Abstract
Objective:
Schizophrenia (SZ) is characterized by neurobiological and associated cognitive and functional deficits, including pronounced cortical thinning, that lead to acute and long-term functional impairment. Research with older adults supports the role of non-pharmacological interventions, such as exercise (E) and cognitive training (CT), for cognitive impairments. This literature influenced the development of combined CT&E treatments for individuals with SZ. However, the impact of longer combined treatment duration (6 months) on neuroanatomy has yet to be explored in patients in the early course of the illness. The impact of adding exercise to cognitive training for key brain regions associated with higher-order cognition was examined here using magnetic resonance imaging (MRI) in first-episode psychosis (FEP) patients.
Methods:
UCLA Aftercare Research Program patients with a recent first episode of schizophrenia were randomly assigned to either combined cognitive and exercise training (CT&E) (N = 20) or cognitive training alone (CT) (N = 17) intervention. Cortical thickness was measured longitudinally and analyzed for two regions of interest using FreeSurfer.
Results:
Compared to patients in the CT group, those in the CT&E group demonstrated an increase in cortical thickness within the left anterior cingulate cortex over the six-month treatment period (ACC: F(1, 35) = 4.666, P < .04). Directional tendencies were similar in the left dorsolateral prefrontal cortex (DLPFC: F(1,35) = 4.132, P < .05).
Conclusions:
These findings suggest that exercise and cognitive training may synergistically increase fronto-cingulate cortical thickness to mitigate progressive neural atrophy in the early course of SZ. This combined intervention appears to be a valuable adjunct to standard pharmacologic treatment in FEP patients.
1. Introduction
Debilitating deficits in learning, memory, and executive function are among the core features of schizophrenia (SZ) (Bowie and Harvey, 2006). These higher-order cognitive deficits manifest early in the course of the illness and strongly predict functional outcome (Green et al., 2004; Lindgren et al., 2020). These deficits might be due, in part, to the progressive degeneration and atrophy of brain structures observed in SZ, including key regions associated with cognition, such as the hippocampus, cingulate cortex and the prefrontal cortex (PFC). Remarkable cortical thinning, particularly in the prefrontal-temporal regions of the cortex including the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC), have been observed among SZ patients following their first psychotic episode (Schultz et al., 2010).
The North American Prodrome Longitudinal Study (NAPLS) revealed that high-risk subjects who converted to psychosis had a steeper rate of cortical loss in the DLPFC and orbitofrontal cortex compared to non-converters and controls (Cannon et al., 2015). Two well-replicated and pervasive cognitive deficits in early psychosis are in verbal memory (Aleman et al., 1999; Jordan et al., 2014; Mesholam-Gately et al., 2009; Milev et al., 2005; Nuechterlein et al., 2011; Valli et al., 2012) and executive function (Bilder et al., 2000; Sponheim et al., 2010). These deficits exert a strong negative impact on overall functioning in SZ (Goldberg et al., 2007; Nuechterlein et al., 2011) and are consistent with the neurodegeneration observed in the PFC and ACC. Unfortunately currently approved pharmacological therapies for SZ patients are not effective in reversing the delitrious cognitive deficits that are commonly found in this disorder.
A large body of evidence demonstrates that incorporation of regular, physical exercise into treatment can improve brain structure and cognitive function in both healthy aging populations and those with mild cognitive impairment (Colcombe and Kramer, 2003; Smith et al., 2010). For example, among older adults, participation in a regular exercise program has been linked to significant cortical thickening and reduced atrophy in the frontotemporal region, both in healthy individuals and those diagnosed with mild cognitive impairment (Barha et al., 2020; Haeger et al., 2019; Köbe et al., 2016; Rogge et al., 2018; Tamura et al., 2015). Multiple correlational studies show that physical activity and cardiorespiratory fitness levels are positively correlated with cortical thickness and gray matter volume in regions strongly implicated in cognition, namely the PFC, the hippocampus, and the cingulate gyrus (Gujral et al., 2017; Weinstein et al., 2012).
Until the last decade, exercise was relatively under-recommended as an adjunctive therapeutic intervention to target cognition in SZ (Holley et al., 2011). However, a growing body of research now suggests that regular exercise programs can be an effective treatment for SZ patients, with promising effects on physical health, core symptoms, and cognition (Firth et al., 2017; Kimhy et al., 2015). Although to date there have been few neuroimaging studies of exercise and brain structure in SZ (and only two in first-episode psychosis (FEP)), they suggest physical activity levels correlate with cognitive deficits and volumetric differences in key cortical regions (Falkai et al., 2013; Lee et al., 2013; McEwen et al., 2015; Mittal et al., 2013; Scheewe et al., 2013). Exercise interventions may induce volumetric increases in the hippocampus and cortical thickening in the ACC and the PFC (van der Stouwe et al., 2018). Thus, physical exercise might both reduce cognitive deficits and combat progressive neural atrophy in SZ. However, there is significant variability in these study approaches and their reproducibility needs to be further established (Falkai et al., 2013). In summary, these studies provide initial support for the relationship between physical activity and prefrontal and limbic structural plasticity that subserves cognitive functioning and suggest that exercise might be an adjunctive intervention with beneficial neurobiological effects that help remediate cognitive deficits.
Cognitive training (CT) is another evidence-based therapeutic method of enhancing cognition and reducing cognitive deficits (Vita et al., 2021; Wykes et al., 2011). Unfortunately, the generalizability of CT to cognitive domains beyond those specifically targeted and its impact on real-world outcomes need to be strengthened (Owen et al., 2010). Evidence from preclinical studies suggests that a program that concurrently incorporates both cognitive training and exercise is likely to yield greater results than the sum of their parts. After finding that a combined aerobic exercise and cognitive enrichment living condition yielded 30 % greater neurogenesis in mice relative to either condition alone, Fabel et al. (2009) suggested that physical activity “primes” increased neurogenesis from cognitive enrichment.
Another proposed mechanism is that exercise promotes proliferation and division of neuronal precursors, while CT supports the survival of these cells, implying a synergistic effect between the two processes (Kempermann et al., 2006, 1997; Kronenberg et al., 2003). In other words, new neurons created during physical exercise quickly die off when adequate learning opportunities or novel experiences do not accompany the increased physical activity (Kempermann et al., 2010; Kleim et al., 2007; van Praag et al., 1999). For newly grown neurons to survive in the hippocampus, effortful learning must occur during CT (Curlik and Shors, 2011). Based on this preclinical research, we believe that a treatment program consisting of concurrent physical exercise and cognitive training will lead to greater benefits in neural structure and greater impact on everyday functioning compared to either treatment alone.
Promising intervention research on the combination of CT and exercise is emerging, but most studies have focused on healthy aging individuals who do not have the same degree of neurodegeneration and cognitive impairment as patients with SZ (Eggenberger et al., 2015; Lauenroth et al., 2016; McEwen et al., 2018; Gavelin et al., 2021). It is imperative to further study this hypertrophic effect in SZ, given the major changes observed across the course of illness in brain structures associated with cognition. In a prospective study of female FEP patients, a 12-week aerobic exercise intervention yielded positive volumetric and cortical thickness changes in the medial temporal regions and associations between regional brain increases and both reduced positive symptom severity and improved cognition compared to a Hatha yoga intervention and waitlist group (Woodward et al., 2020).
While the evidence for exercise as a route to cortical volume enhancement in SZ is growing, there is still little research on the impact of combining exercise and cognitive training, and even less so in FEP patients. Identifying treatment protocols that maximize cortical benefits and cognitive gains in FEP is crucial for both ameliorating the deficits that already exist at the time of the first psychotic episode and preventing the further deterioration observed in chronic SZ. Furthermore, early intervention may improve clinical outcome more broadly, given that most FEP patients are in late adolescence or early adulthood and are still developing. Our pilot work with a small sample suggested that FEP patients show cognitive and functional improvements, along with increased BDNF, when exercise is added to CT (Nuechterlein et al., 2016). We demonstrated in a randomized controlled trial (RCT) with a larger FEP sample that large cognitive gains and work/school functioning improvements occur when aerobic exercise is added to cognitive training (Nuechterlein et al., 2022). In the current analyses, we sought to examine the neuroanatomical impact of the combination of physical exercise and CT in FEP.
