Abstract
Introduction:
Electromagnetic radiation (EMR) is widely used nowadays in various fields due to rapid expansion of technology and affects different organs such as endocrine glands. Antioxidants protect the cells and act as a free radical scavenger.
Aim of Work:
The aim of the study was to clarify the effect of EMR emitted from Wi-Fi router on the thyroid gland of adult male albino rats and the possible protective role of combined Vitamin C and zinc.
Materials and Methods:
Thirty adult male albino rats were divided into three groups: Group I (control group), Group II (received combined Vitamin C and Zinc in one tablet called IMMUNO-MASH), and Group III (experimental groups). Group III was divided into two subgroups (A and B) according to the duration of exposure: 6 h and 24 h/day. Each of these groups was divided into two equal subgroups. One was exposed only to EMR while the other was exposed to EMR and received combined Vitamin C and zinc. All rats were weighed at the beginning and at the end of the experiment. The thyroid gland was prepared for general histological, anti-calcitonin immunostaining, and ultrastructural study. Furthermore, measurement of total serum T3, T4, and thyroid-stimulating hormone (TSH) hormone levels and quantitative analysis of immunoreactive C-cells were done. Then, statistical analysis was done on the number of immunoreactive C-cells, data of the body weight, and the hormonal levels.
Results:
A highly significant increase in the body weight in subgroups exposed to EMR for 24 h/day was observed. Furthermore, they showed a highly significant decline in T3 and T4 levels together with a highly significant increase in TSH level. With increasing period of exposure, there was a variable degree of deterioration in the form of congestion and dilatation of blood vessels, cellular infiltration, follicular disintegration, vacuolar degeneration, and desquamated follicular cells in the colloid. The C-cells showed a significant increase in the mean number compared with the control group. Ultrastructural analysis of follicular cells revealed colloid droplets, deteriorations in rough endoplasmic reticulum, degenerating nuclei, and swollen mitochondria according to the dose of exposure. There was apparent improvement with the use of combined Vitamin C and zinc.
Conclusion:
Wi-Fi radiation has a very serious effect on thyroid gland morphology and activity. Moreover, experimentally induced hypothyroidism by radiation resulted in increased C-cell number. Combined Vitamin C and zinc could have a protective role against this tissue damage.
Keywords: Thyroid, Vitamin C, Wi-Fi, zinc
INTRODUCTION
Due to the proliferation of wireless technology, we now inhabit a world permeated by electromagnetic radiation (EMR). Concern about the possible adverse effect of exposure to EMR on health has arisen in response to the fast growth of wireless communication equipment such as mobile phones and Wi-Fi routers.[1,2]
EMR is of two kinds: ionizing and nonionizing radiation which has negative impacts on individuals. The ionizing radiation modulates the normal neutral charge of the atoms of living tissue cells and changes their normal function, whereas the nonionizing radiation does not produce any remarkable changes on the atomic structure and is emitted by different sources at home and workplace.[3]
These radiations have harmful effects on living tissue because they heat the tissue through the transfer of energy from many photons. Ionizing radiations, on the other hand, are those with a high frequency, such as ultraviolet, X-rays, and gamma rays, because their photons have enough energy to ionize molecules or break chemical bonds. As a result of chemical reactions, they are toxic to living organisms.[4]
EMR can affect the normal growth of different cells, viability, and bacterial sensitivity to antibiotics, depending on several factors such as the power level, the duration of exposure, frequency of radiation, pulsed or continuous wave, and the type and characters of exposed tissue.[5,6]
The thyroid gland as one of the most important endocrine glands is more vulnerable to EMR. Chronic exposure to microwaves from Wi-Fi significantly affects the thyroid gland, provoking histopathological changes in its structure through oxidative stress generated in the gland. Alterations in thyroid hormone (T3 and T4) levels and consequent changes in thyroid-stimulating hormone (TSH) have also been reported in experimental animals with chronic exposure to these radiations.[7]
Numerous prophylactic agents have been used to protect cells from the deleterious effects of EMR. Vitamin C is an excellent agent in preventing EMR-induced cell damage. Hence, it is a radioprotective agent and one of the strongest antioxidative agents.[8]
Zinc is a very important trace element needed for different biological procedures. It has a catalytic function in living cells. It is essential for growth of cells, cell proliferation, and differentiation. It so it produces a remarkable role in protecting several biological structures from the harmful-free radicals.[9,10]
This work is performed to study the effect of EMR emitted from Wi-Fi router on the thyroid gland of adult male albino rats at different durations of exposure to EMR and aiming to study the possible protective role of combined Vitamin C and zinc administration.
MATERIALS AND METHODS
Animals and ethical approval
Thirty adult male albino rats ranging in age from 6 to 8 weeks and in weight from roughly 150 to 250 g were obtained from the Tanta Faculty of Medicine’s animal house for this investigation. They were housed in clean properly ventilated separate glass cages under similar environmental condition. The dimensions of each glass cage were 30 cm × 40 cm × 50 cm with fixed longitudinal holes (1 cm in diameter). These were created so that animals might be firmly held in place while yet receiving adequate airflow.[11,12] A glass divider separated each cage into two halves. All the animals were kept in a room heated to between 24°C and 30°C and given free access to food and water. The protocol of the present work was approved by the Faculty of Medicine, Tanta University Research Ethics Committee, approval code 34019, date 12/8/2020.
The animals were randomly allocated into the following groups:
Group I (control group). It consisted of five rats isolated in their cage in a separate room under the same environmental conditions without exposure to any EMR
Group II (received combined Vitamin C and zinc called IMMUNO-MASH from Elite Pharmacy). Five rats were used in this group and isolated in their cage under the same environmental conditions as the control group. IMMUNO-MASH film-coated tablets composed of combined Vitamin C and Zinc in a concentration of 500 mg and 23.9 mg, respectively. Each rat was given a daily dose according to its weight equivalent to the allowed adult human (one tablet) daily dose for 1 month[13]
-
Group III (experimental groups): Included 20 rats were exposed to EMR emitted from the Wi-Fi router device for 30 days at different durations. They were subdivided equally into two subgroups (A and B) according to the duration of EMR exposure: 6 h and 24 h/day.
