Abstract
Recent randomized controlled trials (RCTs) have shown no benefit but dose-dependent harm by early full nutritional support in critically ill patients. Lack of benefit may be explained by anabolic resistance, suppression of cellular repair processes, and aggravation of hyperglycemia and insulin needs. Also early high amino acid doses did not provide benefit, but instead associated with harm in patients with organ dysfunctions. However, most studies focused on nutritional interventions initiated during the first days after intensive care unit admission. Although the intervention window of some RCTs extended into the post-acute phase of critical illness, no large RCTs studied nutritional interventions initiated beyond the first week. Hence, clear evidence-based guidance on when and how to initiate and advance nutrition is lacking. Prolonged underfeeding will come at a price as there is no validated metabolic monitor that indicates readiness for medical nutrition therapy, and an adequate response to nutrition, which likely varies between patients. Also micronutrient status cannot be assessed reliably, as inflammation can cause redistribution, so that plasma micronutrient concentrations are not necessarily reflective of total body stores. Moreover, high doses of individual micronutrients have not proven beneficial. Accordingly, current evidence provides clear guidance on which nutritional strategies to avoid, but the ideal nutritional regimen for individual patients remains unclear. In this narrative review, we summarize the findings of recent studies, discuss possible mechanisms explaining the results, point out pitfalls in interpretation of RCTs and their effect on clinical practice, and formulate suggestions for future research.
Keywords: Nutrition, Macronutrients, Glucose, Amino acid, Micronutrients, Critical illness, Intensive care
Take-home message
| In the acute phase of critical illness, high doses of all macronutrients should be avoided because of anabolic resistance and potential harm through suppression of cellular repair pathways, and increased hyperglycemia and insulin need. Although prolonged underfeeding through avoiding full nutrition likely comes at a price, the time point when anabolic resistance ceases cannot be monitored, precluding true individualized nutrition. |
Introduction
In acute critical illness, catabolism is stimulated resulting in muscle wasting, weakness and failure to wean [1]. A low protein, energy, and micronutrient intake of these patients may aggravate catabolism and is associated with infections, delayed recovery, and increased mortality [2–4]. However, the traditional assumption that nutrients may counteract catabolism and thereby improve clinical outcome in critically ill patients is challenged by cumulative evidence from large randomized controlled trials (RCTs) revealing harm by providing full nutrition in the acute phase [5–8]. Even though a personalized approach has been suggested [9, 10], monitoring tools that accurately quantify the actual energy, protein and micronutrient need for the individual patient are currently not available [4]. In this narrative review, we provide a condensed interpretation of recent RCT evidence, discuss the impact of evidence on clinical practice, and formulate some suggestions for future research.
Medical nutrition therapy in the ICU: evidence from RCTs
Observational studies have associated a cumulative protein and energy deficit with impaired outcome of critical illness [2, 3, 11]. However, the results of these studies might also be explained by feeding intolerance as a marker of severity of illness [2, 3, 12]. In the last decade, several large RCTs have addressed the timing, route and dosage of medical nutrition therapy in critically ill patients. The first RCT that challenged the assumption that early full nutrition would be beneficial was the EPaNIC RCT, published in 2011 [5]. In 4640 adult critically ill patients, initiation of parenteral nutrition to supplement insufficient enteral nutrition prolonged dependency on intensive care as compared to delaying supplemental parenteral nutrition until 1 week after intensive care unit (ICU) admission. Patients receiving early supplemental parenteral nutrition had a prolonged duration of vital organ support, more infections, a higher incidence of ICU-acquired weakness, and impaired recovery herefrom [5, 13]. Supplemental parenteral nutrition also did not improve functional status at hospital discharge, as assessed by the 6-min walking distance and activities of daily living [5]. These results were subsequently confirmed in critically ill children (PEPaNIC RCT, N = 1440), in whom supplemental parenteral nutrition also adversely affected 2- and 4-year neurodevelopmental outcomes [8, 14]. In both RCTs, mortality was unaffected, whereas harm by early parenteral nutrition was present in all studied subgroups, including patients with a high nutritional risk score (NRS score ≥ 5), patients with body mass index < 25 or ≥ 40, patients with sepsis, patients with a contraindication to enteral nutrition, and critically ill neonates [5, 8]. Theoretically, harm in these RCTs could be explained by the higher feeding dose—early overfeeding—or by harm that is specific to the parenteral feeding route. Secondary analyses of these RCTs suggested dose-dependent harm rather than harm by the intravenous feeding route [15, 16], which was corroborated by subsequent RCTs. Indeed, two large RCTs—the CALORIES (N = 2400) [17] and NUTRIREA-2 RCT (N = 2410) [6]—found no difference between the enteral and parenteral nutrition route with a similar energy dose in both groups. In the Nutrirea-2 RCT including ventilated patients with shock, early high-dose enteral nutrition was even more harmful as compared with early high-dose parenteral nutrition, by inducing potentially lethal gastrointestinal complications [6]. In both RCTs, patients were randomized shortly after ICU admission, and the intervention window was 5–7 days. Also in three RCTs that randomized critically ill patients to a lower or a higher dose of enteral nutrition initiated in the acute phase and continued for 6 (EDEN RCT, N = 1000), 14 (PermiT RCT, N = 894) or 28 days (TARGET RCT, N = 3957), a higher dose of enteral nutrition initiated in the acute phase did not provide benefit, and secondary outcomes suggested potential harm [18–20]. Also long-term functional outcome was not improved by higher feeding doses in the EDEN and TARGET RCTs [21–23]. The absence of benefit in the latter RCTs was consistent in all studied subgroups, including patients with high predicted risk of death [20], patients with body mass index (BMI) ≤ 18 [20], and patients with sepsis [19, 20]. In a detailed post hoc analysis of the PermiT RCT, none of the studied baseline nutritional risk markers could identify patients who would benefit from early enhanced enteral nutrition, including the modified Nutrition Risk in Critically Ill (NUTRIC) score, BMI, transferrin, phosphate, urinary urea nitrogen, and nitrogen balance [24]. A low baseline prealbumin level, presumed to indicate high nutritional risk, even associated with significant mortality harm when receiving higher-dose enteral nutrition [24]. Likewise, the recent NUTRIREA-3 RCT, which randomized 3044 ventilated patients with shock to early high-dose nutrition (25 kcal/kg/day and 1.0–1.3 g protein per kg per day) versus low-dose nutrition in the first week (6 kcal/kg/day and 0.2–0.4 g protein/kg/day), found harm by early high-dose nutrition, with prolonged ICU dependency and increased complications in this group [7]. Importantly, in the NUTRIREA-3 RCT, the nutritional target was defined by randomization; medical nutrition therapy could be provided through either the enteral or parenteral route to reach that target [7]. A recent meta-analysis confirmed harm by early full feeding in critically ill patients as compared with permissive underfeeding. Although this meta-analysis did not include the EPaNIC RCT, it likely does not affect the conclusion, as EPaNIC patients were equally harmed by early full feeding [25]. A recent meta-analysis as part of the updated American feeding guidelines did not show benefit by supplemental parenteral nutrition as compared with enteral nutrition alone. Yet, the different design of included RCTs, with different timing of supplemental parenteral nutrition and different co-interventions and control groups, may complicate interpretation of the results [26]. Altogether, recent RCTs have shown dose-dependent harm by early medical nutrition therapy in critically ill patients, independent of the route of feeding and perceived nutritional risk. Table 1 summarizes the RCTs comparing a higher vs. lower amount of energy through the same or a different route, with the intervention initiated early after ICU admission. We report the energy intake on day 3 in these studies, as the higher energy target was reached in the respective high-dose group at that early time point. This time point also approximates the estimated end of the early period of the acute phase, as suggested by the European feeding guidelines [27]. Yet, the duration of the acute phase is debatable and likely variable between patients. American guidelines suggest a duration of 7–10 days for the acute phase, without any distinction between early and late periods [26].
Table 1.
