Abstract
Background
Chronic lung disease (CLD) is common among children with HIV (CWH) including in those taking antiretroviral therapy (ART). Azithromycin has both antimicrobial and anti-inflammatory effects and has been effective in improving lung function in a variety of lung diseases. We investigated lung function trajectories among CWH with CLD on ART enrolled in a randomized controlled trial of adjuvant azithromycin. We also investigated factors that modified the effect of azithromycin on lung function.
Methods
The study used data from a double-blinded placebo-controlled trial conducted in Malawi and Zimbabwe of 48 weeks on azithromycin (BREATHE: ClinicalTrials.gov NCT02426112) among CWH aged 6 to 19 years taking ART for at least six months who had a forced expiratory volume in one second (FEV1) z-score <-1.0. Participants had a further follow-up period of 24 weeks after intervention cessation. FEV1, forced vital capacity (FVC) and FEV1/FVC were measured at baseline, 24, 48 and 72-weeks and z-scores values calculated. Generalized estimating equations (GEE) models were used to determine the mean effect of azithromycin on lung-function z-scores at each follow-up time point.
Results
Overall, 347 adolescents (51% male, median age 15 years) were randomized to azithromycin or placebo. The median duration on ART was 6.2 (interquartile range: 3.8–8.6) years and 56.2% had an HIV viral load < 1000copies/ml at baseline. At baseline, the mean FEV1 z-score was − 2.0 (0.7) with 44.7% (n = 155) having an FEV1 z-score <-2, and 10.1% had microbiological evidence of azithromycin resistance. In both trial arms, FEV1 and FVC z-scores improved by 24 weeks but appeared to decline thereafter. The adjusted overall mean difference in FEV1 z-score between the azithromycin and placebo arms was 0.004 [-0.08, 0.09] suggesting no azithromycin effect and this was similar for other lung function parameters. There was no evidence of interaction between azithromycin effect and baseline age, lung function, azithromycin resistance or HIV viral load.
Conclusion
There was no observed azithromycin effect on lung function z-scores at any time point suggesting no therapeutic effect on lung function.
Trial registration
ClinicalTrials.gov NCT02426112. First registered on 24/04/2015.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-024-03155-x.
Keywords: Chronic lung disease, HIV, FEV1, Africa, Azithromycin, Children, Adolescents
Background
Worldwide, an estimated 2.8 million children and adolescents (0–19 years) live with HIV, with 1.8 million in eastern and southern Africa [1]. The roll-out of antiretroviral therapy (ART) has improved survival of children with HIV (CWH), so that growing numbers are reaching adolescence and adulthood. There is a growing evidence however, that despite ART, CWH experience a range of multisystem chronic comorbidities [2].
Studies in recent years have shown that up to a third of African CWH have chronic lung disease (CLD) typically with cough, hypoxia and significantly reduced exercise tolerance and impaired lung function. Radiological studies are consistent with the aetiology being constrictive obliterative bronchiolitis (COB) [3]. The lack of association between abnormal lung function and ART use and duration or CD4 count, suggests that this form of HIV-related CLD may not be responsive to ART once established [4, 5]. While the underlying drivers of COB in the context of HIV infection are not well understood, it is thought to be a consequence of chronic inflammation either due to HIV-mediated aberrant systemic immune activation or recurrent lung co-infections (which children with HIV are at elevated risk of), with tissue injury followed by aberrant fibro-proliferative remodelling in the small airways [6–12]
Azithromycin is an oral macrolide that has broad-spectrum antibiotic properties and is used to treat a wide range of infections including respiratory tract infections [13]. Azithromycin is also recognised to have immunomodulatory properties [14]. It likely reduces inflammation and has been shown to have therapeutic effects in several chronic lung diseases including improvement in lung function and reduction in infective exacerbations in patients with cystic fibrosis, [15] and improvement in forced expiratory volume in one second (FEV1) in obliterative bronchiolitis syndrome in the context of lung transplantation [16–18].
We hypothesized that azithromycin may therefore also improve lung function and reduce risk of infective exacerbations among children with HIV-associated CLD, through its immunomodulatory and antibiotic properties respectively [19]. We conducted a double-blind, placebo-controlled individually randomised trial (BREATHE) to investigate the impact of 48 weeks of weekly azithromycin on lung function and risk of acute respiratory exacerbations (ARE) in older children with HIV. The main trial results including the safety profile of azithromycin have been published previously [20]. In this secondary analysis, we investigated the effect of adjuvant azithromycin therapy on lung function trajectories (FEV1, forced vital capacity [FVC] and FEV1/FVC z-score) among CWH on ART with CLD and whether any treatment effects persist after cessation of treatment. We also investigated factors that could modify potential therapeutic effects of azithromycin on lung function.
