Abstract
To observe the clinical outcomes of patients diagnosed with metastatic breast cancer undergoing Trastuzumab Deruxtecan (T-DXd) therapy in a real-world setting. The study retrospectively reviewed and collected medical data from 13 patients at Shin Kong Wu Ho-Su Memorial Hospital who underwent T-DXd treatment over a period from April 2022 to June 2023. Demographics, pathological characteristics, treatment patterns, and outcomes were descriptively analyzed. Thirteen patients diagnosed with metastatic breast cancer underwent T-DXd treatment between April 2022 and June 2023. This study observed that T-DXd was effective in patients with high human epidermal growth factor receptor 2 (HER2) levels. In patients with low HER2, the majority also experienced favorable responses. Only 2 patients exhibited poor or no response: one was a BRCA2 carrier with unmanageable disease progression, and the other had a HER2 1 + status with multiorgan metastases whose cancer was not controlled by T-DXd. Additionally, 2 patients with no HER2 expression responded well to T-DXd treatment. T-DXd is a valuable treatment alternative for patients with breast cancer, including those with HER2-high, HER2-low, and HER2-negative statuses. In this study, the majority of patients experienced positive therapeutic effects. However, this evaluation relied on a limited sample size and short-term observations. Additional studies involving larger and more diverse patient groups and long follow-up durations are required.
Keywords: antibody-drug conjugates, breast cancer, bystander effect, HER2, T-DXd, Trastuzumab Deruxtecan
1. Introduction
Trastuzumab Deruxtecan (T-DXd) is a highly effective treatment for human epidermal growth factor receptor 2 (HER2)-positive breast cancer.[1] The United States Food and Drug Administration approved T-DXd in 2021. Scientists and medical professionals worldwide are committed to exploring further treatment possibilities for breast cancer. T-DXd performed extremely well in the DESTINY-Breast04 trial.[2,3] Not only strongly HER2-positive patients but also those with low HER2 levels are benefited from the treatment. The cytotoxic agents effectively reduced the size of the target cell and its surrounding cells through its bystander effect.[4] Thus, this study administered T-DXd to Taiwanese patients to document response to the drug of the Taiwanese population. This study presents the short-term treatment outcomes as a single-center experience with T-DXd therapy across different HER2 subgroups of breast cancer in a real-world setting.
2. Materials and methods
2.1. Population
The Institutional Review Board of Shin Kong Wu Ho-Su Memorial Hospital approved this study. A retrospective review was conducted, and medical data of patients treated with T-DXd were collected over a period between April 2022 and June 2023. The inclusion criteria were
Having a diagnosis of breast cancer and undergoing neoadjuvant chemotherapy, surgery, adjuvant chemotherapy, hormonal therapy, or radiotherapy; and subsequently receiving T-DXd upon the detection of distant metastasis.
Having metastatic breast cancer, not experiencing a visceral crisis, and having an estimated life expectancy of more than 2 years.
Patients who were lost to follow-up during treatment were also excluded from the analysis. A total of 13 patients were included in the study. All were Taiwanese women aged between 45 and 74 years, with the majority aged 50 to 60 years. The median age was 56 years old.
2.2. Clinical assessments
The initial cancer stages of the patients varied from Stage Ia to Stage IIIc, as classified by the American Joint Committee on Cancer (AJCC) AJCC Cancer Staging Manual, 8th edition. The treatment regimen adhered to the National Comprehensive Cancer Network and American Society of Clinical Oncology guidelines for 2023. Treatment commenced soon after diagnosis. T-DXd was administered as a second-line or subsequent therapy upon the observation of disease relapse or metastasis.
The primary objective of this study was to evaluate the efficacy of T-DXd across various HER2 subgroups, including patients with HER2 immunohistochemistry (IHC) 0. Patients with breast cancer were segmented into 4 groups according to HER2 levels: HER2 IHC 3+, HER2 IHC 2+, HER2 IHC 1+, and HER2 IHC 0. Fluorescence in situ hybridization (FISH) analysis was conducted for the HER2 2 + group. Patients in the HER2 2 + category with positive FISH results comprised the HER2-high group, whereas those with negative FISH - results comprised the HER2-low group. The VENTANA HER2 (4B5) antibody (Roche, Basel, Switzerland) was utilized for HER2 assessment. A HER2-negative result (score 0) was identified by the absence of staining or membranous staining in fewer than 10% of tumor cells.
Data, including those on demographic characteristics, clinicopathological characteristics (such as staging and biomarkers), treatment patterns (including the type of treatment, dosage, and duration), and treatment outcomes (reflected in imaging results from positron emission tomography [PET] and computed tomography [CT]), were reviewed and collected.
3. Results
T-DXd was administered to 13 patients with breast cancer (identified as Patients A–M) from April 2022 to June 2023. These patients were segmented into 4 subgroups based on HER2 intensity: 5 patients were HER2 3+, 4 patients were HER2 2+, 2 patients were HER2 1+, and 2 patients were HER2-negative (Table 1).
