Summary
Background
Tight-fitting masks and respirators, in manikin studies, improved aerosol source control compared to loose-fitting masks. Whether this translates to humans is not known.
Methods
We compared efficacy of masks (cloth and surgical) and respirators (KN95 and N95) as source control for SARS-CoV-2 viral load in exhaled breath of volunteers with COVID-19 using a controlled human experimental study. Volunteers (N = 44, 43% female) provided paired unmasked and masked breath samples allowing computation of source-control factors.
Findings
All masks and respirators significantly reduced exhaled viral load, without fit tests or training. A duckbill N95 reduced exhaled viral load by 98% (95% CI: 97%–99%), and significantly outperformed a KN95 (p < 0.001) as well as cloth and surgical masks. Cloth masks outperformed a surgical mask (p = 0.027) and the tested KN95 (p = 0.014).
Interpretation
These results suggest that N95 respirators could be the standard of care in nursing homes and healthcare settings when respiratory viral infections are prevalent in the community and healthcare-associated transmission risk is elevated.
Funding
Defense Advanced Research Projects Agency, National Institute of Allergy and Infectious Diseases, Centers for Disease Control and Prevention, the Bill & Melinda Gates Foundation, and The Flu Lab.
Keywords: Source control, SARS-CoV-2, Surgical masks, Respirators, Exhaled breath aerosols
Research in context.
Evidence before this study
The high transmissibility of SARS-CoV-2 appears in part to be due to a high and increasing ability to be shed into aerosols. Face masks have been shown to be effective in reducing the amount of viral respiratory aerosols emitted by persons infected with respiratory pathogens, including SARS-CoV-2. Lab experiments using manikins demonstrated that N95 respirators were more effective than cotton and surgical masks in reducing viral aerosol emissions. No human study has examined the relative efficacy of different types of masks and respirators as source control for SARS-CoV-2.
Added value of this study
We addressed the relative efficacy knowledge gaps by performing a controlled human study with volunteers having community-acquired SARS-CoV-2 infections. We compared the efficacy of cloth masks, surgical masks, KN95s, and N95 respirators as source control for reducing the viral RNA load in exhaled breath. Each individual served as their own control and provided masked and unmasked samples on the same day, allowing us to show the direct effect of each type of masks and respirators. We defined source-control factor (SCF%) as a percentage reduction in viral load released into the environment when wearing a mask and presented the SCFs for each of the four categories of masks and respirators. After controlling for potential confounders, we demonstrated N95 respirators were significantly more efficacious as source control than all other types of masks and respirators used in this study.
Implications of all the available evidence
Our findings, together with previous studies showing the effectiveness of general masking, support the recommendation for people to wear masks in areas where there is a high risk of transmission of respiratory infections. N95 respirators could become the standard of care for source control in healthcare settings. They should be worn by healthcare workers to protect their vulnerable patients, as well as themselves, when community rates of respiratory infections are high.
Introduction
Development and widespread application of effective vaccines and antiviral drugs decreased the mortality rate of coronavirus disease 2019 (COVID-19).1, 2, 3 However, the emergence of the Omicron variant and its sub-lineages, together with a relaxation of non-pharmaceutical interventions, led to an unprecedented surge in COVID-19 cases and hospitalizations in the United States in 2022.4 The intrinsic transmissibility of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has increased over time,5, 6, 7 which appears in part to be due to an increased ability to be shed into aerosols8, 9, 10 and the ability of the latest Omicron subvariants to evade immunity from vaccinations and prior infections.11,12 The highly transmissible nature of rapidly emerging Omicron variants in the setting of high rates of vaccination and prior infection underscores the critical role of non-pharmaceutical interventions, such as wearing face masks,13 to reduce inhalation (airborne) transmission of the virus by reducing the amount of virus in the air.
Face masks can effectively reduce risk of transmission of respiratory pathogens by limiting the amount of viral respiratory aerosols emitted into the air by infected persons. This is known as source control,14 and has been demonstrated to significantly reduce aerosol emission of influenza, seasonal coronaviruses, and SARS-CoV-28,15,16 and effectively reduce community transmission.13,17, 18, 19 In a controlled trial, loose-fitting face masks reduced the viral load (as measured by qRT-PCR for viral RNA) in fine and coarse aerosol samples by 48% and 77%, respectively, when worn by mildly symptomatic COVID-19 patients while singing and shouting.8
Few studies have examined the relative efficacy of different types of masks and respirators as source control for SARS-CoV-2. A series of laboratory experiments using manikins conducted by the U.S. Centres for Disease Control and Prevention found that tight-fitting, knotted medical masks provided more effective source control for SARS-CoV-2 than unknotted masks.20 When doubled or knotted/tucked, masks were found to block a greater percentage of particles from simulated coughing. Manikin experiments also demonstrated that N95 respirators were more effective than cotton and surgical masks in reducing general and infectious viral aerosol emissions, with the effect being even greater when the N95 respirators was properly fitted.21,22 To our knowledge, no human study has yet compared the efficacy of different mask and respirators as source control for SARS-CoV-2.
Our primary objective was to compare the efficacy of cloth masks, surgical masks, KN95s, and N95 respirators as source control for reducing the viral load (estimated by RNA copy number) in exhaled breath aerosol (EBA) samples collected from individuals with community-acquired SARS-CoV-2 infection. In this human study, individuals served as their own controls. We hypothesized that KN95 and N95 respirators would be superior to cloth and surgical masks.
Methods
Ethics
This controlled human exhaled breath aerosol experimental study was conducted at the University of Maryland, College Park (UMD). It was approved by the University of Maryland Institutional Review Board and the Human Research Protection Office of the Department of the Navy (Reference: 1556127). This study was conducted in accordance with the principles of Good Clinical Practice and the ethical guidelines of the Declaration of Helsinki. Informed consent was obtained from all study participants.