Methods
Study design and setting
Participants were enrolled between June 2016 and September 2018 into the BREATHE trial (ClinicalTrials.gov NCT02426112) from public sector HIV clinics in Blantyre, Malawi and Harare, Zimbabwe, with a follow-up period of up to 72 weeks [20, 21].
Trial procedures
The trial protocol has been published [20, 21]. Briefly, eligibility criteria were age 6–19 years, having perinatally acquired HIV, being on ART for at least six months and an FEV1 z-score < -1.0. Other eligibility criteria were having been disclosed their HIV status (for those aged ≥ 12 years) and having a guardian able to provide consent (for those aged < 18 years).
Exclusion criteria were having a condition likely to be fatal during the study period (e.g. malignancy), acute respiratory tract infection or tuberculosis (screened for using the Xpert™ MTB/RIF (Cepheid, Sunnyvale, CA, USA) on one sputum sample), pregnancy or breastfeeding, history of cardiac arrhythmia, a prolonged QTc interval (> 440 and > 460 milliseconds in males and females respectively), creatinine clearance < 30mls/minute, alanine aminotransferase > 2 times the upper limit of normal, known macrolide hypersensitivity, or use of drugs known to prolong the QTc interval.
Participants were randomised to either receive an oral weekly weight-based dose of azithromycin or placebo for 48 weeks. Following enrolment, participants were followed up at 2, 12, 24 and 36 weeks for ascertainment of incident symptoms, side-effects and adherence and for drug re-supply.
Lung function parameters (FEV1, FVC and FEV1/FVC) were measured by spirometry (with American Thoracic Society quality assessment criteria met pre bronchodilator) at enrolment, 24, 48 and at 72 weeks. HIV viral load was also assessed at enrolment and 48 weeks. All participants were tested for tuberculosis with a sputum sample for Xpert™ MTB/RIF (Cepheid, Sunnyvale, CA, USA) at baseline. Azithromycin sensitivity profiles were assessed using the Kirby–Bauer disk diffusion method.
The trial had 80% statistical power to detect a standardized mean difference in FEV1 z-score of 0.32 SD between the two trial arms at a 95% confidence level at 48 weeks.
Statistical analysis
Data analysis was performed using STATA 16 (StataCorp College Station, TX). Measurements were included in analysis if obtained within 4 weeks either side of the scheduled appointment. The corresponding z-scores of the lung function parameters were calculated using GLI African American spirometric reference equations adjusting for age, sex, height and ethnicity [22, 23].
Participant demographics and clinical assessments were summarized at all time points, reporting means (with standard deviation (SD)) and proportions (with % values) for continuous and categorical variables respectively. Mean and 95% confidence interval trajectories for FEV1, FVC and FEV1/FVC z-scores were visualized to show crude longitudinal trends over time.
A marginal mean regression model of the form Yt = β0 + β1X + β2Yt0 + β3time + β4X* time (where Yt is the lung function z-score outcome measured at three follow-up time points and X is the treatment variable) that considered the time of follow-up and trial arm was developed using the Generalized Estimating Equation (GEE) model, with robust-standard errors and an unstructured covariance structure [24]. The treatment effects at 24, 48 and 72 weeks were considered as β1 and β1 + β4 respectively. Further model adjustments to include factors that were imbalanced by trial arm at baseline were made. An interaction term between treatment and time variables was introduced in the model to constrain no effect for trial arm at baseline [25].
Subgroup analysis for the relationship between the intervention and lung function z-scores were performed in pre-specified sub-groups including (i) baseline severity of lung disease (<-2 vs. ≥-2 z-score) and (ii) baseline viral load (< 1000 copies/ml vs. > = 1000 copies/ml) (iii) age group (< 11 years vs. 11–16 years vs. ≥ 16 years) and (iv) carriage of azithromycin-resistant bacteria. Effect modification was examined by incorporating an interaction term between subgroups, trial arm and time of follow-up.
As a sensitivity analysis, we compared participant characteristics at baseline for those who completed follow up vs. those were lost to follow-up. In addition, multiple imputation with 10 iterations was used to impute viral load (natural log time non-varying variable) at 48-week timepoint where it was missing (5%). The registered variables associated with viral load missingness were baseline viral load, sex, age, weight and height.
