Abstract
Resistance to starvation is a classic complex trait where genetic and environmental variables can greatly modify an animal’s ability to survive without nutrients. Genetic analyses in Drosophila have shown that starvation resistance is highly polygenic with different genetic architectures in different mapping populations. In this study we sought to dissect the genetic basis of starvation resistance among a set of mitonuclear genotypes carrying different mtDNAs paired with specific nuclear genomes from the Drosophila Genetic Reference Panel (DGRP). We focused on differences between one of the most sensitive strains (DGRP-765) and a strain with more moderate resistance (DGRP-315) whose starvation phenotypes appeared to be modified by alternative mtDNAs. Using complementary pooled-sequencing and forward genetic mapping approaches, we identified regions of chromosomes 2L, 3L and 3R contributing to starvation sensitivity and localize a major effect locus modifying starvation resistance to the coding region of phospholipase iPLA2-VIA. These analyses further confirm that the alternative mtDNAs had little influence on variation in starvation resistance between the genotypes studied. The sensitive line shows a starvation-dependent depletion of glucose and glycogen that is modified by hemi- and heterozygosity in the iPLA2-VIA region. These findings contribute to our understanding of the complex genetic relationship between resistance to starvation stress and nutrient metabolism.
Introduction
Coping with periods of low nutrient availability is a fundamental fitness component of all organisms. In animals, multiple metabolic processes are required to convert dietary nutrients into usable resources to successfully cope with periods of starvation. Energy that is not needed immediately has to be converted into a form amenable for efficient storage and that stored energy has to be accessible for utilization during periods of starvation. For these reasons, the genetics of starvation resistance provides a compelling model for understanding the integrative aspects of animal physiology (Brown et al. 2019). Furthermore, a better understanding of the genetic basis of nutrient catabolism and storage will also shed a light on genes relevant to metabolic diseases and fitness traits in nature (Hardy et al. 2018).
Much of the information on the genetics of starvation resistance comes from studies in Drosophila employing either direct selection on experimental outbred populations or genome wide association studies (GWAS) across samples of inbred lines. Variation in starvation resistance is an appealing trait for studies of quantitative and evolutionary genetics due to its highly polygenic nature and its correlation with other fitness and metabolic traits (Service and Rose 1985; Chippindale et al. 1996; Harshman et al. 1999; Hardy et al. 2018; Everman et al. 2019). GWAS approaches using either the Drosophila Genetic Reference Panel (DGRP;(Mackay et al. 2012)) or the Drosophila Synthetic Population Resource (DSPR;(Everman et al. 2019)) have identified a number of candidate loci influencing variation in starvation resistance confirming the complexity of its polygenic nature (Harbison et al. 2004; Sorensen et al. 2007; Huang et al. 2012; Mackay et al. 2012; Everman and Morgan 2018; Everman et al. 2019). Importantly, there are clear differences among studies in the location of QTLs and the putative candidate loci identified (Huang et al. 2012; Mackay et al. 2012; Everman and Morgan 2018; Everman et al. 2019). These differences have been attributed to the distinct genetic composition of the mapping populations and presumably unique epistatic interactions among loci influencing starvation resistance (ops. cit.). Notably, none of the QTL identified in the two subsets of the mapping lines used by (Everman et al. 2019) overlapped, likely due to the different founder lines used to build each subset (DSPR pA and pB). Despite these differences, there are shared loci that emerge from a comparison of multiple studies (table S9 in (Everman et al. 2019)).
A growing literature has identified a role for mitochondrial functions in stress responses at multiple levels (Uma Naresh et al. 2022; Erkosar et al. 2025). Given the central roles that mitochondria play in both anabolic and catabolic functions, the complex interactions between mitochondrial and nuclear genomes may play important roles in adaptive traits, and particularly in metabolically demanding conditions such as starvation, oxidative stress and animal performance (Rand et al. 2004; Hill 2015). In previous work, we demonstrated the impact of mitonuclear epistasis in development time among a panel of 72 mitonuclear genotypes constructed from 6 mtDNAs introgressed into 12 nuclear genomes from the Drosophila Genetic Reference Panel (DGRP) (Mossman et al. 2016). We selected a subset of these lines to examine starvation resistance based on significant differences in development time between mtDNAs in each of several DGRP backgrounds. These analyses revealed a putative mitonuclear epistatic interaction influencing starvation resistance and its relation to diet composition. In the current study, we sought to use a combination of quantitative genetic and classical genetic mapping approaches to examine the genetic basis of starvation resistance between two DGRP lines carrying different mtDNAs (DGRP-765 and -315). Genomic sequencing of sensitive and resistant pools of adults from advanced intercross populations derived from these lines identified regions of allelic differentiation. Chromosome segregation and recombination mapping identified major factors on chromosome 3L, 3R and 2L, but not chromosome X, 2R or the mitochondrial genome. Deficiency mapping indicated that the starvation sensitivity in DGRP-315 localizes in part to the phospholipase iPLA2-VIA gene with associated effects of carbohydrate metabolism. The correspondence of these approaches contributes to our understanding of the polygenic nature of starvation resistance and points to polymorphisms in the iPLA2-VIA region that motivate further study of the associations between starvation sensitivity, lipid metabolism and carbohydrate metabolism.
Materials and Methods
Fly stocks
The current study focuses on the differences in starvation resistance between specific mitonuclear genotypes carrying alternative mtDNAs placed onto nuclear genomic backgrounds of the Drosophila Genetic Reference Panel (DGRP;(Mackay et al. 2012)). In a previous study we constructed a panel of 72 mitonuclear genotypes using balancer chromosome substitution to place six mtDNAs onto each of 12 DGRP nuclear backgrounds to study mitonuclear epistasis for development time and its dietary modification (Mossman et al. 2016). From these 72 lines we quantified starvation resistance of 12 mitonuclear genotypes consisting of four different mtDNAs placed on three DGRP nuclear backgrounds (lines DGRP-765, -315 and -820). The four mtDNAs included two from D. simulans (siI and siII) and two from D. melanogaster (OregonR = OreR and Zimbabwe53 = Zim53). These mtDNAs were chosen to test for the impact of mtDNA polymorphism and divergence on starvation resistance. The DGRP backgrounds were chosen based on published starvation data showing that DGRP-765, -315 and -820 had low, medium and high values of starvation resistance, respectively (Ayroles et al. 2009; Mackay et al. 2012). The genotype notation used is mtDNA;nuclearDNA, e.g., Zim53;315 carries the D. melanogaster Zim53 mtDNA on a homozygous DGRP-315 chromosomal background.
The results of this starvation assay are shown in Figure 1. The resistance levels were consistent with previously published rankings of the three nuclear genotypes (820 > 315 > 765). The results further showed that one unique combination, siI;765, (a D. simulans siI mtDNA on the DGRP-765 nuclear background) had a significant increase in starvation resistance compared to the other mitonuclear genotypes in the DGRP-765 background. The goal of the current study was to determine whether the apparent ‘rescue’ of starvation resistance was due to the introgression of the siI mitochondrial haplotype or due to a nuclear factor or factors. The focal mitonuclear genotypes are shown in Table 1. Additional genotypes for segregation, recombination and deficiency mapping are listed in Table S1.
Figure 1. Starvation time among mitonuclear genotypes on different diets.

The x-axis lists the genotypes with the notation: mtDNA;nucDNA-Diet, with Diets I, IV and V being balanced, low and high sugar:yeast ratios, respectively (10g:10g, 5g:15g, 15g:5g; see Methods).
Table 1. Mitonuclear stocks used for genetic analyses in this study.
