Abstract
Background:
Trait mindfulness, the tendency to attend to present-moment experiences without judgement, is negatively correlated with adolescent anxiety and depression. Understanding the neural mechanisms underlying trait mindfulness may inform the neural basis of psychiatric disorders. However, few studies have identified brain connectivity states that correlate with trait mindfulness in adolescence, nor have they assessed the reliability of such states.
Methods:
To address this gap in knowledge, we rigorously assessed the reliability of brain states across 2 functional magnetic resonance imaging (fMRI) scan from 106 adolescents aged 12 to 15 (50% female). We performed both static and dynamic functional connectivity analyses and evaluated the test-retest reliability of how much time adolescents spent in each state. For the reliable states, we assessed associations with self-reported trait mindfulness.
Results:
Higher trait mindfulness correlated with lower anxiety and depression symptoms. Static functional connectivity (ICCs from 0.31–0.53) was unrelated to trait mindfulness. Among the dynamic brains states we identified, most were unreliable within individuals across scans. However, one state, an hyperconnected state of elevated positive connectivity between networks, showed good reliability (ICC=0.65). We found that the amount of time that adolescents spent in this hyperconnected state positively correlated with trait mindfulness.
Conclusions:
By applying dynamic functional connectivity analysis on over 100 resting-state fMRI scans, we identified a highly reliable brain state that correlated with trait mindfulness. The brain state may reflect a state of mindfulness, or awareness and arousal more generally, which may be more pronounced in those who are higher in trait mindfulness.
Keywords: Brain states, dynamics, hyperconnectivity, mindfulness, adolescents, trait mindfulness
Introduction
Adolescence is a time of rapid social, emotional, and brain maturation, and a critical time for the onset of mental illness. A meta-analysis of epidemiological studies found that 38% of adolescents with anxiety or fear-related disorders were diagnosed before the age of 14 (1). In the United States, 15% of adolescents experienced a major depressive episode in 2018 (2). Many adolescents continue to suffer from anxiety and/or depression into adulthood (3,4). There is a need to understand protective factors for mental illness in adolescence.
Trait mindfulness may be one such protective factor, defined as the tendency or disposition to pay attention to present moment experiences in a non-judgmental, accepting way (5). Trait mindfulness constructs were derived from mindfulness meditation practices and training (e.g., mindfulness-based stress reduction; (5)), which aim to not just cultivate mindfulness in the moment during meditation practice (‘state’ mindfulness) but extend the benefits to daily life (‘trait’ mindfulness). Trait mindfulness is typically measured using self-report scales which inquire about daily experiences. For example, the Child and Adolescent Mindfulness Measure (CAMM) has questions about emotional awareness: “I keep myself busy so I don’t notice my thoughts or feelings” (7). In adults, higher trait mindfulness scores have been consistently found to correlate with positive mental health outcomes, e.g. emotional well-being, and reduced psychopathology, e.g. reduced rumination and catastrophizing (8–10). In the last ten years, trait mindfulness scales have been validated for use with children and adolescents. These scales, e.g., the Mindful Attention Awareness Scale-Adolescents (11), and the Child and Adolescent Mindfulness Measure (CAMM) (7), have been found to likewise correlate with positive mental health status (12,13).
Despite the value of trait mindfulness for emotional well-being, little is known about the neural correlates of trait mindfulness in children and young adolescents (in contrast to numerous studies of adults; (14)). Three studies in children and adolescents have examined trait mindfulness correlates with task-based functional magnetic resonance imaging (fMRI activations) (15,16) and structural MRI (17). In consideration of traits that are supposedly consistent across contexts, resting-state fMRI is appealing because it measures functional connectivity of brain regions and networks that are independent of specific task demands. Resting-state fMRI contains both intrinsic, static components and time-varying, dynamic components (18–20). Static connectivity typically involves correlations between brain regions or networks over the course of an entire scan, whereas dynamic connectivity involves computing correlations within windows that are moved across the scan.
Only two studies have examined the relation of trait mindfulness to resting-state functional connectivity in children and adolescents. One study examined static connectivity in 23 adolescents who were remitted for MDD and 10 healthy controls, and found greater trait mindfulness to be inversely correlated with connectivity between the dorsolateral prefrontal cortex and the inferior frontal gyrus (two regions within the central executive network, CEN) (21). In the second study, we examined both static and dynamic resting-state functional network connectivity in relation to trait mindfulness in 42 children and adolescents (ages 6–17 years) with a focus on three brain networks: the CEN, the default mode network (DMN), and the salience network (SN) (22). Differing from the first study, we extracted functional networks using a group independent component analysis (ICA), which is a data-driven approach to finding spatially independent signals, each including a set of brain voxels sharing co-varying patterns (23). We found that more mindful children (as measured by the CAMM), showed less time in a brain state characterized by salience network (SN) anticorrelations with the other networks, a finding opposite from that found in adults (24). In addition, children who retrospectively reported more present-moment focused thoughts (less mind-wandering) during the scan showed less time in this brain state. Lastly, static functional network connectivity was not associated with trait mindfulness, suggesting the dynamic measures were more sensitive to trait mindfulness in this youth sample.
In the present study, we comprehensively investigated the functional brain bases of trait mindfulness in the largest sample (N>100) of adolescents to-date. We assessed the neural correlates of two trait mindfulness scales – the MAAS-A, which focuses on measuring day-to-day lapses of attention, and the CAMM, which focuses on emotional regulation and awareness. Our preregistered aim was to evaluate whether mindful adolescents show more or less time in an anticorrelated brain state, for instance, the DMN-SN anticorrelated brain state found in our previous study. We extracted functional brain networks using ICA, in keeping with evidence that ICA captures brain functional organization while retaining meaningful within-subject variability (25). We assessed 6 networks across the whole brain with both static and dynamic connectivity.