RCTs providing different amount of calories by day 3 independent of the route
| Study or author, year, design | N of patients, population | Route in “high” vs. “low” | Duration of intervention | Energy on day 3 in “high” | Energy on day 3 in “low” | Non-nutritional calories included | Proportional protein restriction in “low” | Improved outcomes with “high” | Impaired outcomes with “high” |
|---|---|---|---|---|---|---|---|---|---|
| EPaNIC 2011, single-center[5] | 4640 mixed ICU patients | EN + PN vs. EN | 7 days | 20 kcal/kg/day | 5 kcal/kg/day | No | Yes | None | ICU and hospital LOS, MV and RRT duration, Infections, Muscle weakness, Cholestasis |
| EDEN 2011, multi-center [18] | 1000 pt with ARDS | EN in both groups | 6 days | 1300 kcal/day | 400 kcal/day | No | Yes | None | GI complications |
| Arabi 2011, single-center [28] | 240 ICU patients | EN | 7 days | 1250 kcal/day | 1070 kcal/day | Yes | No | None | Hospital mortality |
| Rice 2011, single-center [29] | 200 patients with ARF | EN | 6 days | 1400 kcal/day | 300 kcal/day | No | Yes | None | GI complications |
| Charles 2014, single-center [30] | 83 ICU patients | EN or PN | Not reported | 17 kcal/kg/daya | 12 kcal/kg/daya | No | No | None | None |
| Petros 2014, single-center [31] | 100 MV patients | EN + PN | 7 days | 24 kcal/kg/day | 14 kcal/kg/day | No | Yes | Nosocomial infections | GI complications, higher insulin needs |
| PermiT 2015, multi-center [19] | 894 ICU patients | EN | 14 days | 1300 kcal/day | 840 kcal/day | Yes | No | None | Need for renal replacement therapy |
| INTACT 2015, single-center [32] | 78 ICU patients with ALI | EN (PN allowed after 72-96 h) | Hospital stay | 90% of target (target 30 kcal/kg/day) | 65% of target | Yes | Yes | None | Hospital mortality |
| Rugeles 2016, single-center [33] | 187 ICU patients (120 analyzed) | EN | 7 days | 19 kcal/kg/day | 12 kcal/kg/day | No | No | None | Higher insulin needs |
| EAT-ICU 2017, single-center [34] | 199 MV patients | EN + PN vs. EN + PN after day 7 | ICU stay (max 90 days) | 24 kcal/kg/day | 13 kcal/kg/day | Yes | Yes | None | Hyperglycemia, Higher insulin needs, ICU LOS |
| TARGET 2018, multi-center [20] | 3957 MV patients | EN | 28 days | 30 kcal/kg/day | 22 kcal/kg/day | Yes | No | None | GI complications |
| Nutrirea-3 2023, multicenter [7] | 3044 patients receiving MV and vasopressors | EN or PN in both groups | 7 days | 25 kcal/kg/day | 8 kcal/kg/day | Yes | Yes | None | Readiness for ICU discharge, GI complications, Liver dysfunction |
Only RCTs studying a feeding intervention initiated in the first 2–3 days were included in the table. Some data are extracted from figures in original papers, where calories on day 3 were not presented in precise numbers. Definition of day 3 may differ between the studies depending on (1) counting from day 0 or day 1 (we used the third day reported); (2) counting from ICU admission or randomization. Estimation of energy requirements was different between the studies
ALI acute lung injury, ARDS acute respiratory distress syndrome, ARF acute respiratory failure, EN enteral nutrition, GI gastrointestinal, ICU intensive care unit, LOS lenght of stay, MV mechanical ventilation, PN parenteral nutrition, RCT randomized controlled trial, RRT renal replacement therapy,
aAverage, not day 3
The above-mentioned RCTs have been criticized for administering relatively low doses of proteins and for calculating the energy target by a fixed formula [35, 36]. Recent trials do not support these critiques, however. Indeed, apart from increased energy intake, also high protein supply initiated early in critical illness did not provide benefit. The largest RCT on protein supplements, the EFFORT Protein RCT, which randomized 1329 critically ill patients to receive a high (≥ 2.2 g/kg/day) or a usual (≤ 1.2 g/kg/day) protein dose, initiated within 96 h after ICU admission and continued for up to 28 days did not show benefit by high protein doses [37]. On the contrary, an early high protein dose associated with prolonged ICU dependency and increased mortality in the most severely ill patients and in patients with acute kidney injury [37]. Also the Nephro-Protective RCT (N = 474) did not find benefit by intravenous amino acid supplementation started on day 1 or 2 and continued until ICU discharge [38], and secondary analyses of the EPaNIC and PEPaNIC RCTs attributed harm by early parenteral nutrition specifically to the higher protein doses administered [15, 16]. A recent meta-analysis on the effect of higher versus lower protein doses in critically ill patients confirmed no benefit of higher-dose protein. The meta-analysis suggested potential heterogeneity of treatment, however, with significant mortality harm restricted to patients with acute kidney injury, which requires further investigation [39].
A second reason why recent nutritional RCTs could have failed to show any benefit of early enhanced medical nutrition therapy has been suggested to be the absence of indirect calorimetry. Indeed, exact quantification of energy expenditure requires indirect calorimetry, as predictive equations do not accurately estimate energy expenditure in all patients [40]. However, there is no solid evidence that early indirect calorimetry-based feeding would be superior to nutrition based on a calculated energy target. Although a meta-analysis suggested a potential reduction in 28-day mortality in critically ill adults, there was no impact on 90-day mortality, and also morbidity outcomes were similar [41]. Such transient mortality difference could be explained by confounding in unblinded RCTs. Moreover, the perceived mortality difference at 28 days was borderline significant, as the statistical significance would have been lost if one patient would have had a different 28-day mortality outcome (fragility index of 1). In addition, the meta-analysis included RCTs with different design and co-interventions, which further complicates interpretation of these results. The largest RCT comparing calculated versus measured energy target feeding, the TICACOS-International RCT (N = 580), found no benefit of indirect calorimetry-based feeding initiated early after ICU admission [42]. Moreover, the study was stopped prematurely because of slow recruitment, which may question the feasibility of routine use of indirect calorimetry. Evidently, these findings cannot necessarily be extrapolated to patients with prolonged critical illness.
Impact of evidence on clinical practice and guidelines
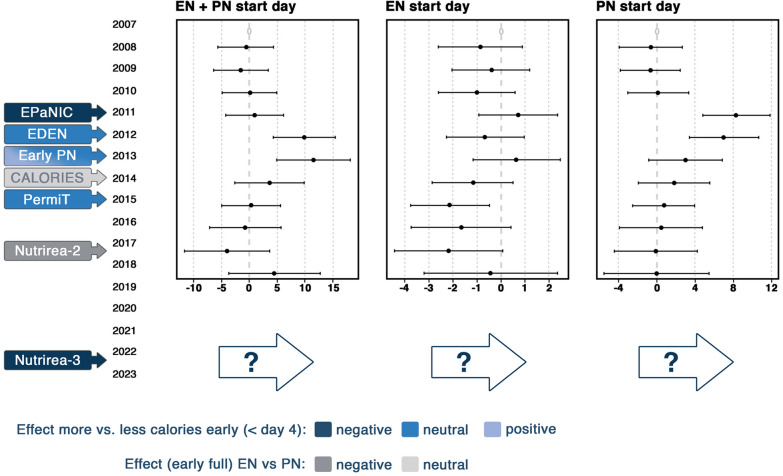
Published studies on the route, timing, and dose of nutrition may have changed practice in Europe remarkably as suggested by Veraar et al. [43]. The reported time point to start enteral and/or parenteral nutrition moved to a later time point soon after the EPaNIC study was published (Fig. 1). Nevertheless, a trend back to an earlier start slowly occurred over several years thereafter, with somewhat larger variation between respondents. The CALORIES RCT showing no difference between isocaloric enteral and parenteral nutrition may have contributed to this apparent change. Obviously, the trend only takes into account the timing and not the dose, which may have decreased during the time period presented in Fig. 1. If not, one might expect that at least the dose of nutrition in the acute phase would decrease in future years, since the Nutrirea-3 RCT recently showed significant harm by early high-dose nutrition, regardless of the route [7]. Studies addressing dosage and route by interventions initiated beyond the first days in ICU are scarce. One RCT (N = 305) starting supplemental parenteral nutrition in patients not tolerating 60% or more of measured energy needs via enteral route by day 4 after ICU admission did not show harm as compared with withholding supplemental parenteral nutrition until day 9 [44]. In this RCT, supplemental parenteral nutrition led to significantly less infections between day 9 and day 28, although this potential late protective effect was counteracted by more infections between day 4 and day 9 [45].