A table of additional stocks used for chromosome substitution, recombination mapping and deficiency mapping are listed in supplemental file 1.
| Stock Name (mtDNA;nuclearDNA) | Mitochondrial haplotype | Nuclear haplotype | Phenotype |
|---|---|---|---|
| Zim53;765 | D. melanogaster Zim53 | DGRP-765 | Starvation sensitive |
| siI;765 | D. simulans siI | DGRP-765 recombinant | Starvation resistant |
| DGRP-765 | From original DGRP-765 line | DGRP-765 | Starvation sensitive |
| Zim53;315 | D. melanogaster Zim53 | DGRP-315 | Starvation resistant |
Starvation Stress
Survival during starvation stress was measured by placing approximately 125 female flies per genotype in each of three replicate demography cages. Flies were collected and sorted over CO2 and allowed to recover in quart-sized plastic demography cages affixed with a 25×95 mm vial containing one of three different isocaloric food media: high sugar food (15% sucrose, 5% Yeast, 2% agar), balanced food (10% sucrose, 10% yeast, 2% agar) or low sugar food (5% sucrose, 15% yeast, 2% agar; referred to as food type V, II and IV, respectively, in (Zhu et al. 2014)). The three replicate cages for each genotype were conditioned on these diets for 7 days unless otherwise indicated, with new food vials added every other day. After 7 days, any dead flies were removed, and the vial for each demography cage was replaced with 2% Difco Bacto-agar prepared with deionized H20 to initiation starvation. The number of dead flies was recorded every 12 hours and removed from each demography cage until all flies were dead. As starvation resistance values for the different genotypes were most distinct on the high sugar diet “V”, most of the genetic mapping was carried out on that diet. The survival data from each cage were generally consistent so counts from each replicate cage were pooled and the combined data modeled using a Kaplan-Meir survival model implemented in R (Therneau and Grambsch 2000). Significant differences in survivals were determined using a log rank test.
Forward genetic mapping of starvation sensitivity
Chromosomal localization of resistance.
To determine if different mtDNAs were associated with the difference in starvation resistance reciprocal crosses were done between siI;765 and Zim53;765 as follows: virgin female siI;765 × male Zim53;765 and virgin female Zim53;765 × male siI;765). Maternal inheritance of mtDNA provides a test for the role of the siI mtDNA in the rescue of starvation sensitivity. To localize nuclear factors associated with starvation sensitivity we generated genotypes that were selectively heterozygous for different combinations of Zim53;765 autosomes. Twenty Zim53;765 virgin females were first crossed to 20 males from the double-balancer stock (wgSp-1/CyO; Dr1/TM3, Sb1). Twenty F1 male progeny with both the 2nd chromosome CyO and 3rd chromosome Dr markers present were sorted over CO2 and crossed to 20 Zim53;765 virgin females in eight replicate 20-female × 20-male crosses. Progeny from this second cross were segregated into four groups based on the presence or absence of the CyO and Dr markers. The four groups were: reconstituted wild-type Zim53;765, chromosome 2 heterozygotes (CyO/765), chromosome 3 heterozygotes (Dr/765) and heterozygotes for both chromosome 2 and 3 (CyO/765;Dr/765). Flies from each group were moved to 3 replicate demography cages with approximately 125 flies each. The survival of each group under starvation stress was assayed as described earlier.
Whole genome sequencing of sensitive and resistant pools from Advanced Intercross Populations
Population Cages.
Five replicate advanced intercross populations (AIPs) were established between the sensitive (Zim53;765) and resistant (Zim53;315) lines. Each replicate population was initiated using 25 Zim53;315 virgin females crossed to 25 Zim53;765 males, in addition to the reciprocal cross, to yield a total number of 50 male and 50 female parents of each line as the founders of each of the five replicate cages. These parents were introduced into Plexiglas cages (20 × 20 × 20cm) and allowed to lay eggs in four 125mL culture bottles with 30mL of Drosophila medium in each bottle. The four bottles in each cage were left with their tops open for 3 days, and then each bottle was plugged to allow flies to develop. Prior to eclosion, the plugs were removed from each bottle, and all flies were allowed to emerge into the cage and mate at random. Four new culture bottles replaced the old bottles in each replicate cage, and the adults were allowed to lay eggs for 48 hours. After egg laying, adults were cleared from each bottle, plugs were inserted, and cultures were allowed to develop for 7 days following the termination of egg laying, after which the plugs were removed from all bottles to allow eclosion of the next generation into each cage. Population sizes exceeded 1000 individuals after the first generation. All five cages were maintained at large population sizes (>2000 adults) for 10 generations to allow recombination to take place between the sensitive (Zim53;765) and resistant (Zim53;315) DGRP nuclear genotypes. These two DGRP strains are homozygous for the standard (ST) chromosome arrangement (http://dgrp2.gnets.ncsu.edu/) so this lack of inversions should have allowed free recombination across the genome in these populations.
Starvation Stress.
The female flies from each generation-10 population cage were collected over CO2 and moved to demography cages with a density of approximately 150 flies per cage. The flies were allowed to recover for 7 days with access to high sugar food (Type V: 15% sucrose, 5% Yeast, 2% agar) and then subjected to starvation conditions by replacing the food vials with a 2% agar and water mix. Every six hours from the start of starvation, all the dead flies were collected, flash frozen and stored at −80C. The high sugar treatment enhanced the difference among genotypes in starvation resistance. The genotypic differences in starvation were also evident with moderate or low sugar treatments but mapping experiments were more effective with the high sugar treatment so that was used throughout these analyses.
Pool-sequencing.
Pools of approximately 50 dead flies were made by combining flies from the two earliest time points when deaths were first observed (24 hour and 30 hour) and the last two time points when the last live flies were present (96 hour and 102 hour). DNA from each replicate pool was isolated using the DNeasy DNA purification kit from Qiagen (Cat No 69506). Each sample was RNase treated prior to DNA elution using a 10-minute RNase A (Cat No 19101) treatment. Libraries were prepared and sequenced using the DNBseq platform by the Beijing Genomics Institute (BGI).
Estimation of genetic differentiation using Popoolation2.
Raw reads were mapped to the dm6 build of the Drosophila genome using bwa version 0.7.15 (Li and Durbin 2009). Variants were called using samtools version 1.9 (Li et al. 2009). Allele frequency differences and estimates of population subdivision (FST) were determined using Popoolation2 (Kofler et al. 2011), and the significance of differences in allele frequencies between the early-dying and late-dying sample across the five replicate populations was determined using the Cochran-Mantel-Haenszel test. P-values were corrected using the Bonferroni method and differences with FDR-corrected p-values < 0.05 were considered to be significant. Linkage disequilibrium was estimated from paired end reads, following the procedure described in (Feder et al. 2012). The expectation of this AIP pool-seq experiment is that recombination would segregate major factors responsible for the difference in starvation resistance between these strains so that extreme-phenotype mapping by pooled sequencing might identify chromosomal regions associated with this difference. We acknowledge that 10 generations, moderate populations sizes (~2000) and founding samples of 50 extreme-phenotype adults may not have the power to generate high resolution recombinants for localizing individual loci, but our intention was to use this as a means to complement other classical mapping approaches (chromosome segregation, marker-recombination assays and deficiency mapping). The use of five replicate populations provided some level of replication that was appropriate for the budget available at the time.
Chromosome 3 recombination
The chromosome segregation experiments indicated that the majority of the difference in starvation resistance between the lines mapped to chromosome 3 and the pooled sequence analyses of the AIP cages identified two broad candidate regions on chromosome 3, one each on 3L and 3R. We performed recombination mapping using three successive crosses to marked mapping stocks to further localize candidate regions (cross 1, 2, 3; see Fig. S1). For cross 1 we attempted to generate Zim53;765 chromosome 3 recombinants by crossing 20 Zim53;765 virgin females to 20 males from a multiply marked recessive stock obtained from the Bloomington Stock Center (BSC_576 = ru1 hry1 Diapth-1 st1 cu1 sr1 es ca1 hereafter referred to as BSC_576 for convenience). The progeny from this cross however failed to eclose. As an alternative cross 1, we crossed 20 siI;765 virgin females to 20 BSC_576 males in an effort to allow recombination across the DGRP-765 3rd chromosomes that are present in the siI;765 stock. The siI;765 genotype was suspected to be heterozygous for the DGRP-765 3rd chromosome and a 3rd chromosome from the TM3 balancer stock that may have recombined with the DGRP-765 3rd chromosome during the construction of the siI;765 original stock since siI;765 lacked the TM3 marker Sb1 (Sb is at 89B4, band 58). This suspicion of balanced heterozygosity was based on the observation that several generations of backcrossing of male DGRP-765 to virgin female siI;765 did not reduce starvation resistance implying that the siI;765 carried some heterozygosity and was in some way resistant to the impact of recurrent backcrossing.