Table 1.
Patient age, the breast cancer subtype, the disease profiles, the lines of T-DXd treatment and the treatment outcomes.
| No. total 13 | |
|---|---|
| Age | |
| 40–50 | 1 |
| 51–60 | 8 |
| 61–70 | 3 |
| 71–80 | 1 |
| Breast cancer subtype | |
| HR + | 7 |
| HER 2 0 | 2 |
| HER2 1+ | 2 |
| HER2 2 + FISH+ | 2 |
| HER2 2 + FISH- | 2 |
| Her2 3+ | 5 |
| Pathological stage | |
| PCR | 2 |
| I | 6 |
| II | 3 |
| III | 2 |
| IV | 0 |
| Metastasis site | |
| Local LN | 4 |
| Distant organs | 9 |
| Lines of T-DXd | |
| 1st | 1 |
| 2nd | 1 |
| 3rd | 1 |
| >3rd | 10 |
| Response | |
| Progression | 1 |
| Equivocal | 1 |
| Partial regression | 8 |
| Almost complete regression | 3 |
FISH = fluorescent in situ hybridization, HER2 = human epidermal growth factor receptor 2, T-DXd = Trastuzumab Deruxtecan.
All patients commenced treatment after completing neoadjuvant therapy, chemotherapy, dual-target therapy, hormonal therapy, or surgery. Due to financial constraints, treatment plans varied among individuals, with most starting on a full dose (5.4 mg/kg), with adjustments made based on tolerance (Table 2).
Table 2.
Details of disease, treatment, and outcome of the 13 patients.
| Patient | Age | Diagnosis date | Receptor | Stage | Treatment line | Trastuzamab Deruxtecan (T-DXd) duration | Dose | Response | |
|---|---|---|---|---|---|---|---|---|---|
| 3+ | Patient A | 60 | 2016/04/09 | ER- PR- HER2 3+ | cT2N1M0->T2N1miM0 | neoH + P -> TH ->-> Xeloda±TDM-1->-> H-> TDM-1 > Xeloda±Tykerb-> HP + Navelbine -> TDM-1 (2021/02~2022/04) | 2022/04/27 ~ now | 200 mg (46.5 kg) 4.3 mg/kg |
Almost complete |
| 3+ | Patient B | 52 | 2017/11/01 | ER + PR- HER2 3+ | cT2N1M0->pT1cN0M0 | neoH + P-> hormone-> TPH->Navelibin + P-> P -> ENHERTU x3 -> Vinorelbine | 2022/06/22-2022/08/03 | 300 mg (59 kg) 5.1 mg/kg |
Partial |
| 3+ | Patient C | 55 | 2019/11/06 | ER- PR- HER2 3+ | cT2N1M0-> pT0NxM0 | Pharmorubicin + endoxan-> TPH-> H -> TDM-1 (2021/10) -> ENHERTU (2022/05) × 3 -> Xeloda | 2022/05/11-2022/06/22 | 291 mg (54.7 kg) 5.3 mg/kg |
Partial |
| 3+ | Patient D | 56 | 2005/05/29 | ER + PR- HER2 3+ | cT2N1M0 ->pT1bN0M0 | Taxotere + Epirubicin-> H ->TDM-1(2015/03/18) -> Lapatinib(2015/06/03)->Halaven (2015/8/03) -> xeloda + lapatinib(2016/01) -> xeloda + lapatinib + AI (2018/01) -> kadcyla (2021/04) ->Perjeta(2021/06) ->capcitabin + AI -> Vinorelbin + AI (2023/05) ->ENHERTU (2023/06) | 2023/06/21 ~ still using (one cycle) |
300 mg (65 kg) 4.61 mg/kg |
Partial |
| 3+ | Patient E | 51 | 2019/01/03 | ER + PR + HER2 3 | cT4bN3aM1-> pT3N3aM0 | Neo Kadcyla (2019/01) -> Neo H + P (2019/04) -> op(2019/09) -> TPH (2019/11) -> H (2020/05) -> HP + Navelbine (2020/07) -> H (2021/06) -> Enhertu (2023/06) | 2023/06/04 ~ still using (2 cycles) |
300 mg (63 kg) 4.7 mg/kg |
Partial |
| 2+/ FISH + | Patient F | 56 | 2017/06/05 | ER-PR- HER2 2+ | cT2N1M0->pT1cN0M0 | FAC -> TH (2017) -> H -> ENHERTU (2022/10) | 2022/10/21 ~ still using | 297 mg (55 kg) 5.4 mg/kg |
Almost complete |
| 2+/ FISH + | Patient G | 61 | 2018/10/24 | ER-PR- HER2 2+ | cT2N1M0 -> pTxN0M0 | neoHP + T-> H -> ENHERTU(2022/05–09)-> Nerlynx(2022/10 - now) | 2022/05/25-2022/09/07 | 300 mg (67 kg) 4.48 mg/kg |
Partial |
| 2+/FISH− | Patient H | 59 | 2021/09/02 | ER + PR- HER2 2+ | cT2N3M0->pT2N0M0 | neoEC + T -> Femera + RT -> Ibrance (2022/06)-> ENHERTU (2022/07) | 2022/07/20 ~ still using | 300 mg (52 kg) 5.77 mg/kg |
Almost complete |
| 2+/ FISH− | Patient I (BRCA2+) | 62 | 2012/12/04 | ER + PR + HER2 2+ | cT2N0M0 ->pT2N0M0 | TC + RT-> Hormone(T-F) ->Ibrance (2019/02) ->Halaven(2020/10) → Talazoparib(2022/02-2022/12) -> ENHERTU(2023/02)-> Talazoparib(2023/05) | 2023/02/02-2023/04/12 | 200 mg (53 kg) 3.77 mg/kg |
