Abstract
This study aims to figure out the worldwide prevalence of anticancer therapy-associated acute kidney injury (AKI) and tubulointerstitial nephritis (TIN) and the relative risk of each cancer drug. We conducted an analysis of VigiBase, the World Health Organization pharmacovigilance database, 1967–2023 via disproportionate Bayesian reporting method. We further categorized the anticancer drugs into four groups: cytotoxic therapy, hormone therapy, immunotherapy, and targeted therapy. Reporting odds ratio (ROR) and information component (IC) compares observed and expected values to investigate the associations of each category of anticancer drugs with AKI and TIN. We identified 32,722 and 2056 reports (male, n = 17,829 and 1,293) of anticancer therapy-associated AKI and TIN, respectively, among 4,592,036 reports of all-drug caused AKI and TIN. There has been a significant increase in reports since 2010, primarily due to increased reports of targeted therapy and immunotherapy. Immunotherapy exhibited a significant association with both AKI (ROR: 8.92; IC0.25: 3.06) and TIN (21.74; 4.24), followed by cytotoxic therapy (7.14; 2.68), targeted therapy (5.83; 2.40), and hormone therapy (2.59; 1.24) for AKI, and by cytotoxic therapy (2.60; 1.21) and targeted therapy (1.54; 0.61) for TIN. AKI and TIN were more prevalent among individuals under 45 years of age, with a female preponderance for AKI and males for TIN. These events were reported in close temporal relationship after initiation of the respective drug (16.53 days for AKI and 27.97 days for TIN), and exhibited a high fatality rate, with 23.6% for AKI and 16.3% for TIN. These findings underscore that kidney-related adverse drug reactions are of prognostic significance and strategies to mitigate such side effects are required to optimize anticancer therapy.
Keywords: Anticancer drugs, Acute kidney injury, Tubulointerstitial nephritis, World Health Organization
Subject terms: Kidney, Cancer
Introduction
The recent strides in cancer therapeutics have been remarkable, reflecting persistent endeavors in drug development across the medical domain, despite cancer still ranking as the second leading cause of death globally1. From 2000 to 2019, the global life expectancy increased by 6 years, a trend largely attributable to the substantial rise in the global incidence of cancer and the utilization of diverse anticancer agents for therapeutic interventions2,3 The introduction of novel targeted therapies, immunotherapies and hormone therapies over the past decades has enhanced patient survival rates compared to standard conventional chemotherapies for specific types of cancers4.
It is well established that cancer patients, especially those with advanced cancer, are more susceptible to acute kidney injury (AKI) and tubulointerstitial nephritis (TIN)5,6. AKI in cancer patients impacts prognosis and management, including reduced rates of cancer remission7 and subsequently elevated mortality rates8 as administration of effective therapies is withheld due to concerns about kidney injury. Although advanced cancer itself could be a risk factor for AKI, there is a possibility that anticancer treatment could be a potential inducer of AKI and TIN5.
In particular, the various side effects associated with traditional cytotoxic therapies such as nephrotoxicity, are well-documented, especially with agents like cisplatin9.
Nonetheless, substantial concerns remain due to the relatively limited accumulation of experience with these novel drugs, especially concerning the diverse patient population, which encompasses variations in age, gender, and ethnicity. As the mechanisms of action of each drug vary widely, the mechanisms causing side effects are also diverse. While some studies have attempted to elucidate the nephrotoxicity of novel drugs, research on the long-term and large-scale effects of various medications remains insufficient10.
Our study focuses on analyzing the global risk of AKI and TIN linked to 165 types of anticancer medications. To assess the association between anticancer treatment and AKI and TIN, we have compared the risk to that of other drugs. Utilizing data from the World Health Organization (WHO) global pharmacovigilance database, our objective is to enhance patient safety following anticancer treatment and to establish effective monitoring guidelines for healthcare professionals. Vigibase, the WHO global database of adverse drug reactions (ADRs) reports, is essential resource for pharmacovigilance research due to its extensive coverage. Through the provision of comprehensive data which was observed in patients, our study aims to aid healthcare professionals in selecting safer and more appropriate medications for cancer patients in clinical settings.
Methods and materials
Study design and data sources
This retrospective pharmacovigilance study conducted a disproportionality analysis based on ADRs reported in the WHO database, VigiBase. VigiBase has amassed adverse case reports from over 170 countries, covering more than 25,000 drugs and compiling reports since 1967. It serves as a database system where adverse events can be reported by physicians, pharmacists, healthcare professionals, and patients11,12. Each incoming report undergoes scrutiny based on predefined quality criteria, and is regularly reviewed and analyzed. The Uppsala Monitoring Centre (UMC) in Uppsala, Sweden, manages the database, and thoroughly reviewed according to predefined quality standards. Reported adverse reactions are classified according to the preferred terms of the Medical Dictionary for Regulatory Activities (MedDRA) 26.0. Anticancer drug-related cases were extracted from November 14, 1967, to July 26, 2023. This study received approval from the Institutional Review Board at Kyung Hee University Medical Center and the Uppsala Monitoring Centre (WHO Collaborating Centre) and involved the utilization of de-identified patient data. The requirement for informed consent was waived in this study, as VigiBase does not contain personal information.
Definition of exposure groups
We compiled a list of anticancer drugs included in our research, which was analyzed based on the anticancer drugs reported to the WHO in relevant literature13. Anticancer drugs were classified into four categories: (1) cytotoxic therapies, (2) hormone therapies, (3) immunotherapies including programmed cell death protein 1 (PD-1) inhibitors, programmed cell death ligand 1 (PD-L1) inhibitors, and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors, and (4) targeted therapies including kinase inhibitors. We excluded certain drugs from our study, even though they fell into one of the four categories for classifying anticancer agents. For instance, drugs like cyclophosphamide have dual roles, serving as both therapeutic agents for conditions like glomerulonephritis that may cause AKI and as anticancer agents14,15. This dual usage complicates distinguishing whether AKI results from their therapeutic use or their role as anticancer agents. Additionally, drugs like interferon have various components, such as alpha and beta, introducing potential confusion16,17.
All anticancer drugs are considered ‘suspected’ only when showing disproportionate association with kidney-related ADRs based on the WHO causality assessment recommendations. Each case report includes patient characteristics (sex and age), general information (region and reporting year), anticancer drug details (indication, start and end dates of administration, and dosage), and kidney-related adverse event information (time-to-onset and end date, seriousness, and final outcome). Time to onset refers to the number of days from the date of drug administration to the date when the adverse reaction occurred. The outcome of each event was classified as either "fatal" or "recovered/recovering," and severity, including hospitalization, lasting disabilities, life-threatening situations, and death, was assessed by physicians.