2. Methods
2.1. Participants
The study participants in this RCT were enrolled in the UCLA Aftercare Research Program from March 2013 through April 2016. This research was approved by the UCLA IRB and was consistent with international ethical standards. All participants provided written informed consent. Participants were recruited from a variety of Los Angeles psychiatric hospitals and clinics and referrals from clinicians in independent practices. Inclusion criteria for entry into the Aftercare Research Program were: (1) a recent onset of psychotic illness, with the beginning of the first psychotic episode within the last 24 months; (2) a DSM-IV diagnosis of SZ, schizoaffective disorder, depressed type, or schizophreniform disorder; (3) 18 to 45 years of age; (4) sufficient acculturation and fluency in the English language to avoid invalidating research measures; and (5) residence within commuting distance of the UCLA Aftercare Research Program. Study exclusion criteria were: (1) a known neurological disorder or significant head injury; (2) significant and habitual drug abuse or alcoholism in the 6 months prior to hospitalization, or psychosis that was accounted for by substance abuse; and (3) estimated premorbid IQ <70. The participants had an age, educational level, and sex distribution typical of individuals with a first episode of psychosis, and a racial and ethnic breakdown that was representative of the Greater Los Angeles area (Table 1).
Table 1.
Participant descriptive statistics.
| Cognitive training (n = 17) | Cognitive training & exercise (n = 20) | Combined (n = 37) | |
|---|---|---|---|
|
| |||
| Mean age at program entry, years (SD) | 23.5 (5.4) | 21.8 (3.8) | 22.7 (4.7) |
| Mean education, years (SD) | 12.4 (0.7) | 12.9 (1.2) | 12.6 (0.8) |
| Mean months from psychosis onset to program entry (SD) | 9.3 (7.2) | 7.7 (4.9) | 8.4 (6.0) |
| Mean months from psychosis onset to baseline test (SD) | 13.2 (7.6) | 11.9 (5.5) | 12.5 (6.4) |
| Mean total 24-item BPRS | 44.4 (11.1) | 37.0 (8.9) | 41.2 (10.6) |
| Sex (Male) | 89 % | 57 % | 73 % |
| Race | |||
| Caucasian | 33 % | 29 % | 31 % |
| Asian | 11 % | 14 % | 13 % |
| Pacific Islander | 0 % | 0 % | 0 % |
| Native American | 11 % | 0 % | 6 % |
| African American | 33 % | 14 % | 25 % |
| Mixed | 11 % | 43 % | 25 % |
| Ethnicity (Hispanic) | 67 % | 57 % | 63 % |
| Diagnosis | |||
| Schizophrenia | 56 % | 57 % | 56 % |
| Schizophreniform | 22 % | 43 % | 31 % |
| Schizoaffective, depressed type | 22 % | 0 % | 13 % |
| Handedness (right) | 89 % | 86 % | 88 % |
| Cognitive training: number of sessions attended, mean (SD) | 64.7 (24.9) | 67.5 (21.9) | 66.2 (23.1) |
| Bridging group: number of sessions attended, mean (SD) | 18.5 (7.5) | 20.4 (7.3) | 19.5 (7.4) |
| Exercise in-clinic: number of sessions attended, mean (SD) | 37.1 (14.3) | ||
| Exercise at home: number of sessions attended, mean (SD) | 27.4 (12.2) | ||
| Exercise total: number of sessions attended, mean (SD) | 64.4 (13.3) | ||
After entry into the UCLA Aftercare Research Program, FEP patients in the study were stabilized on antipsychotic medication and randomized to Cognitive Training and Exercise (CT&E) or Cognitive Training (CT). This process required about 3 months, followed by baseline assessments, including a baseline MRI. Immediately after the assessment battery was completed, 1:1 randomization to CT&E vs. CT occurred. Both treatments lasted 6 months. A second MRI scan took place at the end of the 6-month randomized treatment. Medication treatment response did not exclude patients from the trial, but rather was used as a regression covariate.
2.2. Interventions
In addition to the interventions described below, all patients received treatment with a second-generation antipsychotic medication, and had regular visits with the treating psychiatrist and individual case manager. We compared outcomes for a patient group that received CT with a patient group that received CT&E. Participants were selected from the UCLA Aftercare Research Program so that the groups’ demographic and clinical characteristics would be comparable.
2.2.1. Cognitive training
CT and CT&E groups participated in the same systematic Internet-based CT program. Training was administered at the Aftercare Research Program, with the first 12 weeks focused on neurocognitive training and the second 12 weeks focused on social cognitive training. Patients attended the clinic 2 days/week to complete 4 h/week of computerized CT. Thus, each patient was assigned 48 h of neurocognitive training and 48 h of social cognitive training, thereby equaling the CT amount found by Fisher and colleagues to lead to gains across multiple cognitive domains (Fisher et al., 2010). For the neurocognitive training, we used Posit Science’s BrainHQ program components aimed at improving auditory discrimination, processing speed, working memory, verbal memory, and verbal reasoning. For social cognitive training we used Posit Science’s SocialVille, which focuses on facial recognition, social perception, processing of emotions, and interpretation of social information. See Nuechterlein et al. (2022) for more details.
2.2.2. Exercise program
In addition to the CT sessions, participants randomized to the CT&E group participated in a 24-week progressive aerobic and strength conditioning exercise program designed by MRI study PI and NSCA-certified personal trainer (S.C.M.). The CT&E exercise program, in use at the clinic since 2013, was designed to meet ACSM (American College of Sports Medicine) and AHA (American Heart Association) recommendations for adults of at least 150 min of moderate aerobic exercise and at least 2 days of muscle conditioning exercises per week. We used an exercise dosage designed to be both feasible and effective in improving cognition in FEP (Firth et al., 2018). Two 45-min in-clinic exercise sessions/week and two 30-min sessions/week of exercise homework were assigned. A certified exercise instructor led the in-clinic exercise group. The exercise sessions included a dynamic warm-up (7.5 min) and cool-down after exercise (7.5 min). The exercise sessions included 30 min of combined moderate-intensity aerobic conditioning (1-min intervals) and moderate-to-high-intensity strength and calisthenic conditioning (1-min intervals). Participants were instructed on proper form and technique for five different exercises at the start of each session and completed three rounds of the five sets of aerobic and strength training intervals. Aerobic exercise intensity was monitored via a wireless activity and heart rate monitor (Fitbit Charge HR) to ensure patients were exercising in their individualized 60–80% target heart rate zone and to remotely track homework. The exercise program was individually tailored based on the patient’s current physical abilities, with intensity incrementally increased. A comprehensive approach was used to address FEP patients’ exercise barriers (specifically amotivation), including individual text message reminders, enthusiastic and experienced exercise trainers, monetary and social incentives, meetings with family to provide support at home to increase homework compliance, individualized homework plans, and goal setting during a weekly clinic group.
2.2.3. Bridging group
The Bridging Group occurred 1 h/wk., separately for the CT and CT&E groups, and provided an opportunity for patients to learn strategies for directly applying the CT to their daily lives. Discussions emphasized how the computerized training exercises could help patients achieve work and school goals, as well as improve social interactions. For example, computerized training to improve one’s ability to recall a sequence of instructions through chunking information can help one to better remember an employer’s directions and instructions. In the CT&E group, there were also discussions about the benefits of aerobic exercise for brain functioning and cognition.
2.3. MRI acquisition
Structural imaging data were collected on a Siemens 3 T Trio Scanner (Siemens Medical Solutions USA, Inc., Malvern, PA) with a 12-channel head coil at the UCLA Staglin Center for Cognitive Neuroscience (CCN). Subject head movement was minimized with foam padding. High-resolution three-dimensional structural images were obtained with a sagittal T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence (time repetition [TR] = 2300 ms, time echo [TE] = 2.91 ms, inversion time [TI] = 900 ms, 160 slices, field of view [FOV] = 256 mm, voxel size = 1.0 mm × 1.0 mm × 1.2 mm, flip angle [FA] = 9°) and co-localized T2-weighted turbo spin-echo (TSE) sequence (TR/TE = 6310/67 ms, FOV = 220 mm, matrix = 256 mm × 256 mm, flip angle = 149°, 30 transversal slices, slice thickness = 4.0 mm). A neuroradiologist confirmed that all MRI scans were free of gross structural abnormalities.