Results
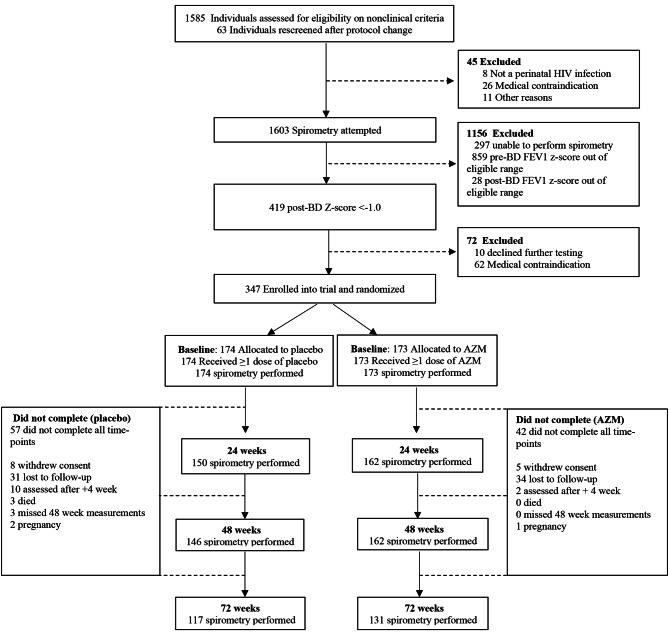
In total, 347 (51% (n = 177) male) participants were recruited of whom 308 (88.8%) were retained in follow-up by 48 weeks and 248 (71.5%) by 72 weeks. More participants in the placebo arm (n = 54/174) were lost to follow-up as compared to intervention arm (n = 39/173) during the study period (Fig. 1).
Fig. 1.
Participant enrolment flow diagram
Participant characteristics at baseline
Median age was 16.7 (IQR:14.2–19.2] years and participants had been taking ART for a median 6.2 (IQR: 3.8–8.6) years, with 55.9% of participants having a VL < 1000copies/ml at enrolment. Almost half (175/347) of participants had stunting (defined as height for age z-score <-2) and underweight (181/347: defined as weight for age z-score <-2). Furthermore, 44.7% of participants had an FEV1 z-score <-2. The distribution of participants with an FEV1 z-score <-2 were balanced between the two arms although mean absolute FEV1 values were lower in the azithromycin group [mean: 1.59 (SD: 0.50)] than placebo [mean: 1.71 (SD: 0.53). Overall, no participant had a sputum positive TB culture and one in 10 (35/347) participants were azithromycin resistant at baseline (Table 1). In addition, baseline characteristics were similar between participants who completed follow up (n = 248) vs. those who were lost to follow up (n = 99) (Supplementary Table 1).
Table 1.
Characteristics of participants at baseline by trial arm
| Variables | AZM (N = 173) | Placebo (N = 174) |
|---|---|---|
| Demographic characteristics, n (%) | ||
| Age categories at baseline | ||
|
<11 years 11–15 years ≥16 years |
25 (14.5) 87 (50.3) 61 (35.3) |
22 (12.6) 73 (42.0) 79 (45.4) |
| Sex | ||
|
Male Female |
93 (53.8) 80 (46.2) |
84 (48.3) 90 (51.7) |
| Country | ||
|
Malawi Zimbabwe |
53 (30.6) 120 (69.4) |
53 (30.5) 121 (69.5) |
| Clinical history | ||
| Viral load (< 1000 copies/ml), n (%) | 100 (58.5) | 94 (54.0) |
| Duration taking ART, median (IQR) | 5.9 (3.8-9.0) | 6.4 (3.9–8.2) |
| Anthropometry, n (%) | ||
| Height for age | ||
|
<-2 ≥-2 |
95 (54.9) 78 (45.1) |
80 (46.0) 94 (54.0) |
| Weight for age | ||
|
<-2 ≥-2 |
98 (56.7) 75 (43.4) |
83 (47.7) 91 (52.3) |
| Spirometry, mean (SD) | ||
| FEV1 | 1.59 (0.50) | 1.71 (0.53) |
| FEV1 z-score | -2.01 (0.76) | -2.0 (0.74) |
| FVC, | 1.89 (0.59) | 2.04 (0.63) |
| FVC z-score | -1.77 (0.97) | -1.71 (0.89) |
| FEV1/FVC | 0.85 (0.08) | 0.84 (0.08) |
| FEV1/FVC z-score | -0.66 (1.14) | -0.74 (1.13) |
| Severity of lung disease, n (%) | ||
| FEV1 z-score | ||
|
<-2 ≥-2 |