equivocal |
| 1+ | Patient J | 45 | 2016/09/20 | ER + PR + HER 2 1+ | cT3N1M0->pT2N1M0 | neoEC -> neoTE->Ibrance(2020/12)-> Halaven(2021/04)-> Ixempra(2021/10)->-Vinorelbine(2022/06)->ENHERTU(2022/11) | 2022/11/16~2023/01/18 | 300 mg (56 kg) 5.3 mg/kg |
No response |
| 1+ | Patient K | 69 | 2017/01/23 | ER + PR + HER2 1+ | cT3N1M0->pT1cN2M0 | neoEC + T -> RT + Nolvadex→Femara -> KISQALI + Femara (2021/09 -2023/02) -> ENHERTU(2023/02) | 2023/02/22~ still using | 286 mg (53 kg) 5.39 mg/kg |
Partial response |
| 0+ | Patient L | 53 | 2017/03/20 | ER-PR- HER2 - | cT1bN0M0->pT1bN0M0 | FAC ->IORT->RT ->TDM-1(2020/10)->H + P (2020/12)-> C + T (2021/02)-> TDM-1 (2021/09)->H + P(2022/03)-> ENHERTU (2022/05) | 2022/05/04-2022/12/23 | 300 mg (54.8 kg) 5.47 mg/kg |
Partial response |
| 0+ | Patient M | 74 | 2012/01/05 Lt BC. 2022/03 R’t BC |
ER + PR - Her 2 3+ -----------ER- PR- HER2 - |
cT2N0M0 -> pT1N0M0 -------------- pT2N3aM0 |
Cysplatin + T + kytruda + RT -> Enhertu (2023/06) | 2023/6/28~ | 300 mg (58 kg) 5.17 mg/kg |
Partial response |
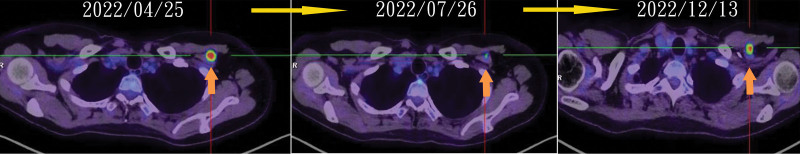
The 5 HER2 3 + group patients ranged in age from 51 to 60 years. Three patients (A, B, and C) exhibited axillary lymph node metastasis only, whereas the remaining 2 had bone and brain metastases. Before initiating T-DXd treatment, each patient had undergone at least 2 prior lines of therapy. Financial burdens led 2 patients to modify their treatment regimen after 3 cycles, resulting in disease progression for Patients B and C approximately 6 to 8 months later (Fig. 1). After completing 3 treatment cycles, Patient A demonstrated almost complete disease regression. Patient D started treatment in June 2023 and did not undergo follow-up imaging; however, remarkable improvements were observed in neurological symptoms associated with brain metastasis.
Figure 1.
Illustration of the patient disease regression during T-DXd and progression after switching to other agents. T-Dxd = Trastuzumab Deruxtecan.
Patients in the HER2 2 + group were further divided based on FISH results into HER2-high (FISH+) and HER2-low (FISH−) categories, with ages ranging from 56 to 62 years. The HER2-high group comprised 2 patients (F and G, whereas Patients H and I constituted the HER2-low group). Diagnosed in 2017 and 2018, respectively, both patients in the HER2-high group initially presented with Stage IIb disease. Before receiving T-DXd, Patient F experienced local recurrence and new ductal carcinoma in situ in the opposite breast, and liver and spine metastases were identified in October 2022. T-DXd, as a third-line treatment, led to an almost complete regression of her metastases after 4 cycles. Patient G had only local axillary lymph node metastases in May 2022, with regression observed in magnetic resonance imaging (MRI) in October 2022.
In the HER2-low (FISH−) group, Patient H underwent neoadjuvant therapy in March 2022 and began T-DXd treatment following surgery in July 2022. PET in April 2023 indicated regression of the brain metastasis. Patient I, having a diagnosis of BRCA2-positive breast cancer with liver, bone, and lung metastases, received T-DXd therapy from February 2023 to April 2023. Her PET scan in April 2023 revealed progression of the liver metastases, equivocal interval changes in lung metastases, and regression of bone metastases. Prior to T-DXd treatment, she had undergone chemotherapy, radiotherapy, hormonal therapy, and targeted therapy.
