Abstract
Chronic wasting disease (CWD) is a prion disease affecting deer, elk and moose in North America and reindeer, moose and red deer in Northern Europe. Pathogenesis is driven by the accumulation of PrPSc, a pathological form of the host’s cellular prion protein (PrPC), in the brain. CWD is contagious among North American cervids and Norwegian reindeer, with prions commonly found in lymphatic tissue. In Nordic moose and red deer CWD appears exclusively in older animals, and prions are confined to the CNS and undetectable in lymphatic tissues, indicating a sporadic origin.
We aimed to determine transmissibility, neuroinvasion and lymphotropism of Nordic CWD isolates using gene-targeted mice expressing either wild-type (138SS/226QQ) or S138N (138NN/226QQ) deer PrP. When challenged with North American CWD strains, mice expressing S138N PrP did not develop clinical disease but harbored prion seeding activity in brain and spleen. Here, we infected these models intracerebrally or intraperitoneally with Norwegian moose, red deer and reindeer CWD isolates. The moose isolate was the first CWD type to cause full-blown disease in the 138NN/226QQ model in the first passage, with 100% attack rate and shortened survival times upon second passage. Furthermore, we detected prion seeding activity or PrPSc in brains and spinal cords, but not spleens, of 138NN/226QQ mice inoculated intraperitoneally with the moose isolate, providing evidence of prion neuroinvasion. We also demonstrate, for the first time, that transmissibility of the red deer CWD isolate was restricted to transgenic mice overexpressing elk PrPC (138SS/226EE), identical to the PrP primary structure of the inoculum.
Our findings highlight that susceptibility to clinical disease is determined by the conformational compatibility between prion inoculum and host PrP primary structure. Our study indicates that neuroinvasion of Norwegian moose prions can occur without, or only very limited, replication in the spleen, an unprecedented finding for CWD.
Author summary
Chronic wasting disease (CWD) is a prion disease of cervids that is expanding its global footprint. The pathogenesis of prion disease is driven by the accumulation of PrPSc, a misfolded isoform of the cellular prion protein (PrPC). CWD prion strains from North America are lymphotropic, while Norwegian moose and red deer prions are not, and therefore considered non-contagious, sporadic CWD forms.
We studied the propagation of Norwegian CWD prions in gene-targeted mice carrying cervid PrPC variants. We reveal that the Norwegian moose isolate induces clinical disease in mice expressing a PrPC variant previously shown to only display subclinical infection upon challenge with North American CWD. We report the first instance of red deer CWD transmission exclusively to mice overexpressing elk PrPC.
Notably, our findings suggest a neuroinvasion route for Norwegian moose CWD prions that potentially bypasses spleen replication, underscoring the complexity of prion disease transmission, and the need for continued research into the behavior of prions across different species and protein variants.
Introduction
Chronic wasting disease (CWD) is the prion disease of free-ranging and captive cervid populations in the United States, Canada, South Korea, Norway, Sweden, and Finland [1–3]. The disease is primarily caused by the aggregation and accumulation of PrPSc, a misfolded and infectious isoform of the host-expressed cellular prion protein (PrPC). PrPSc is thought to be the main, if not the only, component of prions [4,5]. CWD prions can replicate and accumulate in peripheral tissues of infected animals, particularly in the lymphatic system. Infected animals shed infectious prions into the environment through saliva, blood, urine, and feces [6–8], and environmental prion contamination can facilitate prolonged indirect transmission [9–11]. CWD in North America is highly contagious, and prevalence has reached up to ~19% within endemic regions and as high as ~87% in localized areas [12].
Several distinct CWD prion strains have been identified in North America [13–17]. They are all lymphotropic, contagious, and considered to be acquired by infection. By contrast, Norwegian CWD has distinct features; CWD in moose and red deer was identified in very old animals, with PrPSc detectable in the brain but not in lymphatic tissues, while lymphotropism was only evident in cases of reindeer CWD [18–21]. This gave rise to the hypothesis that CWD in Norwegian moose and red deer represent sporadic, atypical forms of CWD, reminiscent of atypical/Nor98 scrapie and atypical H- and L-BSE strains [22–24]. On the host’s side, previous studies have suggested that animals harboring PrPC allelic variants were less prone to contracting CWD compared to their wild-type counterparts [25–27]. However, certain prion strains have been shown to break these genotypic transmission barriers, e.g., the H95+ and 116AG CWD strains from white-tailed deer were able to produce terminal prion disease in Tg60 mice expressing the 96S deer PrPC, a mouse model resistant to transmission from other CWD isolates [14,16].
The objective of our study was to delve deeper into the characteristics of Norwegian CWD prion isolates, particularly their propagation dynamics in hosts that carry cervid PrPC variants known for their reduced susceptibility to CWD, e.g., S138N in reindeer/caribou. We were also interested in exploring their ability to infiltrate the brain following infection from peripheral sites. To this end, we characterized the propagation of three Norwegian CWD isolates, i.e., the M-NO3 moose isolate, R-NO16 reindeer isolate, and H-NO1 red deer isolate in gene-targeted mice expressing either the wild-type deer representative for mule deer, white-tailed deer and reindeer/caribou (138SS/226QQ) or the 138NN (138NN/226QQ) PrPC variant found in caribou [28,29], or transgenic mice overexpressing elk (138SS/226EE) PrPC [30–32]. Our findings reveal that the M-NO3 moose isolate was able to induce clinical disease in gene-targeted mice expressing the 138NN-PrPC variant, an experimental host that previously only showed subclinical infection, i.e., prions were detected by in vitro amplification, but infected mice did not display clinical signs, upon infection with North American CWD isolates [29]. In addition, while M-NO3 did not elicit clinical disease in our mouse models following intraperitoneal inoculation, we detected prion seeding activity using RT-QuIC in several mouse brains and spinal cords, as well as proteinase K (PK)-resistant PrPSc by western blot in the spinal cord of one Prnp.Cer.138NN mouse. Notably, there was no prion seeding activity detected in their spleens. This suggests a route of neuroinvasion for M-NO3 prions that may bypass or only transiently involve splenic prion replication. Furthermore, we report the novel finding that the H-NO1 red deer isolate can transmit disease exclusively to transgenic mice overexpressing elk PrPC.
Results
Propagation of Norwegian CWD isolates in Prnp.Cer.Wt mice
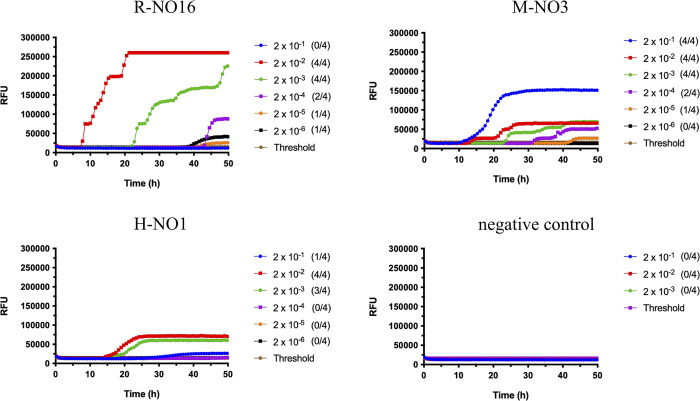
Prior to inoculation, brain homogenates (BH) from R-NO16, M-NO3 [20], and H-NO1 [21] were tested for prion seeding activity in RT-QuIC using mouse recombinant PrP as a substrate and compared based on endpoint dilution and lag phase. Endpoint dilution is the highest sample dilution resulting in a positive reaction [33]. Ten percent BH from R-NO16 and M-NO3 were still positive for seeding activity when diluted up to 2 x 10−4, while H-NO1 was positive only up to 2 x 10−3 dilution, suggesting lower amounts of seeding-capable prions in the H-NO1 brain (Fig 1). Lag phase represents the time required to cross the threshold [33]. R-NO16 had the shortest lag phase with ~5 hours reaction time, followed by M-NO3 at ~10 hours, and H-NO1 at ~15 hours (Fig 1). This suggests that R-NO16 had the highest prion seeding activity, followed by M-NO3 and H-NO1.
Fig 1. Comparison of Norwegian isolates seeding activity in RT-QuIC.
Ten percent brain homogenates were subjected to serial dilutions from 2 x 10−1 to 2 x 10−6 in RT-QuIC seed dilution buffer. Samples were considered positive when a minimum of two out of four wells crossed the threshold relative fluorescence unit (RFU). Threshold is the average RFU of all negative control reactions plus five times their standard deviation. Negative control was a CWD-negative mule deer. R-NO16 and M-NO3 were positive for prion seeding activity up to 2 x 10−4 dilution (upper panels), and H-NO1 up to 2 x 10−3 dilution (bottom left panel). The y-axis represents the RFU, and the x-axis represents time in hours (h). Mouse recombinant PrP was used as substrate for the RT-QuIC reactions.