Fig. 1.
Evolution in nutritional practice in relation to large-scale randomized controlled trials. This graph presents multivariable regression of the start day of EN alone, PN alone, and EN in combination with PN from 2007 to 2018 in Europe, with 2007 as the reference year. Studies potentially influencing the actual dynamics of feeding practices are shown in the left. Data based on NutritionDay (16,032 patients admitted to 1389 intensive care units ([43], reproduced with permission). The reference (zero days) is set at the intercept of a multivariate model for year 2007 and does not represent real days since admission. EN enteral nutrition, PN parenteral nutrition, x axis days, y axis years
In response to RCTs performed in the last decade, international nutritional guidelines have changed. European guidelines have shifted toward recommending less aggressive nutrition in the acute phase (Table 2) [40]. Whereas the 2006/2009 guidelines recommended to start enteral nutrition within 24 h and to reach the caloric target (25 kcal/kg/day) within 2–3 days [46, 47], the 2019 and partially updated 2023 guidelines recommend to initiate low-dose enteral nutrition within 48 h after ICU admission, unless contraindicated, and to advance toward energy target within 3–7 days [40]. If enteral nutrition is insufficient, parenteral nutrition is suggested to be initiated between days 4 and 7 instead of within the first 2 days [40, 47] With regard to protein doses, a grade B recommendation to infuse protein at 1.3–1.5 g/kg/day was reformulated into a grade 0 recommendation stating that 1.3 g/kg/day can be delivered progressively [40, 47]
Table 2.
Comparison of European and American nutrition guidelines, and considerations for future recommendations
| European guidelines (2019; partially updated in 2023) [37] | American guidelines (2022) [46] | Considerations for future recommendations | |
|---|---|---|---|
| Primary feeding route | Enteral (grade A recommendation) | Enteral or parenteral (strong recommendation) | Probably enteral |
| Feeding dose, time of initiation and mode of progression of nutrition | Early initiation of enteral nutrition (within 48 h) (grade B recommendation). Start with hypocaloric nutrition (grade B recommendation), progressive increase toward target within 3–7 days (grade A recommendation) | 12–25 kcal/kg/day during the first 7–10 days; dose depending on clinical judgement (weak recommendation) | Avoid full feeding in the first days. The ideal time point of initiation and mode of progression of nutrition is unknown (to be studied). Suggestion to initiate medical nutrition support progressively and to accept below-target feeding for at least several days. Suggestion to alter nutritional intake depending on clinical evolution (higher doses and faster progression in recovering patients, temporary tapering in case of new severe insult), and to consider also non-nutritional calories when calculating the energy intake |
| Timing of supplemental parenteral nutrition (in case of insufficient enteral nutrition) | Case-by-case evaluation (grade GPP); suggestion to initiate supplemental parenteral nutrition between day 4 and 7 | No supplemental parenteral nutrition prior to day 7 (strong recommendation) | Below-target feeding can be accepted for at least several days. The ideal time point to start supplemental parenteral nutrition is unclear and likely varies between patients, depending on the duration of anabolic resistance. Harm by too early initiation of supplemental parenteral nutrition is dose-related rather than route-related |
| Protein target | 1.3 g/kg can be delivered progressively (grade 0 recommendation) | 1.2–2 g/kg/day (weak recommendation) | Avoid high protein doses in the acute phase. Ideal dose in the post-acute phase unclear (to be studied) |
Also the American nutritional guidelines changed over time, yet to a different extent. Whereas the 2009 guidelines recommended early initiation of enteral nutrition, and no medical nutrition therapy if enteral nutrition is not feasible [26], the 2022 guidelines recommend either enteral or parenteral nutrition as primary feeding modality in the first 7–10 days [48]. Despite a lower suggested feeding target than in the 2009 guidelines (12–25 kcal/kg/day in the first 7–10 days instead of 25–30 kcal/kg/day), the wider range still includes the full target immediately after ICU admission [26, 48]. However, this recommendation was made before Nutrirea-3 RCT results were available, which showed harm by such high energy dose started early after ICU admission when compared to a dose twice lower than the lowest dose recommended by the American guidelines. If enteral nutrition is insufficient, the latest guidelines still recommend no supplemental parenteral nutrition prior to day 7 [48]. Regarding the protein target, the American guidelines contain a weak recommendation to administer 1.2–2 g protein/kg/day. However, this suggestion was made before the EFFORT Protein RCT was published [37], and one could anticipate that an updated version of the guidelines will no longer suggest such high doses.
The differences between the European and American guidelines reflect the persistent uncertainty regarding the optimal nutritional strategy in critically ill patients. Indeed, since recent large-scale RCTs were mainly negative, it has become clear which feeding strategies should be avoided, but not what one should do. We suggest that the varying level of evidence should be better reflected in the guidelines. In this regard, we suggest that guidelines contain strong recommendations what to avoid, and that suggestions what to do could be more imprecise. It has become clear that high doses of all macronutrients should be avoided in the acute phase. However, the duration of the acute phase remains unclear. This is illustrated by differences between the European and American guidelines, which allow below-target feeding for 3–7 days, or for maximum 7–10 days, respectively [40, 48]. Differences between international guidelines may reflect different appreciations of the potential harm by a long period of relative starvation, versus the potential harm by starting full nutritional support too early. Also within guidelines, some recommendations may be confusing. Whereas the American guidelines allow early feeding doses up to 25 kcal/kg/day—which may need to be updated after Nutrirea-3—and suggest either enteral or parenteral nutrition as primary feeding modality, there is a strong recommendation against supplemental parenteral nutrition during the first 7 days. As of today, the optimal nutritional dose and feeding regimen in the acute phase and beyond remain unclear, as well as the ideal time point to start medical nutrition therapy. No RCT has evaluated whether low-dose feeding in the acute phase is superior as compared with progressive medical nutrition therapy, intermediate-dose feeding, or even no nutrition.
In addition, no large RCT has studied whether early enteral nutrition is actually superior to delayed enteral nutrition [49]. When medical nutrition therapy is initiated, however, we suggest that enteral nutrition is the primary feeding modality, unless contraindications. Although RCTs have not shown superiority of the enteral feeding route as compared with the parenteral route, the intervention window was short. When administered for a prolonged period of time, parenteral nutrition by itself can induce morbidity, as observed in patients with short bowel [50]. Moreover, costs of enteral nutrition are lower. A potential pragmatic feeding strategy is suggested in Fig. 2.
Fig. 2.
