Abstract
Background
The National Comprehensive Cancer Network (NCCN) guideline recommends consideration of weekly cisplatin as an alternative option for patients with head and neck cancer undergoing definitive chemoradiation. However, in a recent phase III trial (ConCERT), 20% of patients treated with weekly cisplatin could not receive a total of 200 mg/m2, and the association of low adherence to weekly cisplatin and cancer control outcomes remains unclear. To fill this knowledge gap, we performed an observational cohort study of patients with head and neck cancer undergoing definitive chemoradiation with weekly cisplatin.
Methods
Our institutional database was queried for patients with non-metastatic head and neck cancer who underwent definitive chemoradiation with weekly cisplatin (40 mg/m2) between November 2007 and April 2023. Adherence to weekly cisplatin was defined as receiving at least 5 cycles with a total cumulative dose of 200 mg/m2. Survival outcomes were evaluated using Kaplan–Meier method, log-rank tests, Cox proportional hazard multivariable (MVA) analyses. Logistic MVA was performed to identify variables associated with low adherence to weekly cisplatin. Fine-Gray MVA was performed to analyze failure outcomes with death as a competing event.
Results
Among 119 patients who met our criteria, 51 patients (42.9%) had low adherence to weekly cisplatin. Median follow up was 19.8 months (interquartile range 8.8–65.6). Low adherence to weekly cisplatin was associated with worse overall survival (adjusted hazards ratio [aHR] 2.94, 95% confidence interval [CI] 1.58–5.47, p < 0.001) and progression-free survival (aHR 2.32, 95% CI 1.29–4.17, p = 0.005). It was also associated with worse distant failure (aHR 4.55, 95% CI 1.19–17.3, p = 0.03), but not locoregional failure (aHR 1.61, 95% CI 0.46–5.58, p = 0.46). KPS < 90 was the only variable associated with low adherence to weekly cisplatin (adjusted odds ratio [aOR] 2.67, 95% CI 1.10–6.65, p = 0.03).
Conclusion
Our study suggested that over 40% of patients underwent fewer than 5 weekly cisplatin cycles and that low adherence to weekly cisplatin was an independent, adverse prognostic factor for worse survival and distant failure outcomes. Those with reduced adherence to weekly cisplatin were more likely to have poor performance status. Further studies are warranted to improve the adherence to chemotherapy and outcomes.
Keywords: Adherence, Compliance, Concurrent chemotherapy, ChemoRT, Head and neck cancer, Weekly cisplatin
Introduction
Definitive chemoradiation remains one of the standard of care treatment options for locally advanced head and neck cancer [1]. High-dose cisplatin given every 3 weeks is currently a category 1, preferred concurrent chemotherapy regimen based on the National Comprehensive Cancer Network (NCCN) guideline [1]. However, given its toxicity profiles, nearly a third of patients could not receive the 3rd cycle in the RTOG 0129 trial [2].
Alternatively, the national guideline also recommends low-dose weekly cisplatin as an additional treatment option [1]. In a prior meta-analysis and recently completed ConCERT trial, there was no statistically significant difference in locoregional control and overall survival between high- and low-dose cisplatin [3, 4] as similarly seen in the postoperative setting [5]. Despite such favorable outcomes, adherence to low-dose cisplatin remains challenging. The meta-analysis suggested comparable chemotherapy interruptions for high- versus low-dose cisplatin [3], and up to a third of patients with low-dose cisplatin could not receive a total of 200 mg/m2among patients with head and neck cancer, comparable to those with cervical cancer [4, 6–9]. However, the impact of poor adherence to low-dose cisplatin on cancer control outcomes remains unclear. To fill this knowledge gap, we performed an observational cohort study of patients with head and neck cancer undergoing definitive chemoradiation with low-dose cisplatin, evaluating the association between the adherence to low-dose cisplatin and outcomes.
