Abstract
Previous studies have shown that the nonstructural glycoprotein NSP4 plays a role in rotavirus pathogenesis by functioning as an enterotoxin. One prediction of the mechanism of action of this enterotoxin was that it is secreted from virus-infected cells. In this study, the media of cultured (i) insect cells infected with a recombinant baculovirus expressing NSP4, (ii) monkey kidney (MA104) cells infected with the simian (SA11) or porcine attenuated (OSU-a) rotavirus, and (iii) human intestinal (HT29) cells infected with SA11 were examined to determine if NSP4 was detectable. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis–Western blotting, immunoprecipitation and N-terminal amino acid sequencing identified, in the early media from virus-infected cells, a secreted, cleavage product of NSP4 with an apparent molecular weight of 7,000 that represented amino acids 112 to 175 (NSP4 aa112–175). The secretion of NSP4 aa112–175 was not affected by treatment of cells with brefeldin A but was abolished by treatment with nocodazole and cytochalasin D, indicating that secretion of this protein occurs via a nonclassical, Golgi apparatus-independent mechanism that utilizes the microtubule and actin microfilament network. A partial gene fragment coding for NSP4 aa112–175 was cloned and expressed using the baculovirus-insect cell system. Purified NSP4 aa112–175 increased intracellular calcium mobilization in intestinal cells when added exogenously, and in insect cells when expressed endogenously, similarly to full-length NSP4. NSP4 aa112–175 caused diarrhea in neonatal mice, as did full-length NSP4. These results indicate that NSP4 aa112–175 is a functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells.
Rotaviruses are major pathogens causing life-threatening dehydrating gastroenteritis in young children and animals. Despite extensive studies of different animal models, rotavirus pathogenesis remains incompletely understood. A nonstructural protein, NSP4, encoded by rotavirus genome segment 10, is a transmembrane, endoplasmic reticulum (ER)-specific glycoprotein with pleotropic functions in viral replication and pathogenesis (15). NSP4 serves as an intracellular receptor for newly made double-layered particles and interacts with viral capsid proteins during viral morphogenesis (1). NSP4 has been shown previously to be an enterotoxin that causes diarrhea in mouse pups, suggesting a role for NSP4 in rotavirus pathogenesis (3, 21). Mutations in NSP4 have also been associated with altered virus virulence by comparing the sequences and biological activities of NSP4 from two pairs of virulent and avirulent porcine rotaviruses, thus supporting a role for NSP4 in rotavirus pathogenesis (46). Increasing evidence indicates that this enterotoxin functions to activate a signal transduction pathway that increases intracellular calcium levels in cells by mobilizing calcium from the ER and ultimately resulting in chloride secretion (3, 11, 33, 38, 39). Recent studies have shown that NSP4 induces diarrhea by activating an age-dependent, calcium-sensitive anion (probably chloride) permeability in the small and large intestinal mucosa in both normal mice and mice with cystic fibrosis that lack the cystic fibrosis transmembrane regulator (cystic fibrosis transmembrane regulator chloride channel). These properties of NSP4 indicate that it is a novel secretory agonist since other secretagogues fail to function in mice with cystic fibrosis (33). The effects of NSP4 are specific, and an avirulent form of NSP4 does not induce diarrhea in mice (46).
It has been postulated elsewhere that the enterotoxin activity of NSP4 may be responsible for the profuse diarrhea observed early after rotavirus infections of animals prior to the detection of histologic changes in the intestine that contribute to subsequent malabsorption (5, 8, 31, 41). One model for the mechanism of action of NSP4 is that this enterotoxin is released from virus-infected enterocytes and extracellular NSP4 functions in a paracrine fashion to stimulate secretion from adjacent epithelial crypt cells (3, 18). This model requires that either NSP4 is released by cell lysis or a novel pathway for secretion of NSP4 must exist. Extracellular NSP4 was not detected in early work that characterized NSP4 as a transmembrane, ER-specific glycoprotein (14, 23).
Release or secretion of a viral protein product into the medium is one approach used by viruses to exert their pathogenic effect on the host. Many viruses code for proteins that counteract host immune defenses (19). The T2 protein (40) and a serine protease inhibitor (28) of myxoma virus, a 35,000-molecular-weight (MW) (35K) protein of vaccinia virus (26), human immunodeficiency virus type 1 Tat (44), and a glycoprotein from Ebola virus (43) all are actively secreted from virus-infected cells and have autocrine or paracrine effects on host cells.
This paper reports studies designed to test the hypothesis that NSP4 is released from virus-infected cells in the absence of cell lysis. This idea was strengthened by the report that rotavirus can reach the cell surface by a nonconventional vesicular exocytic pathway that bypasses the Golgi apparatus and results in virus release from nonlysed, polarized epithelial cells (22). This current study indicates that a cleavage product of NSP4 that retains enterotoxin activity is secreted from rotavirus-infected cells, and this could be the active form that causes the early, profuse diarrhea prior to the detection of histologic changes in the intestine.
MATERIALS AND METHODS
Cells and viruses.
Spodoptera frugiperda (Sf9) insect cells were grown and maintained in TNM-FH (Hinks) medium (Gibco, Grand Island, N.Y.) with 10% fetal bovine serum (FBS) (16). The human intestine cell line HT29 clone 19A (HT29 cells) was routinely cultured in Dulbecco modified Eagle medium (Gibco) with 4.5 g of glucose per liter, supplemented with 4 mM l-glutamine–10% FBS (2). The monkey kidney MA104 cell line (MA104 cells) was maintained in medium 199, and simian rotavirus SA11 cl3 (SA11) was maintained in MA104 cells, as previously described (17). The HT29 cells were used at passages between 25 and 40. Tissue culture-attenuated porcine rotavirus OSU (OSU-a) was kindly provided by Linda Saif, Ohio State University, and grown in MA104 cells (46). Baculovirus recombinants pAc461/SA11-10 encoding SA11-NSP4 (1) and pFastBac/SA11-10aa112–175 encoding SA11-NSP4 aa112–175 (this paper) were used to express NSP4 and NSP4 aa112–175, respectively.
Analyses of NSP4 products, SA11 structural proteins, ER transmembrane protein calnexin, and human interleukin 8 (IL-8).
