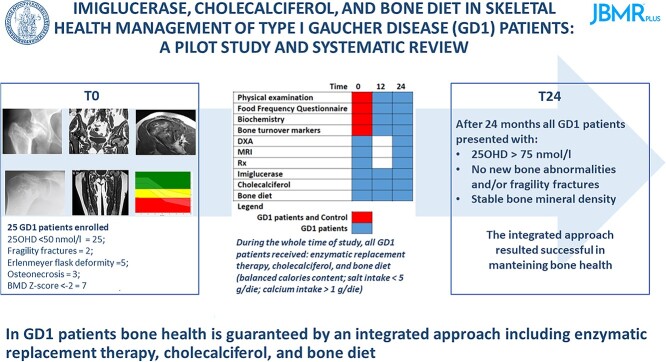
Abstract
Skeletal anomalies represent a characteristic feature of type 1 Gaucher disease (GD1). Here we evaluated the impact of an integrated therapy comprising enzyme-replacement therapy (ERT), cholecalciferol, and a normocalcemic-normocaloric-hyposodic diet (bone diet) on bone health in GD1 patients. We also performed a systematic review to compare our results with available data. From January 1, 2015 to February 28, 2019, all GD1 patients referred to Federico II University were enrolled and treated with the integrated therapy. Bone turnover markers and bone mineral density (BMD) were evaluated at baseline (T0) and after 24 months (T24). We enrolled 25 GD1 patients, all showing 25-hydroxy vitamin D (25OHD) levels < 50 nmol/l (hypovitaminosis D) at T0. Response to cholecalciferol treatment was effective, showing a direct relationship between 25OHD levels before and after treatment. At T0, 2 GD1 patients showed fragility fractures, 5 the Erlenmeyer flask deformity, 3 osteonecrosis, and 7 a BMD Z-score ≤ –2. Overall, GD1 patients with bone anomalies showed higher C-terminal telopeptide levels compared with those without bone anomalies. No new bone anomalies occurred during 2 years of follow-up. At T24, BMD remained stable across the entire study cohort, including in patients with bone anomalies. The systematic review showed that our study is the first that evaluated all bone health parameters. Hypovitaminosis D is prevalent in GD1 patients. The response to cholecalciferol treatment was effective but different to healthy subjects and in patients with metabolic bone disorders. Integrated therapy including ERT, cholecalciferol, and bone diet guarantees bone health.
Keywords: vitamin D, Gaucher disease, bone markers, enzyme-replacement therapy
Graphical Abstract
Graphical Abstract.
Introduction
Gaucher disease (GD) is a rare lysosomal storage disorder usually caused by mutations in the GBA1 gene, encoding the lysosomal enzyme beta-glucocerebrosidase, also known as acid beta-glucosidase (GCase). Other very rare forms of GD are caused by mutations in the PSAP gene that encodes the GCase activator protein, Saposin C.1 GCase deficiency leads to intra-lysosomal accumulation of glucosylceramide in the cells of the reticuloendothelial system of the liver, the spleen, and the bone marrow (Gaucher cells).1 GD is a proteiform disease and, conventionally, 3 main forms are described. GD type 1 (GD1, OMIM #230800) is the most common GD form (>90%), characterized by organomegaly (spleen and liver), hematologic manifestations (anemia and thrombocytopenia), and skeletal abnormalities, without a classical neurological impairment. Skeletal involvement is described in >80% of GD1 patients. Indeed, the 2023 version of nosology of genetic skeletal disorders classifies GD as a lysosomal storage disease with skeletal involvement (group 22).2 GD type 2 (GD2, OMIM#230900), or the acute neuropathic form, is characterized by early onset of neurological impairment, whereas GD type 3 (GD3, OMIM #231000), or the sub-acute neuropathic form, is similar to GD2 but with less severe neurologic and clinical impairment. Although these definitions are useful clinically, the 3 phenotypes are a continuum.3 Formal diagnosis of GD requires the measurement of GCase activity, while the genotyping confirms the diagnosis.1,4 Enzyme-replacement therapy (ERT) and substrate-reduction therapy (SRT) are gold-standard treatments for GD.5 ERT overcomes blocks in the catabolic pathway and reduces accumulated substrates, influencing the clinical features and life expectancy of GD patients. ERT significantly impacts on organomegaly and blood count alterations, but it does not completely prevent and/or treat bone involvement although it gradually reduces bone marrow infiltration, osteopenia, bone pain, and bone crises.6-8 Bone health is closely related to 25-hydroxy vitamin D (25OHD) status and an appropriate diet, characterized by low salt intake (<5 g/die), adequate calcium intake (>1 g/die), and balanced calorie content.9 Indeed, vitamin D deficiency and/or unhealthy diet worsen bone health.9,10 Due to a lack of data regarding the prevalence and management of hypovitaminosis D and bone response to integrated treatment in GD1 patients, the aims of this prospective study were to evaluate: (1) the vitamin D status and prevalence of GD1 skeletal anomalies, including alterations in bone mineral density (BMD); (2) the efficacy of cholecalciferol treatment in the correction of vitamin D deficiency; and (3) the efficacy of an integrated therapy comprising ERT, normocalcemic, a normocaloric and hyposodic diet (bone diet), and cholecalciferol on GD1 skeletal anomalies and bone turnover markers (BTMs).
We also performed a systematic review to evaluate the up-to-date status of vitamin D and management of bone health in GD1 patients.
Materials and methods
Study populations
We consecutively enrolled adult GD1 patients from Southern Italy referred to the Department of Clinical Medicine and Surgery of the Federico II University (FedII-DCMS), Naples, a level 3 centre for GD1 management (https://www.orpha.net/consor/cgi-bin/Clinics_Search.php?lng=IT&data_id=98631&Centri%20specializzati=Centre-of-Expertise-for-inborn-errors-of-metabolism&title=Centre%20of%20Expertise%20for%20inborn%20errors%20of%20metabolism&search=Clinics_Search_Simple) from January 1, 2015 to February 28, 2019. An alpha-numeric code, composed by “GD” and a number, was assigned to each GD1 patients based on enrollement time. The study included males and females older than 18 years who were affected by GD1 confirmed by both enzymatic and genetic assays. Exclusion criteria for GD1 patients: (1) age ≤ 18 years, (2) chronic kidney disease [estimated glomerular filtration rate (GFR) <60 mL/min/1.73 m2; according to Kidney Disease Improving Global Outcomes (KDIGO) classification (https://kdigo.org/wp-content/uploads/2024/03/KDIGO-2024-CKD-Guideline.pdf)],11 and (3) personal history of nephrolithiasis. Two healthy control subjects age-, sex-, and body mass index (BMI)-matched for all GD1 patients were enrolled among employers, fellows, and undergraduates of the Federico II University, in-law and non–genetically related relatives of GD1 patients, and family reference panel. Exclusion criteria for healthy controls included: (1) personal or familial history of GD, rickets,12 bone deformity, or short stature (≤–2.0 SD below a population’s mean for age and gender),13 (2) personal history of nephrolithiasis, defined as calcification in the kidney collecting system with a diameter higher than 2 millimeters, evaluated with ultrasound or evaluated using a fixed sequence questionnaire aimed at detecting a history of upper urinary tract stones,14 chronic kidney disease,11 or gouty diathesis, described as the formation of urinary stones in primary gout in the presence or absence of gouty arthritis,11,15 or (3) biochemical evidence of secondary bone disorders. These latter were excluded according to first- and second-tier investigations proposed by the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS).16 Controls were evaluated only at baseline.