Methods
This study was approved by Roswell Park Comprehensive Cancer Center institutional review board (EDR 103707) and follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. A waiver of consent was obtained from the institutional review board given the retrospective nature of our study. Consent process would not be feasible, and obtaining consents in retrospect would pose a greater risk than the waiver.
Our institutional database was queried for patients with non-metastatic head and neck cancer who underwent definitive chemoradiation with weekly cisplatin (40 mg/m2) between November 2007 and April 2023. All patients underwent 69.96–70 Gy/33–35 fractions using intensity modulated radiation therapy [10], and treatment volumes were previously described [10] based on several guidelines [11–13]. Of note, in our institutional practice, we routinely covered levels II-V unilaterally for well-lateralized tonsils and bilaterally for other primary oropharyngeal sites, with level Ib included for those with invasion into oral cavity or select level II lymphadenopathy per treating physician’s discretion. All patients underwent baseline positron emission tomography/computed tomography (PET/CT) which guided the staging and contouring. No dose or volume reduction was performed for those with human papillomavirus (HPV)-associated head and neck cancer. Excluded patients were those who underwent surgery, radiation alone, other chemotherapy regimens, or had metastatic cancer.
Supportive care measures for skin, oral mucositis, and pain control have been previously described [14–16]. In our institutional practice, high-dose cisplatin given every 3 weeks was routinely given for those with excellent performance status, normal renal function, and limited medical comorbidities. Otherwise, for other patients with normal renal function who had a reasonable performance status with some medical comorbidities, it was a treating physician’s discretion to give weekly cisplatin, fine-tune its schedule such as delaying or discontinuing certain cycles, and coordinate other necessary infusions as clinically appropriate. For example, given the nature of weekly cisplatin, weekly peripheral blood tests were routinely performed to rule out metabolic abnormalities. Antibiotics and escalated care were performed as clinically necessary in the setting of neutropenic fever or other infectious etiologies.
Adherence to low-dose cisplatin was defined as receiving at least 5 cycles with a total cumulative dose of 200 mg/m2. A total of 200 mg/m2was chosen as a benchmark since there were no statistical differences in survival between 2 versus 3 high-dose cisplatin cycles in the RTOG 0129 [2]. Other relevant variables included age, gender, smoking history, Karnofsky Performance Status (KPS), race, body mass index (BMI), primary disease site, cancer staging based on the American Joint Committee on Cancer (AJCC) 7th edition, and HPV status. All missing variables were coded as unknown for analysis. Race was self-reported by patients. Non-Caucasian patients were grouped into a single category prior to our analysis given small subgroup sample sizes, and they included African American, American Indian or Alaska Native, Asian, Hispanic, and others who were unknown or declined to answer.
The primary endpoint was overall survival (OS) and progression-free survival (PFS). OS was defined as the time interval from diagnosis to death by any cause or last follow up. PFS was defined similarly as OS, except that PFS included any tumor progression in addition to death by any cause or last follow up. Other endpoints were locoregional failure (LRF) and distant failure (DF), defined as the time intervals from diagnosis to tumor recurrences within and outside head and neck, respectively.
Statistical analysis
Baseline characteristics were compared using Fisher exact test and Mann–Whitney U tests as appropriate. Survival outcomes were evaluated using Kaplan–Meier method, log-rank tests, Cox proportional hazard multivariable (MVA) analyses. Logistic MVA was performed to identify variables associated with poor adherence to low-dose cisplatin. Fine-Gray MVA was performed to analyze LRF and DF outcomes with death as a competing event. All MVA models were built based on all baseline variables as listed previously.
All p values were two-sided, and p values less than 0.05 were considered statistically significant. All analyses were performed using R (version 4.2.1, R Project for Statistical Computing, Vienna, Austria).