- Group III A (it was divided into two subgroups) – Subgroup III A1: It included five rats exposed to EMR s for 6 h/day for 30 days. Subgroup III A2 consisted of five rats exposed to EMR s for 6 h/day for 30 days and received IMMUNO-MASH (combined Vitamin C and zinc) in the same dose once daily for 30 days. The first dose was given 24 h before the experiment
- Group III B (it was divided into two subgroups) – Subgroup III B1: It included five rats exposed to EMRs for 24 h daily for 30 days. Subgroup III B2 consisted of five rats exposed to EMRs for 24 h/day for 30 days and received IMMUNO-MASH (combined Vitamin C and zinc) in the same dose once daily for 30 days. The first dose was given 24 h before the experiment.
Sample collection
All rats were weighed at the beginning and at the end of the experiment before scarification. At the end of the experiment, they were anesthetized using thiopental sodium and then sacrificed. The thyroid gland was dissected, extracted gently, and prepared for histological and ultrastructural study. Each specimen was divided into two lobes; the right lobes were fixed in 10% buffered formalin for light microscopic examination (hematoxylin and eosin [H and E] stain and anti-calcitonin immunostaining) and the left ones were fixed in glutaraldehyde buffer solution for electron microscopic examination.
Light microscopic study
The right lobes of thyroid glands were fixed in 10% neutral-buffered formalin for 24 h, then dehydrated in progressively stronger alcohols, cleaned in xylene, and finally embedded in paraffin. After that, gland sections ranging in thickness from 5 to 7 µm were prepared and stained using the following dyes:
Hematoxylin and eosin stain
It was used for studying the general histological structure of the thyroid gland in all groups and subgroups. The nuclei of cells appeared blue while cytoplasm appeared with variable degrees of pink coloration.[14]
Immunohistochemical study (anti-calcitonin immunostaining)
The right lobes of thyroid glands were fixed in Bouin’s fluid for 1 day at 4°C; then, they were washed in 0.1 M phosphate buffer (pH = 7.4) at 4°C and then embedded in paraffin and 5-µm-thick sections were cut. Blocking reagent (Dako Poland) was used for blocking of the endogenous peroxidase activity over 10 min, and a specific antibody against calcitonin (Dako Poland) was used. After washing with distilled water and 0.05 MTRIS-HCl pH = 7.4, three times for 5 min, the sections were incubated with the antibody for 15 min at room temperature, and then, sections were washed three times in TRIS buffer. The Labeled Streptavidin–Biotin 2 System method was applied according to the protocol for identification of the immunocytochemical reaction. The sections were counterstained with Mayer’s hematoxylin. The specific antibody was omitted in the staining procedure in the negative control. Positive control was done for specific tissue recommended by the producer. The slides from different subgroups were individually mounted onto Superfrost Plus glass slides. The cytoplasm of immunopositive C-cells appeared brownish in color.[15,16]
Transmission electron microscopic study
Just after extraction of the left lobes of the thyroid glands, the specimens were divided by a sharp glass knife using an ultramicrotome into small pieces about 1 mm3 in size. Then, they were processed for preparation of semithin and ultrathin sections.[17]
Hormonal assay
Blood samples were drained from the rat tail vein at the end of the experiment before scarification. They were collected into glass tubes (without anticoagulant) to clot followed by centrifugation and preserved at −20°C. Total T3, T4, and serum TSH hormone levels were measured using radio-immuno assay (RIA) Kit (Diagnostic Products Corporation, LA, USA).
Morphometric study
The morphometric measurement was performed at the central laboratory in the Faculty of Medicine in Tanta University using Leica Qwin 500 ImageJ Analyzer computer system (Germany). Ten different nonoverlapping randomly selected fields from each slide were quantified for the number of immunoreactive C-cells in anti-calcitonin-stained sections (at ×400).
Statistical analysis
Data of the body weight, the number of anti-calcitonin immunoreactive C–cells, and the hormonal levels were analyzed in all groups and subgroups. For multiple comparisons, the statistical difference among all groups and subgroups was assessed by using one-way analysis of variance, followed by Tukey test (t-test) using the Statistical Package for the Social Sciences version 16 (SPSS Inc., Chicago, IL, USA).[18] The mean ± standard deviation was used expressing all collected values. The difference was considered significant when probability of differences (P ≤ 0.05) and highly significant if P < 0.001. If P ≥ 0.05, the difference was considered nonsignificant.
RESULTS
Light microscopic results
Both group I and II were similar and revealed the normal structure of the thyroid gland in the form of packed follicles of variable sizes, and their lumen contained homogeneous eosinophilic colloid with active peripheral vacuolation. Each follicle was lined by a single layer of cuboidal epithelial follicular cells [Figure 1a and d]. The follicles were lined by cuboidal follicular cells. The follicular cells had rounded basophilic nuclei and acidophilic cytoplasm and were separated by thin connective tissue septa containing parafollicular cells and blood capillaries. The parafollicular cells (C-cells) had large spherical nuclei and lightly stained cytoplasm [Figure 1b and e]. Toluidine blue semithin sections of both groups showed the follicular cells with oval-to-round basophilic nuclei, and the parafollicular cells present in the connective tissue between the thyroid follicles [Figure 1c]
Figure 1.
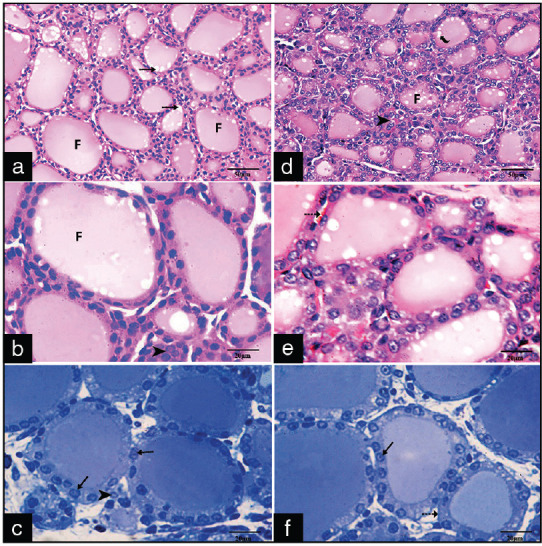
Photomicrographs of sections of the control group (a-c) and Group II (d-f) showing: (a and b) Closely packed follicles (F) lined by a single layer of follicular cells with eosinophilic colloid and active peripheral vacuolations (arrows), The parafollicular cells (arrowheads) lie in between the follicles (H and E, ×400, ×1000), (c) Cuboidal follicular cells with rounded vesicular basophilic nuclei (arrows) (Toluidine blue, ×1000), (d-f) The normal follicles and colloid active peripheral vacuole. The follicular cells (arrows), para-follicular cells (arrow heads) and the basement membrane and capillaries (interrupted arrow)
In the experimental group (Group III) according to the duration of exposure, the results were as follows: in Group III A1, H- and E-stained sections revealed areas of cellular infiltration and some follicles appeared distended with colloid and lined by flat follicular cells. Microcystic follicles appeared with absent or scanty amount of colloid and were lined by cuboidal cells [Figure 2a]. Follicular cells showed that vacuolated cytoplasm and congested blood vessel were noticed between the follicles [Figure 2b]. Toluidine blue semithin sections revealed vacuolated cuboidal follicular cells with an irregular and discontinuous basement membrane [Figure 2c]. In Group III A2, the sections appear nearly normal except some congested blood capillaries were noticed between the follicles and few cells show some cytoplasmic vacuoles [Figure 2d-f].