Definition of outcomes
The primary outcome was the disproportionate measures of kidney-related ADRs after prescription of each anticancer drug classes. We divided kidney-related ADRs into AKI and TIN. Secondary outcomes included subgroup analyses of kidney-related ADRs following different types of anticancer drugs based on age (0–17, 18–44, 45–64, and ≥ 65 years) and sex. Disproportionality analysis was also conducted for each individual anticancer drug.
Statistical analysis
We assessed whether suspected cases of AKI and TIN, classified based on the four types of anticancer drugs mentioned earlier, were differentially reported when compared to the entire pharmacovigilance database drugs. Two common pharmacovigilance measures of disproportionate analysis, the reporting odds ratio (ROR) and information component (IC) were calculated based on anticancer drugs. We used the ROR as a measure of association to estimate the frequentist disproportionality association. ROR is a statistical measure derived from a contingency table based on the number of ADRs. It compares the probability of a specific event occurring with a particular drug to the probability of the same event occurring with all other drugs not related to that specific event18. A lower 95% confidence interval (CI) of the ROR ≥ 1 is deemed statistically significant, indicating an association between the drug and a particular ADR.
IC is calculated using Bayesian methods for case-non-case analysis, comparing the adverse event rate of a specific drug to that of all other drugs18,19. The formula for calculating the IC is as follows: IC = log2([Nobserved + 0.5]/[Nexpected + 0.5]). Nobserved is the case reports for a specific adverse reaction with a drug, Nexpected is the expected cases for that drug-effect combination, calculated as [Ndrug x Neffect]/Ntotal. Ndrug stands for the number of case reports involving a specific drug, Neffect signifies the number of case reports for a particular reaction, and Ntotal encompasses the overall count of case reports within the database. IC0.25 is the lower limit of IC's 95% CI and a positive IC0.25 indicates a statistical signal20. A more detailed description is described in the Supplementary Methods. Disproportionality analysis and subgroup analysis of reports from health professionals were conducted to validate our results. All analyses were performed using the SAS version 9.4 (SAS Inc., Cary, NC, USA) in this study21,22.
Ethical statement
Approval for the use of confidential and electronically processed patient data was granted by the Institutional Review Board of Kyung Hee University Medical Center and the Uppsala Monitoring Centre (WHO Collaborating Centre).
Results
Overall analysis
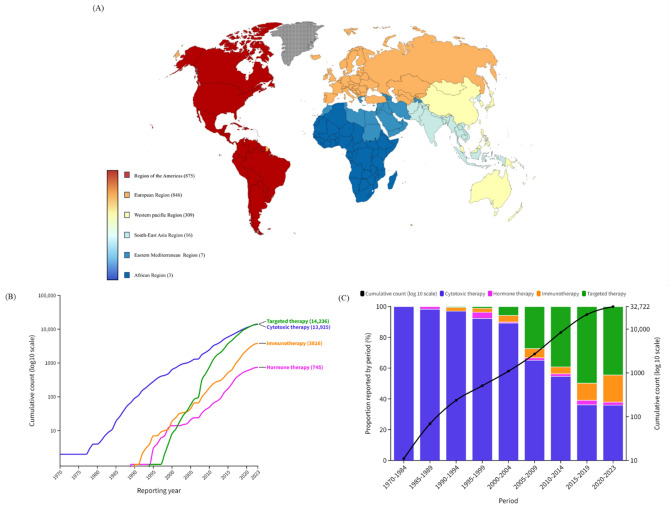
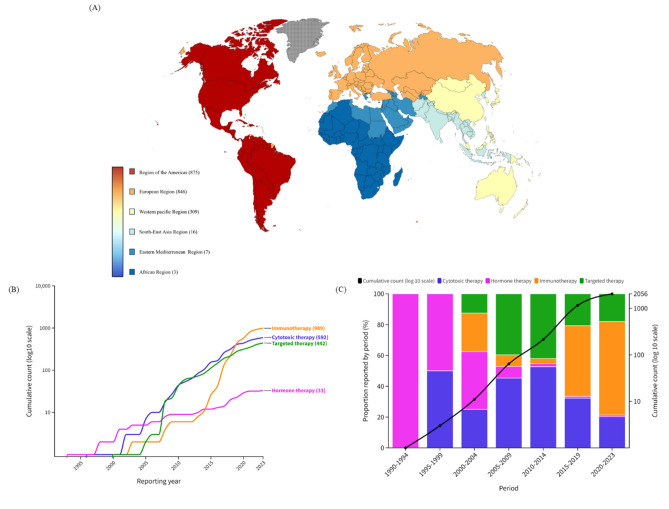
Of the 131,255,418 reports in the full database (Table 1), 32,722 reports (male, n = 17,829) and 2056 reports (male, n = 1293) of AKI and TIN, respectively, in the Vigibase database between 1967 and 2023 were identified. As depicted in Figure 1, the American region accounted for nearly half of the reports, followed by European, Western Pacific, Southeast Asia, Eastern Mediterranean, and African regions. Targeted therapy (43.5%) and cytotoxic therapy (42.6%) accounted for the highest number of reported cases of AKI, while immunotherapy (48.1%) was associated with the highest number of reported cases of TIN. The majority of reports for both AKI and TIN occurred in the age group of over 65 years (42.6% and 41.2%, respectively). The median time from initiation of the offending drug to onset was approximately 16 days for AKI and 27 days for TIN. The rate of fatal outcomes was 23.6% for AKI and 16.3% for TIN.
Table 1.
Overall baseline characteristics of cases of anticancer drugs-associated AKI and TIN in the VigiBase, a WHO pharmacovigilance database, between 1967 and 2023.