Vitamin D status and cholecalciferol treatment
To evaluate the vitamin D status in GD1 patients, we referred to criteria proposed by SIOMMMS for management of diseases with bone phenotype.17 In particular, in GD1 patients, 25OHD levels < or ≥75 nmol/L identified vitamin D deficiency and sufficiency, respectively.17 The vitamin D deficiency was treated with 50 000 UI (1.25 mg) of cholecalciferol every week for 8 weeks. In case of non-correction of vitamin D deficiency, GD1 patients carried out a second cholecalciferol treatment, according to dosage previously exposed (50 000 UI of cholecalciferol every week for 8 weeks).10 After vitamin D deficiency correction, a maintenance therapeutic protocol was started and maintained for the entire follow-up, using cholecalciferol 50 000 UI monthly.10 GD1 patients with vitamin D levels ≥75 nmol/L were treated with cholecalciferol at the maintenance dosage during the entire follow-up. The adherence to cholecalciferol treatment was assessed by 25OHD serum levels dosage every 6 months. The eventually side effects were evaluated according to published criteria.18
ERT treatment
All enrolled GD1 patients were treated with ERT (Imiglucerase), obtaining the therapeutic goals expressed by Pastores and colleagues.19 The ERT dosage at enrolment is reported in Table 1.
Table 1.
Clinical characteristics of patients with Gaucher disease type 1 at enrolment.
| Cd | Sex | GBA mutation | Age at enrollment |
Age at
GD1 diagnosis a (years) |
ERT Duration
(years) |
ERT dose
(U/kg/3wks) |
Bone involvement |
|---|---|---|---|---|---|---|---|
| GD01 | F | R353G/R353G | 49 | 17 | 32 | 28 | |
| GD02 | F | R353G/R353G | 41 | 11 | 30 | 54 | FF |
| GD03 | M | N370S/L444P | 44 | 28 | 16 | 22 | |
| GD04 | M | W312C/W312C | 40 | 14 | 26 | 28 | |
| GD05 | F | N370S/L444P | 56 | 37 | 19 | 29 | Z-score < –2 |
| GD06 | M | N370S/L444P | 57 | 39 | 18 | 20 | |
| GD07 | F | N370S/L444P | 47 | 24 | 23 | 28 | |
| GD08 | M | L444P/S364T | 29 | 19 | 10 | 24 | |
| GD09 | F | L444P/S364T | 25 | 9 | 16 | 30 | |
| GD10 | M | N370S/N188K | 60 | 54 | 6 | 30 | EFD |
| GD11 | F | N370S/L444P | 59 | 33 | 26 | 36 | |
| GD12 | M | N370S/L444P | 50 | 25 | 25 | 26 | ON - Z-score < –2 |
| GD13 | F | N370S/W393R | 29 | 4 | 25 | 34 | ON |
| GD14 | F | N370S/N370S | 69 | 45 | 24 | 20 | FF |
| GD15 | F | L444P /N370S | 20 | 4 | 16 | 30 | Z-score < –2 |
| GD16 | M | N370S/L354X | 48 | 28 | 20 | 28 | Z-score < –2 |
| GD17 | F | N370S/L354X | 49 | 30 | 19 | 35 | |
| GD18 | F | N370S/L354X | 38 | 17 | 21 | 44 | Z-score < –2 |
| GD19 | F | N370S/Mut AH006907.2:g. 1942_7678 with100042_14165 | 19 | 3 | 16 | 27 | EFD |
| GD20 | F | N370S/N370S | 68 | 57 | 11 | 43 | EFD |
| GD21 | F | N370S/D283N | 36 | 16 | 20 | 24 | |
| GD22 | M | N370S/F259L | 48 | 44 | 4 | 33 | EFD – Z-score < –2 |
| GD23 | F | N370S/F259L | 56 | 33 | 23 | 25 | Z-score < –2 |
| GD24 | F | R170C/589-12C > G | 36 | 36 | 0 | 0 | |
| GD25 | M | N370S/W393R | 50 | 50 | 0 | 0 | EFD – ON |
Age at GD1 diagnosis is the same age of treatment initiation.
Abbreviations: bone involvement, occurrence of skeletal anomalies including frailty fractures (FF), Erlenmeyer flask deformity (EDF), bone crises (BC), osteonecrosis (ON), and acute osteomyelitis (aOM); Cd, Enrolment code (alpha-numeric code assigned to each GD1 patients based on enlistment time); ERT dose, enzyme replacement therapy dose at enrolment; ERT duration, Years of treatment with ERT; F, female; GBA, gene encoding the lysosomal enzyme beta-glucocerebrosidase; GD1, Gaucher disease type 1; M, male; Z-score, bone mineral density (BMD) Z-score. GD01, GD02, GD10, and GD14 are splenectomised.
Bone diet
A normocalcemic (calcium intake ≥1 g/day), normocaloric and hyposodic (sodium intake ≤5 g/day) diet (bone diet) was suggested to all GD1 patients at enrolment (T0).11 Twenty-four hours urinary sodium excretion was used to assess the compliance to bone diet. GD1 patients, that have obtained a reduction in urinary sodium excretion ≥44 mmol/24 h (corresponding to a difference in sodium dietary intake ≥2.6 g/24 h), and/or a urinary sodium excretion ≤100 mmol/24 h, were defined as “diet compliant.”20
Menopause definition
According to criteria proposed by the World Health Organization, menopause was defined as permanent cessation of ovarian function, diagnosed after 12 consecutive months of secondary amenorrhea (www.who.int/news-room/fact-sheets/detail/menopause).
Clinical, biochemical, and instrumental parameters
The following clinical, biochemical, and instrumental parameters were measured at T0 and T24 in GD1 patients: weight, height, BMI, blood count, ferritin, calcium (Ca), phosphate, creatinine, 24 h urinary sodium, total alkaline phosphatase (tALP), bone alkaline phosphatase (bALP), 25OHD, 1,25-dihydroxyvitamin D [1,25(OH)2D3], chitotriosidase (CHT), C-terminal telopeptide released from type I collagen (CTx), total procollagen type 1 N-terminal propeptide (P1NP), parathyroid hormone (PTH), hepatic and splenic volume (expressed as multiples of normal volumes), and bone mineral density (BMD). The concentration of CTx [assay range (AR) 0.033–6.000 ng/mL, detection limit (DL) 0.023 ng/mL, inter-assay coefficients of variation (CV): 6.16%, intra-assay CV: 3.22%], intact P1NP (AR 2 – 230 ng/mL, DL 1 ng/mL, inter-assay CV 7.5%, intra-assay CV 2.86%), 1,25(OH)2D3 (AR 7.5–150 pg/mL; DL 4.2 pg/mL, inter-assay CV 4.6%, intra-assay CV 10.8%); bALP (AR 1–75 mcg/L, DL 0.4 mcg/L, inter-assay CV 7.28%, intra-assay CV 1.56%) were measured by IDS-iSYS analyser, which uses competitive [1,25(OH)2D3] or direct (CTx, P1NP, bALP) immunoassay and for quantification of reaction uses Chemiluminescent Acridinium Ester technology. Measurement of 25OHD (AR 4–150 ng/mL, DL 4 ng/mL, inter-assay CV 7.8%, intra-assay CV 2.3%) was conducted by means of competitive chemiluminescence immunoassay using the DiaSorin LIAISON 25OHD TOTAL assay (DiaSorin, Inc., Stillwater, Minnesota) and analyzed with LIAISON XL. The hepatic and splenic volume were estimated by magnetic resonance imaging (MRI) and expressed as multiples of normal volume.21 The BMD was measured by Dual-Energy X-ray Absorptiometry (DXA; General Electric’s Lunar Prodigy encore) at the lumbar spine (L1 to L4) and hip (total hip and femoral neck). All data were evaluated as part of research clinical protocol validated by the Ethical Committee of University of Naples, Federico II (EC protocol number 195/17).