For cross 2, 20 F1 virgin siI;765 / BSC_576 females were collected and crossed to 20 males from a multiply marked recessive stock with the same recessive markers as BSC_576 in addition to a dominant marker present on one copy of chromosome 3 balanced over the TM6 balancer (BSC_1711: ru1 hry1 Diapth-1 st1 cu1 sr1 es Pri1 ca1 / TM6B, Bri1, Tb1). This cross was done in three replicate 20-female × 20-male vials. F1 males from this second cross were collected and segregated into distinct recombinant classes based on the recessive and dominant markers present.
For cross 3, males from each of these recombinant categories were each mated to virgin starvation resistant siI;765 females and to virgin starvation sensitive Zim53;765 females to generate offspring with different segments of the 3rd chromosome that was homozygous for the starvation-sensitive DGRP-765 chromosome. Comparison of the offspring from these two different mitonuclear lines that showed significant differences in starvation (siI;765 and Zim53;765) could further resolve regions of chromosome 3 associated with starvation sensitivity. Due to the presumed heterozygosity of the siI;765 3rd chromosome we expected that recombination between the multiple-marked 3rd chromosome with the siI;765 3rd chromosomes would be confounded if: 1) the chromosome suspected to be the homologue of the 3rd chromosome in siI;765 was a broken balancer chromosome, or 2) if that homologue was some other wild type chromosome that had been recombining with the DGRP-765 3rd chromosome. This would reduce the efficiency of the recombination mapping as only an unknown fraction of the offspring would have been derived from the actual pairing of the siI;765 3rd chromosome derived from DGRP-765 and the recombinant-marked chromosomes derived from the F1 female parent. We acknowledge that this was not ideal but collected the mapping data to see if it provided useful information consistent with the independent chromosome segregation mapping and AIP pool-seq data.
Five classes of recombinant offspring phenotypes were designated based on the visible markers present reflecting different breakpoints along the chromosome: class i: ru, h; class ii: ru, h, Diap, st; class iii: ru, h, Diap, st, cu; class iv: ru, h, Diap, st, cu, sr; and class v: ru, h, Diap, st, cu, sr, e. Twenty males from each group were crossed to 20 Zim53;765 virgin females and allowed to mate for 48 hours in three replicate 20-female × 20-male crosses (the Zim53;765 genotype was confirmed to be homozygous for the DGRP-765 chromosome). The males from each of these crosses were removed and then re-mated to siI;765 virgin females. Female offspring from each of these two sets of crosses were collected over CO2. Sorted by recombinant class and conditioned for starvation assays on high sugar food. Sufficient flies for a starvation stress survival assay were only available from the class i, class ii and class v crosses, so only these were considered. The survival of these females under starvation stress was measured as described above.
Chromosome 3L Deficiency Screen
The recombination mapping and the AIP pool-seq data implicated chromosome 3L as harboring a major starvation factor, so deficiency mapping was pursued. Twenty males from each deficiency line (Supplemental Table 1) were crossed to 20 Zim53;765 virgin females in eight replicate 20-female × 20-male crosses. F1 females were collected and sorted over CO2. Females were separated based on the presence of the respective balancer marker (flies heterozygous for the deficiency and the 765 chromosome do not have the balancer present) and moved to 3 replicate demography cages with approximately 125 flies each, for each genotype. The flies were allowed to recover for 7 days on high sugar food and survival under starvation stress measured as described above.
iPLA2-VIA Complementation
The chromosome 3L deficiency mapping, in conjunction with the available DGRP sequences, identified iPLA2-VIA as a candidate gene based on nucleotide polymorphisms private to DGRP-765 and starvation sensitivity phenotypes of iPLA2-VIA alleles (Lin et al. 2018). Deficiencies within that gene were obtained from the Bloomington Stock Center for further complementation mapping: BSC_80133 = y1 w*; iPLA2-VIADelta174 and BSC_80134 = y1 w1; iPLA2-VIADelta192 hereafter 80133 and 80134 respectively. These deficiencies were generated by imprecise excision of a P-element from iPLA-VIA that removed segments of 5’ noncoding sequence and exons 1–5 and -7 (Lin et al. 2018). Twenty Zim53;765 flies were crossed in reciprocal to 20 flies from each of these two iPLA-VIA coding region deficiencies. Each cross was done in replicates of 4. As a control, males from each iPLA-2-VIA deficiency stock were also crossed to Zim53;315 females with the expectation that the DGRP-315 nuclear genome would complement the iPLA2-VIA deficiencies and restore starvation to normal levels . F1 females were collected and sorted over CO2 and moved to 3 replicate demography cages with approximately 125 flies each. The flies were allowed to recover for 7 days on high sugar food and survival under starvation stress measured as described above.
Glucose Assay
The free glucose levels, as described in (Tennessen et al. 2014), were measured for five genotypes: Zim53;765, Zim53;315, iPLA2-VIADelta174, Zim53;315 crossed to iPLA2-VIADelta174 and Zim53;765 crossed to iPLA2-VIADelta174. 120 female flies from each genotype were collected and sorted over CO2 and allowed to recover in demography cages affixed with 25×95 mm vials of high sugar food (15% sucrose, 5% Yeast, 2% agar) for five days. Three replicates were done for each genotype. After five days, any dead flies were removed and half of the remaining live flies were removed from the demography cage, immediately flash frozen in liquid N2 and stored at −80°C. The food supplied to each demography cage was replaced with 2% Difco Bacto-agar prepared with deionized H20. After 12 hours, dead flies were discarded and the remaining live flies were removed from the demography cage, immediately flash frozen in liquid N2 and stored at −80°C. Six frozen flies from each replicate were transferred to a 1.5uL micro-centrifuge tube and homogenized in 120uL of Phosphate Buffered Saline (PBS) using a steel bead and Qiagen TissueLyser II set to 30Hz for 2 minutes. A 10uL aliquot was removed to measure protein content using the Pierce™ BCA Protein Assay Kit (23225). The remaining homogenate was heat treated for 10 minutes at 70°C. The homogenate was diluted 1:4 with PBS. The free glucose was measured with the Glucose Oxidase (GO) kit (MAK097–1KT) by following the protocol for free glucose measurement laid out in (Tennessen et al. 2014).
Data Availability
Drosophila strains, code and raw data are available at the Rand Lab GitHub site for this publication (https://github.com/DavidRandLab/Williams-et-al.-Starvation-Genetics-2021). The authors affirm that all analyzed data necessary for confirming the conclusions of the article are present within the article, figures, tables and GitHub site. Raw sequences for the pool-seq mapping are available at BioProject accession number: PRJNA1130456.
Results
Starvation resistance among mitonuclear genotypes
Twelve mitonuclear genotypes from a panel of 72 previously-reported genotypes (Mossman et al. 2016) were chosen for starvation assays as described in the Methods section. Each of these 12 mitonuclear introgression lines was subjected to starvation assays on three different isocaloric diets: high sugar food (15% sucrose, 5% Yeast, 2% agar), balanced food (10% sucrose, 10% yeast, 2% agar) or low sugar food (5% sucrose, 15% yeast, 2% agar; referred to as food type V, II and IV, respectively (Zhu et al. 2014)). The mitonuclear genotypes on the DGRP-765 nuclear background were of particular interest because the DGRP-765 line represents one of the least resistant lines in terms of starvation resistance in the DGRP, and it appeared that introgression of the siI mitochondrial DNA haplotype significantly improved resistance to starvation only in the DGRP-765 nuclear background (Figure 1). To test whether the rescue of starvation resistance mapped to a cytoplasmic or nuclear factor, we performed reciprocal crosses to examine if maternal inheritance of mtDNA was the source of the rescue of starvation sensitivity. When we crossed Zim53;765 to siI;765, the direction of the cross (Zim53;765 female crossed siI;765 males vs. siI;765 females to Zim53;765 males) made no difference to starvation resistance of its progeny. This implied that the siI mtDNA haplotype was not the source of the rescue, as would be expected if the trait was being inherited maternally (Fig. S2).
