Abstract
The scarcity of reliable devices for diagnosis of Animal African trypanosomiasis (AAT) presents a limitation to control of the disease. Existing high-sensitivity technologies such as PCR are costly, laborious, time-consuming, complex, and require skilled personnel. Hence, utilisation of most diagnostics for AAT is impracticable in rural areas, where the disease occurs. A more accessible point-of-care test (POCT) capable of detecting cryptic active infection, without relying on expensive equipment, would facilitate AAT detection. In turn, early management, would reduce disease incidence and severity. Today, several ongoing research projects aim at modifying complex immunoassays into POCTs. In this context, we report the development of an antigen (Ag) detection sandwich ELISA prototype for diagnosis of T. congolense infections, which is comprised of nanobody (Nb) and monoclonal antibody (mAb) reagents.
The Nb474H used here, originated from a past study. Briefly, the Nb was engineered starting from mRNA of peripheral blood lymphocytes of an alpaca immunized with soluble lysate of Trypanosoma congolense (TC13). T. congolense glycosomal fructose-1,6-bisphosphate aldolase (TcoALD) was discovered as the cognate Ag of Nb474H. In this study, splenocytes were harvested from a mouse immunized with recombinant TcoALD and fused with NS01 cells to generate a hybridoma library. Random screening of the library on TcoALD retrieved a lone binder, designated IgM8A2. Using Nb474H as Ag-capture reagent in combination with the IgM8A2 monoclonal antibody Ag-detection reagent resulted in a tool that effectively detects native TcoALD released during infection by T. congolense parasites.
Hitherto, development of POCT for detection of active trypanosome infection is elusive. The Nanobody/Monoclonal Antibody (Nb/mAb) “hybrid” sandwich technology offers prospects for exploration, using the unique specificity of Nb as a key determinant in Ag capturing, while using the versatility of monoclonal Ab to adapt to various detection conditions.
Author summary
Animal African trypanosomiasis is a parasitic wasting disease, which mainly affects livestock of poor households in remote rural areas of sub-Saharan Africa, Asia and Latin America. Affordable interventions are primary to eradication campaigns. The latter is mandated to the Pan African Tsetse and Trypanosomiasis Eradication Campaign (PATTEC). PATTEC conducts activities including mass screening of human population for Human African trypanosomiasis (HAT) and treating positive cases. This strategy has dramatically reduced cases of HAT over the last two decades. Highly sensitive and specific current tests for AAT are technically demanding, confining their use to well-equipped reference laboratories in urban centres. Inaccessibility of tests for AAT by the locals living in remote areas, hinders case finding and delays treatment intervention, eventually perpetuating disease transmission and severity. Hence, the aim of our ongoing work is to ultimately convert a complex laboratory-based test for AAT caused by T. congolense, developed earlier by our research team, into a simple hand-held device suitable for use in the field. The current test format employs a nanobody-monoclonal antibody sandwich combination to detect T. congolense infections, through detection of trypanosome Fructose-1,6-bisphosphate aldolase that is present in the blood of infected animals. The diagnostic capability of the assay was successfully demonstrated on experimental samples.
Introduction
African trypanosomiasis (AT) is a devastating disease of humans and animals. Elimination of Human African Trypanosomiasis (HAT) of sleeping sickness, as a public health threat, is being targeted by the year 2030 [1]. The realization of this elimination would be a milestone in the fulfilment of the United Nations’s Sustainable Development Goals of eradicating poverty, ending hunger, and promoting good-health and well-being of the people [2]. Persistence of AT has affected development in communities where the disease occurs. Whereas trypanosome species responsible for HAT are restricted to Africa, some of the species causing the Animal African Trypanosomiasis (AAT) are prevalent far beyond the borders of Africa [3,4]. Hence, AT cases have been reported in South America [5], the Mediterranean Europe [6], and Asia [7,8] qualifying it among the most widely spread animal diseases in the world. Generally, AT is responsible for direct loss of lives [9,10], and it has a negative impact on the economy [11]. Employing chemicals to control the tsetse fly vector for AT, has raised environmental concerns including indiscriminate killing of non-target animals [12]. While elimination of AT would relieve the affected communities from the disease burden, it is both complex and highly demanding. An integrated approach involving vector control, case detection and treatment, has seen a near-elimination of gambiense-HAT cases in the disease endemic regions [13] holding promise for a future elimination altogether.
Deployment of accessible low-cost diagnostics that readily reveal trypanosomes in the host, as well as reservoir species, would be valuable for guiding treatment decision, monitoring control program and surveillance. Unfortunately, current tests for trypanosomiasis do not meet the ASSURED criteria required by the World Health Organization [14]. Largely, the existing tests for trypanosomes are costly, less sensitive, less specific, inherently complex, laborious and time-consuming, require qualified personnel, and rely on electricity making them unsuitable for use in resource-deprived rural communities. Deployment of reliable point-of-care tests (POCT) [15] for AAT on farms, would enable case finding and allow prompt treatment intervention. The only field POCT for AAT are card agglutination test (CATT/T. evansi) for detection of T. evansi [16], and VerY Diag (CEVA), which is a Lateral Flow Assay (LFA) for multiplex detection of T. congolense and T. vivax species. While these tests have aided epidemiological investigations of the target trypanosome species, they are Ab detection tests incapable of differentiating active infections from past exposures, hence restricting their scope. Where the test result is needed to inform treatment decisions, deployment of a confirmatory test is crucial. Microscopy and polymerase chain reaction (PCR) are valuable tests employed by reference laboratories to confirm AAT. However, microscopy is ineffective when specimen examination is delayed, time-consuming, laborious, not always field adapted, and it requires technical expertise. PCR on the other hand, is a high throughput sensitivity test; however, the technique requires reliable access to electricity and it is technically demanding. Therefore, conventional PCRs are not ideally suited for field operations. In contrast, Ag detection tests are amiable tool of choice for development of POCT for trypanosomiases, because they confirm active infection as well as drug failure. The deployment of Ag detection POCT has facilitated the control of malaria [17] and COVID-19 [18] among other infectious diseases. However, past efforts to develop similar mAb-based Ag detection tests for trypanosomiasis have been futile [19]. The mAb-based Ag detection test prototype for trypanosomiasis suffers from low sensitivity, mostly attributed to low Ag loads in specimens resulting from sequestration of Ag in immune complex, and an inherently low pathogen loads [20]. The first description of Nb technology 30 years ago by Hamers-Casterman et al [21] reinvigorated discovery research, targeting the technology, for the development of Ag detection tests for AAT [22,23]. Typically, Nbs possess a relatively extended complementarity determining region three (CDR3), compared to conventional Abs [24], thus allowing their preferential bindings to cryptic epitopes [25,26]. The fact that Nbs can bind Ag-Ab immune complexes through a unique epitope recognition, motivated its exploration as a tool of choice for outwitting low sensitivity of Ag-detection tests, caused by parasite-induced host Abs interference.
Whereas developments of several Nb-based diagnostic devices for trypanosomiasis were initiated [22,23,27], commercialization of these tests has not yet been achieved for practical reasons. Firstly, some of these Nb-based devices are detecting their respective target Ags in a homologous sandwich fashion [22] making translation into an Ag capture LFA without loss of sensitivity impossible. Indeed, in an Ag detection LFA, the target Ag is pre-complexed with high amounts of detection Nbs and the complex is driven by capillary action to a line of printed Ag-capture Nbs. In a homologous sandwich format, the Ag-capture Nbs would compete for the same binding sites with the already-couped detection Nb. In this case, most of the complexes would escape capture, causing a drastic reduction in signal intensity and consequently lowering the assay sensitivity. A second limitation affecting development of Nb-based test devices is the small size of Nbs, which often compromise conjugation to Gold (Au) nanoparticles [28]. To mitigate these limitations, but still exploit the highly specific Nb-capturing capacity, we explored here a Nb/mAb “hybrid” heterologous sandwich system. As our previous research has shown that trypanosome aldolase is a target that allows for a highly sensitive detection of active trypanosome infection [22], the target of the new test format was kept the same. Hence, a mouse mAb (IgM8A2) was generated against TcoALD and integrated in the ELISA to substitute the previously described Nb detection reagent, thereby achieving a Nb474H/IgM8A2 “hybrid” sandwich setup. The capability of the “hybrid” sandwich system to detect native TcoALD was demonstrated.
Methods
Ethics statement
Permission for the use of mice was granted by the Ghent University Global Campus Institutional Animal Use and Care committee (Project number: IACUC 2022–014).
Mice
Eight-weeks old female mice BALB/c and C57BL/6N were procured from the Korean Animal Technology (KOATEC) Co. Ltd, Republic of Korea. The animals were acclimatized for a fortnight in a facility at the Biomedical Research Centre, the Ghent University Global Campus, Republic of Korea. While the BALB/c mice were used for generation of hybridoma, C57BL/N6 were used for culturing trypanosomes.