Figure 2.
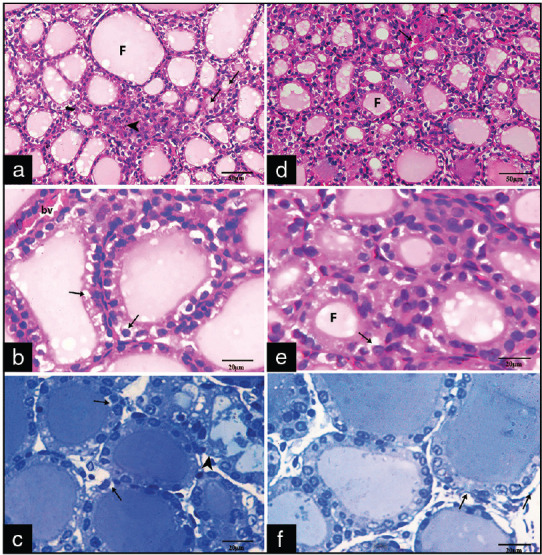
Photomicrographs of sections of Groups III A1 (a-c) and A2 (d-f) showing: (a) Areas of cellular infiltration (arrowhead). Some follicles (F) appear distended with colloid and lined by flat follicular cells, microcystic follicles (arrows) appear with absent or scanty amount of colloid (H and E ×400) (b and c) some follicular cells with vacuolated cytoplasm (arrows) and irregular and discontinuous basement membrane (arrowhead) (H and E ×1000, toluidine blue, ×1000). (d) Congested blood vessels (arrow) (H&E, x400) (e) few follicular cells with cytoplasmic vacuolations (arrow) (H&E x1000). (f) follicular cells with rounded basophilic nuclei with irregular basement membrane (arrows) (Toluidine blue X1000)
In Group III B1 with prolonged duration of radiation exposure (24 h daily), the sections revealed marked disintegration and disorganization of follicles with interrupted follicular wall and dilated blood vessels [Figure 3a]. Other sections showed follicular cells with vacuolated cytoplasm and cells with pyknotic nuclei [Figure 3b]. Toluidine blue semithin sections revealed follicular cells with markedly vacuolated cytoplasm and small darkly stained nuclei and desquamated epithelial cells in the lumen of follicles [Figure 3c]. In Group III B2, the thyroid follicles showed lesser interruption with few follicular cells appeared with cytoplasmic vacuolations, some cystic follicles with flattening of follicular epithelium, and some normal follicles with colloid inside [Figure 3d-f].
Figure 3.
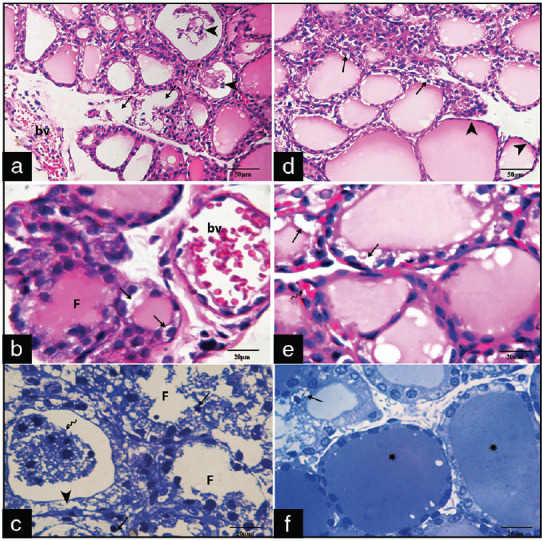
Photomicrographs of sections of groups III B1 (a-c) and B2 (d-f) showing: (a) Markedly distorted follicles (arrows) with interrupted follicular wall and desquamated epithelial follicular cells inside their lumens (arrowheads) and markedly dilated blood vessels (bv) (H and E, ×400), (b) Highly dilated and congested bv, follicular cells with vacuolated cytoplasm (arrows) (H and E, ×1000). (c) Are distorted follicles (F) lined by follicular cells with vacuolated cytoplasm (arrowheads) and small darkly stained nuclei and desquamated epithelial cells (arrowheads) in their lumen(wavy arrow) (Toluidine blue X1000).(d,e,f) cystic follicles with flattening of follicular epithelium (arrowheads) vacuolated cytoplasm of some follicular cells (arrows) and some normal follicles with colloid (Asterixes). (H&E, x400, x1000, Toluidine blue X1000)
Immunohistochemical results
Immunohistochemically stained sections from both group I and II revealed a faint positive cytoplasmic immunohistochemical reaction of the parafollicular cells in the form of a brownish coloration and immuo-negative follicular cells [Figure 4a and b]. In Group III A1, there was a mild positive reaction in the cytoplasm of parafollicular cells in the stroma between follicles, whereas in Group III A2, there was an obvious decrease in the brownish positive cytoplasmic reaction in the parafollicular cells [Figure 4c and d]. In Group III B1, there were strong positive cytoplasmic immunoreactive parafollicular cells, whereas in Group III B2, it showed a lesser positive cytoplasmic immunoreactive parafollicular cells [Figure 4e and f].
Figure 4.