2.4. MRI analysis
High resolution T1- and T2-weighted images were screened for image quality to ensure absence of artifacts using MRIQC toolbox software (version 0.16.1; https://mriqc.readthedocs.io) (Esteban et al., 2017). Image processing was subsequently performed with the FreeSurfer software suite (version 6.0; http://surfer.nmr.mgh.harvard.edu) to derive measures of cortical thickness (Fischl and Dale, 2000). Cortical surface area and thickness maps were constructed according to the Destrieux atlas (Destrieux et al., 2010) in an automated manner using default parameters within the recon-all pipeline in FreeSurfer (Fischl et al., 2004). The details of these processing pipelines have been previously described in detail (Dale et al., 1999; Fischl et al., 1999). To measure changes in cortical thickness in patients between baseline and follow-up scans and between groups, and to account for within-subject correlations and avoid asymmetry-induced bias in longitudinal images, images were subjected to further processing with the FreeSurfer longitudinal stream (Reuter et al., 2012; Reuter and Fischl, 2011). The quality of the cortical reconstruction and segmentation was assured by visual inspection by a trained examiner (B.J.) and manually corrected as necessary with the support of quality assurance tools in FreeSurfer (https://surfer.nmr.mgh.harvard.edu/fswiki/QATools).
Between-group cortical thickness comparisons were performed in standard space. For the cortical thickness analysis we analyzed intervention effects for a priori regions of interest in the left PFC and left cingulate structures, which are key executive functioning regions (Takahashi et al., 2020). These regions also appear sensitive to change with exercise interventions in SZ patients (Scheewe et al., 2013; Takahashi et al., 2020), and excessive cortical thinning in these regions is specifically targeted in FEP patients (Schultz et al., 2010). For this study, data were analyzed from two cortical ROIs (Fig. 1). The first, was the left rostral middle frontal gyrus, which includes Brodmann area 46 (the core component of the DLPFC; Rajkowska and Goldman-Rakic, 1995), which was chosen as the ROI representative of the DLPFC. The second, was the left rostral middle-anterior part of the cingulate gyrus and sulcus (as defined in the Destrieux atlas), which was used as the ROI representative of the ACC with no modifications. Mean cortical thickness values (calculated as the mean distance between the pial and gray/white matter surfaces across the specified ROI) were extracted from these two ROIs for each hemisphere. The MRI data analyses were blinded to the treatment group. All ANOVA and correlational statistical analyses were performed using SPSS v28.0.
Fig. 1.
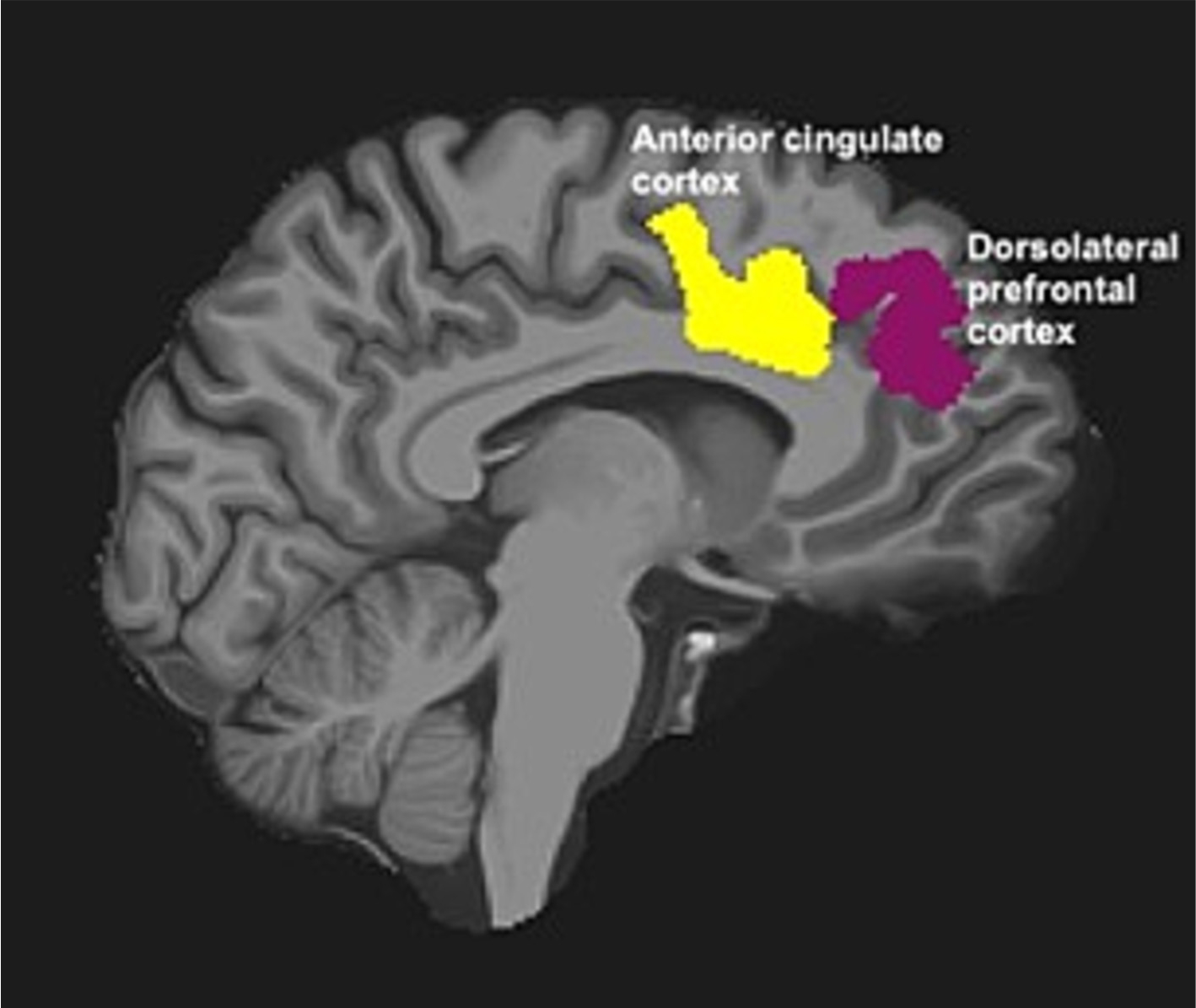
Regions of interest (ROIs) examined in this study. ROIs include the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC) from the Destrieux Atlas in the left hemisphere, overlaid on a sagittal slice from a single participant.
3. Results
The CONSORT diagram for the study is shown in Fig. 2. After screening 133 subjects, 52 were randomized into the study and completed baseline scanning. A total of 15 were not included in the MRI data analysis because they either dropped out during the protocol (N=6 from CT&E and N=5 from CT) or completed the protocol but were unable to have a follow-up scan (N=1 from CT&E and N=3 from CT). Drop-out rate did not differ by treatment arm. Only patients with a baseline and 6-month follow-up scan were included in the longitudinal MRI analysis. No patients were lost to the final analysis due to poor data quality, including no excessive motion (>3 mm) or artifacts. Therefore, there were 37 subjects included in the MRI final analysis (N = 20 CT&E, N = 17 CT).
Fig. 2.