Trypanosomes, trypanosome lysate, and sera
Trypanosomes used in the study were Trypanosoma congolense TC13, T. b. brucei An Tat 1.1E, T. vivax ILRAD 700, and T. evansi STIB 816. Trypanosoma congolense was propagated, purified, and homogenized into lysate according to the protocol described elsewhere [22]. Briefly, aliquots of frozen trypanosome stocks (50 μl) were each reconstituted in 1xPBS (500 μl). The viability of cells was checked by wet smear and live parasites were quantified by a haemocytometer (Improved Neubauer China, Cat. No. 1103). For infection, 200 μl solution containing 5000 trypanosomes (T. congolense, T. b. brucei, T. vivax or T. evansi) was inoculated per mouse via intraperitoneal route. Mice were euthanized by CO2 gas at the first peak of parasitaemia (1x108 trypanosomes/ml) and bled by cardiac puncture using a one ml syringe (Kovax-syringe, Korea Vaccine Co. Ltd) prefilled with heparin solution (40 μl). Obtained blood was pooled in a 15 ml centrifuge tube followed by centrifugation (1224 x g, 10 mins, 22°C). The buffy coat was collected and passed through a PD-10 column (Cytiva, Cat. No. 17043501) packed with DEAE Sepharose Fast Flow matrix (Cytiva, Cat. No. 17070901). The DEAE Sepharose Fast Flow matrix was pre-equilibrated with Phosphate Saline (PS) solution (NaCl, 36.5 mM; NaH2PO4, 3.6 mM; Na2HPO4, 59.5 mM) at either pH 7.5 (for T. congolense and T. vivax), or pH 8.0 (for T. b. brucei and T. evansi). Trypanosomes were eluted from the column using PS solution containing D-Glucose (88.8 mM) at either pH 7.5 or pH 8.0 for the respective species of trypanosomes. Eluted cells were centrifuged (1736 x g, 15 mins, 22°C) and obtained pellet was dissolved in 1xPBS (1000 μl). The lysate was prepared by resuspending the pellet followed by three rounds of freeze and thaw cycles alternating between -80°C and thaw 37°C, respectively. While on ice, the partially lysed cells were homogenized by sonication (Ultrasonic Processor K-SuperSonic KSS-N900DT, Korea Process Technology Co., Ltd.), and centrifuged (27,237 x g, 30 mins, 4°C). The supernatant was collected and the concentration of protein in the soluble lysate was estimated by a NanoDrop spectrophotometer and stored at—20°C.
Sera used in the experiment were harvested from uninfected (naïve) as well as trypanosome-infected mice. In brief, mice were bled into a 1.5 ml centrifuge tube and blood was stored at 4°C for 2 days followed by centrifugation (9425 x g, 10 mins, 4°C). Afterwards, sera were harvested by a micropipette and stored at -20°C.
Recombinant proteins
All recombinant proteins used in this study originated from past studies. Nb474 fused with hisx6 peptide tag (Nb474H), Nb474 fused with both hisx6 and haemagglutinin (HA) peptide tags (Nb474HA), TcoALD, T. vivax aldolase (TvALD), and Leishmania mexicana aldolase (LmALD) originated from [22]; T. congolense pyruvate kinase (TcoPYK) from [23]; and T. evansi enolase (TevENO) and Nb77 fused with hisx6 peptide tag (Nb77H) from [27]. These proteins were produced, and purified by nickel affinity chromatography. The levels of production and purity of the recombinant proteins were analysed by SDS-PAGE. The concentration of the purified protein in the sample was measured by a NanoDrop and stored, in aliquots, at -20°C.
Mice immunization and analysis of immune response
Prior to immunization, blood (2.5 μl) was collected by tail-snip from the BALB/c mice (n = 3), and it was diluted (1/200) in 1xPBS. The diluted blood samples were stored at -20°C until analysed by Ab-ELISA. On the day of immunization, TcoALD was diluted to a desired concentration in a sterile distilled water and then emulsified in Gerbu Adjuvant (Biotechnik GmbH, Cat. No. 3001-1mL) following the manufacturer’s guideline (Table 1). The Ag preparation was successively administered subcutaneously six times into scruff of the neck of the mice. Two days after the last booster shot, blood (2.5 μl) was collected from each of the immunized mice and it was diluted (1/200) in 1xPBS. The Ab levels in the blood sample preparations was analysed by ELISA.
Table 1. The immunization schedules per mouse.
| Day | 0 | 14 | 21 | 28 | 29 | 30 |
|---|---|---|---|---|---|---|
| TcoALD (μg) | 100 | 50 | 50 | 50 | 50 | 50 |
| Adjuvant (μl) | 40 | 20 | 20 | 20 | - | - |
| Distilled H 2 O (μl) | 34 | 17 | 17 | 17 | 37 | 37 |
| Total dose (μl) | 100 | 50 | 50 | 50 | 50 | 50 |
Construction of hybridoma library
A hybridoma library was generated using the ClonaCell-HY Hybridoma Kit (STEMCELL Technologies, Cat. No. 03800). A mouse with the highest Ab response was euthanized on day eight post last booster shot, and the spleen was harvested in 5 mL ClonaCell-HY Medium B (STEMCELL Technologies, Cat. No. 03802) followed by pulverisation in Medium B (5 ml) using a gentleMACS Dissociator (Miltenyi Biotec, Cat. No. 130-093-235). The macerated cell suspension was sieved through a 70 μm strainer (SPL cell strainer, Cat. No. 93070). The filtrate was diluted (1/6) in Medium B followed by centrifugation (316 x g, 10 mins, 22°C). This step was repeated twice. The washed cell pellet was resuspended in Medium B (25 ml) and cells were enumerated by a haemocytometer. Next, 1x108 splenocytes were obtained for fusion with NS01 parental myeloma. For preparation of NS01 cells, the Medium A passaged cells (1x107) were inoculated into a T-250 flask prefilled with Medium A (148 ml) a day before fusion. The cell suspension was later evenly distributed over 15 culture dishes (SPL Life Sciences Co., Ltd. Cat. No. 20100) followed by an overnight incubation in a humidified CO2 incubator (5% CO2, 37°C). On the day of fusion, the cells were harvested by centrifugation (316 x g, 10 mins, 22°C) when they were in early-mid log phase (8.2x104 cells/ml). The pellet was resuspended in Medium B (30 ml) followed by centrifugation (316 x g, 10 mins, 22°C). The washing step was repeated twice and the pellet was resuspended in Medium B (25 ml) followed by enumeration using a haemocytometer. For fusion, the splenocytes (9.5x107cells in 19 ml) were mixed with the NS01 cells (1.6 x107 cells in 25 ml) in a 50 ml centrifuge tube followed by centrifugation (316 x g, 10 mins, 22°C). The pellet was disrupted by gentle tapping. ClonaCell-HY PEG (1 ml) was added dropwise to the pellet by a one ml sterile transfer pipette (VWR, Cat. No. VWRI612-1747) over a period of one minute without stirring. The cells were resuspended by the tip of a serological pipette for one minute by a continuous gentle stirring. Then Medium B (4 ml) was dispensed, dropwise over a period of four minutes, into the fusion mixture while stirring in-between the additions until all the solution was ejected. Additional Medium B (10 ml) was slowly added to the fusion mixture followed by incubation (15 mins, 37°C) in a water bath. Afterwards, Medium A was added twice to the cells (starting with 30 ml and then 40 ml), and each of these additions was followed by centrifugation (316 x g, 7 mins, 22°C). The supernatant was carefully drained after the last wash leaving behind the pellet, which was then slowly resuspended in ClonaCell-HY Medium C (10 ml) and transferred into a T-75 cm2 cell culture flask (CellStar cell culture flask Greiner bio one, Cat. No. 658170) prefilled with Medium C (20 ml). The cell mixture was incubated in a humidified incubator (5% CO2, 16 hrs, 37°C). The following day, fused cell suspension was transferred into a 50 ml centrifuge tube and centrifuged (316 x g,10 mins, 22°C). Obtained pellet was resuspended in Medium C (12 ml), and transferred into ClonaCell-HY Medium D (90 ml). The two solutions were mixed by gently inverting the bottle several times followed by incubation (5 mins, 37°C). The semi-solid medium was distributed (10 ml/plate) over ten 100 mm cell culture dishes. The seeded dishes were arranged in large square plates for a prolonged incubation. Each of the large square plates received at most three seeded dishes and a fourth dish filled with water only to provide local humidity. The assembled cultures were incubated for 10 days, without disturbance, in a humidified CO2 incubator (5% CO2, and 37°C).
Hybridoma library screening and isotype characterization
The hybridoma library was screened for anti-TcoALD mAb producing clones when the colonies were visible on the semi-solid medium eleven days after plating. During screening, a colony was drawn into a 10 μl micropipette tip and subsequently inoculated into a well of a 96-well cell culture (SPL Life technologies, Cat. No. 31096) prefilled with Medium E (100 μl). When all the wells were seeded, additional Medium E (50 μl) was dispensed into each of the seeded wells followed by incubation in a humidified CO2 incubator (4 days, 37°C, 5% CO2). On day four, supernatant (50 μl) was harvested per mini-culture and probed by ELISA for the presence of mAbs against TcoALD. For the ELISA, Half-Area ELISA plate (Corning, Cat. No. 3690) was coated with TcoALD (0.25 μg/well) diluted in 1xPBS for an overnight at 4°C. The wells were washed thrice with PBS-T. Blocking solution, 5% skimmed milk (Oxoid, Cat No: LP00338) in 1xPBS, was added (160 μl/well) to washed wells followed by incubation (2 hrs, 22°C). The blocking solution was discarded and the wells were washed thrice. Thereafter, the mini-culture supernatant (50 μl), previously harvested, was added into each of the wells followed by incubation (1 hr, 22°C). The supernatant was discarded and the wells were washed thrice. Goat anti-mouse immunoglobulin (Ig) horseradish peroxidase (HRP) (SouthernBiotech SBA Clonotyping System-HRP, Cat. No. 5300–05) diluted (1/1000) in 2.5% milk solution was added into the wells (50 μl/well) followed by incubation (1 hr, 22°C). The unbound antibodies were washed four times. Thereafter, 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate (Sigma, T0440-100 ml) was added (50 μl/well) followed by incubation (15 mins, 22°C). The reaction was stopped by adding 1M H2SO4 (50 μl/well).