| Variables | AKI (n = 32,722) | TIN (n = 2056) |
|---|---|---|
| Region reporting, n (%) | ||
| African Region | 15 (0.1) | 3 (0.2) |
| Region of the Americas | 16,786 (51.3) | 875 (42.6) |
| South-East Asia Region | 361 (1.1) | 16 (0.8) |
| European Region | 11,910 (36.4) | 846 (41.2) |
| Eastern Mediterranean Region | 75 (0.2) | 7 (0.3) |
| Western Pacific Region | 3575 (10.9) | 309 (15.0) |
| Reporting year, n (%) | ||
| 1969–1979 | 4 (0.01) | 0 (0.00) |
| 1980–1989 | 65 (0.2) | 0 (0.00) |
| 1990–1999 | 440 (1.3) | 3 (0.2) |
| 2000–2009 | 2201 (6.7) | 61 (3.0) |
| 2010–2019 | 18,813 (57.5) | 1092 (53.1) |
| 2020–2023 | 11,199 (34.2) | 900 (43.8) |
| Reporter qualification, n (%) | ||
| Health Professional | 29,049 (88.8) | 1924 (93.6) |
| Non-Health Professional | 2231 (6.8) | 64 (3.1) |
| Unknown | 1442 (4.4) | 68 (3.3) |
| Studies, n (%) | ||
| Study related | 12,230 (37.4) | 383 (18.6) |
| Non-study related | 20,241 (61.9) | 1659 (80.7) |
| Unknown | 251 (0.8) | 14 (0.7) |
| Sex, n (%) | ||
| Male | 17,829 (54.5) | 1293 (62.9) |
| Female | 12,384 (37.9) | 661 (32.2) |
| Unknown | 2509 (7.7) | 102 (5.0) |
| Age, years, n (%) | ||
| < 18 | 866 (2.7) | 54 (2.6) |
| 18–44 | 2164 (6.6) | 319 (15.5) |
| 44–64 | 9603 (29.4) | 530 (25.8) |
| ≥ 65 | 13,952 (42.6) | 847 (41.2) |
| Unknown | 6137 (18.8) | 306 (14.9) |
| Delay (TTO), days, mean (SD) | 16.53 (80.1) | 27.97 (98.1) |
| Drug class, n (%) | ||
| Cytotoxic therapy | 13,925 (42.6) | 592 (28.8) |
| Hormone therapy | 745 (2.3) | 33 (1.6) |
| Immunotherapy | 3816 (11.7) | 989 (48.1) |
| Targeted therapy | 14,236 (43.5) | 442 (21.5) |
| Fatal outcomes, n (%) | ||
| Recovered/recovering | 14,561 (44.5) | 1053 (51.2) |
| Fatal | 7719 (23.6) | 334 (16.3) |
| Unknown | 10,442 (31.9) | 669 (32.5) |
| Single drug suspected, n (%) | 32,518 (99.4) | 2024 (98.4) |
AKI, acute kidney injury; SD, standard deviation; TIN, tubulointerstitial nephritis; TTO, time to onset; WHO, World Health Organization.
Figure 1.
World map (A) and cumulative counts (B and C) of AKI cases per year in association with different anticancer drugs. AKI, acute kidney injury.
Cumulative report analysis
The number of reports has seen a dramatic increase since 2010 (Figs. 1 and 2). While cytotoxic therapy has consistently been reported as the leading cause of AKI since the 1970s, the advent of targeted therapy in the early 2000s led to a steep rise in AKI attributed to targeted therapy, particularly evident in the early 2010s (Fig. 1B and C). In contrast, reports of TIN were predominantly associated with hormone therapy in the 1990s. However, following the development of immunotherapy, there has been a notable surge in TIN reports since the 2010s, with hormone therapy only accounting for a minimal number of TIN reports at present (Fig. 2B and C).
Figure 2.
World map (A) and cumulative counts (B and C) of TIN cases per year in association with different anticancer drugs. TIN, tubulointerstitial nephritis.
Disproportionality analysis of AKI and TIN reports by anticancer therapies
The analysis of anticancer therapy-associated AKI and TIN reports indicated that most anticancer drugs are associated with AKI and TIN (Tables 2 and S1). Immunotherapy was associated with the most AKI reports (ROR: 8.92 [95% CI, 8.63–9.21]; IC: 3.11 [IC0.25: 3.06]), followed by cytotoxic therapy (ROR: 7.14 [95% CI, 7.01–7.26]; IC: 2.71 [IC0.25: 2.68]), targeted therapy (ROR: 5.83 [95% CI, 5.73–5.93]; IC: 2.42 [IC0.25: 2.40]), and hormone therapy (ROR: 2.59 [95% CI, 2.41–2.79]; IC: 1.37 [IC0.25: 1.24]). Similarly, immunotherapy has been reported as the most strongly associated factor with TIN (ROR: 21.74 [95% CI, 20.39–23.18]; IC: 4.34 [IC0.25: 4.24]), followed by cytotoxic therapy (ROR: 2.60 [95% CI, 2.40–2.82]; IC: 1.35 [IC0.25: 1.21]), and targeted therapy (ROR: 1.54 [95% CI, 1.40–1.69]; IC: 0.61 [IC0.25: 0.45]). Hormone therapy was found to have no significant association with TIN.
Table 2.
Disproportionality analysis of anticancer-associated AKI and TIN cases.
| Total | AKI | TIN | |||||
|---|---|---|---|---|---|---|---|
| Observed | ROR (95% CI) | IC (IC0.25) | Observed | ROR (95% CI) | IC (IC0.25) | ||
| Sex | |||||||
| Male | 2,012,633 | 17,829 | 6.71 (6.60–6.83) | 2.42 (2.40) | 1293 | 4.56 (4.30–4.84) | 1.98 (1.89) |
| Female | 2,228,455 | 12,384 | 8.14 (7.98–8.30) | 2.75 (2.72) | 661 | 3.62 (3.34–3.93) | 1.75 (1.62) |
| Anticancer drugs | |||||||
| Cytotoxic therapy | 1,771,906 | 13,925 | 7.14 (7.01–7.26) | 2.71 (2.68) | 592 | 2.60 (2.40–2.82) | 1.35 (1.21) |
| Hormone therapy | 240,728 | 745 | 2.59 (2.41–2.79) | 1.37 (1.24) | 33 | 1.05 (0.74–1.47) | 0.06 (-0.52) |
| Immunotherapy | 367,813 | 3816 | 8.92 (8.63–9.21) | 3.11 (3.06) | 989 | 21.74 (20.39–23.18) | 4.34 (4.24) |
| Targeted therapy | 2,211,589 | 14,236 | 5.83 (5.73–5.93) | 2.42 (2.40) | 442 | 1.54 (1.40–1.69) | 0.61 (0.45) |
AKI, acute kidney injury; CI, confidence interval; IC, information component; ROR, reported odds ratio; TIN, tubulointerstitial nephritis.
Number in bold indicates statistical significance (P < 0.05).
Overall, a sex disproportion has been observed for both, AKI and TIN. Females showed slightly higher association with AKI (male IC [IC0.25]: 2.42 [2.40], female IC [IC0.25]: 2.75 [2.72]), while males appeared to have a slightly higher association with TIN (male IC [IC0.25]: 1.98 [1.89], female IC [IC0.25]: 1.75 [1.62]). However, the degree of association between males and females varied widely across different age groups (Tables 2 and 3). Similar associations were observed between anticancer drug and AKI and TIN (Table S2 and S3).
Table 3.