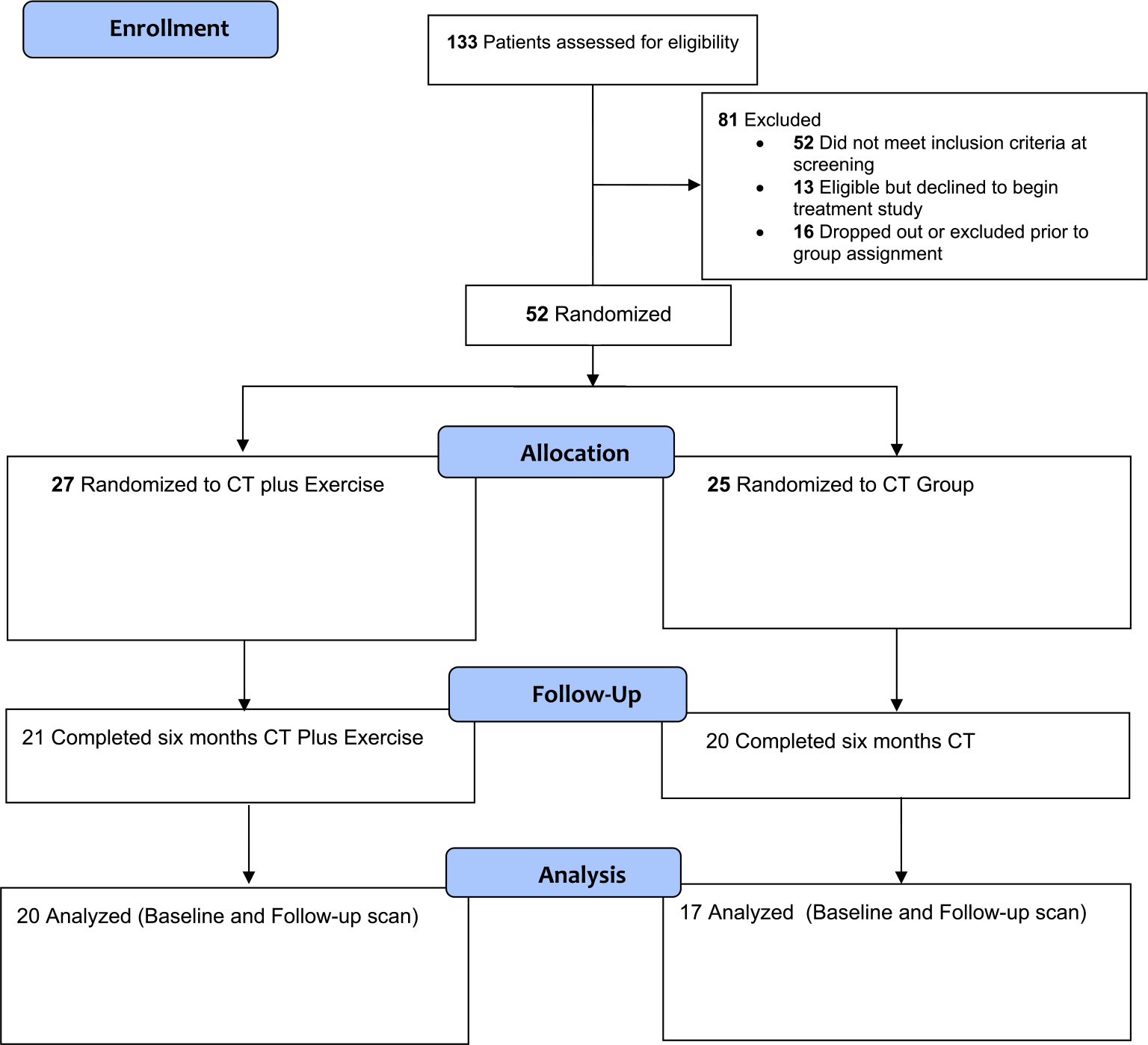
The CONSORT diagram for the participants in the study.
No significant differences were found between CT&E and CT groups for age, gender, ethnicity, handedness, personal years of education, symptom severity, or duration of illness (Table 1). The two treatment groups did not differ over the 6-month period in their attendance of the cognitive training sessions (CT&E mean = 67.5 (SD = 21.9); CT mean = 64.7 (SD = 24.9), t36 = 0.14, P = .71). Similarly, they did not differ in their attendance at the weekly bridging group (CT&E mean = 20.4 (SD = 7.3); CT mean = 18.5 (SD = 7.5), t36 = 0.59, P = .45). The CT&E group completed a mean of 37.1 (SD = 14.3) exercise sessions in the clinic and 27.4 (SD = 12.2) home exercise sessions, with a mean of 64.4 (SD = 13.3) total exercise sessions for the duration of the intervention.
3.1. Cortical thickness
There was a significant between group x time interaction in the left ACC (F(1, 35) = 4.666, P < .04) and left prefrontal (F(1,35) = 4.132, P < .05) regions. Follow-up within-group analyses for the left ACC showed that the CT&E group increased significantly in cortical thickness (mean change = 0.56 mm, t19 = 2.81, P = .01), while the CT group did not change significantly (mean change = −0.02 mm, t16 = 0.71, P = .49). As shown in Fig. 3, in the CT&E group the ACC mean baseline = 2.76 mm (SD = 0.18) and mean follow-up = 2.82 mm (SD = 0.19). For the CT group, the ACC mean baseline = 2.82 mm (SD = 0.17) and mean follow-up = 2.79 mm (SD = 0.16).
Fig. 3.
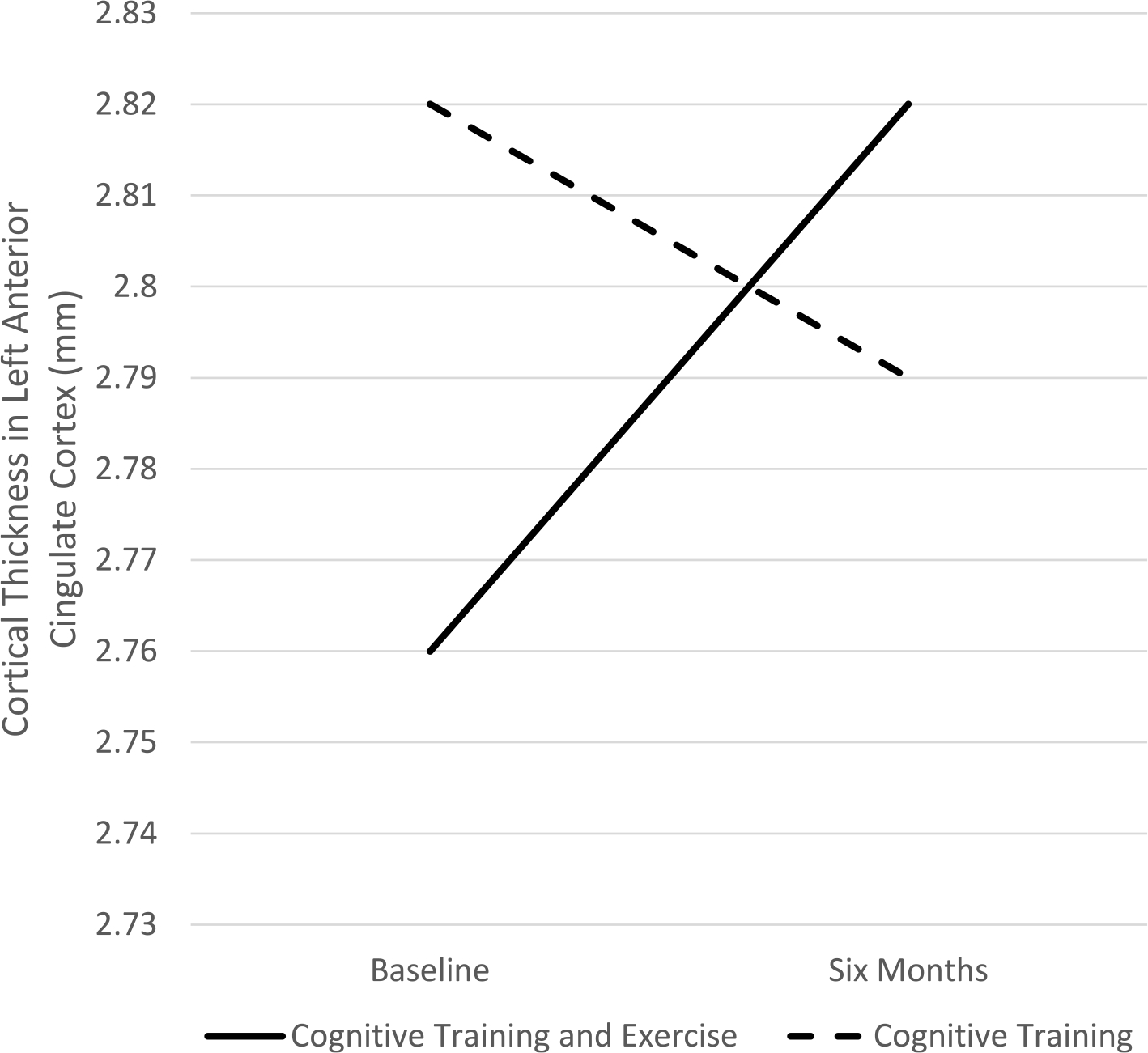
Differential effects of Cognitive Training Plus Exercise versus Cognitive Training alone on cortical thickness in the left anterior cingulate cortex.