Results
A total of 119 patients (101 male [84.9%], median [interquartile range] age, 63.5 [56–71] years) met our criteria (Table 1). The majority patients were former smoker (n = 64, 53.8%) with good KPS of 90–100 (n = 81, 68.1%) who underwent definitive chemoradiation for oropharyngeal cancer (n = 66, 55.5%). Of 119 patients, 51 patients (42.9%) had poor adherence to low-dose cisplatin. When compared between those with or without adherence to low-dose cisplatin, there were more patients with poor KPS and other racial backgrounds (Table 1). Median follow up was 19.8 months (interquartile range 8.8–65.6).
Table 1.
Baseline characteristics
| > / = 5 Cycles | < 5 Cycles | P | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender | 1.00 | ||||
| Male | 58 | 85.3 | 43 | 84.3 | |
| Female | 10 | 14.7 | 8 | 15.7 | |
| Smoker | 0.15 | ||||
| Never | 21 | 30.9 | 9 | 17.6 | |
| Former | 36 | 52.9 | 28 | 54.9 | |
| Current | 11 | 16.2 | 14 | 27.5 | |
| Age | 0.85 | ||||
| < 65 | 38 | 55.9 | 27 | 52.9 | |
| 65 or older | 30 | 44.1 | 24 | 47.1 | |
| KPS | 0.003 | ||||
| 90–100 | 54 | 79.4 | 27 | 52.9 | |
| < 90 | 14 | 20.6 | 24 | 47.1 | |
| Race | 0.02 | ||||
| White | 63 | 92.6 | 39 | 76.5 | |
| Other | 5 | 7.4 | 12 | 23.5 | |
| BMI | 0.68 | ||||
| Normal | 18 | 26.5 | 14 | 27.5 | |
| Underweight | 1 | 1.5 | 3 | 5.9 | |
| Overweight | 27 | 39.7 | 21 | 41.2 | |
| Obese | 21 | 30.9 | 13 | 25.5 | |
| Not available | 1 | 1.5 | 0 | 0.0 | |
| Site | 0.17 | ||||
| Oropharynx | 41 | 60.3 | 25 | 49.0 | |
| Larynx | 12 | 17.6 | 16 | 31.4 | |
| Oral cavity | 0 | 0.0 | 1 | 2.0 | |
| Other | 15 | 22.1 | 9 | 17.6 | |
| T staging | 0.27 | ||||
| 1–2 | 37 | 54.4 | 22 | 43.1 | |
| 3–4 | 31 | 45.6 | 29 | 56.9 | |
| N staging | 0.30 | ||||
| 0–1 | 16 | 23.5 | 17 | 33.3 | |
| 2–3 | 52 | 76.5 | 34 | 66.7 | |
| HPV | 0.46 | ||||
| Negative | 11 | 16.2 | 12 | 23.5 | |
| Positive | 36 | 52.9 | 22 | 43.1 | |
| Not available | 21 | 30.9 | 17 | 33.3 | |
N Number of patients, BMI Body mass index, KPS Karnofsky performance status, HPV Human papillomavirus
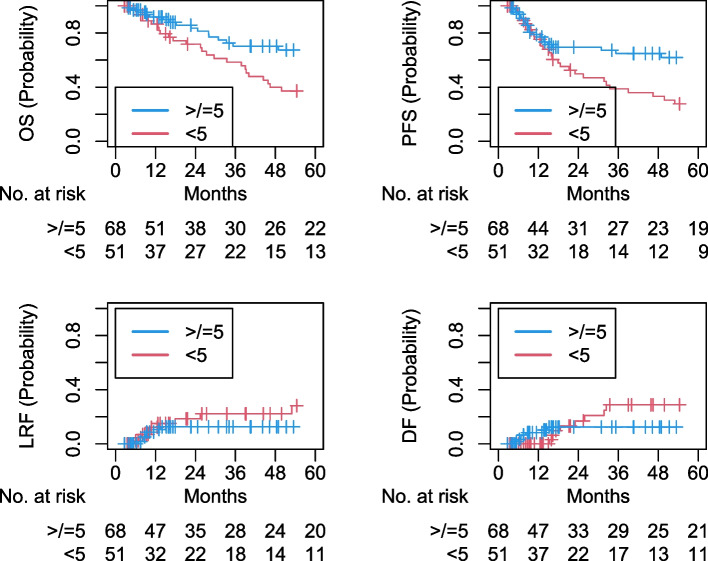
On Cox MVA (Table 2), poor adherence to low-dose cisplatin was associated with worse OS (adjusted hazards ratio [aHR] 2.94, 95% confidence interval [CI] 1.58–5.47, p < 0.001) and PFS (aHR 2.32, 95% CI 1.29–4.17, p = 0.005; Fig. 1). On Fine-Gray MVA (Table 3), it was associated with worse DF (aHR 4.55, 95% CI 1.19–17.3, p = 0.03), but not LRF (aHR 1.61, 95% CI 0.46–5.58, p = 0.46; Fig. 1). On logistic MVA (Table 4), KPS < 90 was the only variable associated with poor adherence to low-dose cisplatin (adjusted odds ratio [aOR] 2.67, 95% CI 1.10–6.65, p = 0.03).