78 (45.1) 95(54.9) |
77 (44.3) 97 (55.7) |
| FVC z-score | ||
|
<-2 ≥-2 |
61 (35.9) 109 (64.1) |
54 (32.0) 115 (68.1) |
|
FEV1/FVC z-score <-2 ≥-2 |
18 (10.6) 152 (89.4) |
22 (13.0) 147 (87.0) |
| Resistance to AZM | 15 (8.7) | 20 (11.5) |
AZM: Azithromycin; IQR: Interquartile range; 4 and 2 missing values for FVC and viral load respectively
Crude spirometry measurements at follow-up (24, 48 and 72 weeks)
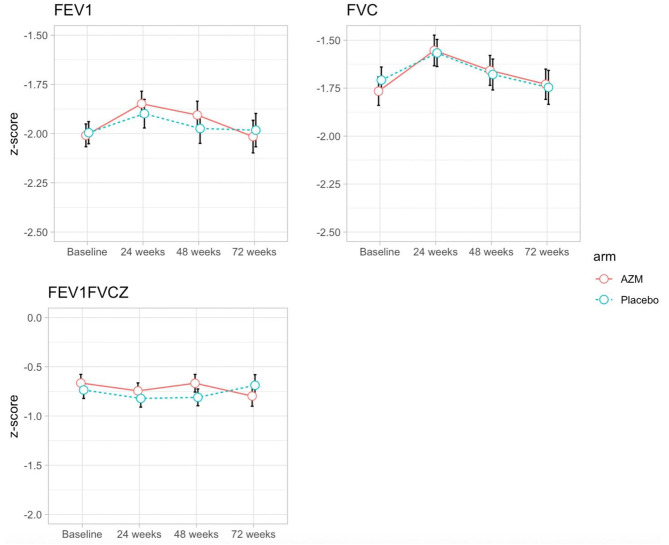
In both arms, absolute FVC and FEV1 measures showed improvements over the follow-up period (Supplementary Fig. 1). In both arms, the corresponding z-score values showed an increase from baseline to 24 weeks (FVC; p value: 0.021, FEV1; p value: 0.037 [Fig. 2]). There was a 5% and 3% reduction in the proportion of children with a z-score < -2 between baseline and 24 weeks for FEV1 and FVC respectively regardless of arm. However, FEV1 and FVC z-scores in both arms at 48 and 72 weeks were comparable to baseline measurements (Fig. 2). There was no change over time in FEV1/FVC ratio in either arm.
Fig. 2.
Unadjusted mean scores and 95% confidence intervals for spirometry measurements at 24, 48 and 72 weeks by treatment arm
Adjusted lung function trajectories by trial arm
In z-score models adjusted for sex, age, site and natural log HIV viral load, there was no statistical evidence for a treatment effect at 24 weeks, with a mean difference between the intervention and control arm in FEV1 of -0.02 (95% CI: -0.05, 0.02), in FVC of -0.03 [95% CI: -0.09, 0.03] and in FEV1/FVC ratio of 0.02 (95% CI: -0.04, 0.08; Table 2). At 48 and 72 weeks, both adjusted FEV1 (48 weeks: 0.02 [95% CI: -0.04, 0.09]; 72 weeks: 0.03 [95% CI: -0.05, 0.10]) and FVC (48 weeks: 0.04 [95% CI: -0.05, 0.13]; 72 weeks: 0.04; [-0.06, 0.13]) z-scores showed a weak positive azithromycin treatment effect, however there was no statistical evidence for a treatment effect. There was no evidence of azithromycin treatment effect on FEV1/FVC z-score at either 48 weeks [-0.01; 95% CI: -0.11, 0.09] or 72 weeks [-0.03; 95% CI: -0.07, 0.14]. Overall, there was no treatment effect on lung function z-scores after factoring the changes across the individual time points; FEV1: 0.004 [95% CI: -0.08, 0.09], FVC: 0.02 [95% CI: -0.07, 0.12] and FEV1/FVC: -0.01 [95% CI: -0.12, 0.09]. Similarly, all adjusted models of absolute lung function measurements showed no evidence of azithromycin treatment effect in crude lung measurements at all time points (Table 2). In the sensitivity analyses, results were similar to unimputed data.
Table 2.