Gene-targeted mice expressing wild-type deer PrP (Prnp.Cer.Wt), were inoculated intracerebrally (i.c.) or intraperitoneally (i.p.) with R-NO16, M-NO3 or H-NO1. Mice inoculated i.c. with R-NO16 and M-NO3 reached terminal prion disease with average survival times of 628.4 ± 25.4 days post-inoculation (dpi) and 700 ± 108.5 dpi, respectively (Table 1). Mice inoculated with R-NO16 displayed signs similar to those inoculated with a Canadian reindeer isolate [29], designated R-CA1 here onwards, the most apparent being ataxia, low-profile walking, and lethargy, while groups inoculated with M-NO3 mostly displayed signs of intense hyperactivity, loss of balance, and pronounced kyphosis. However, neither isolate resulted in a full attack rate, with R-NO16 giving a 71.4% and M-NO3 a 66.7% attack rate (Table 1). Mice inoculated i.c. with H-NO1 did not develop clinical disease up to 820 dpi (Table 1). Upon i.p. inoculation, only mice inoculated with R-NO16 reached terminal disease at 719.4 ± 30.7 dpi with 87.5% attack rate, while those inoculated i.p. with M-NO3 did not develop clinical signs of prion disease up to the experimental endpoint of 779 dpi (Table 1). Of note, experimental endpoints among gene-targeted mice were selected according to the natural lifespan of mouse models and to be comparable between groups of mice with different genotypes inoculated with the same CWD isolate, e.g., Prnp.Cer.Wt and Prnp.Cer.138NN mice inoculated i.p. with M-NO3 (Table 1).
Table 1. Survival times of Prnp.Cer.Wt, Prnp.Cer.138NN and TgElk mice following intracerebral (i.c.) and intraperitoneal (i.p.) inoculation with Norwegian CWD isolates.
| Average survival times ± SD in days (N1/Ntotal) | ||||||
|---|---|---|---|---|---|---|
| Inoculum | Tissue | passage | roi | Prnp.Cer.Wt | Prnp.Cer.138NN | TgElk |
| R-NO16 | Brain | 1 | i.c. | 628.4 ± 25.4 (5/7) | 734 (5/5) b | na |
| Reindeer | 637 (1/7) a, 733 (1/7) b | |||||
| 1 | i.p. | 719.4 ± 30.7 (7/8) | 734 (4/4) b | na | ||
| 703 (1/8) a | ||||||
| M-NO3 | Brain | 1 | i.c. | 700 ± 108.5 (4/6) | 565, 656 (2/5) | 258 (4/4) b |
| Moose | 623, 635 (2/6) a | 720 (1/5) a, 756, 832 (2/5) b | ||||
| 1 | i.p. | 297, 653 (2/5) a, 779 (3/5) b | 600, 651, 720 (3/5) a, 774, 782 (2/5) b | na | ||
| 2 | i.c. | na | 360.6 ± 34.7 (9/9) | na | ||
| H-NO1 | Brain | 1 | i.c. | 572, 656, 720 (3/5) a, 818, 820 (2/5) b | 714 (1/4) a, 784, 798, 832 (3/4) b | 166 (1/4) |
| Red deer | 257 (3/4) b | |||||
| 1 | i.p. | na | 629, 629, 705 (3/3) a | na | ||
| 2 | i.c. | na | na | 149.3 ± 44.5 (4/5) | ||
| 315 (1/5) b | ||||||
a Humane endpoint euthanasia with no clinical signs or confirmed prion disease, defined in methods.
b Experimental endpoint euthanasia with no clinical disease, defined as ≥ 700 dpi for mice inoculated with R-NO16, ≥ 750 dpi for mice inoculated with M-NO3 and H-NO1, and ≥ 250 dpi for the TgElk mouse line independent of inoculum (see methods). N1/Ntotal = number of mice euthanized / number of mice per group; SD = standard deviation; roi = route of inoculation; i.c. = intracerebral; i.p. = intraperitoneal; na = not inoculated.
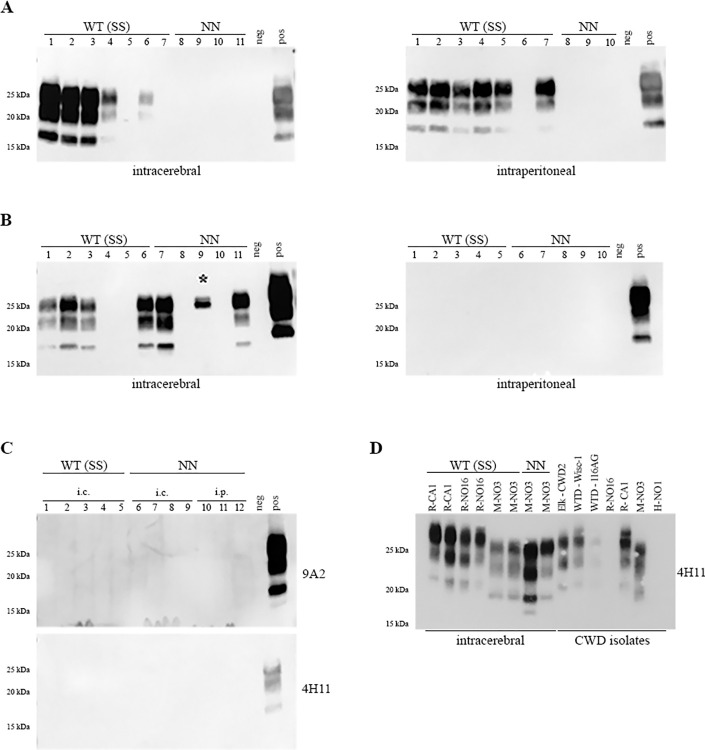
Mice that exhibited clinical prion signs were euthanized upon terminal disease (see materials and methods) and were confirmed to harbor PK-resistant prions (PrPres) in their brains by western blot analysis (Fig 2A and 2B). PrPres patterns, following first passage, were compared to original isolates from Norway used for inoculation, and to previously characterized CWD isolates from North America (Fig 2D). Norwegian isolates had low to moderate seeding activity in RT-QuIC, and only M-NO3 harbored detectable amounts of PrPres by western blot. Despite similar seeding activity, R-NO16 did not show PrPres, possibly due to a higher amount of PK-sensitive PrPSc compared to M-NO3 (Figs 2D and A in S1 Text). Overall, brain homogenates from mice inoculated with M-NO3 harbored PrPres of lower molecular weight, with a pattern comparable to that of the original M-NO3 isolate. PrPres in brain homogenates of mice infected with R-NO16 appears similar to R-CA1, an experimental isolate from reindeer [34], and two isolates from white-tailed deer consisting of different strains (Wisc-1 and 116AG; 14,16). CWD from elk harboring strain CWD2 [17] exhibits PrPres with a molecular weight that is intermediate between that of M-NO3 and other North American CWD strains (WTD-Wisc-1, WTD-116AG and R-CA1). Additionally, the molecular weight of this PrPres differs from any PrPres detected in gene-targeted mice (Figs 2 and A in S1 Text) Mice euthanized due to reasons unrelated to prion disease or due to reaching our experimental endpoint (see materials and methods) did not contain levels of PrPres detectable by western blots in their brains (Fig 2A–2C). However, some of these PrPres-negative mouse brains were positive for seeding activity in RT-QuIC (Table 2 and Fig B in S1 Text), suggesting that they harbor either very low amounts of prions, or PK-sensitive prions. Spleens from these mice were analyzed by RT-QuIC and groups inoculated with R-NO16 were positive, independent of the inoculation route, and, to a lesser extent, those inoculated with H-NO1 (Table 2). Interestingly, Prnp.Cer.Wt mice inoculated i.p. with M-NO3 were the only ones that did not exhibit any seeding activity in either their brains or spleens (Table 2). Additionally, brains and spleens of mice inoculated i.p. were examined for abnormal PrP deposits by immunohistochemistry and only those inoculated with R-NO16 were found positive (Fig C in S1 Text). In contrast to the same mouse model inoculated with R-CA1 [29], mice inoculated with R-NO16 did not harbor PrP deposits in their hippocampus (Fig C in S1 Text).
Fig 2.
PrPres detection in Prnp.Cer.Wt and Prnp.Cer.138NN brain homogenates of mice inoculated i.c. and i.p. with R-NO16 (A), M-NO3 (B), and H-NO1 (C) isolates. Ten percent brain homogenates were digested with 50 μg/mL PK for one hour at 37°C. For (A) and (B), the anti-PrP antibody used was 4H11 (1:500), and for (C) it was 9A2 (1:1000, upper panel) and 4H11 (1:500, lower panel). (D) PrPres-positive brain homogenates of gene-targeted mice as indicated inoculated i.c. with R-CA1, R-NO16, and M-NO3 were analyzed by western blot along with CWD isolates from North America (Elk-CWD2, WTD-Wisc-1, WTD-116AG, R-CA1) and Norway (R-NO16, M-NO3, H-NO1) digested with 50 μg/ml of PK using anti-PrP antibody 4H11 (1:500). R-NO16 and H-NO1 did not harbor detectable amounts of PrPres. Samples from Asterisk (*) in Fig 2B indicates non-specific signal. WT (SS) = Prnp.Cer.Wt; NN = Prnp.Cer.138NN; neg = non-inoculated Prnp.Cer.138NN mouse brain; pos = PrPres-positive Prnp.Cer.Wt control brain; kDa = kilodalton; i.c. = intracerebral; i.p. = intraperitoneal.