GD1 skeletal anomalies – definition of fractures, Erlenmeyer flask deformity, bone crises, osteonecrosis, acute osteomyelitis, and reduced bone mineral density
At baseline and during the entire follow up, the occurrence of fragility fractures, bone crises, osteonecrosis, acute osteomyelitis, and Erlenmeyer flask deformity (EFD) were assessed and recorded. Fragility fractures were defined as bone fractures resulting from low-energy trauma.16 The vertebral fractures occurrence was evaluated by the semiquantitative Genant’s method followed by quantitative vertebral morphometry.16 Bone crises were defined as episodes of deep bone pain, usually located to one extremity or joint, associated with fever and leukocytosis.22 The occurrence of osteonecrosis, caused by bone ischemia causing bone tissue death, was evaluated using the Ficat staging system.7 Acute osteomyelitis was defined as a bone infection, which is often seeded hematogenously.7 Erlenmeyer flask deformity (EFD) was defined as the distal femur and endosteal scalloping based on BM infiltration in GD, which starts before puberty.23 Always at enrolment and after two years, the BMD was measured by DXA. According to Z-score, all GD1 patients were classified as normal BMD, defined as a Z-score > –2.0, and pathological low BMD, defined as a Z-score ≤ –2.0.8,16 For GD1 men older than 50 years and for post-menopausal GD1 women also Z-score values were examined, and GD1 were classified as normal BMD, defined as a T-score between +2.5 and –1, osteopenia, defined as a T-score between –1.0 and –2.4, and osteoporosis (Op), defined as a T-score ≥ –2.5.8,16
Systematic review
A systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines24 (Figure 1). Two independent authors (AV and VA) collected studies in PubMed, Google Scholar, Cochrane, Scopus, Web of Science, and Google Book databases on December 20, 2023, searching keywords included “vitamin D,” “Gaucher,” “Bone Markers,” “Enzyme Replacement Therapy”. All studies were screened for duplicated articles. All selected studies were acquired in full text. No language restriction was applied. We selected only original articles (cross-sectional study, prospective study, clinical trial). Two authors (AV and VA) independently extracted the parameters from the selected studies, and prepared a database. The extracted data included: (1) first author’s last name, (2) publication year, (3) country in which the study was performed, (4) latitude of the city in which patients were enrolled, (5) total number of enrolled patients, (6) gender, (7) mean age, (8) GD1 therapy, (9) prevalence of hypovitaminosis D, (10) treatment to correct hypovitaminosis D, (11) assessment for calcium intake, (12) assessment for BMD, (13) Op assessment, (14) bone anomalies assessment, (15) assessment for BTMs (resorption and formation bone markers), (16) assessment for PTH levels, (17) assessment for menopause occurrence, (18) follow-up duration. All eventually discrepancies related to the extracted data were resolved through a discussion between AV and VA with AB and DR. We selected only studies in which we found at least 4 of the following parameters: hypovitaminosis D, hypovitaminosis D treatment, assessment of calcium dietary intake, methodology used for BMD assessment, Op prevalence, bone anomalies, PTH assessment, BTM evaluation.
Figure 1.
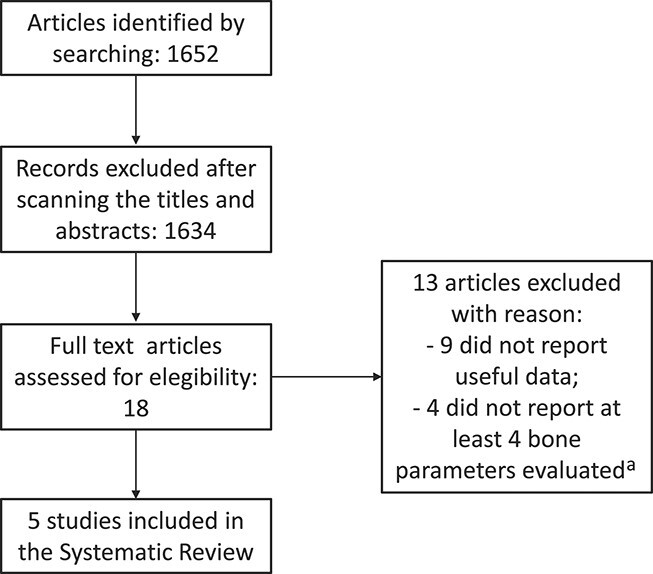
Systematic review flow-chart. aBone data: hypovitaminosis D, hypovitaminosis D treatment, assessment of calcium dietary intake, methodology used for bone mineral density assessment, osteoporosis prevalence, bone anomalies, parathormone assessment, bone turnover marker evaluation.
Statistical analysis
All statistical analyses were performed using the IBM SPSS Statistics software, version 23 (International Business Machines Corporation, Armonk, New York). The Kolmogorov–Smirnov test was used to assess the data distribution. Data are expressed as media ± standard deviation for continuous variables with normal distribution, as median (25°–75°) for continuous variables with not-normal distribution, and as absolute number (percentages) for categorical variables.25 The analysis of variance (ANOVA), the Mann–Whitney test, and the Chi-squared test were used to assess differences in continuous variables with normal distribution, in continuous variables with not-normal distribution, and in categorical variables, respectively. Differences from baseline after pharmacological treatments were examined using the Student’s t-test for paired samples in continuous variables with normal distribution. Pearson’s and Spearman’s correlation coefficient was used to determine relationships between different parameters at baseline and during follow-up. All reported P values are two-sided, and the significant level was set at P <0.05.
Results
We enrolled 25 GD1 patients [male (M): female (F) 9(34.6): 16(65.4); mean age 45.0 ± 13.5 years; BMI: 26.6 ± 5.9 kg/m2, 7 women in menopause], all from Southern Italy. A post-hoc sample size analysis indicated that the enrolled population was representative of all GD1 patients living in Southern Italy, given the estimated GD1 prevalence in Italy 0.89/100 000, according to Carubbi and colleagues,26 and the Southern Italy entire population (https://www.istat.it/it/files/2021/12/CENSIMENTO-E-DINAMICA-DEMOGRAFICA-2020.pdf), accepting a 90% confidence level and a 15% margin of error (https://www.epicentro.iss.it/strumenti/samplesize).
The clinical characteristics of enrolled GD1 patients are summarized in Tables 1 and 2. Fifty control subjects age, sex, and BMI-matched to GD1 patients [M: F 18 (36.0): 32 (64.0), mean age 45.3 ± 12.9 years, BMI: 26.4 ± 5.8 kg/m2, 14 women in menopause] were also enrolled. Biochemical parameters of GD1 patients and controls at T0 are reported in Table 2.
Table 2.