Treatment effect (adjusted mean difference) for spirometry variables at 24, 48 and 72 weeks
| Outcome | Adjusted mean difference (95% CI) | |||
|---|---|---|---|---|
| 24 weeks (n = 312) | 48 weeks (n = 308) | 72 weeks (n = 248) | Overall effect | |
| FEV1 | -0.12 [-0.31, 0.07] | 0.04 [-0.01, 0.09] | 0.05 [-0.07, 0.16] | 0.004 [-0.08, 0.09] |
| FEV1 z-score | -0.02 [-0.05, 0.02] | 0.02 [-0.04, 0.09] | 0.03 [-0.05, 0.10] | 0.004 [-0.08, 0.09] |
| FVC | -0.19 [-0.51, 0.12] | 0.04 [-0.02, 0.09] | 0.06 [-0.07, 0.19] | 0.03 [-0.02, 0.09] |
| FVC z-score | -0.03 [-0.09, 0.03] | 0.04 [-0.05, 0.13] | 0.04 [-0.06, 0.13] | 0.02 [-0.07, 0.12] |
| FEV1/FVC | 0.02 [-0.03, 0.08] | 0.01 [-0.01, 0.02] | -0.01 [-0.03, 0.02] | 0.005 [0.01, 0.02] |
| FEV1/FVC z-score | 0.02 [-0.04, 0.08] | -0.01 [-0.11, 0.09] | 0.03 [-0.07, 0.14] | -0.01 [-0.12, 0.09] |
The models shows adjusted mean differences between the intervention and placebo arms with negative values indicating lower values in the intervention arm as compared to the placebo. The xtgee models were adjusted for sex, age, site and natural log HIV viral load
Stratification of models by lung function severity, age, drug resistance and viral load
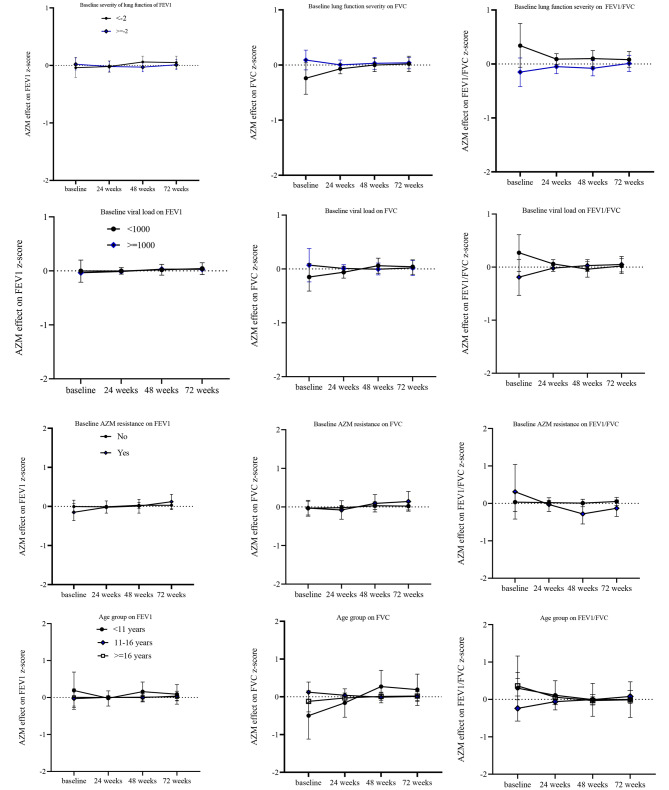
Further adjustments on fully adjusted FEV1, FVC and FEV1/FVC z-score models showed no interaction of azithromycin treatment by baseline lung function severity, baseline viral load, age and azithromycin drug resistance on lung function (Fig. 3).
Fig. 3.
Interaction between AZM with baseline lung function severity, viral load, AZM resistance and age group on lung function outcomes
Safety
There were no severe adverse events related to the study medication [20]. (Supplementary Table 2).
Discussion
This study investigated the trajectories of lung function in CWH. Overall, the study showed no improvements in any lung function parameter (FEV1, FVC and FEV1/FVC z-scores) after 48 weeks of azithromycin.
