SUMMARY
The integration of genomic testing into clinical care enables the use of individualized approaches to the management of rare diseases. We describe the use of belzutifan, a potent and selective small molecule inhibitor of the protein hypoxia-inducible factor 2α (HIF2α), in a patient with polycythemia and multiple paragangliomas (the Pacak–Zhuang syndrome). The syndrome was caused in this patient by somatic mosaicism for an activating mutation in EPAS1. Treatment with belzutifan led to a rapid and sustained tumor response along with resolution of hypertension, headaches, and long-standing polycythemia. This case shows the application of a targeted therapy for the treatment of a patient with a rare tumor-predisposition syndrome. (Funded by the Morin Family Fund for Pediatric Cancer and Alex’s Lemonade Stand Foundation.)
INTRODUCTION
The Pacak-Zhuang syndrome is a rare tumor predisposition syndrome caused by gain-of-function mutations in the gene encoding HIF2α (EPAS1, also known as HIF2A)1–3. Persons with this syndrome have polycythemia at an early age, with the subsequent development of multiple, recurrent, and occasionally metastatic paragangliomas that produce predominantly norepinephrine2,4. Other reported manifestations include somatostatinomas, vascular malformations, and ocular manifestations1,2,5–7. Fewer than 25 cases of Pacak-Zhuang syndrome (defined to include the co-occurrence of polycythemia and paraganglioma or somatostatinoma) have been reported, although many more cases of isolated polycythemia or paragangliomas have been reported in the context of EPAS1 mutations. Most often, this syndrome is caused by somatic mosaicism due to a post-zygotic event8,9 and is rarely reported in the context of germline inheritance10. Activating mutations in EPAS1 lead to decreased degradation of HIF2α and consequent transcriptional upregulation of hypoxia-related genes, including the gene encoding erythropoietin (EPO)1,11. Beginning in childhood and adolescence, affected persons often receive complex treatment, including serial phlebotomy for polycythemia, antihypertensive medications, and repeated surgical resection of functional paragangliomas. These interventions address the consequences of persistently increased HIF2α activity, but therapeutic options to target the underlying genetic cause of the syndrome have been limited. We report here the use of a potent and selective HIF2α inhibitor, belzutifan (MK-6482, previously known as PT2977; Merck) in an adolescent who had the Pacak-Zhuang syndrome with polycythemia and paragangliomas.
METHODS
Genetic Testing
Somatic panel testing12 and whole exome sequencing were performed under a research protocol approved by the Dana–Farber Cancer Institute (DFCI) institutional review board. The patient’s parents gave written informed consent for her participation. After she turned 18 years of age, the patient provided written informed consent to additional genetic analyses under a research protocol approved by the Boston Children’s Hospital institutional review board.
Single-patient protocol
A single-patient protocol for the evaluation of belzutifan was approved by the DFCI institutional review board. Written informed consent for participation was given by the patient’s parents; written assent was provided by the 17-year-old patient. The dose of belzutifan (120 mg daily) was based on the recommended phase 2 dose chosen for the dose-expansion cohort in a phase 1 trial involving adults with advanced renal cell carcinoma13.
Safety was assessed every 1 to 4 weeks for the first 10 months and every 4 to 12 weeks thereafter, with adverse events recorded according to the Common Toxicity Criteria, version 5.0, of the National Cancer Institute14. Circulating markers of potential syndromic manifestations of polycythemia (blood counts and erythropoietin level), paraganglioma (plasma levels of metanephrines and chromogranin A), and somatostatinoma (plasma levels of somatostatin) were assessed every 1 to 3 months. Tumor response was evaluated with the use of both magnetic resonance imaging (MRI) and combined positron emission tomography and computed tomography (PET–CT) with 18F-fluorodeoxyglucose (FDG) or gallium-68 (68Ga)–dotatate.
CLINICAL PRESENTATION
Initial Presentation and Treatment
The female patient first presented at 6 years of age with weakness on her left side and double vision that were attributed to a transient ischemic attack. Severe polycythemia was present (hemoglobin level, 24 g per deciliter) with an inappropriately elevated erythropoietin level (54 mIU per milliliter; reference range 4 to 21); serial phlebotomy was performed. Germline genetic testing for causes of polycythemia, including testing for variants in EPAS1, was negative.