Study design
Beginning in May 2020, we actively recruited COVID-19 cases and their close contacts from UMD and the surrounding community for sample collection.8,9 Upon identifying a volunteer with an active SARS-CoV-2 infection, we invited them to a research clinic to provide 30-min EBA samples with a Gesundheit-II (G-II) human exhaled bioaerosol collector,23 as previously described.8,9 We tested all volunteers with SARS-CoV-2 infections that we were able to recruit to provide samples.
To assess the efficacy of masks as source control, volunteers were asked to provide paired breath samples at each visit, first with a mask on and then without, so they served as their own matched control. Volunteers received no prior training in the proper use of respirators. Among the volunteers included here, two cases studied before September 2020 were asked to repeat the alphabet three times within the 30-min sampling period, whereas subsequent cases were asked to shout “Go Terps” 30 times and sing “Happy Birthday” loudly three times at 5, 15, and 25 min into each 30-min sampling period. In instances where volunteers were too unwell to provide two sets of samples, a single 30-min breath sample was collected.
Mask and respirator assignment and instructions
Between June 2020 and December 2021, volunteers were assigned to wear either their own mask (mostly cloth masks), if available, or a surgical mask (Kimberly–Clark Professional™ Kimtech™ M3) for the masked session during their first shedding visit. If a second visit occurred, volunteers would wear the alternate mask type. The cloth masks were all brought by the volunteers, included a wide variety of types and materials, and information about brands and sources was frequently not available. Some participants came in with double masks, which included a mix of different mask types such as double cloth masks or a cloth mask combined with a surgical mask or KN95 respirator. Because each sub-category had very few samples, we excluded double masks from our analysis.
In December 2021, we stopped testing cloth masks and introduced testing with KN95 respirators from the supply of Powecom KN95s being distributed by our campus. We also include in the analysis KN95s brought by participants when used without another layer. Participants were randomized to wear a surgical mask or KN95 respirator in the masked session for their first shedding visit and the other option at a possible second visit. Starting in March 2022, inexpensive duckbill N95 respirators (ACI 3120 Surgical N95, Armbrustusa.com) popular with our lab staff were added to the mask categories. We then randomized participants to wear either a surgical mask or a respirator (KN95 or N95) at their first shedding visit until March 10, when we stopped testing surgical masks. After March 11, we only tested N95s. Most surgical masks, KN95 respirators, and all the N95 respirators used in the study were provided by our research team, while some volunteers used their own masks if study personnel judged them to be well-fitted. Volunteers who were provided with respirators having a nose wire were instructed to pinch it to fit their nose. Those wearing N95 respirators were instructed to place one strap around their neck and another over the top of their head. No other respiratory protection training was provided.
We previously reported both masked and unmasked data from participants enrolled up to April 30, 2021,8 and the unmasked data only for those enrolled up until March 11, 2022.9 The prior publications included the unmasked samples from those wearing cloth/surgical masks, KN95 respirators, and two persons wearing N95 respirators, and a portion of the masked samples from those wearing cloth masks (7/8), surgical masks (16/23), and KN95 respirators (1/12). The previously published data are included here for comparison with the N95 and most of the KN95 masked sample data that are reported here for the first time.
Laboratory analysis
The viral load in EBA was estimated by measurement of SARS-CoV-2 RNA, quantified using a multiplex real-time RT-PCR assay–TaqPath COVID-19 Combo Kit (Applied Biosystems # A47814). RNA copy numbers were reported per sample for the EBA samples, with a limit of detection (LOD, 95% probability of detection) as 75 copies/sample. Genome sequencing was carried out using mid-turbinate swab samples with an Illumina MiSeq platform (RRID:SCR_016379) for cases enrolled up until April 30, 2021 and an Oxford Nanopore Technologies MinION sequencing system (RRID:SCR_017985) for cases enrolled afterwards. Additional details on sample processing and laboratory analyses were described in our previous publications.8,9
Statistics
We included all the participants who provided paired masked and unmasked samples on the same day, with at least one sample having detectable SARS-CoV-2 RNA in each pair. We analysed the two aerosol size fractions collected by the G-II, fine (≤5 μm in diameter) and coarse (>5 μm in diameter) and combined them for analysis of total EBA (Fine + Coarse EBAs).
Descriptive analyses were conducted for all sample pairs and participants. We used box plots to visualize the viral load in EBA and Wilcoxon signed rank tests in crude paired analyses to compare the viral load between masked and unmasked samples for different masks and respirators. A Mann–Whitney U test was used for the pair-wise comparison of unmasked samples among different variants. We assigned a value of 1 to samples with viral load below the limit of detection for visualizations and in the crude analyses as done in previous studies.8,9
We used a linear mixed effect model for censored responses (R package ‘lmec’, version 1.024), if some of the viral loads were below the limit of detection, and a linear mixed effect model, if all the viral loads were above the limit of detection, to calculate the geometric mean of the viral load in separate models and data subsets for each aerosol size, mask type, and mask wearing condition. We also used lmec to estimate the effect of masks and respirators (exposure) on EBA viral RNA load (outcome) using the full paired dataset for each aerosol size including all mask types (with “no mask” serving as the reference group). To account for the effect of cough and other potential confounders on EBA, we controlled for the number of coughs during the 30-min sampling period, age, sex, BMI, and SARS-CoV-2 variants as confounders in the comparison model. These models accounted for censoring of the outcome by the limit of detection and nested random effects of participants and samples within participants. In these models, the viral RNA load was log-transformed, and the estimates from the models were exponentiated to get the ratios.
