliable understanding of the effectiveness and safety of a therapy afterward.12 Exploring the underlying mechanisms behind patient accrual, retention, and motivation to participate in clinical trials is, therefore, of significance.
Previous studies have focused on investigating barriers to and facilitators of trial participation in other therapeutic areas, including oncology, cardiology, stroke, and human immunodeficiency virus research.4,13-19 Numerous factors have been identified that influence trial participation. These encompass a range of considerations, such as personal motivations and factors related to the study design (e.g., placebo use, time consumption).4,13 However, because patients with neurodegenerative diseases often face a far more devastating future combined with functional or cognitive disabilities, factors that drive trial participation may differ. Therefore, we systematically reviewed the literature to identify factors that influence trial participation in 4 major neurodegenerative disease areas: Alzheimer disease (AD), amyotrophic lateral sclerosis (ALS), Huntington disease (HD), and Parkinson disease (PD). By combining a qualitative thematic analysis with a quantitative meta-analysis, we aim to provide a comprehensive overview of key factors that contribute to trial participation and thus inform investigators how to align their recruitment and retention strategies better.
Methods
Search Strategy
The objective of the systematic review was to search for all original research articles that aim to identify and/or evaluate barriers to and facilitators of clinical trial participation in patients with AD, ALS, HD, or PD. Studies were identified in the PubMed and Embase databases, as well as by screening reference lists from relevant reviews. The search was limited to the most common neurodegenerative diseases: AD, ALS, HD, and PD.20,21 Search terms included “barriers,” “facilitators,” “clinical trials,” “participation,” “Alzheimer's disease,” “amyotrophic lateral sclerosis,” “Huntington's disease,” “Parkinson's disease,” and their synonyms (eAppendix 1, all supplementary material is also available on GitHub). The search was discussed with an information specialist and was limited to qualitative and quantitative studies written in English. The final reference list was generated in May 2023.
Study Selection
After removal of duplicates, the reference list was analyzed using Automated Systematic Review (ASReview)—an active (machine) learning framework that ranks titles and abstracts, based on the relevance of the record, using prior information entered into the software by the reviewer.22 For each disease group, 1 relevant record and 1 irrelevant record were selected to serve as prior information. During the review, ASReview presents an article to the reader based on prior information (i.e., which articles were relevant and which were irrelevant) and the reviewer decides whether the title and abstract fulfill the criteria, and thus, whether the article is relevant or irrelevant. Title and abstract reviewing with ASReview was halted when the number of consecutive irrelevant articles equaled 10% of the total.
Initially, a set of 200 ranked abstracts was created and screened by 2 reviewers. These were discussed until consensus was reached (eAppendix 2.1). The subsequent titles and abstracts were screened by 1 reviewer. References were eligible for full-text screening if the abstract described that the aim of the study was to identify and/or evaluate barriers to and/or facilitators of trial participation in clinical trials for neurodegenerative diseases. Full-text articles had to meet the following criteria to be used in the thematic analysis and meta-analysis (eAppendix 2.2): original research published in a peer-reviewed journal, evaluating barriers to and/or facilitators of participation in a clinical trial with a drug therapy (either symptomatic or disease modifying), and concerning patients with AD, ALS, HD, or PD. To assess and ensure the quality of the qualitative studies, the Critical Appraisal Skills Program (CASP) checklist for qualitative studies was used (eAppendix 3).23
Data Extraction
Data on barriers and facilitators were independently extracted following a prespecified extraction scheme. The first 10 articles and the corresponding study extraction schemes were discussed in-depth and compared between 2 reviewers. Studies solely reporting demographics, people at risk of a neurodegenerative disease (e.g., healthy older patients), outcomes represented by <5% of the sample size, analyses without descriptive statistics, or articles where the patient population with neurodegenerative diseases was ≤50% of the total sample size were excluded from the analysis.
Qualitative Synthesis
Thematic analysis was employed to integrate and interpret the qualitative data from the studies that were included. An inductive approach was used to determine the final themes. First, 2 researchers (T.K. and D.N.W.) independently coded the extracted data; then, the coding schemes were compared. The extracted data consisted of first-order constructs (i.e., direct patient quotes reported in the included studies) and second-order constructs (i.e., statements made by the authors of the included studies).24 The coded data represented the main idea or feeling that was expressed. After initial coding, all codes were collated and grouped. Finally, patterns were identified to create themes and subthemes based on the codes. As each theme can consist of both negative (barriers) and positive (facilitator) statements (e.g., for financial compensation, a positive statement would be that someone was glad they received compensation for their effort, whereas a negative statement would be that they would have appreciated some compensation for their time), we hereafter refer to them as “enablers” of trial participation.
The identified enablers were clustered into 3 overarching factors: patient-, study-, or HCP-related factors. The study- and HCP-related enablers were considered modifiable factors that can be altered before or during a clinical trial and could potentially benefit recruitment strategies. Patient-related enablers were appraised as motivators or beliefs—intrinsic factors that cannot be directly amended to improve participation in clinical trials but may facilitate better education of patients. To ensure trustworthiness of the analysis process and of the results, several consensus meetings were held with D.N.W., A.B., T.K., and R.P.A.v.E. to discuss the created codes, the grouping into patient-, study-, and HCP-related factors, and the final (sub)themes. For transparency, the review process is available on GitHub.