Studies have investigated azithromycin therapy in a number of chronic lung diseases with discrepant findings. In a study of patients with non-cystic fibrosis bronchiectasis, azithromycin did not result in significant improvements in FEV1 or FVC compared to placebo [26]. A randomised controlled trial in individuals with chronic obstructive pulmonary disease showed no differences in lung function parameters between azithromycin-treated and placebo groups [27]. Other studies have reported improvements in lung function parameters [28], reduced respiratory symptoms, [29] and decreased rate of exacerbations in various chronic lung diseases conditions with azithromycin. In a meta-analysis of ten studies of patients with bronchiolitis obliterans syndrome (BOS), azithromycin was shown to improve FEV1 [30]. Also, longitudinal meta-analysis of studies among cystic fibrosis patients reported gains in percentage predicted FEV1 and FVC values with azithromycin [31, 32]. These studies suggest that azithromycin’s anti-inflammatory and immunomodulatory properties may contribute to its beneficial effects on lung function. It should be noted that none of these studies were conducted among individuals with HIV infection.
Our study found azithromycin supplementation had no effect on FEV1/FVC z-scores for participants with either severe lung or mild lung function impairment. One study in the United States identified patients with moderately impaired lung function to benefit more than the severely ill ones though airway microbiology was not systematically evaluated in the study [27, 33]. Similarly, in a study to understand the effect of low-dose azithromycin therapy on acute exacerbations of chronic obstructive pulmonary disease (COPD), the study identified improvement in lung function among those with severe exacerbations in the previous year [34].
The lack of effect may be due to the fact that the lung injury in HIV-associated chronic lung disease, once established is not reversible. In constrictive obliterative bronchiolitis, inflammation is followed by fibrosis occurring predominantly in the walls and contiguous tissues of membranous and respiratory bronchioles, with resultant narrowing of their lumens [35]. It may be that at the stage that treatment was started, most children have already developed fibrosis which may not be amenable to immunomodulatory therapy.
Another possible reason for the lack of effect on lung function is insufficient levels of adherence. We have previously reported that adherence was higher for those randomised to AZM (73.4%) than placebo (68.4%) and declined over the 48 weeks of the study (Score test for trend < 0.02). Those with unsuppressed HIV viral load at baseline had 2.08 (95% CI: 1.19, 3.63) times the odds of non-adherence than those with viral suppression [36]. A statistically significant effect of azithromycin on risk of acute respiratory exacerbations was however, observed in the BREATHE trial, with a 50% reduction in the risk of ARE. The impact of azithromycin on reducing AREs was greater in participants with chronic respiratory symptoms at baseline, those on 1st line ART, with a FEV1 score >-2 and participants without baseline resistance to azithromycin [29].
The study used data from a well-powered clinical trial and spirometry was performed to ATS standards with inbuilt quality control, and microbiological azithromycin resistance data were available. We had long-term follow-up data enabling tracking of longitudinal trajectories of lung function. Note that by 72 weeks, 28.5% were lost to follow-up, reducing statistical power.
In summary, our study showed no beneficial effect of azithromycin supplementation on lung function in CHW who had CLD, overall or in any subgroup.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to all research staff and study participants.
Abbreviations
- HIV
Human Immunodeficiency Virus
- ART
Antiretroviral Therapy
- CWH
Children living with HIV
- CLD
Chronic Lung Disease
- OB
Obliterative Bronchiolitis
- FVC
Forced Vital Capacity
- FEV1
Forced Expiratory Volume in 1 s
- COPD
Chronic Obstructive Pulmonary Disease
- GLI
Global Lung Function Initiative
- GEE
Generalized Estimating Equation
Author contributions
Conception: RAF, AMR. Design: Data acquisition: RAF, GM, CG-M, LN, RS, MN. Data Management: TB, BM, VS. Data analysis: TM, AMR, VS. Interpretation: TM, RAF, AMR, VS. Manuscript drafting: TM, RAF, AMR. Manuscript revision: TM, RAF, AMR, CC, GM, CG-M, LN, RS, MN, VS, JOO, BM. All authors take responsibility for their contributions as outlined above and have read and approved the final manuscript.
Funding
The study was funded through the Global Health and Vaccination Programme of the Norwegian Research Council. RAF is funded by the Wellcome Trust through a Senior Research Fellowship (Grant no 206316_Z_17_Z). MN is supported by an Australian National Health and Medical Research Council Investigator Grant. VS and AMR are partly funded by grant MR/R010161/1 from the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement.
Data availability
The datasets used and/or analysed during the current study are available at the London School of Hygiene and Tropical Medicine repository (Data Compass) on reasonable request to the corresponding author.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The trial registration is ClinicalTrials.gov Identifier: NCT02426112. Ethical approval was granted by the Malawi College of Medicine Research Ethics Committee, the Medical Research Council of Zimbabwe, the Biomedical Research and Training Institute Institutional Review Board, Zimbabwe, the London School of Hygiene and Tropical Medicine ethics committee, the University of Cape Town Research Ethics Committee and the Regional Ethics Committee for Medical and Health Research, Norway. Written informed consent was obtained from all participants.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World AIDS, UNICEF DATA. Day Report 2020 [Internet]. 2020 [cited 2021 Jul 16]. https://data.unicef.org/resources/world-aids-day-report-2020/.
