Abstract
The relationship between the triglyceride glucose-body mass index (TyG-BMI) index and Alzheimer’s disease (AD) pathology, cognition, and brain structure remains unclear. This study aimed to investigate these associations, focusing on cerebrospinal fluid (CSF) biomarkers, cognitive measures, and brain imaging data. Eight hundred and fifty-five non-demented participants were included. Linear regression was used to explore associations between the TyG-BMI index and AD pathology, cognition, and brain structure. The association between the TyG-BMI index and AD risk was assessed using Kaplan–Meier and Cox proportional hazards models. Longitudinal relationships were assessed using linear mixed-effects models. Mediation analyses were conducted to examine AD pathology’s potential mediating role between the TyG-BMI index and cognition as well as brain structure. In the linear regression analyses, higher TyG-BMI levels were associated with increased Aβ42 and decreased Tau, pTau, Tau/Aβ42, pTau/Aβ42, and pTau/Tau. Positive correlations were observed with mini-mental state examination (MMSE), memory (MEM), executive function (EF), and the volumes of the hippocampus, entorhinal cortex, and middle temporal regions, while negative correlations were found with Alzheimer’s Disease Assessment Scale (ADAS). Longitudinally, the TyG-BMI index was inversely associated with ADAS, and positively with MMSE, MEM, EF, hippocampus, entorhinal, and middle temporal. High TyG-BMI levels were correlated with lower AD risk (HR 0.996 [0.994, 0.999]). Mediation analyses revealed AD pathology mediated the association between TyG-BMI index and cognition as well as brain structure. Additionally, the TyG-BMI index could mediate cognitive changes by influencing brain structure. The TyG-BMI index is associated with AD pathology, cognition, and brain structure.
Keywords: Alzheimer’s disease, Triglyceride glucose-body mass index, Pathology, Cognition, Brain structure, Insulin resistance
Subject terms: Cognitive ageing, Neurogenesis
Introduction
Alzheimer’s disease (AD) is a prevalent neurodegenerative disorder profoundly impacting cognition and functionality in affected individuals1. With the aging population, AD poses an increasingly significant burden on individuals, families, and society. Research underscores the critical role of metabolic disturbances, such as insulin resistance (IR) and obesity, in the pathogenesis of AD2,3. Insulin resistance, characterized by impaired cellular response to insulin, has emerged as a pivotal risk factor for AD development, disrupting normal neuronal function and precipitating neuroinflammation, amyloid plaque accumulation, and neurofibrillary tangle formation4–6.
Traditional insulin sensitivity assays, such as the insulin tolerance test, are commonly used to assess insulin sensitivity7. However, these methods require stringent laboratory conditions and prolonged monitoring. The triglyceride glucose (TyG) index offers a simpler and more cost-effective alternative for evaluating IR8. Calculated from fasting blood glucose (FBG) and triglyceride (TG), this index provides a comprehensive reflection of metabolic health9. Prior studies have highlighted an association between the TyG index and AD risk, but establishing a causal relationship remains challenging in observational studies10–12. Furthermore, existing research lacks systematic evidence examining all three aspects of AD pathology, cognitive measures, and brain structure.
To better understand the relationship between metabolic dysfunction and AD, we employed the composite indicator triglyceride glucose-body mass index (TyG-BMI) index, which combines the TyG index with BMI, enhancing its predictive power for IR and metabolic syndrome13. By incorporating BMI, which reflects obesity, the TyG-BMI index captures both systemic metabolic disturbances and IR associated with obesity14. Previous studies have shown that the TyG-BMI index has a good concordance with IR assessments such as homeostasis model assessment of insulin resistance, and the area under the curve for evaluating IR is greater with TyG-BMI compared to TyG alone14,15.
However, there is a lack of studies on the association between TyG-BMI index and AD. Our main objective was to examine the associations between the TyG-BMI index and AD pathology, cognition, and brain structure. We hypothesized that TyG-BMI levels would be associated with specific AD pathological biomarkers, such as Aβ42, Tau, and pTau. These pathological changes could potentially affect cognition, including memory and executive function, and result in volume changes in brain regions such as the hippocampus, entorhinal cortex, and middle temporal regions. Additionally, we sought to investigate whether the TyG-BMI index influences cognition and brain structure through its effects on AD pathology.
Methods
Study sample
A total of 855 non-dementia adults were gathered from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. Patients without available BMI, TG, FBG, blood pressure, history of diabetes, history of cardiovascular disease (CVD), AD pathology, global cognition and cognitive domain measures, and dementia were excluded. These participants, ranging in age from 54 to 91 years, provided us with comprehensive data from the ADNI study, including essential clinical characteristics, biochemical biomarkers of AD, imaging data, and cognitive assessment data. More information is available at http://adni.loni.usc.edu.
Measurements of laboratory parameters
The ADNI database provided all laboratory and anthropometric parameters and medical history data. FBG and TG levels were measured. APOEε4 genotyping was conducted at the ADNI Biomarker Core Laboratory, University of Pennsylvania, to identify participants with at least one APOEε4 allele, designating them the APOEε4 positive status16. Height and weight were measured at baseline for all participants. BMI was computed utilizing the equation: body weight (kilograms) divided by height (meters)2. The TyG-BMI index was calculated applying the following formula: ln [FBG (mg/dL) × TG (mg/dL)/2] × BMI14. The included population lacked a diagnosis of diabetes mellitus and hypertension, and we used FBG ≥ 126 mg/dL as the basis for diabetes mellitus17, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg as the basis for the diagnosis of hypertension18.
Definition of incident AD and cognitive measures
AD diagnosis was confirmed in individuals meeting the established criteria for probable AD set by the US National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria19. The ADNI utilized various scales to evaluate cognitive abilities, encompassing global cognition assessed through the Mini-Mental State Examination (MMSE) and Alzheimer’s Disease Assessment Scale (ADAS), along with specific cognitive domains like executive function (EF) and memory function (MEM)20.
Measurements of AD biomarkers
Cerebrospinal fluid (CSF) specimens were obtained via lumbar puncture and promptly transferred to 10-mL polypropylene tubes. Within a two-hour window, these samples underwent transportation to the laboratory. Subsequently, the CSF samples underwent centrifugation at 2000 g for 10 min. Samples not detected in time for thawing and freezing procedures did not exceed two cycles prior to analysis16. The INNO-BIA AlzBio3 immunoassay (Innogenetics-Fujirebio, Ghent, Belgium) was employed to quantify CSF concentrations of Aβ42, Tau, and pTau (pg/mL). We used Tau/Aβ42 and pTau/Aβ42 because they are better predictors of brain Aβ42 deposition and cognitive decline than tau and pTau expressed alone21,22.