The retrieved positive clones were subjected to a secondary screening to identify potential “false positive” clones producing mAbs against either hisx6-tag on recombinant TcoALD immunizing antigen, or E. coli expression host proteins, which co-purified with the recombinant TcoALD. For this ELISA, all the positive clones were probed for binding unpurified recombinant TcoPYK and Nb474H crude protein extracts (both proteins are fused with hisx6-tag and were not purified from the crude E. coli lysate), or purified Nb474H protein (a protein fused with hisx6-tag), while the purified recombinant TcoALD served as a positive control.
The clones that bound TcoALD specifically were next (iso)typed (SouthernBiotech SBA Clonotyping System-HRP, Cat. No. 5300–05). For (iso)typing, TcoALD was coated (0.25 μg/well) followed by addition of supernatants (50 μl/well). Each of the supernatants was probed with assortment of goat anti-mouse HRP conjugates against mouse IgG1, IgG2a, IgG2b, IgG3 and IgM.
Finally, a third screening was done to verify if the characterized mAbs would detect TcoALD, TcoPYK or 1xPBS in ELISA plate coated with Nb474H or Nb77H as capturing reagents.
Production and purification of monoclonal antibody
The production of the only mAb clone (IgM8A2) that showed specific binding to TcoALD was upscaled after adapting to Hybridoma-Serum Free Medium (SFM) (gibco, Cat. No. 12045–084) and assessing its binding to T. congolense lysate (TcoLys). During adaptation, the IgM8A2 expressing clone cultured in Medium E was progressively exposed to Medium A. For this, Medium E was gradually reduced, from the Medium E-A mixture, by 25% until attaining 0% while Medium A was increased by 25% until reaching 100%. At the final stage of adaptation to 100% Medium A, the culture (2 ml) was first pelleted by centrifugation (316 x g, 10 mins, 22°C) and then resuspended in 100% Medium A (3 ml). The cell suspension was dispensed into a well on cell culture plate (SPL Life technologies, Cat. No. 30006) and incubated in a humidified CO2 incubator (37°C, 5% CO2). Afterwards, cells in Medium A were gradually adapted to SFM. As such, for complete adaptation to SFM, two cultures of 3 ml each, in a mixture of Medium A (25%) and SFM (75%) were pooled in a 15 ml centrifuge tube followed by centrifugation (316 x g, 10 mins, 22°C). The pellet was resuspended in SFM (6 ml) and the cell suspension was dispensed into two culture dishes (3 mL/dish) prefilled with SFM (12 ml/plate) followed by incubation in a humidified CO2 incubator (37°C, 5% CO2). At 1.74x106/ml cell density, the culture (15 ml) was inoculated into a vented tissue flask-T 175 prefilled with SFM (30 ml) and incubated in a humidified incubator (37°C, 5% CO2). On day 4 post-inoculation when the culture has attained a stationary growth phase, characterized by yellowish coloration of the medium, the supernatant was harvested and centrifuged (316 x g, 10 mins, 22°C) followed by storage at -20°C. The purification of mAb was done by AKTA Start (Cytiva) employing UNICORN start 1.1 (Build1.1.0.2) software. During purification, 50 mL of the supernatant was defrosted and clarified by filtration through a 0.2 μM sieve (Sartorius Minisart, S6534). The filtrate was dialysed in 1M (NH4)2SO4, pH 7.5 (500 ml) at 4°C using a dialysis tubing (Spectra/Por Dialysis Membrane Standard RC tubing) of pore size 6-8kD. The dialysate (50 mL) was loaded (0.5ml/min) onto a HiTrap IgM purification column (Cytiva, Cat. No. 17-5110-01), which was pre-equilibrated with five column volume of the binding buffer [20 mM Sodium Phosphate, 1M (NH4)2SO4, pH 7.5]. Thereafter, copious quantity of binding buffer was passed through the column to wash-off the unbound impurities until A280 returned to baseline. The retained Ab was eluted out of the column by washing with elution buffer (20 mM Sodium Phosphate Buffer, pH 7.5) at 0.5 ml/min. The eluted Ab was dialysed in 1xPBS pH 7.4 at 4°C. The concentration of Ab in the sample was estimated by a NanoDrop spectrophotometer and the sample was stored at -20°C.
Biotin-labelling of monoclonal antibody
The IgM8A2 monoclonal antibody was labelled with biotin, using the EZ-Link Sulfo-NHS-Biotin kit (Thermo Scientific, Cat. No. 21217). Biotin powder (4 mg) in the kit was reconstituted in distilled water (500 μl) and the solution (40 μl) was added into a IgM8A2 solution (1000 μl), which was at a concentration of 1 mg/ml. The mixture was incubated on ice for an overnight. Thereafter, the biotin-labelled IgM8A2 (IgM8A2-B) was dialysed in 1xPBS pH 7.4 using Slide-A-Lyzer 3.5 K Dialysis Cassettes (Thermo Scientific, Cat. No. 66330). The concentration of IgM8A2-B in the solution was measured by a NanoDrop spectrophotometer and the sample was stored at -20°C. The success of IgM8A2 biotin-labelling was ascertained by ELISA employing the HRP Streptavidin (Strep-HRP) (Biolegend, Cat. No. 405210 / 1 ml) and TMB reporter system.
Assessing binding properties of retrieved monoclonal antibody
Binding of IgM8A2 to a denatured aldolase was assessed by western blot and an indirect ELISA. For western blot, aldolase was resolved by SDS-PAGE under denaturing condition and electroblotted onto nitrocellulose membrane (Thermo scientific, Cat. No. 88018). The blotted protein was probed with a solution of an unlabelled IgM8A2 (4.47 μg/ml) in 2.5% milk followed by goat anti-mouse IgM HRP (SouthernBiotech, Cat. No. 5300–05) diluted (1/1000) in 2.5% milk. The retention of conjugated anti-mouse IgM by IgM8A2 was revealed by incubation in HRP substrate solution [10 ml 99% methanol (10 ml), 4-Chloro-1-napthol powder (45 mg), 1xPBS (45 ml), and 30% hydrogen peroxide (100 μl)]. An indirect ELISA was used to investigate the binding of IgM8A2 to a heat-denatured aldolase. Briefly, recombinant TcoALD or TvALD was diluted to 200 μg/ml, and aliquoted (120 μl/vial) followed by incubation at 55°C for different time lengths (0, 10, 20, 30, 40, 50, and 60 mins). The samples were coated (10 μg/well) on ELISA plate for an overnight at 4°C. The coating was discarded and wells were blocked with 5% milk solution (160 μl/well) for 2 hrs at 22°C. The wells were washed thrice and IgM8A2 diluted to 4.74 μg/ml in blocking buffer was added (50 μl/well) followed by incubation (1 hr, 22°C). Wells were washed 4 times and a dilution (1/1000) of goat anti-mouse IgM HRP was added (50 μl/ well) followed by incubation (1 hr, 22°C). Wells were washed-off excess unbound HRP conjugate five times. TMB substrate (Sigma, T0440-100 ml) was added (50 μl/well) and incubation was allowed for 15 min. The reaction was stopped with 1M H2SO4 (50 μl/well) and OD450nm was read.
Next, the binding of IgM8A2 to the aldolases of other livestock infective trypanosome species, besides T. congolense, was assessed by an indirect immunofluorescent assay. Fixed-permeabilized trypanosomes were incubated in IgM8A2-B solution (2.5 μg/ml) or Biotin anti-mouse IgM Antibody (Biolegend, Cat. No. 406504/500 μg) solution (2.5 μg/ml) followed by Cy3 Streptavidin (Biolegend, Cat. No. 405215) solution (1 μg/ml) as described (S1 Materials and Methods).
The specificity of Nb474H/IgM8A2-B “hybrid” sandwich system for detection of aldolase was assessed on recombinant TcoALD, TevENO or 1xPBS by ELISA employing the Strep-HRP and TMB reporter system.
Finally, to asses the competition for binding TcoALD between IgM8A2 (Ag-detection reagent) and Nb474 (Ag-capture reagent), a competition ELISA was conducted as described (S2 Materials and Methods).
