Abstract
Background
Critical-illness survivors may experience post-traumatic stress disorder (PTSD) and quality-of-life impairments. Resilience may protect against psychological trauma but has not been adequately studied after critical illness. We assessed resilience and its associations with PTSD and quality of life, and also identified factors associated with greater resilience.
Methods
This prospective, multicentre, study in patients recruited at 41 French ICUs was done in parallel with the NUTRIREA-3 trial in patients given mechanical ventilation and vasoactive amines for shock. Three months to one year after intensive-care-unit admission, survivors completed the Connor-Davidson Resilience Scale (CD-RISC-25), Impact of Event-Revised scale for PTSD symptoms (IES-R), SF-36 quality-of-life scale, Multidimensional Scale of Perceived Social Support (MSPSS), and Brief Illness Perception Questionnaire (B-IPQ).
Results
Of the 382 included patients, 203 (53.1%) had normal or high resilience (CD-RISC-25 ≥ 68). Of these resilient patients, 26 (12.8%) had moderate to severe PTSD symptoms (IES-R ≥ 24) vs. 45 (25.4%) patients with low resilience (p = 0.002). Resilient patients had higher SF-36 scores. Factors independently associated with higher CD-RISC-25 scores were higher MSPSS score indicating stronger social support (OR, 1.027; 95%CI 1.008–1.047; p = 0.005) and lower B-IPQ scores indicating a more threatening perception of the illness (OR, 0.973; 95%CI 0.950–0.996; p = 0.02).
Conclusions
Resilient patients had a lower prevalence of PTSD symptoms and higher quality of life scores, compared to patients with low resilience. Higher scores for social support and illness perception were independently associated with greater resilience. Thus, our findings suggest that interventions to strengthen social support and improve illness perception may help to improve resilience. Such interventions should be evaluated in trials with PTSD mitigation and quality-of-life improvement as the target outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-04989-x.
Keywords: Critical illness, Resilience, Post-traumatic stress disorder, Quality of life, Social support, Illness perception
Background
With advances in intensive care medicine and increasing numbers of patients admitted to intensive care units (ICUs), the number of critical-illness survivors is growing steadily. Studies have shown that these patients are at high risk for physical, cognitive, and psychological impairments that may persist for months or years. One of the main adverse psychological effects of critical illness is post-traumatic stress disorder (PTSD), which affects 4–62% of patients [1–4]. Clinical symptoms of PTSD include intrusive thoughts and memories of the traumatic event, avoidance of reminders of the event, and hyperarousal symptoms such as irritability, impaired concentration, and hypervigilance [5]. These symptoms can persist for over five years after ICU discharge and are associated with impaired quality of life [6].
Psychological resilience is the ability to adapt positively to traumatic and stressful events, thereby protecting against mental ill-health [7]. Resilient people are more likely to develop effective coping strategies for handling adverse situations [8]. Studies in patients with cancer have shown that greater resilience was associated with less anxiety and depression [9, 10]. Resilience can change throughout life and is influenced by both external factors, such as social support, and internal factors, such as perception of the illness and treatment [11–15].
Although the psychological burden of critical illness has been extensively investigated in ICU survivors and their relatives, few studies have focussed on patient resilience. Among patients having survived critical illness or trauma, the proportion with normal-to-high resilience varied widely, from 28 to 76%, and greater resilience was associated with less mental ill-health, pain, physical complaints, and self-care difficulties [16]. These data raise the possibility that promoting resilience in critical-illness survivors may improve psychological and quality-of-life outcomes [17]. However, only a few small studies have assessed the prevalence and determinants of resilience in this population, and they varied regarding the tools used to measure resilience [18].
The primary objective of the prospective, multicentre, observational RESIREA cohort study reported here was to assess resilience in a large cohort of survivors of severe critical illness, using the well-validated 25-item Connor-Davidson Resilience Scale (CD-RISC-25). The secondary objectives were to assess potential associations linking resilience to PTSD symptoms and quality of life and to identify factors associated with the level of resilience.
Methods
Study design and oversight
RESIREA was a planned study conducted in parallel with the randomised controlled multicentre open-label NUTRIREA-3 trial designed to evaluate whether low-calorie low-protein feeding decreased day-90 mortality and/or time to ICU discharge readiness, compared to standard calorie-protein supplies, in adults receiving invasive mechanical ventilation and vasopressor support for shock (ClinicalTrials.gov Identifier: NCT03573739) [19, 20]. The NUTRIREA-3 study was supported by the Nantes University Hospital and funded by a 2017 Programme Hospitalier de Recherche Clinique National grant from the French Ministry of Health (#PHRC-17-0213). NUTRIREA-3 was approved by the competent ethics committee (Comité de Protection des Personnes Sud-Méditerranée 2, #2018-A00424-51).