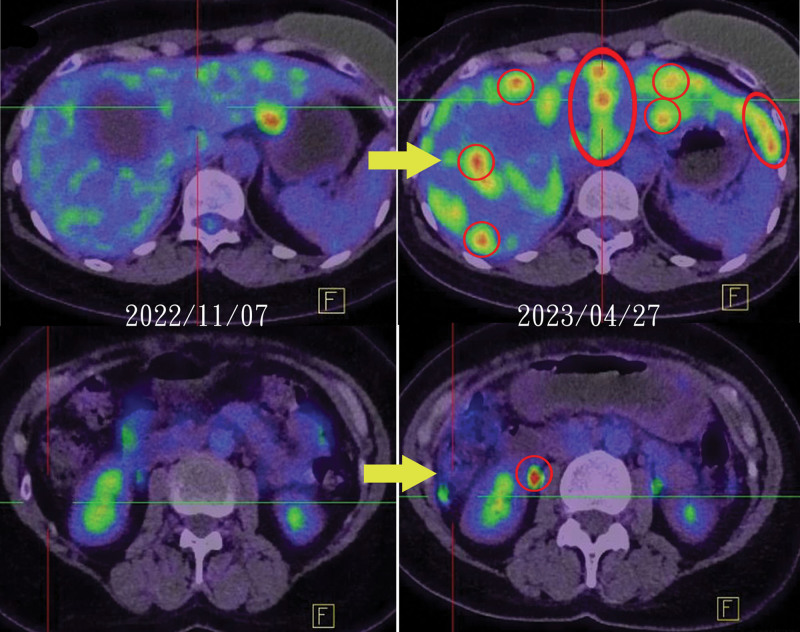
Patients J and K, aged 45 and 69 years, respectively, constituted the HER2 1 + group. Patient J, who had multiple metastases to the myocardium, lungs, brain, bone, peritoneum, liver, ovary and uterus, received 6 lines of therapy before T-DXd treatment. All treatments, except for ixabepilone (Ixabepilone), yielded unsatisfactory results. She discontinued T-DXd after 4 cycles due to financial constraints, and a follow-up PET scan revealed a recurrence of liver and uterine metastases, indicating that T-DXd was ineffective (Fig. 2).
Figure 2.
Patient J experienced recurrent liver and uterine metastases during T-DXd treatment. T-Dxd = Trastuzumab Deruxtecan.
Patient K presented with multiple metastases in the liver, bones, and lungs and had undergone 5 lines of therapy prior to T-DXd, none of which were effective. After 4 cycles of T-DXd treatment, a PET scan displayed regression in liver and bone lesions, with lung metastases remaining unchanged. Patient K accordingly continued under T-DXd management.
The HER2-negative group comprised Patients L and M, aged 53 and 74, respectively. Patient L, who presented with bilateral lung metastasis, responded well to T-DXd; however, a CT scan in March 2023, following treatment cessation in December 2022, revealed a slight progression of lung metastasis. Patient M was initially diagnosed with left breast cancer (estrogen receptor [ER]-positive, progesterone receptor [PR]-negative, HER2 3+) in January 2012, undergoing successful treatment and regular follow-up until June 2018. In March 2022, triple-negative right breast cancer was diagnosed with subsequent imaging confirming metastasis in the right axilla, neck lymph nodes, and brain. After the first cycle of T-DXd treatment in June 2023, Patient M experienced marked improvements in aphasia and lower limb weakness. Further treatment led to further improvements. Both patients had T-DXd treatment alone during our observation.
4. Discussion
In recent years, antibody-drug conjugates (ADCs) have been employed to treat both HER2-positive and HER2-negative breast cancers. Prominent ADCs include Trastuzumab Emtansine (T-DM1), T-DXd, and Sacituzumab Govitecan.