Table 2. Number of mouse brains and spleens positive for PrPres on WB and/or prion seeding activity in RT-QuIC per total number of samples tested within each respective group.
| Number of positive samples / number of samples tested | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prnp.Cer.Wt | Prnp.Cer.138NN | |||||||||||||||
| Brain | Spleen | Brain | Spleen | |||||||||||||
| Inoculum | Tissue | roi | WB | RT-QuIC | RT-QuIC | WB | RT-QuIC | RT-QuIC | ||||||||
| PrPres | -1 | -2 | -3 | -1 | -2 | -3 | PrPres | -1 | -2 | -3 | -1 | -2 | -3 | |||
| R-NO16 | Brain | i.c. | 5/7 | – | – | – | 2/7 | 3/7 | 3/7 | 0/4 | 0/4 | 2/4 | 2/4 | 0/4 | 0/4 | 0/4 |
| Reindeer | ||||||||||||||||
| i.p. | 6/7 | – | – | – | 2/6 | 4/6 | 4/6 | 0/3 | 1/3 | 1/3 | 0/3 | 2/3 | 2/3 | 2/3 | ||
| M-NO3 | Brain | i.c. | 4/6 | – | – | – | 0/5 | 0/5 | 0/5 | 2/5 | 2/5 | 3/5 | 1/5 | 0/5 | 0/5 | 0/5 |
| Moose | ||||||||||||||||
| i.p. | 0/5 | 0/5 | 0/5 | 0/5 | 0/4 | 0/4 | 0/4 | 0/5 | 2/5 | 4/5 | 1/5 | 0/4 | 0/4 | 0/4 | ||
| H-NO1 | Brain | i.c. | 0/5 | 0/5 | 5/5 | 0/5 | 0/3 | 0/3 | 1/3 | 0/4 | 0/4 | 3/4 | 2/4 | 0/4 | 0/4 | 0/4 |
| Red deer | ||||||||||||||||
| i.p. | na | na | na | na | na | na | na | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | ||
The numbers -1, -2, and -3 refer to 2 x 10−1, 2 x 10−2, and 2 x 10−3 RT-QuIC seed dilutions, respectively. Samples were considered positive in RT-QuIC when a minimum of two out of four replicates crossed the threshold relative fluorescence unit (RFU, see methods). Brain homogenates positive for PrPres on WB (Fig 2) were not tested in RT-QuIC, except for Prnp.Cer.138NN mouse brains inoculated i.c. with M-NO3. Brain homogenates from Prnp.Cer.Wt mice inoculated i.c. and i.p. with R-NO16 and i.c. with M-NO3 that were negative for PrPres on WB (Fig 2) were tested in RT-QuIC but not included in this table (data shown in Fig B in S1 Text). roi = route of inoculation; WB = western blot; i.c. = intracerebral; i.p. = intraperitoneal;– = not tested; na = not inoculated.
The absence of clinical disease, PrPres, abnormal PrP deposits, and prion seeding activity in the brains of Prnp.Cer.Wt mice inoculated i.p. with M-NO3, as well as the absence of seeding activity in the spleens of both i.c. and i.p. inoculated groups, provide evidence that M-NO3 prions are not able to propagate in lymphatic tissue, regardless of inoculation route, in animals expressing wild-type deer PrPC (138SS/226QQ).
Norwegian moose CWD isolate causes clinical disease in Prnp.Cer.138NN mice
A gene-targeted mouse line harboring caribou-Prnp homozygous for asparagine at codon 138 (Prnp.Cer.138NN), was inoculated i.c. and i.p. with the R-NO16, M-NO3 and H-NO1 CWD isolates from Norway. The Prnp.Cer.138NN mouse line was resistant to clinical prion disease upon inoculation with various CWD isolates and strains from North America, including reindeer and white-tailed deer prions [29].
Here, the M-NO3 moose isolate was able to break this transmission barrier and produced clinical disease in two out of the five Prnp.Cer.138NN mice inoculated i.c. (40% attack rate; Table 1). PrPres with a low molecular weight similar to that of the original M-NO3 isolate, but slightly different between the two mice, was detectable in their brains upon PK digestion and western blotting (Figs 2B, 2D and A in S1 Text). One other sample had a signal at around 25 kDa, which was confirmed to be unspecific/non-PrP related after deglycosylation. No clinical signs of CWD infection or PrPres were detected in the brains of Prnp.Cer.138NN mice inoculated i.p. with M-NO3 or inoculated by either route with R-NO16 or H-NO1 (Table 1 and Fig 2A–2C).
When the PrPres-negative brains of Prnp.Cer.138NN mice and their spleens were tested for prion seeding activity in RT-QuIC, between 50 and 75% of the brains from i.c.-inoculated animals were positive, regardless of inoculum, whereas all spleens were negative (Table 2). This is different from North American CWD prions, which had detectable seeding activity in the spleen upon i.c. inoculation in this mouse model [29].
Divergent PrPres patterns in brains of Prnp.Cer.138NN mice inoculated i.c. with M-NO3
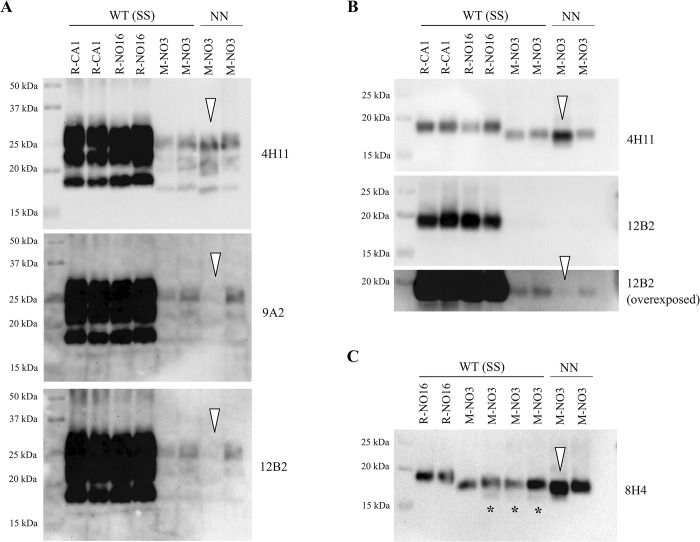
PrPres profiles of R-CA1, R-NO16, and M-NO3 in Prnp.Cer.Wt mice, and M-NO3 in Prnp.Cer.138NN mice were further analyzed by western blot and compared using various monoclonal anti-PrP antibodies (Fig 3). Unglycosylated PrPres in Prnp.Cer.Wt mouse brains inoculated with R-CA1 and R-NO16 had similar molecular weights at ~19 kDa, while PrPres from mice inoculated with M-NO3 migrated slightly faster at ~18 kDa (Fig 3). Interestingly, of the two PrPres-positive Prnp.Cer.138NN mice inoculated i.c. with M-NO3 prions, one mouse had an unglycosylated PrPres band with a similar size as its Prnp.Cer.Wt counterparts, while the other showed a slightly faster migrating band at ~17 kDa (Fig 3). Upon probing with monoclonal antibodies raised against the N-terminus of PrP, 9A2 and 12B2, none of the PrP glycoforms in the sample with the ~17 kDa PrPres (white arrowheads in Fig 3A) seem to be detected, and only faint signals were detectable in the three samples harboring the ~18 kDa PrPres band (Fig 3A). After deglycosylation, PrPres from all four mice inoculated with M-NO3 were still detected with 4H11, but not with 12B2 (Fig 3B). Overexposure of the 12B2 blot revealed faint PrPres bands for the ~18 kDa samples, but none for the ~17 kDa sample (Fig 3B). This indicates that the N-terminal cleavage site of PrPres is different in these two mouse brains. Interestingly, in three out of the four Prnp.Cer.Wt mice, an additional faint PrPres band at approximately ~16 kDa was detectable with 8H4, which was absent in the samples from Prnp.Cer.138NN mice (Fig 3C). These findings confirm previously described strain differences between R-NO16 and M-NO3 [18,35], and indicate the divergence of M-NO3 into PrPres signatures with ~18 or ~17 kDa unglycosylated fragments in Prnp.Cer.138NN mice.
Fig 3. Comparison of PrPres in brain homogenates of Prnp.Cer.Wt and Prnp.Cer.138NN mice inoculated i.c. with R-CA1, R-NO16, or M-NO3.
(A) Ten percent brain homogenates were digested with 50 μg/mL PK for one hour at 37°C. Anti-PrP antibodies used were 4H11 (1:500), 9A2 (1:1000) and 12B2 (1:1000). (B) PK-digested brain homogenates were then subjected to deglycosylation using the PNGase-F enzyme and probed with 4H11 and 12B2 and (C) with 8H4 (1:2000). Open arrowheads indicate the Prnp.Cer.138NN sample with a ~17 kDa PrPres band that is not detectable by 12B2. Asterisks (*) indicate the ~16 kDa PrPres band detected with 8H4 in Prnp.Cer.Wt samples. WT (SS) = Prnp.Cer.Wt; NN = Prnp.Cer.138NN; kDa = kilodalton.
