Abstract
Background
In people with haemophilia or other congenital bleeding disorders undergoing surgical interventions, haemostatic treatment is needed in order to correct the underlying coagulation abnormalities and minimise the bleeding risk. This treatment varies according to the specific haemostatic defect, its severity and the type of surgical procedure. The aim of treatment is to ensure adequate haemostatic coverage for as long as the bleeding risk persists and until wound healing is complete.
Objectives
To assess the effectiveness and safety of different haemostatic regimens (type, dose and duration, modality of administration and target haemostatic levels) administered in people with haemophilia or other congenital bleeding disorders for preventing bleeding complications during and after surgical procedures.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Coagulopathies Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched the reference lists of relevant articles and reviews.
Date of the last search: 20 November 2014.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing any hemostatic treatment regimen to no treatment or to another active regimen in children and adults with haemophilia or other congenital bleeding disorders undergoing any surgical intervention.
Data collection and analysis
Two authors independently assessed trials (eligibility and risks of bias) and extracted data. Meta‐analyses were performed on available and relevant data.
Main results
Of the 16 identified trials, four (112 participants) were eligible for inclusion.
Two trials evaluated 59 people with haemophilia A and B undergoing 63 dental extractions. Trials compared the use of a different type (tranexamic acid or epsilon‐aminocaproic acid) and regimen of antifibrinolytic agents as haemostatic support to the initial replacement treatment. Neither trial specifically addressed mortality (one of this review's primary outcomes); however, in the frame of safety assessments, no fatal adverse events were reported. The second primary outcome of blood loss was assessed after surgery and these trials showed the reduction of blood loss and requirement of post‐operative replacement treatment in people receiving antifibrinolytic agents compared with placebo. The remaining primary outcome of need for re‐intervention was not reported by either trial.
Two trials reported on 53 people with haemophilia A and B with inhibitors treated with different regimens of recombinant activated factor VII (rFVIIa) for haemostatic coverage of 33 major and 20 minor surgical interventions. Neither of the included trials specifically addressed any of the review's primary outcomes (mortality, blood loss and need for re‐intervention). In one trial a high‐dose rFVIIa regimen (90 μg/kg) was compared with a low‐dose regimen (35 μg/kg); the higher dose showed increased haemostatic efficacy, in particular in major surgery, with shorter duration of treatment, similar total dose of rFVIIa administered and similar safety levels. In the second trial, bolus infusion and continuous infusion of rFVIIa were compared, showing similar haemostatic efficacy, duration of treatment and safety.
Authors' conclusions
There is insufficient evidence from randomised controlled trials to assess the most effective and safe haemostatic treatment to prevent bleeding in people with haemophilia or other congenital bleeding disorders undergoing surgical procedures. Ideally large, adequately powered, and well‐designed randomised controlled trials would be needed, in particular to address the cost‐effectiveness of such demanding treatments in the light of the increasing present economic constraints, and to explore the new challenge of ageing patients with haemophilia or other congenital bleeding disorders. However, performing such trials is always a complex task in this setting and presently does not appear to be a clinical and research priority. Indeed, major and minor surgeries are effectively and safely performed in these individuals in clinical practice, with the numerous national and international recommendations and guidelines providing regimens for treatment in this setting mainly based on data from observational, uncontrolled studies.
Keywords: Humans; Surgical Procedures, Operative; Tooth Extraction; Aminocaproic Acid; Aminocaproic Acid/therapeutic use; Antifibrinolytic Agents; Antifibrinolytic Agents/therapeutic use; Blood Loss, Surgical; Blood Loss, Surgical/prevention & control; Factor VIIa; Factor VIIa/therapeutic use; Hemophilia A; Hemophilia A/drug therapy; Hemophilia B; Hemophilia B/drug therapy; Hemostasis, Surgical; Hemostasis, Surgical/methods; Randomized Controlled Trials as Topic; Recombinant Proteins; Recombinant Proteins/therapeutic use; Tranexamic Acid; Tranexamic Acid/therapeutic use
Plain language summary
Preventing bleeding in people with congenital bleeding disorders during and after surgery
In haemophilia and other congenital bleeding disorders blood does not clot properly, which can cause excessive bleeding. This is particularly relevant during surgery, when the risk of bleeding depends on the type and severity of the clotting disorder and on the type of surgery. Therefore, during and after surgery, these individuals should receive treatment to improve the ability of their blood to clot and so prevent bleeding. Clotting factor concentrates (when available and appropriate in those individuals missing specific clotting proteins) or other non‐specific drugs for clotting, or a combination of both, are administered. It is not known what is the optimal dose or duration or method of administration of these treatments in these circumstances.
We searched for randomised controlled trials comparing the efficacy (mortality, blood loss, need for re‐intervention, subjective assessment of efficacy, duration and dose of therapy) and the safety of any type of treatment given to people with congenital bleeding disorders during any type of surgery. We found four trials to be included in this review. Two trials evaluated 59 people with haemophilia A or B receiving antifibrinolytic drugs (agents that reduce the breakdown of clots) or placebo in addition to the initial standard treatment before dental extractions. The remaining two trials evaluated 53 people with haemophilia A or B and inhibitors (antibodies that act against the factor concentrate therapy) receiving an different clotting concentrate, recombinant activated factor VII, both during and after surgery. These two trials evaluated different treatment options: high‐dose compared with low‐dose and a single large (bolus) infusion compared with continuous infusion.
The trials included in this review provide some information in two specific situations in people with congenital bleeding disorders undergoing surgery. However, on the whole, there is not enough evidence from trials to define the best treatments for the various types of disease and types of surgery. Further trials would be useful to improve our knowledge but are difficult to carry out and currently do not appear to be a clinical priority. Indeed, both major and minor surgery are safely performed in clinical practice in these individuals based on local experience and data from uncontrolled studies.
Background
Description of the condition
Congenital bleeding disorders (CBDs) are inherited conditions characterized by the clinical tendency to bleed spontaneously or after haemostatic challenges (trauma, invasive procedures), due to genetically determined quantitative or qualitative (or both) abnormalities of components of the coagulation system. The X‐linked deficiencies of coagulation factor VIII (FVIII) and IX (FIX), haemophilia A and B, are the most represented CBDs, with a similar worldwide prevalence of 1 in 10,000 and 1 in 60,000 male births, respectively (Mannucci 2001). However, the most common CBD is von Willebrand disease (VWD), the prevalence of which is under‐reported because of frequent undiagnosed cases with mild bleeding symptoms. Approximately 1% of the general population show a quantitative or functional deficiency of von Willebrand factor (VWF) (Rodeghiero 1987), whereas the prevalence of clinically significant VWD is much lower, and affects both men and women carrying homozygous or single or double heterozygous abnormalities of VWF gene on chromosome 12 (Federici 2006). Less common CBDs include autosomal recessively inherited deficiencies of:
other coagulation factors (fibrinogen, factor II (FII), factor V (FV), factor VII (FVII), factor X (FX), factor XI (FXI), factor XIII (FXIII);
proteins regulating their biosynthesis or expression (combined FV and FVIII or vitamin K‐dependent factor deficiencies) (Mannucci 2004);
platelet function (defective platelet receptors, enzymes or other components) (Nurden 2005); and
fibrinolysis proteins (α2‐antiplasmin, type 1 plasminogen activator inhibitor‐ PAI‐1) (Aoki 1989).
The prevalence of these rare CBDs vary from 1 in 500,000 to 1 in 2,000,000 births, with higher figures in populations in which consanguineous marriages are common (Mannucci 2004).
In haemophilia A and B, an inverse relationship between the severity of bleeding symptoms and the plasma level of the deficient clotting factor is generally recognized. Accordingly, the definitions given by the International Society for Thrombosis and Haemostasis are based on the residual plasmatic concentration of the missing protein:
severe: FVIII or FIX less than 0.01 units per millilitre (u/ml);
moderate: FVIII or FIX ranging from 0.01 u/ml to less than 0.05 u/ml;
mild: FVIII or FIX greater than 0.05 u/ml up to 0.4 u/ml) (White 2001).
Spontaneous bleeding, typically involving joints with consequent progressive deterioration, is largely confined to people with severe haemophilia (Aledort 1994). The bleeding phenotype is more heterogeneous and not easily predictable by laboratory data in VWD and, particularly, in recessively inherited CBDs (Mannucci 2004). As a consequence of the rarity of these deficiencies, the type and severity of bleeding symptoms are not as well established as for haemophilia and a severity classification has only recently been proposed (Peyvandi 2012).
Bleeding severity and treatment are influenced by the development of neutralising antibodies (inhibitors) which are directed against the therapeutically administered coagulation factors in some people with a CBD. This complication occurs usually within the first 20 exposure days in up to 30% of people with haemophilia A (Wight 2003), particularly in those with severe factor deficiency and genetic abnormalities (null mutations) (Gouw 2007; Gouw 2013). Other factors, both genetic (family history, ethnicity, polymorphisms in genes of FVIII and of immune‐regulatory cytokines) (Astermark 2010) and non‐genetic (initial intensive treatment) (Gouw 2007; Gouw 2013), have been identified as contributing to this abnormal immune response. A much lower inhibitor rate (approximately 1%) is reported in previously treated individuals with severe haemophilia A (Xi 2013). The inhibitor risk has been shown to be associated with exposure days and some missense mutations in people with non‐severe haemophilia A (Eckhardt 2013). Inhibitors are found more rarely (1.5% to 5%) in people with severe haemophilia B (DiMichele 2007) and type 3 VWD (James 2013), and usually in people carrying large gene deletions. Moreover, the additional morbidity risk of severe anaphylactic reactions has been reported in these cases (DiMichele 2007; James 2013). Inhibitors are only occasionally reported in rare CBDs.
Description of the intervention
Haemostatic treatment in people with a CBD is administered for bleeding episodes or for preventing bleeding and related complications (prophylaxis), including when invasive procedures and surgery are needed (Srivastava 2013).
Treatment options vary according to the possibility of specifically correcting the underlying hemostatic deficiency (Table 1). Indeed, the mainstay of treatment of coagulation factor deficiencies is the replacement of the missing protein by intravenous administration of the appropriate factor concentrate (when available) or of factor‐containing products (cryoprecipitate, prothrombin complex concentrate, fresh‐frozen plasma). For example, a variety of FVIII plasma‐derived and recombinant concentrates are available, but no specific FV concentrate has yet been developed (Brooker 2012). Replacement product availability also depends on the regulations in individual countries (fibrinogen, FX, FXI and FXIII concentrates are licensed only in some countries) and, particularly, on economic resources (Stonebraker 2010). When needed, or if no specific replacement is available, blood products (red blood cell, platelet concentrates, fresh frozen plasma) and non‐specific haemostatic drugs are therapeutic options (Mannucci 1998). The latter include the antifibrinolytic lysine analogues, epsilon‐aminocaproic acid and tranexamic acid, and desmopressin. Desmopressin is a synthetic analogue of the antidiuretic hormone that raises the plasma levels of FVIII and VWF, and is administered when treating mild haemophilia A and responsive VWD. It is also successfully used in FXI deficiency (Franchini 2009) and platelet function disorders (Coppola 2008) and its use in pregnancy in women with a CBD is the subject of another Cochrane Review (Karanth 2012).
1. Treatment options in people with CBD according to the possibility of specifically correcting the haemostatic deficiency.
| Bleeding Disorder | Specific replacement | Other haemostatic treatment |
| Haemophilia A | FVIII concentrates* | |
| Haemophilia B | FIX concentrates° | |
| Haemophilia with inhibitors | ‐ | aPCC, rFVIIa |
| Von Willebrand disease | Endogenous: DDAVP; Exogenous: VWF‐containing concentrates# | |
| Fibrinogen deficiency | Fibrinogen concentrates^ | Cryoprecipitate, FFP |
| FII deficiency | ‐ | PCC, FFP |
| FV deficiency | ‐ | FFP |
| FV+FVIII deficiency | ‐ | FFP |
| FVII deficiency | FVII concentrates° | FFP, PCC |
| FX deficiency | FX concentrate^ | PCC |
| FXI deficiency | FXI concentrate^ | FFP, DDAVP |
| FXIII deficiency | FXIII concentrates§ | FFP, cryoprecipitate |
| VKD factor deficiency | ‐ | FFP, PCC |
| Platelet function abnormalities | Platelet concentrates | DDAVP, rFVIIa |
| Fibrinolysis abnormalities | ‐ | FFP, antifibrinolytics |
aPCC: activated prothrombin complex concentrate DDAVP: desmopressin FFP: fresh frozen plasma PCC: prothrombin complex concentrates rFVIIa: activated recombinant factor VII VKD: vitamin K‐dependent VWF: von Willebrand factor
*Various intermediate‐ and high‐purity plasma‐derived concentrates and recombinant products available °Various high‐purity plasma‐derived concentrates and a single recombinant product currently available ^Plasma‐derived concentrate, not licensed in all countries #A series of plasma‐derived intermediate‐ and high‐purity FVIII concentrates, with different VWF/FVIII ratio, and a single concentrate of virtually VWF alone available
§ One plasma‐derived concentrate and a recently developed recombinant FXIII (A subunit) product, not available in all countries
Given that standard replacement is partially or completely ineffective in people with haemophilia with inhibitors, the use of the so‐called bypassing agents (activated prothrombin complex concentrates, aPCC and activated recombinant FVII, rFVIIa) is required (Collins 2013). This is particularly true in those with high‐responding inhibitors (anamnestic levels above 5 BU/ml). People with inhibitors experience higher morbidity and a poorer quality of life than those without inhibitors, mainly due to their poor orthopaedic status, because of the non‐optimal management of bleeds and non‐standardised prophylaxis regimens (Gringeri 2003; Scalone 2006).