ELISA titration of antigen capture Nanobody against detection monoclonal antibody
Nb474H (5 μg/ml) was serially diluted (two-fold) in 1x PBS until a final concentration of 0.005 μg/mL. Except for the wells on column 12, which were filled with 1xPBS (50 μl/well), the dilutions were coated (50 μl/well) on respective wells (S1 Table) followed by overnight incubation at 4°C. Next, wells were emptied, washed thrice, and SuperBlock blocking buffers (thermoscientific, Cat. No. 37516) was added in coated wells (160 μl/well) for 2 hrs at 22°C with an hourly buffer refreshment. Afterward, the blocking buffer was emptied and the wells were washed thrice. A constant amount of TcoALD antigen was added across the plate (0.25 μg/well) followed by incubation for an hour at 22°C. Wells were washed thrice. Next, two-fold serial dilutions of IgM8A2-B, starting from 5 μg/mL until 0.08 μg/ml, were added row-wise in a decreasing concentration except for wells in row H, which received 1xPBS only (S2 Table). The reaction was incubated for an hour at 22°C and the wells were washed four times. Strep-HRP diluted to 0.5 μg/ml in SuperBlock was added (0.025 μg/well) into the wells followed by incubation for an hour at 22°C. The enzyme conjugate was emptied and the wells were washed five times. TMB substrate (Sigma, T0440-100 ml) was added (50 μl/well) and color development was allowed for 15 mins. The reaction was stopped by adding 1M H2SO4 (50 μl/well) and OD450nm was read.
Analytical sensitivity of N474H/IgM8A-B “hybrid” sandwich ELISA
A fixed amount of Nb474H was coated (0.1 μg/well) in wells on rows B-E, columns 2–11 and incubated for an overnight at 4°C. The following morning, coating solution was emptied and the wells were washed thrice. Washed wells were blocked by adding SuperBlock (160 μl/well) for 2 hrs at 22°C with an hourly refreshment. The blocking solution was discarded and wells were washed thrice. Serial dilutions (two-fold) of TcoALD (100–0.0004 μg/ml) were added into duplicate wells with control wells (D11E11) receiving 1xPBS only (S3 Table). The reaction was incubated for an hour at 22°C. The unbound antigens were discarded and the wells were washed thrice. The IgM8A2-B diluted to 2.5 μg/mL in SuperBlock was added into the wells (50 μl/well) followed by incubation for an hour at 22°C. Wells were washed four times and Strep-HRP diluted to 0.5 μg/ml in blocking buffer was added (50 μl/well) followed by an hour of incubation at 22°C. The HRP conjugate was discarded and wells were washed five times. TMB substrate (Sigma, T0440-100 ml) was added (50 μl/well) into the wells and incubated for 15 mins. The reaction was stopped by adding an equal volume of 1M H2SO4 solution and the absorbance was read at 450nm.
Detection of T. congolense infections by Nb474H/IgM8A2-B “hybrid” sandwich ELISA
Sera collected from the naïve or mice experimentally infected with a panel of trypanosome species including T. congolense TC13 at parasitemia 1x108 trypanosomes/ml, T. b. brucei AnTat1.1E at parasitemia 4.8x108 trypanosomes/ml, T. evansi STIB 816 at parasitemia 2.7x108 trypanosomes/ml, or T. vivax ILRAD 700 at parasitemia 4.1x108 trypanosomes/ml were examined with the Nb474H/IgM8A2-B “hybrid” sandwich ELISA. Nb474H was coated (0.1 μg/well) and incubated for an overnight at 4°C. Wells were emptied and washed thrice. Washed wells were blocked with 10% BSA (BOVOGEN, Cat. No. BSAS 0.1) at 160 μl/well and incubated for an hour at 22°C. The blocking buffer was refreshed and incubation was continued for an hour before discarding. Undiluted sera were added (50 μl/well) in duplicate into emptied wells. Both recombinant TcoALD (10 μg/well) and T. congolense lysate (5 μg/well) were used as positive controls for the ELISA. Incubation was performed for one hour at 22°C. The unbound antigens were discarded and the wells were washed thrice. Next, IgM8A2-B diluted to 2.5 μg/ml in 10%BSA was added into the wells (50 μl/well) followed by incubation for an hour at 22°C. The wells were washed four times. A strep-HRP solution diluted to 0.25 μg/ml in 10% BSA was added into the wells (50 μl/well) followed by incubation for 30 mins at 22°C. The conjugate was discarded and the wells were washed six times. Thereafter, color development was initiated by adding TMB substrate (Sigma, T0440-100 ml) at 50 μl/well. Incubation was allowed for 15 mins and the reaction was stopped by adding 1M H2SO4 (50 μl/well) followed by OD reading at 450nm.
Binding of native T. congolense aldolase by Nb474H/IgM8A2-G sandwich in a dot blot system
The potential for translation of Nb474 and Gold-labelled IgMA82 (IgM8A2-G) “hybrid” sandwich technology into a LFA device was demonstrated in a dot-blot assay. The IgM8A2 was labelled with Gold 20nm using a Gold Conjugation Kit (abcam, Cat. No. ab188215) according to the manufacturer’s protocol. Specifically, IgM8A2 was dialysed in 2-(N-Morpholino) ethanesulfonic acid hydrate, 4-Morpholineethanesulfonic acid (MES hydrate) buffer at pH 7.4. The dialysed mAb was later diluted to 0.25 mg/ml in an Ab diluent provided by the kit. Thereafter, an aliquot (12 μl) of the diluted mAb was mixed with the kit’s Reaction Buffer (42 μl). The mixture (45 μl) was dispensed into a vial of lyophilized Gold 20 nm followed by reconstitution and then incubation (15 mins, 22°C). A Quencher solution, provided by the kit, was added (5μl) into the mixture. To wash off unlabelled mAb, a solution of the Quencher diluted (1/10) in distilled water was added to the mixture followed by centrifugation (9000 x g, 22°C). The supernatant was carefully drawn and the resultant pellet was resuspended in a fresh diluted Quencher (50 μl). Thereafter, the binding of IgM8A2-G to test proteins including TcoALD, T. congolense lysate (TcoLys), LmALD, and TevENO was examined. The test proteins were either blotted direct on a nitrocellulose membrane (S4 Table) or anchored onto a nitrocellulose membrane by a blotted Nb474H capture reagent (S5 Table). To examine IgM8A2-G binding to test or control proteins blotted direct on the nitrocellulose membrane, each of the test samples was spotted (30 μg/spot) into a “well” encircled by an adhesive label (Chengu 400 Pieces Self-Adhesive Reinforcement Labels). When the sample spots were damp-dried, the membrane was blocked by immersing into a 5% milk solution and incubated with gentle rocking for an overnight at 22°C. The blocking buffer was rinsed thrice with PBS-T and IgM8A2-G solution was spotted (10 μl/spot) on the “wells” previously blotted with the samples. The reaction was allowed to proceed for 45 mins at 22°C. The membrane was washed thrice with PBS-T with each wash lasting for 30 mins. The membrane was blot-dried and photographed. To examine IgM8A2-G binding to proteins captured by blotted Nb474H, the Nb474H was spotted on the membrane (150 μg/spot) and allowed to damp-dry. Afterwards, the membrane was blocked with 5% milk for an overnight at 22°C. The membrane was rinsed thrice. Thereafter, each of the test samples was separately spotted (90 μg/spot) in one of the “wells” previously spotted with Nb474 followed by incubation (45 mins, 22°C). The membrane was washed thrice with PBS-T and each of the washings lasted 30 mins. Afterward, the membrane was blot-dried and the photograph was acquired by a camera.