We analyzed the reads from an RNA-sequencing data set from each fly line and compared the genotype calls for each SNP present in Zim53;765 reads (restricted to the coding regions) and the genotype calls at the same locations in siI;765. For the vast majority of these sites, the siI;765 was heterozygous for the DGRP-765 allele and an alternative allele (in most cases the reference allele) (Fig. S2A,B). We did a similar comparison of siI;765 to an alternate mitonuclear genotype (Zim53;315) and did not see a similar overlap between the siI;765 allele and the Zim53;315 allele (Fig. S2A,B). Based on these results we concluded that the siI;765 genotypes was heterozygous across the DGRP-765 chromosomes. This suggested to us that starvation sensitivity in Zim53;765, and the two other mito;765 combinations (Ore;765 and siII;765), were homozygous recessive and the improvement in resistance to starvation seen in siI;765 was due to its nuclear heterozygosity. Indeed, we observed that the offspring from a Zim53;765 to Zim53;315 cross were significantly more resistant to starvation than Zim53;765 (see Figure 6).
Figure 6. Zim53;765 fails to complement iPLA2-VIA null lines.
Survival of female offspring from indicated crosses following starvation stress. Each panel represents the male parent, and colored lines identify the female parent. Zim;765 is starvation sensitive as a homozygote and as a heterozygote over two different iPLA2-VIA deficiency null alleles. Differences in survival were determined using a log-rank test (implemented in the R survival package) and included in Table S5_iPLA-VIA complementation).
Chromosome 3 heterozygosity partially suppresses Zim53;765 starvation sensitivity
Based on the suppression of starvation sensitivity seen in Zim53;765 heterozygotes, (Zim53;765 × Zim53:315 F1 hybrids) we reasoned that starvation sensitivity phenotypes in Zim53;765 and other genotypes were homozygous recessive traits and that we could localize the effect locus/loci to chromosomes using chromosome segregation tests and observing which chromosome segregants masked the trait. Virgin females from three different genotypes (the original DGRP-765, Zim53;765 and Zim53;315) were mated to males from the marked double-balancer stock (wgSp-1/CyO; Dr1/TM3, Sb1). F1 males heterozygous for the maternal autosomes paired with the paternal CyO and Dr1 markers were mated back to virgin females from the three maternal genotypes, generating the four 2-autosome segregant populations of F1 flies as follows: heterozygous for chromosome 2, heterozygous for chromosome 3, heterozygous for both chromosome 2 and 3, or neither (the latter being reconstituted homozygous wild type 2nd and 3rd chromosomes of the original maternal genotypes: DGRP-765, Zim53;765 and Zim53;315). DGRP-765 and Zim53;765 flies heterozygous for chromosome 3 had a ~200% increase in mean survival relative to the original stock (Figure 2A; Table S2:SummaryTable_figure2a) and flies heterozygous for both chromosomes 2 and 3 realized additional increases in mean survival. Heterozygosity for chromosome 2 alone contributed ~75% increase (range 50–116%; see Table S2:SummaryTable_figure2a). For Zim53:315, chromosome 3 heterozygosity contributed nothing alone but a small effect in combination with chromosome 2.
Figure 2. Chromosome 3 suppresses Zim53;765 starvation sensitivity.
(A) DGRP-765 and Zim53;765 flies are starvation sensitive while Zim;315 flies are more resistant. In both DGRP-765 and Zim53;765, heterozygosity for chromosome 3 (chr3) rescues the majority of starvation sensitivity with chromosome 2 heterozygotes (chr2) contributing additional resistance. In Zim53;315 heterozygosity on chromosome 2 is the primary source of increases in starvation resistance, with little additional contribution from chromosome 3. (B) A replicate experiment with Zim53;765 shows a similar major effect of chromosome 3 with additional effect of chromosome 2, while the siI;765 stock with nuclear heterozygosity has increased starvation resistance and distinct chromosomal contributions. Differences in survival between pairs of genotypes were determined using a log-rank test (implemented in the R survival package) and included in Table_S2_Chromosome Segregation Mapping.
A repeat segregation experiment with Zim53;765 and the heterozygous siI;765 genotype was conducted and revealed comparable results for Zim53;765 (80–100% increase from chromosome 3 heterozygosity with additional effects of chromosome 2) and very different chromosomal influences for siI;765, as expected from its heterozygous condition (Figure 2B; see Table S2:SummaryTable_figure2b; raw data are in other tabs of Table S2). Taken together, these results suggest that the main effect locus/loci controlling Zim53;765 and DGRP-765 starvation sensitivity are present on chromosome 3 with a secondary locus/loci present on chromosome 2.
Sites on chromosome 2R and 3 have significant differences in allele frequency between early and late dying flies.
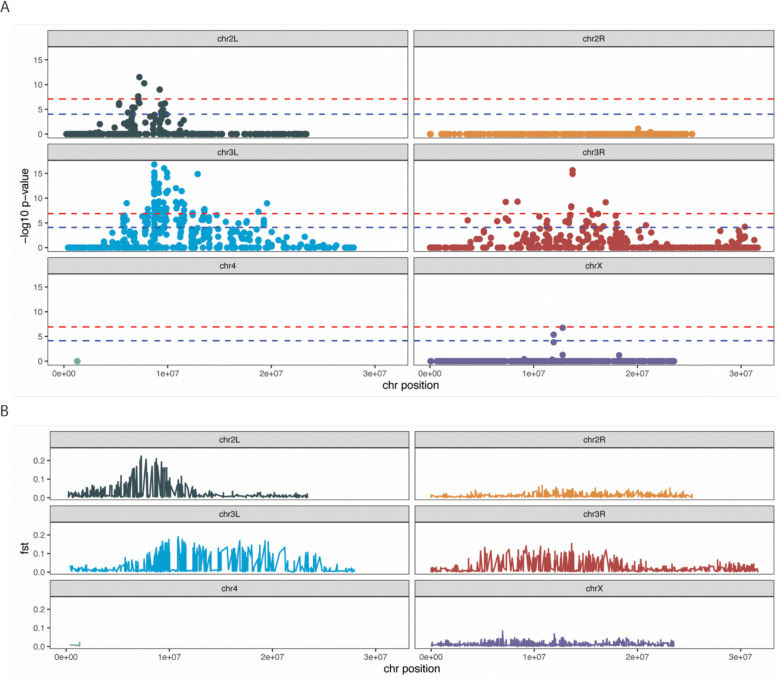
To understand the genetic basis of 765 starvation sensitivity, we sought to identify genetic differences characteristic of starvation sensitive flies. We created replicate advanced intercross populations (AIPs) in which Zim53;765 was allowed to recombine with Zim53;315. We set up five independent populations where Zim53;765 flies were crossed to Zim;315 in reciprocal and the populations maintained for 10 generations at population sizes >1000 adults. Following this, females from each population were subjected to starvation stress and dead flies were collected every six hours until all flies were dead. Flies that died during the first three and last two six-hour intervals were separately combined into early- and late-dying pools of adult females (18–24, 24–30 and 30–36 hours vs. 90–96 and 96–102 hours ). Total genomic DNA was sequenced from each of the five replicates from these two pooled samples, hereafter ‘early’ and ‘late’ dying pools. Consistent differences in allele frequencies across the early and late dying populations were determined using the Cochran-Mantel-Haenszel test implemented in Popoolation2 (Kofler et al. 2011). There was an enrichment in significantly different variants across chromosome 3 and a localized region of chromosome 2R (Figure 3). These results suggest that Zim53;765 and as a consequence DGRP-765, starvation sensitivity is based on variants present on chromosome 3 and chromosome 2. Importantly, these advanced intercross pool-seq analyses are consistent with the chromosome segregation analyses where factors on chromosomes 3 and 2 were responsible for starvation sensitivity of the Zim;765 genotype.