How the intervention might work
Surgical procedures may be safely performed in people with a CBD, preferably by an experienced team in the management of haemophilia, providing there is adequate haemostatic coverage for as long as the bleeding risk persists and until wound healing is complete (Srivastava 2013). Although the classification is not clearly established, major and minor surgery are usually distinguished according to the type of procedure and surgery‐related risk. Among major procedures, orthopaedic surgery is commonly needed in people with haemophilia due to their joint disease. Tonsillectomy, dental surgery, central venous access implantation and liver biopsy are minor surgical procedures frequently required in the management of people with a CBD (Hermans 2009).
Guidelines for the management of CBDs by national scientific organizations or expert panels include recommendations for haemostatic coverage of surgery and invasive procedures (Berntorp 1998; Bolton‐Maggs 2006; Collins 2013; Mannucci 2004; Mannucci 2009a; Mauser‐Bunschoten 2001; Santagostino 2000; Srivastava 2013; Teitel 1998; UKHCDO 2003). Treatment should be administered according to the type and severity of the CBD, the presence of inhibitors, and to the target haemostatic level (when appropriate). Treatment choices should reflect the bleeding risk related to the type of procedure and also the possible specific modifiers of the risk or severity, or both, of bleeding complications. For example, adeno‐tonsillectomy, considered minor surgery, is associated with a high rate of bleeding complications; on the other hand, bleeding may be highly dangerous when occurring in confined spaces, as in neurosurgery, spinal surgery and surgery of the posterior chamber of the eye (Cosmi 2009). However, the optimal duration and target levels of haemostatic treatment (pre‐operative and during the post‐operative course) required to safely prevent bleeding complications have not been definitively established, even in people with haemophilia and VWD, for which more clinical experience and published data are available (Hermans 2009). Moreover, the individual bleeding risk, the need for, or the intensity of, haemostatic coverage (or both) are often difficult to predict. This is because in most cases, with the exception of haemophilia A and B (and in part of VWD), residual factor levels do not completely reflect bleeding phenotype (Pavlova 2013; Peyvandi 2012). Factor concentrates may be administered by intermittent bolus infusions according to the half‐life of the missing protein, or by continuous infusion. The stability of plasma factor levels by continuous infusion may represent an advantage in the surgical setting, eliminating unnecessary factor peak levels immediately after a bolus infusion, and the subsequent trough levels, possibly associated with inadequate haemostatic coverage (Martinowitz 1997). Moreover, continuous infusion may considerably reduce the overall requirement for factor concentrates, thus being more cost‐effective than bolus administration; even more so when an 'adjusted dose' is used in accordance with the factor daily clearance (Martinowitz 1992).
For individuals without a CBD, the stratification of the risk of surgery‐provoked venous thromboembolism and the consequent decision to undertake thromboprophylaxis, based on to the type of surgery and on the related degree of the individual's mobility, is well established and evidence‐based clinical practice guidelines are available (Gould 2012). People with a CBD have long been thought to be protected from thromboembolic disease. The need (and modality) of thromboprophylaxis in these individuals, particularly in high‐risk orthopaedic surgery, is currently disputed. This is mostly a consequence of the prolonged life‐expectancy of people with a CBD, due to the advances in treatment and general care, which implies a growing need for both orthopaedic and general surgery in an ageing population (Mannucci 2009b).
Some research evidence suggests that surgery, combined with intensive treatment with factor concentrates, is associated with a risk of inhibitor development in people with severe haemophilia A. This is particularly true at first exposure and for more than five exposure days (Eckardt 2011). This risk has been reported even in people with non‐severe haemophilia, with conflicting data concerning a higher likelihood when factor concentrates are administered by continuous infusion (Eckhardt 2012; Sharathkumar 2003).
Why it is important to do this review
This review aims to investigate the most effective and safe treatment to prevent bleeding in people with a CBD undergoing surgery.
Objectives
To assess the effectiveness and safety of different haemostatic regimens (type, dose and duration, modality of administration and target haemostatic levels) administered in people with a CBD for preventing bleeding complications during and after surgical procedures.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Children and adults with a known CBD (any severity) undergoing any surgical intervention, with any follow up available.
Types of interventions
Any haemostatic treatment regimen compared to no treatment or to another active regimen.
Types of outcome measures
Primary outcomes
Mortality
-
Blood loss assessed objectively
-
during surgery
by variation of haemoglobin levels
by transfusion requirement (number of red blood cell (RBC) units infused)
-
after surgery
by variation of haemoglobin levels
by transfusion requirement (number of RBC units infused)
-
Need for re‐intervention
Secondary outcomes
Need for additional unplanned dosing of the drug under study
Need for alternative haemostatic treatment
Haemostatic effectiveness (as assessed and rated by the surgeon or the treating physician)
Achievement of sustained target haemostatic levels (as measured by lab test during or after surgery)
Duration of replacement treatment
Concentrate consumption
Thromboembolic adverse events
De novo inhibitor development
Search methods for identification of studies
No restrictions based on dates, language, publication type or status were imposed.
Electronic searches
We identified relevant trials from the Cystic Fibrosis and Genetic Disorders Group’s Coagulopathies Trials Register using the terms: *FVII* OR Factor VII* OR Factor IX* OR Factor XIII* OR factor replacement OR DDAVP OR fresh frozen plasma OR antifibrinolytics OR dental OR surgery.
The Coagulopathies Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), weekly searches of MEDLINE and prospective hand‐searching of one specialized journal, Haemophilia. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the International Society of Haemostasis and Thrombosis Congresses; and the International Congresses of World Federation of Haemophilia. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group Module.
Date of last search: 20 November 2014.
The online registry ClinicalTrials.gov (www.ClinicalTrials.gov) was searched for possible ongoing trials.
Searching other resources
References of the included trials were checked and experts were contacted to retrieve any unpublished trials.
Data collection and analysis
Selection of studies
Two review authors independently read the titles and abstracts of the citations identified by the search to select the trials that fit the inclusion criteria. Where there was disagreement between authors, they reached a decision by consensus or by including a third author of the review in further discussions.
Data extraction and management
Two authors independently extracted characteristics and data from each trial using a customised data extraction form. They searched for the following characteristics and reported in the Characteristics of included studies table, as available for each trial:
trial design;
type of CBD;
severity of CBD;
type of surgical procedure;
regimen of haemostatic treatment (type; dose; modality of administration);
pre‐operative and post‐operative target haemostatic levels;
duration of treatment;
comparison intervention;
mortality;
bleeding (blood loss objectively assessed, variation of hemoglobin levels, transfusion requirement and number of RBC units infused, haemostatic effectiveness judged by the surgeon);
additional or alternative treatment;
need for re‐intervention;
concentrate consumption (where appropriate);
thromboembolic adverse events.
As outlined in the protocol, the authors reported outcomes at any available follow‐up period and the extracted the numbers of participants in each subgroup.
Assessment of risk of bias in included studies
Each author assessed the risk of bias of the selected trials using the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1 (Higgins 2011). They assessed the following domains as having either a: low risk of bias; unclear risk of bias; or high risk of bias.
Randomisation ('low risk': randomisation list generated using a computer, random number table, or similar methods; 'unclear risk': described as randomised, but no details given; 'high risk': non‐random approach, e.g. alternation, use of case record numbers, dates of birth or day of the week).
Concealment of allocation ('low risk': list from a central independent unit, on‐site locked computer, identically appearing treatment; 'unclear risk': not described; 'high risk': if allocation sequence was known to, or could be deciphered by the investigators who assigned participants or if an open allocation schedule was used).
Blinding (‘low risk’: if participants, investigators and outcome assessors were blinded, or if any of these were not blinded but outcome assessment was judged not to influence the outcome; ‘unclear risk’: if this issue was not discussed; ‘high risk’: if none of the parties involved in the trial were blinded).
Incomplete outcome data (‘low risk’: if any withdrawals were described in full and were equal across groups; ‘unclear risk’: if insufficient information was given; ‘high risk’: if the missing data were likely to be directly related to the outcome or if they were uneven across groups).
Selective outcome reporting.
Other potential sources of bias.
Where there was disagreement between the authors on a trial's evaluation, they reached a decision by consensus or by mediation with the third author. The authors present the risk of bias assessment in the 'Risk of bias' tables (Characteristics of included studies).
Measures of treatment effect
For binary outcome measures, the authors aimed to use data on the number of trial participants with each outcome event, by allocated treated group, irrespective of compliance and whether or not the participant was later excluded from treatment or follow up. The authors used different scales for particular outcomes when needed. The authors aimed to calculate a pooled estimate of the treatment effect for each outcome across trials using the odds ratio (OR) (the odds of an outcome among treatment allocated participants to the corresponding odds among controls) and also report the corresponding CIs.
For continuous outcomes, the authors recorded the mean values for each group or mean post‐treatment or intervention values and standard deviation (SD). The authors, where appropriate, aimed to generate a pooled estimate of treatment effects by calculating the mean difference (MD) and the corresponding CIs. If different trials used different scales for the same outcome, the authors would have considered using the standardised mean difference (SMD) and the corresponding CIs.
Unit of analysis issues
The unit of analysis was the treated individual. In the case of data from cluster‐randomised trials, if the information was available, the authors planned to calculate the intracluster correlation coefficient (ICC) according to Donner (Donner 2011). In the case of identifying cross‐over trials, the authors planned an analysis with the marginal probabilities of success method (Becker 1993), rather than by evaluating them as parallel trials.
Dealing with missing data
The authors reported the numbers and reasons for dropouts and withdrawals in all intervention groups (if described), or that the papers specified that there were no dropouts or withdrawals, if that was the case. The authors planned to contact the original Investigators for clarification on any missing information.
Assessment of heterogeneity
If the authors had been able to combine a sufficient number of trials, they planned to assess the degree of heterogeneity between trials through visual examination of the combined data presented in the forest plots and by using the I2 statistic together with Chi2 values and their CIs (Deeks 2011). This measure describes the percentage of total variation across trials that are due to heterogeneity rather than by chance (Higgins 2003). The values of I2 lie between 0% and 100%. The authors planned to use a categorization of heterogeneity according to the following cut‐off values:
not important (I2 values 0% to 40%);
moderate (I2 values 30% to 60%);
substantial (I2 values 50% to 90%); and
considerable (I2 values 75% to 100%).
Assessment of reporting biases
The authors aimed to assess the consistency of measurements and outcomes planned by the original Investigators during the trial and those reported within the published paper by comparing the trial protocols with the information in the final publication. As protocols were not available, the authors compared the 'Methods' and the 'Results' sections of the final papers. They also used their knowledge of the clinical background to identify standard outcome measures usually taken, but not reported by the investigators. In the case of a sufficient number of trials (10 or more) included, the authors planned to attempt the assessment of publication bias by using a funnel plot.
Data synthesis
The authors planned to attempt to pool data by disease and treatment combinations, i.e. combinations of participants with a similar CBD diagnosis (severe or non‐severe haemophilia A and B, severe or non‐severe mild CBD, individuals with and without inhibitors) and of different treatment regimens (dose regimens of the same haemostatic treatment; modalities of administration, duration, types of procedure). However, this was only possible for one combination (Analysis 1.2).
1.2. Analysis.
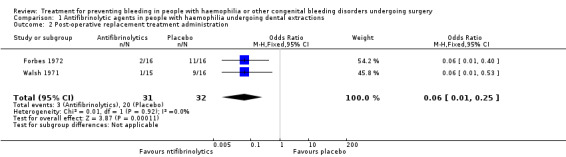
Comparison 1 Antifibrinolytic agents in people with haemophilia undergoing dental extractions, Outcome 2 Post‐operative replacement treatment administration.
For the meta‐analysis, the authors used a fixed‐effect model. If they had identified moderate, substantial or considerable heterogeneity (as defined above), they would have used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Given the limited number of trials included in the review, the authors have not been able to undertake any of the proposed subgroup analyses. They had expected to find trials in different CBDs, in people with different disease severities (or both), in people with and without inhibitors and in different surgical settings (implying significant clinical heterogeneity). If they are able to include a sufficient number of such trials in the future, they plan to undertake subgroup analyses based on:
type and severity of CBD (people with severe and non‐severe haemophilia);
inhibitor status (individuals with inhibitors and without inhibitors);
type of surgery (orthopaedic surgery, major surgery, dental procedures, minor surgery).