Results
Screening hybridoma library retrieved a specific anti-trypanosome aldolase monoclonal antibody
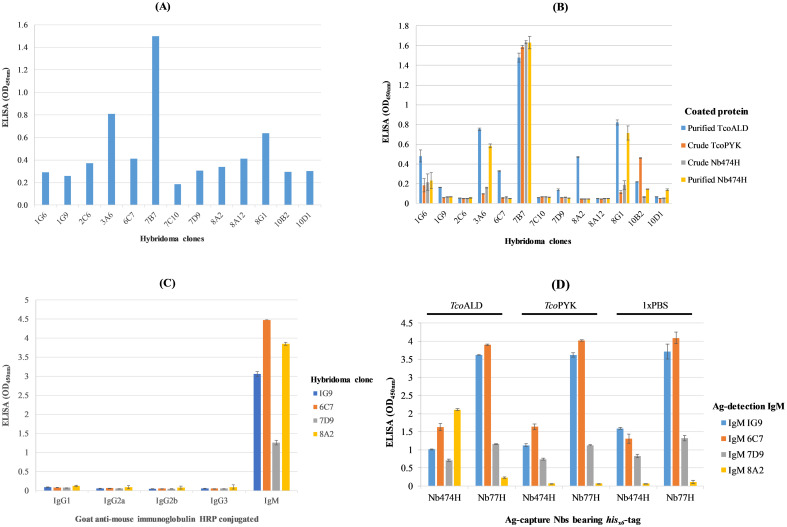
To induce B cell clones expressing anti-TcoALD specific antibodies for construction of hybridoma library, mice were immunized with recombinant TcoALD. Comparing ELISA signals (OD450nm) on blood samples collected before (pre-immune) and after immunization (immune), it was conceivable that immunization of mice with TcoALD evoked Ab response (S1 Fig). Afterwards, hybridoma library was constructed from B-cells of a mouse with the highest Ab response and the library was screened on TcoALD. A primary screening of the hybridoma library retrieved 13 reactive clones. A clone was considered reactive when the OD450nm was ≥ 0.2 units (Fig 1A). Of the 13 clones, two (1G6 and 1G9) originated from plate 1, one (2C6) from plate 2, one (3A6) from plate 3, one (6C7) from plate 6, three (7B7, 7D9, and 7C10) from plate 7, three (8A2, 8A12, and 8G1) from plate 8, and two (10B2 and 10D1) from plate 10. The consistency and specificity of the 13 reactive clones for binding TcoALD was again verified by a secondary screening on TcoALD or irrelevant proteins including hisx6-tag bearing TcoPYK in E. coli BL21 (DE3) crude lysate, hisx6-tag bearing Nb474 (Nb474H) in E. coli WK6 lysate, and purified Nb474H. Four of the clones (1G9, 6C7, 7D9 and 8A2) showed unique TcoALD specificity, while five clones (1G6, 3A6, 7B7, 8G1, and 10B2) produced antibodies that bound both TcoALD as well as irrelevant proteins. In addition, four clones (2C6, 7C10, 8A12, and 10D1), which had scored positive in the primary screening turned out negative in the secondary screening (Fig 1B). To check Ab isotype expressed by each of the four hybridoma clones (1G9, 6C7, 7D9 and 8A2), the clones were probed with goat anti-mouse HRP conjugates against mouse IgG1, IgG2a, IgG2b, IgG3 or IgM. Surprisingly, all the four hybridoma clones 1G9, 6C7, 7D9 and 8A2 were IgM as inferred from specific reactivity with goat anti-mouse IgM HRP (Fig 1C). Next, each of the four IgM producing clones was verified for reactivity with TcoALD, TcoPYK, or PBS added into the ELISA wells precoated with hisx6-tag Nb474 (Nb474H) or hisx6-tag Nb77 (Nb77H) capturing reagent. Unlike the three IgM clones (1G9, 6C7, and 7D9) that showed indiscriminate reactivity, IgM clone 8A2 reacted with TcoALD dispensed into wells precoated with Nb474H only (Fig 1D).
Fig 1. Selection of clones producing anti-TcoALD monoclonal antibody (mAb) from hybridoma library.
(A) Primary screening of the hybridoma library identified 13 positive hybridoma clones. Threshold OD score for positive clones was ≥ 0.2 units. (B) Positive hybridoma clones subjected to a secondary screening on TcoALD or irrelevant proteins. Clones 1G9, 6C7, 7D9 and 8A2 produced TcoALD specific binders; clones 1G6, 3A6, 7B7, 8G1, and 10B2 produced binders of both TcoALD and irrelevant protein; and each of the clones 2C6, 7C10, 8A12, and 10D1 has no TcoALD binder. (C) Isotyping mAbs showing specific binding to TcoALD. Going by their reactivity with the goat anti-mouse immunoglobulin M antibody isotype, all the mAbs belonged to the IgM class. (D) IgM8A2 detects TcoALD in a sandwich combination with Nb474H. IgM clones were analysed for specific detection of TcoALD in a sandwich combination with Nb474H (anti-TcoALD Nb) or irrelevant Nb77H (anti-TevENO Nb). Only IgM8A2 specifically bound TcoALD in a sandwich combination with Nb474H.
Binding characteristics of the anti-trypanosome aldolase monoclonal antibody
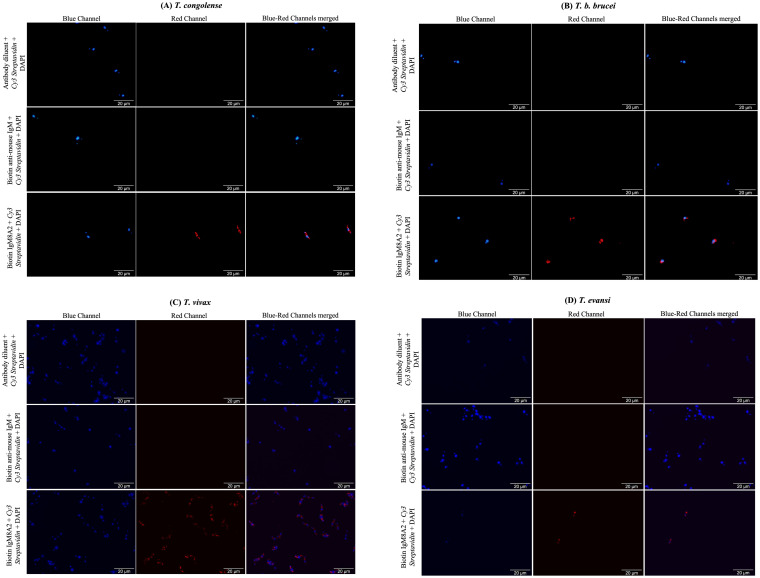
It was necessary to know whether IgM8A2 mAb also binds a denatured aldolase target. This is an attribute required to inform specimen handling and processing prior to testing. Western blot conducted on TcoALD resolved under denaturing conditions did not show binding of the IgM8A2 to a nitrocellulose membrane-blotted TcoALD protein. When binding of IgM8A2 to a heat-denatured recombinant TcoALD or TvALD was also assessed by ELISA, only baseline signals characterised heat-treated samples (S2 Fig). These two experiments indicate denaturation of TcoALD abolish recognition by IgM8A2. Secondly, cross-recognition of native aldolases from other important species of livestock trypanosomes including T. congolense, T. b. brucei, T. vivax and T. evansi by IgM8A2 was analysed by an indirect immunofluorescence assay. Cross-reactivity of IgM8A2 TcoALD Ag-detection reagent may find application in the development of a trypanosome pan-reactive multiplex LFA comprised of multiple trypanosome species specific aldolase Ag-capture reagents and a pan-reactive IgM8A2 trypanosome aldolase Ag-detection reagent. Therefore, cross-recognition of native aldolases from different trypanosomes was assessed in situ by incubating fixed and permeabilized trypanosome cells in a solution of biotin-labelled IgM8A2 (IgM8A2-B), and the binding of IgM8A2 was probed with Cy3 strepatavidin red fluorescein conjugate. It was discovered that IgM8A2 binds fixed and permeabilized trypanosome irrespective of the species T. congolense, T. b. brucei, T. vivax or T. evansi. (Fig 2A–2D).
Fig 2. In situ indirect labelling of trypanosome glycosome with a biotinylated IgM8A2 (IgM8A2-B) primary antibody.
Fixed and permeabilized T. congolense (A), T. b. brucei (B), T. vivax (C) or T. evansi (D) was probed in succesion with IgM8A2-B, biotin anti-mouse IgM or diluent only followed by Cy3 streptavidin red fluorescein conjugate. Red staining of trypansomes was observed across all the species where IgM8A2-B followed by Cy3 streptavidin red fluorescein conjugate was added pointing to cross-reactivity of the IgM8A2.
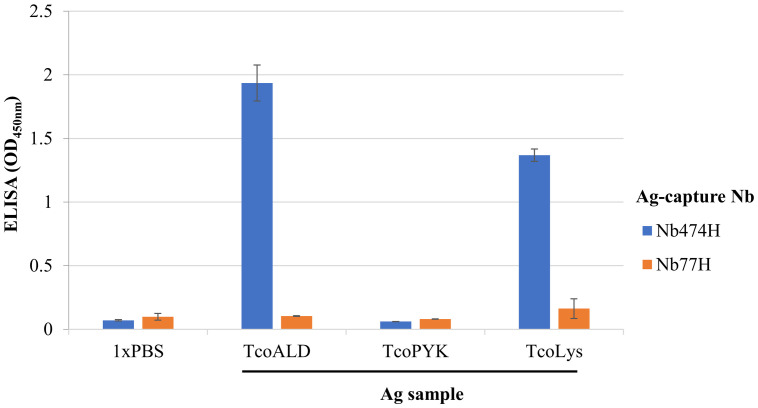
Thirdly, it was necessary to authenticate that IgM8A2, generated against recombinant TcoALD, also binds native TcoALD in a sandwich combination with Nb474H. This information would pave way for further progression with the development of the immunoassay meant for detection of native TcoALD released in blood of animals with ongoing T. congolense infections. For this experiment, a T. congolense lysate (TcoLys) preparation was used as a source of native TcoALD. We demonstrated that IgM8A2 binds native TcoALD, selectively captured by Nb474H, from a repertoire of proteins in the TcoLys. This evidence was adduced from a high signal intensity (OD450nm) observed in the TcoALD-treated well, which was comparable to TcoLys-treated (Fig 3).
Fig 3. Detection of native aldolase in the lysate of T. congolense (TcoLys) by Nb474H/IgM8A2 “hybrid” sandwich ELISA.
Test samples including recombinant T. congolense aldolase (TcoALD), T. congolense pyruvate kinase (TcoPYK) and TcoLy added to wells pre-coated with Nb474H or Nb77H was each probed with IgM8A2. High signal intensity was observed in wells pre-coated with Nb474H capture reagent followed by addition of TcoALD or TcoLys, and then IgM8A2. From this finding it is plausible to conclude that Nb474H/IgM8A2 “hybrid” sandwich ELISA binds recombinant as well as native TcoALD.