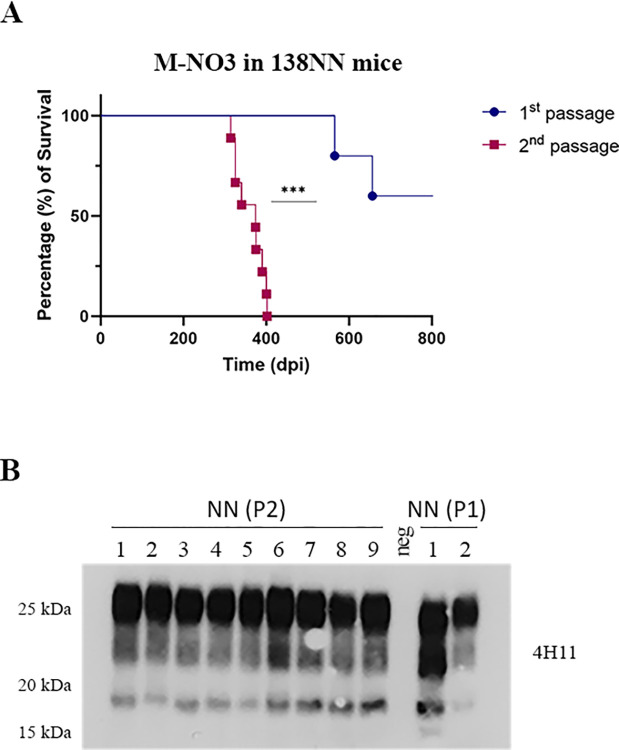
Next, we performed a second passage of M-NO3 in Prnp.Cer.138NN mice. We used a pool of brain homogenates from the two PrPres-positive Prnp.Cer.138NN mice inoculated with M-NO3 for i.c. passage (n = 9). All mice in this group developed terminal, clinical prion disease, with a significantly shortened survival time of 360.6 ± 34.7 dpi compared to the first passage with 565 and 656 dpi, respectively (Fig 4A and Table 2). Brain homogenates of all mice were positive for PrPres in western blot (Figs 4B and D in S1 Text). We compared the PrPres pattern and reactivity with different anti-PrP antibodies in brain homogenates of mice upon 2nd passage with those observed in the 1st passage. Interestingly, all brain homogenates from 2nd passage had PrPres similar to the higher molecular weight pattern in 1st passage, which was detectable with monoclonal antibody 12B2 (Figs 4B and D in S1 Text).
Fig 4. Second passage of M-NO3 in Prnp.Cer.138NN mouse model.
(A) Comparison of survival times of Prnp.Cer.138NN inoculated with M-NO3 (1st passage; n = 5; attack rate = 40%; see Table 1). Equal volumes of 10% brain homogenates of two mice with terminal prion disease in the 1st passage were pooled and used for the 2nd passage using a group of n = 9 Prnp.Cer.138NN mice for i.c. inoculation. Survival times are depicted as Kaplan-Meyer curve, and the log-rank test was used for statistical analysis. *** p-value = 0.0006. (B) Brain homogenates of 1st and 2nd passage were digested with 50 μg/ml of PK and subjected to western blot using anti-PrP antibody 4H11 (1:500), and 12B2 (Fig D in S1 Text).
Prion transport to the CNS in Prnp.Cer.138NN mice inoculated i.p. with M-NO3
Upon i.p. inoculation, brains and spleens of H-NO1-inoculated Prnp.Cer.138NN mice did not harbor detectable prion seeding activity. As expected, seeding activity was detectable in both brains and spleens of mice infected i.p. with R-NO16 (Table 2).
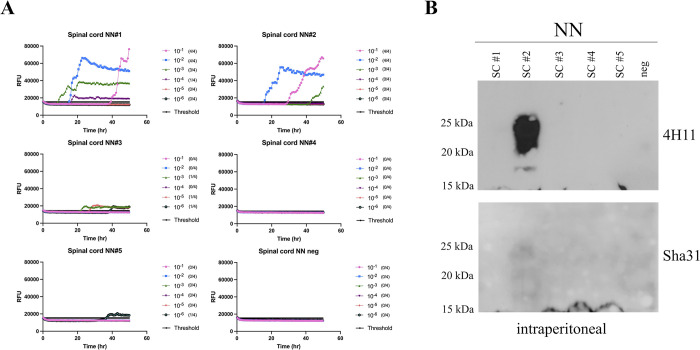
Notably, Prnp.Cer.138NN mice inoculated i.p. with M-NO3 showed prion seeding activity using RT-QuIC, in their brains and spinal cords, but not their spleens (Figs 5, E in S1 Text and Table 2). Serial protein misfolding cyclic amplification (sPMCA) up to 5 rounds was consistently negative when brain or spleen homogenates were analyzed using brain homogenates of either Prnp.Cer.Wt or Prnp.Cer.138NN as a substrate (representative result shown in Fig F in S1 Text), indicating a lower sensitivity of detection by serial PMCA compared to RT-QuIC under the experimental conditions used here. Notably, PrPres was detectable by western blot in the spinal cord sample of the i.p. inoculated Prnp.Cer.138NN mouse displaying the highest seeding activity. This finding supports the RT-QuIC results (Figs 5 and G in S1 Text) and demonstrates the transport of M-NO3 prions to the CNS. These findings suggest that M-NO3 prions are capable of neuroinvasion upon peripheral infection in Prnp.Cer.138NN, but not Prnp.Cer.Wt mice. This was specific to M-NO3 prions, since Prnp.Cer.138NN mice inoculated i.p. with H-NO1 prions did not produce the same outcome (Table 2).
Fig 5. RT-QuIC and western blot analysis of spinal cord homogenates of Prnp.Cer.138NN mice inoculated i.p. with M-NO3.
(A) Ten percent spinal cord homogenates were subjected to serial dilutions from 2 x 10−1 to 2 x 10−6 in RT-QuIC seed dilution buffer. Samples were considered positive when a minimum of two out of four wells crossed the threshold relative fluorescence unit (RFU). Threshold is the average RFU of all negative control reactions plus five times their standard deviation. Negative control was a naïve spinal cord homogenate of a Prnp.Cer.138NN mouse. The y-axis represents the RFU, and the x-axis represents time in hours (h). Mouse recombinant PrP was used as substrate for the RT-QuIC reactions. (B) Spinal cord homogenates were digested with 50 μg/ml of PK and subjected to western blot analysis using anti-PrP antibodies 4H11 (1:500) and Sha31 (1:10,000); see also overexposed western blot in Fig G in S1 Text).
Norwegian red deer isolate is transmissible to transgenic mice expressing elk PrPC
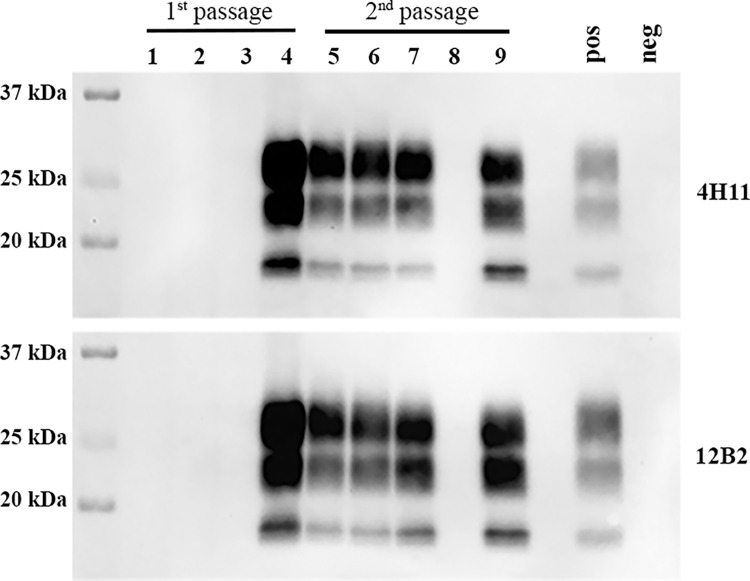
The Norwegian red deer isolate H-NO1 was inoculated i.c. into transgenic mice expressing elk PrPC [30,31], on the basis that this red deer was homozygous for glutamic acid (E) at Prnp codon 226 [21], which is identical to elk Prnp (226EE). The H-NO1 red deer isolate was able to transmit disease in TgElk mice, albeit at a low attack rate of 25%, where only one out of the four mice inoculated developed disease at 166 dpi (Table 1). PrPres was present in the brain of this singular mouse (Fig 6). Upon second passage, the attack rate increased to 80% (four out of five mice) while the average survival time remained comparable at 149.3 ± 44.5 dpi (Table 2). The four terminally ill TgElk mice from the second passage accumulated PrPres in their brains (Fig 6). Of note, M-NO3 was also inoculated into TgElk for comparison, and none of the mice developed disease up to 258 dpi (Table 1). The effective propagation of H-NO1 prions in TgElk mice, along with the subclinical presence of H-NO1 prions with seeding activity in the brains and spleens of Prnp.Cer.Wt, and in the brains of Prnp.Cer.138NN mice, suggests that codon 226 may influence the transmission of Norwegian red deer prions. Specifically, homozygosity for glutamic acid at this codon (226EE) appears to facilitate the development of full-fledged CWD, whereas the presence of glutamine (226QQ) may only allow for subclinical prion propagation upon first passage.
Fig 6. PrPres detection in brain homogenates of TgElk mice inoculated with H-NO1.
Ten percent brain homogenates were digested with 50 μg/mL PK for one hour at 37°C. Anti-PrP antibodies used were 4H11 (1:500, upper panel) and 12B2 (1:1000, lower panel). Lanes 1–4 corresponds to TgElk mice inoculated i.c. with the H-NO1 brain homogenate. Lanes 5–9 correspond to TgElk mice inoculated with brain homogenate from the TgElk in lane 4. pos = PrPres-positive TgElk control; neg = non-inoculated TgElk; kDa = kilodalton.
Discussion
We have previously shown that gene-targeted mice expressing cervid PrPC homozygous for asparagine at codon 138 (Prnp.Cer.138NN) and inoculated with North American CWD isolates do not develop clinical CWD, but harbor seeding-capable prions, mostly in their spleens [29]. Here we report that the Norwegian moose isolate M-NO3 was able to break the genotypic transmission barrier into Prnp.Cer.138NN mice upon i.c. inoculation, resulting in clinical CWD and accumulation of PrPres in their brains.