Sf9 cells were infected with baculovirus recombinant pAc461/SA11-10 or pFastBac/SA11-10aa112–175 at a multiplicity of infection (MOI) per cell of 3 and 5 in T-150 flasks. The inocula were removed 2 h later, and NSP4 products were expressed for 4 days. MA104 cells and HT29 cells in T-150 flasks were infected with SA11 or OSU-a at an MOI of 20. The inocula were removed 1 h later and replaced with 25 ml of medium without FBS. This time was taken as 0 h postinfection (hpi) for all experiments. In trafficking experiments, the medium contained 2.5 μg of the Golgi apparatus-ER-disrupting drug brefeldin A (BFA) per ml, 10 μg of the microtubule-depolymerizing drug nocodazole (NOC) per ml, or 0.5 μg of actin filament-disrupting drug cytochalasin D (Cyt.D) (12, 13) (Sigma, St. Louis, Mo.) per ml. Infected cells and culture media were harvested at various times postinfection. Cells from each T-150 flask were lysed in 300 μl of lysis buffer (10 mM Tris containing 2% sodium salt of deoxycholic acid, pH 7.4). The culture medium from each T-150 flask was cleared of any cell debris, dialyzed against 50 mM NH4HCO3, lyophilized, and then reconstituted in 300 μl of phosphate-buffered saline (PBS). Proteins in the cell lysates and reconstituted culture media were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blots were probed with appropriate antibodies. Primary antibodies used in this work were rabbit anti-NSP4pep120–147 (J. M. Ball and M. K. Estes, unpublished data) and rabbit anti-NSP4pep114–135 polyclonal antibodies (3) made in this laboratory that detect NSP4 products, mouse anti-SA11cl3 polyclonal antibody made in this laboratory that detects SA11 structural proteins (9), rabbit anti-calnexin carboxy terminus polyclonal antibody (Stress Gen Biotechnologies Corp., Victoria, British Columbia, Canada) for detecting the ER transmembrane protein calnexin, and a mouse anti-human IL-8 monoclonal antibody (R & D Systems, Inc., Minneapolis, Minn.) for detecting human IL-8. The secondary antibodies used in this work were alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin (heavy plus light chains) and alkaline phosphatase-conjugated goat anti-mouse immunoglobulin (heavy plus light chains) (Sigma). To visualize the targeted bands, p-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate (American Life Science Inc., Arlington Heights, Ill.) were used as the chromogenic substrates for alkaline phosphatase. Prestained SDS-PAGE standards for a low range of molecular weights (MW) were obtained from Bio-Rad Laboratories (Hercules, Calif.). Due to variations in the migration of different batches of commercial prestained standards, fully glycosylated and nonglycosylated NSP4 proteins were used as internal controls on gels. Cell lysates containing fully glycosylated and nonglycosylated NSP4 proteins were made in this laboratory by infecting MA104 cells with SA11 and maintaining the infected cells in the absence (−TM) and presence (+TM) of 2 μg of tunicamycin (Sigma) per ml.
The NSP4 cleavage product released into the MA104 cell medium was also analyzed by immunoprecipitation. MA104 cells grown in a T-75 flask were infected with SA11 at an MOI of 20. The inoculum was removed 1 h later and replaced with 13 ml of Met-free medium for 30 min of starvation. Four hundred microcuries of l-[35S]Met (Redivue Pro-Mix [35S]; Amersham Pharmacia Biotech, Piscataway, N.J.) was added at the end of starvation. Medium (1.4 ml) was collected at 5.5, 6.5, and 7.5 hpi and directly analyzed by immunoprecipitation without being concentrated. Rabbit anti-NSP4pep120–147 (1:500 dilution) was used to react with released [35S]NSP4-related products to form immune complexes. Formalin-fixed Staph A was used to pellet the [35S]NSP4-related products. SDS–15% PAGE was used to resolve the [35S]NSP4-related products, and autoradiography was used to visualize the products.
N-terminal amino acid sequence analysis.
A 7K NSP4-related product in the medium of baculovirus recombinant pAc461/SA11-10-infected Sf9 cells and of SA11-infected MA104 cells was partially purified by an anti-NSP4 immunoaffinity column and resolved by SDS–15% PAGE. After SDS-PAGE, the protein bands were electroblotted from the gel onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.) and visualized with Coomassie blue for sequencing (30, 45).
Production and purification of the NSP4 cleavage product NSP4 aa112–175.
The portion of SA11 gene 10 that encodes NSP4 aa112–175 was cloned into the baculovirus expression vector pFastBac1 (Life Technologies, Baltimore, Md.). The sequence of the resulting baculovirus recombinant DNA was confirmed by dideoxy sequencing. A recombinant baculovirus expressing NSP4 aa112–175 was generated as described by the manufacturer, and the recombinant virus stock was plaque purified. NSP4 aa112–175 was produced from insect cells infected at an MOI of 5 with the recombinant baculovirus expressing NSP4 aa112–175 in TNH-FH (Hinks) medium containing 10% FBS. NSP4 aa112–175 was released into the medium. Six days postinfection, the medium containing NSP4 aa112–175 was harvested and clarified. This clarified NSP4 aa112–175-containing medium was further purified by using an agarose immunoaffinity column onto which rabbit immunoglobulin G against SA11 NSP4 had been immobilized (3, 11, 37). The bound NSP4 aa112–175 was eluted with 0.1 M glycine-HCl buffer at pH 2.8, neutralized with 4 M K2HPO4 immediately, and then passaged through an immunoaffinity column containing rabbit immunoglobulin G against wild-type baculovirus proteins made in this laboratory. NSP4 aa112–175 was eluted in the flowthrough containing unbound protein. The final purified NSP4 aa112–175 was dialyzed exhaustively against 50 mM NH4HCO3, using a dialysis membrane with an MW cutoff of 3,500 (Spectrum, Houston, Tex.), and aliquots were lyophilized. The purity of NSP4 aa112–175 was examined by SDS–15% PAGE, followed by silver staining with a kit (Sigma) for verifying the purity.
Measurement of intracellular calcium concentration ([Ca2+]i).
[Ca2+]i in HT29 cells was measured by calcium imaging using the fluorescent Ca2+ indicator fura-2/AM as previously described (11, 46). Sf9 cells grown on coverslips were infected with a recombinant baculovirus expressing NSP4 or NSP4 aa112–175, at an MOI of 20, and loaded with fura-2/AM at 36 hpi (38). [Ca2+]i in Sf9 cells was measured according to essentially the same procedures as used for HT29 cells, except at room temperature. Cells loaded with fura-2/AM were superfused continuously with Na-HEPES (containing 1 mM Ca2+) to remove extracellular dye. Intracellular Ca2+ was measured by ratio imaging. The averaged ratio signal obtained from each cell was digitally saved as a log file. The collected values from cells imaged within a single experiment (6 to 10 cells) were then averaged to give an experimental observation of one (n = 1).
Diarrhea induction in neonatal mice.
Purified NSP4 aa112–175 was inoculated intraperitoneally into 6- to 7-day-old CD1 mice (Charles River Labs, Wilmington, Mass.) in a total volume of 50 μl of endotoxin-free PBS. The severity of diarrhea was scored using a scale of 1.0 to 4.0 as previously described (3). All animal studies were done using coded samples.
RESULTS
An NSP4 cleavage product, NSP4 aa112–175, is produced in both gene 10-recombinant baculovirus-infected Sf9 cells and SA11-infected MA104 cells.