Prior to assessing correlations with mindfulness, we systematically investigated the scan-to-scan reliability of the functional connectivity measures. Reliability is an important component in brain-based individual differences research (26). If differences in functional imaging measures (e.g., connectivity) between individuals are not stable across imaging sessions, that is, lacking in consistency and/or agreement across sessions (which can be measured using intraclass coefficients (ICC) (27,28), then these measures cannot be predictively useful objective markers of traits of interest. A meta-analysis of static FC studies reporting reliability found ICCs for single connections were relatively low (29). Reliability issues could underlie discrepant results in studies of functional connectivity and trait mindfulness in adults (13). By focusing on reliability in this study, we hope to contribute to more replicable brain-behavior findings.
Methods and Materials
Preregistration
The analyses reported below were preregistered on the Open Science Framework (https://osf.io/wesu4).
Participants
A total of 127 young adolescents (12.04–14.69 years) were recruited using social media, flyers, and local schools. Inclusion and exclusion criteria are found in Supplement. One hundred and six of the 127 participants (50% M) had at least one usable resting-state fMRI run (n=100 had 2 usable runs), the remainder were excluded for sleep (n=3), failure to complete the scan (n=12), or excessive head motion (n=6). Procedures were approved by the Massachusetts Institute of Technology Committee on the Use of Human Subjects. All adolescents and their legal guardians provided assent and consent. Parents and adolescents were compensated for their time.
Self-report measures
Parents reported on household income, parental education, as well as their child’s race/ethnicity, medications, and medical conditions. Medications are listed in Table S1. Adolescents completed the remaining questions, including the self-report short pubertal scale (30), which provides two metrics: pubertal stage (categorical) and pubertal development (continuous).
We collected two trait mindfulness measures from the adolescents, the Mindful Attention and Awareness Scale for Adolescents (MAAS-A; hereafter ‘MAAS’) (11), and the Child and Adolescent Mindfulness Measure (CAMM) (7) (for details see Supplement). We collected the following additional self-report scales: depression (the MFQ; (31)), state and trait anxiety (STAI-C; (32)) and mind-wandering (the MWQ; (33)).
Self-report analysis
We imputed missing item data (0.5% of responses) for the self-report questionnaires using predictive mean matching in the mice data package (34). There were no significant demographic differences between participants with usable and unusable rest data (Supplement). We also examined associations between participant demographics and mindfulness scores, to identify possible confounds before the brain imaging data analysis. Lastly, we examined the bivariate relationships between the self-report measures, to assess the external validity of the mindfulness questionnaires.
Brain Imaging
Collection
Images were collected using a Siemens Prisma 3-T scanner with a 64-channel head coil. The protocol consisted of a T1-weighted scan, two fieldmaps (AP-PA and PA-AP), and then two resting-state scan runs (Supplement). Two resting-state scan runs of 4 minutes in duration each and TR of 0.8s were collected back-to-back. During the scans, participants were instructed to stare at a fixation cross. The T2*-weighted, gradient-recalled, multiband echo-planar imaging scan parameters were as follows for each of the two runs: multiband acceleration factor (8), TE (37 ms), flip angle (52°), echo spacing (0.58 ms), slice number (72), resolution (2 mm isotropic).
Preprocessing and denoising
Data were first preprocessed in fMRIPrep (v22.1.1), including T1 bias-field correction, fieldmap correction, brain extraction, normalization to the ICBM 152 nonlinear template, tissue segmentation, and motion correction procedures (35). We then spatially smoothed the functional data using a Gaussian kernel of 6 mm, and denoised the data in the CONN toolbox (36). We used standard denoising (37) (Supplement), with the exception of despiking instead of removing high motion frames (18).
Network extraction and dynamic functional connectivity analysis
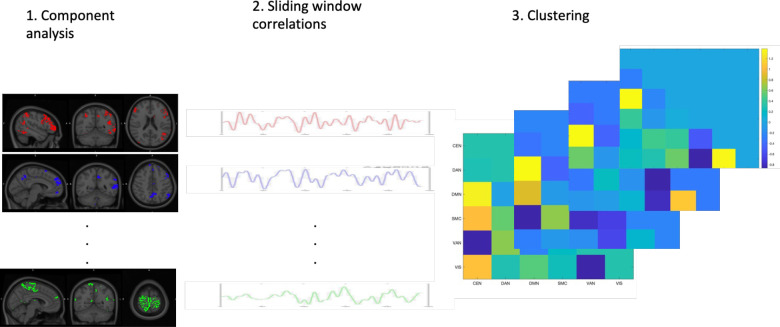
The procedure is outlined in Figure 1, and pipeline branches are shown in Table 2. We extracted networks using ICA, a data-driven analysis approach that finds spatially and temporally independent components (38). ICA was run on each individual separately, concatenating across the two runs, using FSL’s Melodic (39) (the Individual ICA), and on all participants together (the Group ICA in GIFT (v4.0c) (40) (Supplement). For Melodic, an automated network finding pipeline was executed with spatially cross-correlated networks from the 7-network Yeo atlas (41) and the components using FSL’s fslcc tool. As anticipated in our pre-registration, the limbic network often did not match any component closely (for ~ 1 in 4 participants), whereas the other 6 networks were consistently found (See Supplementary File 1 detailing the average correlations coefficients for each network). For this reason, the limbic network was omitted from analyses, resulting in 6 networks per participant: central executive network (CEN), dorsal attention network (DAN), default mode network (DMN), somatomotor cortex (SMC), ventral attention network (VAN), and visual network (VIS). Bilateral networks were treated as single networks (41). Network maps are provided in Supplement, as well as methods and network maps for Group ICA.