We used forest plots to show effect estimates, the ratio of masked to unmasked viral load (i.e., fraction of unmasked aerosol detected in masked samples). We defined source-control factor (SCF%) as a percentage reduction in viral load released into the environment when wearing a mask similar to a fit factor,25 computed from the outward leak fraction (i.e., masked/unmasked viral load):
The improvement (%) was defined as the relative efficacy of one mask type in source control compared to the other, computed from the ratio of outward leak fractions:
We conducted all analyses in R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria) and RStudio.
Role of funders
The funders had no role in study design, data collection, analysis, interpretation of data, preparation of the manuscript, or decision to publish.
Results
Study population
From June 2020 to May 2022, among 106 volunteers who provided EBA samples, 44 provided 60 same-day paired (with and without mask or respirator) EBA samples where at least one of the paired samples contained detectable SARS-CoV-2 RNA (Supplementary Fig. S1). There was no significance difference between our study population and those excluded volunteers in terms of their characteristics except that those excluded were younger and had a higher proportion of SARS-CoV-2 variants other than Alpha, Delta, and Omicron.
The analysed paired samples included 8 with cloth masks, 26 with surgical masks (25 Kimtech™ M3 and 1 unknown brand), 13 with KN95 respirators (10 Powecom and 3 unknown brands), and 13 with N95 respirators (Table 1; Supplementary Tables S1 and S2). Among the 44 volunteers, 16 volunteers provided two sample pairs on different days, while the remaining volunteers provided only one pair.
Table 1.
Characteristics of the volunteers at the level of sample pairs.a
| Cloth Mask | Surgical Mask | KN95 | N95 | All sample pairs | |
|---|---|---|---|---|---|
| Number of positive exhaled breath sample pairs | 8 | 26 | 13 | 13 | 60 |
| Variant, N (%)b | |||||
| Other | 6 (75) | 17 (65) | 0 (0) | 0 (0) | 23 (38) |
| Alpha | 1 (12) | 2 (8) | 1 (8) | 0 (0) | 4 (7) |
| Delta | 1 (12) | 1 (4) | 2 (15) | 0 (0) | 4 (7) |
| Omicron BA.1 | 0 (0) | 5 (19) | 8 (62) | 1 (8) | 14 (23) |
| Omicron BA.2 | 0 (0) | 1 (4) | 2 (15) | 12 (92) | 15 (25) |
| Sex, N (%)c | |||||
| Female | 2 (25) | 10 (38) | 4 (31) | 9 (69) | 25 (42) |
| Male | 6 (75) | 16 (62) | 9 (69) | 4 (31) | 35 (58) |
| Age, mean ± SD | 28.4 ± 14.9 | 23.3 ± 8.4 | 38 ± 19.5 | 33.7 ± 17.5 | 29.4 ± 15.3 |
| Age group, N (%) | |||||
| <18 | 0 (0) | 0 (0) | 0 (0) | 2 (15) | 2 (3) |
| 18–45 | 7 (88) | 25 (96) | 8 (62) | 7 (54) | 47 (78) |
| >45 | 1 (12) | 1 (4) | 5 (38) | 4 (31) | 11 (18) |
| White | 7 (88) | 20 (77) | 12 (92) | 7 (54) | 46 (77) |
| BMI, mean ± SD | 23.4 ± 2.5 | 23.5 ± 3.1 | 27.9 ± 6.8 | 22.7 ± 2.4 | 24.3 ± 4.4 |
| Chronic respiratory illness, N (%) | 0 (0) | 7 (27) | 5 (38) | 1 (8) | 13 (22) |
| Vaccination status, N (%) | |||||
| Boosted | 0 (0) | 2 (8) | 8 (62) | 13 (100) | 23 (38) |
| Fully vaccinated, not boosted | 1 (12) | 5 (19) | 4 (31) | 0 (0) | 10 (17) |
| Partially vaccinated | 1 (12) | 4 (15) | 1 (8) | 0 (0) | 6 (10) |
| Unvaccinated | 6 (75) | 15 (58) | 0 (0) | 0 (0) | 21 (35) |
| Symptomatic, N (%) | 8 (100) | 26 (100) | 13 (100) | 13 (100) | 60 (100) |
| Days post symptom onset, mean ± SD (range) | 4 ± 2 (1–6) | 4 ± 1 (1–7) | 3 ± 1 (1–5) | 3 ± 1 (1–5) | 4 ± 1 (1–7) |
| Coughs per 30 min, mean ± SD (range) | 1 ± 2 (0–6) | 4 ± 14 (0–69) | 13 ± 17 (0–64) | 6 ± 10 (0–32) | 6 ± 13 (0–69) |
| Median upper respiratory symptoms (IQR) | 2.5 (1.8–4.2) | 3 (2–4) | 2 (1–5) | 4 (2–7) | 3 (2–5) |
| Median lower respiratory symptoms (IQR) | 0 (0–0.2) | 1 (0–2) | 2 (1–2) | 2 (1–3) | 1 (0–2) |
| Median systemic symptoms (IQR) | 3 (0–6.5) | 3 (1–6) | 5 (1–7) | 3 (1–6) | 3 (1–6) |
| Median gastrointestinal symptoms (IQR) | 1 (0–2) | 0 (0–1) | 2 (0–3) | 0 (0–2) | 0 (0–1.2) |
| Temperature (C), mean ± SD | 37.2 ± 0.2 | 37.1 ± 0.2 | 37.3 ± 0.4 | 36.9 ± 0.2 | 37.1 ± 0.3 |
| Oxygen saturation (SpO2), mean ± SD | 97.8 ± 1 | 98 ± 0.8 | 97.2 ± 0.8 | 97.8 ± 1 | 97.8 ± 0.9 |
Among the 44 volunteers included in this study, 16 volunteers provided two sample pairs, while the remaining volunteers provide 1 pair. Characteristics of the 44 volunteers are presented in Supplementary Table S1.
