The NICO (non-invasive airway management in comatose patients) randomized controlled trial found that, in acute poisoned comatose patients with a Glasgow Coma Scale < 9, withholding intubation was associated with a significant clinical improvement in the hierarchical composite primary end point of in-hospital death, length of stay in intensive care unit (ICU), and length of hospital stay compared to routine practice [1]. The trial-based economic evaluation estimated the incremental cost-effectiveness ratio (ICER) at 28 days, using the outcome of in-hospital death and adverse events related to intubation (mechanical ventilation, admission to the ICU, and rapid-onset pneumonia).
The economic evaluation was performed using patient-level data collected alongside the trial [1, 2]. Data for the economic analysis, covering medical resource use and major events, were collected prospectively (electronic supplementary material [ESM]). The perspective was the healthcare provider, restricted to hospitals. The end point of the economic evaluation was the ICER expressed as the difference in costs divided by the difference in composite outcome. Total costs in 2023 euros (€) were estimated from the time of recruitment until the earliest of death, withdrawal and 28 days, not discounted [3]. Differences in mean costs and frequency of adverse events were estimated using separate generalized linear-regression mixed models, with a gamma distribution and logarithmic link for costs, and a Bernoulli distribution (logit link) for frequency. All models were adjusted for the strategy (fixed effect) and the hospital (random effect). The ICER indicates the additional investments needed for the intervention to gain one extra unit of effect compared with usual care. The uncertainty surrounding these point estimates was examined using a stratified for hospital non-parametric bootstrapping technique with 1000 replications [3]. The net monetary benefit for different levels of willigness to pay was calculated. Detailed methods are included in the electronic supplementary material.
The hierarchical composite primary end point was improved in the restricted intubation compared with the control group, with a win ratio of 1.85 (95% confidence interval [CI] 1.33–2.58; P < 0.001) and 1.83 (95% CI 1.29–2.60; P = 0.001) after stratification by center. The total mean cost in the restricted intubation group was €3803 (7399) vs €5407 (9316) in the control group, a not statistically significant difference of €1613 (95% CI −€3797 to €572). The main driver of the cost difference was higher ICU costs, with a statistically significant difference (€ − 1463; 95% CI € − 2658 to € − 267) (ESM).
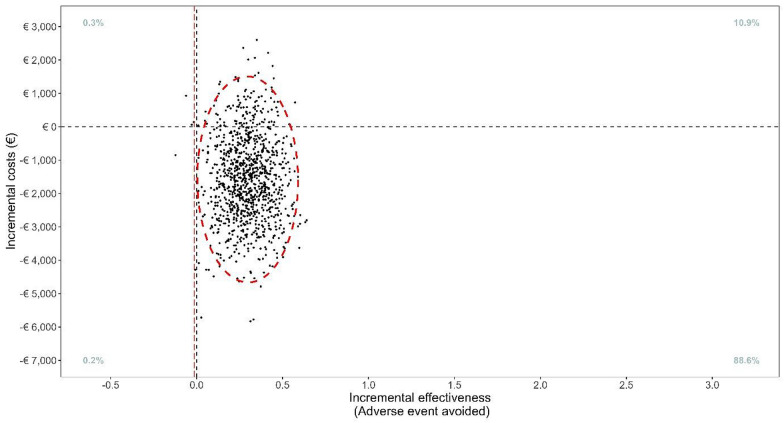
The point estimate of the ICER was − €5578 per event averted indicating that the withholding of intubation was both cost saving and event reducing. The uncertainty in the incremental costs and effect represented on the cost-effectiveness plane in Fig. 1 shows that in 88.6% of bootstrap replications, restrictive intubation was both more effective and less costly. The net monetary benefit ranged from €1670 to €16,021 (ESM).
Fig. 1.