Suggested pragmatic feeding strategy for ICU patients. In the hyperacute phase of critical illness, no nutrition is necessary. In patients developing spontaneous hypoglycemia, intravenous glucose is initiated to treat hypoglycemia. After initial stabilization, EN is started, when possible, considered “early” if started within 48 h. If EN is not possible and no non-nutritional energy is provided, low-dose glucose may need to be considered. If tolerated, EN is progressively increased toward target over several days. If EN is not possible or insufficient, PN should likely be initiated between days 4 and 8, and progressively increased toward target. The exact duration of the different phases shown in the figure is not known and likely varies individually. As full feeding should be avoided in the first days after ICU admission, it seems prudent to ensure sufficient micronutrient intake by maintenance doses of micronutrients, provided as long as the patient does not receive sufficient macronutrient intake via oral or enteral nutrition (unlike PN, standard commercial EN formulations contain micronutrients). If a patient develops hypophosphatemia upon initiation or increase of feeding, temporarily reducing macronutrient intake while correcting electrolyte abnormalities (in particular potassium and phosphate) is advised. Similarly, in patients developing a new severe insult (e.g., a new septic shock) during ICU stay while receiving full feeding, temporarily reducing or stopping macronutrient intake seems prudent. 1Reasons to delay EN [40]. 2No nutrition if high non-nutritional intake (e.g., propofol, citrate, glucose-containing solutions). 3Consider non-nutritional intake. 4A drop in phosphate by at least 0.16 mmol/l to below 0.65 mmol/l after initiating medical nutrition therapy. 5Coverage of increased basal needs according to ESPEN micronutrient guidelines [51]. EN enteral nutrition, PN parenteral nutrition, ICU intensive care unit
Micronutrient administration in critical illness
Without external replenishment, most micronutrient stores become depleted within weeks to months in healthy persons [51]. In critically ill patients, micronutrient stores could decrease much faster. Additionally, patients may be malnourished upon ICU admission, and have increased micronutrient utilization and increased losses bodily fluids and continuous renal replacement therapy [52, 53]. Moreover, the recent shift in feeding guidelines toward more restrictive feeding in the acute phase may contribute to development of micronutrient deficiencies. Micronutrients are crucial for vital functions such as ATP production, antioxidant and immune defenses, gene transcription and as cofactors for numerous enzymes [54]. Symptoms of micronutrient deficiencies are unspecific and resemble those of critical illness [55]. They may only become unmasked when medical nutrition therapy is started, manifesting as refeeding syndrome. Indeed, refeeding increases the need and intracellular uptake of several micronutrients (especially thiamine, but also other B-vitamins and trace elements) and electrolytes (predominantly phosphate and potassium). In case of low total body stores, this may lead to life-threatening arrhythmias, cardiac depression, lactic acidosis and severe muscle weakness. There are no validated standard criteria for diagnosing refeeding syndrome. A drop in phosphate levels by at least 0.16 mmol/l to below 0.65 mmol/l has been used as possible surrogate [56, 57]. The risk of refeeding is increased when transitioning from prolonged fasting or prolonged low-dose feeding to full feeding. In the Refeeding RCT (N = 339), temporarily restricting macronutrient intake in patients developing refeeding hypophosphatemia associated with decreased mortality as compared with continuing and increasing nutritional intake, while electrolytes were corrected with similar efficiency in both groups [57]. These data support cautious build-up of medical nutrition therapy in patients at risk of refeeding syndrome. Whether the risk of refeeding could be reduced with progressive medical nutrition therapy and/or by administering sufficient amounts of micronutrients and electrolytes in the acute phase to prevent micronutrient and electrolyte deficiencies has not been confirmed in studies. Nevertheless, administration of maintenance doses of micronutrients appears prudent. Ideal doses remain unclear, however. Plasma micronutrient concentrations cannot guide therapy, since they are also affected by redistribution due to inflammation. Hence, plasma micronutrient concentrations not necessarily reflect body stores and no accurate correction formulas for inflammation are available [58]. When the patient receives 1500 kcal via commercial enteral nutrition formula, this contains enough micronutrients to cover DRI (Dietary Reference Intake) for healthy people [51]. However, critically ill patients may have higher requirements to cover basal needs. Recommendations for presumed optimal intakes can be found in the ESPEN micronutrient guideline published in 2022 [51]. The suggested maintenance doses for critically ill patients are generally based on low-level evidence. Apart from maintenance doses, also much higher pharmacological doses have been suggested for individual micronutrients including vitamin C, vitamin D and selenium. Such practice should probably be separated from nutritional interventions and considered as pharmacological intervention aiming for a therapeutic effect of a drug. However, administration of such high pharmacological dose of any micronutrient has not been shown beneficial and is not recommended [51, 59].
Mechanisms explaining lack of benefit of early high-dose nutrition
Several mechanisms may explain the lack of benefit of early high-dose nutrition, including anabolic resistance (inability to use nutrients for anabolism), suppression of cellular repair processes including autophagy and ketogenesis, and aggravation of stress hyperglycemia and insulin need (see Fig. 3) [4]. It appears that in acute critical illness, macronutrients cannot be used as in health [4]. Although medical nutrition therapy has been promoted to limit endogenous catabolism, recent evidence supports the opposite. Indeed, increased feeding intake did not prevent muscle loss in the EPaNIC RCT [13, 60]. Moreover, several RCTs showed increased ureagenesis by increased protein or amino acid intake, suggesting futile catabolism of the extra provided amino acids [16, 34, 37, 38, 61–63].
Fig. 3.
Potential mechanisms for the lack of benefit by early full feeding in critical illness. This figure is a reproduction and adaptation from [4], under the Creative Commons Attribution 4.0 International License, (http://creativecommons.org/licenses/by/4.0/)
Medical nutrition therapy also powerfully suppresses autophagy and ketogenesis, which may be detrimental [4]. Indeed, autophagy is an important cellular recovery process that is able to clear macromolecular damage including damaged cell organelles, protein aggregates and intracellular microorganisms [64, 65]. Increasing evidence has implicated activated autophagy as crucial pathway for recovery from critical illness-induced organ failure [64]. In normal physiology, autophagy is powerfully suppressed by feeding and insulin, and experimental studies confirm autophagy suppression by enhanced nutrition in critical illness [13, 64, 66]. In a subset of patients included in the EPaNIC RCT, muscular autophagy suppression by early parenteral nutrition associated with increased weakness, confirming the functional relevance of these findings [13]. Also suppression of ketogenesis has been implicated as potential mediator of harm by early high-dose nutrition. Apart from being an efficient energy source, ketones may stimulate autophagy and muscle regeneration, and have anti-inflammatory effects [67]. Experimental studies have shown that exogenous ketone administration protected septic mice against muscle weakness [68, 69]. In a secondary analysis of the EPaNIC and PEPaNIC RCTs, withholding early parenteral nutrition activated ketogenesis, especially in critically ill children, in whom the effect on ketogenesis statistically mediated part of the outcome benefit of the intervention [70, 71]. Hence, anorexia and enteral feeding intolerance could be adaptive in acute critical illness by activating cellular repair processes induced by relative fasting.
Early medical nutrition therapy also increases the degree of (stress) hyperglycemia, which is associated with poor outcome, although the ideal blood glucose target remains debated [72]. In contrast to the initial Leuven RCTs that showed morbidity and mortality benefit by tight glucose control in patients receiving early parenteral nutrition (N = 3448) [73–75], the recent TGC-fast RCT (N = 9230) in patients not receiving early parenteral nutrition did not show a benefit on mortality, although secondary endpoints suggested a potential benefit on morbidity [76]. A striking difference between the initial Leuven RCTs and the TGC-fast RCT is the much lower severity of hyperglycemia in the TGC-fast RCT, explained by considerably lower energy provision due to the omission of early parenteral nutrition [76]. Hence, excess mortality in the original Leuven RCTs seems explained by more severe hyperglycemia evoked by early parenteral nutrition that led to overfeeding in the first week [76, 77]. Mechanistic studies have attributed harm by severe iatrogenic hyperglycemia to glucose overload in vital organs, and not to lack of insulin effect [78]. Indeed, insulin by itself may have unwanted side effects on organ recovery, as insulin is a powerful suppressor of autophagy and ketogenesis [79, 80]. This may explain why lower glucose levels independently associated with improved outcome in RCTs, whereas increased insulin doses independently associated with harm [81]. Hence, avoiding early full feeding may be beneficial by avoiding iatrogenic severe hyperglycemia and by lowering the insulin need.
At present, no validated metabolic monitor can identify the time point when anabolic resistance switches into feeding responsiveness. Potential signs of overfeeding, including hyperglycemia and increased insulin need, hyperuremia, increased urea-over-creatinine ratio, and hypertriglyceridemia, are non-specific [82]. Moreover, also underfeeding could lead to hyperuremia and an increased urea-over-creatinine ratio [83]. Indirect calorimetry does not determine the amount of endogenous energy production that is not suppressible by nutrition [4]. Measurements of resting energy expenditure (REE) using indirect calorimetry after the acute phase could be helpful to guide the dose of nutrition; however, validation is needed, as well as certain expertise to interpret the results beyond one value of REE only [84]. It must be borne in mind that overfeeding leads to increased REE (not reflecting the actual needs) due to increased diet-induced thermogenesis which could erroneously prompt physicians to increase the nutritional dose, whereas underfeeding does not lead to decreased REE. Likely, the duration of anabolic resistance and of undesirable effects of nutritional support differ between patients, as the untoward response to feeding likely accompanies the acute stress response and its associated inflammatory and endocrine changes [4]. In the absence of a metabolic monitor and in view of the above insights, it may be prudent to temporarily restrict macronutrients again in patients confronted with a new severe insult in ICU, including de novo septic shock occurring later in ICU stay.