Sensitivity analysis
For future updates of this review, if the authors include a sufficient number of trials, they plan a sensitivity analysis based on the risk of bias of trials, including or excluding trials assessed as having an overall high risk of bias (i.e. where the proportion of information from trials at high risk of bias is sufficient to affect the interpretation of the results).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
Two authors (AC and MNDDM) independently evaluated the literature search, retrieving 344 unique studies after screening of titles and abstracts. Overall, 16 studies were then identified as potentially relevant (Avanoglu 1999; Batorova 2000; Forbes 1972; Jiménez‐Yuste 2002; Lee 2005; NCT00357656; Pruthi 2007; Rakocz 1993; Ramstrom 1975; Sindet‐Pedersen 1986; Shapiro 1998; Stajcic 1985; Stajcic 1989; Tavenner 1972; Waly 1995; Walsh 1971), as detailed in Figure 1. Of these, four trials were considered eligible for this review,10 studies were excluded for reasons reported below, one awaits classification and a further study is ongoing.
1.
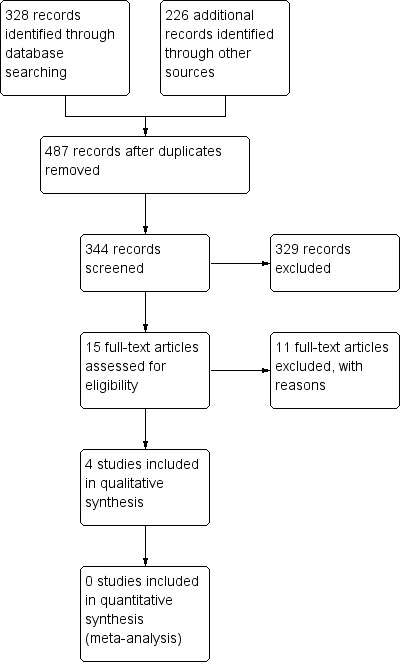
Study flow diagram.
Included studies
There are four trials included in this review (Forbes 1972; Pruthi 2007; Shapiro 1998; Walsh 1971).
Trial design
Three of the four included trials were RCTs (Forbes 1972; Pruthi 2007; Shapiro 1998), of which two were double blinded (Forbes 1972; Shapiro 1998) and one had an open‐label design (Pruthi 2007). The remaining trial was a double‐blind controlled trial, which we regarded as a quasi‐RCT (Walsh 1971). All trials had two arms; however, in the Pruthi trial, a third participant group was also included for safety assessment (Pruthi 2007). Two trials were multicentre (Pruthi 2007; Shapiro 1998), whereas one of the remaining two trials was carried out at a single centre (Forbes 1972) and one at two centres (Walsh 1971).
Participants
All four included trials enrolled adults and children with haemophilia A and B. In two trials, participants were people with haemophilia (all severities) without inhibitors (Forbes 1972; Walsh 1971). The remaining two trials specifically evaluated people with haemophilia and inhibitors (Pruthi 2007; Shapiro 1998).
Haemophilia A or B without inhibitors
In both trials of people with haemophilia A and B without inhibitors, participants were undergoing dental extractions. The Walsh trial evaluated 31 participants (24 with haemophilia A, 7 with haemophilia B) from two centres in the UK (Walsh 1971), whereas the Forbes trial evaluated 28 participants (20 with haemophilia A, 8 with haemophilia B) from a single UK centre with 32 separate procedures overall (Forbes 1972).
Haemophilia A or B with inhibitors
In the two trials in participants with haemophilia with inhibitors, major or minor elective surgical procedures were considered. In the Shapiro trial, 29 participants with inhibitors (25 haemophilia A, three haemophilia B, one acquired haemophilia A) from four centres in the USA underwent 11 major and 18 minor surgical procedures (Shapiro 1998). In the Pruthi trial 24 males with haemophilia and inhibitors from 11 centres in the USA underwent 22 major and two minor surgical procedures. Additionally, in this second trial, 12 haemophilia participants without inhibitors undergoing 12 major surgical procedures were included for safety comparative purposes (Pruthi 2007).
Interventions
Haemophilia A or B without inhibitors
In both of these placebo‐controlled trials, the role of antifibrinolytic agents given in addition to replacement treatment was evaluated in participants undergoing dental extractions (Forbes 1972; Walsh 1971). In the Walsh trial, participants received an intravenous infusion of 6 g of epsilon‐aminocaproic acid (EACA) in 250 ml isotonic saline (experimental arm) or isotonic saline (control arm), immediately after a pre‐operative (one hour) loading dose of human antihaemophilic globulin concentrate (or cryoprecipitate) or factor‐IX concentrate calculated to raise their level of the deficient factor to 50% average normal (Walsh 1971). The participants in the experimental arm then received EACA orally in orange squash every six hours, whereas participants in the control group received a drink with a similar taste. The duration of treatment and follow up was 10 days or seven days in the two different participating centres (Walsh 1971). In the Forbes trial, participants were randomised to either tranexamic acid (1 g three‐times‐a‐day) or placebo tablets starting two hours before extraction and continuing for five days. All participants then received the factor VIII or IX equivalent of 1000 ml of human plasma intravenously one hour before extraction (Forbes 1972).
Haemophilia A or B with inhibitors
In both of these trials, treatment regimens of recombinant activated factor VII (rFVIIa) for attaining and maintaining effective haemostasis during and after elective surgical procedures were evaluated (Pruthi 2007; Shapiro 1998). In the Shapiro trial, participants were randomised to receive a low‐dose (35 μg/kg) or high‐dose (90 μg/kg) rFVIIa bolus regimen, starting immediately prior to surgical incision, intra‐operatively as needed, every two hours for the first 48 hours and every two to six hours for the following three days. These five days of blinded dosing represented the primary trial period, after which open‐label (90 μg/kg) rFVIIa was available for maintenance (Shapiro 1998). In the Pruthi trial, all participants received a pre‐operative bolus of 90 μg/kg rFVIIa, then were randomised to continuing bolus infusions (90 μg/kg every two hours during surgery and through post‐operative day (POD) five, then every four hours from POD six to 10) or at receiving rFVIIa continuous infusion (50 μg/kg/hour through POD five, then 25 μg/kg/hour from POD six to 10). Due to such a different modality of administration, participants were unblinded (Pruthi 2007).
Outcomes
The included trials had different outcome definition and assessment.
Haemophilia A or B without inhibitors
Both included trials of participants undergoing dental extractions aimed to provide an objective estimation of bleeding and the efficacy of experimental treatment in reducing the need for replacement treatment and its safety (Forbes 1972; Walsh 1971). The Walsh trial evaluated the frequency of intra‐oral bleeding by daily report of occurrence of bleeding and measurement of haemoglobin levels at post‐operative days 1, 3, 5 and 7, and the requirement for post‐operative therapeutic material. Safety was evaluated by reporting the side effects of treatment (Walsh 1971). In the Forbes trial, blood loss was measured with detection of 51Cr‐labelled red cells in oral secretions and faeces over 24‐hr collections for five days and the fall in haemoglobin levels. Requirement for further replacement therapy after the initial dose was recorded. Safety was monitored by renal and liver function tests, electrocardiogram, and the reporting of side effects (Forbes 1972).
Haemophilia A or B with inhibitors
In both trials evaluating rFVIIa treatment regimens for haemostatic coverage of surgical procedures in people with inhibitors, the primary efficacy endpoint was the subjective assessment of haemostasis by the investigators, at different times and using different scales (Pruthi 2007; Shapiro 1998). In the Shapiro trial the assessment was referred to the surgical site during the operation, at wound closure, at 8, 24 and 48 hours after wound closure and daily from days three to five after wound closure (Shapiro 1998). In the Pruthi trial, haemostasis was assessed at wound closure, at eight, 24, 48, 72 hours following wound closure and daily until discharge or POD 10 (Pruthi 2007). In both trials, total dosage and length of rFVIIa treatment, together with safety (adverse events), were also evaluated.
Excluded studies
Ten trials were excluded as they were not RCTs or quasi‐RCTs (Avanoglu 1999; Batorova 2000; Jiménez‐Yuste 2002; Rakocz 1993; Ramstrom 1975; Sindet‐Pedersen 1986; Stajcic 1985; Stajcic 1989; Tavenner 1972; Waly 1995). Clinical setting and treatment comparisons of the excluded trials are detailed in an additional table (Table 2).
2. Clinical settings and treatment comparisons in excluded studies*.
| [Study ID] | [Bleeding Disorders] | [Setting] | [Treatment comparisons] |
| Tavenner 1972 | Haemophilia A and B | Dental surgery | Participants treated with blood/plasma only vs epsilon aminocaproic acid with plasma as required vs tranexamic acid with blood/cryoprecipitate as required. |
| Ramstrom 1975 | Haemophilia A and B and von Willebrand disease | Dental surgery | Participants treated with replacement treatment (pre‐operative target level 30% ‐ 50%) vs replacement treatment (pre‐operative target level 30% ‐ 50%) supported by tranexamic acid and antibiotics vs replacement treatment (pre‐operative target level 5% ‐ 10%) supported by tranexamic acid, antibiotics and local measures (local haemostatics and acrylic splints). |
| Stajcic 1985 | Haemophilia A | Dental surgery | Participants treated with replacement treatment receiving epsilon aminocaproic acid systemically (IV) or locally (wound irrigation and soaked gauze) or both systemically and locally. |
| Sindet‐Pedersen 1986 | Haemophilia A and B and von Willebrand disease | Dental surgery | Participants treated with replacement treatment (pre‐operative levels ˜60%) and systemic (IV) tranexamic acid vs replacement treatment and epsilon aminocaproic acid given systemically and as mouthwashes vs the same latter treatment with lower FVIII dose (pre‐operative levels ˜15%). |
| Stajcic 1989 | Haemophilia A | Dental surgery | Observational study of participants receiving a single FVIII infusion and systemic and local (soaked gauze) epsilon aminocaproic undergoing suture of the extraction wound or not. |
| Rakocz 1993 | Haemophilia A and B, von Willebrand disease, FVII and FXI deficiency, Glanzmann's thrombasthenia | Dental surgery | Participants treated with fibrin glue alone or with the latter and tranexamic acid mouth washes. |
| Waly 1995 | Haemophilia A | Dental surgery | Participants treated with replacement treatment and systemic IV tranexamic acid, receiving or not local tranexamic acid (irrigation of operative field and mouth washes). |
| Avanoglu 1999 | Haemophilia A and B | Circumcision | Participants treated with fibrin glue, oral tranexamic acid and replacement treatment after first bolus infusion (target level > 50%) as bolus administrations for 4 days or continuous infusion (4 IU/kg per hour) for 2 days. |
| Batorova 2000 | Haemophilia A | General and orthopaedic surgery | Participants receiving replacement treatment after first bolus infusion as bolus administrations or continuous infusion according to preoperative PK study, in both cases aimed to maintain trough FVIII levels > 50%. |
| Jiménez‐Yuste 2002 | von Willebrand disease | Otolaryngologic surgery | Participants receiving desmopressin and tranexamic acid or FVIII concentrate. |
*All studies but one (Lee 2005) are not randomised or quasi‐randomised trials, but retrospective or prospective observational studies.
FVIII: factor VIII IV: intravenous PK: pharmacokinetic vs: versus
Studies awaiting classification
One trial is awaiting classification (Lee 2005). This has a randomised controlled design, however, outcome data were not clearly reported, we plan to contact the authors for further information.
Ongoing studies
One ongoing trial addressing the comparison between treatment by bolus infusions and continuous infusions of a recombinant full‐length factor VIII concentrate in people with haemophilia A undergoing major orthopaedic surgery was identified (NCT00357656).
Risk of bias in included studies
Please refer to Figure 2.
2.
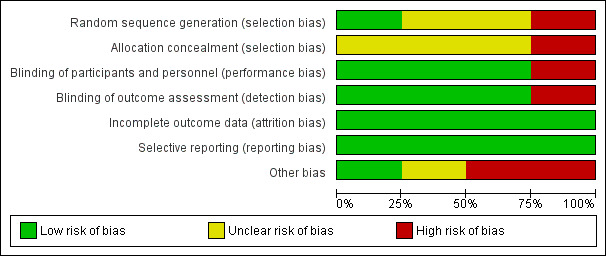
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Generation of randomisation sequence
Only one trial provided details about the generation of the randomisation sequence (Shapiro 1998). This trial reported a 1:1 randomisation within surgical categories to receive either treatment, thus the risk of bias was considered low (Shapiro 1998). In two trials, in which no information about the method of randomisation was reported, we considered the risk of bias to be unclear (Forbes 1972; Pruthi 2007). In the remaining trial, participants were assigned to the treatment groups according to a pair‐matching technique. The risk of bias in this trial was therefore considered high (Walsh 1971).