Clinical and biochemical parameters evaluated in patients with Gaucher disease type 1 at the enrolment and after 24 months.
| T0 | T24 | Control | |||||
|---|---|---|---|---|---|---|---|
| All (n = 25) | Z score ≤ –2 (n = 7) | Z score > –2 (n = 18) | All (n = 25) | Z score ≤ –2 (n = 7) | Z score > –2 (n = 18) | Values c | |
| BMI (kg/m2) | 25.4 ± 3.1 | 24.8 ± 5.1 | 25.6 ± 4.2 | 27.0 ± 4.9 | 25.2 ± 4.3 | 27.7 ± 5.2 | 25.7 ± 3.6 |
| CHT (nmol/mL/h) | 276.0 (211.3–421.0) | 255.5 (110.0–345.0) | 286 (229.0–451.5) | 164.0 (122.0–334.0) | 210.0 (63.75–315.0) | 164.0 (123.5–290.5) | |
| Calcium (mmol/L) | 2.39 ± 0.02 | 2.40 ± 0.03 | 2.39 ± 0.02 | 2.40 ± 0.03 | 2.45 ± 0.02 | 2.38 ± 0.03 | 2.40 ± 0.02 |
| Phosphate (mmol/L) | 0.97 ± 0.15 | 0.99 ± 0.16 | 0.97 ± 0.15 | 1.01 ± 0.16 | 0.99 ± 0.17 | 1.01 ± 0.15 | 0.99 ± 0.16 |
| Creatinine (μmol/L) | 69.9 ± 10.6 | 70.7 ± 10.7 | 69.6 ± 10.1 | 72.7 ± 10. 5 | 74.9 ± 11.3 | 71.8 ± 9.8 | 70.1 ± 10.2 |
| Hb (mmol/L) | 9.22 ± 0.91 | 9.22 ± 1.02 | 9.21 ± 0.87 | 9.11 ± 0.92 | 9.05 ± 0.97 | 9.13 ± 0.86 | 9.17 ± 0.89 |
| PLT (109/L) | 225.9 ± 53.8 | 207.8 ± 62.6 | 232.9 ± 57.6 | 179.5 ± 64.8 | 185.5 ± 63.0 | 177.2 ± 67.8 | 238.8 ± 58.7 |
| Ferritin (μg/L) | 154.0 (60.3–468.8) | 347.5 (42.75–542.25) | 154.0 (66.0–435.0) | 187.0 (77.0–313.5) | 432.5 (50.25–662.25) | 187.0 (87.0–263.0) | 128.7 ± 87.6 |
| Liver volume (MN) | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.39 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | |
| Splenic volume (MN)d | 3.2 ± 0.5 | 2.9 ± 0.6 | 3.3 ± 0.3 | 3.0 ± 0.5 | 3.2 ± 0.3 | 3.0 ± 0.6 | |
| Femur BMD (g/cm2) | 0.81 ± 0.17 | 0.80 ± 0.21 | 0.81 ± 0.16 | 0.82 ± 0.17 | 0.81 ± 0.19 | 0.82 ± 0.15 | |
| Femur Z-score | –0.29 ± 1.11 | –0.30 ± 1.19 | –0.29 ± 1.09 | –0.46 ± 1.07 | –0.38 ± 1.17 | –0.49 ± 1.01 | |
| Femur T-score | –0.80 ± 1.26 | –0.78 ± 1.31 | –0.81 ± 1.25 | –0.87 ± 1.23 | –0.77 ± 1.30 | –0.91 ± 1.21 | |
| Lumbar BMD (g/cm2) | 0.96 ± 0.17 | 0.98 ± 0.19 | 0.95 ± 0.16 | 0.95 ± 0.19 | 0.98 ± 0.20 | 0.94 ± 0.18 | |
| Lumbar Z-score | –0.25 ± 1.33 | –0.32 ± 1.38 | –0.22 ± 1.32 | –0.43 ± 1.38 | –0.35 ± 1.39 | –0.46 ± 1.31 | |
| Lumbar T-score | –0.92 ± 1.50 | –0.96 ± 1.52 | –0.90 ± 1.48 | –0.99 ± 1.48 | –0.96 ± 1.50 | –1.00 ± 1.47 | |
| tALP (U/L) | 75.9 ± 21.7 | 87.5 ± 10.8 | 71.4 ± 22.1 | 81.8 ± 21.2 | 92.5 ± 18.7 | 77.6 ± 22.9 | 78.8 ± 16.5 |
| bALP (μg/L) | 16.6 ± 10.5 | 13.4 ± 10.7 | 17.8 ± 10.5 | 16.6 ± 9.4 | 19.4 ± 9.3 | 15.5 ± 10.6 | 15.3 ± 9.8 |
| CTX (ng/mL) | 0.29 ± 0.22 | 0.58 ± 0.18b | 0.18 ± 0.22 | 0.26 ± 0.12 | 0.39 ± 0.15 | 0.21 ± 0.11 | 0.25 ± 0.12 |
| P1NP (ng/mL) | 57.7 ± 23.2 | 52.6 ± 27.2 | 59.7 ± 23.1 | 59.1 ± 23.5 | 59.9 ± 25.1 | 58.8 ± 19.2 | 50.3 ± 18.3 |
| PTH (pmol/L) | 5.4 ± 1.2a | 5.6 ± 1.3a | 5.3 ± 1.1a | 3.9 ± 1.0 | 3.7 ± 1.1 | 4.0 ± 1.0 | 3.6 ± 1.0 |
| 1,25(OH)2D (pmol/L) | 130.2 ± 55.9 | 138.6 ± 60.1 | 126.9 ± 55.1 | 139.5 ± 46.8 | 138.7 ± 59.9 | 139.8 ± 15.6 | 124.6 ± 42.2 |
| 25(OH)D (nmol/L) | 44.0 ± 14.6a | 42.8 ± 13.8a | 44.5 ± 14.6a | 85.2 ± 15.0 | 86.1 ± 16.4 | 84.9 ± 14.8 | 75.2 ± 19.7 |
Data are expressed as media ± standard deviation for continuous variables with normal distribution at Kolmogorov–Smirnov test and median (25°-75°) for continuous variables with not-normal distribution at Kolmogorov–Smirnov test. T0: data at enrolment. T24: data 24 months after the integrated therapy with (1) enzyme replacement therapy, (2) normocalcic, normocaloric, and hyposodic diet, and (3) cholecalciferol.
Significantly different compared to controls (P < 0.05, ANOVA).
Significantly different compared to GD1 patients with Z score > –2 (P < 0.05, ANOVA).
Control values measured in 2 healthy control subjects age-, BMI-, and sex-matched for each enrolled patient with Gaucher type 1 disease.
Four patients with Z-score > –2 were splenectomised.
Abbreviations: 1,25(OH)2D, 1-25 dyhydroxyvitamin D; 25(OH)D, 25 Hydroxyvitamin D; bALP, bone isoenzyme of alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; Calcium, total calcium corrected for albumin; CHT, chitothriosidase; CTX, C-terminal telopeptide of type I collagen; MN, multiples of normal volumes for spleen (0.2% of body weight) and liver (2.5% of body weight); P1PNP, total procollagen type 1 N-terminal propeptide; tALP, total alkaline phosphatase.
All GD1 patients were analyzed at T0 and T24. At T0, 2 GD1 patients reported a history of fragility fractures, 5 showed EFD, 3 reported a history of osteonecrosis, 7 a BMD Z-score ≤ –2.0 and 18 a BMD Z-score > –2 (Table 1). Two post-menopausal women (GD05 and GD23) and the man aged > 50 (GD12) with a BMD Z-score ≤ –2.0 showed a BMD T-score ≤ –2.5. Fourteen GD1 patients affected by skeletal anomalies (FF + EFD + Osteonecrosis + Op) had significantly higher CTx levels compared to GD1 patients without skeletal anomalies (0.42 ± 0.23 vs 0.24 ± 0.11 ng/mL, respectively; P <0.05). The age at initiation and the duration of ERT treatment were not different between GD1 patients with and without skeletal anomalies. No significant relationship was found between different GBA1 mutations and the occurrence of skeletal anomalies in GD1 patients. Moreover, GD1 patients with BMD Z-score ≤ –2.0 showed higher CTx levels compared to GD1 patients with BMD Z-score > –2 (Table 2).