Figure 1:
Dynamic functional connectivity analysis. In 1) functional networks are extracted from participants resting-state fMRI scans using ICA. In 2) timecourses of the functional networks are correlated within sliding windows to make connectivity matrices. In 3) the time-varying connectivity matrices are clustered into distinctive states using k-means.
Table 2:
Reliabilities of dynamic and static functional network connectivity. Details run1-run2 reliabilities of ‘proportion of time’ in brain states as well as sFNC strengths for different pipelines.
| ICA | Sliding Window | Cluster Criterion | Number states | Reliabilities (ICC) |
|---|---|---|---|---|
| Individual | No convolution | Cal. | 2 | 0.65 |
| Elbow | 4 | 0.23–0.38 | ||
| Gap | 9(7) | 0.27–0.54 | ||
| Convolution | Cal. | 2 | 0.37 | |
| Elbow | 4(3) | 0.27–0.45 | ||
| Gap | 11(7) | 0.12–0.36 | ||
| Individual STATIC | NA | NA | NA | 0.31–0.53 (PS=0.70) |
| Group | No convolution | Cal. | 2 | 0.48 |
| Elbow | 4(3) | 0.24–0.39 | ||
| Gap | 11(5) | 0.07–0.33 | ||
| Convolution | Cal. | 2 | 0.33 | |
| Elbow | 4 | 0.18–0.34 | ||
| Gap | 11(8) | 0.10–0.32 | ||
| Group STATIC | NA | NA | NA | 0.01–0.50 (PS=0.57) |
NA: not applicable. Number of states vary based on clustering solutions (in parentheses are denoised selection). See methods for details on each branch. PS: pattern similarity, taking correlations between the sFNC patterns across runs.
We then extracted the mean time-courses of each network. First, we implemented a standard sliding window analysis using icatb_compute_dfnc from the GIFT toolbox (18). We took tapered windows with widths of 30 TRs (24 seconds) convolved with a Gaussian (3 TRs), and slid in steps of 1 TR (following our previous study (22)) resulting in 270 windows, to construct windowed correlation matrices (network connectivity, over time). We also explored a windowed approach without the Gaussian convolution. The windowed correlation matrices for all participants were then concatenated separately for run 1 and 2, resulting in two concatenated matrices (one for each run) that aggregated data across subjects.
To identify common patterns of connectivity (i.e., states) across participants and runs, k-means clustering was applied to these concatenated matrices in Matlab (scripts will be made available upon publication at https://osf.io/3gwt9/). We determined the optimal number of connectivity states by comparing the results from three separate cluster optimization techniques (Supplement).
Connectivity state analysis
Statistical analyses were performed using R. In rare cases, we removed connectivity states for signs of noise including extremely skewed distributions across participants (see Table 2), reclassifying windows using the remaining states. We calculated the proportion of time a participant spent in the connectivity state, the number of episodes in each state, and the average dwell time in each state, along with the total number of transitions. To examine the reliability of these dynamic measures, we conducted intra-class correlations (see below) across runs (e.g., does dwell time in state 1 in run 1 correlate with dwell time in state 1 in run 2?).
Finally, for those dynamic measures that were reliable, we calculated the average across the runs, and then assessed the relationships with the CAMM and MAAS scales individually. We controlled for framewise displacement, as well as pubertal stage (which we found correlated with mindfulness), given their possible impacts on functional connectivity (42–44). We controlled for multiple comparisons using false discovery rate (FDR) correction within each clustering solution for the set of reliable measures.
Static FNC analyses
Static functional network connectivity (sFNC) was calculated as the correlations between the networks across the entire time-courses (no windowing) of each run separately.
Reliability
The two separate resting-state runs allowed assessment of the consistency of individual brain measures across runs. We implemented this using intra-class correlation coefficients (ICCs) for two dynamic measures: connectivity state dwell time and overall proportions of time, as well as for sFNC edges (network-network correlations). This reliability measure, specifically ICC(2,1) has been used previously in test-retest applications in brain imaging (45). In the case of comparisons of sFNC patterns across runs, we calculated edge-wise ICCs, and also a metric of pattern similarity (Supplement) (46).
Results
Self-Report and Demographics
We assessed relationships between demographics and the two mindfulness variables of interest, the MAAS (M = 68.46, SD = 13.03) and the CAMM (M= 31.25, SD = 4.66) questionnaires. The CAMM and the MAAS were positively correlated (r = 0.53), and both scales were negatively correlated with pubertal development (B = −1.91, p = 0.033 and B = −5.15, p = 0.049, respectively) when controlling for all other demographic variables. These relationships were not explained fully by age (Tables S3 and S4). As there was a relatively small range of ages (12–15) and a wider range of pubertal development, pubertal development was included as a covariate in further analyses.
In addition, the self-report measures showed a series of negative bivariate relationships between mindfulness and mental health symptoms and mind-wandering (see Table S5).