T-DXd has a stable linker and a significantly higher payload compared with T-DM1. Regarding the drug-to-antibody ratio (DAR), T-DXd has approximately double the DAR of T-DM1 (DAR 8 compared with 3–4).[5] With high membrane permeability, T-DXd demonstrates potent cytotoxicity against both strongly HER2-positive cancer cells and those with lower HER2 expression levels. This characteristic was not observed in studies involving T-DM1.[5,6]
In the DESTINY-Breast04 trial,[2] T-DXd had superior cytotoxic ability in treating HER2-low breast cancer. Its high membrane permeability and potent payload cytotoxicity make it potent against target cells and those in the surrounding environment, a phenomenon known as the bystander effect. This effect has yielded remarkable results both in vitro and in vivo.[4,5]
4.1. T-DXd is effective in treating nearly all HER2 intensities
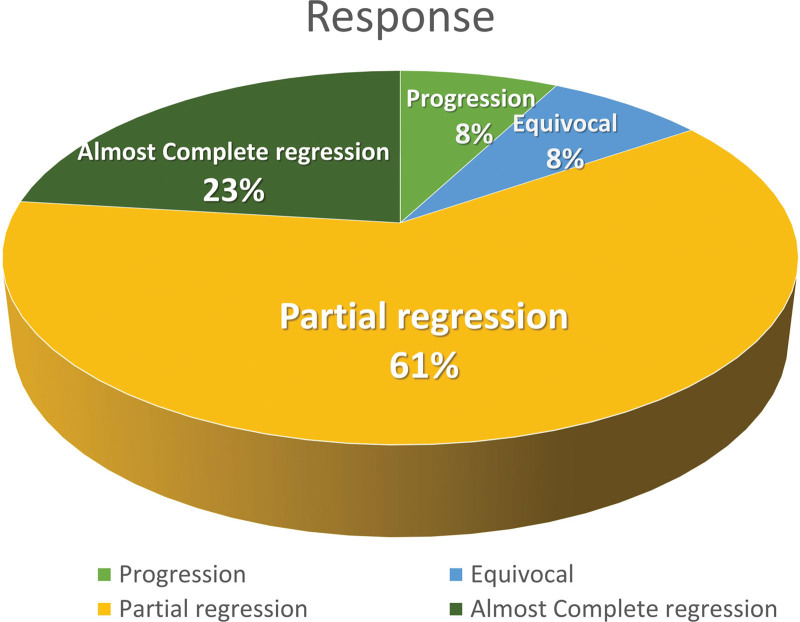
Among the patients treated with T-DXd, 3 (3/13) exhibited almost complete regression, 8 (8/13) exhibited partial regression, 1 (1/13) had an equivocal (poor) response, and 1 (1/13) demonstrated no response to disease (Fig. 3). T-DXd was effective in most HER2-intense patients and HER2-negative patients.
Figure 3.
Treatment response of the 13 patients with breast cancer to T-DXd treatment. T-Dxd = Trastuzumab Deruxtecan.
Regarding the patients with poor responses, one had HER2 2+/FISH- status and was a BRCA2 carrier, and the other was HER2 1+. These cases are discussed individually in the following sections.
4.2. Promising effects in HER2-positive group
In the HER2-positive metastatic breast cancer group, the complete response rate to T-DXd therapy was approximately 28.6% (2/7). This result is similar to that of the DESTINY-Breast03 study,[7] in which the complete response rate was approximately 21.1%. In our observation, the administration of trastuzumab with nalvelbine after T-DXd resulted in a progression-free survival of approximately 8 months. Although the progression-free survival (PFS2) outcomes in the DESTINY-Breast03 study are not definitive, this suggests a potential treatment option following T-DXd therapy.[7]
T-DXd exerted beneficial effects in the HER2 3 + group. Two patients (Patients B and C) switched medications, and their disease progressed, as revealed in follow-up PET scans 6 and 8 months later, respectively. Patient A, who continued the treatment at a dosage of 4.3 mg/kg for approximately 15 months, experienced no disease progression. In the DESTINY-Breast01 study,[3] previously treated HER2-positive patients with breast cancer had a 60.9% response rate and approximately 16.4 months of progression-free survival. These results may explain why T-DXd was highly effective for our HER2 3 + patients during the treatment.
4.3. T-DXd effectively treats breast cancer with brain metastasis
Patient D, from the HER2 3 + group, had been undergoing various treatment regimens for breast cancer for approximately 18 years. She developed brain metastasis in 2015, with disease progression observed in 2023. She commenced T-DXd treatment in June 2023. After 1 treatment cycle, a marked improvement in the weakness of her right upper limb was observed—an outcome not observed for this patient during her previous outpatient clinic visits.
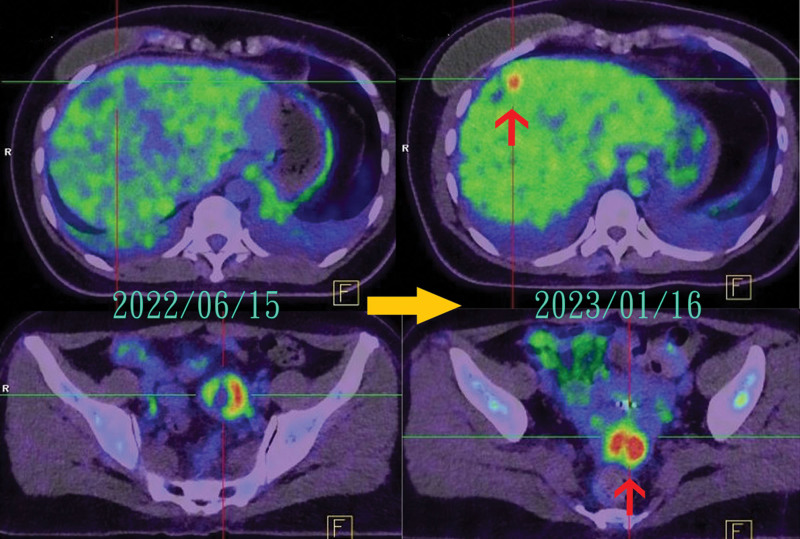
Patient H, categorized as HER2 2 + FISH-, began T-DXd treatment in July 2022 following the detection of new brain metastasis in June 2022. After 6 months of treatment, almost complete remission was evident in a brain MRI in December 2022, with a similar outcome observed in a PET image in April 2023. An approximately 9-month progression-free period was observed (Fig. 4). This 9-month progression-free period was comparable to the results of the DESTINY-Breast04 trial[2] involving HER2-low patients with advanced metastatic breast cancer.