In the left prefrontal ROI, follow-up within-group analyses revealed that the two treatment groups moved in opposite directions to create the significant between group x time interaction, but neither group changed significantly by itself. The CT&E group showed a non-significant tendency to increase in cortical thickness (mean change = 0.05 mm, t19 = 1.76, P < .095), while the CT group showed a slight and non-significant decrease (mean change = −0.04 mm, t16 = 1.19, P = .25). As shown in Fig. 4, the CT&E mean baseline = 2.28 mm (SD = 0.18) and mean follow-up = 2.32 mm (SD = 0.18). For the CT group, the mean baseline = 2.21 mm (SD = 0.19) and mean follow-up = 2.17 mm (SD = 0.22).
Fig. 4.
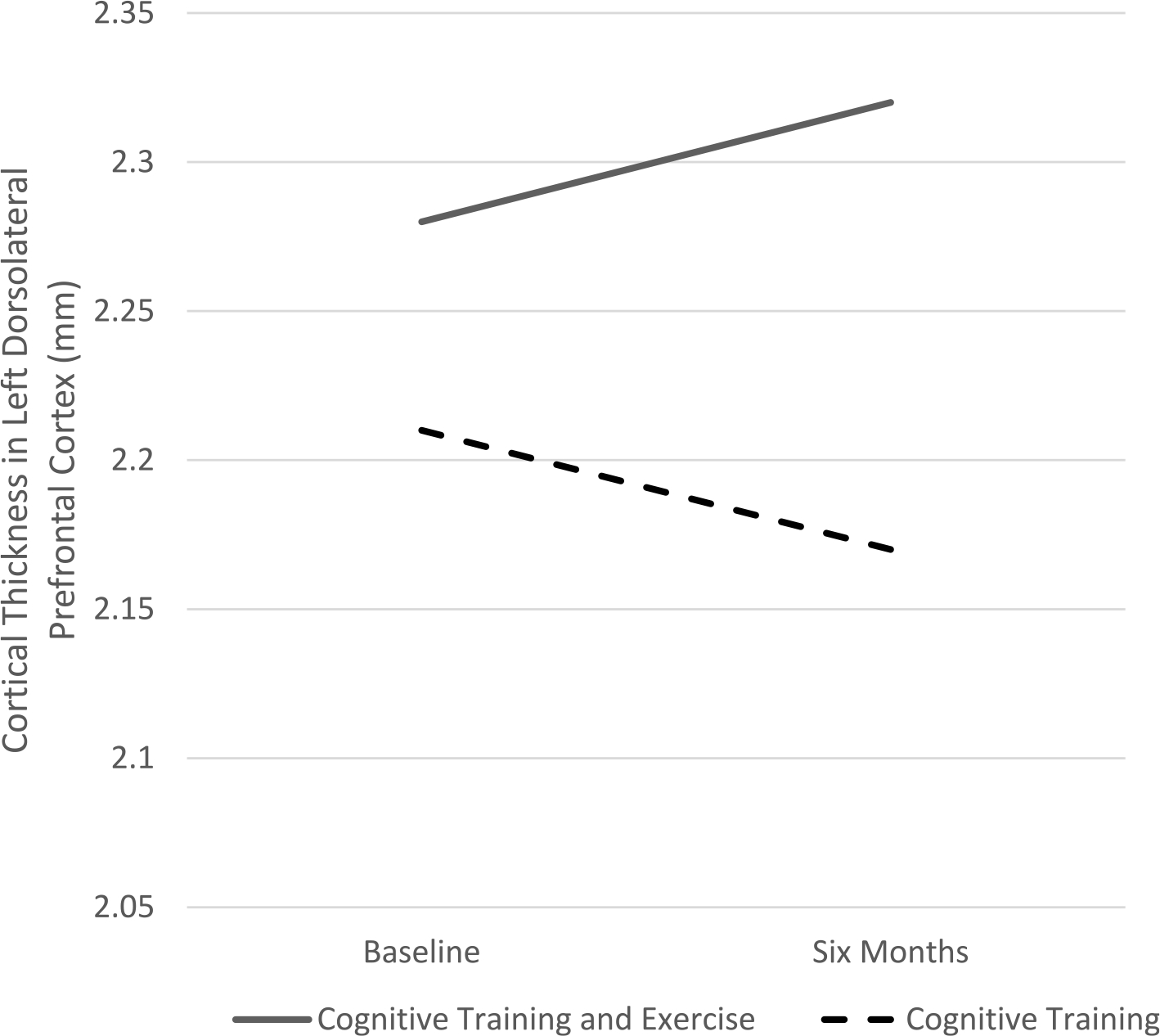
Differential effects of Cognitive Training Plus Exercise versus Cognitive Training alone on cortical thickness in the left dorsolateral prefrontal cortex.
3.2. Correlates of cortical thickness changes
Exploratory correlational analyses were completed to examine whether the cortical thickness changes in six months in the two ROIs were associated with changes in cardiorespiratory endurance, overall cognitive performance, and everyday functioning. Within the CT&E group, in which cardiorespiratory changes would be expected to be impacted by the exercise sessions, increases in cardiorespiratory fitness (lower recovery heart rate in the YMCA 3-min step test) correlated r = 0.52 with increased cortical thickness in the left ACC, but this was only a non-significant trend (P = .07) due to the small sample size with complete data (N = 13). Similarly in the CT&E group, increased cortical thickness in left prefrontal ROI was correlated r = 0.34 with gains in the overall cognitive performance (MATRICS Consensus Cognitive Battery Overall Composite T score), but this was not significant. One significant relationship of increased cortical thickness in the left ACC was improved work/school functioning when evaluated in the full sample to maximize sample size, r = 0.43, P < .02, N = 29.
4. Discussion
In the present study, we investigated structural changes in the cortex associated with a combined cognitive training and physical exercise intervention in patients with a recent first episode of psychosis. We discovered that the combined CT&E group experienced a significant increase in cortical thickness in the left ACC and a tendency toward left DLPFC increase over the 6-month intervention period. Conversely, the CT only group, which did not receive the structured exercise intervention, experienced a non-significant tendency toward cortical thinning in the ACC and DLPFC over the 6-month interventional period. Thinning of prefrontal cortical regions is regularly observed in the early course of SZ (Cannon et al., 2015). These initial findings suggest that exercise, in the context of cognitive training, may be a plasticity-enhancing adjunctive protocol that can address the cortical neurodegeneration observed in the early course of SZ. This expands our previous work in which we reported evidence for a correlation between physical activity levels and brain structure using a subset of the baseline FEP structural MRI scans (McEwen et al., 2015). In that study we found focal cortical thickness reduction in DLPFC, orbitofrontal cortex and hippocampal volume in the low physical activity FEP group compared to the high physical activity FEP group before assignment to a cognitive-enhancement-focused intervention.