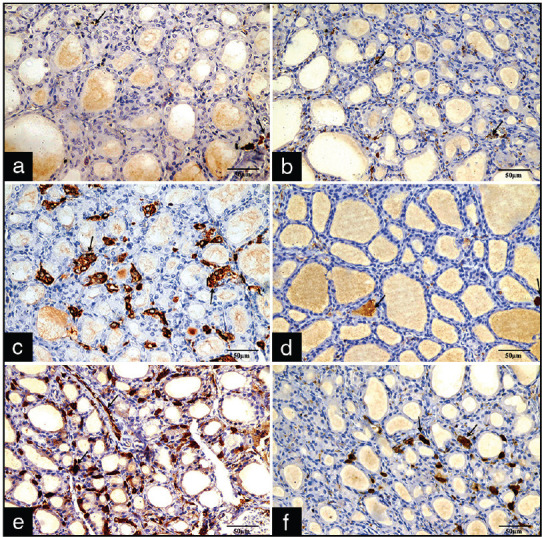
Photomicrographs of sections stained with anti-calcitonin immunostaining: (a) Control group and (b) Group II with normal brownish positive cytoplasmic immunoreactive parafollicular cells (arrows) and immunonegative follicular cells. (c) Mild positive reaction in the cytoplasm of parafollicular cells in the stroma between follicles. (d) Obvious decrease in the brownish positive cytoplasmic reaction in the parafollicular cells. (e) Strong positive cytoplasmic immunoreactive parafollicular cells (f) Less positive cytoplasmic immunoreactive parafollicular cells (x400)
Statistical analysis of the average number of anti-calcitonin-positive C-cells in the thyroid gland of different groups and subgroups in male albino rats revealed a highly significant increase in the mean number of anti-calcitonin-positive C-cells (P ≤ 0.001) in the Wi-Fi irradiated subgroup III B1 (exposed to radiations for 24 h/day) as compared to control (Group I) and Group II. On the other hand, there was there was no significant change in the number of anti-calcitonin-positive C-cells between subgroup III A1 when compared with subgroup III A2. However, there was only a significant increase in the number of anti-calcitonin-positive C-cells between subgroup III B1 when compared with those subgroup III B2 [Table 3].
Table 3.
The quantitative measurements of the number of anti-calcitonin positive C-cells of the thyroid gland and their statistical comparison in different groups and subgroups
| Group I | Group II | Sub group III A1 | Sub group III A2 | Sub Group III B1 | Sub group III B2 | ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| F test | P | ||||||||||||||
| C-cells | 27.6±2.3 | 22±2.7 | 43.2±8.6 | 35.6±3.8 | 142±31.2 | 68±16.8 | 14.56958 | <0.001** | |||||||
|
| |||||||||||||||
| Tukey’s Test | |||||||||||||||
|
| |||||||||||||||
| I & II | I & III A1 | I & III A2 | I & III B1 | I & III B2 | II & III A1 | II & III A2 | II & III B1 | II & III B2 | III A1 & III A2 | III A1 & III B1 | III A1 & III B2 | III A2 & III B1 | III A2 & III B2 | III B1 & III B2 | |
| C-cells | 0.3 | 0.05* | 0.3 | <0.001** | 0.005* | 0.01* | 0.05* | <0.001** | 0.004* | 0.3 | 0.003* | 0.05* | 0.001** | 0.01* | 0.01* |
P>0.05 no significant difference. P≤0.05 significant difference (*). P≤0.001 highly significant difference (**)
Transmission electron microscopic results
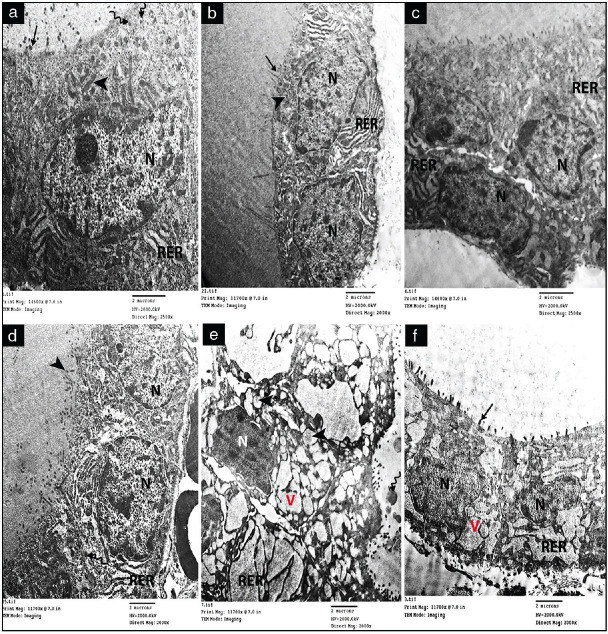
Electron microscopic examination of the thyroid gland of both the control group (Group I) and Group II revealed the normal follicular cells with a rounded euchromatic nucleus, prominent nucleolus, and peripheral clumps of heterochromatin. Elongated electron-dense mitochondria appeared in their cytoplasm with tubular cisternae of rough endoplasmic reticulum (RER) and dense lysosomal granules. The apical surface revealed numerous microvilli [Figure 5a and b]. In Group III A1, electron microscopic examination showed dilated cisternae of RER as compared with Group IIIA2 which showed slight dilatation of the cisternae [Figure 5c and d]. In Group III B1, some follicular cells appeared with irregular dense indented shrunken nuclei, markedly dilated RER, numerous vacuolations, and swollen mitochondria with destructed cristae [Figure 5e]. ln Group IIIB2 the RER of follicular cells showed moderately dilated cristae, cytoplasmic vacuoles and detached micro villi [Figure 5f]
Figure 5.
Electron micrographs of sections of the thyroid gland showing: (a and b) Control (Group I) and Group II showing normal follicular cells with a rounded euchromatic nucleus (N), prominent nucleolus and peripheral clumps of heterochromatin, elongated electron-dense mitochondria (arrowhead), tubular cisternae of rough endoplasmic reticulum (RER), and dense lysosomal granules (wavy arrows). Its apical surface shows numerous microvilli (arrows). (c) Group III A1 shows dilated cisternae of rough endoplasmic reticulum (RER). (d) group III A2 show slightly dilated rough endoplasmic reticulum (RER). (e) group III B1, a follicular cell with irregular dense indented shrunken nucleus (N), markedly dilated rough endoplasmic reticulum (RER), numerous vacuolations (V) and swollen mitochondria (arrowheads) with destructed cristae. (f) group III B2, dilated cisternae of rough endoplasmic reticulum (RER), cytoplasmic vacuoles(V), and detached microvilli (arrow) x(2000).