M-NO3 was shown to propagate in bank voles and several transgenic mouse models, and to possess different properties compared to North American and other Norwegian CWD isolates [18,35]. Here, in all Prnp.Cer.Wt and one of the clinical Prnp.Cer.138NN mice, M-NO3 retained an ~18 kDa unglycosylated PrPres fragment, detectable by the N-terminal 12B2 antibody [20,35]. Interestingly, although we did not consistently observe other unglycosylated PrPres fragments reported previously [18,20], we mainly detected a ~17 kDa fragment in the second PrPres-positive Prnp.Cer.138NN mouse, not recognized by 12B2 antibody. Similar to previous reports [14,36], this implies the existence of a minor sub-strain within M-NO3, which selectively propagated in one Prnp.Cer.138NN mouse, while Prnp.Cer.Wt mice and the other clinically affected Prnp.Cer.138NN mouse only propagated the major strain. Alternatively, the variant with the lower molecular weight might have evolved upon passage in the Prnp.Cer.138NN mice. However, in 2nd passage, the majority of PrPres detected in the mouse brains appeared comparable to the ~18 kDa variant detectable by 12B2, indicating that the ~17 kDa variant might be less stable upon passage, or replicating less efficiently, considering that the group of mice was inoculated with a pool of the two PrPres-positive brain homogenates from the 1st passage.
We previously demonstrated that North American CWD strains propagated in spleens of both Prnp.Cer.Wt and Prnp.Cer.138NN mice inoculated i.c. and i.p. [29]. Propagation of the Norwegian moose isolate M-NO3 was confined to the brain in both mouse lines, with no seeding activity detectable in the spleen by RT-QuIC, even upon i.p. inoculation, at the endpoints of our experiments. This corroborates previous reports that M-NO3 is not lymphotropic [18,20] and extends these findings for the first time to peripheral infection. Intraperitoneal inoculation of both Prnp.Cer.Wt and Prnp.Cer.138NN mice with M-NO3 did not result in clinical disease up to ~750 dpi, but Prnp.Cer.138NN mice had seeding activity in their brains and spinal cords, as well as detectable PrPres in the spinal cord of one mouse. Prion seeding activity was not evident in Prnp.Cer.Wt mice infected i.p. with M-NO3, and we argue that 138N-PrPC might be more efficiently converted by M-NO3 prions than 138S-PrPC. This is supported by the shorter survival times of i.c.-inoculated Prnp.Cer.138NN mice, compared to their wild-type (138SS) counterparts.
Our results suggest that M-NO3 prions are capable of neuroinvasion from peripheral sites of infection. The lack of detectable prion seeding activity in the spleens of these mice suggests that replication in the spleen either does not occur during pathogenesis and therefore, does not play a role in neuroinvasion, or that replication in the spleen is transient and may not necessarily contribute to neuroinvasion. The absence of prion seeding activity in spleen contrasts with findings in the majority of CWD cases and our gene-targeted mouse models. In these instances, prion seeding activity and/or PrPres are consistently detected in lymphatic tissue, e.g., tonsil, lymph node, spleen, at the terminal stage of the disease [29,37,38]. This discrepancy suggests variations in prion/strain behavior and disease progression across different models. Furthermore, prions are frequently detected in the lymphatic tissues of infected cervids but not their brain, and not vice versa [37,39]. Overall, these reports suggest that in CWD, lymphatic tissue replication precedes neuroinvasion and that detectable levels of prions persist in lymphatic tissue until terminal disease. Additional experiments to study kinetics of prion replication and transport in different tissues throughout the incubation period in Prnp.Cer.138NN mice are needed to rule out the possibility of early or rapid prion clearance in the spleen following neuroinvasion [40–42]. It is important to note though that a minor pathway of neuroinvasion independent of the spleen or the immune system with direct spread from the peritoneum via visceral nerve fibers has been described [43,44], consistent with our detection of prion seeding activity and PrPres in spinal cords. These data do not contradict the proposition that CWD in Norwegian moose started spontaneously at an advanced age but may suggest that infected animals propagate prions at peripheral sites other than lymphatic tissues [41]. It also indicates that environmental contamination from deceased infected moose might result in transmission to other cervids, even though in an inefficient manner and restricted to certain PrPC genotypes [9,10,45–47]. The Prnp of European fallow deer (Dama dama) is not polymorphic at codon 138 and encodes asparagine (138NN), in contrast to serine in the wild-type PrP of the majority of cervid species, and harbors glutamate at position 226 (226EE; 27,48). Fallow deer were resistant to mule deer CWD upon natural exposure, but not to intracerebral inoculation [47–49]. It remains to be determined whether fallow deer are more susceptible to peripheral infection with Norwegian moose CWD, or whether they would be protected by the glutamate at residue 226 (226EE), as shown in gene-targeted mice expressing cervid 138SS/226EE (elk) PrPC [18]. Furthermore, the presence of prions within uncharacterized peripheral reservoirs in the Norwegian moose might provide a source of prion exposure if consumed [50–52].
For R-NO16, the PrPres profile was identical to R-CA1 upon passaging into Prnp.Cer.Wt mice. However, R-NO16 resulted in prolonged incubation periods, lower attack rates, as well as the absence of hippocampal abnormal PrP deposition, confirming previous findings that R-NO16 and R-CA1 are different CWD strains [18,35]. Of note, a higher attack rate in animals inoculated i.p. was observed. Although this could be due to enhanced peripheral replication resulting in more ‘efficient’ prion neuroinvasion or selection of more ‘replicable’ prions [53,54], it could also simply be due to individual mouse differences, given the small difference in the number of animals testing positive, i.e., 7 out of 8 in i.p.- vs 5 out of 7 in i.c-inoculated groups. Upon i.c. and i.p. inoculation with R-NO16, most Prnp.Cer.Wt mice showed positive seeding activity in their spleens. However, this was not the case for Prnp.Cer.138NN mice inoculated i.c., indicating a possible restriction in prion spread within these animals when inoculated with R-NO16. This might be due to inefficient anterograde transport, very low prion replication in the brain, or prion degradation [40–42]. It is crucial to note that this observation is unique to the Norwegian CWD isolates examined in this study, as the same mouse line readily replicated North American CWD prions in their spleens following i.c. inoculation [29]. The majority of the Prnp.Cer.138NN mice inoculated i.p. with R-NO16 harbored seeding activity in their spleens and brains, demonstrating that R-NO16 can replicate within the spleens of this mouse line, and is transported efficiently to the brain.
We also report for the first time that the Norwegian red deer isolate H-NO1 is infectious and transmissible. Identical PrPSc and PrPC genotypes were required for H-NO1 to induce clinical disease. The differential transmissibility of M-NO3 and H-NO1 to TgElk indicates that these two isolates harbor different CWD strains; however, further characterization is needed for confirmation. A genotype match between the host PrPC and incoming prions often results in the most efficient prion disease transmission [55–58]. While H-NO1 (226EE) successfully transmitted full-fledged CWD to TgElk mice (226EE), albeit with incomplete attack rates, it failed to cause clinical prion disease in both Prnp.Cer.Wt (226QQ) and Prnp.Cer.138NN (226QQ) mice. It is important to note that 226QQ hosts tend to generate fewer stable strains than those expressing 226EE [17], suggesting more stable H-NO1 prion propagation in TgElk mice, which was confirmed upon second passage. Nevertheless, we detected prion seeding activity in the brains of both mouse lines inoculated i.c., and in the spleen of one Prnp.Cer.Wt mouse. Peripheral infection of Prnp.Cer.138NN mice with H-NO1 was even less effective, with no detectable prions in their brains and spleens. Unfortunately, we did not have the Prnp.Cer.Wt group inoculated i.p. to directly compare, due to limited resources. Nevertheless, these findings still support previous reports that H-NO1 is poorly, if at all, lymphotropic, indicated by the absence of abnormal PrP deposits in this red deer’s lymph nodes and tonsils [21].
In summary, we confirmed that the transmission of CWD prions is not merely a matter of host genotype. Rather, it is the unique traits of each prion strain guiding the course of transmission. One of our most intriguing findings is that neuroinvasion of Norwegian moose CWD prions can occur possibly without, or only with minor/transient, involvement of prion replication in the spleen, which has not been reported for CWD prions [37–39,59,60]. Even though it is, arguably, possible that spleen prion clearance occurred early on, the absence of detection upon terminal disease is still an uncommon trait of CWD prions [37,38], which also suggests the absence of prion dissemination from the CNS to the periphery and subsequent shedding. These observations broaden the spectrum of atypical features of Norwegian moose CWD prions. They underscore the intricate interplay between prion strains and host PrP genotypes that define transmission barriers, thus reminding us of the diversity and complexity inherent in the world of prions.
Materials and methods
Ethics statement
This study was performed under animal care protocols AC19-0047 and AC22-0015 approved by the University of Calgary Health Sciences Animal Care Committee and in compliance with the guidelines of the Canadian Council for Animal Care.
CWD isolates
Reindeer isolate R-NO16 (ID #18-CD2788) was from a free-ranging reindeer in the Nordfjella region and was not characterized previously. R-NO16 had the A/C PrP genotype, i.e., alleles A (Ser225)/C (deletion) and 109KK [61]. Moose isolate M-NO3 (ID #17-CD11399) was described previously [18,20,35]. Both the reindeer and moose isolates were homozygous for serine and glutamine at codons 138 (138SS) and 226 (226QQ), respectively [18,20,35,61]. Red deer isolate H-NO1 (ID #17-CD14501) was from a free-ranging animal from the Gjemnes region, Norway, and was homozygous for serine and glutamic acid at codons 138 (138SS) and 226 (226EE), respectively [21]. WTD-Wisc-1, WTD-116AG, and R-CA1 isolates were described previously [14,16,34]. Brains were homogenized as 10% (w/v) in sterile 1X PBS using the gentleMACS Dissociator (Miltenyi Biotec) and stored at -80°C.