MRI assessment
The protocol of the ADNI FreeSurfer-based pipeline has been detailed in previous publications23. Initial preprocessing of the MRI T1-weighted image involved intensity normalization and gradient expansion. Following this, non-brain tissue removal was performed using a hybrid watershed/surface deformation approach. Automatic Talairach transform was then employed to segment subcortical white matter and deep gray matter structures. We selected the hippocampus, entorhinal cortex, and middle temporal regions for our analysis due to their critical involvement in AD pathology, as they are among the first regions to show signs of atrophy. These regions are crucial for memory formation and have been extensively studied in AD research. Hippocampus, entorhinal and middle temporal volumes were extracted for this study, and 744, 740, 740 participants were included in the analysis of TyG-BMI index with brain imaging, respectively.
Statistical analyses
For continuous variables, mean (standard deviation) or median (interquartile range) were used to represent normal or non-normal distributions, respectively. Analysis of Variance or the Kruskal–Wallis test was employed for analysis accordingly. Categorical variables were depicted as numbers (n) and percentages (%), with chi-square tests employed for assessment. Outliers exceeding three standard deviations were eliminated from the statistical analysis involving the TyG-BMI index.
Initially, relationships between the TyG-BMI index (independent variable) and AD pathology, cognition, and brain structure (dependent variable) were explored via multivariable linear regression models. Analyses were conducted both with and without adjustment for covariates to assess the robustness of the findings. To identify potential interaction effects, we examined interaction terms between TyG-BMI and various covariates. For covariates where the interaction term was significant (P < 0.05) or indicated a potential interaction (P < 0.1), we conducted stratified analyses to further explore the associations within these subgroups.
The TyG-BMI index was also categorized into tertiles (low [T1], medium [T2], high [T3]). Using multivariable logistic regression (MLR), we compared the associations of the second and third tertiles with the first tertile (reference) in relation to AD pathology, cognition, and brain structure. Additionally, to explore the connection between the TyG-BMI index and the likelihood of AD development, we calculated the cumulative incidence rate through the Kaplan–Meier method and estimated the hazard ratio (HR) with a 95% confidence interval (CI) for AD using the Cox proportional hazards model. Both continuous TyG-BMI values and tertile groups were analyzed to assess their impact on AD risk.
We employed linear mixed-effects models to delineate the longitudinal relationships between baseline TyG-BMI index and longitudinal measures of AD pathology, cognition, and brain structure. Interaction terms between TyG-BMI index and time were included to assess changes over time.
For mediation analyses, we followed Baron and Kenny’s approach to ascertain whether AD pathology mediated the association of the TyG-BMI index with cognition and brain structure, and whether the TyG-BMI index partially affected cognition through brain structure24. We established a mediating effect if the following conditions were met: (1) the independent variable (IV) correlated with mediator variable (MV); (2) the TyG index correlated with dependent variable (DV); (3) MV correlated with DV; and (4) the correlation between the IV and cognition was attenuated after introducing MV as mediators into the regression model. In addition, the magnitude of attenuation or indirect effects was estimated and significance was ascertained via 10,000 self-directed iterations.
In all analyses, adjustments were made for age, sex, ethnicity, education, APOEε4 carrier status, cognitive diagnosis, smoking, drinking, hypertension, and CVD as covariates. Additionally, intracranial volume was included as a covariate in MRI measurements analyses. Given that all outcome variables were standardized to z-scores in the model, the coefficient represents the standardized effect. Statistical analyses were performed using R version 4.2.0, with statistical significance set at P < 0.05 for all analyses.
Ethical approval
The entire approval for this study was obtained from the Eisai Ethics Committee (2017-0433). Following the Declaration of Helsinki, written informed consent was obtained from all participants or their guardians.
Results
Participants’ characteristics
Eight hundred and fifty-five non-demented participants were included, with a mean age of 73.03 ± 7.13 years, 44.0% female, and a maximum follow-up of 16 years. Individuals displaying elevated TyG-BMI index levels exhibited several distinct demographic and clinical characteristics. They tended to be younger, had lower levels of education, were less likely to be of whites, and were less likely to carry the APOEε4 gene. Additionally, they showed a higher prevalence of CVD, diabetes, and hypertension. Significant difference in Aβ42, Tau, pTau, Tau/Aβ42, pTau/Aβ42, pTau/Tau, ADAS hippocampus, entorhinal between groups. The T2, T3 group had higher CSF Aβ42 and lower Tau and pTau compared to the T1 group. For brain structure, the hippocampus, entorhinal, and middle temporal were larger in the group with high TyG-BMI index levels (Table 1).
Table 1.
Characteristics based on the TyG-BMI index tertiles of 855 participants.