Statistical Analysis
The study characteristics are summarized as frequency (percentage), with the thematic analysis being presented visually as a flowchart. The flowchart illustrates the distribution of data points for each enabler, reflecting the number of quotes or statements extracted from the included studies. The quantitative data, namely the proportion of responders reporting a certain enabler in each study, were synthesized using meta-analysis to obtain pooled effect size estimates across studies. A meta-analysis was conducted for each modifiable enabler separately. From each quantitative study, we calculated a proportion of responders reporting a certain enabler; these were subsequently logit transformed. Mixed-effects logistic models were fitted to account for the binomial structure of the data and to account for between-study heterogeneity.25 The weighting of the meta-analyses was indirectly affected by the number of information sources and the number of responses in that more information contributed to better estimation of the overall effect size. The pooled estimated percentages and their 95% CIs were reported for each modifiable enabler in a forest plot. We anticipated that the diversity in question formulation regarding trial participation would introduce heterogeneity. To quantify between-study heterogeneity, we report τ2 together with the Q and I2 statistics.
As an exploratory analysis, we evaluated whether study heterogeneity was driven by disease population, especially as AD data originate primarily from caregivers.3 As such, we added an indicator variable for AD vs non-AD as covariate in a meta-regressive model; the odds ratio and 95% CI were reported, together with the reduction in between-study heterogeneity. All quantitative analyses are performed in R (version 4.2.1).26 The “metafor” package was used for the meta-analyses.27 All data and code for this manuscript are available on GitHub.
Standard Protocol Approvals, Registrations, and Patient Consents
As this study is a systematic review and did not involve direct participation of human participants, approval by an ethical committee was not applicable.
Data Availability
The data used in both thematic analysis and meta-analysis are accessible through a GitHub repository (github.com/daphneweemering/trial-participation). This repository remains indefinitely accessible. In the event of data unavailability on GitHub, it can be obtained upon request.
Results
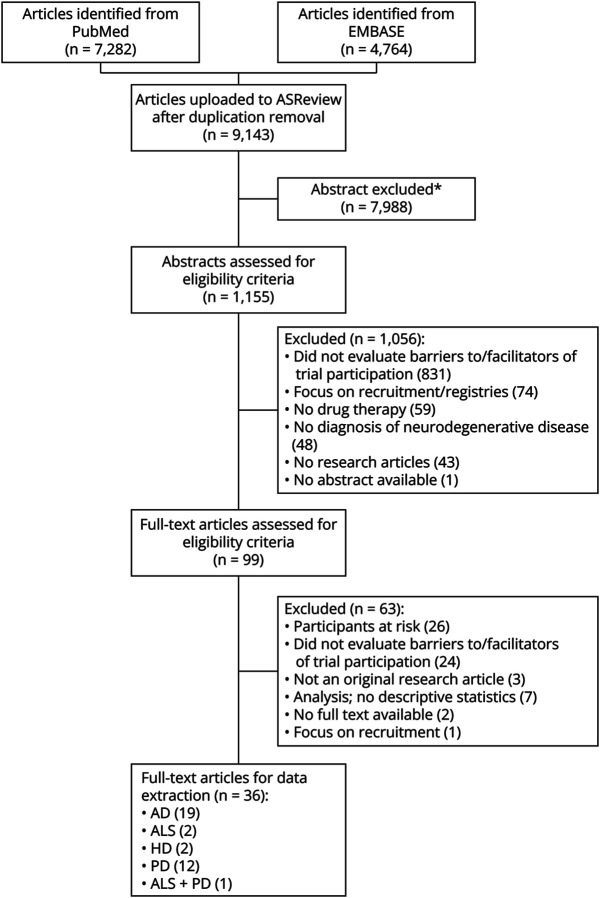
In total, 9,143 unique citations were identified; of these, 1,155 abstracts were screened in ASReview, and 99 full-text articles were included for the eligibility review (Figure 1). Inter-rater agreement was assessed for a fixed batch of 200 abstracts. Specifically, the kappa statistic (κ) was used to evaluate the degree of concordance between the reviewer assessments of the abstracts. This showed strong agreement (κ = 0.86) between 2 independent reviewers. Of the 99 articles assessed, 63 were excluded on the basis of predetermined criteria. Articles were excluded if they focused solely on patients at risk of a neurodegenerative disease (n = 26), lacked an assessment of barriers and/or facilitators of trial participation (n = 24), were not classified as an original research article (n = 3), performed an analysis and did not provide descriptive statistics (n = 7), were unavailable in full text (n = 2), or solely concentrated on recruitment (n = 1). An example of an excluded study is the study by Linger et al.,28 who evaluated the effect of several recruitment strategies on the likelihood of trial participation. No studies were excluded on the basis of the CASP, as the overall score for each study was adequate (eAppendix 3). Hence, 36 articles were used for data extraction and analysis (AD: n = 19, ALS: n = 2, HD: n = 2, PD: n = 12, ALS + PD: n = 1). The references to the included articles are given in eAppendix 4.
Figure 1. PRISMA Flowchart of Systematic Review.