At T0, all GD1 patients showed 25OHD levels <75 nmol/L, without significant differences between women and men (dns). Furthermore, GD1 patients showed lower 25OHD and higher PTH serum levels compared to controls (Table 2). According to criteria previously exposed, GD1 patients were treated with cholecalciferol (50 000 UI of cholecalciferol every week for 8 weeks10). After 8 weeks (T2), 25OHD increased significantly in all patients (P <0.01, Student’s t-test for paired samples) up to 75.2 ± 23.0 nmol/L. GD1 patients who did not achieve 25OHD levels ≥75 nmol/l received a second cholecalciferol treatment at the same dosage (50 000 UI of cholecalciferol every week for 8 weeks10). A significant and direct relationship was found between T0 and T2 25OHD levels (P <0.05; r = 0.569) (Figure 1A). In addition, the 25OHD levels at T2 directly correlate to the increase in 25OHD (P <0.05; r = 0.631) (Figure 1B). Clinical and biochemical parameters of GD1 patients who obtained 25OHD levels ≥75 nmol/L after the first cholecalciferol treatment were not significantly different compared to GD1 patients obtaining 25OHD levels >75 nmol/L after the second cholecalciferol treatment (dns). As reported in Table 2, at T24, PTH levels were not significant different between GD1 patients and controls.
After vitamin D deficiency correction, GD1 patients with BMD Z-score ≤ –2.0 were treated with alendronate 70 mg/week. During the 2-year follow-up, no changes in ERT dosage was made, and all GD1 patients were compliant to bone diet. In particular, 17 GD1 patients showed urinary sodium excretion ≤100 mmol/24 h and 8 a reduction in urinary sodium excretion ≥44 mmol/24 h.
At T24, clinical and radiological investigation did not show the occurrence of any new fragility fracture, EFD, bone crisis, osteonecrosis, or acute osteomyelitis, in the entire study population. No significant reduction in BMD was observed in GD1 patients both with BMD Z-score > and ≤–2. These results were observed in naïve GD1 patients (GD24 and GD25) and in those with long-term ERT treatment (GD01-GD23, mean ERT duration 19.4 ± 7.1 years). The differences in CTx levels, observed at T0 in GD1 patients affected by skeletal anomalies compared to those without skeletal anomalies, disappeared at T24. Finally, no significant differences were found between different GBA1 mutations and the response to ERT, cholecalciferol, and bone diet. No patients developed kidney stones.
The systematic review, performed according to criteria previously exposed, selected 5 studies,27-31 that included at least 4 of the following parameters: hypovitaminosis D, hypovitaminosis D treatment, assessment of calcium dietary intake, methodology used for BMD assessment, Op prevalence, bone anomalies, PTH assessment, BTM evaluation. As reported in Table 3, this is the first and only study contextually evaluating the prevalence of vitamin D deficiency in GD1 patients and their response to an integrated treatment, including ERT, cholecalciferol, and bone diet. This latter was evaluated using contextually biochemical, instrumental, and clinical parameters.
Table 3.
Studies evaluating bone health, vitamin D status, and hypovitaminosis D treatment in GD1 patients.
|
First
Author |
Year | Cnt | Lat | n | M | F | Mean age (years) | GD1T | HypoD N(%) | HypoD-T | Ca-I (g/d) | BMD | Op N(%) | BA N(%) | PTH | BTM | Meno | FU (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schiffmann | 2002 | USA | 42.36 | 29 | 16 | 13 | 36.4 ± 8.2 | ERT | 0 (0)a | n.a.b | 0.6/1.0b | QCT | n.a.c | n.a. | Yesa | n.a. | n.a. | 2 |
| Ciana | 2005 | Ita | 45.63 | 12 | 6 | 6 | 30.9 ± 5.5 | ERT | n.a. | n.a.d | n.a. | DXA | n.a | HS 1: (58.4)e HS 2: (33.3)e HS 3: (8.3)e |
Yes | BF, BR | n.a. | 4.5 |
| Parisi | 2008 | Arg | -34.60 | 9 | 4 | 5 | 26.9 ± 6.9 | ERT | 9 (100) | n.a. | n.a. | TB-DXA | n.a | n.a. | n.a. | BF, BR | n.a. | n.a. |
| Mikosch | 2009 | Eng | 51.50 | 60 | 34 | 26 | 47.6 ± 17.8 | ERT | 44 (73.7)f | n.a. | n.a. | DXAg | n.a. | n.a. | Yes | n.a. | n.a. | n.a. |
| Zimmermann | 2018 | Rom | 46.77 | 50 | 19 | 31 | 40 (26-51) | ERT | n.a.h | n.a.i | n.a. | DXA | 13 (34)j | FF: 9 (28) BC: 3 (8) ON: 9 (28) |
Yes | BF, BR | n.a. | n.a. |
| Barbato | 2023 | Ita | 40.85 | 25 | 9 | 16 | 45.0 ± 13.5 | ERT | 25 (100) | Chol | 1.0k | DXA | 7 (28) | FF: 2 (8) EFD: 4 (20) ON: 2(8) |
Yes | BF, BR | Yes | 2 |
Year: publication year. Cnt: Country. Lat: latitude. USA: United States of America. Ita: Italy. Arg: Argentina. Eng: England. Rom: Romania. Latitude: Latitude of city in which the study was performed. N: number of GD1 patients enrolled in the study. M: men. F: women. Mean age: mean of GD1 patients at enrolment. Data are expressed as mean ± standard deviation or median (quartiles otherwise) according to data provided by authors. GD1T: Therapy for GD1.
All enrolled GD1 patients showed normal values of 25OHD, 1,25(OH)2D and PTH at enrolment.
GD1 patients were randomized in 3 groups (1, 2, 3). Groups 1 received Calcitriol (0.25-3.0 g/day) and calcium (0.6 g/d) during months 1-6 and calcitriol, calcium and ERT during months 7-24; Groups 2 received Calcitriol (0.25–3.0 mg/day), calcium (0.6 g/d) and ERT during months 1-24; Group 3 received calcium (1.0 g/d) and ERT during months 1-24.
GD1 patients with QCT ≤ 100 mg/cm3 were excluded from the study.
Calcitriol treatment (0.25-0.50 μg/day) was administered before ERT treatment to all GD1 patients.
Evaluated using modified Hermann score (HS).
Hypovitaminosis D is defined as 25OHD serum levels < 80 nmol/L.
BMD was assessed by DXA in 38 GD1 patients.
25OHD serum levels were measured [24.6 (18.8; 28.3) ng/mL], no data were available regarding prevalence of hypovitaminosis D.
GD1 patients treated with vitamin D supplementation were excluded from the study.
Osteoporosis was diagnosed with Z score < –2.5. No post-menopausal status was assessed.
A normocalcic (calcium intake ≥ 1 g/day), normocaloric (1800 kcal) and hyposodic (sodium intake ≤ 5 g/day) diet, also called bone diet, was prescribed to all GD1 patients at study enrolment. The adherence to diet was assed monitoring 24 h urinary sodium excretion.
Abbreviations: BA, Bone anomalies [Fractures (FF), Enlenmeyer flask deformity (EFD), bone crises (BC), osteonecrosis (ON), and acute osteomyelitis(aOM)] prevalence; BF, bone formation; BMD, methodology used for BMD assessment; BR, bone resorption; BTM, bone turnover markers; Ca-I, assessment of calcium dietary intake expressed as grams/day; Chol, cholecalciferol; DXA, Dual Energy X-ray Absorptiometry; ERT, Enzyme replacement therapy; FU, follow up; HypoD, prevalence of vitamin D deficiency; HypoD-T, vitamin D deficiency treatment; Meno, menopause assessment; n.a., data not available; Op, Osteoporosis prevalence (using BMD Z-score); QCT: quantitative computed tomography; TB-DXA, Total body Dual Energy X-ray Absorptiometry.
Discussion
Skeletal manifestations in GD1 patients are often under-diagnosed and under-treated. Our study results demonstrate that: (1) hypovitaminosis D is highly prevalent in GD1 patients; (2) the proposed cholecalciferol treatment10 is effective to correct vitamin D deficiency and to reduce PTH levels, maintaining euvitaminosis D and PTH in the normal range in GD1 patients; (3) GD1 patients with skeletal anomalies, showed elevated CTx levels compared to GD1 patients without skeletal anomalies; (4) the integrated treatment with ERT, cholecalciferol, bone diet, and alendronate (only in GD1 patients with BMD Z-score ≤ –2.0) guarantees BMD stability and the occurrence of no new skeletal manifestations, without any side effects.