Fourthly, we investigated if Nb474 (TcoALD Ag-capture reagent) and IgM8A2 (TcoALD Ag-detection reagent) bind a common epitope. In the event that both Nb474 and IgM8A2 bind a common epitope on TcoALD, translation of the immunoassay from an ELISA to a LFA would be technically impossible. A prior exposure of TcoALD to IgM8A2 Ag-detection reagent, as it is the case with LFA, would lead to epitope masking by IgM8A2 such that only a few or even no will be left on TcoALD for the binding of Nb474 Ag-capture reagent. The likelihood of common epitope binding by the two reagents was assessed by a competitive ELISA. The ELISA showed that Nb474 and IgM8A2 do not interfere with each other’s binding to TcoALD. Premixing of Nb474HA (serving as a TcoALD detection reagent) with a varying concentration (high to low) of IgM8A2 (serving as a competing reagent) followed by addition of the mixture (Nb474HA and IgM8A2) to TcoALD did not interfere with the binding of Nb474HA to TcoALD, and vice versa. In both ELISA, a constant high signal (OD450nm) was recorded across all the different concentration of the competing reagents (S3A and S3B Fig).
A Nb474H/IgM8A2-B “hybrid” sandwich ELISA was developed and evaluated for detection of aldolase
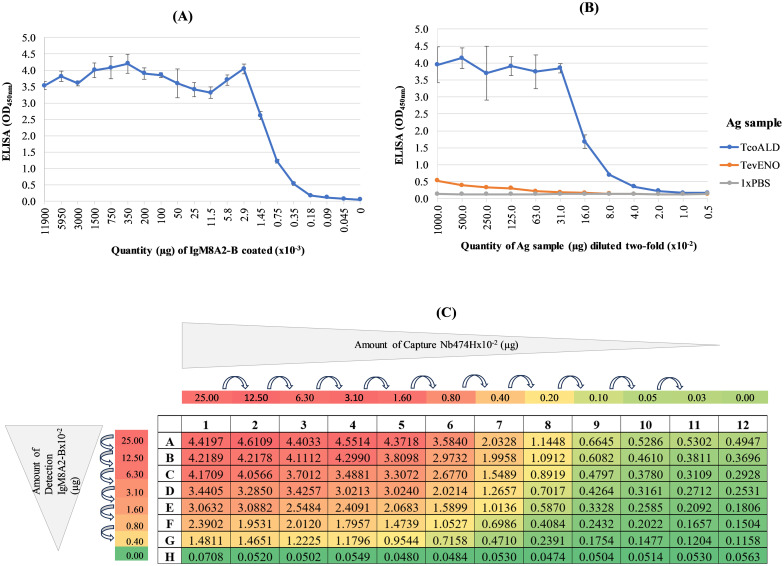
A Nb474H/IgM8A2-B “hybrid” sandwich ELISA was developed, and the working concentration of each of the Ag binding reagents, Nb474H Ag-capturing reagent and biotin-labelled IgM8A2 (IgM8A2-B) Ag-detection reagent, was optimized on TcoALD prior to evaluation of the “hybrid” immunoassay for detection of trypanosome infections in mice. As a first step, recognition of IgM8A2-B Ag-detection reagent by Strep-HRP was assessed by ELISA. Signal intensity (OD450nm) observed on probing a coated IgM8A2-B with Strep-HRP and TMB substrate reporter system was proportional to the concentration of the coated biotin-labelled mAb (Fig 4A) signifying that, indeed, Strep HRP was binding the coated biotin-conjugate. Next, the detection of TcoALD by Nb474H/IgM8A2-B “hybrid” sandwich ELISA was assessed against TevENO or 1xPBS controls. Higher signal intensity (OD450nm) was recorded in wells treated with TcoALD sample than in the control wells treated with TevENO or 1xPBS (Fig 4B) proving that Nb474H/IgM8A2-B “hybrid” sandwich ELISA ably detects TcoALD. Subsequently, the working concentration of each of the Nb474H and IgM8A2-B test reagents required for detection of TcoALD was determined by a checkerboard titration. Qualitative (heatmap) as well as quantitative (OD450nm) data obtained from the titration experiment suggests the working concentration for each of the Nb474H and IgM8A2-B reagents falls about 0.25 μg/ml per 50 μl diluent (Fig 4C). Thereafter, the analytical detection limit of the optimized “hybrid” sandwich ELISA was also determined by titration on an in-house recombinant TcoALD. The least amount of TcoALD, which could be detected by the assay was 78.13 ng. This quantity of TcoALD was the least that gave a signal intensity (OD450nm) two-fold above the negative control (S4 Fig).
Fig 4. Development of the Nb474H/IgM8A2-B “hybrid” sandwich ELISA prototype.
(A) IgM8A2 was labelled with biotin (IgM8A2-B) and validated by ELISA employing Strep-HRP TMB substrate reporter system. A high signal intensity (OD450nm) observed at higher concentrations of IgM8A2-B tapered with the decreasing concentrations of the mAb indicating that Strep-HRP was binding coated IgM8A2-B. (B) Nb474H/IgM8A2-B “hybrid” sandwich ELISA prototype binds TcoALD. Compared to TevENO-treated wells where signal intensity remained baseline throughout different concentrations of the protein, a higher signal intensity proportional to the concentrations of TcoALD was observed indicating that the prototype ELISA was binding TcoALD. (C) Nb474H titrated against IgM8A2-B to find effective matching working concentration. The signal intensity was proportional to the amount of Nb474H and IgM8A2-B reagents used. High OD450nm readings were consistent with wells treated with high concentrations of the Nb or IgM and vice versa. A standard working concentration of both Nb474H and IgM8A2-B was deduced to be 0.25 μg/ml per 50 μl because it was the least concentration of reagents, which was still giving a relatively high signal.
A Nb474H/IgM8A2-B “hybrid” sandwich ELISA detects active T. congolense infections
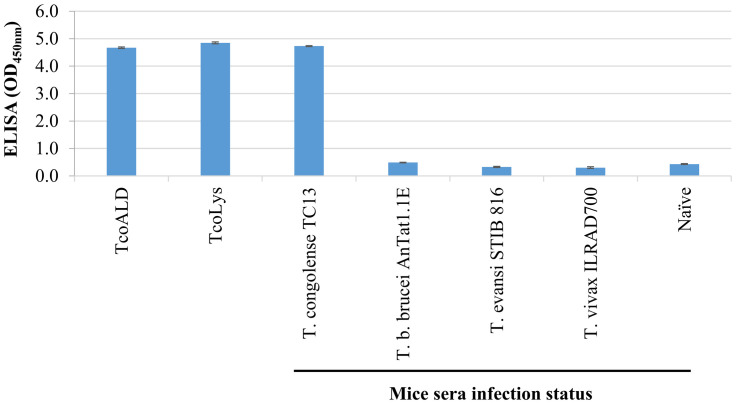
A proof-of-concept detection of native TcoALD circulating in sera of mice infected with T. congolense by the optimized Nb474H/IgM8A2-B “hybrid” sandwich ELISA was demonstrated to establish potential application of the assay for detection of T. conglense infections. A panel of mice sera collected from naïve animals or trypanosome infected (T. congolense TC13, parasitaemia 1x108 trypanosomes/ml; T. b. brucei AnTat1.1E, parasitaemia 4.8x108 trypanosomes/ml; T. evansi STIB 816, parasitaemia 2.7x108 trypanosomes/ml; or T. vivax ILRAD700, parasitaemia 4.1x108 trypanosomes/ml) was probed with Nb474H/IgM8A2-B “hybrid” sandwich ELISA. Signal intensity (OD450nm) recorded from wells treated with T. congolense infected mice sera was 10-fold higher than those wells treated with naïve mice sera or sera collected from mice infected with other trypanosome species (Fig 5) suggesting capability of the “hybrid” sandwich ELISA for specific detection of T. congolense infected animals.
Fig 5. Nb474H/IgM8A2-B “hybrid” sandwich ELISA specifically detects T. congolense infection in mice.
A panel of specimens including recombinant T. congolense aldolase (TcoALD), T. congolense lysate (TcoLys), and sera collected from trypanosome infected or naïve mice were probed with Nb474H/IgM8A2 sandwich ELISA. High signal intensity, comparable to TcoALD or TcoLys, was recorded in wells treated with sera collected from T. congolense TC13 infected mice (1x108 trypanosomes/ml) indicating capability of the ELISA for detection of T. congolense infections. No reaction was observed in wells treated with naïve or sera collected from mice infected with T. b. brucei AnTat1.1E (4.8x108 trypanosomes/ml), T. evansi STIB 816 (2.7x108 trypanosomes/ml) or T. vivax ILRAD700 (4.1x108 trypanosomes/ml).