Allocation concealment
No details about concealment of allocation were reported in three trials, therefore the risk of bias was considered unclear (Forbes 1972; Pruthi 2007; Shapiro 1998). In the remaining double‐blinded trial, allocation was administered by a single physician on the basis of pair matching, therefore the risk of bias was considered high (Walsh 1971).
Blinding
The risk of bias was considered low in three out of the four included trials which were described as being double‐blinded and in which identically appearing treatments were given: tablets (Forbes 1972); intravenous 250 ml infusions and orange‐flavoured drink (Walsh 1971); or intravenous drug bolus (Shapiro 1998). In one trial, the lack of knowledge of laboratory results by the clinicians was also stated (Forbes 1972). In the Pruthi trial, participants and physicians could not be blinded due to the major difference between the modality of administration of treatment (bolus infusions or continuous infusion). The risk of bias was thus high in this trial (Pruthi 2007).
Incomplete outcome data
Intention‐to‐treat populations for the efficacy analysis were used in all of the included trials. No withdrawals were reported in the Forbes trial (Forbes 1972), whereas one participant withdrew from the Walsh trial; however, this participant was included in the efficacy analysis on the basis of the original treatment group (Walsh 1971). In the Shapiro (n = 5) and Pruthi (n = 6) trials, all participants who did not complete the trial because of haemostatic treatment failures were included in the trial efficacy analysis (Shapiro 1998; Pruthi 2007). This is also the case for a further participant in the Shapiro trial who withdrew due to an adverse event (Shapiro 1998). Whereas Pruthi excluded one participant from the analysis because he suffered from acquired (not congenital) haemophilia (Pruthi 2007). Overall, the risk of bias has been judged to be low for all trials.
Selective reporting
We were not able to compare the trial protocols and reports for all included trials. We did compare the outcomes reported in the methods to those reported in the results, and we found that outcomes were reported in full in all cases. Outcomes and timing of assessments were among those commonly used in this field and defined as outcome measures for this review. On this basis, we considered the risk of bias for all trials to be low (Forbes 1972; Pruthi 2007; Shapiro 1998; Walsh 1971).
Other potential sources of bias
The Walsh trial was conducted at two centres, adopting different methods for calculating replacement therapy and, in particular, different follow up and duration of the investigational treatment. Moreover, participants showed differences in mean factor levels and mean number of teeth removed per patient in the two treatment groups at one centre. However, results were reported separately for the two participating centres (Walsh 1971). No relevant other source of bias was detected in the Forbes trial (Forbes 1972). Two trials used subjective assessments rather than an objective measurement for the primary efficacy endpoint, thus carrying an inherent risk of bias (Pruthi 2007; Shapiro 1998). In both of these trials, additional haemostatic drug administration was allowed with different timings. Only the Pruthi trial clearly reported the number of additional doses, with a different distribution between the two treatment arms (Pruthi 2007). No adjustment is reported for age, which is likely to be different in the two treatment groups in both trials (in particular in the Pruthi trial) or inhibitor titres (data not provided). Both trials included major and minor surgical procedures, with adjustment of analysis for surgery type reported only in one trial (Shapiro 1998).
Effects of interventions
Haemophilia A or B without inhibitors
Two trials (n = 59) evaluated the role of antifibrinolytic agents given in addition to replacement treatment in people undergoing dental extractions (Forbes 1972; Walsh 1971). Data on outcomes and assessments from these trials are reported below, in the analyses (Analysis 1.1; Analysis 1.2), and in an additional table (Table 3).
1.1. Analysis.
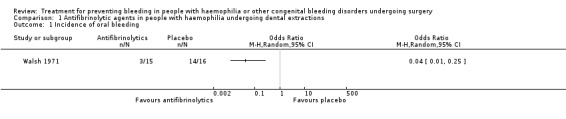
Comparison 1 Antifibrinolytic agents in people with haemophilia undergoing dental extractions, Outcome 1 Incidence of oral bleeding.
3. Additional data on efficacy and safety outcomes in studies with antifibrinolytic agents in people with haemophilia undergoing dental extractions (Walsh 1971; Forbes 1972).
| Outcome | Walsh 1971 | Forbes 1972 | ||
|
EACA n = 15 |
Placebo n = 16 |
Tranexamic acid n = 16 |
Placebo n = 16 |
|
| Blood loss per participant, mean (range), ml | n.e. | n.e. | 61.2 (1 ‐ 749) | 84.1 (4 ‐ 323) |
| Reduction in Hb levels | 3.9/3.7%* | 6.3/6.3 %* | 0.3^ | 1.4^ |
| Post‐operative replacement treatment administration, number of participants (%) |
1 (6.7) | 9 (56.3) | 2 (12.5) | 11 (68.8) |
| Serious side effects, number of participants (%) | 1 (6.7) | 0 (0) | 0 (0) | 0 (0) |
| Any side effect, number of participants (%) | 12 (80) | 8 (50) | 0 (0) | 0 (0) |
*percentage reduction from the baseline, values reported at the 2 participating centres; ^reduction in g/100 ml
n: number n.e.: not evaluated
Primary outcomes
1. Mortality
Neither trial specifically addressed this outcome. In the frame of safety assessments, no fatal adverse event was reported.
2. Blood loss assessed objectively
a. during surgery
i. by variation of haemoglobin levels
Neither trial reported on this outcome.
ii. by transfusion requirement (number of red blood cell (RBC) units infused)
This outcome was not considered specifically by either trial. The lack of blood transfusion requirement was clearly stated in one trial (Walsh 1971), whereas no information was provided in the second trial (Forbes 1972).
b. after surgery
i. by variation of haemoglobin levels
This outcome was reported in both trials, but by different assessments (Table 3). In the Forbes trial, the drop in haemoglobin levels in the five‐day follow up was higher in the placebo group than in the tranexamic acid group (1.4 g/100 ml versus 0.3 g/100 ml). However, the main objective measure of blood loss was provided by the assessment of 51Cr‐labelled red cells in oral secretions and faeces. Over the five‐day evaluation, blood lost per participant was 84.1 ml (range 4 to 323) and 61.2 ml (range 1 to 749) in the placebo and tranexamic acid group, respectively (Mann‐Whitney U test, P < 0.025) (Forbes 1972). In the Walsh trial, a slightly greater (but not statistically significant) fall in haemoglobin occurred, on average, in the placebo group (6.3% at both participating centres) than in the EACA group (3.9% and 3.7% at the two different participating centres (Walsh 1971). Moreover, in this trial, the number of participants reporting intra‐oral bleeding was significantly higher in the placebo group than in participants receiving EACA, OR 0.04 (95% CI 0.01 to 0.25) (Analysis 1.1) (Figure 3).
3.
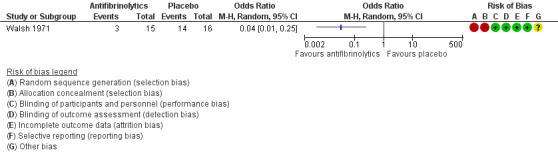
Forest plot of comparison: 1 Antifibrinolytic agents in people with haemophilia undergoing dental extractions, outcome: 1.1 Incidence of oral bleeding.
ii. by transfusion requirement (number of RBC units infused)
This outcome was not specifically reported in either trial. Again, the lack of blood transfusion requirement was clearly stated in the Walsh trial (Walsh 1971), whereas no information was provided in the Forbes trial (Forbes 1972).
3. Need for re‐intervention
This outcome was not reported in either trial.
Secondary outcomes
1. Need for additional unplanned dosing of the drug under study
This outcome was not addressed in either of the trials (Forbes 1972; Walsh 1971). The investigational drug was administered according to the trial protocols and no additional dosing was clinically considered.
2. Need for alternative haemostatic treatment
This outcome was not considered in either of the trials. However, the need for additional replacement treatment was reported (Forbes 1972; Walsh 1971) (Analysis 1.2). In the Forbes trial, the number of participants requiring additional plasma or concentrate infusions during the five‐days trial was higher in the placebo group than in the tranexamic acid group (11 out of 16 versus 2 out of 16) (Forbes 1972). Similarly, the number of participants requiring post‐operative replacement material was significantly higher in the placebo groups than in the EACA‐treated groups (7 out of 12 and 2 out of 4 versus 1 out of 11 and zero out of 4) over the 10‐day and seven‐day follow up at the two centres participating in the Walsh trial (Walsh 1971). Combined data from these trials significantly favoured the treatment group, OR 0.06 (95% CI 0.01 to 0.25) (Analysis 1.2).
3. Haemostatic effectiveness (as assessed and rated by the surgeon or the treating physician)
Subjective assessments of haemostatic efficacy were not considered in either trial.
4. Achievement of sustained target haemostatic levels (as measured by lab test during or after surgery)
This outcome is not relevant to trials focused on antifibrinolytic agents.
5. Duration of replacement treatment
Again, this outcome is not relevant to trials focused on antifibrinolytic agents. As regards the administration of these agents, duration of treatment was defined according to the trial protocols, five days (Forbes 1972) and 10 or seven days in the two participating centres (Walsh 1971), respectively.
6. Concentrate consumption
These trials reported the effect of antifibrinolytic agents in reducing the need for replacement concentrate. The number of units of antihaemophilic globulin infused per root extracted was lower in the participants receiving tranexamic acid than those receiving placebo; in the trial report this was represented in a figure, with no absolute or mean values provided (Forbes 1972). Similarly, at the two centres participating in the Walsh trial, lower mean numbers of units (145 and 0 versus 2331 and 1881) and of doses (1.75 and 1.25 versus 0.09 and 0) of post‐operative replacement concentrate given per participant were reported in those on antifibrinolytic therapy (Walsh 1971).
7. Thromboembolic adverse events
This outcome was not reported in either trial.
8. De novo inhibitor development
Neither trial considered this outcome in their methods or reported this in the results. However, the trial follow‐up period was quite short (five to 10 days) for such an assessment (Forbes 1972; Walsh 1971).
Haemophilia with inhibitors
Two trials (n = 53) considered people undergoing major or minor elective surgery and compared treatment regimens of rFVIIa in this setting (Pruthi 2007; Shapiro 1998). Data and assessments are reported below and in the analyses and additional tables (Analysis 2.1; Analysis 3.1; Analysis 4.1; Analysis 5.1) (Table 4; Table 5; Table 6; Table 7).
2.1. Analysis.
Comparison 2 Low‐dose vs high‐dose rFVIIa in people with haemophilia and inhibitors undergoing surgery, Outcome 1 Need for additional/alternative therapy.
3.1. Analysis.
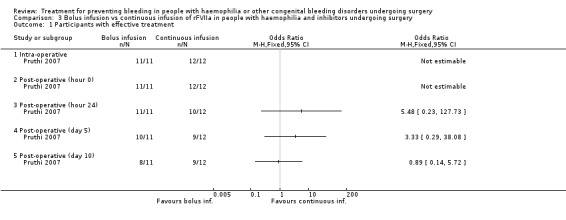
Comparison 3 Bolus infusion vs continuous infusion of rFVIIa in people with haemophilia and inhibitors undergoing surgery, Outcome 1 Participants with effective treatment.
4.1. Analysis.
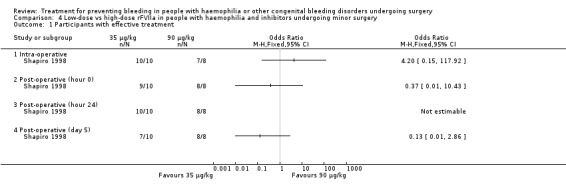
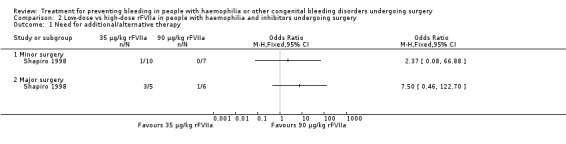
Comparison 4 Low‐dose vs high‐dose rFVIIa in people with haemophilia and inhibitors undergoing minor surgery, Outcome 1 Participants with effective treatment.
5.1. Analysis.
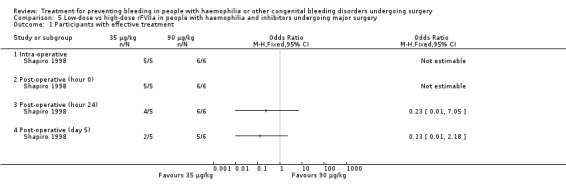
Comparison 5 Low‐dose vs high‐dose rFVIIa in people with haemophilia and inhibitors undergoing major surgery, Outcome 1 Participants with effective treatment.