Based on prior information (i.e., marking a relevant and an irrelevant article before title and abstract reviewing) and reviewer choices, the most relevant articles are pushed forward for review. Title and abstract reviewing was halted when the number of consecutive irrelevant articles equaled 10% of the total. *Abstracts are excluded based on ASReview systematic reviewing software. AD = Alzheimer disease; ALS = amyotrophic lateral sclerosis; ASReview = Automated Systematic Review; HD = Huntington disease; PD = Parkinson disease; PRISMA = Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Study Characteristics
Articles reporting different qualitative and/or quantitative methods, diverse responder types, or differing disease groups were treated as unique information sources, and data were extracted separately for each part of the article. As a result, the total number of unique information sources is greater than the number of articles. Overall, among the 36 articles, we identified 45 unique information sources enrolling a total sample size of 5,269 patients, caregivers, and HCPs, of which 18 sources were qualitative by design, 25 sources quantitative, and 2 sources combined qualitative and quantitative methods. The characteristics of the distinct information sources are summarized in Table 1; the characteristics of the individual studies are provided in the supplementary material (eAppendix 5). Of note, most information sources provided data from patients with either AD or PD (87%, 39 of 45). In addition, compared with the other disease areas, data for AD originated in 70% (16 of 23) from caregivers or patient–caregiver dyads; this was only 14% (3 of 22) for non-AD sources. For the non-AD sources, most data originated from patients.
Table 1.
Summary of the Unique Information Sources
| AD (n = 23) | ALS (n = 4) | HD (n = 2) | PD (n = 16) | Overall (n = 45) | |
| Total sample size | 1,734 | 535 | 273 | 2,727 | 5,269 |
| Data collection method | |||||
| Survey | 12 (52) | 4 (100) | 1 (50) | 8 (50) | 25 (56) |
| (Semi)structured interview | 7 (30) | — | 1 (50) | 4 (25) | 12 (27) |
| Focus group | 3 (13) | — | — | 2 (13) | 5 (11) |
| (Semi)structured interview and focus group | 1 (4) | — | — | 1 (6) | 2 (4) |
| (Semi)structured interview, focus group, and survey | — | — | — | 1 (6) | 1 (2) |
| Responder type | |||||
| Patients | 5 (22) | 3 (75) | 1 (50) | 11 (69) | 20 (44) |
| Patients and caregivers | 7 (30) | — | 1 (50) | 1 (6) | 9 (20) |
| Caregivers | 9 (39) | — | — | 1 (6) | 10 (22) |
| Patients, caregivers, HCPs | — | — | — | 2 (13) | 2 (4) |
| HCPs | 2 (9) | 1 (25) | — | 1 (6) | 4 (9) |
Abbreviations: AD = Alzheimer disease; ALS = amyotrophic lateral sclerosis; HCP = health care professional; HD = Huntington disease; PD = Parkinson disease.
For the data collection method and responder type, data are N (% of total information sources in disease group). This table includes the number of stand-alone information sources, not the number of articles. Articles combining qualitative and/or quantitative data collection methods, diverse responder types, or disease groups were analyzed separately.
Emerged Enablers
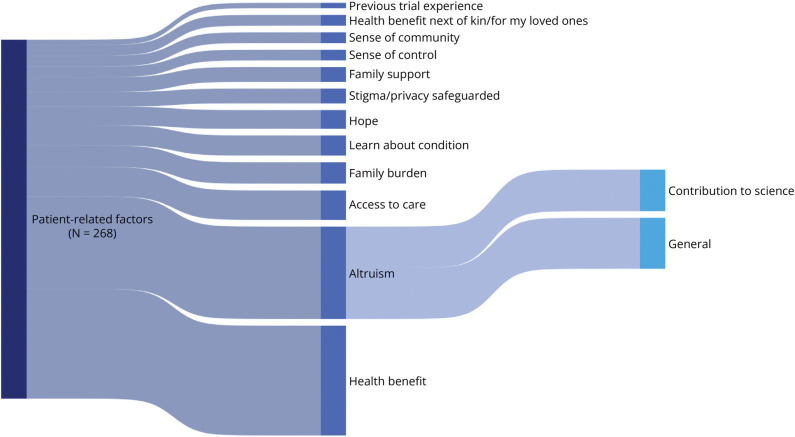
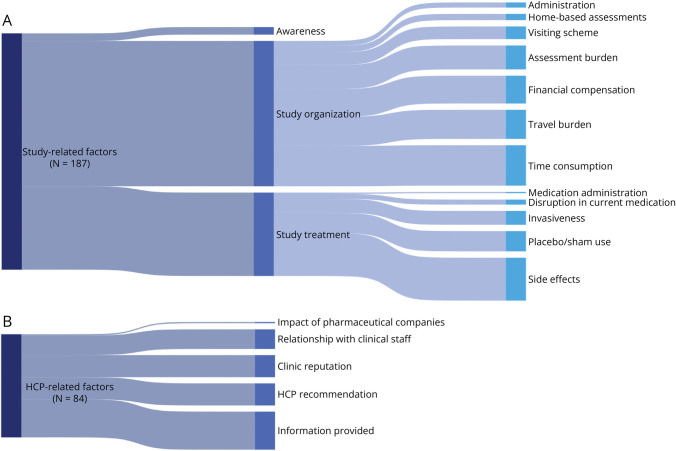
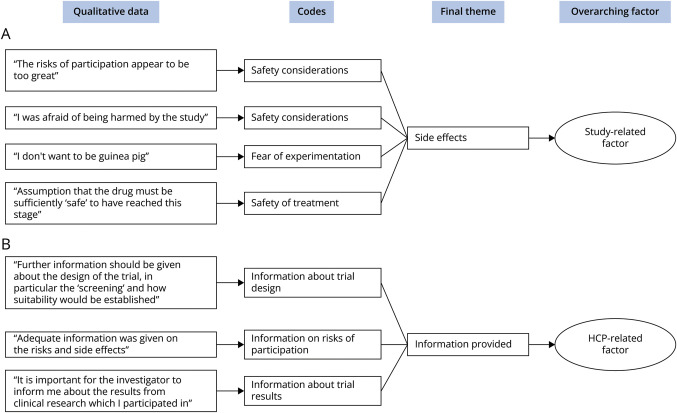
A total of 539 qualitative data points (i.e., text segments including quotes and statements from the qualitative studies) were extracted from the enrolled study population. Of these data points, 268 (50%) concerned patient-related enablers, 187 (35%) concerned study-related enablers, and 84 (16%) concerned HCP-related enablers. These data could be deduced to 31 unique enablers, which are depicted in dynamic flowcharts. Figure 2 displays the identified patient-related enablers; Figure 3 displays the modifiable study- and HCP-related enablers. A thicker “flow” indicates a higher frequency of data allocated to a certain enabler. Enablers standing out in terms of frequency are personal health benefit (15% of total number of qualitative data points), general altruism (7%), side effects (6%), time consumption (6%), contribution to science (6%), and information provided by HCPs (6%). The complete code tree with qualitative data and frequencies is available in the GitHub repository. An illustrative example of the coding process is shown in Figure 4.