Figure 3. Significant allelic frequency differences between the tails of replicate advanced intercross populations subjected to starvation stress.
An advanced intercross population was created by crossing Zim53;765 and siI;315 in reciprocal. The hybrids were allowed to recombine freely for 10 generations before being subjected to starvation stress. Dead flies were collected every 6 hours and dead flies from the first 3 timepoints were combined and subjected to sequencing analysis. The same was done with the last two timepoints. In total 5 replicates AIP populations were generated and sequenced. (A) Consistent differences in allele frequencies across replicates were determined using the Cochran-Mantel-Haenszel (CMH) test and Popoolation2. The y-axis is the negative log-transformed, Bonferroni corrected p-value of each SNP (represented by each circle). The x-axis is the position of each SNP along the indicated chromosome. Blue and red lines highlight corrected p-values above 1×10−5 and 1×10−6 respectively. (B) FST between pools for each site averaged across replicate populations. The location of elevated FST values is generally concordant with significant CMH test statistics.
Heterozygosity of chromosome 3L region partially suppresses Zim53;765 starvation sensitivity
To further localize the main effect loci on chromosome 3 we used recombination and deficiency mapping across chromosome 3. As there are over 200 deficiencies spanning chromosome 3, we first generated a series of recombinants with break points at different locations along chromosome 3. Cross 1 used siI;765 virgin females mated to a multiply marked recessive stock (ru1 hry1 Diapth-1 st1 cu1 sr1 es ca1 hereafter referred to as BSC_576 for convenience). Cross 2 used heterozygous F1 virgin females from cross 1 mated to males from a similarly marked recessive stock with one dominant marker added to distinguish progeny (BSC_1711: ru1 hry1 Diapth-1 st1 cu1 sr1 es Pri1 ca1 / TM6B, Bri1, Tb1). The F1 offspring from cross 2 revealed recombinant males that were pooled into three classes of recombinants (classes i, ii, v) that were selected based on the presence of visible recessive markers and sufficient flies for starvation analyses (Figure 4A). Recombinant class i is likely heterozygous for the region of 3L from the telomere up to at least the regions marked by h, with recombination breakpoints existing anywhere between h and the cu gene. Recombinant class ii is heterozygous for most of 3L and up to cu gene on 3R, with breakpoints existing anywhere between the cu gene and the ca gene. Recombinant class v is likely heterozygous for most of chromosome 3 with breakpoints existing from the ca gene up to the telomere of 3R. Other classes of recombinants did not emerge in sufficient numbers to conduct the final mapping crosses. It is important to note that because these recombinants were generated from siI;765, the pool of recombinants will carry both the DGRP-765 alleles from that chromosome which was substituted onto the D. simulans siI mtDNA background during original stock construction and alternate, unknown alleles from a chromosome that was likely introduced during the balancer substitution process during original line construction. A TM3 Sb1 3rd chromosome balancer was used in these crosses and may have recombined with segments of the DGRP-765 3rd chromosome during the replacement crosses (see Methods).
Figure 4. Recombination mapping localizes the majority of Zim53;765 starvation sensitivity to chromosome 3L.
(A) Cartoon representation of recombination breakpoints for each recombinant class generated through meiotic recombination of the chromosome 3 mapping line. (B) Survival of female offspring produced by crossing each recombinant class to Zim53;765 homozygotes or heterozygotes. Differences in survival were determined using a log-rank test (implemented in the R survival package; *** = p , 0.001, * = p<0.05) and included in Table_S3_Meiotic Mapping Survival.
To reveal the localization, cross 3 used males from the three recombinant classes emerging from cross 2, each of which were mated to virgin Zim53;765 females, and then the same males were mated to virgin siI;765 as a control. The starvation sensitivity of the female offspring of the Zim53;765 and recombinant males was compared to the female offspring of the siI;765 and recombinant male controls. While we cannot discern genotype at the individual fly level, DGRP-765 chromosome 3 alleles will be enriched in the progeny from the Zim53;765 vs recombinant cross, while the alternative will be true for the siI;765 vs recombinant cross. We expected that flies homozygous for the causal Zim53;765 allele would die earlier during starvation stress, thus revealing the region of Zim53;765 chromosome 3 responsible for its starvation sensitivity. Although the heterogeneity of respective offspring populations will mask this effect, the enrichment of the DGRP-765 allele in the Zim53;765 vs recombinant cross population and the alternate allele in the siI;765 vs recombinant cross population should result in appreciable differences in deaths early on during starvation stress. Indeed, we observed a significant difference between the starvation sensitivity of recombinant class i offspring, but not in the case of the class ii offspring; class v offspring showed a small but significant difference (see Figure 4B; Table S3). These results indicate that heterozygosity of a region between the h marker of recombinant class i and the cu marker of recombinant class ii carries a factor required to suppress Zim53;765 starvation sensitivity.
Hemizygosity of 3L: 9812381 – 9899255 prevents partial suppression of Zim53;765 starvation sensitivity
We employed a deficiency screen across the region between recombinant class i and recombinant class ii markers. The objective of this screen was to identify the region(s) of chromosome 3L that failed to complement Zim53;765 starvation sensitivity. Zim53;765 virgin females were crossed to a series of flies deficient for various parts of chromosome 3L (Table S1). The deficient region of each line is maintained over a balancer chromosome so the resulting offspring can be positive for the balancer, indicating complete heterozygosity of chromosome 3, or offspring lacking the balancer are hemizygous across the deficiency region and heterozygous for the rest of chromosome 3. Significant differences in the starvation resistance of the two classes of offspring indicates that heterozygosity of the deficiency region is required for the suppression of starvation sensitivity. We initially screened 15 deficiency lines and identified nine where the starvation sensitivity was not suppressed (failed to complement) in the partial chromosome 3L hemizygote (Table S4A and Fig.S3). Several of the deficiency stock crosses with the most significant difference between Df and wt chromosome pairings shared a common region of chromosome 3 between 3L:9812381 – 3L:9899255 (Fig. S3 and Figure 5), indicating that heterozygosity in this region is required for the suppression of Zim53;765 starvation sensitivity. Two deficiencies mapped to similar locations but showed conflicting evidence for suppression of starvation sensitivity (Df282 and Df 283; Fig. S3 and Table S1). The latter contained additional genes (lncRNA:CR45878, CG46387) in the proximal region of the deficiency; the functional consequences of these mapping differences were unclear. Three deficiencies on either side of the putative overlap region also showed significant suppression of sensitivity (BSC113, 4470 and 4488). Despite the variation among the deficiency data, they were consistent with the three broader-scale approaches of chromosome segregation, pool-seq and recombination mapping in identifying candidate genes in this region of chromosome 3L.
Figure 5. Deficiency mapping identifies the 3L: 9812381 – 9899255 region as a component of Zim53;765 starvation sensitivity.