Statistical analysis results
Statistical analysis of the average body weight of male albino rats in different groups revealed a significant increase in body weight in Group III A1 and Group III B2. However, there was a highly significant increase in body weight in subgroup III B1 (exposed only to radiation for 24 h/day) [Table 1]
-
Total serum tri-iodothyronine (T3)
There was a significant (P value ≤ 0.05) decrease in serum T3 level in subgroup III A1 when compared with groups I & II. On the other hand, there was a highly significant decrease in serum T3 level in subgroup III B1 when compared with groups I & II. There was a significant increase in serum T3 level between subgroup III A1 & III A2. There was a highly significant increase in serum T3 level between subgroup III B1 & III B2.
Total serum thyroxine (T4)
There was a significant decrease in serum T4 level in subgroup III A1 when compared with groups I & II.
On the other hand, there was a highly significant decrease in serum T4 level in subgroup III B1 when compared with groups I & II.
There was a significant increase in serum T4 level between subgroup III A1 & III A2. However, there was a highly significant increase in serum T4 level between subgroup III B1 & III B2.
Serum thyroid stimulating hormone (TSH)
There was a significant increase in serum TSH level in subgroup III A1 when compared with groups I & II.
However, there was a highly significant increase in serum TSH level in subgroup III B1 when compared with groups I & II.
There was a significant decrease in serum TSH level between subgroup III A1 & III A2. However, there was a highly significant decrease in serum TSH level between subgroup III B1 & III B2 [Table 2].
Table 1.
Comparison of the body weight of different groups and subgroups between the beginning and the end of the experiment
| Group I | Group II | Sub group III A1 | Sub group III A2 | Sub Group III B1 | Sub group III B2 | ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| F test | P | ||||||||||||||
| Beginning of experiment | 211. 8±38.97 | 203.8±27.78 | 206.7±28.30 | 205.6±28.08 | 208.6±38.16 | 205.4±28.69 | 0.054 | 0.994 | |||||||
| End of experiment | 215.40±11.39 | 210.60±12.42 | 238.20±11.37 | 220.20±13.01 | 334.60±27.31 | 287±14.40 | 46.235 | <0.001** | |||||||
|
| |||||||||||||||
| Tukey’s Test | |||||||||||||||
|
| |||||||||||||||
| I & II | I & III A1 | I & III A2 | I & III B1 | I & III B2 | II & III A1 | II & III A2 | II & III B1 | II & III B2 | III A1 & III A2 | III A1 & III B1 | III A1 & III B2 | III A2 & III B1 | III A2 & III B2 | III B1 & III B2 | |
| End | 0.46 | 0.025* | 0.27 | <0.001** | 0.004* | 0.012* | 0.12 | <0.001** | 0.001* | 0.02* | <0.001** | 0.001* | <0.001** | <0.001** | 0.001** |
P>0.05 no significant difference. P≤0.05 significant difference (*). P 0.001 highly significant difference (**)
Table 2.
Hormonal analysis of total serum T3, T4 and TSH in different groups and subgroups
| Group I | Group II | Sub group III A1 | Sub group III A2 | Sub Group III B1 | Sub group III B2 | ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| F test | P | ||||||||||||||
| T3 | 0.492±0.049 | 0.498±0.043 | 0.4±0.015 | 0.46±0.023 | 0.218±0.008 | 0.37±0.016 | 54.57 | 0.001** | |||||||
| T4 | ±39.14 3.30 | 40.66±3.62 | 33.92±2.43 | 38.48±1.29 | 17.32±1.281 | 31.02±2.109 | 2.31736 | 0.04* | |||||||
| TSH | 2.302±0.451 | 2.218±0.448 | 3.2±0.449 | 2.524±0.36 | 7.444±0.412 | 5.538±0.634 | 9.20595 | <0.001** | |||||||
|
| |||||||||||||||
| Tukey’s Test | |||||||||||||||
|
| |||||||||||||||
| I & II | I & III A1 | I & III A2 | I & III B1 | I & III B2 | II & III A1 | II & III A2 | II & III B1 | II & IIIB2 | III A1 & IIIA2 | III A1 & III B1 | III A1 & IIIB2 | III A2 & IIIB1 | III A2 & IIIB2 | III B1 & III B2 | |
| T3 | 0.74 | 0.03* | 0.06 | <0.001** | 0.01* | 0.02* | 0.12 | 0.001** | 0.008* | 0.03* | <0.001** | 0.07 | <0.001** | 0.03* | 0.001** |
| T4 | 0.6 | 0.008* | 0.5 | <0.001** | 0.005* | 0.03* | 0.3 | <0.001** | 0.005* | 0.03* | <0.001** | 0.1 | <0.001** | 0.008* | 0.001** |
| TSH | 0.7 | 0.002* | 0.06 | <0.001** | 0.004* | 0.002* | 0.08 | <0.001** | 0.002* | 0.03* | <0.001** | 0.01* | <0.001** | <0.001** | 0.001** |
P>0.05 no significant difference. P≤0.05 significant difference (*). P≤0.001 highly significant difference (**)
DISCUSSION
The different durations (6 h and 24 h) used in this work were similar to the conditions that most people may be exposed to Wi-Fi radiation. The 6-h duration resembles the same duration that the employees are exposed to radiation at work. The 24-h group was exposed to Wi-Fi for 24 h/day which is the same duration that most people are exposed in their homes. The public type of Wi-Fi router (2.45 GHz) device was used as it is commonly used nowadays and simulates the actual condition that most people were exposed to radiation at work.[1,10,19]
Our results revealed a significant increase in the rats’ body weight in the subgroup exposed to EMR for 6 h/day and a highly significant (P ≤ 0.001) increase in the subgroup exposed to EMR for 24 h/day at the end of the experiment when compared to their weight at the beginning. A similar finding was reported by Fahmy et al.[18] who reported a direct correlation between the increase in rats’ body weight and the dose of radiation. This body weight increase can be explained by the associated decrease in T3 and T4 serum levels and increased TSH serum level which acts as a growth factor producing weight gain.[20,21]
On the other hand, the subgroup exposed to EMR for 6 h/day and received combined Vitamin C and zinc at the same time in this study showed a significant decrease (p>0.05) in body weight, while those exposed to EMR for 24h/day and received combined Vitamin C and zinc showed a mild decrease in their body weight when compared to subgroups that were exposed only to EMR. These findings coincide with those reported by Elwakeel and Zaki,[22] who explained these results to be due to a decrease in the serum level of TSH.