When the patient was 9 years of age, amlodipine therapy was initiated for persistent hypertension caused by right renal artery stenosis with possible thrombosis that was presumed to be related to underlying polycythemia. At 14 years of age, she had a hypervascular right suprarenal mass, measuring 2.9 cm by 3.2 cm, that extended into the infrahepatic inferior vena cava and three additional retroperitoneal masses. The plasma normetanephrine levels were elevated (17 nmol per liter; reference value, <0.9). Concurrently, increasing hypertension led to the use of additional antihypertensive medications, and the patient underwent phlebotomy more frequently. The patient underwent resection of the dominant right suprarenal mass, right nephrectomy, partial resection and reconstruction of the inferior vena cava, and resection of the other three retroperitoneal masses. Pathological examination showed at least eight discrete paragangliomas and an atrophic right kidney with renal artery stenosis.
Diagnosis of the Pacak-Zhuang Syndrome
The patient was referred to our pediatric cancer genetic risk program. A three-generation family history was negative for polycythemia or paragangliomas, and additional germline testing was unrevealing. The co-occurrence of polycythemia with new diagnosis of paraganglioma led to the clinical diagnosis of the Pacak-Zhuang syndrome. Whole exome sequencing of the suprarenal paraganglioma revealed an EPAS1 variant, c.1589C>A (p.A530E), in 49% of 239 reads; this variant was absent from peripheral blood and adjacent normal adipose tissue (Fig. 1). Subsequent Sanger sequencing revealed the same EPAS1 variant in four additional paragangliomas but not in adipose tissue, lymph node (Fig. 1), or kidney (not shown). The presence of the EPAS1 mutation in multiple tumors confirmed the diagnosis.
FIGURE 1. Genetic Diagnosis Based on EPAS1 Sequencing.
Shown are the results of DNA sequencing of tumors, normal adipose and lymph node tissues, and peripheral blood samples obtained from the patient. The image from the Integrative Genomics Viewer, version 2.9.4, shows the patient’s whole exome sequencing read alignments against the human reference genome hg19 (left). A snapshot of the sequencing reads from the tumor and normal adipose tissue and peripheral blood samples is shown. The c.1589C>A (p.A530E) variant in EPAS1 (the gene encoding HIF2α; also known as HIF2A) was detected in 49% of 239 reads from the tumor and was absent from the adipose tissue and peripheral blood samples. Targeted Sanger sequencing shows the c.1589C>A variant in five tumors; the variant was not present in normal adipose tissue and lymph node samples (right).
Clinical Progression and Initiation of Belzutifan Therapy
After the initial paraganglioma resection, antihypertensive agents were discontinued. The plasma normetanephrine levels remained slightly elevated (0.94 to 1.2 nmol per liter) (Fig. 2A) and began to increase further at approximately 2 years after surgery. At 16 years of age, the patient underwent whole-body MRI followed by a dedicated abdominal MRI, which revealed a new left suprarenal mass (1.5 cm by 1.0 cm) and a lesion in the aortocaval space (<1 cm in diameter). Central nervous system venous malformations were not seen on the whole-body MRI, and subsequent ophthalmologic examination did not show any ocular manifestations.
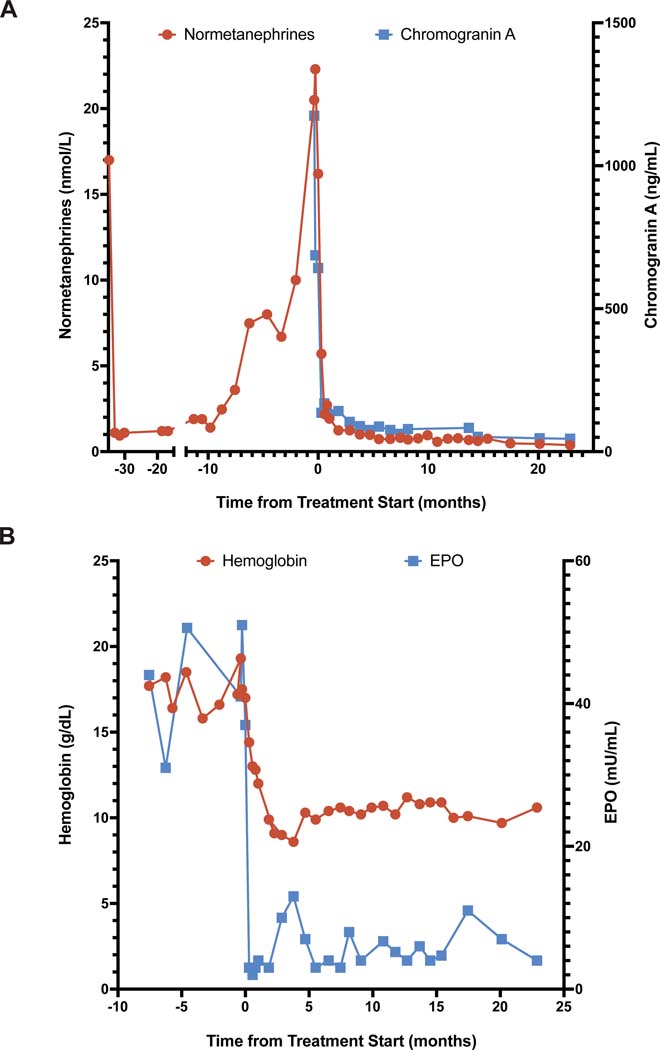
FIGURE 2. Blood Biomarker Response Profiles.