(A) Survival of offspring from the cross between female Zim53;765 and male deficiency stocks following starvation stress. Heterozygous refers to progeny that inherited the marked balancer chromosome, hemizygous refers to progeny that inherited the deficient chromosome. Line numbers correspond to Bloomington Stock Center names (see table S1). Differences in survival were determined using a log-rank test (implemented in the R survival package) and included in Table S4_Deficiency mapping: summary and statistics tabs. (B) Linear representation of hemizygous regions of respective deficiency lines. Highlighted region is common to the three deficiency regions which failed to suppress Zim53;765 starvation sensitivity. See Fig. S3 for additional crosses
Variation in iPLA2-VIA as candidates for Zim53;765 starvation sensitivity
Next, we sought to identify Zim53;765 variants in the chromosome 3L region that might underlie its sensitivity to starvation. We utilized the available genomic sequencing data of each DGRP line to identify nucleotide variants unique to the DGRP-765 sequence (Mackay et al. 2012). As the causal variant is deleterious, we focused our initial analysis on low frequency variants (present in less than 10 % of the 200 DGRP2 sequences). We identified 83 variants which fit this frequency cutoff and only thee SNPs were predicted to lead to non-synonymous changes (Fig. S4 and Table 2). A C-to-A mutation, predicted to result in a G-to-V amino acid residue change in the conserved GXGXXG DNA binding motif of the phospholipase iPLA2-VIA, appeared as a strong candidate. Mutations in iPLA2-VIA have previously been shown to cause starvation sensitivity and reduced lifespan in Drosophila (Kinghorn et al. 2015; Kinghorn and Castillo-Quan 2016; Lin et al. 2018). In addition, PolyPhen-2 predicted the mutation as probably damaging, with a score of 0.999 out of 1 (Adzhubei et al. 2010). Based on this analysis, we performed finer resolution deficiency mapping in the iPLA2-VIA gene.
Table 2. Sequence details of candidate SNPs in the genomic region identified by classical mapping approaches.
See text and Fig. S4 for details.
| Chr. | start | end | REF | ALT | CONSEQUENCE | REFDNA | VARDNA | REFAA | VARAA | SYMBOL |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| chr3L | 9824166 | 9824166 | C | T | nonsynonymous | CCA | TCA | P | S | CG43897 |
| chr3L | 9833935 | 9833935 | G | A | synonymous | AGC | AGT | S | S | RasGAP1 |
| chr3L | 9854881 | 9854881 | C | A | nonsynonymous | GGC | GTC | G | V | iPLA2-VIA |
| chr3L | 9880027 | 9880027 | A | G | synonymous | TTT | TTC | F | F | Taf2 |
| chr3L | 9880564 | 9880564 | G | T | synonymous | ATC | ATA | I | I | Taf2 |
| chr3L | 9881643 | 9881643 | C | A | nonsynonymous | GAG | GAT | E | D | Taf2 |
| chr3L | 9884329 | 9884329 | A | T | synonymous | ATA | ATT | I | I | CalpB |
| chr3L | 9884605 | 9884605 | A | G | synonymous | GGA | GGG | G | G | CalpB |
iPLA2-VIADel174 fails to complement Zim53;765/ DGRP-765 starvation sensitivity and glucose homeostasis
To determine if the starvation phenotype of Zim53;765 and as a consequence DGRP-765, results from a defective copy of iPLA2-VIA, we sought to determine if the Zim53;765 copy of iPLA2-VIA gene could complement iPLA2-VIA null mutations. We crossed Zim53;765 virgin females to two previously characterized iPLA2-VIA null lines (iPLA2-VIADel174, iPLA2-VIADel192), generated by imprecise excision of a mobile element; Table S1 (Lin et al. 2018). To rule out the potential effects of mtDNA haplotype on starvation resistance, we also utilized the original DGRP-765 strain obtained from the Bloomington Stock center. We found that progeny from DGRP-765 crossed to iPLA2-VIADel174 or iPLA2-VIADel192 were also starvation sensitive (Figure 6). In contrast, crossing iPLA2-VIADel174 or iPLA2-VIADel192 to the starvation resistant strain Zim53;315 resulted in the suppression of starvation sensitivity (i.e., restored starvation resistance; Figure 6).
Depletion of glucose and glycogen are associated with starvation sensitivity in Zim53;765 and DGRP-765.
Ablation or pharmaceutical inhibition of calcium independent phospholipases have been shown to reduce insulin secretion in response to a glucose challenge (Song et al. 2005; Ali et al. 2013). This raised the question of whether Zim53;765 and iPLA2-VIA share defects in glucose metabolism. We observed that following 12 hours of starvation, Zim53;765 and the original DGRP-765 line significantly deplete their levels of free glucose and glycogen in contrast to Zim53;315, suggesting improper regulation of glucose during acute starvation (Figure 7). Interestingly, this phenotype was not observed in iPLA2-VIADel174 homozygotes (Figure 7). However, we found that progeny from Zim53;765 crossed to iPLA2-VIADel174 also significantly deplete their available free glucose following 12 hours of starvation (Figure 7; Table S6a,b,c). Taken together, these results indicate shared genetic bases of iPLA2-VIA and Zim53;765 in starvation sensitivity but distinct roles in glucose metabolic effects on starvation. Notably these effects follow a week of conditioning on high sucrose foods that may influence the time course of sugar depletion during starvation and its impact on survival during starvation.
Figure 7. iPLA2-VIA[Delta174] fails to complement glucose and glycogen depletion phenotype of Zim53;765.
The free glucose and total glycogen levels were measured for Zim53;765, Zim53;315, iPLA2-VIA[Delta174], Zim53;315 crossed to iPLA2-VIA[Delta174] and Zim53;765 crossed to iPLA2-VIA[Delta174]. Values in the un-starved (control) was compared to the values in the starved samples (12 hours) for 3 replicates of 6 flies using a student’s t-test. Significant differences are indicated by * <0.05, ** <0.01.
Discussion
In the current study we sought to identify the genetic basis of a striking difference in starvation resistance between one particular genotype among a set of related mitonuclear genotypes (see Figure 1). Given the central role that mitochondria play in both anabolic and catabolic processes related to energy stores, the possibility that combinations of mitochondrial and nuclear genes might contributed to quantitative variation for starvation resistance warranted additional genetic analysis. This is further motivated by the highly polygenic nature of starvation resistance and its correlation with other fitness and metabolic traits (Service and Rose 1985; Chippindale et al. 1996; Harshman et al. 1999; Hardy et al. 2018; Everman et al. 2019). Our early crosses ruled out an effect of the mtDNA on the strong differences in starvation between siI;765 and Zim53;765 which refocused the analyses to the genetic architecture of starvation resistance between distinct nuclear genomes. Because the nuclear genomes used to build the mitonuclear lines were part of the well-characterized DGRP we sought to use the classical tools of the Drosophila model and the genomic information available for the DGRP to carry out a quantitative trait locus search between two distinct homozygous lines from the DGRP. The motivation was twofold: the difference between the sensitive mitonuclear lines (in DGRP-765 backgrounds) and resistance lines (in DGRP-315 or -820 backgrounds) was very distinct, and the sensitive mitonuclear-765 lines also appeared less sensitive to the high sugar:protein ratio in the food used prior to the starvation assay (Figure 1). Applying forward genetic approaches to this classic quantitative genetic system offered the opportunity to test the hypothesis that starvation sensitivity was associated with sugar metabolism.
Polygenic basis of Zim53;765 starvation sensitivity
The chromosomal segregation analyses and the Advanced Intercross Population (AIP) pool-seq analyses show parallel results at a chromosomal scale and further confirm the polygenic basis of the differences between the DGRP-315 and -765 genomes. Figure 2 shows a clear main effect of chromosome 3 and some additive or epistatic effects from chromosome 2. Chromosome 3 heterozygosity was necessary to suppress Zim53;765 starvation sensitivity but heterozygosity of chromosome 2 and 3 together further improved starvation resistance. Notably, chromosome 2 heterozygosity in isolation had limited effect on suppressing Zim53;765 starvation sensitivity (Figure 2A, B). This indicates that the chromosome 2 locus/loci acts as a modifier that genetically interacts with the chromosome 3 locus/loci. Importantly, Zim53;315 has a distinct genetic basis of starvation resistance, with chromosome3 having little effect (Figure 2A)
The pool-seq results from the AIP design (Figure 3) show corresponding patterns for chromosomes 2 and 3: strong evidence on chromosome 3 for allele frequency differences between early-and late dying pools of flies, but limited evidence for this on chromosome 2. Figure 2 shows a weak signal of starvation suppression by chromosome 2 for the very last-dying flies but it is not significant. This could reflect heterozygosity of the few loci that just pass significance on chromosome 2L in the pool-seq data (Figure 3), but this remains speculative.