Gold-labelled IgM8A2 binds native TcoALD in a dot blot assay
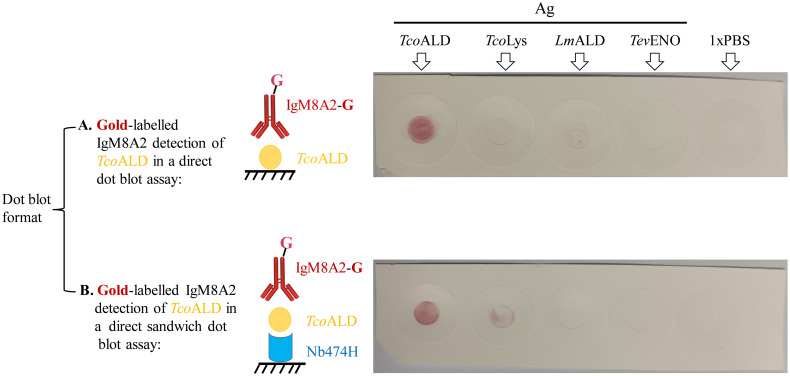
To assess if, indeed, an opportunity exists for translation of the Nb/mAb “hybrid” sandwich ELISA prototype into a LFA, a dot blot assay employing Au-labelled IgM8A2 (IgM8A2-G) as an Ag-detection reagent was performed. First, the binding of IgM8A2-G to a recombinant TcoALD or its native form in T. congolense lysate (TcoLys) was assessed on proteins that were directly blotted on a nitrocellulose membrane. Next, the detection by IgM8A2-G of recombinant TcoALD or its native form in TcoLys was assessed on proteins anchored onto the nitrocellulose membrane by a spotted Nb474H Ag-capture reagent. Staining of nitrocellulose membrane occurred on the spot directly blotted with recombinant TcoALD only (Fig 6A). On the second membrane where recombinant TcoALD or its native form in TcoLys samples was anchored by Nb474H, staining occurred on spots treated with recombinant TcoALD and TcoLys (Fig 6B). These results show that IgM8A2-G, in a sandwich combination with Nb474H, binds recombinant as well as native TcoALD.
Fig 6. Dot blot detection of aldolase using Gold-labelled IgM8A2 (IgM8A2-G).
(A) Binding of IgM8A2-G to antigen (Ag) including recombinant T. congolense aldolase (TcoALD), T. congolense lysate (TcoLys), recombinant L. mexicana aldolase (LmALD), recombinant T. evansi enolase (TevENO) or 1xPBS directly blotted on the nitrocellulose membrane. IgM8A2-G specifically labelled the spot blotted with TcoALD. (B) A sandwich setup showing the binding of IgM8A2-G to Ag captured by Nb474H. Signal was observed in wells spotted with the TcoALD and TcoLys indicating the ability of IgM8A2-G for binding both recombinant as well as native aldolase.
Discussion
The Pan African Tsetse and Trypanosomiasis Eradication Campaign (PATTEC) coordinates the implementation of activities aimed at eliminating the tsetse fly vector and trypanosomiasis from the African continent [29]. A key factor undermining the eradication campaign is the inadequacies of current diagnostics for trypanosomiasis, which may make achievement of the set target unrealistic, in particular when it comes to the animal disease variants of trypanosomiasis. Undoubtedly, not many livestock farms have integrated trypanosomiasis diagnosis in their system. The deployment of ideal POCT for AAT in endemic areas for routine diagnosis, would change the trajectory. Unfortunately, such a test is yet non-existent. For this reason, clinicians often rely on clinical signs to arrive at a tentative diagnosis. Diagnosis of trypanosomiasis relying on clinical signs only, is grossly imperfect because the disease does not have pathognomonic signs for discrimination from other diseases that present similar signs. The elimination campaign would succeed faster with the deployment of effective POCT for active screening, monitor therapeutic efficacy, and disease surveillance.
Abs have been used for the development of several commercial Ag detection POCT devices for infectious diseases [30]; however, no such commercial Ag detection test exists for AAT [31] despite decades of active research. Hope for realization of the Ag detection tests for AAT has been raised with the invention of Nb technology, as a potential substitute for the conventional Abs. Although it has numerous desirable attributes [32,33], Nbs have not been completely free of hurdles [33]. Related to diagnostic applications, conjugation of Nbs to high molecular weight reporter molecules, including nanoparticles, enzymes and biotin for employment as Ag detection reagents in immunoassay, often presents a challenge. However, we have now shown herein that this challenge could be side-stepped by incorporation of a mAb as a detection reagent, ending up with a Nb/mAb “hybrid” Ag detection test device for trypanosomiasis. Specifically, a prototype antigen detection ELISA unit for detection of T. congolense infections was derived from a Nb (Nb474) and a mAb (IgM8A2) heterologous reagents targeting aldolase of the parasite. While Nb474H originated from an alpaca, IgM8A2 is of mouse origin.
A three-stage screening of the hybridoma library recovered an anti-TcoALD specific IgM (IgM8A2). The primary screening recovered 13 positive clones, re-screening of the 13 positive clones recovered four clones, and a final re-screening of these four clones, with Nb474H employed as an antigen capture reagent, revealed IgM8A2 as a TcoALD specific binder. The multi-stage screening employed identified among the positive clone non-specific binders, which were subsequently discontinued from downstream experiments. Although no further investigation was carried to ascertain the precise targets of these non-specific binders, binding to hisx6-tag was postulated, given their consistent reaction with recombinant hisx6-tag proteins. Noted were a few clones that scored positive in the primary screening and negative in the secondary screening. False positive reactions, characterized by high background signals, offer explanation for the observed status change. The fact that only a single anti-TcoALD mAb producing clone could be retrieved from a large collection, suggests the immunized mouse evoked a low immune response to the Ag. Aldolase is a glycolytic enzyme of eukaryotes [34] meaning that TcoALD partly qualifies as a self-Ag in a mouse model, potentially explaining its poor immunogenicity [35]. It is suggested that the developers of trypanosome Ag detection tests could pursue targeting high copy number low immunogenic Ags, as a way of overcoming low sensitivity resulting from immune complex formation [20]. Incidentally, aldolase Ag brings us closer to the realization of such an anticipated target.
The IgM8A2 binding characteristics were investigated to find additional insights on the mAb. An indirect immunofluorescent assay revealed that IgM8A2 binds T. congolense, T. b. brucei, T. evansi and T. vivax. Cross-reactivity of IgM8A2 was not surprising, given that the TcoALD primary sequence is identical to those of T. b. brucei, T. evansi and T. vivax [22]. However, it can be seen that the cross-reactivity of the IgM8A2 does not affect the specificity of the assay, as the latter is determined by the Nb (Ag-capture reagent). Perhaps, the sensitivity of a LFA for diagnosis of T. congolense developed from a cross-reactive IgM8A2 (Ag detection reagent) may be affected when confronted with T. congolense mix infection with T. b. brucei, T. vivax, or T. evansi. A practical solution is to impregnate large quantities of IgM8A2 into a conjugate pad, in order to sequester competing aldolases. On the other hand, a cross-reactive IgM8A2 would work well for a multiplex immunoassay for multi-species detection. In this case, a trypanosome species test line could be printed with a species-specific Nb, adopting a strategy reviewed in Anfossi et al [36]. It was also observed that denaturation of aldolase completely abolish detection by IgM8A2, suggesting the mAb binds a conformational epitope. Results of a competition ELISA, conducted to verify whether Nb474 and IgM8A2 compete for the same epitope, suggested nothing of that kind, as the binding of Nb474 to TcoALD was not affected by IgM8A2 and vice versa. The heterologous binding site recognition of the two reagents solves the technical challenge, which affected the translation of Nb474 homologous sandwich ELISA into a LFA device.
Assessment of the Nb/mAb “hybrid” sandwich ELISA for cross-reactivity on naïve or trypanosome-infected mouse sera revealed the assay’s specificity for T. congolense, further reiterating the contribution of Nb (Ag capturing reagent) as a determinant of the assay’s specificity. To assess if, indeed, opportunity exists for translation of Nb/mAb “hybrid” sandwich ELISA prototype into a lateral flow assay, a dot blot employing IgM8A2-G was performed. IgM8A2-G specifically detected recombinant TcoALD as well as TcoLys, albeit with a stronger signal intensity on spots blotted with the recombinant protein. Taken together, Au-labelling did not affect the binding of IgM8A2 to aldolase. High signal intensity observed on recombinant TcoALD compared to the lysate could have been influenced by variation in the Ag concentration, being higher in the recombinant protein preparation than in the lysate.
Given that the new technology has removed a bottle-neck encountered with the nanoparticle-labelling of Ag detection Nb, proliferation of Nb/mAb “hybrid” Ag detection tests for infectious diseases including African trypanosomiasis is envisaged. Hitherto, labelling of Nb with Au nanoparticles for incorporation as Ag detection reagent in a LFA has proven a daunting task. The latter is among the challenges that have hindered the progression of Nb-based test devices from development to commercialization. The current “hybrid” technology, in which detection Nb was replaced by a mAb, offers a relief to the obstacle. Furthermore, this innovation demonstrates how mAb could be exploited to synergize Nbs in the development of analytical devices and vice versa. The “hybrid” sandwich technology reported here, sets stage for rapid advancement of Nb-based LFA device development in all spheres. Construction of a LFA from the recently developed Nb/mAb “hybrid” solid-phase ELISA prototype reported should be first next step in this direction.