Bootstrap distribution of 1000 incremental cost–effectiveness ratio comparing restrictive intubation to control in the intention-to-treat population
Withholding intubation had an 88.6% probability of dominance vs routine practice with lower hospitalization and ICU costs. Limitation: we used a fixed emergency department cost per patient. Policymakers should explore how these monetary benefits can be appropriately utilized in critical care.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
NICO study group members:
Tabassome Simon; Damien Viglino; Marine Cachanado; Clementine Cassard; Emmanuel Montassier; Benedicte Douay; Jeremy Guenezan; Pierrick Le Borgne; Youri Yordanov; Armelle Severin; Melanie Roussel; Matthieu Daniel; Adrien Marteau; Nicolas Peschanski; Dorian Teissandier; Richard Macrez; Julia Morere; Tahar Chouihed; Damien Roux; Frederic Adnet; Ben Bloom; Anthony Chauvin.
Yonathan Freund, MD, PhD; IMProving Emergency Care FHU, Paris, France; Emergency Department and Service Mobile d’Urgence et de Reanimation (SMUR), Hopital Pitie-Salpetriere, Assistance Publique–Hopitaux de Paris (AP-HP), Paris, France. Damien Viglino, MD, PhD; Emergency Department, Grenoble-Alpes University Hospital, and University Grenoble-Alpes, HP2 Laboratory INSERM U 1300, Grenoble, France. Marine Cachanado, MSc; Department of Clinical Pharmacology and Clinical Research Platform Paris-East, AP-HP, Sorbonne University, St Antoine Hospital, Paris, France. Clementine Cassard, MD; Emergency Department and Service Mobile d’Urgence et de Reanimation (SMUR), Hopital Pitie-Salpetriere, Assistance Publique–Hopitaux de Paris (AP-HP), Paris, France. Emmanuel Montassier, MD, PhD; Emergency Department and SMUR, Nantes Universite, CHU Nantes, INSERM UMR 1064, Nantes, France. Benedicte Douay, MD; Emergency Department and SMUR, Hopital Beaujon AP-HP, Clichy, France. Jeremy Guenezan, MD, PhD; Emergency Department, University Hospital of Poitiers, Poitiers, France. Pierrick Le Borgne, MD; Emergency Department, Hopitaux Universitaires de Strasbourg, Strasbourg, France and INSERM UMR 1260, Regenerative NanoMedicine, Federation de Medecine Translationnelle, University of Strasbourg, Strasbourg, France. Youri Yordanov, MD, PhD; IMProving Emergency Care FHU, Paris, France; Emergency Department, Hopital Saint Antoine AP-HP, INSERM, Institut Pierre Louis d’Epidemiologie et de Sante Publique, UMR-S 1136, Paris, France. Armelle Severin, MD; SAMU 92–SMUR Raymond Poincare, Raymond Poincare Hospital, AP-HP, Paris, France. Melanie Roussel, MD; Emergency Department, Univ Rouen Normandie, CHU Rouen, Rouen, France. Matthieu Daniel, MD; Emergency Department, SAMU-SMUR et Secours en Milieu Perilleux, CHU de La Reunion Site Nord Felix Guyon, La Reunion, France. Adrien Marteau, MD; Emergency Department, Centre Hospitalier Universitaire Sud Reunion, Saint Pierre, La Reunion, France. Nicolas Peschanski, MD, PhD; Emergency Department and SAMU35-SMUR, Hopital Pontchaillou, Centre Hospitalier Universitaire de Rennes, Rennes, France. Dorian Teissandier, MD; Faculte de Medecine, Universite de Rennes, Rennes, France (Peschanski); Emergency Department, CHU Clermont-Ferrand, Clermont-Ferrand, France; Universite Clermont Auvergne, INRAE, UNH, Clermont-Ferrand, France. Richard Macrez, MD, PhD; Emergency Department, University hospital of Caen, UNICAEN, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders, GIP Cyceron, Institut Blood and Brain Normandie University, Caen, France. Julia Morere, MD, PhD; Emergency Department and SMUR, Hopital Edouard Herriot, Lyon, France. Tahar Chouihed, MD, PhD; Emergency Department, University Hospital of Nancy, INSERM, UMR_S 1116, University Hospital of Nancy, Nancy, France. Damien Roux, MD, PhD; Universite Paris Cite, AP-HP, Hopital Louis Mourier, DMU ESPRIT, Service de Medecine Intensive Reanimation, Colombes, France. Frederic Adnet, MD, PhD; Emergency Department and Service Mobile d’Urgence et de Reanimation SMUR, Hopital Avicenne, AP-HP, Bobigny, France. Ben Bloom, MD; Emergency Department, Royal London Hospital, London, United Kingdom. Anthony Chauvin, MD, PhD; Emergency Department, Hopital Lariboisiere AP-HP, Paris, France and INSERM U942 MASCOT, University of Paris, Paris, France. Tabassome Simon, MD, PhD; IMProving Emergency Care FHU, Paris, France; Department of Clinical Pharmacology and Clinical Research Platform Paris-East, AP-HP, Sorbonne University, St Antoine Hospital, Paris, France.