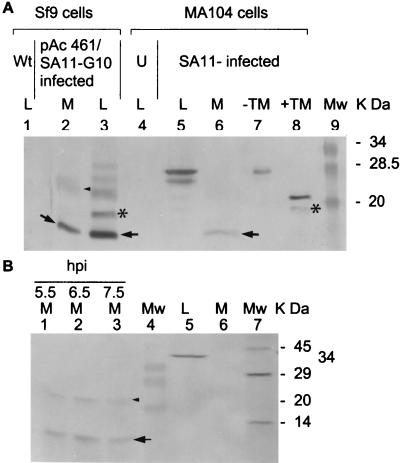
Recombinant baculovirus pAc461/SA11-10-infected Sf9 cells and the medium were harvested 4 days postinfection. The cell lysate and concentrated medium were then analyzed for expressed NSP4 products by SDS–15% PAGE and Western blotting with rabbit anti-NSP4pep120–147 polyclonal antibody. In addition to the regular forms of NSP4 with apparent molecular weights of 28K, 26K, 20K, and 15K*, a 7K band was seen in the cell lysate (Fig. 1A, lane 3, arrow) and medium (Fig. 1A, lane 2, arrow). Rotavirus-infected MA104 cells and the medium were harvested at 7.5 hpi and analyzed by the same approach. NSP4-related major bands that were 28K and 26K were detected in cell lysates (Fig. 1A, lane 5), and a 7K NSP4-related product was seen in the medium (Fig. 1A, lane 6, arrow). The same results were obtained when a rabbit anti-NSP4pep114–135, instead of rabbit anti-NSP4pep120–147, polyclonal antibody was used (data not shown). The appearance of the 7K NSP4-related product in the medium was not affected by the presence of 2 μg of tunicamycin per ml that completely inhibited glycosylation of NSP4 (data not shown). A 23K band detected in the medium (Fig. 1A, lane 2, arrowhead) was shown to be an oligomer of the 7K band (see below). The 15K* NSP4-related band (Fig. 1A, lane 3; Fig. 2A) was always seen in the lysates. This seems to be another cleavage product of the NSP4 cytoplasmic terminus because it was detectable by the rabbit polyclonal antibodies to NSP4pep114–135 and to NSP4pep120–147. Characterization of the 15K* band was not pursued because this form was not detected in the medium. In the presence of protease inhibitors (0.5 μg of aprotinin per ml plus 0.5 μg of leupeptin per ml), the 15K* and 7K bands were not seen in the culture media or cell lysates of either recombinant gene 10-infected insect cells or SA11-infected MA104 cells (data not shown).
FIG. 1.
A secreted form of NSP4 is present in the medium of virus-infected cells. (A) Detection of NSP4 aa112–175 in concentrated culture medium. Baculovirus-infected Sf9 cells and culture medium were harvested at 96 hpi. SA11-infected MA104 cells and medium were harvested at 7.5 hpi. Proteins in 15 μl of the cell lysates and concentrated culture medium were analyzed by SDS–15% PAGE and Western blotting using rabbit anti-NSP4pep120–147 polyclonal antibody. The NSP4-related bands from baculovirus-infected Sf9 cells are shown in lanes 1 to 3. The NSP4-related bands from uninfected and SA11-infected MA104 cells are shown in lanes 4 to 6. Fully glycosylated (−TM, 28K) and nonglycosylated (+TM, 20K) NSP4 proteins are shown as standards in lanes 7 and 8 and in subsequent figures. The lower band in lane 8 (+TM) corresponds to the 15K* cleavage product. Wt, wild-type baculovirus-infected Sf9 cell lysate (lane 1). U, uninfected MA104 lysate (lane 4). (B) Direct detection of [35S]NSP4 aa112–175 in MA104 cell medium. The radiolabeled proteins in the medium collected at 5.5, 6.5, and 7.5 hpi were immediately detected by immunoprecipitation using rabbit anti-NSP4pep120–147 (1:500 dilution) (lanes 1 to 3). 35S-calnexin in the lysate (lane 5) and medium (lane 6) at 7.5 hpi was also immediately detected by immunoprecipitation using rabbit anticalnexin (1:20 dilution). [35S]NSP4-related bands and 35S-calnexin-related bands were resolved by SDS–15% PAGE. Lane 4, prestained MW markers. Lane 7, 14C-methylated protein MW markers (Sigma). Abbreviations: M, medium. L, lysate. TM, tunicamycin. Arrows indicate the 7K cleavage product, subsequently characterized as NSP4 aa112–175 (see text). Arrowheads indicate the 23K oligomer of the 7K cleavage product. Asterisks indicate a 15K uncharacterized cleavage product detected only in cell lysates. Large amounts of the 7K product are detected in the insect cell system compared to virus-infected mammalian cells, probably due to higher levels of protein expression from the recombinant baculovirus at later time points after infection.
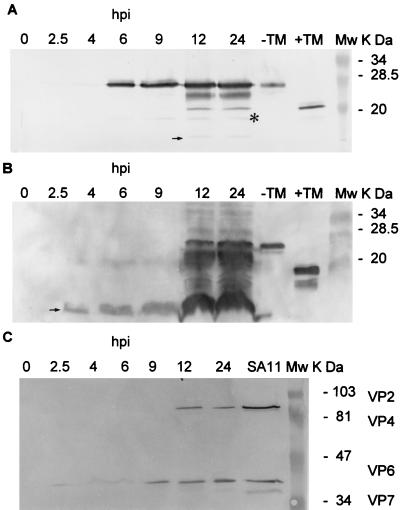
FIG. 2.
Detection of NSP4 aa112–175, full-length NSP4, and SA11 structural proteins in SA11-infected MA104 or HT29 cells at various time points postinfection. Infected cells and culture medium were harvested at 2.5, 4, 6, 9, 12, and 24 hpi. Proteins in 15 μl of cell lysates and of concentrated cultured medium were analyzed by SDS-PAGE and Western blotting with appropriate antibodies. (A) NSP4 and NSP4 aa112–175 in SA11-infected MA104 cell lysates were analyzed by SDS–15% PAGE. (B) NSP4 aa112–175 and NSP4 in concentrated culture medium from SA11-infected MA104 cells were analyzed by SDS–15% PAGE. A larger quantity of NSP4-related proteins was loaded in panel B than in panel A, and so these data cannot be used for direct precursor-product quantitation. (C) SA11 structural proteins in concentrated culture medium from SA11-infected MA104 cells analyzed by SDS–10% PAGE. Mw, molecular weight markers; TM, tunicamycin.