4. Low‐dose vs high‐dose rFVIIa in people with haemophilia and inhibitors undergoing surgery ‐ Treatment efficacy time assessments (Shapiro 1998).
| Time of assessment | Number (%)* of participants with satisfactory haemostasis | ||||
| Minor surgery | Major surgery | P for difference^ | |||
|
35 µg/kg rFVIIa n = 10 |
90 µg/kg rFVIIa n = 8 |
35 µg/kg rFVIIa n = 5 |
90 µg/kg rFVIIa n = 6 |
||
| Intra‐operative | 10 (100) | 7 (88) | 5 (100) | 6 (100) | NS |
| Hour 0 | 9 (90) | 8 (100) | 5 (100) | 6 (100) | NS |
| Hour 8 | 9 (90) | 8 (100) | 4 (80) | 6 (100) | NS |
| Hour 24 | 10 (100) | 8 (100) | 4 (80) | 6 (100) | NS |
| Hour 48 | 9 (90) | 8 (100) | 3 (60) | 6 (100) | NS |
| Day 3 | 8 (80) | 8 (100) | 2 (40) | 6 (100) | 0.014 |
| Day 4 | 7 (70) | 8 (100) | 2 (40) | 6 (100) | 0.008 |
| Day 5 | 7 (70) | 8 (100) | 2 (40) | 5 (83) | 0.030 |
*rated by the investigators; ^calculated across both surgery categories.
NS: not significant rFVIIa: recombinant activated factor VII
5. Low‐dose vs high‐dose rFVIIa in in people with haemophilia and inhibitors undergoing surgery ‐ Total dose and duration of treatment (Shapiro 1998).
| Minor surgery | Major surgery | |||
|
35 µg/kg rFVIIa n = 10 |
90 µg/kg rFVIIa n = 7* |
35 µg/kg rFVIIa n = 5 |
90 µg/kg rFVIIa n = 6 |
|
| Median duration of dosing, days (range) | 4.0 (3‐6) | 6.0 (3‐6) | 15.0 (2‐26) | 9.5 (8‐17) |
| Median number of injections (range) | 29.5 (24‐44) | 38.0 (26‐67) | 135.0 (11‐186) | 81.0 (71‐128) |
| Median total dose of rFVIIa, mg (range) | 45.5 (14 ‐ 171) | 67.0 (31 ‐ 122) | 656 (31 ‐ 839) | 569 (107 ‐ 698) |
*excluding one outlier participant who required 13 days' dosing and a total dose of rFVIIa of 706 mg. If this patient was included, median duration of dosing was unchanged (6.0), but median rFVIIa total dose increased to 80 mg.
rFVIIa: recombinant activated factor VII
6. Bolus infusion vs continuous infusion of rFVIIa in people with haemophilia and inhibitors undergoing surgery ‐ Treatment efficacy time assessments (Pruthi 2007).
| Number of participants with effective treatment (%)* | ||
| Time of assessment |
Bolus infusion n = 11 |
Continuous infusion n = 12 |
| Intra‐operative | 11 (100) | 12 (100) |
| Hour 0 | 11 (100) | 12 (100) |
| Hour 8 | 11 (100) | 11 (92) |
| Hour 24 | 11 (100) | 10 (83) |
| Hour 48 | 9 (82) | 11 (92) |
| Hour 72 | 8 (73) | 10 (83) |
| Day 4 | 10 (91) | 9 (75) |
| Day 5 | 10 (91) | 9 (75) |
| Day 6 | 10 (91) | 9 (75) |
| Day 7 | 9 (82) | 9 (75) |
| Day 8 | 9 (82) | 9 (75) |
| Day 9 | 8 (73) | 9 (75) |
| Day 10 | 8 (73) | 9 (75) |
*rated by the investigators.
7. Bolus infusion vs continuous infusion of rFVIIa in people with haemophilia and inhibitors undergoing surgery ‐ Total dose, duration of treatment, need for additional/alternative therapy and safety (Pruthi 2007).
| Outcome measure |
Bolus infusion n = 11 |
Continuous infusion n = 12 |
| Median duration of therapy, days | 10 | 9 |
| Treatment for > 10 days, number of participants | 5 | 6 |
| Mean total dose of rFVIIa, mg | 237.5 | 292.2 |
| Total number of additional bolus* | 9 | 8 |
| Need for alternative haemostatic therapy | 3 | 3 |
| Serious adverse events^, number of participants | 8 | 7 |
*greater than the 2 allowed by the protocol in any 24‐hour period; ^as assessed by the investigators, including therapeutic failures.
Primary outcomes
1. Mortality
Neither trial specifically addressed this outcome in the methods. However, no fatal adverse event was reported in safety assessments. The Pruthi trial clearly stated that there were no participant deaths (Pruthi 2007).
2. Blood loss assessed objectively
a. during surgery
i. by variation of haemoglobin levels
Neither of the included trials assessed this outcome in the methods or reported any results.
ii. by transfusion requirement (number of RBC units infused)
This outcome was not specifically addressed. In the Shapiro trial, blood transfusion requirement was reported in 6 out of 29 (21%) participants; however, timing of transfusion (intra‐operative or post‐operative) was not clearly reported (transfusion requirement both during and after the operation was described for one participant), as well the number of RBC units infused and the distribution of participants requiring transfusions in the two treatment groups (Shapiro 1998).
b. after surgery
i. by variation of haemoglobin levels
This outcome was not evaluated in either trial.
ii. by transfusion requirement (number of RBC units infused)
Again, this outcome was not considered in these trials. As reported above, the Shapiro trial reported that 6 out of 29 (21%) participants required blood transfusion, without details about timing of transfusion. However, it is likely that transfusions were given after surgery in all cases (as above mentioned, intra‐ and post‐operative need for transfusions is stated in one participant only). The number of RBC units infused and the distribution of participants requiring transfusions in the two treatment groups were not clearly reported (Shapiro 1998). No information is provided in the Pruthi trial (Pruthi 2007).
3. Need for re‐intervention
This outcome was not stated in the methods of either trial. However, the need for re‐intervention was reported in one participant for each trial: evacuation of surgical‐site intracranial haematoma in one participant who underwent excision of an astrocytoma on continuous infusion of rFVIIa (Pruthi 2007); and debridement and re‐closure of the wound in a participant who underwent major surgery in the low‐dose rFVIIa arm (Shapiro 1998).
Secondary outcomes
1. Need for additional unplanned dosing of the drug under study
This outcome was considered in both trials (Table 5; Table 7). The Shapiro trial reported five participants with haemostatic failure after they had received the repeated blinded rFVIIa scheduled dose (Shapiro 1998). Of these, four received escape rFVIIa doses (up to 180 μg/kg) and the fifth was given alternative therapy without receiving an escape dose. All but one of the participants were in the low‐dose rFVIIa treatment arm (Analysis 2.1). In the Pruthi trial, supplemental doses of rFVIIa in the intra‐operative period were given to six out of 11 (55%) participants in the rFVIIa bolus infusion arm and to five out of 12 (40%) participants in the continuous infusion group, with a mean number of supplemental doses of 2.6 (range 0 to 5) and 1.4 (range 0 to 3) for the bolus and continuous infusion arms, respectively (Pruthi 2007). The total number of additional rFVIIa bolus injections beyond the two allowed by the protocol in any 24‐hour period was similar (nine versus eight) in the bolus and continuous infusion treatment arms (Pruthi 2007). None of these results was statistically significant.
2. Need for alternative haemostatic treatment
This outcome was reported in both trials (Table 5; Table 7). As reported above, in the Shapiro trial, five participants with haemostatic failure on the assigned rFVIIa regimens were moved to alternative haemostatic therapy (two activated prothrombin complex concentrate, one porcine FVIII, one recombinant FVIII and one EACA), all but one after receiving escape rFVIIa doses (Shapiro 1998). Four out of these five participants were in the low‐dose rFVIIa treatment arm (Analysis 2.1) (Shapiro 1998). In the Pruthi trial, six participants with ineffective haemostasis on rFVIIa were then treated with activated prothrombin complex concentrate (n = 3) and/or EACA (n = 3), porcine FVIII (n = 1), recombinant FVIII (n = 1). These participants were equally distributed in the two treatment arms (three out of 11, 27% in the bolus infusion and three out of 12, 25% in the continuous infusion groups) (Pruthi 2007).
3. Haemostatic effectiveness (as assessed and rated by the surgeon or the treating physician)
This outcome was the primary efficacy end‐point in the included trials, with different timing and rating assessments. We have considered the main clinically relevant assessments to be: intra‐operative wound closure (hour 0), 24 hours, end of trial (discontinuation of treatment or POD 5 and 10, in the Shapiro and Pruthi trials, respectively (for Pruthi, an intermediate POD 5 assessment was also included)). Data and analyses were reported separately. In the Pruthi trial, the overall haemostatic efficacy at the end of the trial period was similar in the rFVIIa bolus and continuous infusion arms. Accordingly, all time‐point efficacy assessments were comparable in the two treatment groups (Analysis 3.1; Table 6) (Pruthi 2007). In the Shapiro trial, no difference in the percentage of participants with satisfactory haemostasis was found between the low‐dose and high‐dose rFVIIa groups in the intra‐operative period, at wound closure and in the following 24 hours (Shapiro 1998). A higher rate of efficacy was reported in favour of the high‐dose group at post‐operative assessments from day three to day five, with a more pronounced effect in major than in minor surgery (Table 4) (Shapiro 1998). Main time assessments of haemostatic effectiveness are reported separately for minor (Analysis 4.1) and major surgeries (Analysis 5.1).
4. Achievement of sustained target haemostatic levels (as measured by lab test during or after surgery)
Laboratory measurements of FVII activity levels (FVII:C) were reported in both trials, although it is known that such assessments may not be directly correlated with haemostatic efficacy in inhibitor participants. Data from the Pruthi trial were available for 12 participants (seven in the bolus and five in the continuous infusion arms), with a higher (but not significantly different) mean plasma FVII:C in participants receiving continuous infusion than in those on bolus treatment through to 72 hours (Pruthi 2007). In participants with haemostatic failures, FVII:C levels were in excess of 30 IU/ml at the time the therapy was declared to be ineffective (Pruthi 2007). In the Shapiro trial, the mean (SD) increment in FVII:C levels was higher in those participants receiving the high‐dose rFVIIa treatment than in those on low‐dose treatment (13.64 (3.48) IU/ml versus 30.6 (12.02), respectively), with comparable mean levels at subsequent evaluations (Shapiro 1998). As no blood sampling for FVII:C was obtained at the time at which investigators categorised a participant as a treatment failure, FVII:C could not be analysed with respect to the haemostatic outcome (Shapiro 1998).
5. Duration of replacement treatment
In the Shapiro trial it was documented that there was a clear reduction in the number of days of dosing (and consequently of drug injections) required in the high‐dose group than in the low‐dose group in participants undergoing major surgery. In minor surgery participants, the number of days dosing was similar in both groups (Table 5) (Shapiro 1998). The median duration of rFVIIa treatment was similar in the bolus and continuous infusion arms of the Pruthi trial, as was the number of participants requiring rFVIIa treatment for longer than 10 days (5 out of 11 and 6 out of 12), respectively (Table 7) (Pruthi 2007).
6. Concentrate consumption
In the Shapiro trial, the median total amount of rFVIIa given to participants undergoing both major surgery and minor surgery was similar, irrespective of dose arm (Table 5) (Shapiro 1998). In the Pruthi trial, the mean total dose of rFVIIa administered during the intra‐operative period and up to 72 hours after surgery was greater in the continuous infusion arm than in the bolus treatment arm (Table 7) (Pruthi 2007).
7. Thromboembolic adverse events
This outcome was not specifically considered in either trial (Pruthi 2007; Shapiro 1998). However, one thromboembolic adverse event was reported in each trial. In the Shapiro trial, one participant in the low‐dose rFVIIa arm reported a thrombosis of the right internal jugular vein on day three following central venous catheter placement (Shapiro 1998). In the Pruthi trial, one participant receiving rFVIIa bolus infusion for left total knee arthroplasty developed thrombosis of the left popliteal vein and the proximal peroneal vein on POD 10. Interestingly, in the included studies, only the Pruthi trial provided information about the adoption of venous thromboembolism prophylaxis, which consisted of mechanical methods per institutional protocol. In this multicentre trial, no site used pharmacological thromboprophylaxis (Pruthi 2007).
8. De novo inhibitor development
This outcome is not relevant to these trials.
Discussion
Summary of main results
This systematic review found three randomised controlled trials (RCTs) and one quasi‐RCT investigating treatment for preventing bleeding in people with haemophilia or other CBDs undergoing surgery. These trials refer to two specific clinical settings and therapeutic interventions. The first being in people with haemophilia A or B undergoing dental extractions with the use of antifibrinolytic agents as haemostatic support to the initial replacement treatment (two trials, reporting on 59 participants and 63 procedures (Forbes 1972; Walsh 1971). The second clinical setting related to participants with haemophilia A and B with inhibitors treated with different regimens of rFVIIa (low‐dose versus high‐dose, bolus infusion versus continuous infusion) for haemostatic coverage of surgery (two trials, reporting on the whole 53 participants undergone 33 major and 20 minor surgical interventions) (Pruthi 2007; Shapiro 1998).