Figure 2. Weighted Flowchart of the Identified Patient-Related Enablers.
This figure depicts the identified patient-related enablers. The vertical blocks indicate the (sub)themes and enablers. The thickness of the “flows,” that is, the curved/smooth bars between the (sub)themes, is proportional to the number of data points for that (sub)theme. Hence, thicker flows indicate a higher frequency. The sample size (N) indicates the total number of data points.
Figure 3. Weighted Flowchart of the Modifiable Enablers: Study and HCP Related.
This figure depicts the identified study- (A) and HCP-related (B) enablers. The vertical blocks indicate the (sub)themes and enablers. The thickness of the “flows,” that is, the curved bars between the (sub)themes, is proportional to the number of data points for that (sub)theme. Hence, thicker flows indicate a higher frequency. The sample size (N) indicates the total number of data points. HCP = health care professional.
Figure 4. Illustrative Example of the Thematic Analysis Process.
In this figure, we illustrate the coding process of the enablers “side effects” (A) and “information provided” (B). Initially, data were collected from the included studies. Subsequently, an initial coding phase was undertaken. These codes were then organized into final themes. In the last step, the themes were grouped into patient-, study-, or HCP-related factors. HCP = health care professional.
Quantitative Synthesis
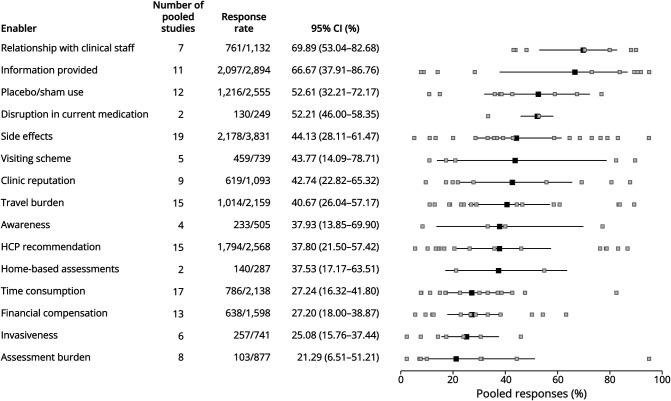
Quantitative data on the modifiable enablers were reported in 27 of the 45 information sources (25 quantitatively designed sources and 2 qualitative sources reporting frequencies); a meta-analysis was conducted for each of these enablers (Figure 5). Overall, the relationship with the clinical staff was the preeminent enabler reported by 70% of the respondents (95% CI 53%–83%), followed by the availability of study information (67%, 95% CI 38%–87%) and the use or absence of a placebo or sham-control arm (53%, 95% CI 32%–72%). The disruption or continuation of usual care was also reported frequently among respondents (52%, 95% CI 46%–58%) but was only based on 2 studies primarily enrolling patients with PD.
Figure 5. Forest Plot of Meta-Analyzed Reported Proportions of the Modifiable Enablers of Trial Participation.
The black boxes indicate the overall estimate of the generalized linear mixed-effects models with 95% CIs based on the Gaussian distribution. The gray boxes indicate the response rate of the individual studies. Articles including different responders, responder types, disease groups, or data collection methods were separately included in the analysis. HCP = health care professional.
Of note, for all enablers, there was considerable between-study heterogeneity (I2 ranging from 84% to 99%, all p < 0.001, eAppendix 6), likely because of the divergent types of survey methods used and the enrolled survey respondents (Table 1). Funnel plots of the 4 enablers with the largest pooled effect sizes (and where the number of information sources is greater than 2) also show large variation in the reported proportions and the precision of these proportions (eAppendix 7). In an attempt to explain the between-study heterogeneity, we evaluated whether responses were different for AD vs non-AD studies (Table 2). Significant differences between the AD responders and not-AD responders were observed for the relationship with the clinical staff (odds ratio [OR] 0.2, 95% CI 0.1–0.3), the visiting scheme (OR 0.1, 95% CI 0.0–0.6), and HCP recommendation (OR 0.1, 95% CI 0.0–0.4), which could explain 48%–90% of the between-study heterogeneity, although significant heterogeneity remained (eAppendix 6).
Table 2.