To directly identify the 7K cleavage product of NSP4 in the medium, l-[35S]Met was used to label proteins produced in SA11-infected MA104 cells. [35S]NSP4-related products in the medium were directly analyzed by immunoprecipitation without any dialysis and concentration of the medium. The major band precipitated by the rabbit anti-NSP4pep120–147 serum was the NSP4 cleavage product of 7K (Fig. 1B, lanes 1 to 3, arrow). 35S-calnexin, an ER transmembrane protein (see below), could not be detected by immunoprecipitation from the same medium using a rabbit anti-calnexin carboxy terminus antibody (Fig. 1B, lane 6), although calnexin was detected in the cell lysate (Fig. 1B, lane 5). A minor band of 23K positioned above the 7K major band (Fig. 1B, lanes 1 to 3, arrowhead) was also detected in the medium. This minor band was also seen elsewhere (Fig. 1A, lane 2, arrowhead; Fig. 2B, 4 to 9 hpi; see also Fig. 4, lanes 1 and 2, and Fig. 5, lanes 2 to 4). Detailed observations showed that (i) the migration of this 23K band was intermediate between the nonglycosylated NSP4 (20K) and monoglycosylated NSP4 (26K), (ii) the 23K band did not contain the N terminus of NSP4 because it did not react with a mouse anti-NSP4 aa2–22 antibody, (iii) the 23K band shifted to a 7K position was seen after treatment with strong detergent (data not shown), and (iv) the 23K band was sometimes detected in purified preparations of the baculovirus-expressed NSP4 aa112–175 (see below). Therefore, this minor 23K band appears to be an oligomer of the secreted NSP4 7K band.
FIG. 4.
Effect of Golgi apparatus-ER- and cytoskeleton-disrupting drugs on NSP4 aa112–175 secretion. MA104 and HT29 cells were infected with SA11 in the absence and presence of trafficking drugs. The culture medium and cells were harvested at 7.5 hpi. Proteins in 15μl of concentrated medium or lysates were resolved by SDS–15% PAGE and Western blotting with rabbit anti-NSP4pep120–147 antibody for probing the various forms of NSP4. (A) NSP4 aa112–175 and NSP4 in the concentrated medium and lysates of SA11-infected MA104 cells. (B) NSP4 aa112–175 and NSP4 in the concentrated medium and lysates of SA11-infected HT29 cells. Arrows indicate NSP4 aa112–175. I, infection; Mw, molecular weight markers; TM, tunicamycin.
FIG. 5.
Comparison of the migration of NSP4 aa112–175 expressed from pFastBac/SA11-10aa112–175 to the 7K products from pAc461/SA11-10-infected Sf9 cells and SA11-infected MA104 cells. (A) Migration of NSP4 aa112–175 from various sources, resolved by SDS–15% PAGE and Western blotting with a rabbit anti-NSP4pep120–147 polyclonal antibody. The arrow indicates NSP4 aa112–175. Lanes: 1, pAc461/SA11-10-infected Sf9 lysate; 2, SA11-infected MA104 cell medium; 3, pFastBac/SA11-10aa112–175-infected Sf9 cell medium; 4, purified NSP4 aa112–175 from the medium in lane 3. (B) NSP4 aa112–175 (500 ng) purified from pFastBac/SA11-10aa112–175-infected Sf9 cell medium, resolved by SDS–15% PAGE and silver staining. Mw, molecular weight markers.
The N-terminal sequence of the 7K products in Fig. 1A, lanes 2 and 6, from both the insect cells and MA104 cells was MIDKLTTRE, indicating that both began at Met112 of NSP4. The apparent MW of the 7K product was consistent with the cleavage product being the cytoplasmic tail of NSP4 containing amino acids (aa) 112 to 175. The amount of NSP4 aa112–175 released from MA104 cells at 7.5 hpi into the medium ranged from 10 to 20 μg per 106 cells, around 20% of the total NSP4 molecules, based on comparisons by Western blotting using purified NSP4 aa112–175 expressed from a recombinant baculovirus (see below) as a standard to semiquantify the amount of NSP4 aa112–175. The expected N-terminal cleavage product NSP4 aa1–111 was not detected when lysates or the medium was probed with various anti-NSP4 antibodies, including antibody to full-length NSP4, suggesting that the N terminus is quickly degraded.
NSP4 aa112–175 is released into the medium early during rotavirus infection of cells.
To determine the kinetics of secretion of NSP4 from SA11-infected cells, the medium from the infected cells was examined at various times postinfection for the presence of NSP4-related products and SA11 structural proteins. SDS–15% PAGE and Western blot analyses with rabbit anti-NSP4pep120–147 showed that full-length NSP4 could be detected as early as 2.5 hpi in cell lysates (Fig. 2A). NSP4 aa112–175 could be detected as early as 4 hpi in the medium (Fig. 2B). When the same medium was examined by SDS–10% PAGE and Western blot analysis with mouse anti-SA11 cl3 polyclonal antibody to detect SA11 structural proteins, VP2, VP4, and VP7 were not detectable in the early medium before 12 hpi, but unexpectedly VP6, the most abundant and soluble capsid protein of rotavirus, was seen in the medium as early as 2.5 hpi (Fig. 2C). Testing of the same medium by Western blot analysis with a rabbit antiserum that contains antibodies to NSP1, NSP2, NSP3, and NSP5 did not detect these nonstructural proteins prior to 12 hpi (data not shown). The experiments described so far were all carried out with MA104 cells infected with SA11. To determine if the release of NSP4 aa112–175 into the medium was a general phenomenon, SA11-infected HT29 cells and OSU-a-infected MA104 cells were also investigated. Results identical to those shown in Fig. 2A and B were obtained, and a product comigrating with NSP4 aa112–175 was seen (data not shown; also see Fig. 4B). Detection of the cleavage product in the medium of cells infected with the avirulent OSU-a was not surprising, since the mutation in this virus does not affect the Met112 cleavage site (46).
SA11 infection of cells in the early stages does not disrupt the ER membrane and ER-Golgi apparatus pathway.
NSP4 was previously characterized as an ER transmembrane protein. To determine if the detection of NSP4 aa112–175 in the medium reflected a general disruption of the ER membrane caused by viral infection, we tested to see if another ER transmembrane protein, the chaperone calnexin, which interacts with NSP4 (32, 35), was present in the medium. MA104 cells were infected with SA11, and the cells were incubated in the absence or presence of BFA, NOC, or Cyt.D. At 7.5 hpi, the concentrated media did not contain calnexin, while calnexin and several apparent cleavage products were detected in the lysates (Table 1), as seen in Fig. 1B, lanes 5 and 6. These data indicated that (i) a general disruption of the ER membrane at 7.5 hpi was not responsible for the detection of ER transmembrane proteins in the medium of virus-infected cells and (ii) disruption of the ER-Golgi apparatus with BFA, or of the cytoskeleton with NOC and Cyt.D, did not contribute to the detection of ER transmembrane proteins in the medium.
TABLE 1.