Subgroups analysis of anticancer-associated AKI and TIN cases.
| IC (IC0.25) based on age, years | ||||||||
|---|---|---|---|---|---|---|---|---|
| AKI | TIN | |||||||
| 0–17 year | 18–44 years | 45–64 years | ≥ 65 years | 0–17 year | 18–44 years | 45–64 years | ≥ 65 years | |
| Sex | ||||||||
| Male | 4.28 (4.14) | 2.95 (2.86) | 2.32 (2.27) | 1.92 (1.88) | 2.88 (2.41) | 3.48 (3.27) | 1.59 (1.41) | 1.63 (1.49) |
| Female | 3.27 (3.06) | 3.42 (3.32) | 2.76 (2.70) | 2.19 (2.15) | − 0.48 (− 2.24) | 2.45 (2.08) | 1.61 (1.38) | 1.52 (1.32) |
| Anticancer drugs | ||||||||
| Cytotoxic therapy | 3.92 (3.80) | 3.33 (3.24) | 2.47 (2.42) | 2.15 (2.10) | 2.41 (1.96) | 3.12 (2.87) | 0.83 (0.56) | 0.63 (0.34) |
| Hormone therapy | NA | 1.38 (0.65) | 0.83 (0.47) | 1.11 (0.96) | NA | 1.10 (− 0.97) | − 0.62 (− 2.69) | 0.40 (− 0.28) |
| Immunotherapy | 3.01 (2.59) | 3.28 (3.08) | 3.44 (3.35) | 2.85 (2.77) | NA | 4.35 (4.00) | 4.48 (4.28) | 4.59 (4.44) |
| Targeted therapy | 4.26 (3.96) | 2.98 (2.83) | 2.58 (2.53) | 2.03 (1.99) | NA | 2.35 (1.87) | 0.63 (0.30) | 0.58 (0.35) |
| Total | ||||||||
| IC (IC0.25) | 7.62 (7.51) | 5.52 (5.44) | 4.54 (4.50) | 4.38 (4.35) | 5.33 (4.88) | 5.44 (5.26) | 3.61 (3.47) | 3.94 (3.83) |
| ROR (95% CI) | 18.34 (17.03–19.76) | 10.54 (10.08–11.03) | 7.49 (7.32–7.67) | 5.31 (5.21–5.41) | 4.42 (3.36–5.82) | 10.60 (9.42–11.93) | 3.49 (3.19–3.82) | 3.64 (3.38–3.92) |
AKI, acute kidney injury; CI, confidence interval; IC, information component; ROR, reported odds ratio; TIN, tubulointerstitial nephritis.
Number in bold indicates statistical significance (P < 0.05).
From an age-specific perspective, reports associated with AKI were most prevalent in the age group of 0–17 years (IC [IC0.25]: 7.62 [7.51]), followed by the age groups of 18–44 years (IC [IC0.25]: 5.52 [5.44]), 45–64 years (IC [IC0.25]: 4.54 [4.50]), and over 65 years (IC [IC0.25]: 4.38 [4.35]). In contrast, reports associated with TIN were most significant in the age group of 18–44 years (IC [IC0.25]: 5.44 [5.26]), followed by the age groups of 0–17 years (IC [IC0.25]: 5.33 [4.88]), over 65 years (IC [IC0.25]: 3.94 [3.83]), and 45–64 years (IC [IC0.25]: 3.61 [3.47]). Cytotoxic therapy, immunotherapy, and targeted therapy exhibited association with AKI and TIN across all age groups, whereas hormone therapy showed no association with TIN across all age groups.
Top anticancer drugs associated with kidney-related adverse reactions
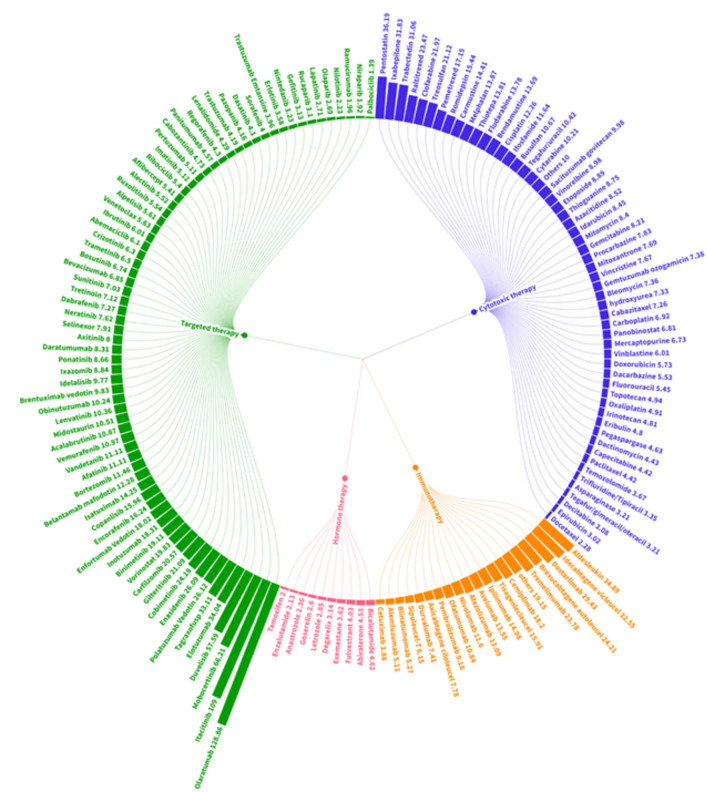
The disproportionality analysis of AKI cases associated with all specific medications is depicted in Fig. 3 and Table S1. Among conventional cytotoxic therapy agents, pentostatin (ROR: 36.19; IC0.25: 3.01), ixabepilone (ROR: 31.83; IC0.25: 3.81) and trabectedin (ROR: 31.06; IC0.25: 4.48) exhibited significantly elevated ROR and IC0.25 values. Indicating substantial disproportionality. Among all anticancer drug classes, targeted therapy showed the highest number of associated specific medications. Especially medications such as olaratumab (ROR: 128.86; IC0.25: 5.30), itacitinib (ROR: 109.00; IC0.25: 3.20), mobecertinib (ROR: 66.21; IC0.25: 3.41), and duvelisib (ROR: 57.59; IC0.25: 3.01) showed significant disproportionality. Immunotherapy emerged as another notable factor contributing to the increasing incidence of AKI cases associated with anticancer drugs. Aldesleukin, a recombinant form of human interleukin (IL)-2 (ROR 34.89; IC0.25: 4.45), and chimeric antigen receptor (CAR) T cell therapy drugs such as idecabtagene vicleucal (ROR: 32.55; IC0.25: 3.03), brexucabtagene autoleucel (ROR: 24.21; IC0.25: 2.74), and tisagenlecleucel (ROR 15.91; IC0.25: 2.74) were prominently represented among the top disproportionality signals within the immunotherapy class. Additionally, PD-1 inhibitors such as dostarlimab (ROR 29.43; IC0.25: 2.97), and CTLA-4 inhibitors such as tremelimumab (ROR 23.78; IC0.25: 2.81) showed significant associations with AKI.