Future perspectives
Although recent evidence has shown benefits associated with relative fasting in acute critical illness, prolonged fasting will likely come at a price. Large nutritional RCTs have focused on initiation of medical nutrition therapy in the first days, and the intervention window was often restricted to the first week in ICU. In the RCTs with a longer intervention window, any benefit of a higher nutritional dose in a post-acute phase may have been counteracted by harm in the acute phase. Future RCTs should focus on interventions initiated beyond the acute phase, and interventions that extend into the recovery period [4]. In this regard, a RCT in hospitalized, non-critically ill patients (N = 2088) found mortality benefit by protocol-guided individualized nutritional support as compared with standard feeding, suggesting that optimized nutrition may improve hard clinical endpoints when provided at the right dose to the right patient [85]. Yet, feeding optimization in this RCT was mainly achieved by optimizing oral intake, which is hardly possible in most ICU patients. Moreover, the protocol also ensured sufficient micronutrient intake in the intervention group. Hence, it remains unclear whether mortality benefit is explained by optimizing macronutrient intake, avoiding micronutrient deficiencies, or both. As consequences of over- and underfeeding are not readily visible, future RCTs should include outcomes beyond ICU discharge. Also, there may be an interaction between optimizing nutritional intake in the post-acute phase and exercise, which needs further study [86, 87]. Future studies should explore whether indirect calorimetry may be helpful to guide energy dosing after the acute phase of critical illness, and evaluate the respiratory quotient as a variable to differentiate between over- and underfeeding, as suggested by some experts [84]. Also macronutrient composition has been scarcely studied, yet likely important based on physiology and indirect evidence. A metabolic monitor allowing monitoring of utilization of nutrients during its provision would be warranted to develop and validate nutritional interventions based on metabolic responses to feeding [4]. Scores monitoring gastrointestinal dysfunction should also be validated in future studies, as these may be useful in exploring this aspect in a complex response to enteral nutrition (EN).
As mechanistic studies suggest benefit by activating fasting responses in critical illness [4], future research should investigate whether these fasting-associated benefits could be exploited in novel feeding strategies. In this regard, intermittent fasting/feeding strategies, ketogenic diets and ketone supplementation have been suggested as alternative strategies [67]. Alternating feeding periods with fasting intervals could intermittently activate a fasting response with increased autophagy and ketogenesis, while avoiding prolonged starvation [67]. Intermittent fasting also improved insulin sensitivity in non-critically ill humans and animals [88]. Moreover, intermittent amino acid provision has been suggested to be more anabolic than continuous amino acid provision by avoiding the so-called muscle-full effect [89–91]. However, large-scale RCT evidence confirming the efficacy and safety of intermittent fasting/feeding, ketone supplementation and ketogenic diets is lacking. RCTs that studied intermittent versus continuous medical nutrition therapy have not shown consistent benefit [92, 93]. However, apart from a lack of power to detect or exclude a benefit, the fasting interval was relatively short in these RCTs (in general only 4–6 h), which may have been too short to activate a full fasting response [94]. Although ketogenic diets have been successfully used in refractory status epilepticus, RCT evidence that would support widespread use in critically ill patients is not available [67, 95]. Moreover, achieving ketosis can be cumbersome, as a considerable number of medication contains carbohydrate compounds [96].
With regard to the micronutrients, future research could aim at unraveling the individual basal needs. Most recommendations regarding coverage of basal needs are Good Clinical Practice Points [51]. Improving estimation of real losses and micronutrient status could help to optimize maintenance dosing.
Conclusions
Large-scale RCT evidence has shown harm by high macronutrient doses via any route during the acute phase of the critical illness, which may be explained by anabolic resistance, suppression of autophagy and ketogenesis, and overfeeding with more severe hyperglycemia and insulin need. The time point when anabolic resistance switches into feeding responsiveness remains unclear. Therefore, personalized medical nutrition therapy, even though desirable, is currently not feasible. Validated tools that monitor actual energy, protein and micronutrient needs and potentially allow minimization of both over- and underfeeding, are warranted. As postponing full nutrition may increase the risk of micronutrient deficiencies, and subsequent refeeding syndrome, it seems prudent to cover basal micronutrient needs in all critically ill patients.
Acknowledgements
ARB holds a grant from Estonian Research Council (PRG1255). JG is granted a senior clinical investigator fellowship by Research Foundation—Flanders (1842724N), and has obtained research funding by grants from KU Leuven (STG/23/032) and the European Society of Intensive Care Medicine (Fundamental Research Award 2022).
Author contributions
All three authors equally contributed and approved all versions.
Declarations
Conflicts of interest
ARB has received speaker or consultancy fees from Nestle, Fresenius Kabi, Nutricia and VIPUN Medical. AdM has received a grant from the Netherlands Organisation for Health Research and Development to perform an RCT investigating high-dose vitamin C in patient post cardiac arrest. In addition, she received several reimbursements from congress organizations for travel and hotel expenses as a speaker, JG does not have conflicts of interest related to this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Angelique M. E. de Man, Jan Gunst, and Annika Reintam Blaser have contributed equally to the manuscript.
References
- 1.Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46(4):637–653. doi: 10.1007/s00134-020-05944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux RNM, Delarue J, Berger MM. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24(4):502–509. doi: 10.1016/j.clnu.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Lew CCH, Wong GJY, Cheung KP, Chua AP, Chong MFF, Miller M. Association between malnutrition and 28-day mortality and intensive care length-of-stay in the critically ill: a prospective cohort study. Nutrients. 2017 doi: 10.3390/nu10010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunst J, Casaer MP, Preiser JC, Reignier J, Van den Berghe G. Toward nutrition improving outcome of critically ill patients: how to interpret recent feeding RCTs? Crit Care. 2023;27(1):43. doi: 10.1186/s13054-023-04317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 6.Reignier J, Boisrame-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, Argaud L, Asehnoune K, Asfar P, Bellec F, Botoc V, Bretagnol A, Bui HN, Canet E, Da Silva D, Darmon M, Das V, Devaquet J, Djibre M, Ganster F, Garrouste-Orgeas M, Gaudry S, Gontier O, Guerin C, Guidet B, Guitton C, Herbrecht JE, Lacherade JC, Letocart P, Martino F, Maxime V, Mercier E, Mira JP, Nseir S, Piton G, Quenot JP, Richecoeur J, Rigaud JP, Robert R, Rolin N, Schwebel C, Sirodot M, Tinturier F, Thevenin D, Giraudeau B, Le Gouge A, Investigators N-T, Clinical Research in Intensive C, Sepsis g Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2) Lancet. 2018;391(10116):133–143. doi: 10.1016/S0140-6736(17)32146-3. [DOI] [PubMed] [Google Scholar]