Comparison of AD Studies (1) and Non-AD Studies (0)
| Barrier/facilitator | Estimated percentage (95% CI) | Odds ratio | p Value | |
| Non-AD | AD | |||
| Relationship with clinical staff | 82.72 (76.72–87.43) | 45.86 (35.32–56.79) | 0.18 (0.10–0.32) | <0.01 |
| Information provided | 76.74 (45.59–92.86) | 45.17 (11.51–83.92) | 0.25 (0.03–2.49) | 0.24 |
| Placebo/sham use | 60.69 (36.59–80.51) | 35.53 (11.88–69.27) | 0.36 (0.06–1.99) | 0.24 |
| Disruption in current medicationa | — | — | — | — |
| Side effects | 56.38 (35.73–75.04) | 28.11 (12.37–52.01) | 0.30 (0.08–1.14) | 0.08 |
| Visiting scheme | 69.20 (36.49–89.78) | 13.44 (2.74–46.15) | 0.07 (0.01–0.61) | 0.02 |
| Clinic reputation | 52.65 (28.49–75.63) | 23.81 (6.21–59.62) | 0.28 (0.04–1.81) | 0.18 |
| Travel burden | 50.65 (30.12–70.97) | 30.19 (14.58–52.28) | 0.42 (0.12–1.50) | 0.18 |
| Awareness | 37.08 (10.69–74.38) | 39.97 (5.42–88.55) | 1.13 (0.06–21.06) | 0.93 |
| HCP recommendation | 59.22 (40.83–75.35) | 14.37 (6.08–30.29) | 0.12 (0.03–0.39) | <0.01 |
| Home-based assessmentsa | — | — | — | — |
| Time consumption | 38.72 (22.14–58.41) | 17.10 (7.91–33.12) | 0.33 (0.10–1.07) | 0.06 |
| Financial compensation | 29.87 (19.05–43.53) | 20.46 (8.44–41.81) | 0.60 (0.19–1.97) | 0.40 |
| Invasiveness | 19.27 (8.88–36.89) | 29.41 (18.03–44.11) | 1.75 (0.62–4.89) | 0.29 |
| Assessment burden | 37.07 (9.08–77.65) | 11.05 (2.08–42.10) | 0.21 (0.02–2.58) | 0.22 |
Abbreviations: AD = Alzheimer disease; HCP = health care professional.
Estimates are based on a generalized linear mixed-effects model with AD responder as moderator (1 = AD, 0 = non-AD).
These enablers only have 2 separate studies, rendering the meta-regression uninformative.
Discussion
The primary objective was to systematically identify factors that influence trial participation in neurodegenerative diseases and provide a comprehensive synthesis of the literature. We have shown the considerable diversity in enablers that drive patient participation. Using thematic deduction, key themes were identified that could be attributed to the patient, the study design, or the health care professional (HCP). Although intrinsic patient factors, such as altruism or personal health benefit, may be less directly modifiable, we identified several study and HCP-related factors that may increase the patient's likelihood of trial participation. These results could help to improve recruitment strategies to include a broader range of patients and to better educate and inform patients about participating in future clinical trials.
Understanding why patients do or do not participate in clinical trials is, however, not only relevant to improving accrual rates among clinical trials. It also provides a better understanding of the drug's safety and efficacy profile, and how applicable study results are to all patients with a certain disease.29 It is well known that only a minor fraction of the patients are eligible to participate in clinical trials for neurogenerative disorders.9,10 However, even among eligible patients, only a subset finally decide to participate.11 With eligibility criteria, the aim is to reduce undesirable characteristics in our trial population (e.g., end-stage disease). There may, however, be a second latent selection mechanism that determines whether an eligible patient will participate. This latent mechanism may be driven by another factor (e.g., age). As a result, our actual trial population deviates considerably from our intended one, which could make eligibility criteria ineffective.10
In a recent population-based study in patients with ALS,30 it was shown that of the 473 patients predicted to have been eligible according to the inclusion and exclusion criteria, only 133 (28%) finally participated in the study. Perhaps unsurprisingly, the patients who actually participated were different from the eligible nonparticipants. In our study, we provide a comprehensive list of factors that may drive these decisions and thus influence these latent selection mechanisms. Indeed, intrinsic patient-related factors play a major role, with altruistic reasons and own health benefit being major themes. Although these motivations are opposites, they are not mutually exclusive; many individuals may be driven by a combination of altruistic and self-interested factors. The observed discrepancy between eligible patients and actual participants highlights the complexity of the decision-making process surrounding trial participation. Our study underscores the importance of addressing barriers and misconceptions about the purpose, risks, and benefits of participation in a clinical trial. Development of better educational materials and consent procedures that match all educational levels and diverse needs may alleviate potential barriers to participate.31 Strategies such as interactive, informed consent interventions, such as a test with feedback in addition to a standard informed consent procedure, have been shown to be effective in improving patients' understanding of the implications of trial participation.32 Informing patients may be further improved by involving patient advocates or communication specialists.33
In addition, we show the significant influence of HCP-related factors on the patient's decision to participate in a clinical trial. Specifically, the relationship that patients and caregivers have with the clinical staff, ensuring that the patients understand the study design and that they receive trial results after participation, emerged as the most influential and potentially modifiable contributors to trial participation. These findings are supported by previous studies, further demonstrating that some patients may not fully comprehend the informed consent procedure, especially when it comes to understanding safety, side effects, and randomization.34 This is significant, as it influences the willingness to participate in a clinical trial,35 and patients may participate not being fully aware of the study purpose and risks associated.15 Furthermore, interventions aimed at addressing recruitment barriers have shown promise. For instance, in a clinical trial evaluating an exercise regimen, various recruitment strategies were assessed, with referrals by neurologists and primary care providers proving most effective.36 This underscores the role of HCPs at all stages of trial engagement.