Figure 3.
ROR of AKI cases in association with all specific medications categorized under 4 classes of anticancer drugs. AKI, acute kidney injury; ROR, reported odds ratio.
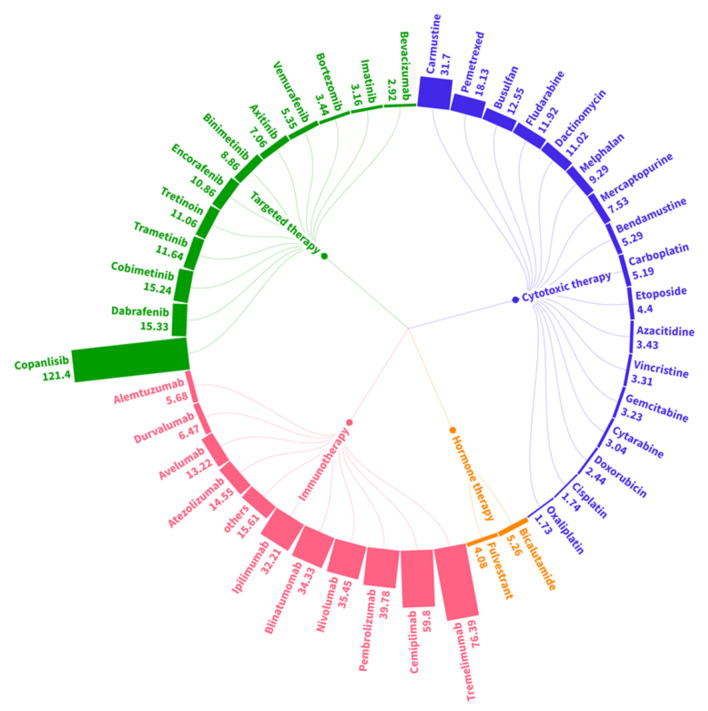
In the case of TIN, fewer cases were reported compared to AKI, resulting in a limited number of drugs showing significant signals (Fig. 4 and Table S1). Among the four classes of anticancer drugs, immunotherapy had the largest number of medications showing a strong association, with ROR values exceeding 30. Tremelimumab (ROR: 76.39; IC0.25: 1.72), cemiplimab (ROR: 59.80; IC0.25: 3.58), pembrolizumab (ROR: 39.78; IC0.25: 5.02), nivolumab (ROR: 35.45; IC0.25: 4.87), blinatumomab (ROR: 34.33; IC0.25: 3.91), and ipilimumab (ROR: 32.21; IC0.25: 4.55) exhibited ROR values exceeding 30, indicating a substantial association with AKI. However, there were no CAR-T cell therapies that exhibited a significantly high disproportionality signal in TIN. Notably, carmustine (ROR: 31.70; IC0.25: 2.46) among cytotoxic therapy, as well as copanlisib (ROR: 121.40; IC0.25: 1.78) among targeted therapy, showed a significant association with AKI.
Figure 4.
ROR of TIN cases in association with all specific medications categorized under 4 classes of anticancer drugs. ROR, reported odds ratio; TIN, tubulointerstitial nephritis.
Discussion
Key findings
In this study, we conducted a large-scale, long-term investigation into the prevalence of all anticancer-associated AKI and TIN, utilizing worldwide data from the WHO international pharmacovigilance database. Since the 2010s, reports of anticancer therapy-associated AKI and TIN have markedly increased, attributed to a sharp rise in reports linked to targeted therapy and immunotherapy. It was evident that almost every drug class could potentially induce AKI and TIN, with immunotherapy being particularly associated with both AKI and TIN, followed by cytotoxic therapy, targeted therapy, and hormone therapy in the case of AKI, and followed by cytotoxic therapy and targeted therapy in the case of TIN. Interestingly, it was observed that immunotherapy displayed a more significant association with AKI and TIN compared to cytotoxic therapy, which is already well-known for its nephrotoxicity. Among the immunotherapy agents, CAR-T cell therapy showed a stronger association with AKI than with TIN. In contrast to previous findings suggesting that hormone therapy contributes to an increased risk of AKI23, our study revealed that hormone therapy did not exhibit a significant association with AKI compared to other drug classes, nor did it show any association with TIN. Unlike most AKI cases attributed to physiological causes occurring more commonly in the older population, anticancer therapy-associated AKI and TIN were more prevalent in younger age groups. Additionally, the fatality rates for anticancer therapy-associated AKI and TIN were found to be 23.6% and 16.3%, respectively. Considering the higher fatality rates and increased occurrence in younger age groups compared to AKI caused by other etiologies, careful consideration is warranted in selecting safer medications for patients who are expected to be more vulnerable to AKI or TIN when choosing anticancer drugs.
Plausible underlying mechanisms
Immunotherapy effectively counteracts immune evasion of tumor cells as it enhances the pro-inflammatory process against the tumor24. However, this process involves interactions with the immune system, leading to certain adverse events through various mechanisms. Among them, IL-2, including aldesleukin, has the potential to trigger a severe capillary leak syndrome, leading to edema, depletion of plasma volume, and a reversible decline in glomerular filtration rate, which is primarily attributed to hypovolemia and can therefore result in AKI25. This provides insight into why aldesleukin shows a stronger correlation with AKI compared to TIN. Furthermore, the majority of immune checkpoint inhibitors (ICPi) operate by suppressing down-regulatory immune pathways, aiming to augment the anti-tumor immune response26. Previous studies have revealed that the majority of lesions in patients with ICPi- induced AKI detected through biopsy were acute TIN24,27. This finding aligns with our observation that ICPi exhibits particularly strong association with TIN. ICPi-induced TIN is characterized by severe inflammation involving infiltrates of inflammatory cells, with or without granuloma formation, primarily mediated by cell-mediated immunity28. It is assumed that ICPi reactivate drug-specific T cells and generate auto-reactive T cells and autoantibodies, which then target tubular epithelial cells, mesangial cells, and podocytes29. These processes contribute to the development of both AKI and TIN as consequences of ICPi treatment. Furthermore, CAR-T therapy, a type of immunotherapy, involves introducing the patient's T cells that specifically target tumor antigens. This process often results in a significant release of cytokines, leading to cytokine release syndrome. Cytokine-mediated capillary leak subsequently causes intravascular volume depletion, resulting in AKI30. This explains why CAR-T therapy shows a particularly significant association with AKI.