| Characteristic | Overall | Tertile1 | Tertile2 | Tertile3 | P |
|---|---|---|---|---|---|
| Number | 855 | 285 | 285 | 285 | |
| Age | 73.03 (7.13) | 73.66 (7.17) | 73.64 (7.09) | 71.79 (6.99) | 0.001 |
| Education | 16.15 (2.71) | 16.64 (2.58) | 16.15 (2.73) | 15.67 (2.76) | < 0.001 |
| Sex (%) | 0.080 | ||||
| Female | 376 (44.0) | 140 (49.1) | 114 (40.0) | 122 (42.8) | |
| Male | 479 (56.0) | 145 (50.9) | 171 (60.0) | 163 (57.2) | |
| Ethnicity (%) | 0.046 | ||||
| Other | 54 (6.3) | 16 ( 5.6) | 12 ( 4.2) | 26 ( 9.1) | |
| White | 801 (93.7) | 269 (94.4) | 273 (95.8) | 259 (90.9) | |
| APOE ε4 (%) | 0.006 | ||||
| APOE ε4 (−) | 495 (57.9) | 150 (52.6) | 159 (55.8) | 186 (65.3) | |
| APOE ε4 (+) | 360 (42.1) | 135 (47.4) | 126 (44.2) | 99 (34.7) | |
| Dignosis (%) | 0.900 | ||||
| CN | 257 (30.1) | 88 (30.9) | 83 (29.1) | 86 (30.2) | |
| MCI | 598 (69.9) | 197 (69.1) | 202 (70.9) | 199 (69.8) | |
| CVD (%) | < 0.001 | ||||
| No | 289 (33.8) | 123 (43.2) | 93 (32.6) | 73 (25.6) | |
| Yes | 566 (66.2) | 162 (56.8) | 192 (67.4) | 212 (74.4) | |
| Diabetes (%) | < 0.001 | ||||
| No | 749 (87.6) | 266 (93.3) | 251 (88.1) | 232 (81.4) | |
| Yes | 106 (12.4) | 19 ( 6.7) | 34 (11.9) | 53 (18.6) | |
| Hypertension (%) | 0.142 | ||||
| No | 506 (59.2) | 182 (63.9) | 163 (57.2) | 161 (56.5) | |
| Yes | 349 (40.8) | 103 (36.1) | 122 (42.8) | 124 (43.5) | |
| Drinking (%) | 0.917 | ||||
| No | 819 (95.8) | 274 (96.1) | 272 (95.4) | 273 (95.8) | |
| Yes | 36 ( 4.2) | 11 ( 3.9) | 13 ( 4.6) | 12 ( 4.2) | |
| Smoking (%) | 0.428 | ||||
| No | 524 (61.3) | 180 (63.2) | 166 (58.2) | 178 (62.5) | |
| Yes | 331 (38.7) | 105 (36.8) | 119 (41.8) | 107 (37.5) | |
| Glucose (mg/dL) | 100.93 (23.64) | 95.78 (14.72) | 99.24 (19.11) | 107.78 (31.96) | < 0.001 |
| Triglycerides (mg/dL) | 146.01 (107.91) | 97.45 (42.74) | 138.28 (66.00) | 202.30 (152.40) | < 0.001 |
| TyG index | 8.73 (0.59) | 8.35 (0.43) | 8.72 (0.47) | 9.12 (0.58) | < 0.001 |
| BMI (kg/m2) | 28.27 (4.42) | 24.14 (1.97) | 27.73 (1.74) | 32.94 (3.53) | < 0.001 |
| TyG-BMI | 247.62 (46.27) | 201.52 (17.37) | 241.26 (10.01) | 300.08 (33.17) | < 0.001 |
| AD pathology | |||||
| Aβ42 (pg/mL) | 1122.10 (606.37) | 1010.84 (582.50) | 1119.42 (608.79) | 1236.03 (608.50) | < 0.001 |
| Tau (pg/mL) | 270.07 (120.10) | 282.23 (124.84) | 279.46 (123.92) | 248.53 (108.31) | 0.001 |
| pTau (pg/mL) | 25.80 (13.45) | 27.35 (13.77) | 26.79 (14.15) | 23.28 (12.01) | < 0.001 |
| Tau/Aβ42 | 0.33 (0.26) | 0.38 (0.26) | 0.34 (0.27) | 0.27 (0.23) | < 0.001 |
| pTau/Aβ42 | 0.03 (0.03) | 0.04 (0.03) | 0.03 (0.03) | 0.03 (0.02) | < 0.001 |
| pTau/Tau | 0.09 (0.01) | 0.09 (0.01) | 0.09 (0.01) | 0.09 (0.01) | < 0.001 |
| Cognition | |||||
| MMSE (IQR) | 29.00 (3.00) | 29.00 (3.00) | 29.00 (3.00) | 29.00 (3.00) | 0.059 |
| ADAS (IQR) | 8.00 (5.67) | 9.00 (5.33) | 8.33 (5.33) | 7.00 (6.00) | 0.017 |
| MEM (IQR) | 0.46 (1.08) | 0.43 (1.20) | 0.43 (1.01) | 0.51 (1.01) | 0.034 |
| EF (IQR) | 0.35 (1.26) | 0.31 (1.33) | 0.35 (1.21) | 0.43 (1.18) | 0.608 |
| Brain structure | |||||
| Hippocampus (mm3) | 7037.83 (1098.63) | 6907.03 (1041.04) | 6958.67 (1150.73) | 7251.97 (1074.47) | 0.001 |
| Entorhinal (mm3) | 3646.17 (707.68) | 3550.51 (714.05) | 3633.67 (711.48) | 3759.56 (683.49) | 0.005 |
| Middle Temporal (mm3) | 20,076.19 (2774.66) | 19,762.92 (2717.40) | 20,228.43 (2878.79) | 20,242.11 (2703.95) | 0.091 |
| Intracranial volume (mm3) | 1,527,091.82 (164,907.44) | 1,515,485.34 (155,151.91) | 1,542,361.67 (175,565.06) | 1,523,482.40 (162,826.57) | 0.138 |
Data are mean (SD), n (%), or median (IQR).
TyG-BMI index, triglyceride glucose-body mass index; AD, Alzheimer’s Disease; MMSE, mini-mental state examination, ADAS, Alzheimer’s Disease Assessment Scale-Cognitive Subscale Question; EF, executive function; MEM, memory function. Aβ42, Amyloid-42; pTau, phosphorylated-tau; Tau, total-tau; APOE ε4, Apolipoprotein E; CN, cognitive normal; MCI, mild cognitive impartment; CVD, cardiovascular disease.
Association of TyG-BMI index with AD biomarkers, cognitive measures and brain structure
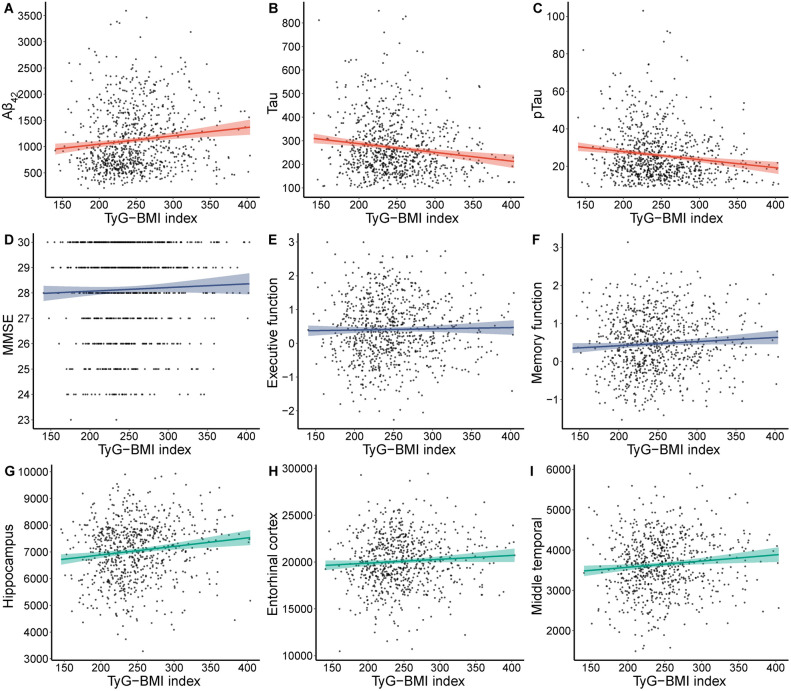
An increased TyG-BMI index showed a significant association with higher levels of Aβ42 (β = 0.096, P = 0.003). Conversely, as the TyG-BMI index increased, Tau (β = − 0.134, P = 6.38E-5), pTau (β = − 0.140, P = 2.62E-5), Tau/Aβ42 (β = − 0.145, P = 3.84E−6), pTau/Aβ42 (β = − 0.144, P = 5.38E−6), and pTau/Tau (β = − 0.127, P = 1.42E−4) gradually decreased (Fig. 1A–C, Supplementary file Table 1). The TyG-BMI index exhibited positive correlations with MMSE (β = 0.071, P = 0.030), MEM (β = 0.095, P = 0.001), EF (β = 0.068, P = 0.042), hippocampus (β = 0.129, P = 3.17E−4), entorhinal (β = 0.098, P = 0.006), and middle temporal (β = 0.077, P = 0.022), and negative correlations with ADAS (β = − 0.093, P = 0.002) (Fig. 1D–I, Supplementary file Table 1). To further understand the association between different levels of TyG-BMI index and AD biomarkers, cognition, and brain structure, we categorized the TyG-BMI index into tertiles and analyzed them using multivariate logistic regression, the results of which are presented in Table 2.