Gathering the patient's input on the study design may be another pathway to promote trial participation.37,38 Patient-centered trial design has gained increasing attention in recent years and has garnered support from regulatory authorities.39,40 Our list of enablers offers valuable insights into which elements of study design are deemed important by patients, supported by other studies,41,42 including the use of placebo, the intensity of the visiting scheme, the travel burden, and the required time investment. Making better use of innovative trial designs that facilitate these patient preferences may well further enhance trial participation, for example, by making more frequent use of platform studies,43-46 interim analyses,47 hybrid designs to reduce placebo arms,30 or by providing a decentralized visiting scheme.48
Our study has several limitations that should be considered. First, the publications analyzed in this study were not specific to individual clinical trials, but rather examined overarching factors influencing trial participation. Given the diverse scope of neurodegenerative clinical trials and the varied focuses of the publications analyzed, it would be a challenge to estimate the proportion of all performed trials represented in the sample of included studies. The overall insights, however, could be valuable as guideline for future studies. Second, the diseases included in our study vary in their symptoms, caregiver involvement, and life expectancy. We have assessed the between-study heterogeneity for each enabler, with a clear difference between AD and non-AD studies, especially in the enablers' “relationship with clinical staff” and “visiting scheme.” This may not be surprising, given that most AD studies were based on the response of caregivers. Third, the quantitative studies assessed only a limited set of enablers relative to the set of enablers identified in the qualitative studies. Hence, subsequent quantitative studies can be improved by incorporating a more comprehensive set of enablers based on qualitative studies, following a standardized study design and survey format. Another avenue of interest may be to explore methodologies such as conjoint analyses to learn how alterations in attributes of a clinical trial (design) improve willingness of patients to participate.49 For instance, a study involving patients with AD and their caregivers employed conjoint analysis to assess which trial design factors would enhance participation. Study findings revealed that factors such as the burden of traveling to the clinic were important barriers, with home visits emerging as most valued alternative to in-clinic follow-up.49 This example underscores the potential value of tailored trial designs, based on patient input, to address specific participation barriers identified in our study.
Finally, it would be of specific interest to do future analyses in populations that are difficult to enroll, such as ethnic minorities, older patients, and those with a lower socioeconomic status.50 Although some studies in this review focused on factors that drive or hinder trial participation in under-represented populations, sample sizes were too limited to identify differential factors influencing trial participation in neurodegenerative diseases. However, given that under-representation of these subgroups in clinical trials profoundly affects the trial's generalizability, efforts are needed to gather specific insights tailored to under-represented subpopulations.
In conclusion, we have identified and quantified the considerable diversity in enablers that drive patient participation in clinical trials for neurodegenerative diseases. Understanding these enablers is critical for designing effective recruitment strategies and optimizing the conduct of clinical trials. These results could help to improve recruitment and retention strategies and be applied to better educate and inform patients about participating in future clinical trials, ultimately advancing the development of effective treatments for neurodegenerative diseases.
Acknowledgment
The authors thank Brenda Vollers-King for assistance with language editing.
Glossary
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- ASReview
Automated Systematic Review
- CASP
Critical Appraisal Skills Program
- HCP
health care professional
- HD
Huntington disease
- OR
odds ratio
- PD
Parkinson disease
Appendix. Authors
| Name | Location | Contribution |
| Daphne N. Weemering, MSc | Department of Neurology, UMC Utrecht Brain Center, University Medical Center Utrecht, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Anita Beelen, PhD | Department of Rehabilitation, Physical Therapy Science & Sports, and Center of Excellence for Rehabilitation Medicine, UMC Utrecht Brain Center, University Medical Center Utrecht; De Hoogstraat Rehabilitation, Utrecht, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Tessa Kliest, MSc | Department of Neurology, UMC Utrecht Brain Center, University Medical Center Utrecht, the Netherlands | Major role in the acquisition of data; study concept or design |