Supporting information
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(XLS)
(XLSX)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
Acknowledgments
We would like to acknowledge the contributions of Mr. Liam Vander Linden and Ms Bolortsetseg Baatar for assisting in the production and purification of recombinant proteins. We also extend our gratitude to Ms Hien Thi Thu Pham for the technical help provided during the immunofluorescence experiments.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This work was funded by the H2020-EU.3.2.1.1 Program for Controlling and progressively minimizing the burden of animal trypanosomosis, a Horizon 2020 project (COMBAT). SM received the grant. COMBAT is a European Union grant and its URL is https://www.combat-project.eu/. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030. Geneva; 2020. https://www.who.int/publications/i/item/9789240010352. [Google Scholar]
- 2.Mishra US, Padhi B. Sustaining the sustainable development goals. Econ Polit Wkly. 2021; 56(34):30–1. [Google Scholar]
- 3.Autyi H, Torr SJ, Michoel T, Jayaraman S, Morrison LJ. Cattle trypanosomosis: The diversity of trypanosomes and implications for disease epidemiology and control. OIE Rev Sci Tech. 2015; 34(2):587–98. [DOI] [PubMed] [Google Scholar]
- 4.Radwanska M, Vereecke N, Deleeuw V, Pinto J, Magez S. Salivarian trypanosomosis: A review of parasites involved, their global distribution and their interaction with the innate and adaptive mammalian host immune system. Front Immunol. 2018; 9:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastos TSA, Faria AM, Couto LFM, Nicaretta JE, Cavalcante ASDA, Zapa DMB, et al. Epidemiological and molecular identification of Trypanosoma vivax diagnosed in cattle during outbreaks in central Brazil. Parasitology. 2020; 147(12):1313–9. doi: 10.1017/S0031182020001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutiérrez C, Juste M.C., Corbera J.A., Magnus E., Verloo D., Montoya J.A. Camel trypanosomosis in the Canary Islands: assessment of seroprevalence and infection rates using the card agglutination test (CATT/T. evansi) and parasite detection tests. Vet Parasitol. 2000; 90:155–9. doi: 10.1016/s0304-4017(00)00225-9 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen VL, Iatta R, Manoj RRS, Colella V, Bezerra-Santos MA, Mendoza-Roldan JA, et al. Molecular detection of Trypanosoma evansi in dogs from India and Southeast Asia. Acta Trop. 2021; 220:1–7. doi: 10.1016/j.actatropica.2021.105935 [DOI] [PubMed] [Google Scholar]
- 8.Asghari MM, Rassouli M. First identification of Trypanosoma vivax among camels (Camelus dromedarius) in Yazd, central Iran, jointly with Trypanosoma evansi. Parasitol Int. 2022; 86:102450. doi: 10.1016/j.parint.2021.102450 [DOI] [PubMed] [Google Scholar]
- 9.Desquesnes M, Holzmuller P, Lai D-H, Dargantes A, Lun Z-R, Jittaplapong S. Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed Res Int. 2013; 2013:194176. doi: 10.1155/2013/194176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco JR, Cecchi G, Priotto G, Paone M, Ebeja AK, Simarro PP, et al. Human African trypanosomiasis cases diagnosed in non-endemic countries (2011–2020). PLoS Negl Trop Dis. 2022; 16(11):1–19. doi: 10.1371/journal.pntd.0010885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw APM, Cecchi G, Wint GRW, Mattioli RC, Robinson TP. Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in Eastern Africa. Prev Vet Med. 2014; 113(2):197–210. [DOI] [PubMed] [Google Scholar]
- 12.Allsopp R, Hursey BH. Insecticidal Control of Tsetse. In: Maudlin I, Holmes PH, Miles MA, editors. The trypanosomiases. London: CABI publishing; 2004. p. 491–507. [Google Scholar]
- 13.Ndung’u JM, Boulangé A, Picado A, Mugenyi A, Mortensen A, Hope A, et al. Trypa-NO! contributes to the elimination of gambiense human african trypanosomiasis by combining tsetse control with “screen, diagnose and treat” using innovative tools and strategies. PLoS Negl Trop Dis. 2020; 14(11):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Diagnostics for the developing world. Nat Rev Microbiol. 2004; 2:231–40. doi: 10.1038/nrmicro841 [DOI] [PubMed] [Google Scholar]
- 15.Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries. PLoS Med. 2012; 9(2012):44111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Songa EB, Hamers R. A Card Agglutination Test CATT for Veterinary use based on an early VAT RoTat 1/2 of Trypanosoma evansi. Ann Soc belge Med trop. 1988; 68: 233–40. [PubMed] [Google Scholar]
- 17.WHO. A Framework for Malaria Elimination. 2017. 94 p. http://apps.who.int/iris/bitstream/handle/10665/254761/9789241511988-eng.pdf?sequence=1.
- 18.WHO. Infection prevention and control in the context of coronavirus disease (COVID-19): A living guideline. 2023. 141 p. https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-guideline-2023.3 [PubMed]
- 19.Nantulya VM. TrypTect CIATT—a card indirect agglutination trypanosomiasis test for diagnosis of Trypanosoma brucei gambiense and T. b. rhodesiense infections. Trans R Soc Trop Med Hyg. 1997; 91(5):551–3. doi: 10.1016/s0035-9203(97)90023-7 [DOI] [PubMed] [Google Scholar]
- 20.Buscher P. Diagnosis of African Trypanosomiasis. In: Magez S, Radwanska M, editors. Trypanosomes and Trypanosomiasis. Springer-Verlag Wien; 2014. p. 189–216. [Google Scholar]
- 21.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993; 363(6428):446–8. doi: 10.1038/363446a0 [DOI] [PubMed] [Google Scholar]
- 22.Odongo S, Sterckx YGJ, Stijlemans B, Pillay D, Baltz T, Muyldermans S, et al. An Anti-proteome Nanobody Library Approach Yields a Specific Immunoassay for Trypanosoma congolense Diagnosis Targeting Glycosomal Aldolase. PLoS Negl Trop Dis. 2016; 10(2):1–24. doi: 10.1371/journal.pntd.0004420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto Torres JE, Goossens J, Ding J, Li Z, Lu S, Vertommen D, et al. Development of a Nanobody-based lateral flow assay to detect active Trypanosoma congolense infections. Sci Rep. 2018; 8(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revets H, De Baetselier P, Muyldermans S. Nanobodies as novel agents for cancer therapy. Vol. 5, Expert Opinion on Biological Therapy. 2005. p. 111–24. [DOI] [PubMed] [Google Scholar]
- 25.Desmyter A, Transue TR, Ghahroudi MA, Thi M-HD, Poortmans F, Hamers R, et al. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat Struct Biol. 1996; 3(9):803–11. doi: 10.1038/nsb0996-803 [DOI] [PubMed] [Google Scholar]
- 26.De Genst E, Silence K, Decanniere K, Conrath K, Loris R, Kinne J, et al. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci U S A. 2006; 103(12):4586–91. doi: 10.1073/pnas.0505379103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Torres JEP, Goossens J, Vertommen D, Caljon G, Sterckx YGJ, et al. An unbiased immunization strategy results in the identification of enolase as a potential marker for nanobody-based detection of trypanosoma evansi. Vaccines. 2020; 8(3):1–20. doi: 10.3390/vaccines8030415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goossens J, Sein H, Lu S, Radwanska M, Muyldermans S, Sterckx YGJ, et al. Functionalization of gold nanoparticles with nanobodies through physical adsorption. Anal Methods. 2017; 9(23):3430–40. [Google Scholar]
- 29.African Union Commission. African Union handbook 2022: a guide for those working with and within the African Union. 2022. 264 p.
- 30.Clerc O, Greub G. Routine use of point-of-care tests: Usefulness and application in clinical microbiology. Clin Microbiol Infect. 2010; 16(8):1054–61. doi: 10.1111/j.1469-0691.2010.03281.x [DOI] [PubMed] [Google Scholar]
- 31.Philippe B, Lejon V. Diagnosis of Human African Trypanosomiasis. In: Maudlin I, Holmes PH, Miles MA, editors. The Trypanosomiases. Oxfordshire: CABI publishing; 2004. p. 203–18. [Google Scholar]
- 32.Muyldermans S. Applications of Nanobodies. Annu Rev Anim Biosci. 2021; 9:401–21. doi: 10.1146/annurev-animal-021419-083831 [DOI] [PubMed] [Google Scholar]
- 33.Jin BK, Odongo S, Radwanska M, Magez S. Nanobodies: A Review of Generation, Diagnostics and Therapeutics. Int J Mol Sci. 2023;24(6):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg JM, Tymoczyko JL, JR GGJ, Stryer L. Biochemistry. 8th ed. W. H. Freeman & Company; 2015. 1056 p. [Google Scholar]
- 35.Murphy K, Weaver C. Janeway’s Immunobiology. 9th ed. New York, NY: Garland Science, Taylor & Francis Group, LLC; 2017. 904 p. [Google Scholar]
- 36.Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Baggiani C. Multiplex lateral flow immunoassay: An overview of strategies towards high-throughput point-of-need testing. Vol. 9, Biosensors. 2018. p. 14. doi: 10.3390/bios9010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(XLS)
(XLSX)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.