These trials showed the efficacy of the addition of antifibrinolytic agents (tranexamic acid or epsilon‐aminocaproic acid) to the initial replacement treatment in people with haemophilia undergoing dental extractions, resulting in the reduction of blood loss and requirement of post‐operative replacement treatment compared with placebo, in the absence of relevant side effects (Forbes 1972; Walsh 1971). As regards people with inhibitors undergoing surgery, a higher post‐operative haemostatic efficacy was revealed when high‐dose (90 μg/kg) regimen of rFVIIa was used as compared with the low‐dose regimen (35 μg/kg), particularly in major surgery, resulting in shorter duration of treatment, lower number of injections but similar total dose of rFVIIa administered and safety (Shapiro 1998). In the same setting, bolus infusion and continuous infusion of rFVIIa showed similar haemostatic efficacy, duration of treatment and safety.
Overall completeness and applicability of evidence
There is a general paucity of well‐designed, rigorous trials reported in the literature on haemophilia and other CBDs, and this review highlights the lack of data concerning treatment for preventing bleeding in people undergoing surgical procedures. The included trials cover relevant specific clinical conditions but do not allow us to present a complete picture of the management of surgery in people with a congenital bleeding disorder (CBD) and to address the objectives of this review. As expected, we found no trials in people with a rare CBD, and even for people with haemophilia A or B, where there is a higher prevalence and greater clinical experience, we were only able to include two trials (in participants without inhibitors) from the 1970s (Forbes 1972; Walsh 1971). The paucity of trials and data about surgery in people with haemophilia could be imputed to challenges in performing surgical interventions in these people until the mid‐1980s, due to the limitations in the availability and safety of replacement products (Franchini 2012). Indeed, the two identified trials aimed to demonstrate the efficacy of antifibrinolytic agents in reducing the need for the limited, difficult to administer and potentially unsafe replacement concentrates available at that time (Forbes 1972; Walsh 1971). In spite of the dramatic changes in replacement treatment since then, the ancillary administration of antifibrinolytics remains a mainstay of treatment in people with a CBD undergoing dental surgery (Srivastava 2013). After the advent of virally‐inactivated plasma‐derived concentrates and, in particular, of recombinant products, general and specific management issues of surgical procedures were increasingly and safely addressed in people with haemophilia. These procedures include adeno‐tonsillectomy, circumcision, placement of central venous access devices, liver biopsies in those with chronic hepatitis and, in particular, major and minor orthopedic surgeries in people with variable stages of arthropathy (Hermans 2009). The large availability (at least in high‐income countries) of factor concentrates and the excellent outcomes in clinical practice, with few bleeding complications, are likely to explain the lack of rigorous trials addressing the optimal treatment in terms of dosing, target factor levels and duration of replacement therapy in the different surgical settings. Therefore, these issues remain substantially unanswered and clinical practice continues to be based on local protocols developed by expert multidisciplinary teams (haematologists, surgeons, anaesthesiologists) and on data from observational, uncontrolled studies or registries. In this respect, a number of national and international documents provide dosing and duration of replacement treatment in various types of surgery, including the recent guidelines from the World Federation of Haemophilia (WFH) (Srivastava 2013). In these guidelines, different regimens and target factor levels are suggested, whether in the presence of resource constraints or not. During the current financial crisis, economic issues have become increasingly important, even in high‐income countries. Costs of treatment were not considered among the outcomes of the included trials. Assessing cost‐effectiveness of treatment should be extensively addressed, in particular in the resource‐demanding setting of surgery in people with a CBD. Another emerging issue refers to the management of the ageing population of people with haemophilia (Mannucci 2009b). The risk of thromboembolic complications (and the need for antithrombotic prophylaxis) has so far been overlooked and largely poorly addressed in clinical studies, even in surgical settings such as orthopaedic surgery, in which such a risk is well recognized in the general population. Therefore, surgical indications and management should be modified in the light of the changing clinical needs of these individuals with an increased life expectancy (Coppola 2013).
The remaining two trials identified were conducted in people with haemophilia and inhibitors receiving rFVIIa. In this setting, the availability of such bypassing agents has greatly encouraged surgical practice since the mid‐1990s. The challenging management, limited experience and pharmaco‐economic implications of treatment supported the need for investigating the most effective and safe regimen of treatment with rFVIIa in surgery. No trials addressed the comparisons between the two available bypassing agents (rFVIIa and activated prothrombin complex concentrate) in this setting. In the included trials, consistent with clinical practice, subjective, rather than objective, assessment of haemostatic efficacy was considered as the primary endpoint. The superiority of the high‐dose rFVIIa regimen illustrated in the Shapiro trial (Shapiro 1998) is well recognized and implemented in clinical practice (Giangrande 2009). The Pruthi trial failed to show advantages of rFVIIa continuous infusion compared with bolus administrations (Pruthi 2007).The continuous infusion approach was considered potentially product‐ and cost‐saving, in particular when complete pharmacokinetic information is used for adjusting dose (Martinowitz 1992; Batorova 2000). However, these uncertainties and the need for specific expertise, mean that in current practice continuous infusion (of rFVIIa and other factor concentrates) is mainly used at just a few specialised centres. The comparison between treatment by bolus or continuous infusion is being further addressed in an ongoing randomised trial with a recombinant protein‐free full‐length factor VIII in people with haemophilia undergoing major orthopaedic surgery (NCT00357656).
Quality of the evidence
The trials included in this review do not allow robust conclusions with respect to the objective(s) of this review. Beyond the limitations of these clinical settings, and in spite of adequate trial designs, the small sample sizes greatly affect the statistical power of the trials. The rarity of the conditions under study, such as haemophilia with inhibitors, often represents an insurmountable barrier, even in multicentre or multinational trials.
Potential biases in the review process
The high likelihood that all relevant trials were identified for this review was provided by the lack of restrictions based on dates, language or publication type in the search strategy. The trial selection and analysis did not result in disagreement among reviewers and all relevant data were obtained from the included trials. On the basis of the retrieved trials, with heterogeneous objectives and evaluated outcomes, It is unlikely that the review process could have introduced bias.
Agreements and disagreements with other studies or reviews
No comparable systematic review is currently available. The lack of robust data supporting treatment recommendations and guidelines was similarly recognised by a literature review on replacement therapy for invasive procedure in people with haemophilia. In this literature review, two trials in dental surgery (also included in this Cochrane Review) were identified as the only RCTs available in haemophilia (Hermans 2009).
Authors' conclusions
Implications for practice.
There is insufficient evidence from RCTs to assess the most effective and safe haemostatic treatment to prevent bleeding in people with a CBD undergoing surgery, currently managed, in most cases, on the basis of local protocols developed by expert clinicians and on information from observational uncontrolled studies. Some conclusions can be drawn, but only in relation to the management of people with haemophilia A or B undergoing dental extractions or of people with inhibitors treated with rFVIIa on surgery.
Implications for research.
Large, adequately‐powered, well‐designed RCTs comparing active treatment to control are ideally needed to provide evidence for the optimal treatment regimen to prevent bleeding in all people with a CBD undergoing different types of surgical procedures. These should address the issues of efficacy, safety and cost‐effectiveness of the different dosing regimens, the duration of treatment, and the strategies for improving outcomes or reducing the need for replacement products (local haemostatic products, antifibrinolytic agents, timing of treatment administration, modalities of administration). Moreover, standardised and commonly agreed outcome measures of efficacy should be identified to facilitate the comparison of results and their pooling. Performing such rigorous studies is always a complex task in this setting and presently does not appear to be a research priority. Indeed, numerous prospective clinical trials are ongoing; however, to our knowledge, only one study will provide a randomised comparison of interventions. Although difficult to pursue, these objectives are important, particularly in the light of the increasing economic constraints and the high healthcare costs of the management of surgery in people with CBDs. Elderly people with haemophilia represent a new challenging population in whom evidence about risk‐benefit assessments in the surgical setting is needed.
Acknowledgements
The authors thank Alfonso Iorio and Tracey Remmington for their valuable support throughout the development of this review.
Data and analyses
Comparison 1. Antifibrinolytic agents in people with haemophilia undergoing dental extractions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of oral bleeding | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Post‐operative replacement treatment administration | 2 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.25] |
Comparison 2. Low‐dose vs high‐dose rFVIIa in people with haemophilia and inhibitors undergoing surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for additional/alternative therapy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Minor surgery | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Major surgery | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Bolus infusion vs continuous infusion of rFVIIa in people with haemophilia and inhibitors undergoing surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with effective treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Intra‐operative | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Post‐operative (hour 0) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Post‐operative (hour 24) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Post‐operative (day 5) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Post‐operative (day 10) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Low‐dose vs high‐dose rFVIIa in people with haemophilia and inhibitors undergoing minor surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with effective treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Intra‐operative | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Post‐operative (hour 0) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Post‐operative (hour 24) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Post‐operative (day 5) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Low‐dose vs high‐dose rFVIIa in people with haemophilia and inhibitors undergoing major surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with effective treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Intra‐operative | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Post‐operative (hour 0) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Post‐operative (hour 24) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Post‐operative (day 5) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Forbes 1972.
| Methods | Single‐centre double‐blind RCT. | |
| Participants | 28 individuals with haemophilia (20 haemophilia A, 8 haemophilia B; 15 severe, 11 moderate, 2 mild) Age: range 13 ‐ 65 years. 32 separate episodes of dental extractions. |
|
| Interventions | Tranexamic acid 1 g 3‐times‐a‐day, starting 2 hours before dental extractions, for 5 days. Placebo tablets given with the same schedule. Both interventions in association with IV factor VIII or IX equivalent of 1000 ml of human plasma 1 hour before extraction and, in the case of excessive bleeding, further administrations sufficient to stop bleeding. |
|
| Outcomes |
Efficacy outcomes: Blood loss measured with 51Cr‐labelled red cells in oral secretions and faeces over 24‐hr collections for 5 days. Need for further replacement therapy after the initial dose. Safety: renal and liver function tests, electrocardiogram, adverse events. |
|
| Notes | Significant increase in ESR and fibrinogen levels in the placebo group were recorded, reflecting the amount of plasma or concentrate infused. Significant depression of urokinase sensitivity after treatment with tranexamic acid was shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation is stated. No further details given. |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind technique and random allocation are stated. No further details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and clinicians did not know which tablet was given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The clinician did not know the treatment type and the results of laboratory assays. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No trial withdrawal or incomplete outcome data stated. |
| Selective reporting (reporting bias) | Low risk | Outcome data reported in the methods and results sections correspond. |
| Other bias | Low risk | No difference in haemophilia severity, mean level of plasma factor VIII/IX and the number of roots extracted in the 2 groups. |
Pruthi 2007.
| Methods | Open‐label multicentre RCT. | |
| Participants | 24 male participants with congenital haemophilia A or B with inhibitors undergoing elective major surgery receiving recombinant FVIIa (rFVIIa) as bolus or continuous infusion for the efficacy outcomes. 12 males with congenital haemophilia A or B without inhibitors undergoing elective major surgery as control group for safety assessment. Age: median (range) 37.4 (10 ‐ 67) years and 26.7 (11 ‐ 53) years in the 2 treatment inhibitor groups; 31 (14 ‐ 76) years in the control non‐inhibitor group. |
|
| Interventions | Bolus infusion: 90 μg/kg rFVIIa every 2 hours during surgery through day 5, then every 4 hours for days 6 ‐ 10. Continuous infusion: 50 μg/kg/hour through day 5, then 25 μg/kg/hour for days 6 ‐ 10. All inhibitor participants received an initial bolus dose of 90 μg/kg rFVIIa. Two additional rFVIIa bolus infusions for breakthrough bleeding allowed in any 24‐hour period. The control group (safety assessment) received FVIII or FIX concentrates per institutional protocols. |
|
| Outcomes |
Primary efficacy outcome
Subjective investigator's assessment of treatment efficacy (2‐level: effective or ineffective) at the time of discontinuation of therapy or at post‐operative day 10. Haemostasis assessment at wound closure (0 hours), at 8, 24, 48, 72 hours following wound closure and daily until discharge or post‐operative day 10. Safety: clinical symptoms; concomitant medications; and adverse events. |
|
| Notes | Sample size not sufficient for adequate statistical power in either efficacy and safety analysis . One participant in the bolus infusion arm excluded from efficacy analysis because of acquired haemophilia. 2 surgeries (1 in each arm) considered as minor. Dosages of rFVIIa, length of treatment and FVII activity levels were also evaluated. Greater mean total dose of rFVIIa was administered during the intra‐operative period and up to 72 post‐operative hours in the continuous infusion arm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central randomisation. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Central randomisation, open‐label treatment. No further details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for physicians and participants because of the different modality of administration. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome was assessed by the investigators, who were not blinded of administered treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT population for the efficacy analysis (one participant in the bolus infusion arm excluded). 6 participants (3 bolus infusion, 3 continuous infusion), who discontinued from the trial because of ineffective haemostasis, were reported in the efficacy analysis. |
| Selective reporting (reporting bias) | Low risk | Outcome data reported in the methods and results sections correspond. |
| Other bias | High risk | Subjective assessment for the primary efficacy endpoint. Number of additional rFVIIa higher in the bolus infusion than in the continuous infusion arm [mean (range) 2.6 (0 ‐ 5) vs 1.4 (0 ‐ 3)]. Trend to younger age of participants in the continuous infusion arm (mean 26.7, range 11 ‐ 53; vs 37.4, 10‐67 in the bolus infusion arm). No data on inhibitor titres provided. |
Shapiro 1998.