Panel A shows plasma levels of normetanephrine and chromogranin A over time (in months) from the start of treatment with belzutifan (time 0). Surgery took place approximately 34 months before the start of belzutifan therapy. Laboratory analysis of chromogranin A at the last three time points was done at the same facility with a change in the assay platform. Panel B shows the blood levels of hemoglobin and erythropoietin over time. Note the differing scales on the two y axes in both panels.
Over a period of 11 months, the plasma level of normetanephrine increased to a peak value of 22.3 nmol per liter and the plasma level of chromogranin A to a peak value of 1175 ng per milliliter (reference range, 0 to 95) (Fig. 2A). MRI and FDG PET–CT revealed progressive growth and increased FDG uptake in the dominant left suprarenal lesion and three additional retroperitoneal lesions, the largest of which, in the aortocaval region, had also increased in size. The patient had recurrent headaches, recurrent hypertension leading to the reinitiation of amlodipine therapy, and worsening polycythemia (peak hemoglobin level, 19.3 g per deciliter) that led to more frequent phlebotomy (Fig. 2B).
The clinical, laboratory, and imaging findings were compatible with new paragangliomas and refractory polycythemia. Given the risks and limitations associated with repeated surgery to resect multiple, recurrent paragangliomas, a targeted treatment option was explored. Treatment with belzutifan at a dose of 120 mg daily was initiated.
RESULTS
Clinical and Biochemical Response
Dramatic decreases in the plasma levels of normetanephrine (from 16.2 to 5.7 nmol per liter) and chromogranin A (from 642 to 136 ng per milliliter) were evident 9 days after the initiation of belzutifan therapy (Fig. 2A). The polycythemia also abated quickly, with the hemoglobin level decreasing into the normal range by day 17 (Fig. 2B). A corresponding clinical response was observed, with the resolution of daily headaches and abatement of hypertension within 10 days after drug initiation. Antihypertensive agents were discontinued after 3 months. After 24 months of belzutifan treatment, there has been no evidence of somatostatinoma, although this condition has been seen in some patients with the Pacak-Zhuang syndrome; this finding may reflect the younger age of our patient. Treatment is ongoing at the time of this report.
Response on Imaging
Eleven days before belzutifan therapy was started, the dominant left adrenal mass measured 2.4 cm by 2.6 cm, the aortocaval nodule measured 1.3 cm by 1.3 cm (Fig. 3), and two smaller soft tissue nodules (<1 cm in diameter) were present. All the lesions were FDG-avid. Other FDG-avid nodules were noted, but new extensive brown fat activation (presumably due to increased norepinephrine) obscured further characterization of these small lesions.