- 7.Reignier J, Plantefeve G, Mira JP, Argaud L, Asfar P, Aissaoui N, Badie J, Botoc NV, Brisard L, Bui HN, Chatellier D, Chauvelot L, Combes A, Cracco C, Darmon M, Das V, Debarre M, Delbove A, Devaquet J, Dumont LM, Gontier O, Groyer S, Guerin L, Guidet B, Hourmant Y, Jaber S, Lambiotte F, Leroy C, Letocart P, Madeux B, Maizel J, Martinet O, Martino F, Maxime V, Mercier E, Nay MA, Nseir S, Oziel J, Picard W, Piton G, Quenot JP, Reizine F, Renault A, Richecoeur J, Rigaud JP, Schneider F, Silva D, Sirodot M, Souweine B, Tamion F, Terzi N, Thevenin D, Thiery G, Thieulot-Rolin N, Timsit JF, Tinturier F, Tirot P, Vanderlinden T, Vinatier I, Vinsonneau C, Voicu S, Lascarrou JB, Le Gouge A, Investigators N-T, Clinical Research in Intensive C, Sepsis G Low versus standard calorie and protein feeding in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3) Lancet Respir Med. 2023;11(7):602–612. doi: 10.1016/S2213-2600(23)00092-9. [DOI] [PubMed] [Google Scholar]
- 8.Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, Debaveye Y, Vlasselaers D, Desmet L, Casaer MP, Garcia Guerra G, Hanot J, Joffe A, Tibboel D, Joosten K, Van den Berghe G. Early versus late parenteral nutrition in critically ill children. N Engl J Med. 2016;374(12):1111–1122. doi: 10.1056/NEJMoa1514762. [DOI] [PubMed] [Google Scholar]
- 9.Wischmeyer PE, Bear DE, Berger MM, De Waele E, Gunst J, McClave SA, Prado CM, Puthucheary Z, Ridley EJ, Van den Berghe G, van Zanten ARH. Personalized nutrition therapy in critical care: 10 expert recommendations. Crit Care. 2023;27(1):261. doi: 10.1186/s13054-023-04539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reintam Blaser A, Rooyackers O, Bear DE. How to avoid harm with feeding critically ill patients: a synthesis of viewpoints of a basic scientist, dietitian and intensivist. Crit Care. 2023;27(1):258. doi: 10.1186/s13054-023-04543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, Heyland DK. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35(10):1728–1737. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 12.Lv C, Jiang X, Long Y, Liu Z, Lin J, Wu C, Ye X, Ye R, Liu Y, Liu M, Liu Y, Chen W, Gao L, Tong Z, Ke L, Jiang Z, Li W. Association between caloric adequacy and short-term clinical outcomes in critically ill patients using a weight-based equation: secondary analysis of a cluster-randomized controlled trial. Front Nutr. 2022;9:902986. doi: 10.3389/fnut.2022.902986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans G, Casaer MP, Clerckx B, Guiza F, Vanhullebusch T, Derde S, Meersseman P, Derese I, Mesotten D, Wouters PJ, Van Cromphaut S, Debaveye Y, Gosselink R, Gunst J, Wilmer A, Van den Berghe G, Vanhorebeek I. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med. 2013;1(8):621–629. doi: 10.1016/S2213-2600(13)70183-8. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs A, Dulfer K, Eveleens RD, Hordijk J, Van Cleemput H, Verlinden I, Wouters PJ, Mebis L, Guerra GG, Joosten K, Verbruggen SC, Guiza F, Vanhorebeek I, Van den Berghe G. Long-term developmental effect of withholding parenteral nutrition in paediatric intensive care units: a 4-year follow-up of the PEPaNIC randomised controlled trial. Lancet Child Adolesc Health. 2020;4(7):503–514. doi: 10.1016/S2352-4642(20)30104-8. [DOI] [PubMed] [Google Scholar]
- 15.Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med. 2013;187(3):247–255. doi: 10.1164/rccm.201206-0999OC. [DOI] [PubMed] [Google Scholar]
- 16.Vanhorebeek I, Verbruggen S, Casaer MP, Gunst J, Wouters PJ, Hanot J, Guerra GG, Vlasselaers D, Joosten K, Van den Berghe G. Effect of early supplemental parenteral nutrition in the paediatric ICU: a preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir Med. 2017;5(6):475–483. doi: 10.1016/S2213-2600(17)30186-8. [DOI] [PubMed] [Google Scholar]
- 17.Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, Bellingan G, Leonard R, Mythen MG, Rowan KM, Investigators CT. Trial of the route of early nutritional support in critically ill adults. N Engl J Med. 2014;371(18):1673–1684. doi: 10.1056/NEJMoa1409860. [DOI] [PubMed] [Google Scholar]
- 18.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, Mehta S, McIntyre L, Solaiman O, Sakkijha MH, Sadat M, Afesh L, Permi TTG. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N Engl J Med. 2015;372(25):2398–2408. doi: 10.1056/NEJMoa1502826. [DOI] [PubMed] [Google Scholar]
- 20.Target Investigators ftACTG. Chapman M, Peake SL, Bellomo R, Davies A, Deane A, Horowitz M, Hurford S, Lange K, Little L, Mackle D, O'Connor S, Presneill J, Ridley E, Williams P, Young P. Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med. 2018;379(19):1823–1834. doi: 10.1056/NEJMoa1811687. [DOI] [PubMed] [Google Scholar]
- 21.Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, Wozniak AW, Colantuoni E, Ely EW, Rice TW, Hopkins RO, Network NNA. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188(5):567–576. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, Hopkins RO, Network NNA. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532. doi: 10.1136/bmj.f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deane AM, Little L, Bellomo R, Chapman MJ, Davies AR, Ferrie S, Horowitz M, Hurford S, Lange K, Litton E, Mackle D, O'Connor S, Parker J, Peake SL, Presneill JJ, Ridley EJ, Singh V, van Haren F, Williams P, Young P, Iwashyna TJ. Outcomes six months after delivering 100% or 70% of enteral calorie requirements during critical illness (TARGET). A randomized controlled trial. Am J Respir Crit Care Med. 2020;201(7):814–822. doi: 10.1164/rccm.201909-1810OC. [DOI] [PubMed] [Google Scholar]
- 24.Arabi YM, Aldawood AS, Al-Dorzi HM, Tamim HM, Haddad SH, Jones G, McIntyre L, Solaiman O, Sakkijha MH, Sadat M, Mundekkadan S, Kumar A, Bagshaw SM, Mehta S, Permi T. Permissive underfeeding or standard enteral feeding in high- and low-nutritional-risk critically ill adults. Post hoc analysis of the PermiT Trial. Am J Respir Crit Care Med. 2017;195(5):652–662. doi: 10.1164/rccm.201605-1012OC. [DOI] [PubMed] [Google Scholar]
- 25.Yue HY, Peng W, Zeng J, Zhang Y, Wang Y, Jiang H. Efficacy of permissive underfeeding for critically ill patients: an updated systematic review and trial sequential meta-analysis. J Intensive Care. 2024;12(1):4. doi: 10.1186/s40560-024-00717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G, Directors ASPENBo, American College of Critical Care M, Society of Critical Care M Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) JPEN J Parenter Enteral Nutr. 2009;33(3):277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 27.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Oczkowski S, Szczeklik W, Bischoff SC. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, Kahoul SH, Brits R. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr. 2011;93(3):569–577. doi: 10.3945/ajcn.110.005074. [DOI] [PubMed] [Google Scholar]
- 29.Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med. 2011;39(5):967–974. doi: 10.1097/CCM.0b013e31820a905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charles EJ, Petroze RT, Metzger R, Hranjec T, Rosenberger LH, Riccio LM, McLeod MD, Guidry CA, Stukenborg GJ, Swenson BR, Willcutts KF, O'Donnell KB, Sawyer RG. Hypocaloric compared with eucaloric nutritional support and its effect on infection rates in a surgical intensive care unit: a randomized controlled trial. Am J Clin Nutr. 2014;100(5):1337–1343. doi: 10.3945/ajcn.114.088609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petros S, Horbach M, Seidel F, Weidhase L. Hypocaloric vs normocaloric nutrition in critically ill patients: a prospective randomized pilot trial. JPEN J Parenter Enteral Nutr. 2016;40(2):242–249. doi: 10.1177/0148607114528980. [DOI] [PubMed] [Google Scholar]