Figure 1.
Associations of TyG-BMI index and Alzheimer’s disease biomarkers, cognition and brain structure. TyG-BMI index, triglyceride glucose-body mass index; Aβ42, Amyloid-42; pTau, phosphorylated-tau; Tau, total-tau; MMSE, mini-mental state examination.
Table 2.
Association of TyG-BMI index and pathology, cognition as well as brain structure.
| Characteristic | Tertile1 | Tertile2 | Tertile3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| AD pathology | ||||||
| Aβ42 | Reference | 1.217 (1.049, 1.413) | 0.001 | 1.353 (1.161, 1.576) | 1.19E−04 | |
| Tau | Reference | 0.971 (0.831, 1.135) | 0.711 | 0.785 (0.669, 0.922) | 0.003 | |
| pTau | Reference | 0.950 (0.815, 1.109) | 0.519 | 0.767 (0.654, 0.899) | 0.001 | |
| Tau/Aβ42 | Reference | 0.871 (0.754, 1.006) | 0.061 | 0.731 (0.614, 0.827) | 9.06E−06 | |
| pTau/Aβ42 | Reference | 0.878 (0.759, 1.106) | 0.081 | 0.717 (0.617, 0.833) | 1.56E−05 | |
| pTau/Tau | Reference | 0.847 (0.726, 0.988) | 0.035 | 0.727 (0.620, 0.852) | 9.32E−05 | |
| Cognition | ||||||
| MMSE | Reference | 0.951 (0.817, 1.107) | 0.516 | 1.157 (0.990, 1.353) | 0.067 | |
| ADAS | Reference | 0.865 (0.745, 1.004) | 0.057 | 0.751 (0.645, 0.876) | 2.75E−05 | |
| MEM | Reference | 1.118 (0.976, 1.282) | 0.109 | 1.300 (1.129, 1.496) | 2.78E−04 | |
| EF | Reference | 1.167 (1.000, 1.362) | 0.051 | 1.238 (1.055, 1.452) | 0.009 | |
| Brain structure | ||||||
| Hippocampus | Reference | 1.064 (0.903, 1.255) | 0.457 | 1.360 (1.148, 1.612) | 4.10E−04 | |
| Entorhinal | Reference | 1.084 (0.920, 1.276) | 0.335 | 1.342 (1.131, 1.592) | 7.76E−04 | |
| MidTemp | Reference | 1.130 (0.970, 1.318) | 0.117 | 1.227 (1.045, 1.439) | 0.013 | |
TyG-BMI index, triglyceride glucose-body mass index; AD, Alzheimer’s Disease; MMSE, mini-mental state examination, ADAS, Alzheimer’s Disease Assessment Scale-Cognitive Subscale Question; EF, executive function; MEM, memory function. Aβ42, Amyloid-42; pTau, phosphorylated-tau; Tau, total-tau.
All factors adjusted for sex, age, ethnicity, Apolipoprotein E4, cognitive dignosis, diabetes, education, smoking, drinking, hypertension and cardiovascular disease, brain structure additionally adjusted for intracerebral volume.
In the interaction analysis, we found significant interactions between age and TyG-BMI index with Aβ42, cognitive diagnosis and TyG-BMI index with Tau, and MMSE and TyG-BMI index. In the subgroup analyses, we observed significant associations between TyG-BMI index and AD pathologies among specific participant subgroups (Supplementary file Table 2). Specifically, we found that in individuals over 60 years old, males, those with mild cognitive impairment, and those not carrying the APOEε4 gene, the associations between TyG-BMI and AD biomarkers were consistent with those observed in the overall population (Supplementary file Table 3). Subgroup analyses examining cognition and brain structure produced findings consistent with those of AD biomarkers. However, the association was notably more significant among participants lacking the APOEε4 gene, and no significant differences were observed by gender (Supplementary file Tables 4 and 5).
Longitudinal relationship between TyG-BMI index with cognitive measures and brain structure
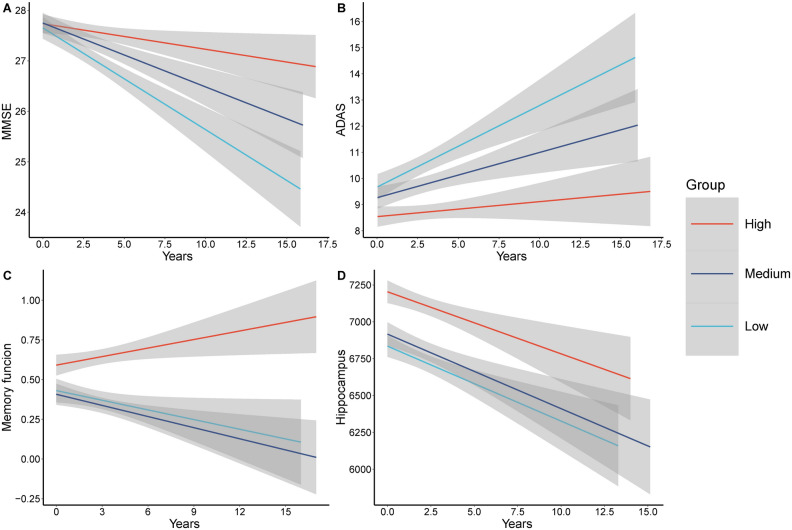
Longitudinally, we found associations between the TyG-BMI index and MMSE (β = 0.045, P = 6.36E-5), ADAS (β = − 0.046, P = 5.88E−6), EF (β = 0.015, P = 0.011), MEM (β = 0.024, P = 3.36E−5), entorhinal (β = 0.014, P = 0.007) and middle temporal volume (β = 0.013, P = 0.036) (Supplementary file Table 6). MMSE scores exhibited a slower decline over time in the medium and high groups compared to the low group. Similarly, ADAS scores showed a slower rate of increase in the medium and high groups. Regarding MEM, there was a gradual decline in the low and medium groups, while the high group demonstrated a gradual improvement. Furthermore, hippocampal volume exhibited a slower rate of decline in the medium and high groups (Fig. 2). The number of AD pathology, cognition, and imaging data included in the analysis during the follow-up time is shown in Supplementary file Table 7.