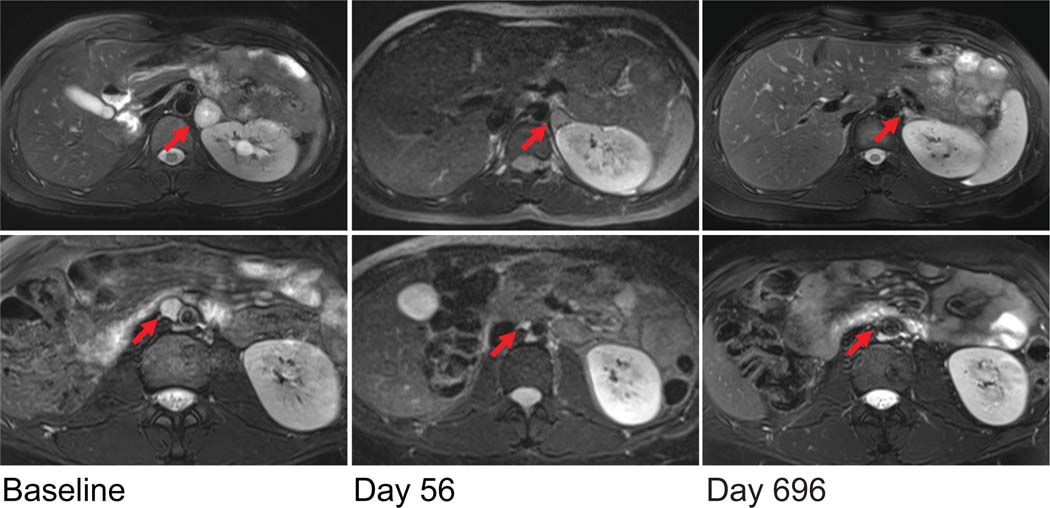
FIGURE 3. Anatomic Imaging Response.
Early and sustained responses of the left adrenal mass (top, red arrows) and the aortocaval nodule (bottom, red arrows) are shown in fat-suppressed, T2-weighted axial MRI scans. By day 56, both the left adrenal mass and the aortocaval nodule had decreased substantially in size; the smaller retroperitoneal nodules were barely discernable (not shown). The most recent MRI scans (from day 696) showed predominantly normal adrenal tissue and no residual retroperitoneal disease.
At 17 days after the initiation of belzutifan therapy, the dominant left adrenal mass had decreased slightly in size. On day 56, MRI revealed substantial shrinkage of all lesions. The left adrenal lesion had decreased in size to 1.4 cm by 1.3 cm, and the aortocaval nodule and other retroperitoneal nodules were now barely discernible (Fig. 3). The most recent MRI, obtained on day 696, revealed further reduction in the size of the left adrenal lesion (to 1.2 cm by 0.7 cm), with predominantly normal adrenal tissue now evident. Corresponding decreases in FDG uptake of the left adrenal mass were observed, from intense FDG avidity at baseline to no evident metabolically active disease by day 531 (Fig. S1 in the Supplementary Appendix).
Side Effects of Treatment
During 24 months of treatment with belzutifan, the patient had minimal side effects, with adverse events of only grade 1 or 2. After the initial normalization of the hemoglobin level, the level remained in the range of 8.6 to 11.2 g per deciliter, and the patient continued to be asymptomatic without an indication for transfusion. In addition to anemia, an expected side effect given the mechanism of action of belzutifan, other grade 2 adverse events included weight gain and a decreased lymphocyte count. Grade 1 events included decreased white cell and neutrophil counts, eosinophilia, maculopapular rash, dry skin, QTc prolongation, hypertension (intermittent), hypotension (two events due to diastolic blood pressures 1 mm Hg below the normal range), hyperkalemia, sinus tachycardia, proteinuria, and hematuria. The patient did not have any interruption or modification of the belzutifan dose, and no grade 3 or 4 adverse events had occurred as of the data cutoff date.
DISCUSSION
The application of targeted molecular therapies that are based on genomic analysis has a prominent place in contemporary oncologic care. The use of somatic tumor testing to identify potential therapeutic targets is frequently incorporated into the treatment of cancers in children, adolescents, and adults.15 Similarly, the value of identifying a predisposition to tumors is increasingly recognized as being central to care, particularly in children. The identification of heritable factors that predispose persons to cancer facilitates risk assessment and surveillance for early tumor detection, in addition to prompting cascade testing for family members.16
The number of identified susceptibility loci for paraganglioma has been increasing, with a growing number of syndromes being known to predispose persons to the development of paragangliomas.17 The molecular characterization of paragangliomas has led to their classification into three major subtypes: pseudohypoxia, kinase signaling, and Wnt-altered.18 Paragangliomas that are associated with EPAS1 mutations fall into the pseudohypoxia subtype, which includes both VHL (von Hippel–Lindau gene)–related and EPAS1-related paragangliomas as well as tricarboxylic acid cycle–related paraganglioma (which is associated with mutations in genes such as SDHA, SDHB, SDHC, SDHD, SDHAF2, and FH).17,18
The EPAS1 variant that was identified in our patient (p.A530E) has been reported in another patient with polycythemia and paragangliomas.19 The same variant was also identified in a tumor sample obtained from a patient with an apparently sporadic pheochromocytoma.20 Other mutations at the same amino acid residue (i.e., p.A530T and p.A530V) were among the first to be described in patients with the Pacak– Zhuang syndrome.1,2 These gain-of-function mutations affect prolyl hydroxylation and decrease the binding of HIF2α to the VHL protein, thus reducing the proteasomal degradation of HIF2α and resulting in a pseudohypoxic state.1 The activation of downstream hypoxia-related genes is thought to lead to polycythemia, paraganglioma, and somatostatinoma.1,2