In our correlational analyses we explored the relationship between these structural changes in the cortex and relevant cognitive and functional changes over a 6-month period in this population of patients known to have severe deficits in a wide range of cognitive functioning. In the CT&E group we found a non-significant tendency for increased left prefrontal cortical thickness to be associated with overall cognitive functioning gains. If demonstrated to be significant in a larger sample, this finding could be indicative of a putative biomarker, specifically DLPFC cortical thickness, mediating improvements in cognitive functioning from the combined intervention. Based on the known structural progressive brain changes in SZ, which are most prominent in the DLPFC in the early course of the illness (Zipursky et al., 2013), increasing structural integrity in this region central to subserving a wide range of cognitive functioning is paramount. This relationship will clearly need to be demonstrated in a larger sample size with appropriate statistical power to detect a significant mediation effect. Across both groups, an increase in cortical thickness in the left ACC was significantly associated with improved work/school performance. This highlights the importance of strengthening the ACC, which is known to support emotion regulation, conflict monitoring and higher order cognitive function, to enable better functional outcomes in patients.
Existing research on the efficacy of cognitive training suggests that by itself, cognitive training does indeed confer therapeutic cognitive benefits in SZ and is associated with improvements in learning-induced cortical plasticity (Genevsky et al., 2010). However, the effect of cognitive training alone does not entirely offset the large deficits induced by SZ. Furthermore, imaging studies examining structural correlates of cognitive training alone have shown only a limited effect in addressing associated neurodegeneration (Eack et al., 2010; Morimoto et al., 2018). The effects, which have been minimally replicated, are largely limited to the hippocampus. In the current study, the observed positive plasticity changes in the CT&E group suggest that exercise can enhance the effects of cognitive training, expanding there neuroplastic effects beyond the hippocampus, and remediate structural degeneration in the other cortical regions intimately associated with the cognitive deficits in SZ.
One potential neurobiological mechanism underlying the observed cortical thickening is elevation in the release of brain-derived neurotrophic factor (BDNF). Increasing cardiovascular activity, as we did in this intervention, has been shown to correlate with increases in peripheral serum BDNF (Håkansson et al., 2017; Kim et al., 2014; Walsh et al., 2018), perhaps by means of sympathetic increase of cerebral blood flow (Alomari et al., 2015; Seifert and Secher, 2011). Thus, one potential mechanism by which exercise complements cognitive training and broadens the neurostructural benefits is by elevating cortical BDNF expression, which has been shown to promote neurogenesis and neuroplasticity (Bathina and Das, 2015; Lu et al., 2014). A meta-analysis, which included both cognitive training and exercise intervention in SZ found BDNF levels were increased in SZ patients undergoing these non-pharmacological interventions (Sanada et al., 2016). Our report of cognitive, functional outcome, and BDNF outcomes in the larger RCT from which the current sample was drawn, supports the BDNF gains in the CT&E group and the tendency for BDNF gain to predict cognitive gain, but again a larger sample will be necessary to demonstrate that these tendencies are statistically reliable (Nuechterlein et al., 2022).
The prefrontal and cingulate cortical regions were selected a priori due to consistent association of the degeneration in these regions with deficits in cognition in SZ. The conflict monitoring and cognitive control model developed by Botvinick et al. (2001) has focused on the roles of the DLPFC and ACC and their key contributions to conflict-monitoring and behavioral adaptation, respectively. When such prefrontal and cingulate brain regions are impaired, affected individuals show predictable deficits in context maintenance, response inhibition and conflict monitoring (MacDonald et al., 2000), which lead to profound impairments in the ability to carry out goal-directed behaviors.
Beyond this, the observed positive changes in the CT&E group suggest that exercise, in the context of cognitive training, may be a valuable adjunctive treatment to standard psychiatric care to address the cognitive deficits of SZ (Nuechterlein et al., 2016; Nuechterlein et al., 2022). While there is an emerging body of research attesting to the efficacy of combining physical exercise with cognitive training in the aging population, this study is among the first to examine the effects of exercise-supplemented cognitive training in early psychosis. Our findings support the view that concurrently supplementing cognitive training with exercise allows the mechanisms underlying these two treatments to act synergistically and produce an effect that may address the cortical degeneration in SZ.
This study has several strengths, including the use of randomization to assign participants to groups that are sufficiently representative and matched for a strong initial RCT, as well as the focus on FEP patients who may have greater potential for cortical structural benefitis. The results have important implications for the treatment of the early course of psychosis. With respect to physical health, exercise may be very valuable to individuals with psychosis, who experience abnormally high rates of concurrent physical ailments such as cardiovascular disease, aberrant metabolic activity, and associated increased early mortality (Kritharides et al., 2017; Penninx and Lange, 2018). This study provides evidence to support the cortical structural benefits of this adjunctive therapy for FEP patients.
The present study has some limitations. Since all patients received cognitive training, the longitudinal effects of exercise alone on brain structure (separate from cognitive training) could not be explicitly studied. Further, although the results of the CT vs. CT&E longitudinal analyses support the conclusion of prevention of cortical thinning due to the synergistic combination of cognitive training and exercise in FEP, an examination of the impact of this treatment combination in age-matched healthy controls or another major psychopathology group would be very useful. Additionally, a priori regions of interest were used in this initial exploratory investigation to maintain sufficient power to detect significant effects, and the analysis was limited to the selected regions, which does not preclude changes in other brain regions that were not explored. Future research should be sufficiently powered to allow the exploration of the whole brain. Another caveat is that the use of automated cortical region constructing used the Destrieux atlas, which may not necessarily match DLPFC and ACC ROIs from other studies.
5. Conclusion
The addition of a regular exercise program to cognitive training has beneficial effects on cortical structure in the ACC and DLPFC. Starting this intervention early in the course of SZ, as close to the first psychotic episode as possible, might maximize the benefits of this type of intervention. Research suggests that adolescence and early adulthood are critical periods for the development of SZ, during which multiple neurobiological processes essential to development are profoundly altered (Hadar et al., 2018; Uhlhaas, 2011). Along with the greater plasticity and general potential for antipsychotic medication response observed in younger individuals, beginning this intervention early enough might reduce disruption of these neurobiological processes and improve functional outcome.
Our intriguing initial findings show the promise of inducing experience-dependent plasticity, as evidenced by structural changes in cortical tissue thickness, along with associated improvements in cognition and functioning through the synergistic addition of regular physical exercise to a structured cognitive training program in patients in the early course of SZ.
Acknowledgements
We thank the UCLA Aftercare Research Program patients for their willing participation and the staff for their consistent dedication to improving treatments for the initial phase of schizophrenia.
Funding
This research was supported by National Institute of Mental Health (NIMH) Center grant P50 MH066286 (K.H.N.) and research grants K01 MH099431 (S.C.M), R34 MH102529 (K.H.N.), and R01 MH110544 (K. H.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health.
Footnotes
Declaration of competing interest
K.H.N. has received research grants from Posit Science and Janssen Scientific Affairs that support other research, and has been a consultant to Astellas, Genentech, Janssen, Medincell, Otsuka, ReCognify, Takeda, and Teva. J.V. has been a paid consultant to Posit Science, Lumosity, and Boehringer Ingelheim and had a research grant from Posit Science. K.L. S. has been a consultant to Otsuka and received a speaker’s honorarium from Janssen. S.C.M. has been a paid employee to atai Life Sciences. B.J., J.N., and S.M.W. report no conflicts of interest. This RCT study is registered with NIH at clinicaltrials.gov (NCT02267070).