Within the realm of targeted therapy, certain medications act by inhibiting factors contributing to vascular formation, such as platelet-derived growth factor and vascular endothelial growth factor, thereby disrupting tumor angiogenesis31. For instance, VEGF receptor antibodies interfere with normal glomerular function and the integrity of the glomerular basement membrane, leading to AKI32. Additionally, phosphoinositide 3-kinase phosphorylates mammalian target of rapamycin33, which plays a pivotal role in signaling renal regeneration and repair in tubular cells and interstitial fibroblasts34. Consequently, inhibitors of phosphoinositide 3-kinase or mammalian target of rapamycin disrupt these processes, contributing to the occurrence of AKI. Interestingly, certain targeted therapies exhibited stronger association with AKI and TIN. For instance, olaratumab, a platelet-derived growth factor receptor antibody, may lead to AKI by preventing angiogenesis and kidney development, as PDGF signaling is crucial for these processes35. Copanlisib, a PI3-kinase inhibitor, may be associated with TIN by blocking the PI3Kγ-Akt pathway, which plays a protective, antiapoptotic role in the kidney, thus leading to increased apoptosis and accelerated renal tubular cell death36.
New aspects and comparison with previous research
AKI is typically more prevalent in older individuals due to age-related declines in kidney function, rendering them more vulnerable to injury37. However, our findings indicate that AKI shows stronger associations in younger age groups. A previous study has linked the occurrence of AKI in critically ill children and young adults to poor outcomes, often resulting in increased mortality rates38. This suggests that AKI occurring in younger age groups can result in poorer prognosis.
While hormone therapy showed an association with AKI, it exhibited no association with TIN. Additionally, AKI showed significantly lower association compared to other drug classes. However, previous studies have reported an increased risk of AKI occurrence with hormone therapy like androgen deprivation therapy23,39. While our study primarily investigated the relationship between various anticancer treatments and AKI, hormone therapy has also been of interest due to its potential association with AKI. Previous research have proposed that hormone therapies, particularly androgen deprivation therapy, might lead to metabolic changes, subsequently reducing glomerular function. Additionally, testosterone has been thought to have a protective effect on renal function by inducing vasodilation of renal vessels23.
In our findings, although hormone therapy showed an association with AKI, the risk of occurrence was markedly lower compared to other drug classes, and the number of reports was minimal, indicating relatively fewer kidney-related side effects.
Clinical and policy implications
Considering kidney-related ADRs when selecting anticancer therapy is crucial for patient mortality and safety. Cancer patients, especially those combining various medications, such as proton pump inhibitors or nonsteroidal anti-inflammatory drugs, are more vulnerable to AKI or TIN24. Our findings indicate that precautions should be taken when administering immunotherapy to patient groups that are expected to be more susceptible to AKI or TIN, given the higher association between immunotherapy and these ADRs compared to the nephrotoxicity caused by traditional chemotherapy. Since AKI due to ICPi is associated with increased rates of chronic kidney disease and mortality, extra vigilance is necessary in groups with a low baseline glomerular filtration rate38. In the event of AKI, drug discontinuation is a sensible approach. However, when TIN is suspected, there is an ongoing debate regarding whether a biopsy is necessary or if empirical treatment with corticosteroids is sufficient40. Some studies suggest that early corticosteroid use is associated with higher odds of kidney function recovery, highlighting the need for appropriate decision-making based on the individual patient's condition27. Our study revealed that the median time to onset for AKI and TIN was 16 days and 27 days, respectively, following the administration of anticancer agents. This finding underscores the critical importance of thorough monitoring and the implementation of effective therapeutic interventions in the weeks following the initiation of anticancer treatment. Healthcare professionals should remain proactive in identifying and managing these ADRs to ensure the safety of cancer patients undergoing treatment.
Strengths and limitations
This study has several limitations. Firstly, we were unable to ascertain the exact denominator of patients exposed to anticancer drugs, which precluded the calculation of actual incidence rates. Therefore, all values are expressed in relative terms, considering that VigiBase aggregates global data, enabling generalization of the findings. Given the limitation in relative reporting and the higher reporting rates in regions such as the Americas, including the United States, and Europe, where easier access to new anticancer drugs is possible, it could be suggested that our study's global distribution pattern also reflected this trend. Although Vigibase encompasses reports from more than 170 countries worldwide, there could be bias between countries due to underreporting in some regions. AKI is known to be more prevalent among Black individuals compared to other races due to various factors, including genetic and socioeconomic risks41. Therefore, cautious interpretation of the data is necessary, as there could be potential bias between races in the reported cases.
Secondly, among the kidney-related ADRs which we focused on, such as TIN, they can only be verified by a diagnostic kidney biopsy. However, data related to renal biopsy results were not consistently included in the WHO's reporting system, making it changing to determine the exact cause of kidney injury. Advancements in diagnostic tools and increased awareness among medical professionals may have contributed to the rise in TIN reports since 2000, compared to AKI, which has been more easily detectable and reported since the 1970s42,43. Thus, as the diagnostic process for TIN is more complex than AKI, it can lead to underdiagnosis, especially in countries with limited medical resources.
Thirdly, preexisting kidney diseases were not collected in the VigiBase database, as only certain drug information and related events were mentioned. Individuals undergoing anticancer treatment often receive various drugs alongside their primary therapy, which may act as AKI inducers. NSAIDs, antimicrobial agents, and certain antibiotics combinations can all contribute to the development of AKI through various mechanisms44,45. Furthermore, previous studies have reported that the co-administration of proton pump inhibitors with immune checkpoint inhibitors is a high-risk factor for sustained AKI46. Other medications, such as diuretics, angiotensin-converting enzyme inhibitors, and angiotensin-receptor blockers, are also associated with AKI47. The effect of these commonly co-administered drugs should be considered as an important confounding factor that may potentially lead to bias in our findings. Although most reports in VigiBase were of single drug suspects, minimizing the influence of non-anticancer medications, we cannot exclude the possibility of bias due to other drugs.
Fourthly, this pharmacovigilance analysis did not categorize the reports based on specific cancer types for each anticancer drug. Also, the increasing use of combination therapies and the prolonged administration of anticancer drugs have made it challenging to thoroughly analyze individual drugs. However, single drugs accounted for close to 99% of AKI and TIN reports (Table 1), thus, we believe that this aspect may not have a significant impact. In addition, given that malignant tumors themselves pose a risk factor for kidney injury48, further research is essential to determine the relative risk of anticancer drugs for ADRs. Due to the lack of data on the number of comorbidities and concomitant prescribed drugs, which can significantly impact kidney function, underscores the need for further investigation. Moreover, our analysis reported high mortality rates due to AKI and TIN following anticancer treatment. However, due to the limitations of VigiBase as a spontaneous reporting database, we acknowledge the potential bias in our results and the need for careful interpretation, as we could not exclude all cases where death may not have been directly caused by AKI and TIN or may have been influenced by underlying malignancies.