- 2.Mutanga JN, Mutembo S, Ezeamama AE, Song X, Fubisha RC, Mutesu-Kapembwa K, et al. Long-term survival outcomes of HIV infected children receiving antiretroviral therapy: an observational study from Zambia (2003–2015) BMC Public Health. 2019;19(1):115. doi: 10.1186/s12889-019-6444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrand RA, Desai SR, Hopkins C, Elston CM, Copley SJ, Nathoo K, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2012;55(1):145–52. doi: 10.1093/cid/cis271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansell DM, Rubens MB, Padley SP, Wells AU. Obliterative bronchiolitis: individual CT signs of small airways disease and functional correlation. Radiology. 1997;203(3):721–6. doi: 10.1148/radiology.203.3.9169694. [DOI] [PubMed] [Google Scholar]
- 5.Iii JPL, Weigt SS, DerHovanessian A, Fishbein MC, Gutierrez A, Belperio JA. Obliterative (Constrictive) Bronchiolitis. Semin Respir Crit Care Med. 2012;33(5):509–32. doi: 10.1055/s-0032-1325161. [DOI] [PubMed] [Google Scholar]
- 6.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the Pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26(1):2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered Microbial communities in Asthmatic Airways. PLoS ONE. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1073–80. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR, et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med. 2012;186(6):536–45. doi: 10.1164/rccm.201204-0693OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willner DL, Hugenholtz P, Yerkovich ST, Tan ME, Daly JN, Lachner N, et al. Reestablishment of recipient-associated microbiota in the lung allograft is linked to reduced risk of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2013;187(6):640–7. doi: 10.1164/rccm.201209-1680OC. [DOI] [PubMed] [Google Scholar]
- 11.Gollwitzer ES, Marsland BJ. Microbiota abnormalities in inflammatory airway diseases - potential for therapy. Pharmacol Ther. 2014;141(1):32–9. doi: 10.1016/j.pharmthera.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Krishna R, Anjum F, Oliver TI. Bronchiolitis Obliterans. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 [cited 2021 Jul 16]. http://www.ncbi.nlm.nih.gov/books/NBK441865/.
- 13.Molyneux E. Bacterial infections in children with HIV/AIDS. Trop Doct. 2004;34(4):195–8. doi: 10.1177/004947550403400403. [DOI] [PubMed] [Google Scholar]
- 14.McMullan BJ, Mostaghim M. Prescribing azithromycin. Aust Prescr. 2015;38(3):87–9. doi: 10.18773/austprescr.2015.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2012;2012(11):CD002203. doi: 10.1002/14651858.CD002203.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain R, Hachem RR, Morrell MR, Trulock EP, Chakinala MM, Yusen RD, et al. AZITHROMYCIN IS ASSOCIATED WITH INCREASED SURVIVAL IN LUNG TRANSPLANT RECIPIENTS WITH BRONCHIOLITIS OBLITERANS SYNDROME. J Heart Lung Transpl. 2010;29(5):531–7. doi: 10.1016/j.healun.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laopaiboon M, Panpanich R, Swa Mya K. Azithromycin for acute lower respiratory tract infections. Cochrane Database Syst Rev. 2015;2015(3):CD001954. doi: 10.1002/14651858.CD001954.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan CTJ, Ward C, Meachery G, Lordan JL, Fisher AJ, Corris PA. Long-term effect of azithromycin in bronchiolitis obliterans syndrome. BMJ Open Respiratory Res. 2019;6(1):e000465. doi: 10.1136/bmjresp-2019-000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verleden GM, Vanaudenaerde BM, Dupont LJ, Van Raemdonck DE. Azithromycin reduces Airway Neutrophilia and Interleukin-8 in patients with Bronchiolitis Obliterans Syndrome. Am J Respir Crit Care Med. 2006;174(5):566–70. doi: 10.1164/rccm.200601-071OC. [DOI] [PubMed] [Google Scholar]