- 32.Braunschweig CA, Sheean PM, Peterson SJ, Gomez Perez S, Freels S, Lateef O, Gurka D, Fantuzzi G. Intensive nutrition in acute lung injury: a clinical trial (INTACT) JPEN J Parenter Enteral Nutr. 2015;39(1):13–20. doi: 10.1177/0148607114528541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rugeles S, Villarraga-Angulo LG, Ariza-Gutierrez A, Chaverra-Kornerup S, Lasalvia P, Rosselli D. High-protein hypocaloric vs normocaloric enteral nutrition in critically ill patients: a randomized clinical trial. J Crit Care. 2016;35:110–114. doi: 10.1016/j.jcrc.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Allingstrup MJ, Kondrup J, Wiis J, Claudius C, Pedersen UG, Hein-Rasmussen R, Bjerregaard MR, Steensen M, Jensen TH, Lange T, Madsen MB, Moller MH, Perner A. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017;43(11):1637–1647. doi: 10.1007/s00134-017-4880-3. [DOI] [PubMed] [Google Scholar]
- 35.Hoffer LJ, Bistrian BR. Nutrition in critical illness: a current conundrum. F1000Res. 2016;5:2531. doi: 10.12688/f1000research.9278.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartl WH, Elke G. Nutrition during the acute phase of critical illness: discussions on NUTRIREA-3. Lancet Respir Med. 2023;11(7):e61. doi: 10.1016/S2213-2600(23)00212-6. [DOI] [PubMed] [Google Scholar]
- 37.Heyland DK, Patel J, Compher C, Rice TW, Bear DE, Lee ZY, Gonzalez VC, O'Reilly K, Regala R, Wedemire C, Ibarra-Estrada M, Stoppe C, Ortiz-Reyes L, Jiang X, Day AG, team EPT The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): an international, multicentre, pragmatic, registry-based randomised trial. Lancet. 2023;401(10376):568–576. doi: 10.1016/S0140-6736(22)02469-2. [DOI] [PubMed] [Google Scholar]
- 38.Doig GS, Simpson F, Bellomo R, Heighes PT, Sweetman EA, Chesher D, Pollock C, Davies A, Botha J, Harrigan P, Reade MC. Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med. 2015;41(7):1197–1208. doi: 10.1007/s00134-015-3827-9. [DOI] [PubMed] [Google Scholar]
- 39.Lee ZY, Dresen E, Lew CCH, Bels J, Hill A, Hasan MS, Ke L, van Zanten A, van de Poll MCG, Heyland DK, Stoppe C. The effects of higher versus lower protein delivery in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Crit Care. 2024;28(1):15. doi: 10.1186/s13054-023-04783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, Mayer K, Montejo-Gonzalez JC, Pichard C, Preiser JC, Szczeklik W, van Zanten ARH, Bischoff SC. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin Nutr. 2023;42(9):1671–1689. doi: 10.1016/j.clnu.2023.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Pertzov B, Bar-Yoseph H, Menndel Y, Bendavid I, Kagan I, Glass YD, Singer P. The effect of indirect calorimetry guided isocaloric nutrition on mortality in critically ill patients-a systematic review and meta-analysis. Eur J Clin Nutr. 2022;76(1):5–15. doi: 10.1038/s41430-021-00919-0. [DOI] [PubMed] [Google Scholar]
- 42.Singer P, De Waele E, Sanchez C, Ruiz Santana S, Montejo JC, Laterre PF, Soroksky A, Moscovici E, Kagan I. TICACOS international: A multi-center, randomized, prospective controlled study comparing tight calorie control versus Liberal calorie administration study. Clin Nutr. 2021;40(2):380–387. doi: 10.1016/j.clnu.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Veraar C, Geilen J, Fischer A, Sulz I, Tarantino S, Mouhieddine M, Mora B, Schuh C, Singer P, Hiesmayr MJ. Timing of parenteral nutrition in ICU patients: a transatlantic controversy. Clin Nutr ESPEN. 2021;46:532–538. doi: 10.1016/j.clnesp.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, Thibault R, Pichard C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381(9864):385–393. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 45.Heidegger CP, Berger MM, Thibault R, Zingg W, Pichard C. Supplemental parenteral nutrition in critically ill patients–authors' reply. Lancet. 2013;381(9879):1716–1717. doi: 10.1016/S0140-6736(13)61072-7. [DOI] [PubMed] [Google Scholar]
- 46.Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, Dgem EC, Hartl W, Heymann C, Spies C, Espen ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25(2):210–223. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C, Espen ESPEN Guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009;28(4):387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 48.Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, McKeever L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2022;46(1):12–41. doi: 10.1002/jpen.2267. [DOI] [PubMed] [Google Scholar]
- 49.Fuentes Padilla P, Martinez G, Vernooij RW, Urrutia G, Roque IFM, Bonfill Cosp X. Early enteral nutrition (within 48 hours) versus delayed enteral nutrition (after 48 hours) with or without supplemental parenteral nutrition in critically ill adults. Cochrane Database Syst Rev. 2019 doi: 10.1002/14651858.CD012340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuglsang KA, Brandt CF, Jeppesen PB. Survival in patients initiating home parenteral support due to nonmalignant short bowel syndrome compared with background population. Clin Nutr ESPEN. 2022;50:170–177. doi: 10.1016/j.clnesp.2022.05.023. [DOI] [PubMed] [Google Scholar]
- 51.Berger MM, Shenkin A, Schweinlin A, Amrein K, Augsburger M, Biesalski HK, Bischoff SC, Casaer MP, Gundogan K, Lepp HL, de Man AME, Muscogiuri G, Pietka M, Pironi L, Rezzi S, Cuerda C. ESPEN micronutrient guideline. Clin Nutr. 2022;41(6):1357–1424. doi: 10.1016/j.clnu.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Koekkoek WA, van Zanten AR. Antioxidant vitamins and trace elements in critical illness. Nutr Clin Pract. 2016;31(4):457–474. doi: 10.1177/0884533616653832. [DOI] [PubMed] [Google Scholar]
- 53.Berger MM, Broman M, Forni L, Ostermann M, De Waele E, Wischmeyer PE. Nutrients and micronutrients at risk during renal replacement therapy: a scoping review. Curr Opin Crit Care. 2021;27(4):367–377. doi: 10.1097/MCC.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koekkoek KWA, Berger MM. An update on essential micronutrients in critical illness. Curr Opin Crit Care. 2023;29(4):315–329. doi: 10.1097/MCC.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 55.Casaer MP, Bellomo R. Micronutrient deficiency in critical illness: an invisible foe? Intensive Care Med. 2019;45(8):1136–1139. doi: 10.1007/s00134-019-05678-y. [DOI] [PubMed] [Google Scholar]
- 56.Doig GS, Simpson F, Heighes PT, Bellomo R, Chesher D, Caterson ID, Reade MC, Harrigan PW, Refeeding Syndrome Trial Investigators G Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med. 2015;3(12):943–952. doi: 10.1016/S2213-2600(15)00418-X. [DOI] [PubMed] [Google Scholar]
- 57.Olthof LE, Koekkoek W, van Setten C, Kars JCN, van Blokland D, van Zanten ARH. Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: a retrospective study. Clin Nutr. 2018;37(5):1609–1617. doi: 10.1016/j.clnu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Berger MM, Talwar D, Shenkin A. Pitfalls in the interpretation of blood tests used to assess and monitor micronutrient nutrition status. Nutr Clin Pract. 2022 doi: 10.1002/ncp.10924. [DOI] [PubMed] [Google Scholar]
- 59.Reintam Blaser A, Alhazzani W, Belley-Cote E, Moller MH, Adhikari NKJ, Burry L, Coopersmith CM, Al Duhailib Z, Fujii T, Granholm A, Gunst J, Hammond N, Ke L, Lamontagne F, Loudet C, Morgan M, Ostermann M, Reinikainen M, Rosenfeld R, Spies C, Oczkowski S. Intravenous vitamin C therapy in adult patients with sepsis: a rapid practice guideline. Acta Anaesthesiol Scand. 2023 doi: 10.1111/aas.14311. [DOI] [PubMed] [Google Scholar]
- 60.Casaer MP, Langouche L, Coudyzer W, Vanbeckevoort D, De Dobbelaer B, Guiza FG, Wouters PJ, Mesotten D, Van den Berghe G. Impact of early parenteral nutrition on muscle and adipose tissue compartments during critical illness. Crit Care Med. 2013;41(10):2298–2309. doi: 10.1097/CCM.0b013e31828cef02. [DOI] [PubMed] [Google Scholar]
- 61.Gunst J, Vanhorebeek I, Casaer MP, Hermans G, Wouters PJ, Dubois J, Claes K, Schetz M, Van den Berghe G. Impact of early parenteral nutrition on metabolism and kidney injury. J Am Soc Nephrol. 2013;24(6):995–1005. doi: 10.1681/ASN.2012070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heyland DK, Wibbenmeyer L, Pollack JA, Friedman B, Turgeon AF, Eshraghi N, Jeschke MG, Belisle S, Grau D, Mandell S, Velamuri SR, Hundeshagen G, Moiemen N, Shokrollahi K, Foster K, Huss F, Collins D, Savetamal A, Gurney JM, Depetris N, Stoppe C, Ortiz-Reyes L, Garrel D, Day AG, Team R-ET A randomized trial of enteral glutamine for treatment of burn injuries. N Engl J Med. 2022;387(11):1001–1010. doi: 10.1056/NEJMoa2203364. [DOI] [PubMed] [Google Scholar]