| Lucie A.G. van Leeuwen, PhD | Department of Neurology, UMC Utrecht Brain Center, University Medical Center Utrecht, the Netherlands | Major role in the acquisition of data |
| Leonard H. van den Berg, MD, PhD | Department of Neurology, UMC Utrecht Brain Center, University Medical Center Utrecht, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content |
| Ruben P.A. van Eijk, MD, PhD | Department of Neurology, UMC Utrecht Brain Center, and Biostatistics & Research Support, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Lang AE. Clinical trials of disease-modifying therapies for neurodegenerative diseases: the challenges and the future. Nat Med. 2010;16(11):1223-1226. doi: 10.1038/nm.2220 [DOI] [PubMed] [Google Scholar]
- 2.Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines. 2019;7(4):97. doi: 10.3390/biomedicines7040097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer's disease clinical trials. Alzheimers Res Ther. 2010;2(6):34. doi: 10.1186/alzrt58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3):245-255. doi: 10.1093/jnci/djy221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raman R, Aisen P, Carillo MC, et al. Tackling a major deficiency of diversity in Alzheimer's disease therapeutic trials: an CTAD task force report. J Prev Alzheimers Dis. 2022;9(3):388-392. doi: 10.14283/jpad.2022.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau YH, Podlewska A, Ocloo J, et al. Does ethnicity influence recruitment into clinical trials of Parkinson's disease? J Parkinsons Dis. 2022;12(3):975-981. doi: 10.3233/JPD-213113 [DOI] [PubMed] [Google Scholar]
- 7.Banzi R, Camaioni P, Tettamanti M, Bertele’ V, Lucca U. Older patients are still under-represented in clinical trials of Alzheimer's disease. Alzheimers Res Ther. 2016;8:32. doi: 10.1186/s13195-016-0201-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carcel C, Harris K, Peters SAE, et al. Representation of women in stroke clinical trials: a review of 281 trials involving more than 500,000 participants. Neurology. 2021;97(18):e1768-e1774. doi: 10.1212/WNL.0000000000012767 [DOI] [PubMed] [Google Scholar]
- 9.Pittock RR, Aakre J, Castillo AM, et al. Eligibility for anti-amyloid treatment in a population-based study of cognitive aging. Neurology. 2023;101(19):e1837-e1849. doi: 10.1212/WNL.0000000000207770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Eijk RPA, Westeneng HJ, Nikolakopoulos S, et al. Refining eligibility criteria for amyotrophic lateral sclerosis clinical trials. Neurology. 2019;92(5):e451-e460. doi: 10.1212/WNL.0000000000006855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiò A, Canosa A, Gallo S, et al. ALS clinical trials: do enrolled patients accurately represent the ALS population? Neurology. 2011;77(15):1432-1437. doi: 10.1212/WNL.0b013e318232ab9b [DOI] [PubMed] [Google Scholar]
- 12.Button KS, Ioannidis JPA, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365-376. doi: 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- 13.Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185-198. doi: 10.1200/EDBK_156686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unger JM, Hershman DL, Till C, et al. “When offered to participate”: a systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. J Natl Cancer Inst. 2021;113(3):244-257. doi: 10.1093/jnci/djaa155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141-148. doi: 10.1016/S1470-2045(06)70576-9 [DOI] [PubMed] [Google Scholar]
- 16.Mills E, Wilson K, Rachlis B, et al. Barriers to participation in HIV drug trials: a systematic review. Lancet Infect Dis. 2006;6(1):32-38. doi: 10.1016/S1473-3099(05)70324-8 [DOI] [PubMed] [Google Scholar]
- 17.Clark LT, Watkins L, Piña IL, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol. 2019;44(5):148-172. doi: 10.1016/j.cpcardiol.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 18.Martin SS, Ou FS, Newby LK, et al. Patient- and trial-specific barriers to participation in cardiovascular randomized clinical trials. J Am Coll Cardiol. 2013;61(7):762-769. doi: 10.1016/j.jacc.2012.10.046 [DOI] [PubMed] [Google Scholar]
- 19.Naidoo N, Nguyen VT, Ravaud P, et al. The research burden of randomized controlled trial participation: a systematic thematic synthesis of qualitative evidence. BMC Med. 2020;18(1):6. doi: 10.1186/s12916-019-1476-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou Y, Dan X, Babbar M, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565-581. doi: 10.1038/s41582-019-0244-7 [DOI] [PubMed] [Google Scholar]
- 21.Zahra W, Rai SN, Birla H, et al. The global economic impact of neurodegenerative diseases: opportunities and challenges. In: Keswani C, ed. Bioeconomy for Sustainable Development. Springer; 2020:333-345. doi: 10.1007/978-981-13-9431-7_17 [DOI] [Google Scholar]
- 22.van de Schoot R, de Bruin J, Schram R, et al. An open source machine learning framework for efficient and transparent systematic reviews. Nat Mach Intell. 2021;3(2):125-133. doi: 10.1038/s42256-020-00287-7 [DOI] [Google Scholar]
- 23.CASP-Qualitative-Checklist-2018_fillable_form.pdf. Accessed March 2, 2023. casp-uk.net/images/checklist/documents/CASP-Qualitative-Studies-Checklist/CASP-Qualitative-Checklist-2018_fillable_form.pdf.