Effect of the Golgi apparatus-ER- and cytoskeleton-disrupting drugs on ER integrity at 7.5 hpi and VP6 secretion
| SA11 infection | Presence of calnexin in:
|
Presence of VP6 in:
|
||
|---|---|---|---|---|
| Medium | Lysate | Medium | Lysate | |
| Alonea | − | + | + | + |
| Plus BFA | − | + | − | + |
| Plus NOC | − | + | + | + |
| Plus Cyt.D | − | + | + | + |
Infection without disrupting drugs.
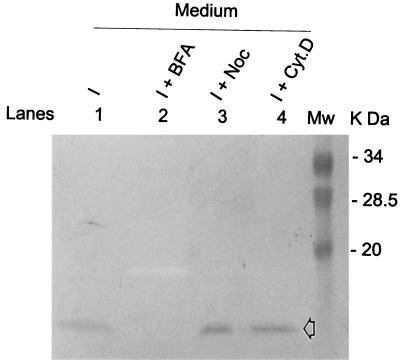
To investigate if the classical ER-Golgi vesicle-mediated secretion pathway was functioning, IL-8 was examined for its detection in the medium of SA11-infected HT29 cells. IL-8 secretion occurs from the Golgi apparatus (42), and it can be induced by diverse inflammatory stimuli in many cells. IL-8 can also be synthesized and secreted by epithelial cells following induction in response to rotavirus infection (6, 36). To compare the secretion pathway of NSP4 aa112–175 with that of human IL-8, HT29 cells were infected with SA11 and incubated in the absence and presence of BFA, NOC, or Cyt.D. The concentrated media were analyzed with a monoclonal anti-human IL-8 antibody to verify the release of IL-8 into the medium. IL-8 was secreted into the medium of SA11-infected HT29 cells at 7.5 hpi (Fig. 3, lane 1), and this secretion was completely abolished by treatment with BFA (Fig. 3, lane 2) but was not affected by treatment with NOC or Cyt.D (Fig. 3, lanes 3 and 4). This result is consistent with IL-8 secretion occurring by a Golgi apparatus-dependent pathway. These data indicated that (i) a general disruption in the cytoskeleton at 7.5 hpi has no detectable influence on the Golgi apparatus-dependent trafficking pathway and (ii) the drugs used to disrupt the trafficking pathways in SA11-infected HT29 cells functioned as expected based on analyses of the location and secretion of IL-8 and calnexin.
FIG. 3.
Effect of the Golgi apparatus-ER- and cytoskeleton-disrupting drugs on IL-8 secretion. HT29 cells were infected with SA11 in the absence and presence of the trafficking-disrupting drugs. The culture medium was harvested at 7.5 hpi. Proteins in 15 μl of concentrated medium were resolved by SDS–15% PAGE and Western blotting with mouse anti-human IL-8 monoclonal antibody. The open arrow indicates IL-8 migration. I, HT29 cells infected with SA11. Mw, molecular weight markers.
The secretion of NSP4 aa112–175 into the medium utilizes a novel microtubule and actin filament network trafficking pathway, rather than the classical ER-Golgi vesicle-mediated secretion pathway.
To investigate if NSP4 aa112–175 was detected in the medium due to trafficking by a classical pathway, SA11-infected MA104 cells and SA11-infected HT29 cells were incubated with medium lacking or containing BFA, NOC, and Cyt.D, and NSP4-related products were detected in cell lysates and concentrated media at 7.5 hpi. In the infected cells lacking the trafficking-disrupting drugs, NSP4 was regularly synthesized in the cells (Fig. 4A, lane 5; Fig. 4B, lane 5) and NSP4 aa112–175 was regularly released into the medium (Fig. 4A, lane 1; Fig. 4B, lane 1). BFA, NOC, and Cyt.D did not affect NSP4 synthesis and glycosylation in the cells (Fig. 4A, lanes 6 to 8; Fig. 4B, lanes 6 to 8). However, the microtubule-depolymerizing drug NOC and actin filament-disrupting drug Cyt.D completely blocked the secretion of NSP4 aa112–175 into the medium (Fig. 4A, lanes 3 and 4; Fig. 4B, lanes 3 and 4), while the Golgi apparatus-disrupting drug and classically mediated secretion inhibitor BFA had no detectable effect on NSP4 aa112–175 secretion (Fig. 4A, lane 2; Fig. 4B, lane 2). These results indicated that (i) the classical ER-Golgi apparatus-dependent vesicle-mediated secretion pathway is not involved in the secretion of NSP4 aa112–175, which is different from that of IL-8, and (ii) the complete inhibition of the secretion of NSP4 aa112–175 independently by NOC and Cyt.D shows that the release of NSP4 aa112–175 from cells utilizes a secretion pathway involving the microtubule and actin filament network. Similar experiments were performed to examine the trafficking pathway of VP6. VP6 secretion was blocked by BFA, but not by NOC or Cyt.D, indicating that in the early stage of infection VP6 was secreted through a classical vesicle-Golgi apparatus-dependent pathway distinct from the pathway followed by NSP4 aa112–175 (Table 1).
Cloning, expression, and purification of NSP4 aa112–175.
We next cloned the fragment of gene 10 that would code for NSP4 aa112–175 (G10aa112–175), inserted this cDNA into a baculovirus expression vector, and made a recombinant baculovirus, pFastBac/SA11-10aa112–175, that expresses NSP4 aa112–175. The expressed NSP4 aa112–175 from pFastBac/SA11-10aa112–175-infected Sf9 cells was purified from the medium by using an immunoaffinity column. The expressed and purified NSP4 aa112–175 was analyzed by SDS–15% PAGE and Western blotting with rabbit anti-NSP4pep120–147 polyclonal antibody for the comparison of comigration. The pFastBac/SA11-10aa112–175-expressed NSP4 aa112–175 comigrated with the NSP4 aa112–175 detected in the medium of pAc461/SA11-10-infected Sf9 cells and SA11-infected MA104 cells, suggesting that the detected cleavage product is the cytoplasmic tail of NSP4 containing aa112–175 (Fig. 5A). Purified NSP4 aa112–175 from medium of pFastBac/SA11-10aa112–175-infected Sf9 cells showed a high purity as stained with silver (Fig. 5B). The pure NSP4 aa112–175 was used in biological function tests and as a standard in semiquantitation of NSP4 aa112–175 secretion.
NSP4 aa112–175 increases [Ca2+]i in Sf9 cells when expressed endogenously and in HT29 cells when added exogenously.