Figure 2.
Longitudinal relationship between different TyG-BMI index levels with cognitive measures and brain structure. MMSE, mini-mental state examination, ADAS, Alzheimer’s Disease Assessment Scale-Cognitive Subscale Question.
Association of TyG-BMI index with survival rates and risk of AD
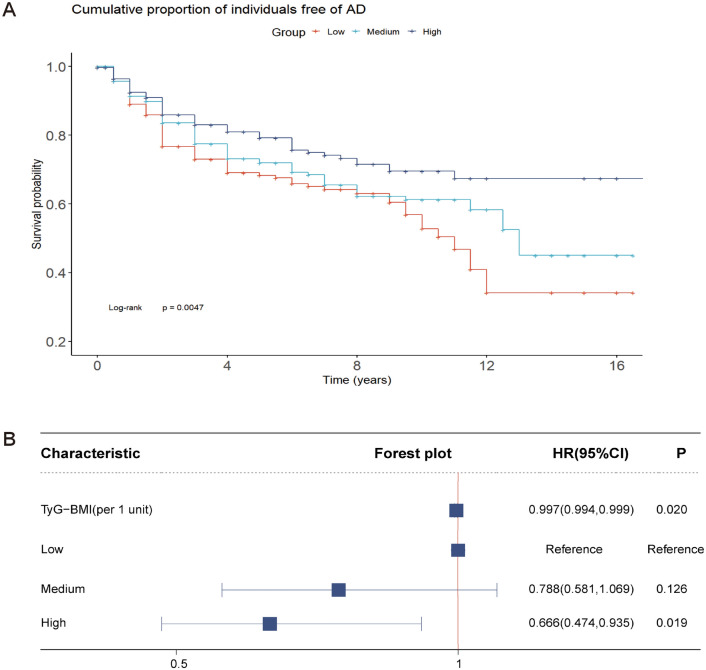
During a follow-up period of up to 16 years, we analyzed patient groups with different TyG-BMI levels using the Kaplan–Meier method. The results showed that the group of patients with high TyG-BMI levels exhibited higher survival rates during the follow-up period, and the survival curves showed longer survival times compared to the group of patients with low TyG-BMI levels (Fig. 3A).
Figure 3.
Association of TyG-BMI index with survival rates and risk of AD. (A) Survival probability of Alzheimer’s Disease over time across different TyG-BMI index levels. (B) The cumulative incidence of Alzheimer’s Disease based on Cox regression of TyG-BMI index.
Adjusted Cox regression was employed to compare the likelihood of developing AD across different TyG-BMI index levels. Findings revealed that in the adjusted model, when considering the TyG-BMI index as a continuous variable, each 1-unit increase in the index correlated with a 0.3% decrease in incident AD risk (HR 0.996 [0.994, 0.999]). Utilizing the TyG-BMI index as a categorical variable, with low group as the baseline, a greater reduction in AD risk was observed in high group (HR: 0.625 [0.444, 0.878]) (Fig. 3B). Additionally, among individuals older than 60 years old, not carrying the APOE ε4 gene, no diabetes or mildly cognitively impaired, the risk of developing AD decreased with each unit increase in the TyG-BMI index. However, these associations were marginally significant (Supplementary file Table 8).
Causal mediation analyses
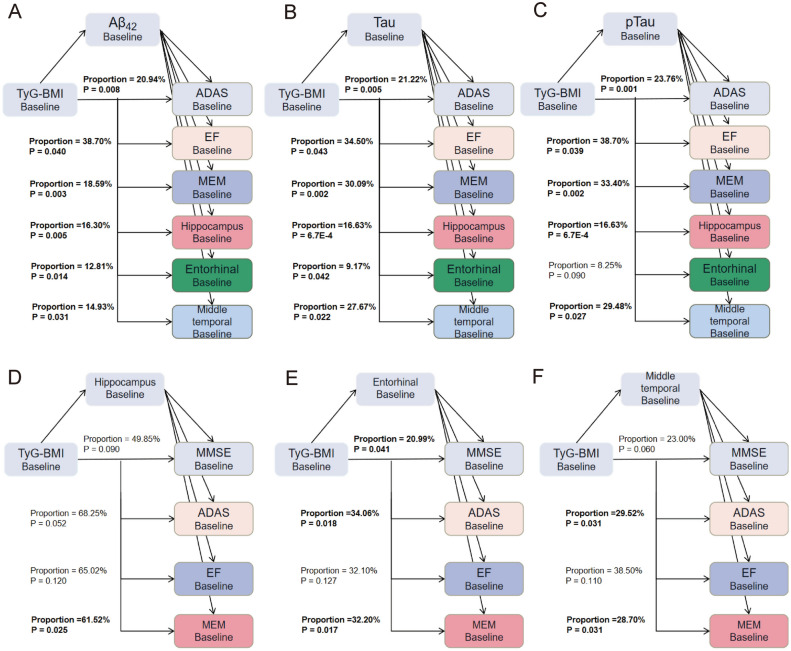
We explored whether the association between TyG-BMI index and cognition as well as brain structure is mediated by AD pathology, and whether the correlation between TyG-BMI index and cognition is mediated by brain structure. Analyses showed that Aβ42, Tau, pTau, and Tau/pTau all mediated changes in cognition and brain structure (Fig. 4A–C, Supplementary file Table 9). Tau proteins as well as pTau proteins have a greater impact on cognition compared to Aβ42. We found that the TyG-BMI index affects the hippocampus and the entorhinal cortex through Tau/Aβ42 and pTau/Aβ42. Specifically, the proportion of the total effect on the hippocampus mediated by Tau/Aβ42 is 32.60%, and by pTau/Aβ42 is 29.70%. The proportion of the total effect on the entorhinal cortex mediated by Tau/Aβ42 is 24.82%, and by pTau/Aβ42 is 21.66%. (Supplementary file Table 9). We also found that the middle temporal mediated the association between TyG-BMI index and ADAS (Proportion = 29.52%) and MEM (Proportion = 28.70%) (Fig. 4D–F, Supplementary file Table 10).