- 24.Butler A, Hall H, Copnell B. A guide to writing a qualitative systematic review protocol to enhance evidence-based practice in nursing and health care. Worldviews Evid Based Nurs. 2016;13(3):241-249. doi: 10.1111/wvn.12134 [DOI] [PubMed] [Google Scholar]
- 25.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29(29):3046-3067. doi: 10.1002/sim.4040 [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2022. Accessed November 27, 2023. R-project.org/. [Google Scholar]
- 27.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 28.Lingler JH, Ren D, Tamres LK, et al. Mechanisms by which cultural-centric narrative influences interest in ADRD research among African American adults. Gerontologist. 2023;63(6):1060-1066. doi: 10.1093/geront/gnac179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan YY, Papez V, Chang WH, Mueller SH, Denaxas S, Lai AG. Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longev. 2022;3(10):e674-e689. doi: 10.1016/S2666-7568(22)00186-6 [DOI] [PubMed] [Google Scholar]
- 30.van Eijk RPA, van den Berg LH, Roes KCB, et al. Hybrid controlled clinical trials using concurrent registries in amyotrophic lateral sclerosis: a feasibility study. Clin Pharmacol Ther. 2023;114(4):883-892. doi: 10.1002/cpt.2994 [DOI] [PubMed] [Google Scholar]
- 31.Juan-Salvadores P, Michel Gómez MS, Jiménez Díaz VA, Martínez Reglero C, Iñiguez Romo A. Patients' knowledge about their involvement in clinical trials. A non-randomized controlled trial. Front Med (Lausanne). 2022;9:993086. doi: 10.3389/fmed.2022.993086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glaser J, Nouri S, Fernandez A, et al. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: an updated systematic review. Med Decis Making. 2020;40(2):119-143. doi: 10.1177/0272989X19896348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz ML, Archer LE, Peppercorn JM, et al. Patient advocates' role in clinical trials: perspectives from Cancer and Leukemia Group B investigators and advocates. Cancer. 2012;118(19):4801-4805. doi: 10.1002/cncr.27485 [DOI] [PubMed] [Google Scholar]
- 34.Pietrzykowski T, Smilowska K. The reality of informed consent: empirical studies on patient comprehension: systematic review. Trials. 2021;22(1):57. doi: 10.1186/s13063-020-04969-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reijula E, Halkoaho A, Pietilä AM, Selander T, Kälviäinen R, Keränen T. Therapeutic misconception correlates with willingness to participate in clinical drug trials among patients with epilepsy; need for better counseling. Epilepsy Behav. 2015;48:29-34. doi: 10.1016/j.yebeh.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 36.Greimel S, Wyman JF, Zhang L, Yu F. Recruitment and screening methods in Alzheimer's disease research: the FIT-AD trial. J Gerontol A Biol Sci Med Sci. 2022;77(3):547-553. doi: 10.1093/gerona/glab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li BT, Daly B, Gospodarowicz M, et al. Reimagining patient-centric cancer clinical trials: a multi-stakeholder international coalition. Nat Med. 2022;28(4):620-626. doi: 10.1038/s41591-022-01775-6 [DOI] [PubMed] [Google Scholar]
- 38.Tong A, Scholes-Robertson N, Hawley C, et al. Patient-centred clinical trial design. Nat Rev Nephrol. 2022;18(8):514-523. doi: 10.1038/s41581-022-00585-w [DOI] [PubMed] [Google Scholar]
- 39.ICH reflection paper: proposed ICH Guideline work to advance Patient Focused Drug Development (PFDD).
- 40.Research C for DE and. FDA Patient-Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient's Voice in Medical Product Development and Regulatory Decision Making. FDA; 2023. Accessed September 29, 2023. fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical. [Google Scholar]
- 41.Beswick E, Johnson M, Newton J, et al. Factors impacting trial participation in people with motor neuron disease. J Neurol. 2024;271(1):543-552. doi: 10.1007/s00415-023-12010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaswani PA, Tropea TF, Dahodwala N. Overcoming barriers to Parkinson disease trial participation: increasing diversity and novel designs for recruitment and retention. Neurotherapeutics. 2020;17(4):1724-1735. doi: 10.1007/s13311-020-00960-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong C, Dakin RS, Williamson J, et al. Motor Neuron Disease Systematic Multi-Arm Adaptive Randomised Trial (MND-SMART): a multi-arm, multi-stage, adaptive, platform, phase III randomised, double-blind, placebo-controlled trial of repurposed drugs in motor neuron disease. BMJ Open. 2022;12(7):e064173. doi: 10.1136/bmjopen-2022-064173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon A, Kivipelto M, Molinuevo JL, Tom B, Ritchie CW; EPAD Consortium. European Prevention of Alzheimer's Dementia Longitudinal Cohort Study (EPAD LCS): study protocol. BMJ Open. 2019;8(12):e021017. doi: 10.1136/bmjopen-2017-021017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bateman RJ, Benzinger TL, Berry S, et al. The DIAN-TU Next Generation Alzheimer's prevention trial: adaptive design and disease progression model. Alzheimers Dement. 2017;13(1):8-19. doi: 10.1016/j.jalz.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paganoni S, Berry JD, Quintana M, et al. Adaptive platform trials to transform amyotrophic lateral sclerosis therapy development. Ann Neurol. 2022;91(2):165-175. doi: 10.1002/ana.26285 [DOI] [PubMed] [Google Scholar]
- 47.van Unnik JWJ, Nikolakopoulos S, Eijkemans MJC, et al. Development and evaluation of a simulation-based algorithm to optimize the planning of interim analyses for clinical trials in ALS. Neurology. 2023;100(23):e2398-e2408. doi: 10.1212/WNL.0000000000207306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erga AH, Alves G, Leentjens AFG. The ePark study protocol: a decentralized trial of individual video-assisted cognitive behavioural therapy for depressive disorder in Parkinson's disease. Contemp Clin Trials Commun. 2023;32:101080. doi: 10.1016/j.conctc.2023.101080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karlawish J, Cary MS, Rubright J, TenHave T. How redesigning AD clinical trials might increase study partners' willingness to participate. Neurology. 2008;71(23):1883-1888. doi: 10.1212/01.wnl.0000336652.05779.ea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franzen S, Smith JE, van den Berg E, et al. Diversity in Alzheimer's disease drug trials: the importance of eligibility criteria. Alzheimers Dement. 2022;18(4):810-823. doi: 10.1002/alz.12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in both thematic analysis and meta-analysis are accessible through a GitHub repository (github.com/daphneweemering/trial-participation). This repository remains indefinitely accessible. In the event of data unavailability on GitHub, it can be obtained upon request.