The biochemical results in this work showed a significant decrease in serum T3 and T4 levels and a significant increase in serum TSH level in the subgroup exposed to EMR for 6 h/day when compared with the control group and a highly significant decrease in serum T3 and T4 level and a significant increase in serum TSH level in subgroup exposed to EMR for 24 h/day. These results coincided with those of Nadol’nik et al. and Misa-Agustiño et al.[23,24] who mentioned that the serum levels of T3, T4, and TSH were used as reliable indicators of the thyroid function in both humans and experimental animals which result from blood protein degeneration and thyroid tissue affection due to irradiation.
The decreased levels of T3 and T4 may be due to the impairment of the processes of exocytosis and endocytosis caused by EMR and the elevated serum levels of TSH after irradiation to be due to tissue heating induced by EMR and as an active feedback mechanism of anterior pituitary after decreasing T3 and T4 levels.[25,26] On the other hand, the subgroups exposed to EMR and received combined Vitamin C and Zinc at the same time showed no or mild decrease in T3 and T4 levels These results coincided with those of Mangge et al., Peepre et al., and Elwakeel and Zaki who mentioned that antioxidants had positive effects on thyroid hormones levels due to direct involvement of antioxidants on thyroid gland or on deiodinase enzyme activity. They also mentioned that antioxidants reduced free radicals and inhibited their harmful effects caused by exposure to EMR.[22,27,28]
In this research, histological results of the thyroid gland revealed little affection of follicles with decrease in their size and interuuption of follicular basement membrane and presence of inflammatory cells in subgroups exposed to EMR for only 6 hours/ day. However, in the subgroups that were exposed to EMR for 24 h/day, there was marked disorganization of follicles. These findings coincided with Misa-Agustiño et al. and Baloch and LiVolsi[23,29] who reported a decrease in the size of follicles, damage of the follicular epithelium with inflammatory infiltration following acute exposure to EMR, and these results are dependent on the dose of radiation exposed. As exposure to EMR causes damage to the macromolecules of the thyroid gland, resulting in marked morphological alterations in the gland and affection the thyroid epithelium, connective tissue, and follicular and interfollicular cells.
In this work, some follicles had vacuolated cytoplasm of follicular cells in the subgroups that were exposed to EMR for 6 h/day. Other follicles showed highly vacuolated cytoplasm of follicular cells with small dark densely stained nuclei with desquamated and proliferated follicular cells in the subgroups that were exposed to EMR for 24 h/day. Mohamed and Elnegris and Pall[20,30] explained the thyroid deterioration produced by EMR due to a chain of biological mechanisms including activation of voltage-gated calcium channel which is triggered by EMR sources, and these histological alterations were due to the stimulatory effects of TSH on the thyroid gland.
The decrease in the size of follicles and their content of colloid in EMR groups is to compensate the decrease in thyroid hormones in blood as endocytosis of colloid proceeds at a rate greater than synthesis, resulting in progressive depletion of colloid.[31] The desquamated follicular cells inside the colloid occur as the degenerated follicular epithelial cells were susceptible to slough off, and the hypertrophied follicles were due to the increased TSH serum level stimulating neovascularization, hyperplasia, and morphological changes in the follicular cells.[32]
In this study, the exposure of EMR resulted in dilated blood vessels noticed between the follicles However, highly dilated and congested blood vessels were observed between the follicles with increased period of exposure. These results were matched with Rajkovic et al. and Aboul-Fotouh et al.[31,33] Who explained these results by the increased needs of the follicular cells to blood as a result of the sustained stimulation by the increased TSH serum level leading to congested blood vessels and more inflammatory precess. The amount of histamine and neuropeptide Y nerve fibers increases after being exposed to EMR. Histamine, like the other mediator, raises capillary permeability, which in turn raises thyroid blood flow. They improve capillary perfusion, which in turn allows chemicals such as TSH to be transported through the circulatory system to the thyroid gland.[34]
Also in this work, the subgroups exposed to EMR and received combined vitamin C and zinc showed little affection of the follicular tissue as compared to groups exposed to only EMR. These results coincided with those of Peepre et al. and Salehi et al.[28,35] who reported that antioxidants can prevent the cellular damage as they reduce the oxidative stress placed on the gland by harmful radiations. Antioxidants such as zinc prevent the lipid peroxidation and reduce free radicals produced by exposure to EMR inhibiting their harmful effects.[10,36]
In the present study, exposure of EMR resulted in an increase in the number of parafollicular cells which is related directly to the length of the period of exposure of EMR. these findings are due to c-cell hyperplasia which is attributed to the influence of the elevated TSH serum level.[16,22] Misa-Agustiño et al. and Faour and Gilloteaux and Pardhan[23,37] mentioned that the follicular cells regulate C-cell activity through the release of regulatory substances such as insulin-like growth factors and fibroblast growth factor. Those substances exert a paracrine influence on C-cells. Furthermore, it was found that hypothyroidism can modify the activity of the parafollicular cells and provoke an increase in the number of parafollicular cells which showed signs of hyperactivity under the induced hypothyroid conditions.