Table 2.
Cox proportional hazards multivariable analysis for overall and progression-free survival outcomes
| Overall Survival | Progression-Free Survival | |||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | P | aHR | 95% CI | P | |
| Cisplatin | ||||||
| > / = 5 Cycles | Reference | Reference | ||||
| < 5 Cycles | 2.94 | 1.58–5.47 | < 0.001 | 2.32 | 1.29–4.17 | 0.005 |
| Gender | ||||||
| Male | Reference | Reference | ||||
| Female | 0.76 | 0.31–1.87 | 0.55 | 0.42 | 0.15–1.18 | 0.1 |
| Smoker | ||||||
| Never | Reference | Reference | ||||
| Former | 1.74 | 0.76–3.98 | 0.19 | 1.89 | 0.81–4.39 | 0.14 |
| Current | 1.61 | 0.58–4.49 | 0.36 | 1.38 | 0.51–3.75 | 0.53 |
| Age | ||||||
| < 65 | Reference | Reference | ||||
| 65 or older | 0.85 | 0.46–1.58 | 0.62 | 0.73 | 0.40–1.33 | 0.3 |
| KPS | ||||||
| 90–100 | Reference | Reference | ||||
| < 90 | 1.93 | 1.02–3.66 | 0.04 | 1.91 | 1.04–3.50 | 0.04 |
| Race | ||||||
| White | Reference | Reference | ||||
| Other | 0.94 | 0.38–2.33 | 0.89 | 1.14 | 0.48–2.70 | 0.76 |
| BMI | ||||||
| Normal | Reference | Reference | ||||
| Underweight | 0.53 | 0.12–2.29 | 0.39 | 2.9 | 0.51–16.59 | 0.23 |
| Overweight | 0.52 | 0.26–1.05 | 0.07 | 0.78 | 0.39–1.56 | 0.48 |
| Obese | 0.88 | 0.38–2.08 | 0.78 | 1.13 | 0.51–2.49 | 0.77 |
| Not available | < 0.001 | < 0.001-Inf | 1 | < 0.001 | < 0.001-Inf | 1 |
| Site | ||||||
| Oropharynx | Reference | Reference | ||||
| Larynx | 0.46 | 0.19–1.12 | 0.09 | 0.6 | 0.26–1.40 | 0.24 |
| Oral cavity | 1.91 | 0.17–21.13 | 0.6 | Jan-84 | 0.17–20.13 | 0.62 |
| Other | 1.1 | 0.49–2.45 | 0.82 | 1.06 | 0.48–2.36 | 0.89 |
| T staging | ||||||
| 1–2 | Reference | Reference | ||||
| 3–4 | 2.54 | 1.30–4.96 | 0.006 | 2.71 | 1.40–5.23 | 0.003 |
| N staging | ||||||
| 0–1 | Reference | Reference | ||||
| 2–3 | 1.37 | 0.58–3.20 | 0.47 | 1.46 | 0.65–3.32 | 0.36 |
| HPV | ||||||
| Negative | Reference | Reference | ||||
| Positive | 0.54 | 0.20–1.46 | 0.23 | 0.76 | 0.29–1.97 | 0.57 |
| Not available | 0.92 | 0.38–2.23 | 0.86 | 1.31 | 0.57–3.03 | 0.52 |
aHR Adjusted hazards ratio, 95% CI 95% Confidence interval, BMI Body mass index, KPS Karnofsky performance status, HPV Human papillomavirus
Fig. 1.