Full-length SA11 NSP4 has been shown previously to increase [Ca2+]i in recombinant baculovirus-infected Sf9 cells when NSP4 is expressed endogenously (39). To determine if the cleavage product NSP4 aa112–175 increases [Ca2+]i in Sf9 cells when expressed endogenously, Sf9 cells were infected with the same MOI of recombinant baculovirus expressing either full-length SA11 NSP4 or NSP4 aa112–175. [Ca2+]i was measured by calcium imaging fluorescence microscopy at 36 hpi. When expressed endogenously, NSP4 aa112–175 increased [Ca2+]i to 4.3-fold over [Ca2+]i in wild-type baculovirus-infected Sf9 cells, while full-length NSP4 increased [Ca2+]i to 6.4-fold (Table 2). The [Ca2+]i levels in Sf9 cells expressing NSP4 aa112–175 and full-length NSP4 were not significantly different, but both were significantly higher than those in wild-type baculovirus-infected cells (P < 0.01, Student t test). Exogenously added SA11 NSP4 also can increase [Ca2+]i in HT29 cells (11), and so we next sought to determine if exogenously added purified NSP4 aa112–175 would mobilize intracellular calcium in these human cells. The purified NSP4 aa112–175 was added exogenously to HT29 cells, and [Ca2+]i was measured by calcium imaging fluorescence microscopy. The basal level of intracellular Ca2+ in HT29 cells was 100 ± 10 (standard error) nM. NSP4 aa112–175 (100 nM) increased [Ca2+]i to 560 ± 40 nM, 5.6-fold over the basal level, while full-length NSP4 (100 nM) increased [Ca2+]i to 690 ± 95 nM, a 7.0-fold increase. The calcium mobilization was transient, lasting approximately 1 to 2 min as previously reported (11, 46) (data not shown). The [Ca2+]i levels in HT29 cells increased by addition of NSP4 aa112–175 and by full-length NSP4 were not significantly different, but both were significantly higher than those in wild-type baculovirus-infected cells (P < 0.01, Student t test).
TABLE 2.
Intracellular [Ca2+]i in Sf9 cells endogenously expressing NSP4 aa112–175
| Baculovirus | Expressed protein | [Ca2+]i ± SE (nM) |
|---|---|---|
| pFastBac/SA11-10aa112–175 | NSP4 aa112–175 | 368 ± 114 (n = 3) |
| pAc461/SA11-10 | Full-length NSP4 | 552 ± 29 (n = 3) |
| Wild type | 86 ± 30 (n = 3) |
NSP4 aa112–175 expressed in baculovirus induces diarrhea in neonatal mice.
To examine if the NSP4 cleavage product could induce diarrhea in neonatal mice, 6- to 7-day-old CD1 mice were inoculated with the purified NSP4 aa112–175 intraperitoneally. Similar numbers of mice developed diarrhea when given the same amount of NSP4 aa112–175 or full-length NSP4 (Table 3). None of the mice given PBS had diarrhea. Although the outbred mice used in these experiments were less sensitive to the effects of the enterotoxin than those in previous experiments (3), these results indicate that truncated NSP4 aa112–175 contains the biologically active domain of NSP4.
TABLE 3.
Pathogenicity of NSP4 aa112–175 given intraperitoneally to 6- to 7-day-old CD1 micea
| Dose (nmol) | No. of mice with diarrhea/total inoculated (%)
|
|
|---|---|---|
| NSP4 aa112–175 | Full-length NSP4 | |
| 0.2 | 3/11 (27) | 3/11 (27) |
| 1.0 | 4/11 (36) | 4/11 (36) |
| 5.0 | 4/10 (40) | NDb |
Additionally, 11 mice were inoculated with 50 μl of PBS alone; none of them developed diarrhea.
ND, not done.
DISCUSSION
Rotavirus NSP4 has been shown previously to function as an enterotoxin (3, 11, 18). A model was proposed for the NSP4 enterotoxic pathway in which NSP4 binds to a putative receptor on intestinal (presumably crypt secretory) cells and triggers a signaling pathway which results in the increase of [Ca2+]i, which leads to stimulation of chloride secretion, resulting in diarrhea. Previously, NSP4 was characterized as an ER-specific transmembrane glycoprotein (14, 23). Therefore, one question related to the model has been: what is the source of functional, exogenous NSP4 in vivo? It had been hypothesized that NSP4 might be released by cell lysis or possibly secreted into the medium of cells, although this had not been detected previously. We report here the identification of a functional enterotoxin cleavage product of NSP4 in the media of both recombinant baculovirus-infected Sf9 cells and rotavirus-infected mammalian cells. This finding provides one possible explanation for the source of NSP4 that functions in pathogenesis. During rotavirus replication in the cells, NSP4 is synthesized, and some NSP4 molecules are cleaved and secreted from the infected cells. The released NSP4 cleavage product is then available to bind the putative receptor on the neighboring secretory cells to trigger the signaling pathway that results in diarrhea.
Release or secretion of a viral protein product into the medium is one approach used by viruses to exert their pathogenic effect on the host. Detection of the cleavage product of NSP4 in the medium early during virus infection indicates the availability of an extracellular biologically functional form of NSP4. The fact that NSP4 aa112–175 was detected in the medium as early as 4 hpi, while the viral structural proteins VP2, VP4, and VP7 and other nonstructural proteins as well as the ER transmembrane protein calnexin, which functions as a chaperone for NSP4, were not detected by 7.5 hpi, indicates that the NSP4 cleavage product in the medium was not derived by cell lysis but rather by an active secretion process. It will be of interest to sort out the cellular components and trafficking pathways involved in the secretion of NSP4 aa112–175 after it is cleaved from glycosylated NSP4, a transmembrane ER-specific protein. The N-terminal cleavage product was not detected in our experiments and may be rapidly degraded.
To investigate the possible trafficking pathway of NSP4 aa112–175, BFA, NOC, and Cyt.D (12, 13, 27) were used in the culture systems of SA11-infected MA104 cells and SA11-infected HT29 cells. To investigate the role of the Golgi apparatus in the secretion of NSP4 aa112–175, BFA, which is known to disrupt the Golgi apparatus and inhibit classical vesicle-mediated secretion, was used. Our results showed that, when Golgi apparatus-dependent IL-8 release was blocked by BFA as expected, NSP4 aa112–175 was efficiently released into the medium. BFA resistance by NSP4 aa112–175 secretion indicates that NSP4 aa112–175 does not require the Golgi apparatus for transportation out of cells. On the other hand, the microtubule-depolymerizing drug NOC and the actin filament-disrupting drug Cyt.D efficiently blocked NSP4 aa112–175 secretion into the medium while IL-8 release was not affected. The cell cytoskeleton provides a pathway between the cell nuclear membrane and cell surface, composed of a network of microtubules, intermediate filaments, and actin filaments. Our results that the secretion of NSP4 aa112–175 is independently blocked by NOC alone and by Cyt.D alone, but not by BFA, indicate that the actin filament and microtubule network is involved in NSP4 aa112–175 trafficking, while the classical ER-Golgi apparatus route is not involved. These results are of interest because rotavirus release from polarized epithelial cells has been reported elsewhere to occur by a nonclassical vesicular transport that bypasses the Golgi apparatus (22). The proposed binding domains of NSP4 with VP4 and VP7 are located on the C terminus from aa 112 to aa 175. The cleavage product NSP4 aa112–175 is now known to be released through the microtubule network. Recently, VP4 and VP7 have also been reported to reach the plasma membrane through the microtubule network in the early stage of viral infection, 3 hpi (34), but these proteins were not detected in the medium. In our study, VP4 and VP7 were not detected in the medium in conjunction with NSP4 aa112–175 as late as 9 hpi. VP6 was secreted into the medium but not by the same pathway as NSP4 aa112–175. Future studies will address if NSP4 aa112–175 interacts with the VP4 and VP7 that reach the plasma membrane and then how NSP4 aa112–175 might release the VP4 and VP7 onto the plasma membrane, while NSP4 aa112–175 itself is secreted into the medium. The kinetics of NSP4 aa112–175 release detected in our study and when VP4 and VP7 reached the plasma membrane (34) were both earlier than the release of virus particles. It is of interest that proteins or peptides released into the medium from rotavirus-infected cells have been recently shown to mobilize [Ca2+]i of other noninfected cells by a phospholipase C-dependent efflux of Ca2+ from the ER and by extracellular Ca2+ influx (4). It seems likely that secreted NSP4 is responsible for these effects.