Bioassays
Gene-targeted mice expressing deer PrPC with either serine (Prnp.Cer.Wt; 138SS/226QQ) or asparagine (Prnp.Cer.138NN; 138NN/226QQ) at residue 138 and transgenic TgElk mice expressing 2.5-fold elk PrPC (138SS/226EE) have been described [29–32].
Eight female mice per group were i.c. inoculated between six to eight weeks of age with 20 μl of 1% BH or i.p. inoculated with 100 μl of 5% BH, as previously described [14]. For 2nd passage of M-NO3 in Prnp.Cer.138NN mice, equal volumes of the two PrPres positive brain homogenates were pooled for i.c. inoculation. Mice were monitored weekly for signs of prion disease and then daily at the onset of at least one confirmatory sign, including rigid tail, hind limb clasping, front and/or hind limb ataxia, loss of righting reflex, circling, occasional tremors, and weight loss. At that point, mice were euthanized by isoflurane overdose followed by cardiac perfusion [29]. Survival time or dpi was defined as the number of days from the date of inoculation to the date of euthanasia. Humane endpoint euthanasia was performed on sick or injured mice showing no clinical signs of prion disease. Animal experiments were terminated at ~700 to 750 dpi for gene-targeted mice, and ~250 dpi for TgElk mice to prevent non-prion related health issues with this mouse line. Mice euthanized prior to 100 dpi due to humane reasons or found dead were excluded from the analyses.
Mouse tissue collection and homogenization
Brains and spleens were harvested, immediately frozen on dry ice, and stored at -80°C, or fixed in formalin. Frozen brains and spleens were homogenized as 20% and 10% (w/v) homogenates, respectively, using the FastPrep-24 Tissue Homogenizer system (MP Biomedicals, Canada).
RT-QuIC
For RT-QuIC, we tested 10% (w/v) brain, spinal cord, or spleen homogenates diluted in a buffer containing 0.1% SDS and 1X N2 media supplement, in serial dilutions from 2 x 10−1 up to 10−6, with four replicates each. Recombinant mouse PrP (aa 23–231) preparation, RT-QuIC reactions, and analyses were performed as previously described [33].
Serial Protein Misfolding Cyclic Amplification (sPMCA)
sPMCA was performed as described previously [29]. Briefly, brains from non-inoculated Prnp.Cer.Wt, and Prnp.Cer.138NN were prepared as 10% (w/v) BH in cold PMCA conversion buffer (4 mM EDTA, 1% Triton X-100 and 1 tablet cOmplete Protease Inhibitor Mini [Roche] in 1X PBS, pH of 7.4). Samples were tested at a 2x10-2 dilution, with R-CA1-infected Prnp.Cer.Wt mouse brain homogenate serving as a positive control. Sonication was set for 30 s at 375–395 W followed by 29.5 min rest and run for 24 h for a total of 48 sonication-rest cycles, corresponding to one round of PMCA. For sPMCA, 10 μl of sample was transferred into 90 μl of fresh PMCA substrate. A total of five rounds were conducted for each substrate.
SDS-PAGE and western blotting
Mouse BH (20% w/v) was diluted at a 1:1 ratio in 2X lysis buffer (100 mM of Tris-HCl pH 7.5, 100 mM of NaCl, 10 mM of EDTA, 1% Triton-X, 1% sodium deoxycholate, H2O). Fifty μg/ml PK was added into the mix, incubated at 37°C for one hour, followed by boiling for 10 min at 95°C in SDS sample buffer. To remove N-linked glycans, PK-digested samples were incubated with PNGase-F enzyme for 24 h at 37°C. Samples were subjected to SDS-PAGE and immunoblotting using anti-PrP antibodies 4H11, 9A2 (aa 102–104, sheep PrP numbering), 12B2 (aa 93–97, sheep PrP numbering), 8H4 (aa 145–180, human PrP numbering) and Sha31 (aa 145–152, human PrP numbering) as previously described [29].
Immunohistochemistry
Immunohistochemistry was performed as previously described [16,29]. Briefly, formalin-fixed brains and spleens embedded in paraffin were mounted on positively-charged slides, deparaffinized, autoclaved in antigen retrieval buffer, incubated in 98% formic acid, and treated with 4 M of guanidine thiocyanate. Abnormal PrP deposits were detected using the BAR224 anti-PrP antibody (aa 141–151, human PrP numbering).
Supporting information
(XLSX)
Fig A. PrPres in brain homogenates of gene-targeted mice and various CWD isolates. PrPres-positive brain homogenates of gene-targeted mice as indicated inoculated i.c. with R-CA1, R-NO16, and M-NO3 were analyzed by western blot along with CWD isolates from North America (Elk-CWD2, WTD-Wisc-1, WTD-116AG, and R-CA1) and Norway (R-NO16, M-NO3, and H-NO1) digested with 50 μg/ml of PK using anti-PrP antibodies 12B2. R-NO16 and H-NO1 did not harbor detectable amounts of PrPres. Fig B. Seeding activity in PrPres-negative brains of Prnp.Cer.Wt mice inoculated with R-NO16 and M-NO3. Left column shows RT-QuIC results from individual Prnp.Cer.Wt mice inoculated i.c. (n = 2) and i.p. (n = 1) with R-NO16 that were negative for PrPres on western blotting (Fig 2). Right column shows RT-QuIC results from individual Prnp.Cer.Wt mice inoculated i.p. with M-NO3 (n = 2) that were negative for PrPres on western blotting (Fig 2). Samples were considered positive when a minimum of two out of four reactions crossed the threshold relative fluorescence unit (RFU), indicated by the violet line. Positive dilutions are highlighted in black rectangles. The threshold is the average RFU of all negative control reactions plus five times their standard deviation. Negative control was a non-inoculated Prnp.Cer.Wt mouse brain. The y-axis represents the RFU, and the x-axis represents time in hours (h). Fig C. Representative immunohistochemistry of brain and spleen sections from gene-targeted mice inoculated i.p. with the R-NO16 and M-NO3 isolates. Abnormal PrP deposits were detected in the frontal cortex, cerebellum, and spleen of the Prnp.Cer.Wt mouse inoculated with R-NO16 (row 3, highlighted with the black rectangle), but not M-NO3 (row 5). No abnormal PrP deposits were detected in Prnp.Cer.138NN mice tissues inoculated with both isolates (rows 2 and 4). No abnormal PrP deposits were detected in the hippocampus of all mice tested. Detection of abnormal PrP deposits was performed using the anti-PrP antibody BAR224 (1:2000) with hematoxylin counterstain. Fig D. Western blot analysis of Prnp.Cer.138NN brain homogenates inoculated with M-NO3. Brain homogenates of M-NO3 1st and 2nd passage were digested with 50 μg/ml of PK and subjected to western blot analysis using anti-PrP antibody 12B2. Non-infected Prnp.Cer.138NN brain homogenate served as a negative control. Fig E. RT-QuIC analysis of PrPres-negative brains and spleens of Prnp.Cer.138NN mice inoculated i.p. with M-NO3. (A) Brain (n = 5) and (B) spleen (n = 4) homogenates of Prnp.Cer.138NN mice inoculated i.p. with M-NO3 that were negative for PrPres in western blot (Fig 2) were serially diluted and analyzed by RT-QuIC using mouse recombinant PrP as a substrate. Samples were considered positive when a minimum of two out of four reactions crossed the threshold relative fluorescence unit (RFU), indicated by the black line. The threshold is the average RFU of all negative control reactions plus five times their standard deviation. Negative control was non-inoculated Prnp.Cer.138NN mouse brain and spleen, respectively. The y-axis represents the RFU, and the x-axis represents time in hours (h). Fig F. Serial PMCA of brain and spleen homogenates of Prnp.Cer.138NN mice inoculated i.p. with M-NO3. 2 x 10–2 dilutions of brain (n = 5) and spleen (n = 3) homogenates of Prnp.Cer.138NN mice inoculated with M-NO3 were subjected to 5 rounds of PMCA using naïve brain homogenates of either Prnp.Cer.Wt (A) or Prnp.Cer.138NN (B) mice as a substrate. sPMCA products of rounds 4 and 5 were digested with PK (50 μg/ml) for 1 hour and analyzed by western blot using anti-PrP mAb 4H11 (1:500). Naïve brain homogenate was used as a negative control, brain homogenate of Prnp.Cer.Wt mice inoculated with R-CA1 served as a positive control. Fig G. Western blot analysis of spinal cord homogenates. Spinal cord homogenates of Prnp.Cer.138NN mice inoculated i.p. with M-NO3 were digested with 50 μg/ml of PK and analyzed by western blot using anti-PrP antibody Sha31 (1:10,000). This is an overexposed version of the western blot shown in Fig 4B (lower panel).