Kaplan–Meier and cumulative incidence curves for overall survival, progression-free survival, locoregional failure, and distant failure for greater than or equal to 5 versus fewer than 5 cycles of weekly low-dose cisplatin. OS: overall survival; PFS: progression-free survival; LRF: locoregional failure; DF: distant failure
Table 3.
Fine-Gray multivariable analysis for locoregional and distant failure outcomes
| Locoregional Failure | Distant Failure | |||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | P | aHR | 95% CI | P | |
| Cisplatin | ||||||
| > / = 5 Cycles | Reference | Reference | ||||
| < 5 Cycles | 1.61 | 0.46–5.58 | 0.46 | 4.55 | 1.19–17.3 | 0.03 |
| Gender | ||||||
| Male | Reference | Reference | ||||
| Female | 0.12 | 0.02–0.73 | 0.02 | < 0.001 | < 0.001- < 0.001 | < 0.001 |
| Smoker | ||||||
| Never | Reference | Reference | ||||
| Former | 6.38 | 0.24–170.72 | 0.27 | 5.69 | 0.57–57.1 | 0.14 |
| Current | 0.78 | 0.04–14.22 | 0.87 | 3.34 | 0.34–32.9 | 0.3 |
| Age | ||||||
| < 65 | Reference | Reference | ||||
| 65 or older | 0.49 | 0.07–3.34 | 0.46 | 0.14 | 0.02–0.88 | 0.04 |
| KPS | ||||||
| 90–100 | Reference | Reference | ||||
| < 90 | 1.81 | 0.55–6.00 | 0.33 | 1.74 | 0.23–13.3 | 0.59 |
| Race | ||||||
| White | Reference | Reference | ||||
| Other | 4.78 | 0.69–32.93 | 0.11 | 0.14 | 0.02–0.78 | 0.03 |
| BMI | ||||||
| Normal | Reference | Reference | ||||
| Underweight | 64.4 | 7.90–524.98 | < 0.001 | < 0.001 | < 0.001–0.001 | < 0.001 |
| Overweight | 2.6 | 0.63–10.77 | 0.19 | 23 | 2.54–208 | 0.005 |
| Obese | 2.04 | 0.48–8.76 | 0.34 | 12.1 | 1.31–113 | 0.03 |
| Not available | 0.003 | < 0.001–0.40 | 0.02 | < 0.001 | < 0.001–0.02 | < 0.001 |
| Site | ||||||
| Oropharynx | Reference | Reference | ||||
| Larynx | 0.98 | 0.15–6.52 | 0.99 | 2.75 | 0.27–28.2 | 0.39 |
| Oral cavity | < 0.001 | < 0.001–0.12 | 0.01 | 1500 | 22.2–1.01e5 | < 0.001 |
| Other | 1.53 | 0.20–11.87 | 0.68 | 1.78 | 0.15–20.8 | 0.65 |
| T staging | ||||||
| 1–2 | Reference | Reference | ||||
| 3–4 | 18.7 | 1.80–194.53 | 0.01 | 5.33 | 1.20–23.7 | 0.03 |
| N staging | ||||||
| 0–1 | Reference | Reference | ||||
| 2–3 | 2.34 | 0.28–20.00 | 0.44 | 19.7 | 3.64–106 | < 0.001 |
| HPV | ||||||
| Negative | Reference | Reference | ||||
| Positive | 2.18 | 0.13–36.41 | 0.59 | 1.1 | 0.20–5.95 | 0.91 |
| Not available | 5.39 | 0.33–88.75 | 0.24 | 0.5 | 0.07–3.57 | 0.49 |
aHR Adjusted hazards ratio, 95% CI 95% Confidence interval, BMI Body mass index, KPS Karnofsky performance status, HPV Human papillomavirus
Table 4.