Lastly, VigiBase is established as a spontaneous reporting system and relies on disproportional analyses vulnerable to quantitative evaluation. Although VigiBase is thoroughly managed within the WHO-UMC system, reporting bias exists and several strategies have been used to mitigate it. We utilized two metrics, IC with IC0.25 and ROR with 95% CI, to cross-validate the association of anticancer drugs with AKI and TIN. When anticancer drugs were used singly, adverse effects of AKI were reported at 99.4% and TIN at 98.4%, with TTO information enhancing the reliability and causality of the reported data. Additionally, we conducted an analysis using only reports from health professionals and found consistent results across all findings. Despite these efforts, unpredictable bias cannot be totally ruled out, but robust methodologies and extensive supplementary analyses are expected to minimize the impact of such biases. Further research, including prospective cohort studies, is necessary to substantiate our findings.
Despite these limitations, this study possesses significant strengths due to its utilization of large-scale, long-term data from the WHO VigiBase. Contrary to previous studies that analyzed only certain anticancer drug classes, our study examined the adverse effects of AKI and TIN for all drug classes, enabling comparisons among anticancer drugs. Additionally, by examining the associations of individual medications, we could identify which drugs within the same class are most closely related to kidney-related adverse effects. Furthermore, by scrutinizing the frequency of adverse events based on sex and age, we identified differences from the commonly known risk factors for AKI, emphasizing the need for careful monitoring even in young age groups, where AKI is generally less expected when administering anticancer drugs. This large-scale survey based on the WHO database will assist healthcare workers in selecting the most appropriate and safe medications for patients when administering anticancer drugs.
Conclusions
Our study, utilizing data from the WHO, revealed a significant increase in kidney-related ADRs, particularly AKI and TIN, since 2010, primarily attributed to targeted therapy and immunotherapy. Immunotherapy displayed the strongest association with both AKI and TIN, followed by cytotoxic therapy, targeted therapy, and hormone therapy for AKI, and cytotoxic therapy and targeted therapy for TIN. While most drugs were linked to AKI and TIN, hormone therapy showed no association with TIN and had the lowest association with AKI compared to other drug classes. Regarding age, younger age groups, especially those under 45, exhibited a higher association with AKI and TIN, which contrasts with the common association of AKI with older age. The onset of ADRs typically took a few weeks to manifest, with fatality rates of 23.6% for AKI and 16.3% for TIN. This study underscores the significance of kidney-related ADRs, as they can also be fatal in younger age groups. These findings will aid in the selection of suitable and safe anticancer medications for individuals while also providing more precise information regarding kidney-related ADRs following cancer treatment.
Supplementary Information
Acknowledgements
The authors extend their gratitude to the Uppsala Monitoring Centre for providing and granting permission to utilize the data analyzed in this study. As a result, the authors, reviewers, and editorial team have invested significant effort and deliberation in addressing these complexities. The results and conclusions presented herein reflect the perspectives of the authors and do not necessarily align with those of the Uppsala Monitoring Centre or the World Health Organization. Therefore, the information provided does not represent the official opinions of the Uppsala Monitoring Centre or the World Health Organization.
Author contributions
Dr Dong Keon Yon had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version before submission. Study concept and design: S-YY, SL, KL, Hayeon Lee, KJ, and DKY; Acquisition, analysis, or interpretation of data: S-YY, SL, KL, Hayeon Lee, KJ, and DKY; Drafting of the manuscript: S-YY, SL, KL, Hayeon Lee, KJ, and DKY; Critical revision of the manuscript for important intellectual content: all authors; Statistical analysis: S-YY, SL, KL, Hayeon Lee, KJ, and DKY; Study supervision: DKY. DKY supervised the study and is guarantor for this study. S-YY, SL, KL, and JSK contributed equally. Hayeon Lee, KJ, and DKY contributed equally as a corresponding author. DKY is the senior author. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT; 2022R1F1A1074102). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Data availability
Data are available on reasonable request. Study protocol, statistical code: available from DKY (email: yonkkang@gmail.com). Dataset: available from the Uppsala Monitoring Centre or World Health Organization through a data use agreement.
Competing interests
Andreas Kronbichler received research grants from CSL Vifor and Otsuka, and fees for consultancy from CSL Vifor, Otsuka, Walden Biosciences, Catalyst Biosciences, AstraZeneca, Glaxo Smith Kline, Roche, and Delta4.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Soo-Young Yoon, Sooji Lee, Kyeongmin Lee and Jin Sug Kim.
Contributor Information
Hayeon Lee, Email: wwhy28@khu.ac.kr.
Kyunghwan Jeong, Email: khjeong@khu.ac.kr.
Dong Keon Yon, Email: yonkkang@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-67020-x.
References
- 1.Tran KB, et al. The global burden of cancer attributable to risk factors, 2010–19: A systematic analysis for the global burden of disease study 2019. Lancet. 2022;400:563–591. doi: 10.1016/S0140-6736(22)01438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization, W. H. WHO methods and data sources for life tables 1990-2019, <https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy> (Dec 2020).