- 20.Ferrand RA, McHugh G, Rehman AM, Mujuru H, Simms V, Majonga ED, et al. Effect of once-weekly azithromycin vs Placebo in Children with HIV-Associated Chronic Lung Disease: the BREATHE Randomized Clinical Trial. JAMA Netw Open. 2020;3(12):e2028484. doi: 10.1001/jamanetworkopen.2020.28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Martinez C, Kranzer K, McHugh G, Corbett EL, Mujuru H, Nicol MP, et al. Azithromycin versus placebo for the treatment of HIV-associated chronic lung disease in children and adolescents (BREATHE trial): study protocol for a randomised controlled trial. Trials. 2017;18(1):622. doi: 10.1186/s13063-017-2344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quanjer PH, Stanojevic S, Stocks J, Cole TJ. GLI-2012 All-Age Multi-Ethnic Reference Values for Spirometry.:15. [DOI] [PMC free article] [PubMed]
- 23.Validation of the global lung. initiative 2012 multi-ethnic spirometric reference equations in healthy urban Zimbabwean 7–13 year-old school children: a cross-sectional observational study | BMC Pulmonary Medicine | Full Text [Internet]. [cited 2020 Sep 27]. https://bmcpulmmed.biomedcentral.com/articles/10.1186/s12890-020-1091-4. [DOI] [PMC free article] [PubMed]
- 24.Hayes AF, Cai L. Using heteroskedasticity-consistent standard error estimators in OLS regression: an introduction and software implementation. Behav Res Methods. 2007;39(4):709–22. doi: 10.3758/BF03192961. [DOI] [PubMed] [Google Scholar]
- 25.J T, L B, T H, J R, M W, M H. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun. 2018;10:80–5. [DOI] [PMC free article] [PubMed]
- 26.Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–7. doi: 10.1016/S0140-6736(12)60953-2. [DOI] [PubMed] [Google Scholar]
- 27.Han MK, Tayob N, Murray S, Dransfield MT, Washko G, Scanlon PD, et al. Predictors of Chronic Obstructive Pulmonary Disease Exacerbation reduction in response to Daily Azithromycin Therapy. Am J Respir Crit Care Med. 2014;189(12):1503–8. doi: 10.1164/rccm.201402-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis Uninfected with Pseudomonas aeruginosa: a Randomized Controlled Trial. JAMA. 2010;303(17):1707–15. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 29.Price A, McHugh G, Simms V, Semphere R, Ngwira LG, Bandason T, et al. Effect of azithromycin on incidence of acute respiratory exacerbations in children with HIV taking antiretroviral therapy and co-morbid chronic lung disease: a secondary analysis of the BREATHE trial. EClinicalMedicine. 2021;42:101195. doi: 10.1016/j.eclinm.2021.101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kingah PL, Muma G, Soubani A. Azithromycin improves lung function in patients with post-lung transplant bronchiolitis obliterans syndrome: a meta-analysis. Clin Transplant. 2014;28(8):906–10. doi: 10.1111/ctr.12401. [DOI] [PubMed] [Google Scholar]
- 31.Samson C, Tamalet A, Thien HV, Taytard J, Perisson C, Nathan N, et al. Long-term effects of azithromycin in patients with cystic fibrosis. Respir Med. 2016;117:1–6. doi: 10.1016/j.rmed.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews [Internet]. 2004 [cited 2023 May 29];(2). https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002203.pub2/full. [DOI] [PubMed]
- 33.Welte T, Azithromycin The Holy Grail to Prevent exacerbations in Chronic Respiratory Disease? Am J Respir Crit Care Med. 2019;200(3):269–70. doi: 10.1164/rccm.201903-0706ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadian S, Sin DD, Lynd L, Harrison M, Sadatsafavi M. Benefit–harm analysis of azithromycin for the prevention of acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2022;77(11):1079–87. doi: 10.1136/thoraxjnl-2021-217962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlesinger C, Meyer CA, Veeraraghavan S, Koss MN. Constrictive (obliterative) bronchiolitis: diagnosis, etiology, and a critical review of the literature. Annals Diagn Pathol. 1998;2(5):321–34. doi: 10.1016/S1092-9134(98)80026-9. [DOI] [PubMed] [Google Scholar]
- 36.Rehman AM, Simms V, McHugh G, Mujuru H, Ngwira LG, Semphere R, et al. Adherence to additional medication for management of HIV-associated comorbidities among older children and adolescents taking antiretroviral therapy. PLoS ONE. 2022;17(6):e0269229. doi: 10.1371/journal.pone.0269229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available at the London School of Hygiene and Tropical Medicine repository (Data Compass) on reasonable request to the corresponding author.