BA.1 includes seven BA.1 and seven BA.1.1; BA.2 includes eight BA.2, five BA.2.12.1, and two BA.2.3.
Sex information was self-reported by study volunteers.
Among the sample pairs, four were from Alpha infections, four from Delta, 29 from Omicron (14 from BA.1 and 15 from BA.2), and 23 from other SARS-CoV-2 variants (Table 1). The samples were predominantly collected from young adults, who all had mild symptoms at the time of testing with a mean age of 30 years (range: 17–66 years) (Table 1; Supplementary Table S1). Cloth and surgical mask pairs were mainly collected before the emergence of Delta and Omicron and from unvaccinated volunteers. Conversely, most KN95 and N95 pairs were collected during the Delta and Omicron waves from volunteers who had completed the initial vaccination series (fully vaccinated) and/or had received at least one booster dose (Table 1; Supplementary Fig. S2).
Viral load in the EBA samples
The viral load in the unmasked aerosol samples ranged from non-detect to 1.77 × 107 RNA copies for fine aerosols, and from non-detect to 1.82 × 105 for coarse aerosols, significantly differing by variants, as previous reported9 (Supplementary Fig. S3). Volunteers who provided paired samples for KN95 respirators shed a higher amount of viral RNA when they were not wearing a mask compared to the other three masked groups (Fig. 1; Supplementary Fig. S4). The highest viral RNA shedder in our study was infected with Omicron BA.1.1, and the sample pair was provided on the day when they wore a KN95 respirator.
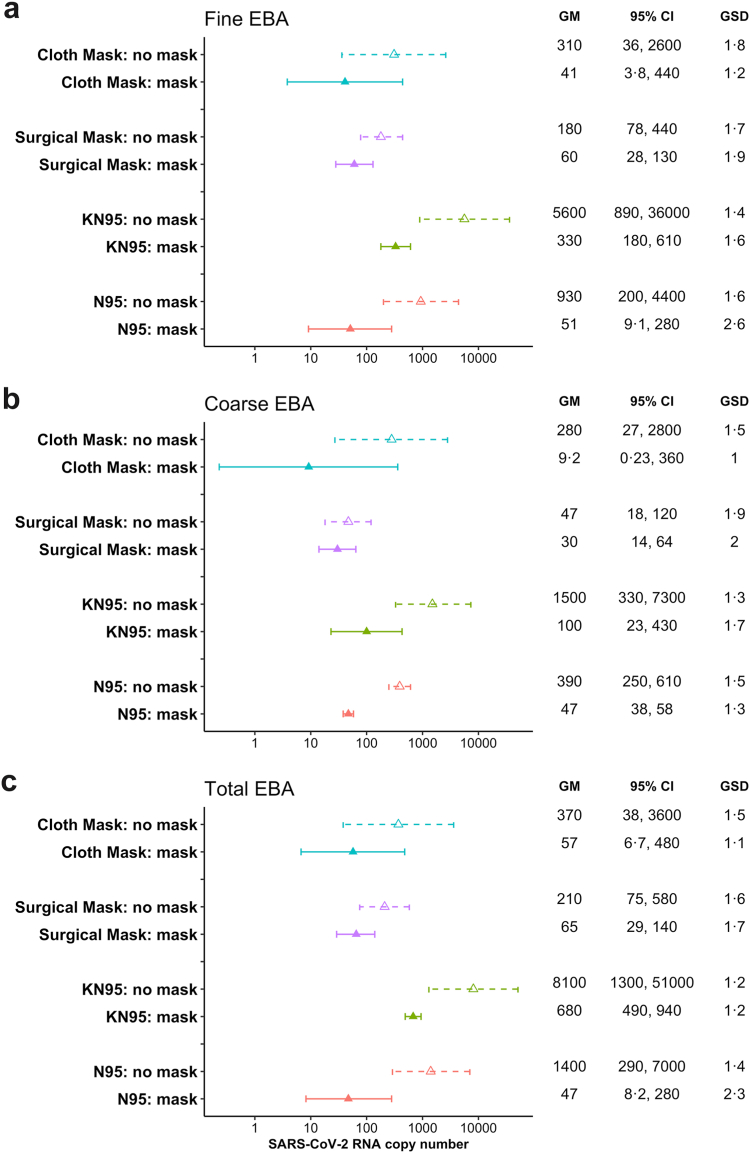
Fig. 1.
Geometric means of viral load in EBA samples. a) Fine aerosol, b) Coarse aerosol, c) Total aerosol. The geometric means and standard deviations were estimated using linear mixed effect models for censored responses (R package ‘lmec’, version 1.024), controlling for the random effects of participants and samples within participant. The triangles denote the geometric means of the SARS-CoV-2 RNA copy numbers in each sample types. The error bars show the 95% confidence interval of each geometric means. Dashed lines and hollow triangles were used for unmasked samples, while solid lines and solid triangles were used for masked samples. GM = Geometric means, and GSD = Geometric standard deviations.
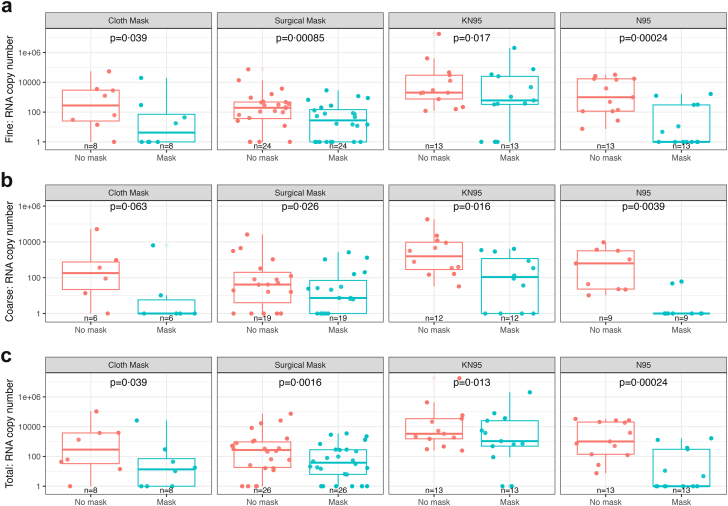
Fig. 1 shows geometric mean viral RNA copy numbers measured in aerosols collected per 30-min sampling period. In the crude paired analysis (Fig. 2), all masks and respirators conferred statistically significant reductions (p < 0.05) in viral load for fine, coarse, and total aerosols, except that cloth mask was only borderline significant (p = 0.063) for coarse aerosols.