On the other hand, anti-calcitonin immunostaining of parafollicular cells of the subgroups that were exposed to EMR and received combined Vitamin C and zinc at the same time in this work showed a nearly normal brownish positive cytoplasmic reaction in the parafollicular cells and obvious mild positive cytoplasmic immunoreactive parafollicular cells in the EMR and received combined Vitamin C and zinc for 24 h/day subgroup. Antioxidant administration in the form of Vitamin C and zinc decreased intracellular superoxide anion and hydrogen peroxide formation produced by exposure to EMR and inhibition of the emitted free radicals.[22,27]
In this work, statistical analysis of the average number of anti-calcitonin-positive C-cells in the thyroid gland of the subgroups which were exposed only to EMR revealed a significant and highly significant increase in the mean number of anti-calcitonin-positive C-cells of subgroups that exposed to EMR for 6 h/day and 24 h/day, respectively, as compared to the control group. These results coincided with those of Gilloteaux and Pardhan and López-Martín et al.[25,37] who found positive immune marking for calcitonin in parafollicular cells that increased in the thyroid tissue in rats exposed to 2.45 GHz radiofrequency. They explained the hyperplasia of immune-positive C-cells by the increased TSH serum level. On the other hand, in this research, statistical analysis of the average number of anti-calcitonin-positive C-cells of the subgroups that were exposed to EMR and received combined Vitamin C and zinc showed a nonsignificant change in number which is explained by the decreased TSH serum level.[37]
In this research, ultrastructural picture of the thyroid gland showed mildly dilated RER in the group exposed to EMR for 6h/day whereas in the group exposed to EMR for 24 h/day showed markedly dilated RER, distorted mitochondria and numerous cytoplasmic vacuolation. These results coincided with those of Esmekaya et al. and Mohamed and Elnegris[20,38] who explained them that EMR produces reactive oxygen species, leading to genomic DNA damage and oxidative deterioration of lipids and proteins and producing a cascade of cellular events including enhanced production of superoxide anion and hydroxyl radicals, DNA fragmentation, and modulation of intracellular oxidized states. The nuclear irregularity may be a result of the markedly dilated cisternae of RER compressing the nuclear membrane and the nucleus. Furthermore, EMR prevents the production of inhibitors to apoptosis and the loss of key proteins involved in cellular homeostasis, leading to the degeneration of cells and their nuclei.[39]
In this research the subgroups exposed to EMR and received vit C and zinc for 6h/day showed minimal affection of the ultrastructural picture of the follicular cells with nearly normal cellular organelles and normal nuclei, while that group received the same vitamin C and zinc but exposed to EMR for 24h/day showed moderate affection of organelles of the follicular cells. This can be explained by the protective and antioxidant role of both Vitamin C and zinc which reduces oxidative stress, local inflammatory reactions, and the degenerative changes of different cellular organelles.[20,22]
CONCLUSION
Accordingly, considering the results in the present research and the relevant previous literature, we can conclude that exposure to 2.45 GHz Wi-Fi exerts deleterious changes of the thyroid gland morphology and physiology through increasing of free radicals and stress oxidative changes. However, there is an apparent prevention from this deleterious effect of EMR with the use of Vitamin C and zinc, so they protect the thyroid gland through reducing the oxidative damage by their antioxidative defense system.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ibrahim RA, Ali AH, Khamis NH, Mohammed HH. Effect of exposure to Wi-Fi router radiation on the lung of adult male albino rats: Histological and immunohistochemical study. Egypt J Histol. 2019;42:1059–69. [Google Scholar]
- 2.Guleria R, Bhushan B, Guleria A, Bhushan A, Dulari P. Non-ionizing radiation and human health. Int J Adv Sci Tech Res. 2020;6:130–5. [Google Scholar]
- 3.Hussein KH, Albderi SA, Hamza ZM, Obaid AK, Hussain HH. Evaluation of health hazards due to the Wi-Fi router on humans. J Phys Conf Ser 1804 012001. 2021 [Google Scholar]
- 4.Calvente I, Fernandez MF, Villalba J, Olea N, Nuñez MI. Exposure to electromagnetic fields (non-ionizing radiation) and its relationship with childhood leukemia: A systematic review. Sci Total Environ. 2010;408:3062–9. doi: 10.1016/j.scitotenv.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Saliev T, Begimbetova D, Masoud AR, Matkarimov B. Biological effects of non-ionizing electromagnetic fields: Two sides of a coin. Prog Biophys Mol Biol. 2019;141:25–36. doi: 10.1016/j.pbiomolbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Amani S, Taheri M, Movahedi MM, Mohebi M, Nouri F, Mehdizadeh A. Evaluation of short-term exposure to 2.4 GHz radiofrequency radiation emitted from Wi-Fi routers on the antimicrobial susceptibility of Pseudomonas aeruginosa and Staphylococcus aureus. Galen Med J. 2020;9:e1580. doi: 10.31661/gmj.v9i0.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai G, Kumar A, Mahobiya P. The effect of radiation on thyroid gland. Int J Biol Res. 2018;3:217–22. [Google Scholar]
- 8.Ozmen O, Kavrik O. Ameliorative effects of Vitamin C against hepatic pathology related to Wi-Fi (2.45 GHz electromagnetic radiation) in rats. Int J Radiat Res. 2020;18:405–12. [Google Scholar]
- 9.Lee SR. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid Med Cell Longev. 2018;2018:22. doi: 10.1155/2018/9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Özsobacı NP, Ergün DD, Tunçdemir M, Özçelik D. Protective effects of zinc on 2.45 GHz electromagnetic radiation-induced oxidative stress and apoptosis in HEK293 cells. Biol Trace Elem Res. 2020;194:368–78. doi: 10.1007/s12011-019-01811-6. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Kesari KK, Saxena VK, Sisodia R. The influence of prenatal 10 GHz microwave radiation exposure on a developing mice brain. Gen Physiol Biophys. 2017;36:41–51. doi: 10.4149/gpb_2016026. [DOI] [PubMed] [Google Scholar]
- 12.Singh KV, Gautam R, Meena R, Nirala JP, Jha SK, Rajamani P. Effect of mobile phone radiation on oxidative stress, inflammatory response, and contextual fear memory in wistar rat. Environ Sci Pollut Res Int. 2020;27:19340–51. doi: 10.1007/s11356-020-07916-z. [DOI] [PubMed] [Google Scholar]