Vitamin D comprises a group of lipid-soluble hormones and prohormones.10 The vitamin D status is evaluated measuring the circulating levels of the 25OHD, while 1,25(OH)2D3 represents the active metabolite.10 The “canonical” properties of 25OHD are associated to the control of calcium-phosphate balance and skeletal homeostasis and, without sufficient 25OHD levels, a low percentage of calcium and phosphate is absorbed, increasing PTH levels.10 The elevated PTH levels in our study population were related to the low vitamin D serum levels at baseline. Considering that GD1 is classified as a lysosomal storage disease characterized by skeletal anomalies,2 in GD1 patients the evaluation of vitamin D status, measuring 25OHD serum levels, is recommended.17 Nevertheless, a previous report highlighted the lack of solid data on assessment of bone health, vitamin D status and management of vitamin D deficiency in GD1 patients,24 and an expert consensus document from the European working group referred the diagnosis and treatment of hypovitaminosis D for GD1 to good clinical practice rather than management goals.32
The systematic review summarizes available studies regarding vitamin D status in GD1 patients.27-31 Our study results show a high prevalence of vitamin D deficiency in GD1 patients from Southern Italy, confirming the results of Mikosch obtained at different latitudes,31 and demonstrate an excellent response to cholecalciferol treatment.10 For the first time, this proves that a regular therapeutic schedule is effective to treat vitamin D deficiency in adult GD1 patients. However, the response curve to the cholecalciferol treatment observed in GD1 patient is different to what observed in healthy subjects33 or in patients affected by Paget’s disease of bone18 (Figure 2). These responses are linked to an increased catabolism of vitamin D induced by cholecalciferol treatment, linked to stimulation of 24-hydroxylase metabolic activities.34 In GD1 patients, we observed a direct relationship between 25OHD serum levels at T0 and T2, and between 25OHD serum levels at T2 and its increase. This unusual response is probably linked to the mitochondrial alteration described in GD, resulting in a reduction in function or in levels of the cytochrome P450 3A4 (CYP3A4), an enzyme involved in catabolism of vitamin D.34
Figure 2.
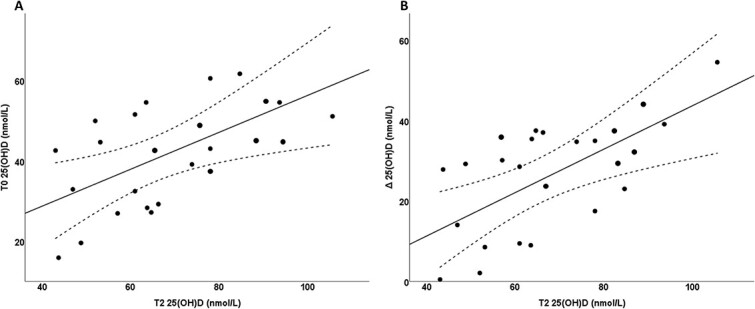
(A) Correlation between 25-hydroxy-vitamin D levels at baseline and 25-hydroxy-vitamin D levels after correction. (B) Correlation between 25-hydroxy-vitamin D levels after correction and its increase.
At T0, GD1 patients and controls showed different 25OHD serum levels, but similar 1,25(OH)2D3 levels. In subjects with normal kidney function, no direct relationship between 1,25(OH)2D3 and 25OHD serum levels were generally observed due to the tight regulation of the actions of cytochrome P450 family 27 subfamily B member 1 (CYP27B1) and of cytochrome P450 family 24 subfamily A member 1 (CYP24A1) enzymes.35
Skeletal anomalies are common at all ages in GD1 patients and represent the main cause of disability and poor quality of life.6,7,36 The proposed integrated treatment of GD1 patients including ERT, cholecalciferol, and bone-diet is effective to stabilize BMD. This result was observed both in naïve GD1 patients and in those with long-term ERT treatment. In addition, no new skeletal anomaly was registered during the 2-year follow-up. Of note, CTx levels at T0 were significantly elevated in GD1 patients affected by skeletal anomalies, and in particular in those with BMD Z-score ≤ –2, compared to GD1 patients without skeletal anomalies. This difference disappeared at T24. CTx is related to bone remodelling and resorption process, and it is considered as reference BTMs for use in fracture risk prediction and monitoring of Op treatment.37 CTx values of our GD1 patients with skeletal anomalies exceed the cut-off value suggested in Op management.38 Literature data are not conclusive regarding the effect of cholecalciferol supplementation on BTMs. The BTMs were reduced by cholecalciferol treatment in patients with vitamin D levels <75 nmol/L.39 Moreover, the association between cholecalciferol supplementation and calcium intake of at least 1 g/day decreased fracture risk and BTMs.40 Grimnes and colleagues demonstrated that the supplementation with both high-dose (6500 IU/day) and standard dose (800 IU/day) of cholecalciferol was effective to reduce BTMs, in particular P1NP and CTx.41 Also, Chen and colleagues observed a negative correlation between vitamin D supplementation and BTMs, in both women with osteopenia and osteoporosis.42 One can argue that, in GD1 patients, cholecalciferol supplementation associated with a proper calcium dietary intake increases calcium and phosphate intestinal absorption and reduces PTH, bone loss and consequently CTx, as reported in Table 2. In addition, alendronate treatment in osteoporotic GD1 could help to reduce CTx levels. Based on this consideration, we suggest the use of CTx as a marker of compliance during the integrated bone health management of GD1 patients.
Both mineralized bone and bone marrow seem to play a role in the pathogenesis of skeletal abnormalities in GD1 patients. The pathogenesis of these abnormalities as the intra-lysosomal sphingolipid accumulation, which induces an alteration of bone vascularity, increased intramedullary pressure, inflammation pattern, and osteoblast, osteoclast, and osteocyte activities.1,7,43 Indeed, some experimental studies emphasize the possible role of pathological osteoblasts and osteoclasts in the development of osteopenia and Op in GD1.44,45
Measuring BMD by DXA cannot differentiate between low BMD due to Op or low BMD due to osteomalacia, caused by hypovitaminosis D. However, none of our patients showed the classical biochemical triade of osteomalacic syndrome (hypocalcemia, hypophosphatemia, and increased ALP),46 as reported in Table 2. Furthermore, the bALP evaluation served to rule out any alterations related to liver manifestations of GD1.
Furthermore, the BMD stability, observed in GD1 patients with BMD Z-score ≤ –2.0 and treated with alendronate, is an expected outcome. Indeed, antiresorptive treatments are able to increase or stabilize BMD.47 On the contrary, the BMD stability in GD1 patients with BMD Z-score > –2.0 confirms the efficacy of our integrated treatment in maintaining bone health.17
Our is a monocentric study. This may be considered a limit but also a strength, because all biochemical parameters were evaluated by the same operators, which are blinded to the disease status, reducing the inter-laboratory inconsistency. Other strengths are: (1) all enrolled GD1 patients came from Southern Italy and were Caucasian; (2) the use of DXA-scan, the gold standard methods for BMD assessment; (3) the contextual evaluation of BTMs and DXA parameters for bone health assessment. Our study was not conceived as a double-blind clinical trial; thus, it cannot prove the superiority of the proposed integrated management of skeletal health in GD1 patients compared to a placebo (on top of ERT), but only its effectiveness and tolerability. Our study results open the way to further clinical trials that may help to clarify these features and to evaluated our integrated approach with different GD treatment, as SRT.