Static Functional Network Connectivity
We assessed correlations (sFNC) between the 6 networks selected in Individual ICA. ICC of the sFNC edges ranged from 0.31–0.53. When assessing the pattern similarity between connectivity matrices across runs, the average was R = 0.70. The z-scored static connectivity for Individual ICA is shown in Figure 2A. Static FNC was characterized by high VAN-SMC correlation, low positive DMN correlations with other networks, and moderately high DAN correlations with other networks. Group ICA resulted in similar reliabilities (Supplement). We assessed the relationships between the individual edges in the sFNC matrices (for both ICA methods) and trait mindfulness, and when controlling for multiple comparisons, none were significant (FDR-corrected ps > 0.25).
Figure 2:
Static and dynamic functional connectivity matrices using the networks from individual ICA. Fisher’s z transform was applied to correlation coefficients. A) Static functional connectivity. B) Dynamic state 1 “static-like”, on left; Dynamic state 2: “hyperconnected”, on right. CEN: central executive network. DAN: Dorsal attention network. DMN: default mode network. SMC: sensorimotor cortex. VAN: ventral attention network. VIS: visual network.
Dynamic Functional Connectivity
Reliability for each pipeline was estimated as a data-driven approach for finding candidates for the dynamic correlates of trait mindfulness. Reliabilities across runs for each combination of networks, convolution method, and optimal clusters are shown in Table 2. Generally, reliability fell in the poor to fair range, where 0–0.4 = poor, 0.4–0.6 = fair, 0.6–0.75 = good and 0.75–1 = excellent (27,28). Reliabilities are shown for the dynamic ‘proportion of time’ metric, and not for dwell time, number of episodes, nor number of transitions, which almost always showed poor reliability. In general, reliability was higher for fewer states, for simple windowing without Gaussian convolution, and for individual ICA (which had fewer networks).
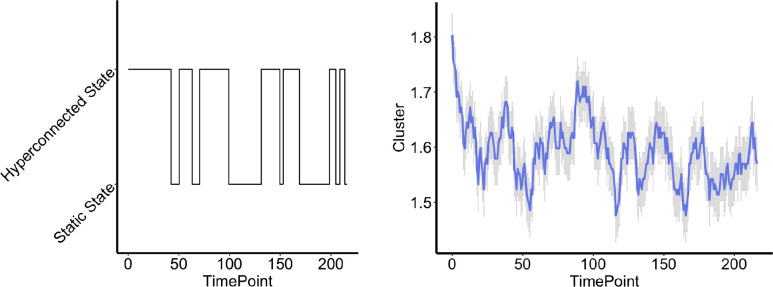
One solution fell into the ‘good’ range of reliabilities. That was individual ICA, using simple windowing, with two clusters. The clusters are shown in Figure 2B, and we term Brain State 1 the ‘static-like’ state (as it closely approximates the static functional connectivity), and Brain State 2 the ‘hyperconnected’ state with high correlations between all networks (Fisher’s zs > 0.6). Reliability of the proportion of time that the adolescents spent in each state was 0.65. Average dwell time in Brain State 1 showed a reliability of 0.56. Participants frequently switched between brain states, but tended to start in the hyperconnected state in both runs, and spent more time in the hyperconnected state overall (Figure 3).
Figure 3:
Timecourses of hyperconnected and static-like brain states. On left, a participant’s timecourse showing the switching between the static-like and hyperconnected brain states (1 and 2). On right, the timecourses are averaged across all participants for run 1. Higher values means more participants were in the hyperconnected brain state (2).
Correlations with Trait Mindfulness
We then ran correlations with trait mindfulness for the reliable dynamic brain states, controlling for average framewise displacement and pubertal development. There was a significant positive relationship between the CAMM and the proportion of time spent in the hyperconnected brain state (B = 0.0076, B = 0.244, p = 0.018; Figure 4), which equates to a negative relationship with the proportion of time spent in the static-like brain state. When controlling for multiple comparisons between the two mindfulness questionnaires and the two reliable dynamic measures, the relationship was trend-level (p = 0.072). We ran the analyses without controlling for puberty, and found significant relationships for hyperconnected state and static-like state proportion time (B = 0.0079, B = 0.255, p = 0.008; B = −0.0079, B = −0.255, p = 0.008), and static-like dwell time (B = −0.29, B = −0.204, p = 0.036). When controlling for multiple comparisons, the relationship was significant for static-like and hyperconnected proportion time (ps = 0.033) and trend-level for static-like dwell time (p = 0.072). There were no significant relationships with the MAAS. In post-hoc exploratory analyses, we examined mental health symptoms, mind-wandering, and composites of the MAAS and CAMM and found no significant relationships to the brain states.
Figure 4:
Brain dynamics correlate with trait mindfulness. CAMM: child and adolescent mindfulness measure. Hyperconnected Proportion (Brain State 2): Proportion of time in the hyperconnected brain state.
Sensitivity Checks
There was no significant relationship between proportion of time in the two brain states and average framewise displacement (p = 0.15). We examined the relationship with instantaneous head motion. First, head motion was not higher at the beginning of runs as compared to the end (Figure S3). Second, there was no zero-order correlation between brain state and head motion. We ran a cross-correlation analysis, and found a significant relationship using bootstrapping between lags 10–21 across both runs, but coefficients were small (R < 0.05; Figure S4). We additionally removed the first 5 timepoints of the runs, and the relationships between mindfulness and the brain states did not change. Robustness checks performed by removing high motion frames (scrubbing) did not change the pattern of results. When controlling for global static functional connectivity (averaged across all the edges), the relationship between hyperconnected proportion of time and mindfulness became trend-level (uncorrected p = 0.093).