Logistic multivariable analysis for poor adherence to weekly low-dose cisplatin
| aOR | 95% CI | P | |
|---|---|---|---|
| Gender | |||
| Male | Reference | ||
| Female | 0.92 | 0.25–3.16 | 0.9 |
| Smoker | |||
| Never | Reference | ||
| Former | 1.70 | 0.59–5.17 | 0.34 |
| Current | 3.25 | 0.81–14.09 | 0.1 |
| Age | |||
| < 65 | Reference | ||
| 65 or older | 1.27 | 0.53–3.07 | 0.59 |
| KPS | |||
| 90–100 | Reference | ||
| < 90 | 2.67 | 1.10–6.65 | 0.03 |
| Race | |||
| White | Reference | ||
| Other | 3.09 | 0.92–11.56 | 0.08 |
| BMI | |||
| Normal | Reference | ||
| Underweight | 6.89 | 0.43–218 | 0.2 |
| Overweight | 1.7 | 0.58–5.23 | 0.34 |
| Obese | 1.7 | 0.49–6.25 | 0.41 |
| Not available | < 0.001 | NA-1.63e122 | 0.99 |
| Site | |||
| Oropharynx | Reference | ||
| Larynx | 1.63 | 0.48–5.61 | 0.43 |
| Oral cavity | 2.04e7 | < 0.001-NA | 0.99 |
| Other | 1.09 | 0.32–3.61 | 0.88 |
| T staging | |||
| 1–2 | Reference | ||
| 3–4 | 1.22 | 0.48–3.06 | 0.67 |
| N staging | |||
| 0–1 | Reference | ||
| 2–3 | 0.72 | 0.22–2.34 | 0.58 |
| HPV | |||
| Negative | Reference | ||
| Positive | 1.88 | 0.48–8.19 | 0.38 |
| Not available | 1.01 | 0.30–3.56 | 0.98 |
aOR Adjusted odds ratio, 95% CI 95% Confidence interval, BMI Body mass index, KPS Karnofsky performance status, HPV Human papillomavirus
Discussion
To our knowledge, this is the largest study of US head and neck cancer patients treated with definitive chemoradiation comparing tumor control and survival outcomes based on the level of adherence to weekly cisplatin. It suggested that over 40% of patients underwent fewer than 5 weekly cisplatin cycles and that low adherence to weekly cisplatin was an independent, adverse prognostic factor for worse survival and distant failure outcomes. Those with reduced adherence to weekly cisplatin were more likely to have poor performance status.
The NCCN guideline [1] and ongoing NRG HN 009 clinical trial protocol (ClinicalTrials.gov Identifier: NCT05050162) do not currently specify the minimum number of weekly cisplatin cycles to be given. Over 40% of patients in our study received less than 5 weekly cisplatin cycles. Such proportion of patients with low adherence to weekly cisplatin was higher than 13–32% reported in the literature for head and nek cancer [4, 6, 7] as well as cervical cancer [8, 9]. This discrepancy may be due to older age in our study with median 63.5 years and more than a quarter of patients with > 70 years of age. Other studies included patients with mean or median age younger than 60 years [4, 6, 7], so it may be possible that our study included more frail patient population.