Figure 4.
Mediation analyses of TyG-BMI index and cognition/brain structure. (A–C) Mediation analyses of TyG-BMI index and cognition as well as brain structure with biomarkers as mediators. (D–F) Mediation analyses of TyG-BMI index and cognition with brain structure as mediators. TyG-BMI index, triglyceride glucose-body mass index; Aβ42, Amyloid-42; pTau, phosphorylated-tau; Tau, total-tau; MMSE, mini-mental state examination; ADAS, Alzheimer’s Disease Assessment Scale-Cognitive Subscale Question; MEM, memory function.
Discussion
In this study, we investigated the association between the TyG-BMI index and AD pathology, cognition, and brain structure. Our findings indicate that higher TyG-BMI levels are significantly associated with lower levels of Tau and pTau, as well as higher levels of Aβ42. These findings suggest that the TyG-BMI index may reflect systemic metabolic disturbances, such as IR and obesity, which are known to influence AD pathology. We observed that higher TyG-BMI index levels were significantly associated with a slower rate of cognitive decline as well as slower atrophy in the entorhinal cortex and middle temporal volume. Additionally, Aβ42, Tau, and pTau significantly mediated the total effect of the TyG-BMI index on cognition and brain structure. These AD pathological proteins may play a crucial role in linking metabolic health to neurodegeneration and cognitive decline.
The association of higher TyG-BMI index levels with lower Tau and pTau levels, as well as higher Aβ42, indicates a potentially protective metabolic effect on AD pathology. Our results are consistent with findings from a cohort study in China, which found a positive association between TyG index and Aβ42, as well as a positive correlation with the Aβ42/Aβ40 ratio, which better reflects the metabolism of β-amyloid11. IR has been found to promote the deposition of Aβ by decreasing Aβ clearance and increasing its oligomerization propensity25,26. Additionally, IR promotes the formation of Aβ fibrils by inducing GM1 ganglioside clustering in presynaptic membranes26. This creates a feedback loop where Aβ oligomers exacerbate brain IR, leading to progressive Aβ deposition27. Moreover, IR promotes Tau hyperphosphorylation through the activity of kinases such as GSK-3β, further establishing the link between IR and Tau pathology28,29. These evidences collectively suggest that metabolic disturbances reflected by the TyG-BMI index may influence AD pathology through mechanisms involving both Aβ and Tau.
We also found that the higher TyG-BMI levels were linked to slower atrophy in the entorhinal cortex and middle temporal volumes, which are critical regions affected in AD. Several investigations have explored the association between IR and brain structure of AD30,31. Studies have shown that IR is associated with cognitive dysfunction and brain atrophy, particularly in the hippocampus and temporal lobe regions, which are crucial for memory and cognitive functions32.
In terms of cognitive measures, we found that higher TyG-BMI index levels were associated with slower cognitive decline. This contrasts with several studies that have reported higher TyG levels are associated with increased cognitive impairment and AD risk33,34. For instance, a meta-analysis indicated that higher TyG levels are significantly associated with a higher risk of cognitive impairment and dementia35. One possible explanation for these contrasting findings is the role of BMI in modulating the effects of IR. While high BMI is generally considered a risk factor for metabolic syndrome, it can also reflect higher muscle mass and better overall nutritional status, which may confer some neuroprotective effects. Research has shown that a higher BMI in older adults can be associated with a protective effect against cognitive decline. For example, a study on Parkinson’s disease patients found that those with higher BMI at diagnosis experienced slower cognitive decline and had a lower risk of developing dementia compared to those with lower BMI36. Additionally, mild IR may trigger compensatory mechanisms that protect brain function in early stages, while severe IR leads to harmful effects37.
In the interaction analysis, we found significant interactions between age and TyG-BMI index with Aβ42. This interaction indicates that the influence of TyG-BMI on Aβ42 levels is more pronounced in older adults. This is supported by findings from previous studies showing that metabolic factors such as IR and BMI have different impacts on AD pathology across age groups. The older adults with better metabolic health may experience a slower accumulation of AD-related pathologies38,39. Similarly, the interaction between cognitive diagnosis and TyG-BMI with Tau suggests that individuals with MCI exhibit a different pattern of Tau accumulation in relation to their TyG-BMI levels compared to cognitively normal individuals. This suggests the need to consider cognitive status when assessing metabolic effects on AD pathology. Previous research has shown that individuals with MCI often have varying degrees of metabolic dysfunction, which can differentially affect the progression of tau pathology40.
Additionally, Aβ42, Tau, and pTau significantly mediated the association of the TyG-BMI index on cognition and brain structure. The TyG-BMI index can mediate cognitive changes by influencing brain structure. We found that the middle temporal lobe mediated the association between TyG-BMI index and ADAS (29.52%) and MEM (28.70%). The middle temporal plays an important role in memory and cognitive function, with increased volume positively associated with MEM scores and negatively associated with ADAS scores. Although studies have shown that IR can affect Aβ deposition and Tau phosphorylation13, leading to neurodegenerative processes38,39. However, the effects of IR vary from person to person. In some cases, IR may induce a range of compensatory mechanisms, such as increasing insulin levels in the brain, which in turn may have a protective effect on neurons32,41.
The association of FBG and TG levels with AD should not be overlooked in the TyG-BMI index as an indicator calculated from them. In a prolonged follow-up of ASPirin in Reducing Events in the Elderly and the UK Biobank cohorts, older individuals exhibiting elevated TG levels experienced a reduced risk of dementia and decelerated cognitive decline42. Besides, the Rotterdam Study revealed that elevated glucose levels were linked to increased IR burden and the risk of AD. However, this association was observed mainly in short-term follow-up results (< 3 years), while in follow-ups exceeding 5.5 years, elevated blood glucose levels and increased IR burden were reduced by 39% and 30%, respectively, suggesting possible time-dependence43.
IR occurs when the cellular response to insulin is weakened, resulting in blood glucose not entering the cell efficiently, which in turn increases insulin levels. Insulin’s protective effect on the brain could explain our results44. Insulin promotes the clearance and degradation of Aβ proteins by activating insulin receptors in glial cells45,46. Besides, IR induces neuronal damage and apoptosis, exacerbating Aβ protein release47. Damaged neurons release more Aβ protein, accelerating Aβ protein aggregation and plaque formation48. Additionally, insulin regulates the transcription and translation of Tau proteins through the PI3K/Akt signaling pathway49. IR interferes with this pathway’s normal function, resulting in abnormal expression and phosphorylation of Tau proteins50. Moreover, IR-induced oxidative stress leads to the accumulation of free radicals and reactive oxidants, causing DNA, lipid, and protein damage in brain cells51,52. This oxidative damage not only induces neuronal degeneration and apoptosis but also accelerates the aggregation of Aβ proteins and the phosphorylation of Tau proteins53.