Other clustering solutions
As a post-hoc analysis, we examined whether there were relationships between mindfulness and the other clustering solutions with lower reliability. No relationships survived multiple comparisons. Example connectivity states can be found in Figures S5 and S6. We examined connectivity states observed in our previous study and found no significant relationships with mindfulness (Supplement).
Discussion
In this preregistered analysis, we investigated the resting-state functional magnetic resonance imaging (fMRI) correlates of trait mindfulness in 106 adolescents. We examined static functional connectivity between networks and applied dynamic functional connectivity methods to identify time-varying connectivity states, or ‘brain states’. Like our previous study with children and adolescents (22), we found no significant relationships between static functional connectivity and trait mindfulness. In terms of dynamic brain states, previous studies have suggested that brain states characterized by anticorrelations between attentional networks may be related to trait mindfulness, linked to more present-focused thinking and less mind-wandering (22,24,47). In our adolescent sample, we did not find any indication of this relationship. Instead, we found a positive correlation between trait mindfulness and time spent in a hyperconnected brain state: adolescents scoring higher on the CAMM measure spent more time in a state with elevated positive connectivity between all brain networks. This is the first observation of this relationship, and there are a number of reasons why this finding should be given consideration.
First, this study was, to our knowledge, the largest study relating trait mindfulness to functional connectivity in adolescents with over a hundred participants. We followed a preregistered analysis pipeline, which may help avoid concerns of false positives due to researcher degrees of freedom (48). In addition, we systematically explored different decision points in dynamic functional connectivity analysis. We examined networks extracted from ICA run separately on every individual compared to networks from Group ICA (23). We examined different windowing methods and different criteria for the clustering of connectivity matrices. We explored these decision points with the goal of optimizing reliability across runs of the resting-state data. Reliability is an important factor to consider in individual differences research (26). If the variance within-individuals is larger than the variance between-individuals, the measure may not be a good candidate to relate to a stable trait. Thus, we optimized reliability across resting-state runs before conducting correlations with mindfulness.
The two-cluster solution identified two reliable brain connectivity states – a ‘hyperconnected’ dynamic brain state, and a ‘static-like’ brain state, as it approximates the static functional connectivity over the course of the scans. The adolescents in this study tended to start in the hyperconnected brain state at the beginning of runs and spent more time in the state overall. Hyperconnected brain states have been reported previously (43,47,49–51), and so have dynamic brain states that are similar in network-network correlations to static functional connectivity (52). In addition, hyperconnectivity throughout many networks may be an emerging signature of the brain’s response to psychedelic drugs (53), and global hypoconnectivity has been found in depression (54,55). A common thread in these findings could be underlying cognitive flexibility and associated neural flexibility of brain states. Like psychedelics, mindfulness meditation may increase cognitive flexibility and lead to a wider range of brain states, including hyperconnected brain states (14,56). However, global hyperconnectivity has also been previously associated with head motion or physiological noise (57–59). Critically, we ruled out the possibility that head motion explained our findings by controlling for average head motion, and examining the relationship within individuals (where HM explained less than 0.25% of variance in brain states). Future studies should examine brain state relationships with physiological signals.
Previous neuroimaging studies have only examined single measures of mindfulness (21,22). We collected two self-report measures of mindfulness – the Child and Adolescent Mindfulness Measure (CAMM)(7), and the Mindful Attention and Awareness Scale – Adolescent (MAAS)(11). Both measures of mindfulness were negatively associated with depression, anxiety, and mind-wandering. The CAMM, but not the MAAS, was found to be correlated with the proportion of time participants spent in the hyperconnected brain state, and the average dwell time (how much time is spent on average per episode in the state). A meta-analysis of resting-state static functional connectivity and mindfulness interventions in adults found increased connectivity after mindfulness interventions, but it was specific to DMN-SN connections (60). The finding that mindfulness was positively related to the amount of time spent in greater connectivity across all examined (reliable) networks is a novel observation. This result is specific to the CAMM and no significant correlations were found when examining the MAAS, or composites of the MAAS and CAMM. Items on the CAMM were adapted from the Kentucky Inventory of Mindfulness Skills (KIMS; 61), specifically from three facets of the KIMS: observing, acting with awareness, and accepting without judgment. The CAMM has questions about emotional awareness, e.g. “I keep myself busy so I don’t notice my thoughts or feelings.” The CAMM shows negative correlations with rumination, stress, negative affect, and emotional and behavioral difficulties (7,12,62). The emotional focus of the CAMM contrasts with the ‘receptive attention’ focus of the MAAS (e.g. “I find myself doing things without paying attention”; 11). Thus our findings indicate that the hyperconnected brain state may be more frequent in individuals better able to notice and regulate their emotions, and contribute to emerging literature distinguishing different brain correlates of aspects of mindfulness (63,64,65).
Limitations
As the hyperconnected state was more present in the beginning of scans, it is possible that it reflects an arousal response present when starting scans. Indeed, prior works have observed that moment-to-moment measures of physiological arousal fluctuate with greater global integration of functional connections (66). We caution attributing this finding to specific cognitive processes (19), as we did not collect any self-report measures about the scans from the adolescents. In addition, global static functional connectivity was correlated with time in the hyperconnected brain state, and the relationship to mindfulness was still present, but weaker, when controlling for global static FC. It is possible that the dynamic outcomes we derived are an index of hyperconnectivity more generally.