References
- Aleman A, Hijman R, De Haan EHF, Kahn RS, 1999. Memory impairment in schizophrenia: a meta-analysis. Am. J. Psychiatry 156, 1358–1366. 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Alomari MA, Khabour OF, Maikano A, Alawneh K, 2015. In: Vascular Function and Brain-derived Neurotrophic Factor: The Functional Capacity Factor, 20, pp. 518–526. 10.1177/1358863X15598390. [DOI] [PubMed] [Google Scholar]
- Barha CK, Best JR, Rosano C, Yaffe K, Catov JM, Liu-Ambrose T, 2020. Sex-specific relationship between long-term maintenance of physical activity and cognition in the health ABC study: potential role of hippocampal and dorsolateral prefrontal cortex volume. <sb:contribution> <sb:title>J. Gerontol. - Ser.</sb: title></sb:contribution><sb:host><sb:issue><sb:series><sb:title>A Biol. Sci. Med. Sci.</sb:title></sb:series></sb:issue></sb:host> 75, 764–770. 10.1093/gerona/glz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathina S, Das UN, 2015. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 11, 1164. 10.5114/AOMS.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JMJ, Woerner MG, Geisler S, Kane JM, Lieberman JA, 2000. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am. J. Psychiatry 157, 549–559. 10.1176/APPI.AJP.157.4.549. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD, 2001. Conflict monitoring and cognitive control. Psychol. Rev. 108 (3), 624–652. 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD, 2006. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2 (4), 531–536. 10.2147/nedt.2006.2.4.531, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, Van Erp TGM, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R, 2015. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry 77, 147–157. 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF, 2003. Fitness effects on the cognitive function of older adults. Psychol. Sci. 14 (2), 125–130. 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Curlik II DM, Shors TJ, 2011. Learning increases the survival of newborn neurons provided that learning is difficult to achieve and successful. J. Cogn. Neurosci. 23, 2159. 10.1162/JOCN.2010.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis: I.Segmentation and surface reconstruction. Neuroimage 9, 179–194. 10.1006/NIMG.1998.0395. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E, 2010. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 53, 1. 10.1016/J.NEUROIMAGE.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KMR, Greenwald DP, Hogarty SS, Keshavan MS, 2010. Cognitive enhancement therapy protects against gray matter loss in early schizophrenia: results from a two-year randomized controlled trial. Arch. Gen. Psychiatry 67, 674. 10.1001/ARCHGENPSYCHIATRY.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenberger P, Schumacher V, Angst M, Theill N, de Bruin ED, 2015. Does multicomponent physical exercise with simultaneous cognitive training boost cognitive performance in older adults? A 6-month randomized controlled trial with a 1-year follow-up. Clin. Interv. Aging 10, 1335. 10.2147/CIA.S87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ, 2017. MRIQC: advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One 12. 10.1371/JOURNAL.PONE.0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G, 2009. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front. Neurosci. 10, 1–7. 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Malchow B, Wobrock T, Gruber O, Schmitt A, Honer WG, Pajonk FG, Sun F, Cannon TD, 2013. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: a randomized controlled MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 263, 469–473. 10.1007/s00406-012-0383-y. [DOI] [PubMed] [Google Scholar]
- Firth J, Stubbs B, Rosenbaum S, Vancampfort D, Malchow B, Schuch F, Elliott R, Nuechterlein KH, Yung AR, 2017. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 43 (3), 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J, Carney R, Elliott R, French P, Parker S, McIntyre R, McPhee JS, Yung AR, 2018. Exercise as an intervention for first-episode psychosis: a feasibility study. Early Interv. Psychiatry 12 (3), 307–315. 10.1111/eip.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM, 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 97, 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM, 1999. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. NeuroImage 9, 195–207. 10.1006/NIMG.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004. Automatically parcellating the human cerebral cortex. Cereb. Cortex 14, 11–22. 10.1093/CERCOR/BHG087. [DOI] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S, 2010. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr. Bull. 36, 869. 10.1093/SCHBUL/SBN170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavelin HM, Dong C, Minkov R, Bahar-Fuchs A, Ellis KA, Lautenschlager NT, Mellow ML, Wade AT, Smith AE, Finke C, Krohn S, Lampit A, 2021. Combined physical and cognitive training for older adults with and without cognitive impairment: a systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 66, 101232. 10.1016/j.arr.2020.101232. [DOI] [PubMed] [Google Scholar]
- Genevsky A, Garrett CT, Alexander PP, Vinogradov S, 2010. Cognitive training in schizophrenia: a neuroscience-based approach. Dialogues Clin. Neurosci. 12 (3), 416–421. 10.31887/DCNS.2010.12.3/agenevsky. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG, 2007. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch. Gen. Psychiatry 64, 1115–1122. 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK, 2004. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 72 (1), 41–51. 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Gujral S, Aizenstein H, Reynolds CF, Butters MA, Erickson KI, 2017. Exercise effects on depression: possible neural mechanisms. Gen. Hosp. Psychiatry. 10.1016/j.genhosppsych.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadar R, Bikovski L, Soto-Montenegro M, Schimke J, Maier P, Ewing S, Voget M, Wieske F, Götz T, Desco M, Hamani C, Pascau J, Weiner I, Winter C, 2018. Early neuromodulation prevents the development of brain and behavioral abnormalities in a rodent model of schizophrenia. Mol. Psychiatry 23, 943–951. 10.1038/MP.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeger A, Costa AS, Schulz JB, Reetz K, 2019. Cerebral changes improved by physical activity during cognitive decline: a systematic review on MRI studies. NeuroImage Clin. 10.1016/j.nicl.2019.101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson K, Ledreux A, Daffner K, Terjestam Y, Bergman P, Carlsson R, Kivipelto M, Winblad B, Granholm A-C, Mohammed AKH, 2017. BDNF responses in healthy older persons to 35 minutes of physical exercise, cognitive training, and mindfulness: associations with working memory function. J. Alzheimers Dis. 55, 645. 10.3233/JAD-160593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley J, Crone D, Tyson P, Lovell G, 2011. The effects of physical activity on psychological well-being for those with schizophrenia: a systematic review. Br. J. Clin. Psychol. 10.1348/014466510X496220. [DOI] [PubMed] [Google Scholar]
- Jordan G, Lutgens D, Joober R, Lepage M, Iyer SN, Malla A, 2014. The relative contribution of cognition and symptomatic remission to functional outcome following treatment of a first episode of psychosis. J. Clin. Psychiatry 75. 10.4088/JCP.13m08606. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH, 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Chesler EJ, Lu L, Williams RW, Gage FH, 2006. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 103, 780. 10.1073/PNAS.0510291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, Wolf SA, 2010. Why and how physical activity promotes experience-induced brain plasticity. Front. Neurosci. 4 10.3389/FNINS.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.jae, Song B.kil, So B, Lee O, Song W, Kim Y, 2014. Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: a pilot study. Psychiatry Res. 220, 792–796. 10.1016/J.PSYCHRES.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Bartels MN, Armstrong HF, Ballon JS, Khan S, Chang RW, Hansen MC, Ayanruoh L, Lister A, Castren E, Smith EE, Sloan RP, 2015. The impact of aerobic exercise on brain-derived neurotrophic factor and neurocognition in individuals with schizophrenia: a single-blind, randomized clinical trial. Schizophr. Bull. 41 (4), 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Markham JA, Vij K, Freese JL, Ballard DH, Greenough WT, 2007. Motor learning induces astrocytic hypertrophy in the cerebellar cortex. Behav. Brain Res. 178, 244. 10.1016/J.BBR.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köbe T, Witte AV, Schnelle A, Lesemann A, Fabian S, Tesky VA, Pantel J, Flöel A, 2016. Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. NeuroImage 131, 226–238. 10.1016/j.neuroimage.2015.09.050. [DOI] [PubMed] [Google Scholar]