(DOCX)
Acknowledgments
Portions of the paper were developed from the thesis of M.I.A. We would like to thank the staff and veterinarians at the Prion-Virology Animal Facility and Clara Christie Centre for Mouse Genomics for excellent animal care. We would also like to acknowledge Keegan McDonald for helping with tissue homogenization, Yo-Ching Cheng for producing the recombinant PrP used in our RT-QuIC runs, and Dr. Gordon Mitchell (Canadian Food Inspection Agency Ottawa, Canada) for providing us with the North American reindeer isolate. We are grateful to Dr. Debbie McKenzie (University of Alberta, Canada) for sharing the TgElk mouse model and the WTD-Wisc-1 isolate, to Dr. Trent Bollinger (Canadian Wildlife Health Cooperative, Canada) for sharing the WTD-116AG isolate, and to Dr. Stefanie Czub (Canadian Food Inspection Agency, Lethbridge, Canada) for providing the Elk-CWD2 isolate.
Data Availability
All data are available in the manuscript and supplementary information.
Funding Statement
Alberta Prion Research Institute (https://albertainnovates.ca/alberta-prion-research-institute/; Grant #201800003 and #212200712 to SG, #201600023 to SG and HMS), Natural Sciences and Engineering Research Council Canada (https://www.nserc-crsng.gc.ca/index_eng.asp; Grant #RGPIN/05309 to SG), Margaret Gunn Endowment for Animal Research (https://research.ucalgary.ca/conduct-research/funding/apply-grants/internal-grants/margaret-gunn-endowment-animal-research-mgear) to SG, Parks Canada Agency (https://parks.canada.ca/agence-agency) to SG, and Genome Canada (https://genomecanada.ca/;Grant #10205 to SG). SG received salary support through the Canada Research Chairs program (https://www.chairs-chaires.gc.ca/home-accueil-eng.aspx). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Koutsoumanis K, Allende A, Alvarez-Ordoňez A, Bolton D, Bover-Cid S, Chemaly M, et al. Update on chronic wasting disease (CWD) III. EFSA J. 2019. Nov 11;17(11):e05863. doi: 10.2903/j.efsa.2019.5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn HJ, Kim JH, Choi KS, Nah JJ, Joo YS, Jean YH, et al. A Case of Chronic Wasting Disease in an Elk Imported to Korea from Canada. Journal of Veterinary Medical Science. 2002;64(9):855–8. doi: 10.1292/jvms.64.855 [DOI] [PubMed] [Google Scholar]
- 3.National Wildlife Health Center. Expanding Distribution of Chronic Wasting Disease [Internet]. 2023 [cited 2023 Apr 6]. Available from: https://www.usgs.gov/centers/nwhc/science/expanding-distribution-chronic-wasting-disease
- 4.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982. Apr 9;216(4542):136–44. doi: 10.1126/science.6801762 [DOI] [PubMed] [Google Scholar]
- 5.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998. Nov 10;95(23):13363–83. doi: 10.1073/pnas.95.23.13363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD Prions in Urine and Saliva of Deer by Transgenic Mouse Bioassay. PLOS ONE. 2009. Mar 18;4(3):e4848. doi: 10.1371/journal.pone.0004848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng YC, Hannaoui S, John TR, Dudas S, Czub S, Gilch S. Early and Non-Invasive Detection of Chronic Wasting Disease Prions in Elk Feces by Real-Time Quaking Induced Conversion. PloS one. 2016;11(11):e0166187. doi: 10.1371/journal.pone.0166187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mammadova N, Cassmann E, Greenlee JJ. Successful transmission of the chronic wasting disease (CWD) agent to white-tailed deer by intravenous blood transfusion. Research in Veterinary Science. 2020. Dec 1;133:304–6. doi: 10.1016/j.rvsc.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 9.Pritzkow S, Morales R, Lyon A, Concha-Marambio L, Urayama A, Soto C. Efficient prion disease transmission through common environmental materials. J Biol Chem. 2018. Mar 2;293(9):3363–73. doi: 10.1074/jbc.M117.810747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental Sources of Prion Transmission in Mule Deer. Emerging Infectious Diseases. 2004. Jun;10(6):1003. doi: 10.3201/eid1006.040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgsson G, Sigurdarson S, Brown P. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. Journal of General Virology. 2006. Dec 1;87(12):3737–40. doi: 10.1099/vir.0.82011-0 [DOI] [PubMed] [Google Scholar]
- 12.Government of Saskatchewan [Internet]. [cited 2020 Apr 20]. CWD map of positive tests | Chronic Wasting Disease. Available from: https://www.saskatchewan.ca/residents/environment-public-health-and-safety/wildlife-issues/fish-and-wildlife-diseases/chronic-wasting-disease/cwd-map
- 13.Otero A, Duque Velasquez C, McKenzie D, Aiken J. Emergence of CWD strains. Cell Tissue Res [Internet]. 2022. Oct 6 [cited 2023 Apr 5]; Available from: doi: 10.1007/s00441-022-03688-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannaoui S, Triscott E, Velásquez CD, Chang SC, Arifin MI, Zemlyankina I, et al. New and distinct chronic wasting disease strains associated with cervid polymorphism at codon 116 of the Prnp gene. PLOS Pathogens. 2021. Jul 26;17(7):e1009795. doi: 10.1371/journal.ppat.1009795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore J, Tatum T, Hwang S, Vrentas C, West Greenlee MH, Kong Q, et al. Novel Strain of the Chronic Wasting Disease Agent Isolated From Experimentally Inoculated Elk With LL132 Prion Protein. Scientific Reports. 2020. Feb 21;10(1):3148. doi: 10.1038/s41598-020-59819-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velásquez CD, Kim C, Herbst A, Daude N, Garza MC, Wille H, et al. Deer Prion Proteins Modulate the Emergence and Adaptation of Chronic Wasting Disease Strains. Journal of Virology. 2015. Dec 15;89(24):12362–73. doi: 10.1128/JVI.02010-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angers RC, Kang HE, Napier D, Browning S, Seward T, Mathiason C, et al. Prion Strain Mutation Determined by Prion Protein Conformational Compatibility and Primary Structure. Science. 2010. May 28;328(5982):1154–8. doi: 10.1126/science.1187107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian J, Kim S, Kane SJ, Crowell J, Sun JL, Christiansen J, et al. Adaptive selection of a prion strain conformer corresponding to established North American CWD during propagation of novel emergent Norwegian strains in mice expressing elk or deer prion protein. PLOS Pathogens. 2021. Jul 26;17(7):e1009748. doi: 10.1371/journal.ppat.1009748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikøren T. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Veterinary Research [Internet]. 2016. Dec [cited 2017 Jan 11];47(1). Available from: http://veterinaryresearch.biomedcentral.com/articles/10.1186/s13567-016-0375-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirisinu L, Tran L, Chiappini B, Vanni I, Bari MAD, Vaccari G, et al. Novel Type of Chronic Wasting Disease Detected in Moose (Alces alces), Norway. Emerging Infectious Diseases Journal [Internet]. 2018. Dec [cited 2020 May 5];24(12). Available from: https://wwwnc.cdc.gov/eid/article/24/12/18-0702_article doi: 10.3201/eid2412.180702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vikøren T, Våge J, Madslien KI, Røed KH, Rolandsen CM, Tran L, et al. First Detection of Chronic Wasting Disease in a Wild Red Deer (Cervus elaphus) in Europe. Journal of Wildlife Diseases. 2019. Oct 9;55(4):970. [PubMed] [Google Scholar]
- 22.Benestad SL, Sarradin P, Thu B, Schönheit J, Tranulis MA, Bratberg B. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Veterinary Record. 2003;153(7):202–8. doi: 10.1136/vr.153.7.202 [DOI] [PubMed] [Google Scholar]
- 23.Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, et al. Identification of a second bovine amyloidotic spongiform encephalopathy: Molecular similarities with sporadic Creutzfeldt-Jakob disease. Proceedings of the National Academy of Sciences. 2004. Mar 2;101(9):3065–70. doi: 10.1073/pnas.0305777101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biacabe AG, Laplanche JL, Ryder S, Baron T. Distinct molecular phenotypes in bovine prion diseases. EMBO reports. 2004. Jan 1;5(1):110–5. doi: 10.1038/sj.embor.7400054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haley NJ, Merrett K, Stein AB, Simpson D, Carlson A, Mitchell G, et al. Estimating relative CWD susceptibility and disease progression in farmed white-tailed deer with rare PRNP alleles. PLOS ONE. 2019. Dec 2;14(12):e0224342. doi: 10.1371/journal.pone.0224342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson C, Johnson J, Vanderloo JP, Keane D, Aiken JM, McKenzie D. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. Journal of General Virology. 2006;87(7):2109–14. doi: 10.1099/vir.0.81615-0 [DOI] [PubMed] [Google Scholar]