- 63.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG, Canadian Critical Care Trials G A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368(16):1489–1497. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 64.Vanhorebeek I, Casaer M, Gunst J. Nutrition and autophagy deficiency in critical illness. Curr Opin Crit Care. 2023;29(4):306–314. doi: 10.1097/MCC.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383(16):1564–1576. doi: 10.1056/NEJMra2022774. [DOI] [PubMed] [Google Scholar]
- 66.Derde S, Vanhorebeek I, Guiza F, Derese I, Gunst J, Fahrenkrog B, Martinet W, Vervenne H, Ververs EJ, Larsson L, Van den Berghe G. Early parenteral nutrition evokes a phenotype of autophagy deficiency in liver and skeletal muscle of critically ill rabbits. Endocrinology. 2012;153(5):2267–2276. doi: 10.1210/en.2011-2068. [DOI] [PubMed] [Google Scholar]
- 67.Gunst J, Casaer MP, Langouche L, Van den Berghe G. Role of ketones, ketogenic diets and intermittent fasting in ICU. Curr Opin Crit Care. 2021;27(4):385–389. doi: 10.1097/MCC.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 68.Weckx R, Goossens C, Derde S, Pauwels L, Vander Perre S, Van den Berghe G, Langouche L. Efficacy and safety of ketone ester infusion to prevent muscle weakness in a mouse model of sepsis-induced critical illness. Sci Rep. 2022;12(1):10591. doi: 10.1038/s41598-022-14961-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goossens C, Weckx R, Derde S, Dufour T, Vander Perre S, Pauwels L, Thiessen SE, Van Veldhoven PP, Van den Berghe G, Langouche L. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care. 2019;23(1):236. doi: 10.1186/s13054-019-2506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Bruyn A, Langouche L, Vander Perre S, Gunst J, Van den Berghe G. Impact of withholding early parenteral nutrition in adult critically ill patients on ketogenesis in relation to outcome. Crit Care. 2021;25(1):102. doi: 10.1186/s13054-021-03519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Bruyn A, Gunst J, Goossens C, Vander Perre S, Guerra GG, Verbruggen S, Joosten K, Langouche L, Van den Berghe G. Effect of withholding early parenteral nutrition in PICU on ketogenesis as potential mediator of its outcome benefit. Crit Care. 2020;24(1):536. doi: 10.1186/s13054-020-03256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gunst J, Verbruggen SC. Insulin resistance in critical illness: consequences for nutrition therapy and glucose management. Curr Opin Crit Care. 2023;29(4):286–292. doi: 10.1097/MCC.0000000000001055. [DOI] [PubMed] [Google Scholar]
- 73.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 74.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 75.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373(9663):547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 76.Gunst J, Debaveye Y, Guiza F, Dubois J, De Bruyn A, Dauwe D, De Troy E, Casaer MP, De Vlieger G, Haghedooren R, Jacobs B, Meyfroidt G, Ingels C, Muller J, Vlasselaers D, Desmet L, Mebis L, Wouters PJ, Stessel B, Geebelen L, Vandenbrande J, Brands M, Gruyters I, Geerts E, De Pauw I, Vermassen J, Peperstraete H, Hoste E, De Waele JJ, Herck I, Depuydt P, Wilmer A, Hermans G, Benoit DD, Van den Berghe G, Collaborators TG-F Tight blood-glucose control without early parenteral nutrition in the ICU. N Engl J Med. 2023;389(13):1180–1190. doi: 10.1056/NEJMoa2304855. [DOI] [PubMed] [Google Scholar]
- 77.Umpierrez GE. Glucose control in the ICU. N Engl J Med. 2023;389(13):1234–1237. doi: 10.1056/NEJMe2309442. [DOI] [PubMed] [Google Scholar]
- 78.Gunst J, Van den Berghe G. Blood glucose control in the ICU: don't throw out the baby with the bathwater! Intensive Care Med. 2016;42(9):1478–1481. doi: 10.1007/s00134-016-4350-3. [DOI] [PubMed] [Google Scholar]
- 79.Gunst J. Recovery from critical illness-induced organ failure: the role of autophagy. Crit Care. 2017;21(1):209. doi: 10.1186/s13054-017-1786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gunst J, De Bruyn A, Casaer MP, Vander Perre S, Langouche L, Van den Berghe G. Impact of tight glucose control on circulating 3-hydroxybutyrate in critically ill patients. Crit Care. 2021;25(1):373. doi: 10.1186/s13054-021-03772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 82.Berger MM, Reintam-Blaser A, Calder PC, Casaer M, Hiesmayr MJ, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Bischoff SC, Singer P. Monitoring nutrition in the ICU. Clin Nutr. 2019;38(2):584–593. doi: 10.1016/j.clnu.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 83.Gunst J, Kashani KB, Hermans G. The urea-creatinine ratio as a novel biomarker of critical illness-associated catabolism. Intensive Care Med. 2019;45(12):1813–1815. doi: 10.1007/s00134-019-05810-y. [DOI] [PubMed] [Google Scholar]
- 84.Achamrah N, Delsoglio M, De Waele E, Berger MM, Pichard C (2021) Indirect calorimetry: The 6 main issues. Clin Nutr 40:4–14 [DOI] [PubMed]
- 85.Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, Kutz A, Tribolet P, Bregenzer T, Braun N, Hoess C, Pavlicek V, Schmid S, Bilz S, Sigrist S, Brandle M, Benz C, Henzen C, Mattmann S, Thomann R, Brand C, Rutishauser J, Aujesky D, Rodondi N, Donze J, Stanga Z, Mueller B. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312–2321. doi: 10.1016/S0140-6736(18)32776-4. [DOI] [PubMed] [Google Scholar]
- 86.Kagan I, Cohen J, Bendavid I, Kramer S, Mesilati-Stahy R, Glass Y, Theilla M, Singer P. Effect of combined protein-enriched enteral nutrition and early cycle ergometry in mechanically ventilated critically ill patients—a pilot study. Nutrients. 2022 doi: 10.3390/nu14081589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. 2012;60(1):16–23. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 88.de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 89.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532(Pt 2):575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92(5):1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- 91.Flower L, Haines RW, McNelly A, Bear DE, Koelfat K, Damink SO, Hart N, Montgomery H, Prowle JR, Puthucheary Z. Effect of intermittent or continuous feeding and amino acid concentration on urea-to-creatinine ratio in critical illness. JPEN J Parenter Enteral Nutr. 2022;46(4):789–797. doi: 10.1002/jpen.2258. [DOI] [PubMed] [Google Scholar]
- 92.Van Dyck L, Casaer MP. Intermittent or continuous feeding: any difference during the first week? Curr Opin Crit Care. 2019;25(4):356–362. doi: 10.1097/MCC.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 93.McNelly AS, Bear DE, Connolly BA, Arbane G, Allum L, Tarbhai A, Cooper JA, Hopkins PA, Wise MP, Brealey D, Rooney K, Cupitt J, Carr B, Koelfat K, Damink SO, Atherton PJ, Hart N, Montgomery HE, Puthucheary ZA. Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial. Chest. 2020;158(1):183–194. doi: 10.1016/j.chest.2020.03.045. [DOI] [PubMed] [Google Scholar]
- 94.Van Dyck L, Vanhorebeek I, Wilmer A, Schrijvers A, Derese I, Mebis L, Wouters PJ, Van den Berghe G, Gunst J, Casaer MP. Towards a fasting-mimicking diet for critically ill patients: the pilot randomized crossover ICU-FM-1 study. Crit Care. 2020;24(1):249. doi: 10.1186/s13054-020-02987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.White H, Venkatesh B. Clinical review: ketones and brain injury. Crit Care. 2011;15(2):219. doi: 10.1186/cc10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katz JB, Owusu K, Nussbaum I, Beekman R, DeFilippo NA, Gilmore EJ, Hirsch LJ, Cervenka MC, Maciel CB. Pearls and pitfalls of introducing ketogenic diet in adult status epilepticus: a practical guide for the intensivist. J Clin Med. 2021 doi: 10.3390/jcm10040881. [DOI] [PMC free article] [PubMed] [Google Scholar]