As described, it is unclear exactly what the hyperconnected brain state detected here and reported in prior studies reflects. In addition, one might question whether other, less reliable, brain states may be related to mindfulness. Researchers have cautioned against optimizing reliability at the cost of validity (67). Indeed, poor reliability does not eliminate the possibility of finding a relationship, but it puts an upper bound on the strength of the relationship (68). In a post hoc analysis, we examined whether any of the lower reliability brain states were related to mindfulness, and none were. It should also be noted that when we examined anticorrelated connectivity states previously identified in the literature (with high face validity), we found no relationship with mindfulness.
Conclusions
We conducted the largest resting-state fMRI study of trait mindfulness in adolescents to-date, examining static and dynamic functional connectivity measures. We identified a reliable hyperconnected brain state that correlated with a trait mindfulness measure related to emotional responding. Future work could examine the state in a wider range of contexts including with momentary self-report assessments.
Supplementary Material
Table 1:
Adolescent demographics as reported by parents. Percentages are shown in parentheses.
| Variable | N = 106 | Variable | N = 106 |
|---|---|---|---|
|
| |||
| Age (years) | Race/Ethnicity (%) | ||
| Mean | 13.46 | White | 65(61.3) |
| Range | 12.04–14.69 | Black | 8(7.5) |
| Sex | Asian | 4(3.8) | |
| Male (%) | 53 (50%) | Hispanic | 10(9.4) |
| Female (%) | 53 (50%) | Pacific | 0(0) |
| Handedness | Amerindian | 0(0) | |
| RH (%) | 92 (86.7%) | Other | 6(5.7) |
| LH (%) | 12 (11.3%) | Mixed | 13(12.3) |
| A (%) | 2 (1.8%) | Pubertal Stage (%) | |
| Income | Pre | 1(0.9) | |
| Mean (SD) | 137,742.20 $ (138,169.90 $) | Early | 8(7.5) |
| Range | 6500$–1.25M $ | Mid | 38(35.8) |
| Late | 16(15.1) | ||
| Post | 36(34.0) | ||
| NR | 5(4.7) | ||
| Conditions (%) | |||
| ADHD | 21(19.8) | ||
| Seizures | 2(1.9) | ||
| Concussions | 8(7.5) | ||
| Anxiety/Depression | 8(7.5) | ||
| On Medication | 25(23.6) | ||
Income: Household Income (USD). M: million. ADHD: Attention Deficit Hyperactivity Disorder. NR: no response. A: ambidextrous.
Funding statement:
This research was supported by the William and Flora Hewlett Foundation (#4429 [J. D. E. G.]).
I.N.T was supported by the Hock E. Tan and K. Lisa Yang Center for Autism Research.
A.K. was supported by the National Institute of Mental Health of the National Institutes of Health under award numbers R21MH127384 and R21MH129630.
NAH was partially supported by the Brain and Behavior Research Foundation (#27970) and the Rural Drug Abuse Research Center (P20GM130461[6026]).
Footnotes
Ethics statement: Procedures were approved by the Massachusetts Institute of Technology Committee on the Use of Human Subjects. All adolescents and their legal guardians provided assent and consent. Adolescents were compensated for their time.
Conflict of interest statement: the authors declare no competing interests
Data availability statement:
Code and data will be uploaded to the following link upon publication: https://osf.io/3gwt9/.
References
- 1.Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. (2022): Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies [no. 1]. Mol Psychiatry 27: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (n.d.). Youth Risk Behavior Survey. Retrieved January 2, 2024, from https://www.cdc.gov/healthyyouth/mental-health/index.html
- 3.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Üstün TB (2007): Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 20: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R (2003): Prior Juvenile Diagnoses in Adults With Mental Disorder: Developmental Follow-Back of a Prospective-Longitudinal Cohort. Arch Gen Psychiatry 60: 709–717. [DOI] [PubMed] [Google Scholar]
- 5.Brown KW, Ryan RM (2003): The Benefits of Being Present: Mindfulness and Its Role in Psychological Well-Being. J Pers Soc Psychol. 10.1037/0022-3514.84.4.822 [DOI] [PubMed] [Google Scholar]
- 6.Kabat-Zinn J (1982): An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen Hosp Psychiatry 4: 33–47. [DOI] [PubMed] [Google Scholar]
- 7.Greco LA, Baer RA, Smith GT (2011): Assessing Mindfulness in Children and Adolescents: Development and Validation of the Child and Adolescent Mindfulness Measure (CAMM). Psychol Assess 23: 606–614. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter JK, Conroy K, Gomez AF, Curren LC, Hofmann SG (2019): The relationship between trait mindfulness and affective symptoms: A meta-analysis of the Five Facet Mindfulness Questionnaire (FFMQ). Clin Psychol Rev 74: 101785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keng SL, Smoski MJ, Robins CJ (2011): Effects of mindfulness on psychological health: A review of empirical studies. Clin Psychol Rev 31: 1041–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomlinson ER, Yousaf O, Vittersø AD, Jones L (2018): Dispositional Mindfulness and Psychological Health: a Systematic Review. Mindfulness 9: 23–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown KW, West AM, Loverich TM, Biegel GM (2011): Assessing Adolescent Mindfulness: Validation of an Adapted Mindful Attention Awareness Scale in Adolescent Normative and Psychiatric Populations. Psychol Assess 23. 10.1037/a0021338 [DOI] [PubMed] [Google Scholar]
- 12.de Bruin EI, Zijlstra BJH, Bögels SM (2014): The Meaning of Mindfulness in Children and Adolescents: Further Validation of the Child and Adolescent Mindfulness Measure (CAMM) in Two Independent Samples from The Netherlands. Mindfulness. 10.1007/s12671-013-0196-8 [DOI] [Google Scholar]