- 27.Arifin MI, Hannaoui S, Chang SC, Thapa S, Schatzl HM, Gilch S. Cervid Prion Protein Polymorphisms: Role in Chronic Wasting Disease Pathogenesis. Int J Mol Sci. 2021;24. doi: 10.3390/ijms22052271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arifin MI, Staskevicius A, Shim SY, Huang Y, Fenton H, McLoughlin PD, et al. Large-scale prion protein genotyping in Canadian caribou populations and potential impact on chronic wasting disease susceptibility. Mol Ecol. 2020. Aug 18;mec.15602. doi: 10.1111/mec.15602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arifin MI, Kaczmarczyk L, Zeng D, Hannaoui S, Lee C, Chang SC, et al. Heterozygosity for cervid S138N polymorphism results in subclinical CWD in gene-targeted mice and progressive inhibition of prion conversion. Proceedings of the National Academy of Sciences. 2023. Apr 11;120(15):e2221060120. doi: 10.1073/pnas.2221060120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaFauci G, Carp RI, Meeker HC, Ye X, Kim JI, Natelli M, et al. Passage of chronic wasting disease prion into transgenic mice expressing Rocky Mountain elk (Cervus elaphus nelsoni) PrPC. Journal of General Virology,. 2006;87(12):3773–80. doi: 10.1099/vir.0.82137-0 [DOI] [PubMed] [Google Scholar]
- 31.Abdelaziz DH, Thapa S, Brandon J, Maybee J, Vankuppeveld L, McCorkell R, et al. Recombinant prion protein vaccination of transgenic elk PrP mice and reindeer overcomes self-tolerance and protects mice against chronic wasting disease. Journal of Biological Chemistry. 2018. Nov 5;jbc.RA118.004810. doi: 10.1074/jbc.RA118.004810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ness A, Zeng D, Kuznetsova A, Otero A, Kim C, Saboraki K, et al. Chronic wasting disease prions in mule deer interdigital glands. PLOS ONE. 2022. Oct 3;17(10):e0275375. doi: 10.1371/journal.pone.0275375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng YC, Hannaoui S, John TR, Dudas S, Czub S, Gilch S. Real-time Quaking-induced Conversion Assay for Detection of CWD Prions in Fecal Material. JoVE (Journal of Visualized Experiments). 2017. Sep 29;(127):e56373. doi: 10.3791/56373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell GB, Sigurdson CJ, O’Rourke KI, Algire J, Harrington NP, Walther I, et al. Experimental Oral Transmission of Chronic Wasting Disease to Reindeer (Rangifer tarandus tarandus). PLOS ONE. 2012. Jun 18;7(6):e39055. doi: 10.1371/journal.pone.0039055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nonno R, Bari MAD, Pirisinu L, D’Agostino C, Vanni I, Chiappini B, et al. Studies in bank voles reveal strain differences between chronic wasting disease prions from Norway and North America. PNAS. 2020. Dec 8;117(49):31417–26. doi: 10.1073/pnas.2013237117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrio T, Filali H, Otero A, Sheleby-Elías J, Marín B, Vidal E, et al. Mixtures of prion substrains in natural scrapie cases revealed by ovinised murine models. Scientific Reports. 2020. Mar 19;10(1):5042. doi: 10.1038/s41598-020-61977-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoover CE, Davenport KA, Henderson DM, Denkers ND, Mathiason CK, Soto C, et al. Pathways of Prion Spread during Early Chronic Wasting Disease in Deer. J Virol. 2017. May 15;91(10):e00077–17. doi: 10.1128/JVI.00077-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox KA, Jewell JE, Williams ES, Miller MW. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). Journal of General Virology,. 2006;87(11):3451–61. doi: 10.1099/vir.0.81999-0 [DOI] [PubMed] [Google Scholar]
- 39.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). Journal of General Virology. 1999;80(10):2757–64. doi: 10.1099/0022-1317-80-10-2757 [DOI] [PubMed] [Google Scholar]
- 40.Shikiya RA, Langenfeld KA, Eckland TE, Trinh J, Holec SAM, Mathiason CK, et al. PrPSc formation and clearance as determinants of prion tropism. PLOS Pathogens. 2017. Mar 29;13(3):e1006298. doi: 10.1371/journal.ppat.1006298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urayama A, Morales R, Niehoff ML, Banks WA, Soto C. Initial fate of prions upon peripheral infection: half-life, distribution, clearance, and tissue uptake. FASEB J. 2011. Aug;25(8):2792–803. doi: 10.1096/fj.11-180729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen B, Soto C, Morales R. Peripherally administrated prions reach the brain at sub-infectious quantities in experimental hamsters. FEBS Lett. 2014. Mar 3;588(5):795–800. doi: 10.1016/j.febslet.2014.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimberlin RH, Walker CA. The role of the spleen in the neuroinvasion of scrapie in mice. Virus Research. 1989. Mar 1;12(3):201–11. doi: 10.1016/0168-1702(89)90039-7 [DOI] [PubMed] [Google Scholar]
- 44.Lasmézas CI, Cesbron JY, Deslys JP, Demaimay R, Adjou KT, Rioux R, et al. Immune system-dependent and -independent replication of the scrapie agent. J Virol. 1996. Feb;70(2):1292–5. doi: 10.1128/JVI.70.2.1292-1295.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, et al. Infectious Prions in Pre-Clinical Deer and Transmission of Chronic Wasting Disease Solely by Environmental Exposure. PLoS One [Internet]. 2009. Jun 16 [cited 2020 Dec 30];4(6). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2691594/ doi: 10.1371/journal.pone.0005916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan Q, Telling G, Bartelt-Hunt SL, Bartz JC. Dehydration of prions on environmentally relevant surfaces protects them from inactivation by freezing and thawing. Journal of Virology. 2018. Jan 31;JVI.02191-17. doi: 10.1128/JVI.02191-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhyan JC, Miller MW, Spraker TR, McCollum M, Nol P, Wolfe LL, et al. Failure of Fallow Deer (Dama dama) to Develop Chronic Wasting Disease When Exposed to a Contaminated Environment and Infected Mule Deer (Odocoileus hemionus). Journal of Wildlife Diseases. 2011. Jul;47(3):739–44. doi: 10.7589/0090-3558-47.3.739 [DOI] [PubMed] [Google Scholar]
- 48.Hamir AN, Kunkle RA, Nicholson EM, Miller JM, Hall SM, Schoenenbruecher H, et al. Preliminary observations on the experimental transmission of chronic wasting disease (CWD) from elk and white-tailed deer to fallow deer. J Comp Pathol. 2008. Apr;138(2–3):121–30. doi: 10.1016/j.jcpa.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 49.Hamir AN, Greenlee JJ, Nicholson EM, Kunkle RA, Richt JA, Miller JM, et al. Experimental transmission of chronic wasting disease (CWD) from elk and white-tailed deer to fallow deer by intracerebral route: Final report. Can J Vet Res. 2011. Apr;75(2):152–6. [PMC free article] [PubMed] [Google Scholar]
- 50.Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, et al. Prions in Skeletal Muscles of Deer with Chronic Wasting Disease. Science. 2006. Feb 24;311(5764):1117–1117. doi: 10.1126/science.1122864 [DOI] [PubMed] [Google Scholar]
- 51.Selariu A, Powers JG, Nalls A, Brandhuber M, Mayfield A, Fullaway S, et al. In utero transmission and tissue distribution of chronic wasting disease-associated prions in free-ranging Rocky Mountain elk. J Gen Virol. 2015. Nov;96(Pt 11):3444–55. doi: 10.1099/jgv.0.000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, Hoover EA. Detection of Chronic Wasting Disease Prions in Salivary, Urinary, and Intestinal Tissues of Deer: Potential Mechanisms of Prion Shedding and Transmission. Journal of Virology. 2011. Jul 1;85(13):6309–18. doi: 10.1128/JVI.00425-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beringue V, Herzog L, Jaumain E, Reine F, Sibille P, Le Dur A, et al. Facilitated Cross-Species Transmission of Prions in Extraneural Tissue. Science. 2012. Jan 27;335(6067):472–5. doi: 10.1126/science.1215659 [DOI] [PubMed] [Google Scholar]
- 54.Igel-Egalon A, Béringue V, Rezaei H, Sibille P. Prion Strains and Transmission Barrier Phenomena. Pathogens. 2018. Mar;7(1):5. doi: 10.3390/pathogens7010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scott M, Groth D, Foster D, Torchia M, Yang SL, DeArmond SJ, et al. Propagation of prions with artificial properties in transgenic mice expressing chimeric PrP genes. Cell. 1993. Jun;73(5):979–88. doi: 10.1016/0092-8674(93)90275-u [DOI] [PubMed] [Google Scholar]
- 56.Scott M, Foster D, Mirenda C, Serban D, Coufal F, Wälchli M, et al. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989. Dec 1;59(5):847–57. doi: 10.1016/0092-8674(89)90608-9 [DOI] [PubMed] [Google Scholar]
- 57.Kurt TD, Jiang L, Fernández-Borges N, Bett C, Liu J, Yang T, et al. Human prion protein sequence elements impede cross-species chronic wasting disease transmission. J Clin Invest. 2015. Apr 1;125(4):1485–96. doi: 10.1172/JCI79408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurt TD, Bett C, Fernández-Borges N, Joshi-Barr S, Hornemann S, Rülicke T, et al. Prion Transmission Prevented by Modifying the β2-α2 Loop Structure of Host PrPC. J Neurosci. 2014. Jan 15;34(3):1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sigurdson CJ. A prion disease of cervids: Chronic wasting disease. Vet Res. 2008. Jul;39(4):41. doi: 10.1051/vetres:2008018 [DOI] [PubMed] [Google Scholar]
- 60.Sigurdson CJ, Barillas-Mury C, Miller MW, Oesch B, van Keulen LJM, Langeveld JPM, et al. PrPCWD lymphoid cell targets in early and advanced chronic wasting disease of mule deer. Journal of General Virology. 2002;83(10):2617–28. doi: 10.1099/0022-1317-83-10-2617 [DOI] [PubMed] [Google Scholar]
- 61.Güere ME, Våge J, Tharaldsen H, Benestad SL, Vikøren T, Madslien K, et al. Chronic wasting disease associated with prion protein gene (PRNP) variation in Norwegian wild reindeer (Rangifer tarandus). Prion. 2020. Jan 1;14(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]