Author contributions
ID-Z and YF had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: ID-Z and YF Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: BFNS, MM, ANO and ID-Z Critical revision of the manuscript for important intellectual content: YF Statistical analysis: BFNS and ANO Obtained funding: YF Administrative, technical, or material support: MM Supervision: ID-Z and YF.
Funding
This study was funded by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique 2019 [Ministere de la Sante, Paris, France]). The study sponsor was the Assistance Publique–Hopitaux de Paris.
Data sharing statement
Data available: Yes. Data types: deidentified participant data. How to access data: data request shall be sent to Isabelle.durand-zaleski@aphp.fr. When available: with publication supporting documents. Document types: statistical/analytic code. How to access documents: request shall be sent to Isabelle.durand-zaleski@aphp.fr. When available: with publication. Who can access the data: researchers whose proposed use of the data has been approved by the steering committee. Types of analyses: any purpose. Mechanisms of data availability: after approval by the steering committee and signed data sharing agreement.
Declarations
Conflicts of interest
ID-Z reported personal fees from Air Liquide, BMS, Pfizer, and Roche, outside the submitted work.
Footnotes
The NICO Study Group details are listed in the acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Isabelle Durand-Zaleski, Email: Isabelle.durand-zaleski@aphp.fr.
on behalf of the NICO Study Group:
Tabassome Simon, Damien Viglino, Marine Cachanado, Clementine Cassard, Emmanuel Montassier, Benedicte Douay, Jeremy Guenezan, Pierrick Le Borgne, Youri Yordanov, Armelle Severin, Melanie Roussel, Matthieu Daniel, Adrien Marteau, Nicolas Peschanski, Dorian Teissandier, Richard Macrez, Julia Morere, Tahar Chouihed, Damien Roux, Frederic Adnet, Ben Bloom, and Anthony Chauvin
References
- 1.Freund Y, Viglino D, Cachanado M, Cassard C, Montassier E, Douay B, et al. Effect of noninvasive airway management of comatose patients with acute poisoning: a randomized clinical trial. JAMA. 2023 doi: 10.1001/jama.2023.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assistance Publique—Hôpitaux de Paris. Non-invasive Airway Management of Comatose Poisoned Emergency Patients [Internet]. clinicaltrials.gov; 2023 juill [cité 1 janv 2023]. NCT04653597. Disponible sur: https://clinicaltrials.gov/study/NCT04653597
- 3.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16(2):e1–5. doi: 10.1016/j.jval.2013.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available: Yes. Data types: deidentified participant data. How to access data: data request shall be sent to Isabelle.durand-zaleski@aphp.fr. When available: with publication supporting documents. Document types: statistical/analytic code. How to access documents: request shall be sent to Isabelle.durand-zaleski@aphp.fr. When available: with publication. Who can access the data: researchers whose proposed use of the data has been approved by the steering committee. Types of analyses: any purpose. Mechanisms of data availability: after approval by the steering committee and signed data sharing agreement.