RESIREA was a multicentre, prospective, observational, cohort study. Of the 61 ICUs participating in the NUTRIREA-3 trial, 41 accepted to also participate in the RESIREA study, including 22 (53.7%) in university hospitals (Additional File 1: RESIREA sites and contributors). Because of organisational constraints, the RESIREA start date was not the same in all 41 ICUs. However, in all ICUs, inclusions in RESIREA stopped at inclusion of the last NUTRIREA-3 trial patient. The RESIREA patients were interviewed by psychologists, who administered five pre-specified questionnaires. The RESIREA study was supported by the Nantes University Hospital. The RESIREA study protocol was approved by the ethics committee of the French Intensive Care Society (CE SRLF 18–19).
Participants
Inclusion in the study occurred in two steps. First, in each participating ICU, consecutive patients included in NUTRIREA-3 were considered for inclusion in RESIREA. Inclusion and exclusion criteria were similar to those for NUTRIREA-3: adults (18 years or older) were eligible if they were receiving invasive mechanical ventilation, with an expected duration of at least 48 h after inclusion and initiation either within 24 h after or within 24 h before ICU admission, concomitantly with vasoactive therapy for shock, and if nutritional support was expected to be started within 24 h after intubation (or within 24 h after ICU admission when intubation occurred before ICU admission). Non-inclusion criteria were specific nutritional needs, such as pre-existing long-term home enteral or parenteral nutrition for chronic bowel disease; dying patient, not-to-be-resuscitated order, or other treatment-limitation decision at ICU admission; pregnancy, recent delivery, or lactation; adult under guardianship; and correctional facility inmate. Specific informed consent for inclusion in the RESIREA study was obtained from the patients, or from their next of kin in patients unable to consent. In the second step, which occurred at ICU discharge, the following non-inclusion criteria were applied: death in the ICU, insufficient fluency in French, persistent severe illness or severe cognitive impairment precluding questionnaire completion, and consent withdrawal.
Data collection
The baseline features of each patient were recorded at inclusion in the NUTRIREA-3 trial. Infectious and non-infectious complications during the ICU stay, dialysis in the ICU, duration of invasive mechanical ventilation, ICU and hospital lengths of stay, and mortality were recorded according to the NUTRIREA-3 trial protocol [19, 20].
Marital status, employment status, number of children, and history of psychiatric disorders were obtained during phone interviews by trained psychologists who had no knowledge of the medical data of the patients. Each interview involved the administration of five scales and lasted about 40 min. The phone numbers used were those in the ICU medical files for each patient. When a call was unanswered, at least two further attempts were made during different days or weeks. Two interviews were planned initially, three months and one year after inclusion in NUTRIREA-3 and RESIREA. However, organisational issues and difficulties experienced by some patients with participating in two long interviews prompted us to aim for at least one interview in each patient. When two interviews were performed, the data obtained during the most recent interview were used for the main analysis. Thus, in all patients, data from a single interview were analysed.
Outcomes
Resilience was assessed with the CD-RISC-25 [21]. Each item is answered using a 0–4 Likert scale. The total score can range from 0 to 100, with higher scores indicating greater resilience. The items are grouped into five sub-scales: personal competence, high standards, and tenacity (eight items; sub-score range, 0–32), trust in one’s instincts, tolerance of negative affect, and strengthening effects of stress (seven items; sub-score range, 0–28), positive acceptance of change and secure relationships (five items; sub-score range, 0–20), control (three items; sub-score range, 0–12), and spiritual influences (two items; sub-score range, 0–8). We defined low, normal, and high resilience as CD-RISC-25 scores ≤ 67, 68–92, and ≥ 93, respectively [21, 22]. Patients designated as “resilient” hereafter are those with scores ≥ 68; patients with scores below this cut-off are designated “non-resilient”.
PTSD symptoms were assessed with the Impact of Event Scale-Revised (IES-R). The symptoms are grouped into three sub-scores: intrusion (sub-score range, 0–32), avoidance (sub-score range, 0–32), and hyperarousal (sub-score range, 0–24). The total score can range from 0 to 88, with higher scores indicating greater symptom severity. We defined severe, moderate, and no PTSD symptoms as IES-R scores ≥ 33, 24–32, and ≤ 23, respectively [23, 24].
Health-related quality of life was assessed with the Short Form-36 (SF-36) [25, 26]. The 36 items investigate eight dimensions: bodily pain, general health, mental health, physical functioning, role emotional, role physical, vitality, and social functioning. Eight sub-scores can be calculated, each of them contributing in different proportions to the calculation of two scores (PCS: physical component summary and MCS: mental component summary), each ranging from 0 to 100. Higher PCS and MCS scores indicate better health-related quality of life.