In conclusion, our study proposes an integrated approach to bone phenotype in GD1 patients, thanks to the effects of a balanced diet combined with vitamin D supplementation on BMD and BTMs in GD1 patients. Indeed, in our GD1 patients, the association between ERT, cholecalciferol and bone-diet obtained BMD stability and an improvement of bone health. In our opinion, a cooperation with a bone specialist in the management of GD1 patients can be improve the prognosis and quality of life.
Contributor Information
Antonio Barbato, Department of Clinical Medicine and Surgery, Federico II University of Naples, 80131 Naples, Italy.
Anita Vergatti, Department of Clinical Medicine and Surgery, Federico II University of Naples, 80131 Naples, Italy.
Alfonso Giaquinto, Department of Clinical Medicine and Surgery, Federico II University of Naples, 80131 Naples, Italy.
Ilaria Libera Pizzulo, Department of Clinical Medicine and Surgery, Federico II University of Naples, 80131 Naples, Italy.
Ludovica Perna, Department of Clinical Medicine and Surgery, Federico II University of Naples, 80131 Naples, Italy.
Giuseppe Perruolo, Department of Translational Medical Sciences, Federico II University of Naples, 80131 Naples, Italy.
Veronica Abate, Department of Clinical Medicine and Surgery, Federico II University of Naples, 80131 Naples, Italy.
Michelina Sibilio, Metabolic Diseases Unit, Santobono-Pausilipon Children's Hospital, 80129 Naples, Italy.
Ciro Mainolfi, Department of Advanced Biomedical Sciences, Federico II University of Naples, 80131 Naples, Italy.
Ernesto Soscia, Institute of Biostructures and Bioimaging of the National Research Council – CNR, 80131 Naples, Italy.
Gianpaolo De Filippo, Assistance Publique-Hôpitaux de Paris, Hôpital Robert-Debré, Service d’Endocrinologie-Diabétologie, 75019 Paris, France.
Pietro Formisano, Department of Translational Medical Sciences, Federico II University of Naples, 80131 Naples, Italy.
Ferruccio Galletti, Department of Clinical Medicine and Surgery, Federico II University of Naples, 80131 Naples, Italy.
Pasquale Strazzullo, Former Professor of Internal Medicine, Federico II University, 80131 Naples, Italy.
Domenico Rendina, Department of Clinical Medicine and Surgery, Federico II University of Naples, 80131 Naples, Italy.
Author contributions
Domenico Rendina, Pasquale Strazzullo (Conceptualization), Domenico Rendina, Pasquale Strazzullo, Antonio Barbato (Methodology), Anita Vergatti, Alfonso Giaquinto, Ilaria Libera Pizzulo (Software), Domenico Rendina, Gianpaolo De Filippo, Pietro Formisano, Pasquale Strazzullo (Validation), Anita Vergatti (Formal analysis), Domenico Rendina, Anita Vergatti, Veronica Abate, Michelina Sibilio, Ernesto Soscia, Ludovica Perna, Giuseppe Perruolo, Ferruccio Galletti (Investigation), Domenico Rendina (Resources, Data curation), Domenico Rendina, Anita Vergatti (Writing—original draft preparation), Anita Vergatti, Gianpaolo De Filippo, Antonio Barbato (Writing—review & editing), Domenico Rendina, Ciro Mainolfi, Gianpaolo De Filippo, Pietro Formisano, Pasquale Strazzullo (Visualization), Pasquale Strazzullo (Supervision), and Domenico Rendina, Antonio Barbato (Project administration). Antonio Barbato and Anita Vergatti contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
None declared.
Conflicts of interest
The study was realized with a partial unconditional support of Sanofi.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
The authors are grateful to the anonymous revisors for enabling us to improve the manuscript.
References
- 1. Pastores GM, Hughes DA. Gaucher disease. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews® [Internet]. University of Washington; 1993–2023. https://www.ncbi.nlm.nih.gov/books/NBK1269/ [PubMed] [Google Scholar]
- 2. Unger S, Ferreira CR, Mortier GR, et al. . Nosology of genetic skeletal disorders: 2023 revision. Am J Med Genet A. 2023;191(5):1164–1209. 10.1002/ajmg.a.63132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cappellini MD, Motta I, Barbato A, et al. . Similarities and differences between Gaucher disease and acid sphingomyelinase deficiency: an algorithm to support the diagnosis. Eur J Intern Med. 2023;108:81–84. 10.1016/j.ejim.2022.11.028 [DOI] [PubMed] [Google Scholar]
- 4. Zampieri S, Cattarossi S, Pavan E, et al. . Accurate molecular diagnosis of Gaucher disease using clinical exome sequencing as a first-tier test. Int J Mol Sci. 2021;22(11):5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leonart LP, Fachi MM, Böger B, et al. . A systematic review and meta-analyses of longitudinal studies on drug treatments for Gaucher disease. Ann Pharmacother. 2023;57(3):267–282. 10.1177/10600280221108443 [DOI] [PubMed] [Google Scholar]
- 6. Charrow J, Andersson HC, Kaplan P, et al. . The Gaucher registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Intern Med. 2000;160(18):2835–2843. 10.1001/archinte.160.18.2835 [DOI] [PubMed] [Google Scholar]
- 7. Marcucci G, Zimran A, Bembi B, et al. . Gaucher disease and bone manifestations. Calcif Tissue Int. 2014;95(6):477–494. 10.1007/s00223-014-9923-y [DOI] [PubMed] [Google Scholar]
- 8. Giuffrida G, Cappellini MD, Carubbi F, Di Rocco M, Iolascon G. Management of bone disease in Gaucher disease type 1: clinical practice. Adv Ther. 2014;31(12):1197–1212. 10.1007/s12325-014-0174-0 [DOI] [PubMed] [Google Scholar]
- 9. Rondanelli M, Faliva MA, Barrile GC, et al. . Nutrition, physical activity, and dietary supplementation to prevent bone mineral density loss: a food pyramid. Nutrients. 2021;14(1):74. 10.3390/nu14010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 11. Rendina D, Mossetti G, De Filippo G, et al. . Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern Italy: role of gender, hypertension and abdominal obesity. Nephrol Dial Transplant. 2009;24(3):900–906. 10.1093/ndt/gfn548 [DOI] [PubMed] [Google Scholar]
- 12. Carpenter TO, Shaw NJ, Portale AA, Ward LM, Abrams SA, Pettifor JM. Rickets. Nat Rev Dis Primers. 2017;3(1):17101. 10.1038/nrdp.2017.101 [DOI] [PubMed] [Google Scholar]
- 13. Pedicelli S, Peschiaroli E, Violi E, Cianfarani S. Controversies in the definition and treatment of idiopathic short stature (ISS). J Clin Res Pediatr Endocrinol. 2009;1(3):105–115. 10.4008/jcrpe.v1i3.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abate V, Vergatti A, Fiore A, et al. . Low potassium intake: a common risk factor for nephrolithiasis in patients with high blood pressure. High Blood Press Cardiovasc Prev. 2023;30(4):343–350. 10.1007/s40292-023-00587-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rendina D, Mossetti G, De Filippo G, Cioffi M, Strazzullo P. Fibroblast growth factor 23 is increased in calcium nephrolithiasis with hypophosphatemia and renal phosphate leak. J Clin Endocrinol Metab. 2006;91(3):959–963. 10.1210/jc.2005-1606 [DOI] [PubMed] [Google Scholar]
- 16. Rossini M, Adami S, Bertoldo F, et al. . Guidelines for the diagnosis, prevention and management of osteoporosis. Reumatismo. 2016;68(1):1–39. 10.4081/reumatismo.2016.870 [DOI] [PubMed] [Google Scholar]