- 13.Treves IN, Li CE, Wang KL, Ozernov-Palchik O, Olson HA, Gabrieli JD (2023): Mindfulness supports emotional resilience in children during the COVID-19 pandemic. Plos One 18: e0278501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treves IN, Bauer CCC, Sacchet MD, Greene KD, Berkovich-Ohana A, Whitfield-Gabrieli S (in press): Toward a Brain Network Science of Mindfulness. The Handbook of Mindfulness and Self-Regulation: Cognitive Neuroscience, Social Personality, Clinical, and Applied Perspectives. New York, NY, US: Springer. [Google Scholar]
- 15.Hehr A, Iadipaolo AS, Morales A, Cohen C, Taub JW, Harper FWK, et al. (2022): Meditation reduces brain activity in the default mode network in children with active cancer and survivors. Pediatr Blood Cancer 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein JA, Bray S, MacMaster FP, Tomfohr-Madsen L, Kopala-Sibley DC (2022): Adolescents with High Dispositional Mindfulness Show Altered Right Ventrolateral Prefrontal Cortex Activity During a Working Memory Task. Mindfulness 13: 198–210. [Google Scholar]
- 17.Friedel S, Whittle SL, Vijayakumar N, Simmons JG, Byrne ML, Schwartz OS, Allen NB (2015): Dispositional mindfulness is predicted by structural development of the insula during late adolescence. Dev Cogn Neurosci 14: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014): Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24: 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kucyi A (2018): Just a thought: How mind-wandering is represented in dynamic brain connectivity. NeuroImage 180: 505–514. [DOI] [PubMed] [Google Scholar]
- 20.Preti MG, Bolton TA, Van De Ville D (2017): The dynamic functional connectome: State-of-the-art and perspectives. NeuroImage 160: 41–54. [DOI] [PubMed] [Google Scholar]
- 21.Peters AT, Burkhouse K, Feldhaus CC, Langenecker SA, Jacobs RH (2016): Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: A pilot study among adolescents with a history of depression. J Affect Disord 200: 178–181. [DOI] [PubMed] [Google Scholar]
- 22.Marusak HA, Elrahal F, Peters CA, Kundu P, Lombardo MV, Calhoun VD, et al. (2018): Mindfulness and dynamic functional neural connectivity in children and adolescents. Behav Brain Res 336: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14: 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim J, Teng J, Patanaik A, Tandi J, Massar SAA (2018): Dynamic functional connectivity markers of objective trait mindfulness. NeuroImage 176: 193–202. [DOI] [PubMed] [Google Scholar]
- 25.Yu Q, Du Y, Chen J, He H, Sui J, Pearlson G, Calhoun VD (2017): Comparing brain graphs in which nodes are regions of interest or independent components: A simulation study. J Neurosci Methods 291: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois J, Adolphs R (2016): Building a Science of Individual Differences from fMRI. Trends Cogn Sci 20: 425–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cicchetti DV (1994): Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 6: 284. [Google Scholar]
- 28.Cicchetti DV, Sparrow SA (1981): Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 86: 127–137. [PubMed] [Google Scholar]
- 29.Noble S, Scheinost D, Constable RT (2019): A decade of test-retest reliability of functional connectivity: A systematic review and meta-analysis. NeuroImage 203: 116157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carskadon MA, Acebo C (1993): A self-administered rating scale for pubertal development. J Adolesc Health 14: 190–195. [DOI] [PubMed] [Google Scholar]
- 31.Kent L, Vostanis P, Feehan C (1997): Detection of Major and Minor Depression in Children and Adolescents: Evaluation of the Mood and Feelings Questionnaire. J Child Psychol Psychiatry 38: 565–573. [DOI] [PubMed] [Google Scholar]
- 32.Spielberger CD, Edwards CD, Montouri J, Lushene R (2012, July 9): State-Trait Anxiety Inventory for Children. 10.1037/t06497-000 [DOI] [Google Scholar]
- 33.Mrazek MD, Phillips DT, Franklin MS, Broadway JM, Schooler JW (2013): Young and restless: validation of the Mind-Wandering Questionnaire (MWQ) reveals disruptive impact of mind-wandering for youth. Front Psychol 4: 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buuren S van, Groothuis-Oudshoorn K (2011): mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 45: 1–67. [Google Scholar]
- 35.Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. (2019): fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield-Gabrieli S, Nieto-Castanon A (2012): Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect 2: 125–141. [DOI] [PubMed] [Google Scholar]
- 37.Kucyi A, Esterman M, Capella J, Green A, Uchida M, Biederman J, et al. (2021): Prediction of stimulus-independent and task-unrelated thought from functional brain networks. Nat Commun 12. 10.1038/s41467-021-22027-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyvärinen A, Karhunen J, Oja E (2001): Independent Component Analysis. Independent Component Analysis. 10.1002/0471221317 [DOI] [Google Scholar]
- 39.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, vol. 23 23. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 40.Correa N, Adali T, Li Y-O, Calhoun VD (2005): Comparison of blind source separation algorithms for FMRI using a new Matlab toolbox: GIFT. Proceedings. (ICASSP ‘05). IEEE International Conference on Acoustics, Speech, and Signal Processing, 2005., vol. 5 5: v/401-v/404 Vol. 5. [Google Scholar]
- 41.Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Zhao X, Zhang X, Liu Y, Zhou P, Ni H, et al. (2018): Age-related early/late variations of functional connectivity across the human lifespan. Neuroradiology 60: 403–412. [DOI] [PubMed] [Google Scholar]