Our study also suggested that low adherence to weekly cisplatin was associated with worse survival and distant metastasis outcomes, but not locoregional control. Our findings in survival outcomes are consistent with studies from Australia and India suggesting survival benefits associated with better adherence to weekly cisplatin [7, 17]. However, while the Australian study did not compare LRF and DF based on the level of adherence to cisplatin, our findings on locoregional control are not consistent with the Indian study suggesting worse LRF associated with poor adherence to cisplatin [7, 17]. Such discrepancy may be in part due to differences in patient populations. For instance, nearly half of patients in our study had p16-positive oropharyngeal cancer, while more than a third of patients in the Indian study had hypopharyngeal cancer, which has been known to have poor prognosis [18, 19].
Limitations of our study include a relatively small sample size of 119 patients, which may be due to the national guideline recommending high-dose cisplatin given every 3 weeks instead as a preferred, category 1 option [1]. As a result, performing subgroup analysis stratified by p16 status was not feasible in our study. Follow up period in our study was also short with median 19.8 months. The number of events, such as death and distant metastasis, were still sufficient to reach statistical significance, highlighting the importance of improved adherence to systemic therapies to prevent early recurrences. Our database also did not fully capture toxicity profiles or medical comorbidities, which may be additional reasons for poor adherence to weekly cisplatin. Other pertinent variables, such as alcohol consumption, were unavailable for analysis,
Conclusion
Our study suggested that low adherence to weekly cisplatin defined as fewer than 5 weekly cycles can be seen in more than 40% of patients. Such poor adherence was an independent, adverse prognostic factor for worse survival and distant metastasis outcomes. Patients with low adherence to cisplatin were more likely to have poor performance status. Further investigations are warranted to improve the adherence to chemotherapy and outcomes.
Acknowledgements
Not applicable.
Prior presentation
This abstract was published as an abstract only during the American Society of Clinical Oncology (ASCO) Annual Meeting in 2024.
Authors’ contributions
J.V. and J.G.: Investigation, Writing – Original Draft. F.F., A.I., M.F., A.A., K.W., V.G., R.M., M.K., M.M., W.H., and A.S.: Writing – Review & Editing, Validation, Supervision. S.M.: Data Curation, Investigation, Formal Analysis, Writing – Original Draft, Writing – Review & Editing, Validation, Supervision.
Funding
This research was supported by the National Cancer Institute Cancer Center Support Grant (P30CA016056). Sponsors had no role in the preparation of this manuscript.
Availability of data and materials
The data cannot be shared publicly due to the privacy of patients who participated in the study. The data are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Roswell Park Comprehensive Cancer Center (EDR-103707). A waiver of informed consent was obtained from the Institutional Review Board of Roswell Park Comprehensive Cancer Center due to the retrospective nature of the study making consent impractical and contacting patients to obtain informed consent would pose a greater risk than the waiver.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sung Jun Ma, Email: SungJun.Ma@osumc.edu.
Anurag K. Singh, Email: Anurag.Singh@RoswellPark.org
References
- 1.Network NCC. Head and Neck Cancers (Version 3.2024). Accessed March 16, 2024, https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 2.Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. 2014;32(34):3858–3866. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Felice F, Belgioia L, Alterio D, et al. Survival and toxicity of weekly cisplatin chemoradiotherapy versus three-weekly cisplatin chemoradiotherapy for head and neck cancer: A systematic review and meta-analysis endorsed by the Italian Association of Radiotherapy and Clinical Oncology (AIRO) Crit Rev Oncol Hematol. 2021;162:103345. doi: 10.1016/j.critrevonc.2021.103345. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Kumar M, Bhasker S, et al. An open-label, noninferiority phase III RCT of weekly versus three weekly cisplatin and radical radiotherapy in locally advanced head and neck squamous cell carcinoma (ConCERT trial). J Clin Oncol. 2022;40(16 Suppl). 10.1200/JCO.2022.40.16_suppl.6004.