We note that the AIP analyses involved recombination between the focal line at the extreme end of the starvation resistance spectrum (Zim53;765, most sensitive) and a line more central in the distribution of starvation scores (Zim;315, with the DGRP-315 nuclear chromosomes). Though a compelling case could be made for recombining Zim;315 with a line at the opposite end of the distribution (most resistant; e.g., Zim;820 with the DGRP-820 nuclear chromosomes), our starvation data did not show a large difference between the −315 and −820 mitonuclear genotypes, but the −315 mitonuclear genotypes had somewhat tighter variance of starvation scores on the different sugar:protein diets, so we chose that background (Figure 1). The AIP pool-seq data show strong allelic differentiation across one broad peak on chromosome 3L and additional peaks on 3R, with fewer significant peaks on chromosome 2L (4 SNPs above threshold). The resolution of these analyses is admittedly low and resolving specific candidate loci was not an expected goal of the AIP pool-seq. It is likely that many of these significant variants are in linkage disequilibrium since the populations recombined over a period of only 10 generations. Nevertheless, the clustering of significant variants on chromosomes 2 and 3 are consistent with a polygenic basis for Zim53;765 starvation sensitivity. The AIP analyses served the important goal of confirming the results of the chromosome segregation analyses and identifying where to focus subsequent higher resolution recombination and deficiency mapping.
Dissecting the 3L candidate region
We sought to narrow the target region, by generating recombinants across chromomere 3 between Zim53;765 and the multiply marked recessive stocks, as this would provide a more localized region for deficiency analyses. We however failed to do this directly as crossing Zim53;765 females to males from each of the multiple marked recessive stocks failed to yield an appreciable number of offspring. We use the siI;765 to generate recombinants because siI;765 was constructed using balancer substitutions and backcrosses to DGRP-765 and thus likely carried DGRP-765 chromosomes, and it produced sufficient offspring to proceed with the remaining two recombination crosses. Despite learning that siI;765 was a hybridized version of 765 nuclear chromosomes, a fact confirmed by comparing the alleles of Zim53;765 variants to siI;765’s, it remained possible that the recombinant offspring would help localize genetic effects between chromosome arms 3L or 3R, which proved true. The 15 different deficiencies used for mapping were chosen to span the regions of chromosome 3L that were most likely to carry a candidate gene or genes contributing to the starvation differences between the DGRP chromosomes present in Zim;765 and Zim;315. The preponderance of the evidence from these data supported the hypothesis that heterozygosity of the region of 3L 9812381 – 9899255 was required for suppression of Zim53;765 starvation sensitivity, with deficiencies BSC392, -394 and -673 being clear examples (Figures 5 and S3). Again, however, the deficiency data included some inconsistent results. When Zim53;765 was crossed with Df(3L)BSC282, there was only a modest difference in the starvation resistance of the heterozygous and homozygous progeny, and when crossed with Df(3L)BSC283, there was no statistical difference in the starvation resistance (Fig. S3). The two additional genes missing in Df(3L)BSC283 relative to Df(3L)BSC282 may explain these conflicting data, but neither gene has any known function reported in Flybase (lncRNA:CR45878, CG46387).
Complementation points to Zim53;765 iPLA2-VIA
There are over 30 genes in the candidate regions defined by 3L 9812381 – 9899255 (see Table S1). Of all the variants in this region, the nonsynonymous mutation present in the calcium-independent phospholipase iPLA2-VIA immediately stood out, due to the location of the mutation in the functional domain of the enzyme, and the phenotypes previously associated with iPLA2-VIA loss of function. Mutations in the human homolog PLA2G6 gene are characteristic of multiple autosomal recessive disease (Guo et al. 2018). The amino acid mutation in the Drosophila iPLA2-VIA protein that is private to DGRP-765 lies in the GXGXXG consensus nucleotide binding motif similar to those found in other protein kinases. This motif contributes to the binding of ATP to iPLA2-VIA which is essential for its catalytic function (Ramanadham et al. 2015). Several studies have independently characterized starvation sensitivity in iPLA2-VIA mutants (Kinghorn et al. 2015; Iliadi et al. 2018; Lin et al. 2018; Mori et al. 2019). In each case, targeted mutations were introduced in iPLA2-VIA to generate null models, with the goal of better understanding its role in neurodegeneration. These studies identified aberrant lipid metabolism, lipid peroxidation and mitochondrial dysfunction as potential causes of stress sensitivity and neurodegeneration. We should not necessarily expect point mutations in iPLA2-VIA to universally have the same effect as these deletion loss of function models, as mutations in PLA2G6 have varying effects on catalytic activity (Engel et al. 2010). However, we can expect that if mutation(s) in iPLA2-VIA do in fact underlie Zim53;765 starvation resistance, an iPLA2-VIA null should fail to complement Zim53;765 starvation sensitivity. We showed that two separate nulls generated by (Lin et al. 2018) do in fact fail to complement Zim53;765 starvation sensitivity, indicating that natural variants in iPLA2-VIA contribute some of the starvation sensitivity of Zim53;765 and DGRP-765.
The correlation between starvation resistance and lipid content has been demonstrated across species and as associated responses to selection for starvation resistance (Chippindale et al. 1996; Harshman et al. 1999; Hardy et al. 2018; Everman et al. 2019). While our current study does not have details on lipid content, there may be a link to the glucose and glycogen utilization we report (Figure 7), and to other possible candidate genes in the 3L 9812381 – 9899255 region: there are several insulin-like peptides (Ilps) ~40–100kb distal to the 3L 9812381 – 9899255 that show polymorphism among the DGRP lines. Some phospholipases have been associated with modulation of insulin secretion in response to glucose by releasing arachidonic acid (AA), and exogenous AA supplementation is sufficient to promote insulin release from rat islet cells (Metz 1988a). This insulin secretion is believed to promote phospholipid hydrolysis and accumulation of phospholipid-derived intermediates (Metz 1988b). This turnover of phospholipids could promote insulin release, creating an interesting dynamic between glucose and lipid metabolism, with phospholipases at the center. The premature depletion of glucose and glycogen in Zim53;765 in the early stages of starvation could be one consequence of the SNP(s) in iPLA2-VIA or an interaction with Ilps. Consistent with this, we found that iPLA2-VIADel174 fails to complement the premature glucose and glycogen depletion phenotypes associated with Zim53;765 (Figure 7). What is especially interesting is that the iPLA2-VIA null itself does not display this phenotype. This raises the possibility that affected pathways which ultimately result in starvation sensitivity differ between the deletion mutation of iPLA2-VIADel174 and the point mutation unique to Zim53;765. It will be an interesting target of a future study to characterize the additional molecular bases underlying the starvation sensitivity of Zim53;765, as the amino acid mutation in the conserved GXGXXG motif of iPLA2-VIA could potentially highlight novel functions of iPLA2-VIA.
Supplementary Material
Acknowledgments
The authors thank the HHMI Summer Fellows Julia Dewey, Tyler Devlin, Brian Franklin, Cynthia Hale-Phillips, Matthew P. McAteer, Zemplen Pataki, Chén Yé, and Denise Yoon who conducted the initial screen for mito-nuclear interactions affecting starvation resistance, which provided the impetus for this work. Dr. Jim Mossman assisted with the analyses of DGRP polymorphisms. Yevgeniy Raynes and Leah Darwin provided helpful edits for R code.
Funding
The work was supported in part by Gilliam Fellowship for Advanced Study awarded to SBW and DMR and NIGMS R01 2R01GM067862 awarded to DMR. DMR acknowledges support of COBRE award P20GM109035 and MIRA R35GM139607
Footnotes
Conflicts of Interest
None declared.