There are several limitations to this study. First, because this study was a single-center study, the results still need to be validated in other larger longitudinal cohorts to ensure generalizability. Second, due to database limitations, we were not able to compare TyG-BMI index with other current IR measurement techniques. Third, we did not consider the effects of cerebrovascular disease, lifestyle, dietary habits, and physical activity. Additionally, the statistical methods used, including MLR, Cox models, and mediation analysis, have inherent limitations. Unmeasured confounders may affect results, and interpretations should be cautious as they suggest potential pathways rather than definitive causality. Mediation analysis, in particular, may have limitations due to model assumptions. These preliminary findings require validation in larger, diverse cohorts and experimental studies.
Conclusions
Based on the presented findings, the TyG-BMI index is associated with AD pathology, cognition and brain structures such as the hippocampus. In addition, the TyG-BMI index may modulate cognitive and structural brain changes by impacting pathologic proteins. These results propose that monitoring the TyG-BMI index could serve as a valuable indicator for assessing AD risk and guiding timely interventions for metabolic disorders, thereby aiding in AD prevention.
Supplementary Information
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.;Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hofmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigate can be found at: https://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Author contributions
Z.H.-Z. contributed to the conception or design of the work. All authors were responsible for the acquisition, analysis and interpretation of data. Z.H.-Z., X.-C. and Z.H.-S. drafted the manuscript. Z.H.-Z. and X.-C. have contributed equally to this work. Critical revision of the manuscript for important intellectual content were performed by all authors. All author agreed with the content of the article to be submitted. All authors reviewed and approved the final manuscript.
Data availability
Raw data supporting the obtained results are available at the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zihao Zhang and Xin Chen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-67052-3.
References
- 1.Wirth M, Villeneuve S, Haase CM, Madison CM, Oh H, Landau SM, Rabinovici GD, Jagust WJ. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 2013;70:1512–1519. doi: 10.1001/jamaneurol.2013.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim. Biophys. Acta. 2009;1792:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Hildreth KL, Van Pelt RE, Schwartz RS. Obesity, insulin resistance, and Alzheimer’s disease. Obesity (Silver Spring) 2012;20:1549–1557. doi: 10.1038/oby.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craft S. Alzheimer disease: Insulin resistance and AD–extending the translational path. Nat. Rev. Neurol. 2012;8:360–362. doi: 10.1038/nrneurol.2012.112. [DOI] [PubMed] [Google Scholar]
- 5.Willette AA, Johnson SC, Birdsill AC, Sager MA, Christian B, Baker LD, Craft S, Oh J, Statz E, Hermann BP, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2015;11:504–510.e501. doi: 10.1016/j.jalz.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: Implications for therapy. Acta Neuropathol. 2013;126:479–497. doi: 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- 7.Vasques AC, Novaes FS, de Oliveira MS, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, Álvarez-Villalobos NA, González-González JG. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: A systematic review. Int. J. Endocrinol. 2020;2020:4678526. doi: 10.1155/2020/4678526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Qian Y, Deng X. Triglyceride glucose index is a significant predictor of severe disturbance of consciousness and all-cause mortality in critical cerebrovascular disease patients. Cardiovasc. Diabetol. 2023;22:156. doi: 10.1186/s12933-023-01893-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J, Xie Z, Wu Y, Liu X, Ma J, Dong Y, Liu C, Ye M, Zhu W. Association of the triglyceride-glucose index with risk of Alzheimer’s disease: A prospective cohort study. Am. J. Prev. Med. 2023;65:1042–1049. doi: 10.1016/j.amepre.2023.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Tian N, Fa W, Dong Y, Liu R, Liu C, Liu K, Mao M, Zhu M, Liang X, Wang N, et al. Triglyceride-glucose index, Alzheimer’s disease plasma biomarkers, and dementia in older adults: The MIND-China study. Alzheimers Dement. (Amst) 2023;15:e12426. doi: 10.1002/dad2.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong S, Han K, Park CY. The insulin resistance by triglyceride glucose index and risk for dementia: Population-based study. Alzheimers Res. Ther. 2021;13:9. doi: 10.1186/s13195-020-00758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y, Fang Z, Zhang X, Wen Y, Lu J, He S, Xu B. Association between triglyceride glucose-body mass index and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: A retrospective study. Cardiovasc. Diabetol. 2023;22:75. doi: 10.1186/s12933-023-01794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One. 2016;11:e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS One. 2019;14:e0212963. doi: 10.1371/journal.pone.0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Classification and Diagnosis of Diabetes Standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S15–s33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 18.Böhm M, Schumacher H, Teo KK, Lonn E, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder R, Weber M, et al. Achieved diastolic blood pressure and pulse pressure at target systolic blood pressure (120–140 mmHg) and cardiovascular outcomes in high-risk patients: Results from ONTARGET and TRANSCEND trials. Eur. Heart J. 2018;39:3105–3114. doi: 10.1093/eurheartj/ehy287. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Ma LZ, Hu H, Wang ZT, Ou YN, Dong Q, Tan L, Yu JT. P-tau and neurodegeneration mediate the effect of β-amyloid on cognition in non-demented elders. Alzheimers Res. Ther. 2021;13:200. doi: 10.1186/s13195-021-00943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racine AM, Koscik RL, Nicholas CR, Clark LR, Okonkwo OC, Oh JM, Hillmer AT, Murali D, Barnhart TE, Betthauser TJ, et al. Cerebrospinal fluid ratios with Aβ42 predict preclinical brain β-amyloid accumulation. Alzheimers Dement (Amst) 2016;2:27–38. doi: 10.1016/j.dadm.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snider BJ, Fagan AM, Roe C, Shah AR, Grant EA, Xiong C, Morris JC, Holtzman DM. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch. Neurol. 2009;66:638–645. doi: 10.1001/archneurol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Jr, Barnes J, Bernstein MA, Borowski BJ, Brewer J, Clegg S, Dale AM, Carmichael O, Ching C, DeCarli C, et al. Magnetic resonance imaging in Alzheimer’s Disease Neuroimaging Initiative 2. Alzheimers Dement. 2015;11:740–756. doi: 10.1016/j.jalz.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 25.Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, Schwartz MW, Plymate S, Craft S. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60:1899–1903. doi: 10.1212/01.WNL.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto N, Matsubara T, Sobue K, Tanida M, Kasahara R, Naruse K, Taniura H, Sato T, Suzuki K. Brain insulin resistance accelerates Aβ fibrillogenesis by inducing GM1 ganglioside clustering in the presynaptic membranes. J. Neurochem. 2012;121:619–628. doi: 10.1111/j.1471-4159.2012.07668.x. [DOI] [PubMed] [Google Scholar]