- 43.Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, et al. (2014): Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clin 5: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Dijk KRA, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taxali A, Angstadt M, Rutherford S, Sripada C (2021): Boost in Test-Retest Reliability in Resting State fMRI with Predictive Modeling. Cereb Cortex N Y N 1991 31: 2822–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubbard NA, Auerbach RP, Siless V, Lo N, Frosch IR, Clark DE, et al. (2023): Connectivity Patterns Evoked by Fearful Faces Demonstrate Reduced Flexibility Across a Shared Dimension of Adolescent Anxiety and Depression. Clin Psychol Sci 11: 3–22. [Google Scholar]
- 47.Mooneyham BW, Mrazek MD, Mrazek AJ, Mrazek KL, Phillips DT, Schooler JW (2017): States of Mind: Characterizing the Neural Bases of Focus and Mind-wandering through Dynamic Functional Connectivity. J Cogn Neurosci 29: 495–506. [DOI] [PubMed] [Google Scholar]
- 48.Nosek BA, Ebersole CR, DeHaven AC, Mellor DT (2018): The preregistration revolution. Proc Natl Acad Sci 115: 2600–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Lacy N, Calhoun VD (2018): Dynamic connectivity and the effects of maturation in youth with attention deficit hyperactivity disorder. Netw Neurosci 3: 195–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS (2013): Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp 34: 2154–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maillet D, Beaty RE, Kucyi A, Schacter DL (2019): Large-scale network interactions involved in dividing attention between the external environment and internal thoughts to pursue two distinct goals. NeuroImage 197: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu Z, Caprihan A, Chen J, Du Y, Adair JC, Sui J, et al. (2019): Altered static and dynamic functional network connectivity in Alzheimer’s disease and subcortical ischemic vascular disease: shared and specific brain connectivity abnormalities. Hum Brain Mapp 40: 3203–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gattuso JJ, Perkins D, Ruffell S, Lawrence AJ, Hoyer D, Jacobson LH, et al. (2023): Default Mode Network Modulation by Psychedelics: A Systematic Review. Int J Neuropsychopharmacol 26: 155–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berman MG, Misic B, Buschkuehl M, Kross E, Deldin PJ, Peltier S, et al. (2014): Does resting-state connectivity reflect depressive rumination? A tale of two analyses. NeuroImage 103: 267–279. [DOI] [PubMed] [Google Scholar]
- 55.Veer IM, Beckmann C, Van Tol M-J, Ferrarini L, Milles J, Veltman D, et al. (2010): Whole Brain Resting-State Analysis Reveals Decreased Functional Connectivity in Major Depression. Front Syst Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Escrichs A, Sanjuán A, Atasoy S, López-González A, Garrido C, Càmara E, Deco G (2019): Characterizing the dynamical complexity underlying meditation. Front Syst Neurosci 13. 10.3389/fnsys.2019.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birn RM (2012): The role of physiological noise in resting-state functional connectivity. NeuroImage 62: 864–870. 10.1016/j.neuroimage.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 58.Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. (2017): Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage 154: 174–187. 10.1016/j.neuroimage.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84: 320–341. 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahrig H, Vago DR, Passarelli M, Auten A, Lynn NA, Brown KW (2022): Disrupting The Resting State: Meta-Analytic Evidence That Mindfulness Training Alters Default Mode Network Connectivity. Sci Rep 1–21. 10.1038/s41598-022-15195-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baer RA, Smith GT, Allen KB (2004): Assessment of mindfulness by self-report: The Kentucky inventory of mindfulness skills. Assessment 11: 191–206. 10.1177/1073191104268 [DOI] [PubMed] [Google Scholar]
- 62.Kuby AK, McLean N, Allen K (2015): Validation of the Child and Adolescent Mindfulness Measure (CAMM) with Non-Clinical Adolescents. Mindfulness 6: 1448–1455. 10.1007/s12671-015-0418-3 [DOI] [Google Scholar]
- 63.Treves IN, Kucyi A, Park M., Kral TRA; Goldberg SB; Davidson RJ; Rosenkranz M; Whitfield-Gabrieli S.; Gabrieli JDE (under review): Connectome predictive modeling of trait mindfulness. [DOI] [PubMed] [Google Scholar]
- 64.Tsai N., Treves I. N., Bauer C. C. C., Scherer E., Caballero C., West M. R., & Gabrieli J. D. E. (2024). Dispositional mindfulness: Dissociable affective and cognitive processes. Psychonomic Bulletin & Review. 10.3758/s13423-024-02462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhuang K., Bi M., Li Y., Xia Y., Guo X., Chen Q., Du X., Wang K., Wei D., Yin H., & Qiu J. (2017). A distinction between two instruments measuring dispositional mindfulness and the correlations between those measurements and the neuroanatomical structure. Scientific Reports, 7(1), 1–9. 10.1038/s41598-017-06599-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shine JM, Bissett PG, Bell PT, Koyejo O, Balsters JH, Gorgolewski KJ, et al. (2016): The Dynamics of Functional Brain Networks: Integrated Network States during Cognitive Task Performance. Neuron 92: 544–554. 10.1016/j.neuron.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finn ES, Rosenberg MD (2021): Beyond fingerprinting: Choosing predictive connectomes over reliable connectomes. NeuroImage 239. 10.1016/j.neuroimage.2021.118254 [DOI] [PubMed] [Google Scholar]
- 68.Nunnally JC (1970): Introduction to psychological measurement. Retrieved January 20, 2024, from https://psycnet.apa.org/PsycINFO/1970-19724-000
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code and data will be uploaded to the following link upon publication: https://osf.io/3gwt9/.