- 17. Bertoldo F, Cianferotti L, Di Monaco M, et al. . Definition, assessment, and management of vitamin D inadequacy: suggestions, recommendations, and warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients. 2022;14(19):4148. 10.3390/nu14194148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rendina D, De Filippo G, Merlotti D, et al. . Vitamin D status in Paget disease of bone and efficacy-safety profile of cholecalciferol treatment in Pagetic patients with hypovitaminosis D. Calcif Tissue Int. 2019;105(4):412–422. 10.1007/s00223-019-00578-1 [DOI] [PubMed] [Google Scholar]
- 19. Pastores GM, Weinreb NJ, Aerts H, et al. . Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41:4–14. 10.1053/j.seminhematol.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 20. Rendina D, De Filippo G, Zampa G, Muscariello R, Mossetti G, Strazzullo P. Characteristic clinical and biochemical profile of recurrent calcium-oxalate nephrolithiasis in patients with metabolic syndrome. Nephrol Dial Transplant. 2011;26(7):2256–2263. 10.1093/ndt/gfq664 [DOI] [PubMed] [Google Scholar]
- 21. Iaccarino Idelson P, Speranza E, Marra M, et al. . Evaluation of the nutritional status of Gaucher disease type I patients under enzyme replacement treatment. Nutrients. 2022;14(15):3180. 10.3390/nu14153180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen IJ. Bone crises in Gaucher disease. Isr Med Assoc J. 2003;5(11):838–839. [PubMed] [Google Scholar]
- 23. Hughes D, Mikosch P, Belmatoug N, et al. . Gaucher disease in bone: from pathophysiology to practice. J Bone Miner Res. 2019;34(6):996–1013. 10.1002/jbmr.3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sainani KL. Dealing with non‐normal data. PM R. 2012;4(12):1001–1005. 10.1016/j.pmrj.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 26. Carubbi F, Nascimbeni F, Levi M, Pecchioli S, Cricelli C, Lapi F. Prevalence of four lysosomal storage diseases in primary care in Italy. Rivista SIMG. 2019;2(26):27–30. [Google Scholar]
- 27. Schiffmann R, Mankin H, Dambrosia JM, et al. . Decreased bone density in splenectomized Gaucher patients receiving enzyme replacement therapy. Blood Cells Mol Dis. 2002;28(2):288–296. 10.1006/bcmd.2002.0517 [DOI] [PubMed] [Google Scholar]
- 28. Ciana G, Addobbati R, Tamaro G, et al. . Gaucher disease and bone: laboratory and skeletal mineral density variations during a long period of enzyme replacement therapy. J Inherit Metab Dis. 2005;28(5):723–732. 10.1007/s10545-005-0032-y [DOI] [PubMed] [Google Scholar]
- 29. Parisi MS, Mastaglia SR, Bagur A, Goldstein G, Zeni SN, Oliveri B. Body composition and bone metabolism in young Gaucher disease type I patients treated with imiglucerase. Eur J Med Res. 2008;13(1):31–38. [PubMed] [Google Scholar]
- 30. Zimmermann A, Popp RA, Rossmann H, et al. . Gene variants of osteoprotegerin, estrogen-, calcitonin- and vitamin D-receptor genes and serum markers of bone metabolism in patients with Gaucher disease type 1. Ther Clin Risk Manag. 2018;14:2069–2080. 10.2147/TCRM.S177480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mikosch P, Reed M, Stettner H, Baker R, Mehta AB, Hughes DA. Patients with Gaucher disease living in England show a high prevalence of vitamin D insufficiency with correlation to osteodensitometry. Mol Genet Metab. 2009;96(3):113–120. 10.1016/j.ymgme.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 32. Biegstraaten M, Cox TM, Belmatoug N, et al. . Management goals for type 1 Gaucher disease: an expert consensus document from the European working group on Gaucher disease. Blood Cells Mol Dis. 2018;68:203–208. 10.1016/j.bcmd.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 33. Wagner D, Hanwell HE, Schnabl K, et al. . The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol. 2011;126(3-5):72–77. 10.1016/j.jsbmb.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 34. Kasarla SS, Garikapati V, Kumar Y, Dodoala S. Interplay of vitamin D and CYP3A4 polymorphisms in endocrine disorders and cancer. Endocrinol Metab (Seoul). 2022;37(3):392–407. 10.3803/EnM.2021.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang JCY, Jackson S, Walsh NP, et al. . The dynamic relationships between the active and catabolic vitamin D metabolites, their ratios, and associations with PTH. Sci Rep. 2019;9(1):6974. 10.1038/s41598-019-43462-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khan A, Hangartner T, Weinreb NJ, Taylor JS, Mistry PK. Risk factors for fractures and avascular osteonecrosis in type 1 Gaucher disease: a study from the international collaborative Gaucher group (ICGG) Gaucher registry. J Bone Miner Res. 2012;27(8):1839–1848. 10.1002/jbmr.1680 [DOI] [PubMed] [Google Scholar]
- 37. Brown JP, Don-Wauchope A, Douville P, Albert C, Vasikaran SD. Current use of bone turnover markers in the management of osteoporosis. Clin Biochem. 2022;109-110:1–10. 10.1016/j.clinbiochem.2022.09.002 [DOI] [PubMed] [Google Scholar]
- 38. Wu CH, Chang YF, Chen CH, et al. . Consensus statement on the use of bone turnover markers for short-term monitoring of osteoporosis treatment in the Asia-Pacific region. J Clin Densitom. 2021;24(1):3–13. 10.1016/j.jocd.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 39. Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94(4):1244–1250. 10.1210/jc.2008-1832 [DOI] [PubMed] [Google Scholar]
- 40. Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis [published correction appears in lancet diabetes Endocrinol]. Lancet Diabetes Endocrinol. 2014;2(4):307–320. 10.1016/S2213-8587(13)70212-2 [DOI] [PubMed] [Google Scholar]
- 41. Grimnes G, Joakimsen R, Figenschau Y, Torjesen PA, Almås B, Jorde R. The effect of high-dose vitamin D on bone mineral density and bone turnover markers in postmenopausal women with low bone mass—a randomized controlled 1-year trial. Osteoporos Int. 2012;23(1):201–211. 10.1007/s00198-011-1752-5 [DOI] [PubMed] [Google Scholar]
- 42. Chen X, Shen L, Gao C, et al. . Vitamin D status and its associations with bone mineral density, bone turnover markers, and parathyroid hormone in Chinese postmenopausal women with osteopenia and osteoporosis. Front Nutr. 2024;10:1307896. 10.3389/fnut.2023.1307896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mikosch P, Hughes D. An overview on bone manifestations in Gaucher disease. Wien Med Wochenschr. 2010;160(23-24):609–624. 10.1007/s10354-010-0841-y [DOI] [PubMed] [Google Scholar]
- 44. Cox TM, Charrow J, Lukina E, Mistry PK, Foster MC, Peterschmitt MJ. Long-term effects of eliglustat on skeletal manifestations in clinical trials of patients with Gaucher disease type 1. Genet Med. 2023;25(2):100329. 10.1016/j.gim.2022.10.011 [DOI] [PubMed] [Google Scholar]
- 45. Pastores GM, Wallenstein S, Desnick RJ, Luckey MM. Bone density in type 1 Gaucher disease. J Bone Miner Res. 1996;11(11):1801–1807. 10.1002/jbmr.5650111125 [DOI] [PubMed] [Google Scholar]
- 46. Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin N Am. 2010;39(2):321–331. 10.1016/j.ecl.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 47. Berger C, Langsetmo L, Joseph L, et al. . Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. CMAJ. 2008;178(13):1660–1668. 10.1503/cmaj.071416 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
The authors are grateful to the anonymous revisors for enabling us to improve the manuscript.