Social support was assessed with the Multidimensional Scale of Perceived Social Support (MSPSS), a 12-item questionnaire measuring the perceived adequacy of social support received from three sources: family, friends, and significant other persons. Each item is rated on a 7-point Likert scale (from 1, strongly disagree to 7, very strongly agree). The total score can range from 12 to 84 and each sub-score from 4 to 28. Higher values indicate greater social support [27].
Perception of illness by the patients was assessed with the Brief Illness Perception Questionnaire (B-IPQ). A 0–10 scale is used to rate eight of the nine B-IPQ items including five items for the cognitive illness representation sub-score (perceived consequences, perception of the timeline, amount of perceived personal control, amount of control of the treatment, and identity; range, 0–50), two items for the emotional illness representation sub-score (concern about the illness and emotional response to the illness; range, 0–20), and one item for the illness comprehensibility sub-score (understanding of the illness; range, 0–10). An additional item assesses causal perceptions by asking patients to list the three most likely causes for their illness. The total B-IPQ score is the sum of the scores on the first eight items and can thus range from 0 to 80, with higher scores reflecting a more threatening perception of the illness [28].
Perceived social support and perception of illness were assessed as dimensions possibly contributing to resilience after critical illness.
Statistical analysis
No reliable data were available for anticipating the difference between groups. Given the observational design, we planned to perform adjusted analyses and, therefore, required sufficient observations for the large number of covariates (about 40 continuous and 10 qualitative covariates). We consequently planned to recruit 400 survivors of severe critical illness.
Baseline characteristics were described as number and percentage for qualitative variables and as mean ± SD and median [interquartile range] for quantitative variables.
For the five questionnaires, responses to at least 75% of items was required for inclusion in the analysis. Mean imputation was performed for missing data.
We determined the percentage of resilient patients (CD-RISC-25 score > 68), with the 95% confidence interval (95%CI). We also determined the percentage of patients with PTSD symptoms, overall and in each severity category (≥ 33 and 24–32), with the 95%CIs. The correlation between the IES-R and CD-RISC-25 scores was assessed by estimating the Spearman correlation coefficient, its 95%CI, and the associated p value. The SF-36, MSPSS, and B-IPQ scores were compared in resilient vs. non-resilient patients by applying the Wilcoxon test.
To identify factors associated with resilience, we first performed univariate analyses using the chi-square or Fisher test for qualitative variables and the Wilcoxon test for quantitative variables. Logistic regression was then used for the multivariate analysis. Variables with significant differences by univariate analysis (p < 0.20) were included in the multivariate analysis.
The analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.3.1 (htpps://www.r-project.org).
Results
Patients
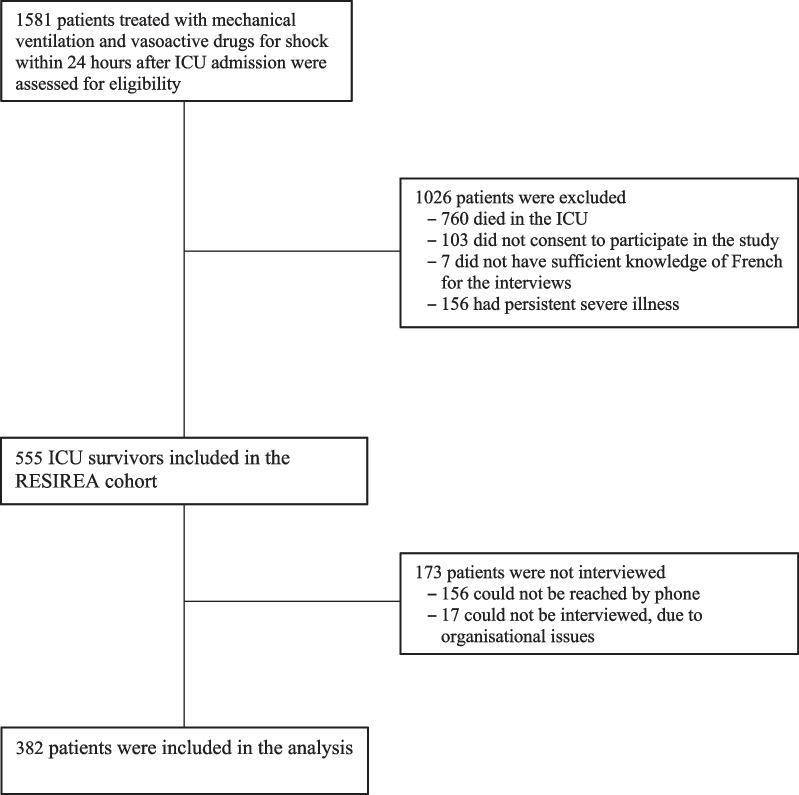
Figure 1 is the patient flow chart. From 23 October 2018 to 8 December 2020, 1581 patients in the 41 participating ICUs were screened for eligibility; among them, 1026 had exclusion criteria, leaving 555 patients eligible for the RESIREA study. Of these, 173 were not interviewed. The remaining 382 (68.8%) patients were included in the analysis (Additional File 2, Table S1). Patients included in the analysis did not differ from patients who were eligible but could not be interviewed (Table 1).