- 13.Pandy V. A simple method for animal dose calculation in preclinical research. EC Pharmacol Toxicol. 2020;8:1–2. [Google Scholar]
- 14.Suvarna KS, Layton C, Bancroft JD. Bancroft's Theory and Practice of Histological Techniques E-Book. London, England: Elsevier Health Sciences; 2018. pp. 121–32. [Google Scholar]
- 15.Zbucki RL, Winnicka MM, Sawicki B, Szynaka B, Andrzejewska A, Puchalski Z. Alteration of parafollicular (C) cells activity in the experimental model of hypothyroidism in rats. Folia Histochem Cytobiol. 2007;45:115–21. [PubMed] [Google Scholar]
- 16.Elkalawy SA, Abo-Elnour RK, El Deeb DF, Yousry MM. Histological and immunohistochemical study of the effect of experimentally induced hypothyroidism on the thyroid gland and bone of male albino rats. Egypt J Histol. 2013;36:92–102. [Google Scholar]
- 17.Wasik A. Electron Microscopy: Methods and Protocols. Acta Biochimica Polonica. 2007;54:887–8. [Google Scholar]
- 18.Fahmy H, Mohammed F, Abdelrahman R, Abu Elfetoh M, Mohammed Y. Effect of radiofrequency waves emitted from conventional WIFI devices on some oxidative stress parameters in rat kidney. J Drug Metab Toxicol. 2015;6:2. [Google Scholar]
- 19.Saili L, Hanini A, Smirani C, Azzouz I, Azzouz A, Sakly M, et al. Effects of acute exposure to WIFI signals (2.45GHz) on heart variability and blood pressure in albinos rabbit. Environ Toxicol Pharmacol. 2015;40:600–5. doi: 10.1016/j.etap.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed DA, Elnegris HM. Histological study of thyroid gland after experimental exposure to low frequency electromagnetic fields in adult male albino rat and possible protective role of Vitamin E. J Cytol Histol. 2015;6:1. [Google Scholar]
- 21.Takahashi N, Misumi M, Niwa Y, Murakami H, Ohishi W, Inaba T, et al. Effects of radiation on blood pressure and body weight in the spontaneously hypertensive rat model. Are radiation effects on blood pressure affected by genetic background?Radiat Res. 2020;193:552–9. doi: 10.1667/RR15536.1. [DOI] [PubMed] [Google Scholar]
- 22.Elwakeel EE, Zaki A. Histological and immunohistochemical alterations of thyroid gland after exposure to low frequency electromagnetic fields and protective effect of Vitamin C in adult male albino rat. J Am Sci. 2019;15:56–64. [Google Scholar]
- 23.Misa-Agustiño MJ, Jorge-Mora T, Jorge-Barreiro FJ, Suarez-Quintanilla J, Moreno-Piquero E, Ares-Pena FJ, et al. Exposure to non-ionizing radiation provokes changes in rat thyroid morphology and expression of HSP-90. Exp Biol Med (Maywood) 2015;240:1123–35. doi: 10.1177/1535370214567611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadol'nik LI, Netsetskaia ZV, Kardash NA, Martynchik DI, Kravchuk RI, Basinskiĭ VA, et al. Functional and morphological characterization of rat thyroid gland at remote periods following single high and low dose radiation exposure. Radiats Biol Radioecol. 2004;44:535–43. [PubMed] [Google Scholar]
- 25.López-Martín E, Jorge-Barreiro FJ, Relova-Quintero JL, Salas-Sánchez AA, Ares-Pena FJ. Exposure to 2.45 GHz radiofrequency modulates calcitonin-dependent activity and HSP-90 protein in parafollicular cells of rat thyroid gland. Tissue Cell. 2021;68:101478. doi: 10.1016/j.tice.2020.101478. [DOI] [PubMed] [Google Scholar]
- 26.Hashish AH, El-Missiry MA, Abdelkader HI, Abou-Saleh RH. Assessment of biological changes of continuous whole body exposure to static magnetic field and extremely low frequency electromagnetic fields in mice. Ecotoxicol Environ Saf. 2008;71:895–902. doi: 10.1016/j.ecoenv.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Mangge H, Becker K, Fuchs D, Gostner JM. Antioxidants, inflammation and cardiovascular disease. World J Cardiol. 2014;6:462–77. doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peepre K, Bhimte B, Deshpandey U, Choudhary P. Antioxidants protect cell damage from free radicals: A research study on thyroid hormones in wistar rats. J Dent Med Sci. 2014;13:75–9. [Google Scholar]
- 29.Baloch ZW, LiVolsi VA. In: Thyroid Toxicity. Oak Park, USA: Bentham Science; 2016. Pathologic effects of radiation on the thyroid gland; pp. 141–63. [Google Scholar]
- 30.Pall ML. Wi-Fi is an important threat to human health. Environ Res. 2018;164:405–16. doi: 10.1016/j.envres.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 31.Aboul-Fotouh GI, El-Nour A, El-Din RK, Farag E, Boughdady WA. Histological study on the possible protective effect of curcumin on potassium dichromate induced hypothyroidism in adult male albino rats. Egypt J Histol. 2018;41:220–35. [Google Scholar]
- 32.Wang H, Yang Z, Zhou B, Gao H, Yan X, Wang J. Fluoride-induced thyroid dysfunction in rats: Roles of dietary protein and calcium level. Toxicol Ind Health. 2009;25:49–57. doi: 10.1177/0748233709102720. [DOI] [PubMed] [Google Scholar]
- 33.Rajkovic V, Matavulj M, Gledic D, Lazetic B. Evaluation of rat thyroid gland morphophysiological status after three months exposure to 50 Hz electromagnetic field. Tissue Cell. 2003;35:223–31. doi: 10.1016/s0040-8166(03)00029-6. [DOI] [PubMed] [Google Scholar]
- 34.Rajkovic V, Matavulj M, Johansson O. Light and electron microscopic study of the thyroid gland in rats exposed to power-frequency electromagnetic fields. J Exp Biol. 2006;209:3322–8. doi: 10.1242/jeb.02375. [DOI] [PubMed] [Google Scholar]
- 35.Salehi B, Martorell M, Arbiser JL, Sureda A, Martins N, Maurya PK, et al. Antioxidants: Positive or negative actors? Biomolecules. 2018;8:124. doi: 10.3390/biom8040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adwas AA, Elsayed A, Azab A, Quwaydir F. Oxidative stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng. 2019;6:43–7. [Google Scholar]
- 37.Gilloteaux J, Pardhan D. Crinophagy in thyroid follicular and parafollicular cells of male obese zucker rat. Ultrastruct Pathol. 2015;39:255–69. doi: 10.3109/01913123.2015.1014611. [DOI] [PubMed] [Google Scholar]
- 38.Eşmekaya MA, Seyhan N, Ömeroğlu S. Pulse modulated 900 MHz radiation induces hypothyroidism and apoptosis in thyroid cells: A light, electron microscopy and immunohistochemical study. Int J Radiat Biol. 2010;86:1106–16. doi: 10.3109/09553002.2010.502960. [DOI] [PubMed] [Google Scholar]
- 39.Mohamed HK, Rateb A. Histoloical and biochemichal study on the toxic effects of bisphenol A on the thyroid gland of adult male albino rats and the possible protection by selenium. Egypt J Histol. 2019;42:667–85. [Google Scholar]