Figure 4.
Markedly regression of brain metastasis of Patient H after T-DXd treatment. T-Dxd = Trastuzumab Deruxtecan.
T-DXd exhibited remarkable and rapid control effects for patients with advanced breast cancer with brain metastasis. In early studies, T-DXd treatment for active brain metastasis demonstrated impressive outcomes (TUXEDO-1: objective response rate [ORR] 73.3%, ROSET-BM: intracranial objective response rate [IC-ORR] 62.7%).[8,9] In the DEBBRAH trial, treatment with T-DXd for both HER2-positive and HER2-low breast cancer with brain metastasis resulted in an IC-ORR of approximately 50%.[10] The observations of the present study are consistent with these results. The response rate and effectiveness of T-DXd are sufficiently high to warrant consideration for patients with brain metastasis.
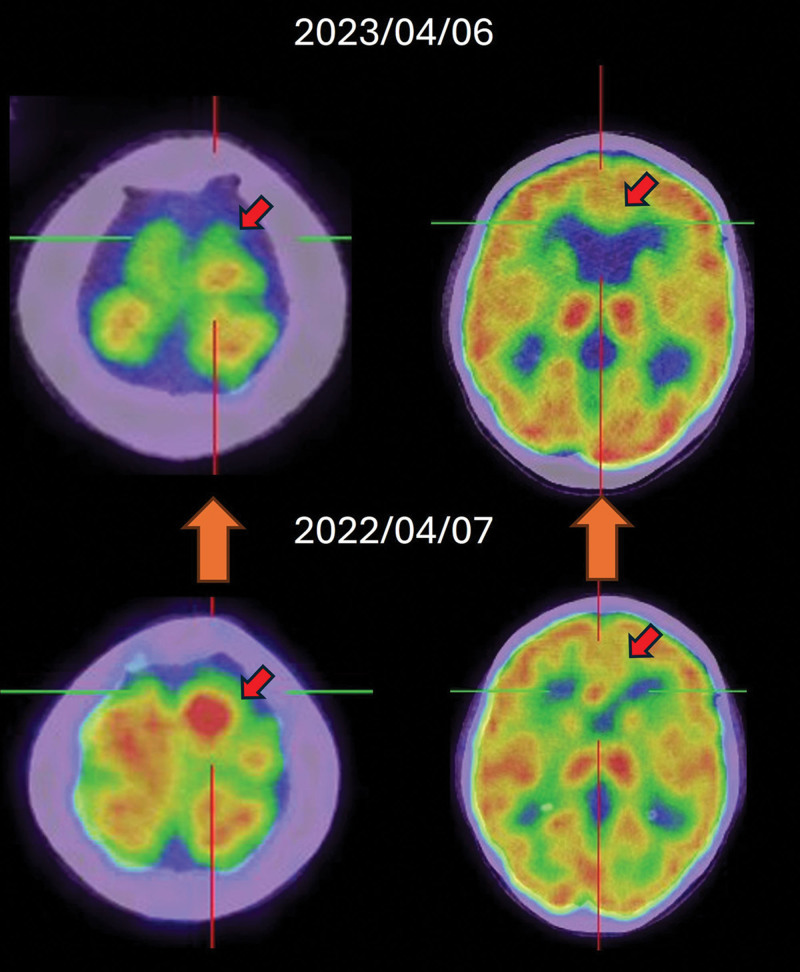
4.4. Poor response of T-DXd in BRCA2 + breast cancer
Patient I, who had a diagnosis of BRCA2-positive breast cancer, underwent various treatments. However, the effectiveness of these treatments, including T-DXd, was limited (Fig. 5). In the OlympiAD and EMBRCA trials,[11,12] talazoparib did not improve overall survival. In these studies, a substantial proportion of patients—43% in OlympiAD and 46% in EMBRCA—underwent platinum-based chemotherapy (PBC) following talazoparib treatment. The outcomes have not been disclosed. In a study on the role of PBC after PARP-inhibitor treatment,[13] the median progression-free survival (PFS) of patients with post-PARP PBC in germline BRCA1 or BRCA2 mutations (gBRCAm) was approximately 1.8 months. The disease control rate was only 14%. This outcome indicates limited response to chemotherapy following multiple regimens (including poly ADP-ribose polymerase inhibitor) in patients with germline BRCA mutations (gBRCAm) and may explain the treatment trajectory observed for Patient I, facilitating adjustments to her care.