Fig. 2.
Viral load in paired masked and unmasked EBA samples for aerosol fraction and total exhaled breath aerosol by mask type. a) Fine aerosol, b) Coarse aerosol, c) Total aerosol. Boxplots present raw data on viral RNA copies in masked and unmasked EBA samples by mask type and aerosol fraction. A value of 1 was assigned to samples with viral load below the limit of detection. N indicates the number of samples included in each boxplot.
Source-control factors
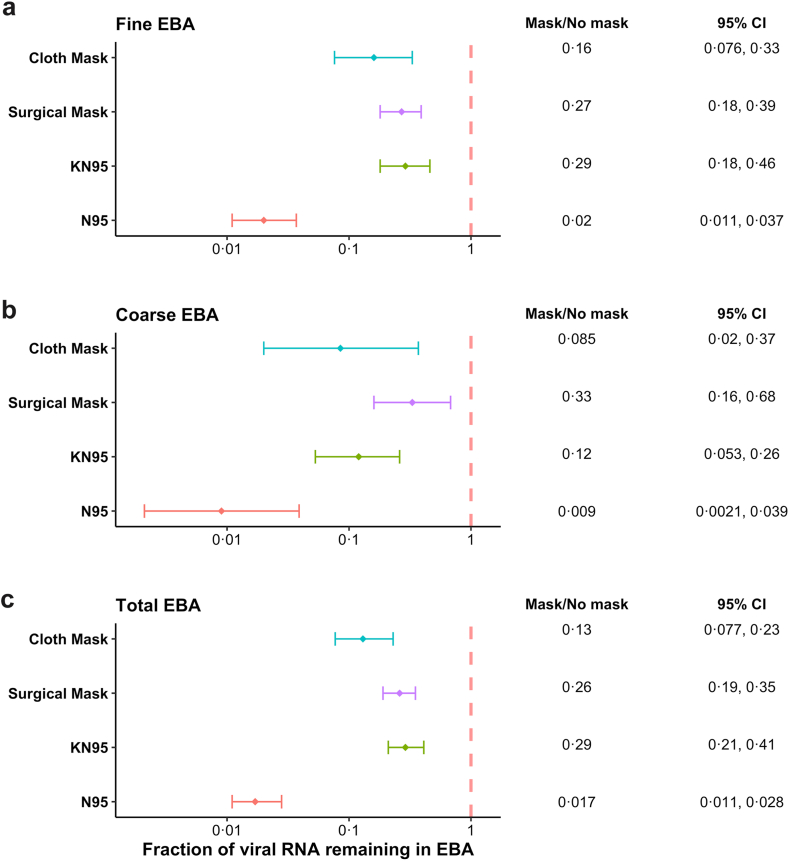
In the paired modelling analyses, all types of masks significantly reduced viral RNA copies in both aerosol fractions and total EBA (Fig. 3; Supplementary Fig. S5). N95 respirators were significantly more efficacious (i.e., produced higher source-control factors) than the other three types of masks for total EBA (Table 2) and for the fine and coarse aerosol fractions (Supplementary Tables S3a and 3b).
Fig. 3.
Ratio of viral RNA in EBA with to without mask controlling for cough, age, sex, BMI, and SARS-CoV-2 variants. a) Fine aerosol, b) Coarse aerosol, c) Total aerosol. The solid diamonds denote the effect estimates, and the error bars show the 95% confidence interval of each estimate. We estimated the ratio of masked to unmasked EBA viral load by aerosol size using linear mixed effect models for censored responses (R package ‘lmec’, version 1.024), controlling for numbers of coughs during the 30-min sampling period, age, sex, BMI, SARS-CoV-2 variants, and random effects of participants and samples within the participant. In a post-hoc test, the difference between N95 and cloth mask in coarse EBA was confirmed to be statistically significant despite a slight overlap in confidence intervals in the lower panel.
Table 2.
Source-control factors and improvement in source control for viral RNA in total exhaled breath aerosol.
| N95 | KN95 | Cloth | Surgical | |
|---|---|---|---|---|
| N95 | 98 (97–99)a | 94 (<10−15)b | 87 (<10−7) | 93 (<10−15) |
| KN95 | 71 (59–79) | −120 (0.014) | −12 (0.61) | |
| Cloth | 87 (77–92) | 49 (0.027) | ||
| Surgical | 74 (65–81) |
Source-control factors (SCF%) are shown in italics on the diagonal (95% confidence interval). They are defined as a percentage reduction in viral load released into the environment when wearing a mask, computed from the outward leak fraction (i.e., masked/unmasked viral load): .
The improvement (%) compares row versus column as percentage increase or decrease (p-value). Statistically significant (p < 0.05) changes are shown in bold. They are defined as the relative efficacy of one mask type in source control compared to the other, computed from the ratio of outward leak fractions: .