Somatic mosaicism is a recognized cause of tumor predisposition but can be challenging to diagnose. A somatic variant may not be detected in DNA extracted from accessible tissues (e.g., blood, saliva, and hair) because the specific cell lineages that are affected depend on the timing of the postzygotic genetic event during embryonic development. We present the case of a patient with somatic mosaicism for an EPAS1-activating mutation that was not detected by germline testing and that was molecularly confirmed only after the development, resection, and somatic sequencing of multiple primary paragangliomas sharing the neural crest cell origin. Belzutifan has recently been approved by the Food and Drug Administration for use in adults with VHL-associated tumors.21 Our patient had rapid and dramatic clinical and imaging responses to belzutifan treatment. Plasma levels of normetanephrine decreased within days after drug initiation, reducing an important cause of complications related to catecholamine excess from functional paragangliomas. Rapid tumor shrinkage was already evident by day 17, and the response has been sustained for 24 months, with treatment ongoing at the data cutoff date. No further tumors have developed. Although belzutifan was given to this patient after a substantial tumor burden was already present, the use of such a targeted agent for earlier tumor “interception” (i.e., intervention at an early step in neoplasm development) in patients with a genetic predisposition is appealing to consider.22 However, further data regarding the long-term effects of HIF2α inhibition in children are needed to assess the risks and benefits of earlier and prolonged administration, particularly in the context of an undefined duration of therapy.
Although anemia due to marked decreases in the erythropoietin level was a common grade 3 adverse event in a phase 1 trial of belzutifan therapy in adults,13 this anticipated pharmacodynamic effect was beneficial in our patient with long-standing, refractory polycythemia. The mechanism of polycythemia in the Pacak–Zhuang syndrome has not been fully elucidated, but both erythropoietin-dependent and erythropoietin-independent mechanisms have been proposed. HIF2α is a known transcriptional regulator of EPO,11,23 and the gain-of-function variant in our patient is expected to lead to increased EPO transcription. The polycythemia in this patient preceded the formation of paragangliomas but was markedly exacerbated in the context of tumor progression. While the patient was receiving belzutifan, her hemoglobin levels stabilized, with mild asymptomatic anemia that has not led to transfusion; the endogenous erythropoietin levels have been appropriate and have not led to replacement.
Precise molecular diagnostic approaches were critical in defining the molecular driver of the syndrome in this patient and for matching her molecular variant to a new therapy that resulted in marked clinical benefits. A similar approach has been shown with the use of alpelisib in persons with mosaic PIK3CA-related overgrowth syndrome.24 The case of our patient broadens the paradigm for the use of targeted therapy for the treatment and potential prevention of tumors in persons with tumor-predisposition syndromes. As the repertoire of molecularly targeted agents expands, the long-term effects of these agents will warrant thorough investigation such that the goals of care for children and adults with tumor-predisposition syndromes may further shift from screening and early detection to cancer interception and prevention.
Supplementary Material
ACKNOWLEDGEMENTS
Supported by the Morin Family Fund for Pediatric Cancer (to Dr. Kamihara) and by an Alex’s Lemonade Stand Foundation Center of Excellence Award in Developmental Therapeutics for infrastructure and personnel support (to Dr. DuBois).
We thank the patient and her family, as well as all the providers involved in her care.
DISCLOSURES
Relevant to this work:
WGK is eligible to receive royalties related to belzutifan, from Merck & Co., Inc.
RFP is an employee of Merck & Co., Inc.
NJZ was an employee of Merck & Co., Inc. at the time of this analysis, owns stock in Merck & Co., Inc. and is eligible to receive royalties related to belzutifan from Merck & Co., Inc.
Other:
JK’s spouse has received consulting fees from ROME Therapeutics, Foundation Medicine, Inc., NanoString Technologies, EMD Millipore Sigma, and Pfizer. JK’s spouse is a founder and has equity in ROME Therapeutics, PanTher Therapeutics and TellBio, Inc. JK’s spouse receives research support from ACD-Biotechne, PureTech Health LLC, and Ribon Therapeutics.