Figure 5.
Uncontrolled disease progression of HER2-low breast cancer with BRCA2 + under T-DXd treatment.
During the treatment of Patient I, the administered dose of T-DXd was only 3.77 mg/kg, which may influence the treatment efficacy, as reported by Dr Doi T. in the phase 1 dose-escalation study of T-DXd.[14]
In one biomarker study on DB-04[15] examining the efficacy of T-DXd in treating various intrinsic subtypes of breast cancer (Luminal A, Luminal B, and HER2-enriched), ESR1 mutations, PIK3CA mutations, or CDK4/6i resistance markers indicated that T-DXd maintained consistent therapeutic efficacy across all intrinsic subtypes. For patients with germline BRCA mutations (gBRCAm) and advanced metastatic breast cancer, T-DXd appears to be a viable treatment option, especially at a full dose (5.4 mg/kg) or higher, despite the lack of studies specifically focusing on the BRCA mutation.
4.5. Same HER2 levels but different responses in the HER2 1 + group
Both patients (Patients J and K) exhibited multiple metastases in the HER2 1 + group. After T-DXd treatment, they had opposite outcomes. In the DESTINY-Breast04 trial subgroup analysis,[2] the effects of T-DXd on PFS did not differ between the low-HER2 groups (IHC1 + or 2+/ISH-). However, this study observed different outcomes, possibly due to variations in real-world patient conditions, including heterogeneity among patients, diverse comorbidities, and pretreatment conditions.
4.6. T-DXd is effective in treating HER2-negative patients with metastatic breast cancer
In the treatment of Patients L and M, who were HER2-negative with multiple metastases, improvements in their condition were observed during T-DXd therapy. Regrettably, Patient L experienced disease progression 3 months after switching her treatment regimen. Patient M exhibited significant improvement in neurological symptoms following the first cycle of T-DXd treatment. She regained the ability to speak, rise from her wheelchair, and walk—all of which she was unable to do before treatment.
In the DAISY trial,[16] T-DXd was effective in treating the HER2 IHC 0 metastatic breast cancer group. The ORR was 30%, and the PFS was 4.2 months. Although this result was the lowest of the 3 cohort groups, it affirmed that T-DXd benefits the HER2 IHC0 group, as evidenced also in our patients. Our observations suggest that the intensity of HER2 expression appears to be associated with treatment efficacy, as in the results of the DAISY trial.[16]
In comparing HER2 1 + and HER2-negative groups, an inconsistent treatment response was observed. Interestingly, the HER2-negative group had a better response than the HER2 1 + group. Upon further examination, it was discovered that both patients in the HER2 1 + group were ER-positive, while the patients in the HER2-negative group were triple-negative. Patient J in HER2 1 + group had the most aggressive metastasis condition among the 2 groups, with metastases in the liver, bone, lung, heart, brain, peritoneum, uterus and ovary. She also had the most extensive treatment before receiving T-DXd. Disease severity with different cancer receptors influence seems to affect the response to T-DXd. To gain a better understanding of these mysteries, large-scale investigations, such as the ongoing DESTINY-Breast15 trial, are necessary. We eagerly await the exciting results that will be forthcoming.
4.7. Side effects
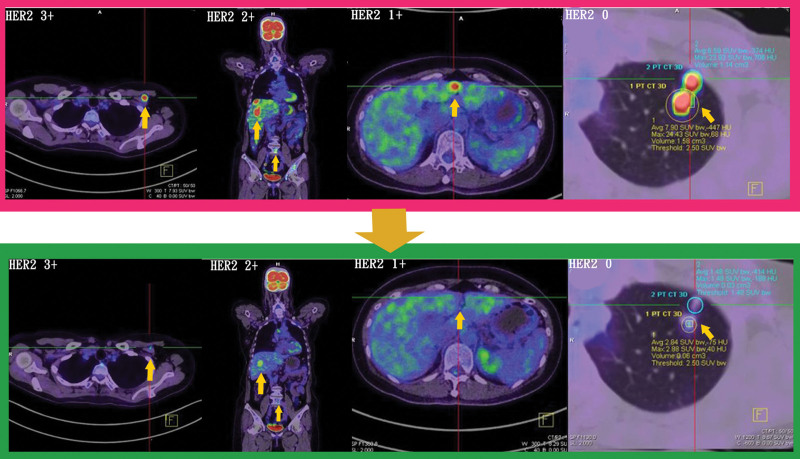
In the 4 groups in the present study, T-DXd was beneficial for most HER2 breast cancer subtypes (Fig. 6). The patients who changed agents did so due to financial constraints rather than side effects. According to earlier studies, the most common side effect of T-DXd is interstitial pneumonitis,[17] with an average onset of 129 days after treatment.[18] In the patients in the present study, the most common side effects were diarrhea, gastrointestinal symptoms (such as nausea, vomiting, and fullness), and grade I alopecia. Interstitial pneumonitis was not observed. None of the patients discontinued treatment due to side effects.
Figure 6.