After controlling for the number of coughs during the sampling period, age, sex, BMI, and SARS-CoV-2 variants, N95 respirators reduced viral load in total exhaled breath aerosol by 98% (95% CI: 97–99%), followed by cloth masks with a reduction of 87% (95% CI: 77–92%) (Table 2). Surgical masks reduced exhaled viral load by 74% (95% CI: 65–81%). KN95 respirators reduced exhaled viral load by 71% (95% CI: 59–79%), and by 76% (95% CI 64–84%) after excluding the three with unknown brands (Supplementary Table S3c).
The source-control factors for total viral RNA aerosol that we observed for cloth masks were superior to those for both surgical masks (p = 0.027) and KN95 respirators (p = 0.014), when including the unknown brand KN95s. The direction of the effect was consistent, but the comparison did not reach statistical significance when analysis was restricted to the individual fine and coarse aerosol size fractions (Supplementary Tables S3a and 3b) and for total aerosol RNA when the off-brand KN95s were excluded (Supplementary Table S3c).
Discussion
Our study demonstrated that N95 respirators were significantly more efficacious as source control than all other types of masks and respirators used in this study. Conversely, the KN95s that we used did not perform any better as source control than loose-fitting cloth and surgical masks. The finding regarding N95 respirators is consistent with a previous experimental study with manikins21 showing that N95 reduced more viral load compared to cotton and surgical masks. Another study also showed that N95 respirators blocked more cough-generated aerosols than cloth and medical procedure masks as well as neck gaiters.22 Importantly, our study further demonstrated that the inexpensive duckbill N95 respirators we used were highly efficacious even when used by untrained study participants who did not undergo respirator training or a prior fit test.
High viral RNA load in exhaled breath is associated with increased probability of breath aerosol containing culturable virus.8,9 A recent study estimated that the Omicron variant, specifically B.1.1.529, required at least 1200 viral RNA copies to infect 63% of a susceptible population.26 This dose, described by Wells27 as a quantum of airborne infection is similar to an infectious dose 50%. Using the geometric means calculated in our study (Fig. 1), our data suggest that a mildly symptomatic person with COVID-19, not wearing a mask or respirator, would exhale on average 2800 RNA copies per hour in their total exhaled aerosol or a little more than two infectious doses, quanta, per hour. However, wearing a N95 respirator would reduce the aerosol shedding rate to less than one tenth of a quantum per hour. This suggests that wearing a N95 respirator can lower the risk of transmission by a factor of 20, provided that the other transmission risk factors (e.g., proximity between the infected and the susceptible, poor ventilation, etc.)28 remain the same.
The majority of the KN95 respirators used in our study (reported by an N95docon.org29 to have consistently high filtration efficiency but variable and high flow resistance) did not outperform loose-fitting masks and when including other brands, KN95s met inferiority criteria compared to cloth masks for total viral aerosol. One possible explanation is that we noted that the KN95 respirators we provided were relatively stiff and did not seal consistently along the entire perimeter of the mask. By contrast, the cloth masks brought by our volunteers tended to wrap farther around the face possibly providing better fit and lower flow resistance. We used one surgical mask brand for these tests so that result may not be representative of all masks30; the same brand used in prior CDC-funded studies of masks for influenza source control.16 The relatively high flow resistance of KN95 filters, compared with surgical and cloth masks, combined with poor fit tended to promote leaks around the face seal. This finding is consistent with a previous human study of exhaled particle counts31 suggesting that surgical masks with a flexible fit are more efficacious than poorly fitting KN95 respirators in reducing micron-scale aerosol particles, with a reduction of particle emission by 90% and 74%, respectively, compared to no mask. Notably, they found that cloth masks did not reduce, or even increased, particle emission, due to particle shedding by the cloth masks themselves.31 This finding points to a strength of measuring viral RNA over particle emission when examining the efficacy of cloth mask use. The effectiveness of negative pressure face coverings as respiratory protection is primarily determined by two factors: filtration efficiency and fit.32 Our finding implies that these two factors are similarly important in the context of source control. However, the KN95 respirator used in this study should not be considered representative of all KN95s—and the duckbill N95 we used should also not be considered representative of all N95s. Additionally, our study focused on source control,14 so these findings cannot be directly applied to personal protection.
All of the masks and respirators used in this study were efficacious as source control for SARS-CoV-2. They all demonstrate a source control factor of over 70, suggesting a substantial reduction in the viral RNA load when wearing a mask or respirator. This is consistent with our previous studies8,15,16 showing that loose-fitting masks reduced the viral load in EBA from volunteers infected with influenza virus, seasonal coronaviruses, and SARS-CoV-2. These findings support strong recommendations that face masks should be worn in settings where there is high risk of inhalation transmission of highly pathogenic organisms.
Our study has several strengths. This study addresses the lack of controlled human studies to directly assess the efficacy of various mask types as source control for SARS-CoV-2 (or any other respiratory virus). We only included participants who provided paired masked and unmasked samples during the same visit. Therefore, individuals acted as their own controls, eliminating the need to control for confounding variables (such as age and sex) other than the number of coughs during each sampling period when we are comparing masked versus unmasked samples. Most importantly, we tried to mimic real-world mask use by not providing volunteers with prior training in proper use of and self-fit tests for the masks and respirators. Additionally, we used a G-II human exhaled bioaerosol collector23 to collect exhaled breath samples and compared the viral RNA in the masked and unmasked EBA samples directly. The impact of masks as source control can be studied with human subjects using aerosol particle counting33,34 and by droplet imaging.35 These methods offer an advantage of not having to work with infectious agents and ill persons. However, the low particle numbers in exhaled breath and low flow of particle measurement devices require testing in a particle free atmosphere. Use of low flows and small cones also requires careful accounting for incomplete capture and orientation of the subject relative to the collector. Imaging is relatively insensitive to smaller aerosols. Here, we employed a combination of high flow rate, large cone, and high sensitivity measurements using molecular assays to avoid the uncertainties inherent in particle counting- and image-based methods.