In the presence of protease inhibitors, the 7K product was not seen in the culture medium or cell lysates. This result indicates that the production of the 7K band is protease dependent rather than being a product of internal initiation of translation at Met112. The protease(s) responsible for the cleavage between aa 111 and aa 112 is not yet clear. Over 100 sequences for NSP4 have been determined (7, 10, 20, 24, 25), and a comparison of NSP4 sequences from available rotavirus strains shows that sequences at aa 111 (mainly E; a few D, A, or T; and rarely R and K residues), 112 (M), 113 (I), and 114 (D, E) are highly conserved. However, no known protease recognizes these specific amino acids, and so the responsible protease may cleave in a sequence-independent manner. Secretion of the 7K protein from the avirulent OSU-a virus shows that mutations in the enterotoxin domain do not affect secretion although they do alter diarrhea induction activity (46). Pulse-chase experiments to demonstrate a precursor product relationship between [35S]Met-NSP4 and the 7K band were not successful. This may be due to a problem in sensitivity of detecting the labeled 7K cleavage product that contains only three methionines while the full-length NSP4 contains nine methionines. Alternatively, there may be two pools of NSP4 in cells and only one of these serves as a precursor pool. These possibilities will be examined in future studies.
Our data demonstrate that NSP4 aa112–175 expressed endogenously is capable of increasing [Ca2+]i mobilization 4.3-fold over the level of wild-type infection in Sf9 cells. Exogenous addition of NSP4 aa112–175 to HT29 cells also increases intracellular [Ca2+]i mobilization to 5.6-fold over the basal level. These results are consistent with previous reports on endogenous expression (39) and exogenous addition (11) of full-length NSP4 mobilizing [Ca2+]i. Purified NSP4 aa112–175 possesses a potential to induce diarrhea in neonatal mice similar to that of full-length NSP4. These properties of NSP4 aa112–175 demonstrate that this cleavage product functions, like full-length NSP4, as an enterotoxin. In fact, this form may be the biologically relevant form of NSP4. In passive protection experiments in mice, antibody to NSP4 aa112–175 significantly reduces the occurrence and severity of diarrhea in pups challenged with rotavirus (C. Q.-Y. Zeng, M. Zhang, M. E. Conner, and M. K. Estes, unpublished data). This soluble, extracellular cleavage product of the enterotoxin could be responsible for directly or indirectly activating the enteric nervous system that has been reported to have a role in rotavirus diarrhea (29).
ACKNOWLEDGMENTS
This work was supported by NIH grant DK 30144 (M. K. Estes) and Texas ATP grant 004949-062 (M. K. Estes and A. P. Morris).
REFERENCES
- 1.Au K S, Chen W K, Burns J W, Estes M K. Receptor activity of rotavirus nonstructural glycoprotein NS28. J Virol. 1989;63:4553–4562. doi: 10.1128/jvi.63.11.4553-4562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augeron C, Laboisse C L. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984;44:3961–3969. [PubMed] [Google Scholar]
- 3.Ball J M, Tian P, Zeng C Q-Y, Morris A P, Estes M K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 4.Brunet J-P, Cotte-Laffitte J, Linxe C, Quero A-M, Géniteau-Legender M, Servin A. Rotavirus infection induces an increase in intracellular calcium concentration in human intestinal epithelial cells: role in microvillar actin alteration. J Virol. 2000;74:2323–2332. doi: 10.1128/jvi.74.5.2323-2332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns J W, Krishnaney A A, Vo P T, Rouse R V, Anderson L J, Greenberg H B. Analyses of homologous rotavirus infection in the mouse model. Virology. 1995;207:143–153. doi: 10.1006/viro.1995.1060. [DOI] [PubMed] [Google Scholar]
- 6.Casola A, Estes M K, Crawford S E, Ogra P L, Ernst P B, Garofalo R P, Crowe S E. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology. 1998;114:947–955. doi: 10.1016/s0016-5085(98)70314-2. [DOI] [PubMed] [Google Scholar]
- 7.Ciarlet M, Liprandi F, Conner M E, Estes M K. Species specificity and interspecies relatedness of NSP4 genetic groups by comparative NSP4 sequence analysis of animal rotaviruses. Arch Virol. 2000;145:371–383. doi: 10.1007/s007050050029. [DOI] [PubMed] [Google Scholar]
- 8.Collins J E, Benfield D A, Duimstra J R. Comparative virulence of two porcine group-A rotavirus isolates in gnotobiotic pigs. Am J Vet Res. 1989;50:827–835. [PubMed] [Google Scholar]
- 9.Crawford S E, Labbé M, Cohen J, Burroughs M H, Zhou Y J, Estes M K. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J Virol. 1994;68:5945–5952. doi: 10.1128/jvi.68.9.5945-5952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunliffe N A, Das B K, Ramachandran M, Bhan M K, Glass R I, Gentsch J R. Sequence analysis demonstrates that VP6, NSP1 and NSP4 genes of Indian neonatal rotavirus strain 116E are of human origin. Virus Genes. 1997;15:39–44. doi: 10.1023/a:1007958914141. [DOI] [PubMed] [Google Scholar]
- 11.Dong Y, Zeng C Q-Y, Ball J M, Estes M K, Morris A P. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-triphosphate production. Proc Natl Acad Sci USA. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott G, O'Hare P. Intracellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 13.Elliott G, O'Hare P. Herpes simplex type 1 tegument protein VP22 induces the stabilization and hyperacetylation of microtubules. J Virol. 1998;72:6448–6455. doi: 10.1128/jvi.72.8.6448-6455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ericson B L, Graham D Y, Mason B B, Estes M K. Identification, synthesis, and modifications of simian rotavirus SA11 polypeptides in infected cells. J Virol. 1982;42:825–839. doi: 10.1128/jvi.42.3.825-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estes M K. Rotavirus and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1625–1665. [Google Scholar]