References
- Adzhubei I. A., Schmidt S., Peshkin L., Ramensky V. E., Gerasimova A. et al. , 2010. A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali T., Kokotos G., Magrioti V., Bone R. N., Mobley J. A. et al. , 2013. Characterization of FKGK18 as inhibitor of group VIA Ca2+-independent phospholipase A2 (iPLA2beta): candidate drug for preventing beta-cell apoptosis and diabetes. PLoS One 8: e71748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroles J. F., Carbone M. A., Stone E. A., Jordan K. W., Lyman R. F. et al. , 2009. Systems genetics of complex traits in Drosophila melanogaster. Nat Genet 41: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. B., Slocumb M. E., Szuperak M., Kerbs A., Gibbs A. G. et al. , 2019. Starvation resistance is associated with developmentally specified changes in sleep, feeding and metabolic rate. J Exp Biol 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippindale A. K., Chu T. J. F. and Rose M. R., 1996. Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution 50: 753–766. [DOI] [PubMed] [Google Scholar]
- Engel L. A., Jing Z., O’Brien D. E., Sun M. and Kotzbauer P. T., 2010. Catalytic function of PLA2G6 is impaired by mutations associated with infantile neuroaxonal dystrophy but not dystonia-parkinsonism. PLoS One 5: e12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkosar B., Dupuis C., Savary L. and Kawecki T. J., 2025. Shared genetic architecture links energy metabolism, behavior and starvation resistance along a power-endurance axis. Evol Lett 9: 150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everman E. R., McNeil C. L., Hackett J. L., Bain C. L. and Macdonald S. J., 2019. Dissection of Complex, Fitness-Related Traits in Multiple Drosophila Mapping Populations Offers Insight into the Genetic Control of Stress Resistance. Genetics 211: 1449–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everman E. R., and Morgan T. J., 2018. Antagonistic pleiotropy and mutation accumulation contribute to age-related decline in stress response. Evolution 72: 303–317. [DOI] [PubMed] [Google Scholar]
- Feder A. F., Petrov D. A. and Bergland A. O., 2012. LDx: estimation of linkage disequilibrium from high-throughput pooled resequencing data. PLoS One 7: e48588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. P., Tang B. S. and Guo J. F., 2018. PLA2G6-Associated Neurodegeneration (PLAN): Review of Clinical Phenotypes and Genotypes. Front Neurol 9: 1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison S. T., Yamamoto A. H., Fanara J. J., Norga K. K. and Mackay T. F., 2004. Quantitative trait loci affecting starvation resistance in Drosophila melanogaster. Genetics 166: 1807–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy C. M., Burke M. K., Everett L. J., Han M. V., Lantz K. M. et al. , 2018. Genome-Wide Analysis of Starvation-Selected Drosophila melanogaster-A Genetic Model of Obesity. Mol Biol Evol 35: 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman L. G., Hoffmann A. A. and Clark A. G., 1999. Selection for starvation resistance in Drosophila melanogaster: physiological correlates, enzyme activities and multiple stress responses. Journal of Evolutionary Biology 12: 370–379. [Google Scholar]
- Hill G. E., 2015. Mitonuclear Ecology. Mol Biol Evol 32: 1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Richards S., Carbone M. A., Zhu D., Anholt R. R. et al. , 2012. Epistasis dominates the genetic architecture of Drosophila quantitative traits. Proc Natl Acad Sci U S A 109: 15553–15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliadi K. G., Gluscencova O. B., Iliadi N. and Boulianne G. L., 2018. Mutations in the Drosophila homolog of human PLA2G6 give rise to age-dependent loss of psychomotor activity and neurodegeneration. Sci Rep 8: 2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn K. J., and Castillo-Quan J. I., 2016. Mitochondrial dysfunction and defects in lipid homeostasis as therapeutic targets in neurodegeneration with brain iron accumulation. Rare Dis 4: e1128616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn K. J., Castillo-Quan J. I., Bartolome F., Angelova P. R., Li L. et al. , 2015. Loss of PLA2G6 leads to elevated mitochondrial lipid peroxidation and mitochondrial dysfunction. Brain 138: 1801–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler R., Pandey R. V. and Schlotterer C., 2011. PoPoolation2: identifying differentiation between populations using sequencing of pooled DNA samples (Pool-Seq). Bioinformatics 27: 3435–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., and Durbin R., 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J. et al. , 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G., Lee P. T., Chen K., Mao D., Tan K. L. et al. , 2018. Phospholipase PLA2G6, a Parkinsonism-Associated Gene, Affects Vps26 and Vps35, Retromer Function, and Ceramide Levels, Similar to alpha-Synuclein Gain. Cell Metab 28: 605–618 e606. [DOI] [PubMed] [Google Scholar]
- Mackay T. F., Richards S., Stone E. A., Barbadilla A., Ayroles J. F. et al. , 2012. The Drosophila melanogaster Genetic Reference Panel. Nature 482: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz S. A., 1988a. Exogenous arachidonic acid promotes insulin release from intact or permeabilized rat islets by dual mechanisms. Putative activation of Ca2+ mobilization and protein kinase C. Diabetes 37: 1453–1469. [DOI] [PubMed] [Google Scholar]
- Metz S. A., 1988b. Membrane Phospholipid Turnover as an Intermediary Step in Insulin-Secretion - Putative Roles of Phospholipases in Cell Signaling. American Journal of Medicine 85: 9–21. [DOI] [PubMed] [Google Scholar]
- Mori A., Hatano T., Inoshita T., Shiba-Fukushima K., Koinuma T. et al. , 2019. Parkinson’s disease-associated iPLA2-VIA/PLA2G6 regulates neuronal functions and alpha-synuclein stability through membrane remodeling. Proc Natl Acad Sci U S A 116: 20689–20699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman J. A., Biancani L. M., Zhu C. T. and Rand D. M., 2016. Mitonuclear Epistasis for Development Time and Its Modification by Diet in Drosophila. Genetics 203: 463–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanadham S., Ali T., Ashley J. W., Bone R. N., Hancock W. D. et al. , 2015. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J Lipid Res 56: 1643–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand D. M., Haney R. A. and Fry A. J., 2004. Cytonuclear coevolution: the genomics of cooperation. Trends Ecol Evol 19: 645–653. [DOI] [PubMed] [Google Scholar]
- Service P. M., and Rose M. R., 1985. Genetic Covariation among Life-History Components - the Effect of Novel Environments. Evolution 39: 943–945. [DOI] [PubMed] [Google Scholar]
- Song K., Zhang X., Zhao C., Ang N. T. and Ma Z. A., 2005. Inhibition of Ca2+-independent phospholipase A2 results in insufficient insulin secretion and impaired glucose tolerance. Mol Endocrinol 19: 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen J. G., Nielsen M. M. and Loeschcke V., 2007. Gene expression profile analysis of Drosophila melanogaster selected for resistance to environmental stressors. Journal of Evolutionary Biology 20: 1624–1636. [DOI] [PubMed] [Google Scholar]
- Tennessen J. M., Barry W. E., Cox J. and Thummel C. S., 2014. Methods for studying metabolism in Drosophila. Methods 68: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau T. M., and Grambsch P. M., 2000. Modeling survival data : extending the Cox model. Springer, New York. [Google Scholar]
- Uma Naresh N., Kim S., Shpilka T., Yang Q., Du Y. et al. , 2022. Mitochondrial genome recovery by ATFS-1 is essential for development after starvation. Cell Rep 41: 111875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. T., Ingelmo P. and Rand D. M., 2014. GxGxE for lifespan in Drosophila: mitochondrial, nuclear, and dietary interactions that modify longevity. PLoS Genet 10: e1004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Drosophila strains, code and raw data are available at the Rand Lab GitHub site for this publication (https://github.com/DavidRandLab/Williams-et-al.-Starvation-Genetics-2021). The authors affirm that all analyzed data necessary for confirming the conclusions of the article are present within the article, figures, tables and GitHub site. Raw sequences for the pool-seq mapping are available at BioProject accession number: PRJNA1130456.