| Methods | Multicentre double‐blind RCT. | |
| Participants | 29 males with haemophilia and inhibitors (25 haemophilia A, 3 haemophilia B, 1 acquired FVIII inhibitor) undergoing elective surgeries (11 major, 18 minor). Age: range 0 ‐ 40 years (0 ‐ 4, n = 9; 5 ‐ 16, n = 13; 17 ‐ 40, n = 7). |
|
| Interventions | Haemostatic treatment with rFVIIa as IV bolus at 35 μg/kg or 90 μg/kg, immediately prior to surgical incision, intra‐operatively every 2 hour or as needed, then every 2 hours from wound closure (time 0) for 48 hours, and every 2 ‐ 6 hours (interval determined by investigators) for additional 3 days. Single additional blinded dose allowed between each 2‐hour interval in the first 48 hours, if needed. Open‐label 90 μg/kg treatment after day 5 on a schedule determined by the investigator. |
|
| Outcomes |
Primary efficacy outcome
Subjective investigator's assessment of treatment efficacy during surgery (3‐level rating, blood loss compared with a participant without haemophilia: as expected, less than expected, more than expected); at wound closure, at 8, 24 and 48 hours after wound closure (3‐level, effective, partially effective, ineffective); daily from days 3 to 5 after wound closure (2‐level, adequate or not adequate). Safety: adverse events |
|
| Notes | Analysis of dose effect adjusted for surgery type. Length of treatment, total dosage and number of injections of rFVIIa, and coagulation tests (PT, FVII, fibrinogen, D‐dimer, platelet count) were also evaluated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised 1:1 within surgical categories to receive either rFVIIa dose. |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind technique. No further details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The rFVIIa dose assigned remained blinded over the 5‐day assessment. The investigators determined the interval of treatment (every 2 ‐ 6 hours) from days 3 to 5. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | rFVIIa dose assigned remained blinded over the 5‐day assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis. 6 participants who did not complete the trial (reasons for withdrawal reported: 5 treatment failures, 1 adverse event) were included in the efficacy analysis. |
| Selective reporting (reporting bias) | Low risk | Outcome data reported in the methods and results sections correspond. |
| Other bias | High risk | Subjective assessment for the primary efficacy endpoint. Trend to younger age of participants in the higher‐dose arm (0 ‐ 4 years: 42.9% vs 20% in the low‐dose arm). No data on inhibitor titres provided. |
Walsh 1971.
| Methods | Double‐blind quasi‐RCT in 2 centres. | |
| Participants | 31 participants with haemophilia (24 haemophilia A, 7 haemophilia B), factor VIII/IX level < 15% (12 severe), undergoing dental extractions. Mean age range: 32.1 ‐ 38.5 years |
|
| Interventions | EACA 6 g IV in 250 ml isotonic saline preoperatively, then orally four times daily in orange squash. Placebo given IV (isotonic saline) and orally (drink with similar taste) with the same schedule. All participants received replacement treatment (antihaemophilc globulin concentrate or cryoprecipitate/factor IX concentrate) calculated (different formulae at the 2 participating centres) to raise factor levels to 50% 1 hour prior to dental extraction. |
|
| Outcomes |
Efficacy outcomes: Intraoral bleeding (daily report of occurrence of bleeding; haemoglobin levels at post‐operative days 1, 3, 5 and 7) Requirement for post‐operative therapeutic material Safety: side effects |
|
| Notes | Different treatment duration and follow up at the 2 participating centres (10 days and 7 days, respectively). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pair‐matching technique on the basis of age, FVIII/IX levels and number or type of teeth to be extracted. |
| Allocation concealment (selection bias) | High risk | Only 1 physician was responsible for the administration of the participant allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and physicians responsible for their care were kept blind to assigned treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians responsible for the care of participants and assessing outcomes were kept blind to assigned treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis. 1 participant withdrew from the trial, but was included in the analysis according to the original treatment group. |
| Selective reporting (reporting bias) | Low risk | Outcome data reported in the methods and results sections correspond. |
| Other bias | Unclear risk | Differences in treatment at the 2 participating centres. Lower mean participants' factor levels and higher mean number of teeth removed per participant in the EACA group at one centre. |
EACA: epsilon‐aminocaproic acid ESR: erythrocyte sedimentation rate ITT: intention‐to‐treat IV: intravenous PT: prothrombin time RCT: randomised controlled trial vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avanoglu 1999 | Not a RCT or quasi‐RCT. Prospective case‐control study. |
| Batorova 2000 | Not a RCT or quasi‐RCT. Observational case‐control study |
| Jiménez‐Yuste 2002 | Not a RCT or quasi‐RCT. Prospective observational study. |
| Rakocz 1993 | Not a RCT or quasi‐RCT. Retrospective case‐control study. |
| Ramstrom 1975 | Not a RCT or quasi‐RCT. Retrospective case‐control study. |
| Sindet‐Pedersen 1986 | Not a RCT or quasi‐RCT. Retrospective case‐control study. |
| Stajcic 1985 | Not a RCT or quasi‐RCT. Observational case‐control study. |
| Stajcic 1989 | Not a RCT or quasi‐RCT. Retrospective case‐control study. |
| Tavenner 1972 | Not a RCT or quasi‐RCT. Retrospective case‐control study. |
| Waly 1995 | Not a RCT or quasi‐RCT. Observational case‐control study. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Lee 2005.
| Methods | Double‐blind, cross‐over RCT. |
| Participants | 13 individuals with haemophilia (11 haemophilia A, 7 severe; 2 haemophilia B, both severe) undergoing dental scaling. |
| Interventions | Experimental treatment: placebo infusion (0.9% saline) within 1 hour prior to the dental procedure; 10 ml 5% tranexamic acid mouthwash up to four times per day for 8 days, according to visual check for gingival bleeding by the participant. Control treatment: factor concentrate infusion according to the type and severity of haemophilia; placebo mouthwash with the same instructions. |
| Outcomes | Post‐scaling haemorrhage (need for factor replacement treatment; reported gingival bleeding). Use of mouthwash (overall use; number of times used to control bleeding; number of times electively not used when bleeding occurred; number of times used despite no bleeding). |
| Notes | Published data did not enable to extract outcomes clearly. Additional information was requested to the authors. |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00357656.
| Trial name or title | Antihaemophilic factor (recombinant) plasma/albumin‐free method (rAHF PFM): A Phase 3/4, prospective, controlled, randomized, multicentre study to compare the efficacy and safety of continuous infusion versus intermittent bolus infusion in participants with severe or moderately severe hemophilia A undergoing major orthopedic surgery. |
| Methods | Randomised multicentre open‐label parallel assignment trial. |
| Participants | People with severe or moderately severe haemophilia A (FVIII < 2%), age 18 ‐ 70 years, previously treated (> 150 exposure days) with factor VIII concentrates, scheduled to undergo an elective unilateral major orthopedic surgery that requires drain placement. |
| Interventions | Recombinant protein‐free factor VIII (rAHF‐PFM). Bolus infusion vs continuous infusion. |
| Outcomes |
Primary outcome Cumulative PRBC volume in the drainage fluid during 24 hours following surgery in subjects receiving rAHF‐PFM by bolus infusion or continuous infusion. Secondary outcomes a. Total amount of hemoglobin in the cumulative drainage fluid during the first post‐operative 24 hours (and until time of drain removal, if drainage continues beyond 24 hours); b. Actual post‐operative blood loss during the first post‐operative 24 hours; c. Number of bleeding episodes during treatment with continuous or bolus infusion. |
| Starting date | June 2006. |
| Contact information | Richard Steinbrugger. |
| Notes | Currently recruiting. Expected to be completed June 2016. |
PRBC: packed red blood cell vs: versus
Contributions of authors
Lead author: AT
First author: AC
Co‐authors: JW, CY, MNDDM
Protocol development and drafting: AC
Protocol revision: JW
Trial search, screening and data extraction: AC, MNDDM
Analysis and interpretation: AC, MNDDM, JW, AT, CY
Manuscript drafting: AC, MNDDM
Manuscript revision and approving: AC, MNDDM, JW, AT, CY
Declarations of interest
Dr Antonio Coppola has acted as paid lecturer or board member to Bayer, Biotest and Kedrion in the previous 36 months.
Dr Matteo Nicola Dario Di Minno acted as paid lecturer or board member and received funds for researches unrelated to the present study from Pfizer, Bayer and Novo Nordisk in the previous 36 months.
Dr Jerzy Windyga received fees as speaker, board member, or consultant and funding for clinical trials not related to the present study from Baxter, Bayer, Biogen Idec, CSL Behring, GlaxoSmithKline, LFB, Novo Nordisk, Octapharma and Pfizer in the previous 36 months.
Dr Antonella Tufano: none known.
Dr Cindy Yeung: none known.
New
References
References to studies included in this review
Forbes 1972 {published data only}
- Forbes CD, Barr RD, Reid G, Thomson C, Prentice CRM, McNicol GP, et al. Tranexamic acid in control of haemorrhage after dental extraction in haemophilia and Christmas disease. British Medical Journal 1972;2:311‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pruthi 2007 {published data only}
- Pruthi RK, Mathew P, Valentino LA, Sumner MJ, Seremetis S, Hoots WK, et al. Haemostatic efficacy and safety of bolus and continuous infusion of recombinant factor VIIa are comparable in haemophilia patients with inhibitors undergoing major surgery. Results from an open‐label, randomized, multicenter trial. Thrombosis and Haemostasis 2007;98:726‐32. [PubMed] [Google Scholar]
Shapiro 1998 {published data only}
- Shapiro AD, Gilchrist GS, Hoots WK, Cooper HA, Gastineau DA. Prospective, randomised trial of two doses of rFVIIa (NovoSeven) in haemophilia patients with inhibitors undergoing surgery. Thrombosis and Haemostasis 1998;80(5):773‐8. [PubMed] [Google Scholar]
Walsh 1971 {published data only}
- Walsh PN, Rizza CR, Matthews JM, Eipe J, Kernoff PBA, Coles MD, et al. Epsilon‐aminocaproic acid therapy for dental extractions in haemophilia and Christmas disease: a double blind controlled trial. British Journal of Haematology 1971;20:463‐75. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Avanoglu 1999 {published data only}
- Avanoglu A, Celik A, Ulman I, Ozcan C, Kavakli K, Nisli G, et al. Safer circumcision in patients with haemophilia: the use of fibrin glue for local haemostasis. BJU International 1999;83:91‐4. [DOI] [PubMed] [Google Scholar]
Batorova 2000 {published data only}
- Batorova A, Martinowitz U. Intermittent injections vs. continuous infusion of factor VIII in haemophilia patients undergoing major surgery. British Journal of Haematology 2000;110:715‐20. [DOI] [PubMed] [Google Scholar]
Jiménez‐Yuste 2002 {published data only}
- Jiménez‐Yuste V, Prim MP, Diego JI, Villar A, Quintana M, Rabanal I. Otolaryngologic surgery in children with von Willebrand Disease. Archives of Otolaryngologologic Head Neck Surgery 2002;128:1365‐8. [DOI] [PubMed] [Google Scholar]
Rakocz 1993 {published data only}
- Rakocz M, Mazar A, Varon D, Spierer S, Blinder D, Martinowitz U. Dental extractions in patients with bleeding disorders. The use of fibrin glue. Oral Surgery Oral Medicine Oral Pathology 1993;75:280‐2. [DOI] [PubMed] [Google Scholar]
Ramstrom 1975 {published data only}
- Ramstrom G, Blomback M. Tooth extractions in haemophiliacs. International Journal Oral Surgery 1975;4:1‐17. [DOI] [PubMed] [Google Scholar]
Sindet‐Pedersen 1986 {published data only}
- Sindet‐Pedersen S, Stenbjerg S. Effect of local antifibrinolytic treatment with tranexamic acid in hemophiliacs undergoing oral surgery. Journal Oral Maxillofacial Surgery 1986;44:703‐7. [DOI] [PubMed] [Google Scholar]
Stajcic 1985 {published data only}
- Stajcic Z. The combined local/systemic use of antifibrinolytics in hemophiliacs undergoing dental extractions. International Journal Oral Surgery 1985;14:339‐45. [DOI] [PubMed] [Google Scholar]
Stajcic 1989 {published data only}
- Stajcic Z, Baklaja R, Elezovic I, Rolovic Z. Primary wound closure in haemophiliacs undergoing dental extractions. International Journal of Oral and Maxillofacial Surgery 1989;18:14‐6. [DOI] [PubMed] [Google Scholar]
Tavenner 1972 {published data only}
- Tavenner RW. Use of tranexamic acid in control of haemorrhage after extraction of teeth in haemophilia and Christmas disease. British Medical Journal 1972;2:314‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waly 1995 {published data only}
- Waly NG. Local antifibrinolytic treatment with tranexamic acid in hemophilic children undergoing dental extractions. Egyptian Dental Journal 1995;41:961‐8. [PubMed] [Google Scholar]
References to studies awaiting assessment
Lee 2005 {published data only}
- Lee AP, Boyle CA, Savidge GF, Fiske J. Effectiveness in controlling haemorrhage after dental scaling in people with haemophilia by using tranexamic acid mouthwash. British Dental Journal 2005;198(1):33‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00357656 {unpublished data only}
- NCT00357656. Phase 3/4 study of a recombinant protein‐free factor VIII (rAHF‐PFM): comparison of continuous infusion versus intermittent bolus infusion in hemophilia A subjects undergoing major orthopedic surgery. http://clinicaltrials.gov/show/NCT00357656 (accessed 17 March 2014).