Images in the pink frame display all HER2 subtypes of breast cancer metastasis before T-DXd treatment, whereas images in the green frame display all HER2 subtype metastases regressing after T-DXd treatment. HER2 = human epidermal growth factor receptor 2, T-DXd = Trastuzumab Deruxtecan.
5. Conclusions
T-DXd is an effective agent for treating various subtypes of HER2 breast cancer. The intensity of HER2 expression appears to be associated with the efficacy of the treatment. The impressive response of brain metastasis to T-DXd observed during the study offers a new treatment option worth considering. Remarkably, positive treatment effects were observed in patients who tested negative for HER2. This result indicates that T-DXd may exert a bystander effect, effectively targeting cancer cells even when HER2 receptor levels are extremely low or undetectable. However, the authors acknowledge that the present study findings are based on a limited sample size of only 13 cases. Consequently, additional studies with larger patient cohorts are essential to comprehensively verify the therapeutic potential of T-DXd.
Acknowledgments
This manuscript was edited by Wallace Academic Editing.
Author contributions
Conceptualization: Fiona Tsui-Fen Cheng.
Data curation: Fiona Tsui-Fen Cheng.
Resources: Fiona Tsui-Fen Cheng.
Supervision: Fiona Tsui-Fen Cheng.
Writing – original draft: Chung-Wei Wu.
Writing – review & editing: Chung-Wei Wu.
Abbreviations:
- CT
- computed tomography
- DAR
- drug-to-antibody ratio
- FISH
- fluorescent in situ hybridization
- gBRCAm
- germline BRCA1 or BRCA2 mutations
- HER2
- human epidermal growth factor receptor 2
- IHC
- immunohistochemistry
- MRI
- magnetic resonance image
- PBC
- platinum-based chemotherapy
- PET
- positron emission tomography
- T-DXd
- Trastuzumab Deruxtecan
The study was presented to and received an exemption from ethical approval due to its retrospective nature by the Ethics Committee of Shin Kong Wu Ho-Su Memorial Hospital (IRB No.20230916R). The study has also been granted formal institutional permission for publication.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Wu C-W, Cheng FT-F. Using Trastuzumab Deruxtecan to treat advanced metastatic breast cancer in patients with varying HER2 expression levels: A single-center experience in Taiwan. Medicine 2024;103:28(e38911).
References
- [1].Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab Deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:816–26. [DOI] [PubMed] [Google Scholar]
- [2].Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med. 2020;382:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS -8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Indini A, Rijavec E, Grossi F. Trastuzumab Deruxtecan: changing the Destiny of HER2 Expressing Solid Tumors. Int J Mol Sci . 2021;22:4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Najjar MK, Manore SG, Regua AT, et al. Antibody-drug conjugates for the treatment of HER2-positive breast cancer. Genes. 2022;13:2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hurvitz SA, Hegg R, Chung WP, et al. Trastuzumab Deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet (London, England). 2023;401:105–17. [DOI] [PubMed] [Google Scholar]
- [8].Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28:1840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yamanaka T, Niikura N, Nomura H, et al. Abstract PD7-01: Trastuzumab Deruxtecan for the treatment of patients with HER2-positive breast cancer with brain and/or leptomeningeal metastases: A multicenter retrospective study (ROSET-BM study). Cancer Res. 2023;83(5_Supplement):PD7–01. [Google Scholar]
- [10].Pérez-García JM, Vaz Batista M, Cortez P, et al. Trastuzumab Deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro-Oncology. 2023;25:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Robson ME, Im SA, Senkus E, et al. OlympiAD extended follow-up for overall survival and safety: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Eur J Cancer. 2023;184:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Litton JK, Hurvitz SA, Mina LA, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31:1526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Valenza C, Trapani D, Gandini S, et al. Platinum-based chemotherapy and PARP inhibitors for patients with a germline BRCA pathogenic variant and advanced breast cancer (LATER-BC): retrospective multicentric analysis of post-progression treatments. Eur J Cancer. 2023;190:112944. [DOI] [PubMed] [Google Scholar]
- [14].Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–22. [DOI] [PubMed] [Google Scholar]
- [15].Modi S, Niikura N, Yamashita T, et al. Trastuzumab Deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with HER2-low, hormone receptor-positive (HR+) unresectable and/or metastatic breast cancer (mBC): Exploratory biomarker analysis of DESTINY-Breast04. J Clin Oncol. 2023;41(16_suppl):1020–1020. [Google Scholar]
- [16].Andre F, Fernanda M, Deluche E, et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat Med. 2023;29:2110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abuhelwa Z, Alloghbi A, Alqahtani A, et al. Trastuzumab Deruxtecan-induced interstitial lung disease/pneumonitis in ERBB2-Positive Advanced Solid Malignancies: a systematic review. Drugs. 2022;82:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guo Z, Ding Y, Wang M, et al. Safety of Trastuzumab Deruxtecan: a meta-analysis and pharmacovigilance study. J Clin Pharm Ther. 2022;47:1837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]