- Kritharides L, Chow V, Lambert TJ, 2017. Cardiovascular disease in patients with schizophrenia. Med. J. Aust. 206, 91–95. 10.5694/MJA16.00650. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G, 2003. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 467, 455–463. 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Lauenroth A, Ioannidis AE, Teichmann B, 2016. Influence of combined physical and cognitive training on cognition: a systematic review. BMC Geriatr. 16 10.1186/S12877-016-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EHM, Hui CLM, Chang WC, Chan SKW, Li YK, Lee JTM, Lin JJX, Chen EYH, 2013. Impact of physical activity on functioning of patients with first-episode psychosis - a 6months prospective longitudinal study. Schizophr. Res. 150, 538–541. 10.1016/j.schres.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Lindgren M, Holm M, Kieseppä T, Suvisaari J, 2020. Neurocognition and social cognition predicting 1-year outcomes in first-episode psychosis. Front. Psychiatry 4 (11), 603933. 10.3389/fpsyt.2020.603933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Lu Y, 2014. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 220, 223–250. 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- MacDonald III AW, Cohen JD, Stenger VA, Carter CS, 2000. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288, 1835–1838. 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McEwen SC, Hardy A, Ellingson BM, Jarrahi B, Sandu N, Subotnik KL, Ventura J, Nuechterlein KH, 2015. Prefrontal and hippocampal brain volume deficits: the role of low physical activity on brain plasticity in first-episode schizophrenia patients. J. Int. Neuropsychol. Soc. 21, 868. 10.1017/S1355617715000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen SC, Siddarth P, Rahi B, Kim Y, Mui W, Wu P, Emerson ND, Lee J, Greenberg S, Shelton T, Kaiser S, Small GW, Merrill DA, 2018. Simultaneous Aerobic Exercise and Memory Training Program in Older Adults with Subjective Memory Impairments. J Alzheimers Dis. 62 (2), 795–806. 10.3233/JAD-170846. Erratum in: J Alzheimers Dis. 2019;67(3):1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ, 2009. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 23, 315–336. 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Andreasen NC, 2005. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry 162, 495–506. 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Gupta T, Orr JM, Pelletier-Baldelli A, Dean DJ, Lunsford-Avery JR, Smith AK, Robustelli BL, Leopold DR, Millman ZB, 2013. Physical activity level and medial temporal health in youth at ultra high-risk for psychosis. J. Abnorm. Psychol. 122, 1101–1110. 10.1037/a0034085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T, Matsuda Y, Matsuoka K, Yasuno F, Ikebuchi E, Kameda H, Taoka T, Miyasaka T, Kichikawa K, Kishimoto T, 2018. Computer-assisted cognitive remediation therapy increases hippocampal volume in patients with schizophrenia: a randomized controlled trial. BMC Psychiatry 18, 1–8. 10.1186/S12888-018-1667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, Yee CM, Gretchen-Doorly D, Mintz J, 2011. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr. Bull. 37 (Suppl. 2), S33–S40. 10.1093/schbul/sbr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Ventura J, McEwen SC, Gretchen-Doorly D, Vinogradov S, Subotnik KL, 2016. Enhancing cognitive training through aerobic exercise after a first schizophrenia episode: theoretical conception and pilot study. Schizophr. Bull. 42 (Suppl. 1), S44–S52. 10.1093/schbul/sbw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, McEwen SC, Ventura J, Subotnik KL, Turner LR, Boucher M, Casaus LR, Distler MG, Hayata JN, 2022. Aerobic exercise enhances cognitive training effects in first-episode schizophrenia: randomized clinical trial demonstrates cognitive and functional gains. Psychol. Med. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, Howard RJ, Ballard CG, 2010. Putting brain training to the test. Nature 465, 775–778. 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BWJH, Lange SMM, 2018. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin. Neurosci. 20, 63. 10.31887/DCNS.2018.20.1/BPENNINX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS, 1995. Cytoarchitectonic definition of prefrontal areas in the normalhuman cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cereb. Cortex 5, 323–337. 10.1093/CERCOR/5.4.323. [DOI] [PubMed] [Google Scholar]
- Reuter M, Fischl B, 2011. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage 57, 19. 10.1016/J.NEUROIMAGE.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B, 2012. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 61, 1402. 10.1016/J.NEUROIMAGE.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge AK, Röder B, Zech A, Hötting K, 2018. Exercise-induced neuroplasticity: balance training increases cortical thickness in visual and vestibular cortical regions. NeuroImage 179, 471–479. 10.1016/j.neuroimage.2018.06.065. [DOI] [PubMed] [Google Scholar]
- Sanada K, Zorrilla I, Iwata Y, Bermúdez-Ampudia C, Graff-Guerrero A, Martínez-Cengotitabengoa M, et al. , 2016. The efficacy of non-pharmacological interventions on brain-derived neurotrophic factor in schizophrenia: a systematic review and meta-analysis. Int. J. Mol. Sci. 17, E1766. 10.3390/ijms17101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheewe TW, van Haren NEM, Sarkisyan G, Schnack HG, Brouwer RM, de Glint M, Hulshoff Pol HE, Backx FJG, Kahn RS, Cahn W, 2013. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur. Neuropsychopharmacol. 23, 675–685. 10.1016/j.euroneuro.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, Nenadic I, Reichenbach JR, Sauer H, Schlösser RGM, 2010. Reduced cortical thickness in first episode schizophrenia. Schizophr. Res. 116, 204–209. 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Seifert T, Secher NH, 2011. Sympathetic influence on cerebral blood flow and metabolism during exercise in humans. Prog. Neurobiol. 95, 406–426. 10.1016/J.PNEUROBIO.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, et al. , 2010. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom. Med. 72 (3), 239–252. 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, Jung RE, Seidman LJ, Mesholam-Gately RI, Manoach DS, O’Leary DS, Ho BC, Andreasen NC, Lauriello J, Schulz SC, 2010. Cognitive deficits in recent-onset and chronic schizophrenia. J. Psychiatr. Res. 44, 421. 10.1016/J.JPSYCHIRES.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Keeser D, Rauchmann BS, Schneider-Axmann T, Keller-Varady K, Maurus I, Dechent P, Wobrock T, Hasan A, Schmitt A, Ertl-Wagner B, Malchow B, Falkai P, 2020. Effect of aerobic exercise combined with cognitive remediation on cortical thickness and prediction of social adaptation in patients with schizophrenia. Schizophr. Res. 216, 397–407. 10.1016/J.SCHRES.2019.11.004. [DOI] [PubMed] [Google Scholar]
- Tamura M, Nemoto K, Kawaguchi A, Kato M, Arai T, Kakuma T, Mizukami K, Matsuda H, Soya H, Asada T, 2015. Long-term mild-intensity exercise regimen preserves prefrontal cortical volume against aging. Int. J. Geriatr. Psychiatry 30, 686–694. 10.1002/gps.4205. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, 2011. The adolescent brain: implications for the understanding, pathophysiology, and treatment of schizophrenia. Schizophr. Bull. 37, 480. 10.1093/SCHBUL/SBR025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli I, Tognin S, Fusar-Poli P, Mechelli A, 2012. Episodic memory dysfunction in individuals at high-risk of psychosis: a systematic review of neuropsychological and neurofunctional studies. Curr. Pharm. Des. 18, 443–458. 10.2174/138161212799316271. [DOI] [PubMed] [Google Scholar]
- van der Stouwe E, van Busschbach JT, de Vries B, Cahn W, Aleman A, Pijnenborg G, 2018. Neural correlates of exercise training in individuals with schizophrenia and in healthy individuals: a systematic review. NeuroImage Clin. 19, 287–301. 10.1016/j.nicl.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH, 1999. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 96, 13427. 10.1073/PNAS.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A, Barlati S, Ceraso A, Nibbio G, Ariu C, Deste G, Wykes T, 2021. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 78 (8), 848–858. 10.1001/jamapsychiatry.2021.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, D’Angiulli A, Cameron JD, Sigal RJ, Kenny GP, Holcik M, Doucette S, Alberga AS, Prud’homme D, Hadjiyannakis S, Gunnell K, Goldfield GS, 2018. Changes in the brain-derived neurotrophic factor are associated with improvements in diabetes risk factors after exercise training in adolescents with obesity: the HEARTY randomized controlled trial. Neural Plast. 2018 10.1155/2018/7169583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein AM, Voss MW, Prakash RS, Chaddock L, Szabo A, White SM, Wojcicki TR, Mailey E, McAuley E, Kramer AF, Erickson KI, 2012. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav. Immun. 26, 811–819. 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ML, Lin J, Gicas KM, Su W, Hui CLM, Honer WG, Chen EYH, Lang DJ, 2020. Medial temporal lobe cortical changes in response to exercise interventions in people with early psychosis: a randomized controlled trial. Schizophr. Res. 223, 87–95. 10.1016/J.SCHRES.2020.05.043. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P, 2011. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am. J. Psychiatry 168 (5), 472–485. 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Reilly TJ, Murray RM, 2013. The myth of schizophrenia as a progressive brain disease. Schizophr. Bull. 39 (6), 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]