- 5.Kiyota N, Tahara M, Mizusawa J, et al. Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J Clin Oncol. 2022;40(18):1980–1990. doi: 10.1200/JCO.21.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal MS, Chaw C, Kovarik J, et al. Primary concurrent chemoradiation in head and neck cancers with weekly cisplatin chemotherapy: analysis of compliance, toxicity and survival. Int Arch Otorhinolaryngol. 2017;21(2):171–177. doi: 10.1055/s-0036-1594020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otty Z, Skinner MB, Dass J, et al. Efficacy and tolerability of weekly low-dose cisplatin concurrent with radiotherapy in head and neck cancer patients. Asia Pac J Clin Oncol. 2011;7(3):287–292. doi: 10.1111/j.1743-7563.2011.01405.x. [DOI] [PubMed] [Google Scholar]
- 8.Ryu SY, Lee WM, Kim K, et al. Randomized clinical trial of weekly vs. triweekly cisplatin-based chemotherapy concurrent with radiotherapy in the treatment of locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e577–81. doi: 10.1016/j.ijrobp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Shrivastava S, Mahantshetty U, Engineer R, et al. Cisplatin Chemoradiotherapy vs Radiotherapy in FIGO Stage IIIB Squamous Cell Carcinoma of the Uterine Cervix: A Randomized Clinical Trial. JAMA Oncol. 2018;4(4):506–513. doi: 10.1001/jamaoncol.2017.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung-Kee-Fung SD, Hackett R, Hales L, Warren G, Singh AK. A prospective trial of volumetric intensity-modulated arc therapy vs conventional intensity modulated radiation therapy in advanced head and neck cancer. World J Clin Oncol. 2012;3(4):57–62. doi: 10.5306/wjco.v3.i4.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biau J, Lapeyre M, Troussier I, et al. Selection of lymph node target volumes for definitive head and neck radiation therapy: a 2019 update. Radiother Oncol. 2019;134:1–9. doi: 10.1016/j.radonc.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Gregoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172–81. doi: 10.1016/j.radonc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Gregoire V, Evans M, Le QT, et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol. 2018;126(1):3–24. doi: 10.1016/j.radonc.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Ma SJ, Iovoli AJ, Wang K, et al. Efficacy of prophylactic high-dose gabapentin and venlafaxine on reducing oral mucositis pain among patients treated with chemoradiation for head and neck Cancer: a single-institution, Phase 2, randomized clinical trial. Int J Radiat Oncol Biol Phys. 2023. 10.1016/j.ijrobp.2023.01.047. [DOI] [PubMed]
- 15.Ma SJ, Wang K, Iovoli AJ, et al. Association of gabapentin use with pain control and feeding tube placement among patients with head and neck Cancer receiving chemoradiotherapy. JAMA Netw Open. 2022;5(5):e2212900. doi: 10.1001/jamanetworkopen.2022.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu ML, Iovoli AJ, Khan M, Farrugia MK, Ma SJ, Singh AK. Prophylactic high-dose gabapentin reduces opiate use during radiation therapy for head and neck squamous cell carcinoma. Cancers (Basel). 2023;15(7). 10.3390/cancers15072003. [DOI] [PMC free article] [PubMed]
- 17.Gupta T, Agarwal JP, Ghosh-Laskar S, Parikh PM, D'Cruz AK, Dinshaw KA. Radical radiotherapy with concurrent weekly cisplatin in loco-regionally advanced squamous cell carcinoma of the head and neck: a single-institution experience. Head Neck Oncol. 2009;1:17. doi: 10.1186/1758-3284-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806–816. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 19.Hall SF, Groome PA, Irish J, O'Sullivan B. The natural history of patients with squamous cell carcinoma of the hypopharynx. Laryngoscope. 2008;118(8):1362–1371. doi: 10.1097/MLG.0b013e318173dc4a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data cannot be shared publicly due to the privacy of patients who participated in the study. The data are available from the corresponding author upon reasonable request.