- 16.Estes M K, Crawford S E, Penaranda M E, Petrie B L, Burns J W, Chan W-K, Ericson B, Smith G E, Summers M D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987;61:1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estes M K, Graham D Y, Gerbra C P, Smith E M. Simian rotavirus SA11 replication in cell cultures. J Virol. 1979;31:810–815. doi: 10.1128/jvi.31.3.810-815.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estes M K, Morris A P. A viral enterotoxin: a new mechanism of virus-induced pathogenesis. In: Paul P S, Francis D H, editors. Mechanism in the pathogenesis of enteric diseases. 2nd ed. New York, N.Y: Kluwer Academic/Plenum Publishers; 1999. pp. 73–82. [PubMed] [Google Scholar]
- 19.Gooding L R. Virus proteins that counteract host immune defenses. Cell. 1992;71:5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- 20.Horie Y, Masamune O, Nakagomi O. Three major alleles of rotavirus NSP4 proteins identified by sequence analysis. J Gen Virol. 1997;78:2341–2346. doi: 10.1099/0022-1317-78-9-2341. [DOI] [PubMed] [Google Scholar]
- 21.Horie Y, Nakagomi O, Koshimura Y, Nakagomi T, Suzuki Y, Oka Y, Sasaki S, Matsuda Y, Watanabe S. Diarrhea induction by rotavirus NSP4 in homologous mouse model system. Virology. 1999;262:398–407. doi: 10.1006/viro.1999.9912. [DOI] [PubMed] [Google Scholar]
- 22.Jourdan N, Maurice M, Delautier D, Quero A M, Servin A L, Trugnan G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vescular transport that bypasses the Golgi apparatus. J Virol. 1997;71:8268–8278. doi: 10.1128/jvi.71.11.8268-8278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabcenell A K, Atkinson P H. Processing of the rough endoplasmic reticulum membrane glycoproteins of rotavirus SA11. J Cell Biol. 1985;101:1270–1280. doi: 10.1083/jcb.101.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkwood C D, Gentsch J R, Glass R I. Sequence analysis of the NSP4 gene from human rotavirus strains isolated in the United States. Virus Genes. 1999;19:113–122. doi: 10.1023/a:1008123123238. [DOI] [PubMed] [Google Scholar]
- 25.Kirkwood C D, Palombo E A. Genetic characterization of the rotavirus nonstructural protein, NSP4. Virology. 1997;236:258–265. doi: 10.1006/viro.1997.8727. [DOI] [PubMed] [Google Scholar]
- 26.Kotwal G J, Isaacs S N, McKenzie R, Frank M M, Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990;250:827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- 27.Lippincott-Schwartz J, Donaldson J G, Schweizer A, Berger E G, Hauri H P, Yuan L C, Klausner R D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 28.Lomas D A, Evans D L, Upton C, McFadden G, Carrell R W. Inhibition of plasmin, urokinase, tissue plasminogen activator, and C1S by a myxoma virus serine proteinase inhibitor. J Biol Chem. 1993;268:516–521. [PubMed] [Google Scholar]
- 29.Lundgren O, Peregrin A T, Persson K, Kordasti S, Uhnoo I, Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287:491–495. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 30.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 31.McAdaragh J P, Bergeland M E, Meyer R C, Johnshoy M W, Stotz I J, Benfield D A, Hammer R. Pathogenesis of rotaviral enteritis in gnotobiotic pigs: a microscopic study. Am J Vet Res. 1980;41:1572–1581. [PubMed] [Google Scholar]
- 32.Mirazimi A, Nilsson M, Svensson L. The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro. J Virol. 1998;72:8705–8709. doi: 10.1128/jvi.72.11.8705-8709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris A P, Scott J, Ball J M, Zeng C Q-Y, O'Neal W, Estes M K. NSP4 enterotoxin elicits age-dependent diarrhea and calcium-mediated iodide influx into intestinal crypts of cystic fibrosis mice. Am J Physiol. 1999;277:G431–G444. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- 34.Nejmeddine M, Trugnan G, Sapin C, Kohli E, Svensson L, Lopez S, Chon J. Rotavirus spike protein VP4 is present at the plasma membrane and is associated with microtubules in infected cells. J Virol. 2000;74:3313–3320. doi: 10.1128/jvi.74.7.3313-3320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou W J, Cameron P H, Thomas D Y, Bergeron J J M. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- 36.Sheth R, Anderson J, Sato T, Oh B, Hempson S J, Rollo E, Mackao E R, Shaw R D. Rotavirus stimulates IL8 secretion from cultured epithelial cells. Virology. 1996;22:251–259. doi: 10.1006/viro.1996.0374. [DOI] [PubMed] [Google Scholar]
- 37.Tian P, Ball J M, Zeng C Q-Y, Estes M K. The rotavirus nonstructural glycoprotein NSP4 possesses membrane destabilization activity. J Virol. 1996;70:6973–6981. doi: 10.1128/jvi.70.10.6973-6981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian P, Estes M K, Hu Y, Ball J M, Zeng C Q-Y, Schilling W P. The rotavirus nonstructural glycoprotein NSP4 mobilizes calcium from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian P, Hu Y, Schilling W P, Lindsay D A, Eiden J, Estes M K. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J Virol. 1994;68:251–257. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Upton C, Macen J L, Schreiber M, McFadden G. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991;184:370–382. doi: 10.1016/0042-6822(91)90853-4. [DOI] [PubMed] [Google Scholar]
- 41.Ward L A, Rosen B I, Yuan L, Saif L J. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77:1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 42.Wolff B, Burns A R, Middleton J, Rot A. Endothelial cell “memory” of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med. 1998;188:1757–1762. doi: 10.1084/jem.188.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Z, Delgado R, Xu L, Todd R F, Nabel E G, Sanchez A, Nabel G J. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- 44.Zauli G, Gibellini D. The human immunodeficiency virus type-1 (HIV-1) Tat protein and Bcl-2 gene expression. Leuk Lymphoma. 1996;23:551–560. doi: 10.3109/10428199609054864. [DOI] [PubMed] [Google Scholar]
- 45.Zeng C Q-Y, Labbé M, Cohen J, Prasad B V V, Chen D, Ramig R F, Estes M K. Characterization of rotavirus VP2 particles. Virology. 1994;201:55–65. doi: 10.1006/viro.1994.1265. [DOI] [PubMed] [Google Scholar]
- 46.Zhang M, Zeng C Q-Y, Dong Y, Ball J M, Saif L J, Morris A P, Estes M K. Mutations in rotavirus nonstructural glycoprotein NSP4 are associated with altered virus virulence. J Virol. 1998;72:3666–3672. doi: 10.1128/jvi.72.5.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]