However, our study does have some limitations. Our study population were mostly young adults, and all had mild symptoms at the time of testing. Therefore, our results may not be generalizable to those who are older or have more symptoms. The types of N95, KN95 respirators, and surgical masks that we included were limited and should not be considered representative of all N95, KN95 respirators, and surgical masks. All of the cloth masks were brought by volunteers and were mostly without brand information or homemade. The numbers of each sub-type were too small to analyse separately, which limited our capacity to assess the efficacy of specific types or brand of masks. We did not assess the efficacy of double masks in this study. As mentioned earlier, those wearing double masks came in with a mix of different types of masks or respirators, and the sample size for each sub-category was too small to have statistical power. In addition, we could not ask people with COVID-19 to provide more than two 30-min breath samples during one visit. So, we could not test different types of face masks at the same visit, although in future studies we could consider collecting paired samples with two different mask types rather than an unmasked control.
We randomly assigned participants to particular types of masks for each visit, however, we changed the types of mask included in the randomization over the course of the pandemic as improved masks and respirators became available. This resulted in greater viral loads in the unmasked samples for those wearing N95 and K95 because most of these samples were collected during Delta and Omicron waves, while most cloth and surgical unmasked samples were collected during earlier waves.
Overall, our findings, together with previous studies showing the effectiveness of general masking,8,13,15, 16, 17, 18, 19 support the recommendation for people to wear masks in areas where there is a high risk of transmission of respiratory infections. The risk of transmission can be minimized through several interventions including air hygiene (ventilation, filtration, and germicidal UV) and source control, including but not limited to wearing masks and respirators.28 Further research is needed to develop improved respiratory protection policies graded and adapted to the risks of infection, morbidity, and mortality. Our results show that N95 respirators were superior to other face coverings, even when worn by untrained individuals. Therefore, N95 respirators could become the standard of care for source control in healthcare settings—employees and visitors are major sources of infection transmission, especially in long-term care facilities.36,37 They should be worn by healthcare workers to protect their vulnerable patients, as well as themselves, when community rates of respiratory infections are high.28
Contributors
DKM conceived the project and obtained funding.
DKM, FH, BA, YE, JL, SST, JG, ISM conceptualized the project and designed the study.
ISM, AS, MO, and NF recruited study volunteers.
DR, KKC, AS, MO, and PK collected exhaled breath samples.
BA, YE, JL, KM, MO, and NF collected clinical samples.
SST, JG, MS, and AAS processed and analysed samples.
FH and JL contributed to data management and curation.
JL, TM, and BJC contributed to data analysis and interpretation.
JL drafted the original manuscript.
JL, KKC, SST, JG, FH, YE, AS, PK, MS, AAS, ISM, TLG, KM, TM, BJC, and DKM participated in editing the manuscript.
JL, FH, and DKM verified the underlying data.
All authors read and approved the final version of the manuscript.
Data sharing statement
Deidentified data and custom code used to analyse the data can be accessed on the Open Science Framework repository: https://osf.io/2jxeg/?view_only=71cc656c3739419796adae1707ddca52.
Declaration of interests
B.J.C. consults for AstraZeneca, Fosun Pharma, GlaxoSmithKline, Haleon, Moderna, Novavax, Pfizer, Roche, and Sanofi Pasteur. D.K.M. consults for A.I.R LLC and holds stock options for Lumen Bioscience, Inc. The authors declare no other competing interests.
Acknowledgements
This work was supported by the Prometheus-UMD, sponsored by the Defense Advanced Research Projects Agency (DARPA) BTO under the auspices of Col. Matthew Hepburn through agreement N66001-18-2-4015, the National Institute of Allergy and Infectious Diseases Centres of Excellence for Influenza Research and Surveillance (CEIRS) Contract Number HHSN272201400008C, and the Centers for Disease Control and Prevention Contract Number 200-2020-09528; by a grant from the Bill & Melinda Gates Foundation; and by a generous gift from The Flu Lab (https://theflulab.org). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or policy of these funding agencies and no official endorsement should be inferred.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105157.
Appendix ASupplementary data
References
- 1.Mohammed I., Nauman A., Paul P., et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccines Immunother. 2022;18(1) doi: 10.1080/21645515.2022.2027160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suthar A.B., Wang J., Seffren V., Wiegand R.E., Griffing S., Zell E. Public health impact of covid-19 vaccines in the US: observational study. BMJ. 2022;377 doi: 10.1136/bmj-2021-069317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen W., Chen C., Tang J., et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54(1):516–523. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iuliano A.D., Brunkard J.M., Boehmer T.K., et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146–152. doi: 10.15585/mmwr.mm7104e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushman M., Kahn R., Taylor B.P., Lipsitch M., Hanage W.P. Population impact of SARS-CoV-2 variants with enhanced transmissibility and/or partial immune escape. Cell. 2021;184(26):6229–6242.e18. doi: 10.1016/j.cell.2021.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Z., Hong H., Wang S., et al. Reproduction number of the omicron variant triples that of the Delta variant. Viruses. 2022;14(4):821. doi: 10.3390/v14040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue C., Song W., Wang L., et al. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect Dis. 2023;23(3):278–280. doi: 10.1016/S1473-3099(23)00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adenaiye O.O., Lai J., de Mesquita P.J.B., et al. Infectious SARS-CoV-2 in exhaled aerosols and efficacy of masks during early mild infection. Clin Infect Dis. 2021;75(1):e241–e248. doi: 10.1093/cid/ciab797. ciab797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai J., Coleman K.K., Tai S.H.S., et al. Exhaled breath aerosol shedding of highly transmissible versus prior severe acute respiratory syndrome coronavirus 2 variants. Clin Infect Dis. 2023;76(5):786–794. doi: 10.1093/cid/ciac846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan K.S., Ong S.W.X., Koh M.H., et al. SARS-CoV-2 Omicron variant shedding during respiratory activities. Int J Infect Dis. 2023;131:19–25. doi: 10.1016/j.ijid.2023.