- 4.Pishvaian MJ, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508–518. doi: 10.1016/s1470-2045(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitchlu A, et al. Acute kidney injury in patients receiving systemic treatment for cancer: A population-based cohort study. J Natl Cancer Inst. 2019;111:727–736. doi: 10.1093/jnci/djy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon SY, et al. National trends in the prevalence of chronic kidney disease among Korean adults, 2007–2020. Sci Rep. 2023;13:5831. doi: 10.1038/s41598-023-33122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canet E, et al. Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: Impact on remission and survival. PLoS One. 2013;8:e55870. doi: 10.1371/journal.pone.0055870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang E, et al. Acute kidney injury predicts all-cause mortality in patients with cancer. Cancer Med. 2019;8:2740–2750. doi: 10.1002/cam4.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 10.Giraud EL, et al. Dose recommendations for anticancer drugs in patients with renal or hepatic impairment: An update. Lancet Oncology. 2023;24:e229. doi: 10.1016/S1470-2045(23)00216-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, et al. Global estimates on the reports of vaccine-associated myocarditis and pericarditis from 1969 to 2023: Findings with critical reanalysis from the WHO pharmacovigilance database. J Med Virol. 2024;96:e29693. doi: 10.1002/jmv.29693. [DOI] [PubMed] [Google Scholar]
- 12.Jeong YD, et al. Global and regional burden of vaccine-associated facial paralysis, 1967–2023: Findings from the WHO international pharmacovigilance database. J Med Virol. 2024;96:e29682. doi: 10.1002/jmv.29682. [DOI] [PubMed] [Google Scholar]
- 13.Salem J-E, et al. Anticancer drug-induced life-threatening ventricular arrhythmias: A World Health Organization pharmacovigilance study. Eur Heart J. 2021;42:3915–3928. doi: 10.1093/eurheartj/ehab362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haubitz M, et al. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int. 2002;61:1495–1501. doi: 10.1046/j.1523-1755.2002.00279.x. [DOI] [PubMed] [Google Scholar]
- 15.Geetha D, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis with renal involvement. Journal of the American Society of Nephrology. 2015;26:976–985. doi: 10.1681/ASN.2014010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ives NJ, et al. Adjuvant interferon-α for the treatment of high-risk melanoma: An individual patient data meta-analysis. Eur J Cancer. 2017;82:171–183. doi: 10.1016/j.ejca.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Kasper LH, Reder AT. Immunomodulatory activity of interferon-beta. Ann Clin Transl Neurol. 2014;1:622–631. doi: 10.1002/acn3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13:519–523. doi: 10.1002/pds.1001. [DOI] [PubMed] [Google Scholar]
- 19.Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22:57–69. doi: 10.1177/0962280211403604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min C. The importance of a world health organization international pharmacovigilance database (VigiBase): Novel methods for safety monitoring and surveillance of medical products. Life Cycle. 2022;2:13. doi: 10.54724/lc.2022.e13. [DOI] [Google Scholar]
- 21.Choi Y, et al. Acute and post-acute respiratory complications of SARS-CoV-2 infection: Population-based cohort study in South Korea and Japan. Nat Commun. 2024;15:4499. doi: 10.1038/s41467-024-48825-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo HG, et al. Global burden of vaccine-associated multiple sclerosis, 1967–2022: A comprehensive analysis of the international pharmacovigilance database. J Med Virol. 2024;96:e29591. doi: 10.1002/jmv.29591. [DOI] [PubMed] [Google Scholar]
- 23.Gandaglia G, et al. Gonadotropin-releasing hormone agonists and acute kidney injury in patients with prostate cancer. Eur Urol. 2014;66:1125–1132. doi: 10.1016/j.eururo.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Cortazar FB, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638–647. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guleria AS, et al. Renal dysfunction associated with the administration of high-dose interleukin-2 in 199 consecutive patients with metastatic melanoma or renal carcinoma. J Clin Oncol. 1994;12:2714–2722. doi: 10.1200/jco.1994.12.12.2714. [DOI] [PubMed] [Google Scholar]
- 26.Izzedine H, et al. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transpl. 2017;32:936–942. doi: 10.1093/ndt/gfw382. [DOI] [PubMed] [Google Scholar]
- 27.Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68:287–291. doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 28.Wanchoo R, et al. Renal toxicities of novel agents used for treatment of multiple myeloma. Clin J Am Soc Nephrol. 2017;12:176–189. doi: 10.2215/cjn.06100616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moturi K, Sharma H, Hashemi-Sadraei N. Nephrotoxicity in the age of immune checkpoint inhibitors: Mechanisms, diagnosis, and management. Int J Mol Sci. 2023;25:414. doi: 10.3390/ijms25010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-Cell (CAR-T) therapy for diffuse large B-Cell lymphoma. Am J Kidney Dis. 2020;76:63–71. doi: 10.1053/j.ajkd.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Board R, Jayson GC. Platelet-derived growth factor receptor (PDGFR): A target for anticancer therapeutics. Drug Resist Updat. 2005;8:75–83. doi: 10.1016/j.drup.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Eremina V, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong J, et al. Nephrotoxicity assessment of podophyllotoxin-induced rats by regulating PI3K/Akt/mTOR-Nrf2/HO1 pathway in view of toxicological evidence chain (TEC) concept. Ecotoxicol Environ Saf. 2023;264:115392. doi: 10.1016/j.ecoenv.2023.115392. [DOI] [PubMed] [Google Scholar]
- 34.Gui Y, Dai C. mTOR signaling in kidney diseases. Kidney. 2020;1:1319–1327. doi: 10.34067/kid.0003782020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandey P, et al. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed Pharm. 2023;161:114491. doi: 10.1016/j.biopha.2023.114491. [DOI] [PubMed] [Google Scholar]
- 36.Kuwana H, et al. The phosphoinositide-3 kinase γ–Akt pathway mediates renal tubular injury in cisplatin nephrotoxicity. Kidney Int. 2008;73:430–445. doi: 10.1038/sj.ki.5002702. [DOI] [PubMed] [Google Scholar]
- 37.O'Sullivan ED, Hughes J, Ferenbach DA. Renal aging: Causes and consequences. J Am Soc Nephrol. 2017;28:407–420. doi: 10.1681/asn.2015121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherer MV, et al. Androgen deprivation therapy and acute kidney injury in patients with prostate cancer undergoing definitive radiotherapy. Prostate Cancer Prostatic Dis. 2023;26:276–281. doi: 10.1038/s41391-021-00415-3. [DOI] [PubMed] [Google Scholar]
- 40.Brahmer JR, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9:002435. doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grams ME, et al. Explaining the racial difference in AKI incidence. J Am Soc Nephrol. 2014;25:1834–1841. doi: 10.1681/asn.2013080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poggio ED, et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol. 2020;15:1595–1602. doi: 10.2215/CJN.04710420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Praga M, González E. Acute interstitial nephritis. Kidney Int. 2010;77:956–961. doi: 10.1038/ki.2010.89. [DOI] [PubMed] [Google Scholar]
- 44.Magee DJ, Jhanji S, Poulogiannis G, Farquhar-Smith P, Brown MRD. Nonsteroidal anti-inflammatory drugs and pain in cancer patients: A systematic review and reappraisal of the evidence. Br J Anaesth. 2019;123:e412–e423. doi: 10.1016/j.bja.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul M, et al. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database Syst Rev. 2013;2013:cd03038. doi: 10.1002/14651858.CD003038.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seethapathy H, et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. 2019;14:1692–1700. doi: 10.2215/cjn.00990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izzedine H, Perazella MA. Anticancer drug-induced acute kidney injury. Kidney Int Rep. 2017;2:504–514. doi: 10.1016/j.ekir.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta S, Gudsoorkar P, Jhaveri KD. Acute kidney injury in critically ill patients with cancer. Clin J Am Soc Nephrol. 2022;17:1385–1398. doi: 10.2215/cjn.15681221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. Study protocol, statistical code: available from DKY (email: yonkkang@gmail.com). Dataset: available from the Uppsala Monitoring Centre or World Health Organization through a data use agreement.