JAP is on an advisory board for Kura Oncology and has previously provided input to Syndax Pharmaceuticals.
WGK has equity interests in the following and is on the Board of Directors of Lilly Pharmaceuticals and LifeMine Therapeutics, Cofounder of Tango Therapeutics and Cedilla Therapeutics, and scientific advisor for Circle Pharma, IconOVir Bio, Fibrogen, and Nextech Invest.
SGD has received consulting fees from Bayer and travel expenses from Loxo Oncology, Roche, and Salarius.
REFERENCES
- 1.Zhuang Z, Yang C, Lorenzo F, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med 2012;367:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacak K, Jochmanova I, Prodanov T, et al. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol 2013;31: 1690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C, Sun MG, Matro J, et al. Novel HIF2A mutations disrupt oxygen sensing, leading to polycythemia, paragangliomas, and somatostatinomas. Blood 2013;121: 2563–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Därr R, Nambuba J, Del Rivero J, et al. Novel insights into the polycythemia-paraganglioma-somatostatinoma syndrome. Endocr Relat Cancer 2016;23:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dmitriev PM, Wang H, Rosenblum JS, et al. Vascular changes in the retina and choroid of patients with EPAS1 gain-of-function mutation syndrome. JAMA Ophthalmol 2020;138:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacak K, Chew EY, Pappo AS, et al. Ocular manifestations of hypoxia-inducible factor-2α paraganglioma-somatostatinoma-polycythemia syndrome. Ophthalmology 2014;121:2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenblum JS, Wang H, Dmitriev PM, et al. Developmental vascular malformations in EPAS1 gain-of-function syndrome. JCI Insight 2021;6(5):e144368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C, Hong CS, Prchal JT, Balint MT, Pacak K, Zhuang Z. Somatic mosaicism of EPAS1 mutations in the syndrome of paraganglioma and somatostatinoma associated with polycythemia. Hum Genome Var 2015;2:15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buffet A, Smati S, Mansuy L, et al. Mosaicism in HIF2A-related polycythemia-paraganglioma syndrome. J Clin Endocrinol Metab 2014;99(2):E369–E373. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo FR, Yang C, Ng Tang Fui M, et al. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med (Berl) 2013;91: 507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Percy MJ, Furlow PW, Lucas GS, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med 2008;358:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 2016;1(19):e87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choueiri TK, Bauer TM, Papadopoulos KP, et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med 2021;27:802–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Common toxicity criteria, version 5.0. Bethseda, MD: National Cancer Institute, November 27, 2017. [Google Scholar]
- 15.DuBois SG, Corson LB, Stegmaier K, Janeway KA. Ushering in the next generation of precision trials for pediatric cancer. Science 2019;363:1175–81. [DOI] [PubMed] [Google Scholar]
- 16.Brodeur GM, Nichols KE, Plon SE, Schiffman JD, Malkin D. Pediatric cancer predisposition and surveillance: an overview, and a tribute to Knudson Alfred G. Jr. Clin Cancer Res 2017;23(11):e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crona J, Taïeb D, Pacak K. New perspectives on pheochromocytoma and paraganglioma: toward a molecular classification. Endocr Rev 2017;38:489–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishbein L, Leshchiner I, Walter V, et al. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 2017;31:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyoda H, Hirayama J, Sugimoto Y, et al. Polycythemia and paraganglioma with a novel somatic HIF2A mutation in a male. Pediatrics 2014;133(6):e1787–e1791. [DOI] [PubMed] [Google Scholar]
- 20.Welander J, Andreasson A, Brauckhoff M, et al. Frequent EPAS1/HIF2α exons 9 and 12 mutations in non-familial pheochromocytoma. Endocr Relat Cancer 2014; 21:495–504. [DOI] [PubMed] [Google Scholar]
- 21.Food and Drug Administration. FDA approves belzutifan for cancers associated with von Hippel–Lindau disease. August 13, 2021 (https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belzutifan-cancers-associated-von-hippel-lindau-disease).
- 22.Blackburn EH. Cancer interception. Cancer Prev Res (Phila) 2011;4:787–92. [DOI] [PubMed] [Google Scholar]
- 23.Rankin EB, Biju MP, Liu Q, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 2007;117:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venot Q, Blanc T, Rabia SH, et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 2018; 558:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.