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uraki R., Ito M., Furusawa Y., et al. Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB. Lancet Infect Dis. 2023;23(1):30–32. doi: 10.1016/S1473-3099(22)00816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X., Chen L.L., Ip J.D., et al. Omicron sublineage recombinant XBB evades neutralising antibodies in recipients of BNT162b2 or CoronaVac vaccines. Lancet Microbe. 2023;4(3) doi: 10.1016/S2666-5247(22)00335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks J.T., Butler J.C. Effectiveness of mask wearing to control community spread of SARS-CoV-2. JAMA. 2021;325(10):998–999. doi: 10.1001/jama.2021.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC . Centers for Disease Control and Prevention; 2020. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic.https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html [cited 2022 Jun 15]. Available from: [Google Scholar]
- 15.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milton D.K., Fabian M.P., Cowling B.J., Grantham M.L., McDevitt J.J. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan C.V., Rose C., Lewis K.N., et al. SARS-CoV-2 incidence in K-12 school districts with mask-required versus mask-optional policies - Arkansas, August-October 2021. MMWR Morb Mortal Wkly Rep. 2022;71(10):384–389. doi: 10.15585/mmwr.mm7110e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowger T.L., Murray E.J., Clarke J., et al. Lifting universal masking in schools - covid-19 incidence among students and staff. N Engl J Med. 2022;387(21):1935–1946. doi: 10.1056/NEJMoa2211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrejko K.L., Pry J.M., Myers J.F., et al. Effectiveness of face mask or respirator use in indoor public settings for prevention of SARS-CoV-2 infection - California, February-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71(6):212–216. doi: 10.15585/mmwr.mm7106e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks J.T., Beezhold D.H., Noti J.D., et al. Maximizing fit for cloth and medical procedure masks to improve performance and reduce SARS-CoV-2 transmission and exposure, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(7):254–257. doi: 10.15585/mmwr.mm7007e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueki H., Furusawa Y., Iwatsuki-Horimoto K., et al. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. mSphere. 2020;5(5) doi: 10.1128/mSphere.00637-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsley W.G., Blachere F.M., Law B.F., Beezhold D.H., Noti J.D. Efficacy of face masks, neck gaiters and face shields for reducing the expulsion of simulated cough-generated aerosols. Aerosol Sci Technol. 2021;55(4):449–457. doi: 10.1080/02786826.2020.1862409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDevitt J.J., Koutrakis P., Ferguson S.T., et al. Development and performance evaluation of an exhaled-breath bioaerosol collector for influenza virus. Aerosol Sci Technol. 2013;47(4):444–451. doi: 10.1080/02786826.2012.762973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F.V., lmec L. Linear mixed-effects models with censored responses. 2012. https://CRAN.R-project.org/package=lmec [cited 2022 Apr 30]. Available from:
- 25.Occupational Safety and Health Administration 1910.134 app A - fit testing procedures (mandatory). | occupational safety and health administration. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA [cited 2024 Feb 28]. Available from:
- 26.Aganovic A., Cao G., Kurnitski J., Wargocki P. New dose-response model and SARS-CoV-2 quanta emission rates for calculating the long-range airborne infection risk. Build Environ. 2023;228 doi: 10.1016/j.buildenv.2022.109924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells W.F. Harvard University Press; Cambridge, MA: 1955. Airborne contagion and air hygiene: an ecological study of droplet infection; p. 423. [Google Scholar]
- 28.Klompas M., Milton D.K., Rhee C., Baker M.A., Leekha S. Current insights into respiratory virus transmission and potential implications for infection control programs: a narrative review. Ann Intern Med. 2021;174(12):1710–1718. doi: 10.7326/M21-2780. [DOI] [PubMed] [Google Scholar]
- 29.N95DECON. High-performance COVID-19 masks. 2021. https://www.n95decon.org/files/high-performance-masks [cited 2021 Sep 12]. Available from:
- 30.Rengasamy S., Miller A., Eimer B.C., Shaffer R.E. Filtration performance of FDA-cleared surgical masks. J Int Soc Respir Prot. 2009;26(3):54–70. [PMC free article] [PubMed] [Google Scholar]
- 31.Asadi S., Cappa C.D., Barreda S., Wexler A.S., Bouvier N.M., Ristenpart W.D. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-72798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberg T., Brosseau L.M. Surgical mask filter and fit performance. Am J Infect Control. 2008;36(4):276–282. doi: 10.1016/j.ajic.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hazard J.M., Cappa C.D. Performance of valved respirators to reduce emission of respiratory particles generated by speaking. Environ Sci Technol Lett. 2022;9(6):557–560. doi: 10.1021/acs.estlett.2c00210. [DOI] [PubMed] [Google Scholar]
- 34.Cappa C.D., Asadi S., Barreda S., Wexler A.S., Bouvier N.M., Ristenpart W.D. Expiratory aerosol particle escape from surgical masks due to imperfect sealing. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-91487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho K.M.A., Davies H., Epstein R., et al. Spatiotemporal droplet dispersion measurements demonstrate face masks reduce risks from singing. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-03519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim W.Y., Tan G.S.E., Htun H.L., et al. First nosocomial cluster of COVID-19 due to the Delta variant in a major acute care hospital in Singapore: investigations and outbreak response. J Hosp Infect. 2022;122:27–34. doi: 10.1016/j.jhin.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawton T., Butler M., Peters C. Airborne protection for staff is associated with reduced hospital-acquired COVID-19 in English NHS trusts. J Hosp Infect. 2022;120:81–84. doi: 10.1016/j.jhin.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.