- 27.Mullins RJ, Diehl TC, Chia CW, Kapogiannis D. Insulin resistance as a link between amyloid-beta and tau pathologies in Alzheimer’s disease. Front. Aging Neurosci. 2017;9:118. doi: 10.3389/fnagi.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Y, Li B, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX. Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: Implication for Alzheimer’s disease. Am. J. Pathol. 2009;175:2089–2098. doi: 10.2353/ajpath.2009.090157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesort M, Johnson GV. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience. 2000;99:305–316. doi: 10.1016/S0306-4522(00)00200-1. [DOI] [PubMed] [Google Scholar]
- 30.Willette AA, Xu G, Johnson SC, Birdsill AC, Jonaitis EM, Sager MA, Hermann BP, La Rue A, Asthana S, Bendlin BB. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36:443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Insulin resistance is associated with the pathology of Alzheimer disease: The Hisayama study. Neurology. 2010;75:764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- 32.Spinelli M, Fusco S, Grassi C. Brain insulin resistance and hippocampal plasticity: Mechanisms and biomarkers of cognitive decline. Front. Neurosci. 2019;13:788. doi: 10.3389/fnins.2019.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Convit A. Links between cognitive impairment in insulin resistance: An explanatory model. Neurobiol. Aging. 2005;26(Suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Xu L, Liu L, Zhan S, Wang S, Song Y. Sex differences in the association between the change in triglyceride-glucose index and cognitive decline: A population-based cohort study. J. Affect. Disord. 2022;316:42–49. doi: 10.1016/j.jad.2022.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Ling Q, Wu Y, Zhang M. Association between the triglyceride glucose index and cognitive impairment and dementia: A meta-analysis. Front. Aging Neurosci. 2023;15:1278730. doi: 10.3389/fnagi.2023.1278730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo HS, Chung SJ, Lee PH, Sohn YH, Kang SY. The influence of body mass index at diagnosis on cognitive decline in Parkinson’s disease. J. Clin. Neurol. 2019;15:517–526. doi: 10.3988/jcn.2019.15.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfova K, Kucera M, Cermakova P. Risk and protective factors of neurocognitive disorders in older adults in Central and Eastern Europe: A systematic review of population-based studies. PLoS One. 2021;16:e0260549. doi: 10.1371/journal.pone.0260549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huo RR, Liao Q, Zhai L, You XM, Zuo YL. Interacting and joint effects of triglyceride-glucose index (TyG) and body mass index on stroke risk and the mediating role of TyG in middle-aged and older Chinese adults: A nationwide prospective cohort study. Cardiovasc. Diabetol. 2024;23:30. doi: 10.1186/s12933-024-02122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang C, Yang R, Kuang M, Yu M, Zhong M, Zou Y. Triglyceride glucose-body mass index in identifying high-risk groups of pre-diabetes. Lipids Health Dis. 2021;20:161. doi: 10.1186/s12944-021-01594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K, He G, Zhang Y, Yin J, Yan Y, Zhang Y, Wang K. Association of triglyceride-glucose index and its interaction with obesity on hypertension risk in Chinese: A population-based study. J. Hum. Hypertens. 2021;35:232–239. doi: 10.1038/s41371-020-0326-4. [DOI] [PubMed] [Google Scholar]
- 41.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Z, Ryan J, Tonkin AM, Zoungas S, Lacaze P, Wolfe R, Orchard SG, Murray AM, McNeil JJ, Yu C, et al. Association between triglycerides and risk of dementia in community-dwelling older adults: A prospective cohort study. Neurology. 2023 doi: 10.1212/WNL.0000000000207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: The Rotterdam Study. Neurology. 2010;75:1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han X, Yang L, Du H, Sun Q, Wang X, Cong L, Liu X, Yin L, Li S, Du Y. Insulin attenuates beta-amyloid-associated insulin/Akt/EAAT signaling perturbations in human astrocytes. Cell Mol. Neurobiol. 2016;36:851–864. doi: 10.1007/s10571-015-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao WQ, Lacor PN, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric aβ. J. Biol. Chem. 2009;284:18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto N, Ishikuro R, Tanida M, Suzuki K, Ikeda-Matsuo Y, Sobue K. Insulin-signaling pathway regulates the degradation of amyloid β-protein via astrocytes. Neuroscience. 2018;385:227–236. doi: 10.1016/j.neuroscience.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Kim B, Sullivan KA, Backus C, Feldman EL. Cortical neurons develop insulin resistance and blunted Akt signaling: A potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxid. Redox Signal. 2011;14:1829–1839. doi: 10.1089/ars.2010.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roos TT, Garcia MG, Martinsson I, Mabrouk R, Israelsson B, Deierborg T, Kobro-Flatmoen A, Tanila H, Gouras GK. Neuronal spreading and plaque induction of intracellular Aβ and its disruption of Aβ homeostasis. Acta Neuropathol. 2021;142:669–687. doi: 10.1007/s00401-021-02345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gabbouj S, Ryhänen S, Marttinen M, Wittrahm R, Takalo M, Kemppainen S, Martiskainen H, Tanila H, Haapasalo A, Hiltunen M, Natunen T. Altered insulin signaling in Alzheimer’s disease brain—Special emphasis on PI3K-Akt pathway. Front. Neurosci. 2019;13:629. doi: 10.3389/fnins.2019.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng M, Wang P. Role of insulin receptor substance-1 modulating PI3K/Akt insulin signaling pathway in Alzheimer’s disease. 3 Biotech. 2021;11:179. doi: 10.1007/s13205-021-02738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ping Z, Peng Y, Lang H, Xinyong C, Zhiyi Z, Xiaocheng W, Hong Z, Liang S. Oxidative stress in radiation-induced cardiotoxicity. Oxid. Med. Cell. Longev. 2020;2020:3579143. doi: 10.1155/2020/3579143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes. 2015;6:456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy RG, Mandal PK, Maroon JC. Oxidative stress occurs prior to amyloid Aβ plaque formation and tau phosphorylation in Alzheimer’s disease: Role of glutathione and metal ions. ACS Chem. Neurosci. 2023;14:2944–2954. doi: 10.1021/acschemneuro.3c00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data supporting the obtained results are available at the corresponding author.