Additional references
Aledort 1994
- Aledort LM, Haschmeyer RH, Pettersson H, the Orthopaedic Outcome Study Group. A longitudinal study of orthopaedic outcomes for severe factor‐VIII‐deficient haemophiliacs. Journal of Internal Medicine 1994;236(4):391‐9. [DOI] [PubMed] [Google Scholar]
Aoki 1989
- Aoki N. Hemostasis associated with abnormalities of fibrinolysis. Blood Reviews 1989;3(1):11‐7. [DOI] [PubMed] [Google Scholar]
Astermark 2010
- Astermark J. Inhibitor development: patient‐determined risk factors. Haemophilia 2010;16(102):66‐70. [DOI] [PubMed] [Google Scholar]
Becker 1993
- Becker MP, Balagtas CC. Marginal modeling of binary cross‐over data. Biometrics 1993;49(4):997‐1009. [PubMed] [Google Scholar]
Berntorp 1998
- Berntorp E. Guidelines on treatment of haemophilia in Sweden. Haemophilia 1998;4(4):425–6. [DOI] [PubMed] [Google Scholar]
Bolton‐Maggs 2006
- Bolton‐Maggs PHB, Chalmers EA, Collins PW, Harrison P, Kitchen S, Liesner RJ, et al. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. British Journal of Haematology 2006;135(5):603‐33. [DOI] [PubMed] [Google Scholar]
Brooker 2012
- Brooker M. Registry of clotting factor concentrates. 9th Edition, 2012. Facts and Figures, available at http://www1.wfh.org/publication/files/pdf‐1227.pdf 2012, issue 6.
Collins 2013
- Collins PW, Chalmers E, Hart DP, Liesner R, Rangarajan S, Talks K, et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: (4th edition). UK Haemophilia Centre Doctors. British Journal of Haematology 2013;160(2):153‐70. [DOI] [PubMed] [Google Scholar]
Coppola 2008
- Coppola A, Minno G. Desmopressin in the treatment of platelet function abnormalities. Haemophilia 2008;14(Suppl 1):31‐9. [DOI] [PubMed] [Google Scholar]
Coppola 2013
- Coppola A, Santoro C, Franchini M, Mannucci C, Mogavero S, Molinari AC, et al. Emerging issues on comprehensive hemophilia care: preventing, identifying and monitoring age‐related comorbidities. Seminars in Thrombosis and Hemostasis 2013;39(7):794‐802. [DOI] [PubMed] [Google Scholar]
Cosmi 2009
- Cosmi B, Alatri A, Cattaneo M, Gresele P, Marietta M, Rodeghiero F, et al. Assessment of the risk of bleeding in patients undergoing surgery or invasive procedures: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thrombosis Research 2009;124(5):e6‐e12. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9 Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DiMichele 2007
- DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. British Journal of Haematology 2007;138(3):305‐15. [DOI] [PubMed] [Google Scholar]
Donner 2011
- Donner A, Piaggio G, Villar J. Statistical methods for the meta‐analysis of cluster randomized trials. Statistical Methods in Medical Research 2001;10(5):325‐38. [DOI] [PubMed] [Google Scholar]
Eckardt 2011
- Eckhardt CL, Bom JG, Naald M, Peters M, Kamphuisen PW, Fijnvandraat K. Surgery and inhibitor development in hemophilia A: a systematic review. Journal of Thrombosis and Haemostasis 2011;9(10):1948‐58. [DOI] [PubMed] [Google Scholar]
Eckhardt 2012
- Eckhardt CL, Mauser‐Bunschoten EP, Peters M, Leebeek FW, Meer FJ, Fijnvandraat K. Inhibitor incidence after intensive FVIII replacement for surgery in mild and moderate haemophilia A: a prospective national study in the Netherlands. British Journal of Haematology 2012;157(6):747‐52. [DOI] [PubMed] [Google Scholar]
Eckhardt 2013
- Eckhardt CL, Velzen AS, Peters M, Astermark J, Brons PP, Castaman G, et al. Factor VIII gene (F8) mutation and risk of inhibitor development in nonsevere hemophilia A. Blood 2013;122(11):1954‐62. [DOI] [PubMed] [Google Scholar]
Federici 2006
- Federici AB. Diagnosis of inherited von Willebrand disease: a clinical perspective. Seminars in Thrombosis and Hemostasis 2006;32(6):555‐65. [DOI] [PubMed] [Google Scholar]
Franchini 2009
- Franchini M, Manzato F, Salvagno GL, Montagnana M, Lippi G. The use of desmopressin in congenital factor XI deficiency: a systematic review. Annals of Hematology 2009;88(10):931‐35. [DOI] [PubMed] [Google Scholar]
Franchini 2012
- Franchini M, Mannucci PM. Past, present and future of hemophilia: a narrative review. Orphanet Journal of Rare Diseases 2012;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giangrande 2009
- Giangrande PLF, Wilde JT, Madan B, Ludlam CA, Tuddenham EGD, Goddard NJ, et al. Consensus protocol for the use of recombinant activated factor VII [eptacog alfa (activated); NovoSeven®] in elective orthopaedic surgery in haemophilic patients with inhibitors. Haemophilia 2009;15(2):501‐8. [DOI] [PubMed] [Google Scholar]
Gould 2012
- Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM, American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e227S‐77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gouw 2007
- Gouw SC, Bom JG, Berg HM. Treatment‐related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood 2007;109(11):4648‐54. [DOI] [PubMed] [Google Scholar]
Gouw 2013
- Gouw SC, Berg HM, Fischer K, Auerswald G, Carcao M, Chalmers E, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood 2013;121(20):4046‐55. [DOI] [PubMed] [Google Scholar]
Gringeri 2003
- Gringeri A, Mantovani LG, Scalone L, Mannucci PM. Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood 2003;102(7):2358‐63. [DOI] [PubMed] [Google Scholar]
Hermans 2009
- Hermans C, Altisent C, Batorova A, Chambost H, Moerloose P, Karafoulidou A, et al. Replacement therapy for invasive procedures in patients with haemophilia: literature review, European survey and recommendations. Haemophilia 2009;15(3):639‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011:available from www.cochrane‐handbook.org.
James 2013
- James PD, Lillicrap D, Mannucci PM. Alloantibodies in von Willebrand disease. Blood 2013;122(5):636‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karanth 2012
- Karanth L, Barua A, Kanagasabai S, Nair NS. Desmopressin acetate (DDAVP) for preventing and treating acute bleeds during pregnancy in women with congenital bleeding disorders. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009824] [DOI] [Google Scholar]
Mannucci 1998
- Mannucci PM. Hemostatic drugs. New England Journal of Medicine 1998;339(4):245‐53. [DOI] [PubMed] [Google Scholar]
Mannucci 2001
- Mannucci PM, Tuddenham EG. The hemophilias ‐ from royal genes to gene therapy. New England Journal of Medicine 2001;344(23):1773–9. [DOI] [PubMed] [Google Scholar]
Mannucci 2004
- Mannucci PM, Duga S, Peyvandi F. Recessively inherited coagulation disorders. Blood 2004;104(5):1243‐52. [DOI] [PubMed] [Google Scholar]
Mannucci 2009a
- Mannucci PM, Franchini M, Castaman G, Federici AB, on behalf of AICE (Italian Association of Hemophilia Centres) other authors are listed at the end of the manuscript. Evidence‐based treatment of von Willebrand disease in Italy. Blood Transfusion 2009;7(2):117‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannucci 2009b
- Mannucci PM, Schutgens RE, Santagostino E, Mauser‐Bunschoten EP. How I treat age‐related morbidities in elderly persons with hemophilia. Blood 2009;114(26):5256‐63. [DOI] [PubMed] [Google Scholar]
Martinowitz 1992
- Martinowitz U, Schulman S, Gitel S, Horoszowski H, Heim M, Varon D. Adjusted dose continuous infusion of factor VIII in patients with haemophilia A. British Journal of Haematology 1992;82(4):729‐34. [DOI] [PubMed] [Google Scholar]
Martinowitz 1997
- Martinowitz U, Schulman S. Coagulation factors concentrations by continuous infusion. Transfusion Medicine Reviews 1997;11(1):56‐63. [DOI] [PubMed] [Google Scholar]
Mauser‐Bunschoten 2001
- Mauser‐Bunschoten EP, Roosendaal G, Berg HM. Product choice and haemophilia treatment in the Netherlands. Haemophilia 2001;7(1):96‐8. [DOI] [PubMed] [Google Scholar]
Nurden 2005
- Nurden AT. Qualitative disorders of platelets and megakariocytes. Journal of Thrombosis and Haemostasis 2005;3(8):1773‐82. [DOI] [PubMed] [Google Scholar]
Pavlova 2013
- Pavlova A, Oldenburg J. Defining severity of haemophilia: More than factor levels. Seminars in Thrombosis and Hemostasis 2013;39(7):702‐10. [DOI] [PubMed] [Google Scholar]
Peyvandi 2012
- Peyvandi F, Michele D, Bolton‐Maggs PHB, Lee CA, Tripodi A, Srivastava A. Classification of rare bleeding disorders (RBDs) based on the association between coagulant factor activity and clinical bleeding severity. Journal of Thrombosis and Haemostasis 2012;10(9):1938‐43. [DOI] [PubMed] [Google Scholar]
Rodeghiero 1987
- Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von Willebrand’s disease. Blood 1987;69(2):454‐9. [PubMed] [Google Scholar]
Santagostino 2000
- Santagostino E, Mannucci PM, for the Italian Association of Haemophilia Centres (AICE). Guidelines on replacement therapy for haemophilia and inherited coagulation disorders in Italy. Haemophilia 2000;6(1):1‐10. [DOI] [PubMed] [Google Scholar]
Scalone 2006
- Scalone L, Mantovani LG, Mannucci PM, Gringeri A. Quality of life is associated to the orthopaedic status in haemophilic patients with inhibitors. Haemophilia 2006;12(2):154‐62. [DOI] [PubMed] [Google Scholar]
Sharathkumar 2003
- Sharathkumar A, Lillicrap D, Blanchette VS, Kern M, Leggo J, Stain AM, et al. Intensive exposure to factor VIII is a risk factor for inhibitor development in mild hemophilia A. Journal of Thrombosis and Haemostasis 2003;1(6):1228‐36. [DOI] [PubMed] [Google Scholar]
Srivastava 2013
- Srivastava A, Brewer AK, Mauser‐Bunschoten P, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia 2013;19:e1‐e47. [DOI] [PubMed] [Google Scholar]
Stonebraker 2010
- Stonebraker JS, Brooker M, Amand RE, Farrugia A, Srivastava A. A study of reported FVIII use around the world. Haemophilia 2010;16(1):33‐46. [DOI] [PubMed] [Google Scholar]
Teitel 1998
- Teitel JM. National haemophilia treatment protocols: Canada. Haemophilia 1998;4(4):422–3. [DOI] [PubMed] [Google Scholar]
UKHCDO 2003
- United Kingdom Haemophilia Centre Doctors’ Organisation. Guidelines on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. Haemophilia 2003;9(3):1–23. [Google Scholar]
White 2001
- White II GC, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thrombosis and Haemostasis 2001;85(3):560. [PubMed] [Google Scholar]
Wight 2003
- Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A. A systematic review. Haemophilia 2003;9(4):418‐35. [DOI] [PubMed] [Google Scholar]
Xi 2013
- Xi M, Makris M, Marcucci M, Santagostino E, Mannucci PM, Iorio A. Inhibitor development in previously treated hemophilia A patients: a systematic review, meta‐analysis, and meta‐regression. Journal of Thrombosis & Haemostasis 2013;11(9):1655‐62. [DOI] [PubMed] [Google Scholar]