Fig. 1.
Flow chart. ICU: intensive care unit
Table 1.
Comparison of the main baseline characteristics between patients included in the analysis and patients who were eligible for inclusion but were not interviewed
| Included patients N = 382 |
Eligible non-included patients N = 173 |
p value | |
|---|---|---|---|
| Age, years, mean ± SD | 61.4 ± 12.4 | 61.7 ± 14.5 | 0.55 |
| Males, n (%) | 257 (67.3) | 115 (66.5) | 0.85 |
| McCabe score, n (%) | 0.08 | ||
| 0 (no fatal underlying disease) | 312 (81.7) | 127 (73.4) | |
| 1 (death expected within 5 years) | 60 (15.7) | 39 (22.5) | |
| 2 (death expected within 1 year) | 10 (2.6) | 7 (4.0) | |
| Pre-existing illness at ICU admission, n (%) | 222 (58.6) | 103 (60.2) | 0.71 |
| Chronic renal failure | 21 (5.5) | 19 (11.1) | 0.02 |
| Liver disease | 28 (7.4) | 17 (10.0) | 0.30 |
| Cardiovascular disease | 55 (14.6) | 28 (16.6) | 0.54 |
| Chronic respiratory failure | 25 (6.6) | 19 (11.2) | 0.07 |
| Neurologic disease | 34 (9.0) | 19 (11.1) | 0.43 |
| Cancer or immune deficiency | 85 (22.5) | 25 (14.6) | 0.03 |
| Oesophageal, gastric, or duodenal ulcer | 19 (5.0) | 7 (4.1) | 0.64 |
| Diabetes mellitus | 73 (19.3) | 44 (25.7) | 0.09 |
| Weight, kg, median [IQR] | 80.0 [68.0–92.0] | 77.0 [67.0–89.5] | 0.14 |
| BMI, kg/m2, median [IQR] | 27.3 [24.2–31.7] | 26.6 [22.7–31.2] | 0.15 |
| SAPS IIa, median [IQR] | 55 [43–69] | 59.0 [44.0–70.0] | 0.32 |
| SOFA scoreb, median [IQR] | 10.0 [8.0–12.0] | 10.0 [8.0–12.0] | 0.06 |
| Medical diagnosis at admission, n (%) | 301 (78.8) | 137 (79.2) | 0.92 |
| Acute illness at ICU admission, n (%) | 0.09 | ||
| Cardiac arrest | 59 (15.5) | 17 (9.8) | |
| Acute heart failure | 65 (17.0) | 28 (16.2) | |
| Acute central nervous system failure | 25 (6.5) | 13 (7.5) | |
| Acute respiratory failure | 157 (41.1) | 70 (40.5) | |
| Trauma | 8 (2.1) | 11 (6.4) | |
| Miscellaneous | 68 (17.8) | 34 (19.7) | |
| Cause of shock, n (%) | 0.03 | ||
| Cardiac | 84 (22.0) | 22 (12.7) | |
| Sepsis | 206 (53.9) | 102 (59.0) | |
| Other | 92 (24.1) | 49 (28.3) | |
| Ongoing treatments at inclusion, n (%) | |||
| Randomised in the Low group of NUTRIREA-3c | 176 (46.1) | 95 (54.9) | 0.05 |
| Prone position | 21 (5.5) | 9 (5.2) | 0.89 |
| Sedative agents | 348 (91.1) | 156 (90.2) | 0.73 |
| NMBA | 139 (36.4) | 46 (26.6) | 0.02 |
| Insulin | 147 (38.5) | 73 (42.2) | 0.41 |
| Anti-microbial treatmentd | 326 (85.3) | 152 (87.9) | 0.43 |
| Dialysis | 30 (7.9) | 19 (11.0) | 0.23 |
| Outcomes | |||
| RRT during the ICU stay, n (%) | 76 (19.9) | 45 (26.0) | 0.11 |
| At least one complicatione during the ICU stay, n (%) | 50 (13.1) | 20 (11.6) | 0.62 |
| Duration of mechanical ventilation, days, median [IQR] | 6.0 [2.0–11.0] | 6.0 [3.0–10.0] | 0.95 |
| ICU length of stay, days, median [IQR] | 9.0 [6.0–16.0] | 9.0 [5.0–15.0] | 0.91 |
| Hospital length of stay, days, median [IQR] | 21.0 [13.0–34.0] | 20.0 [13.0–40.0] | 0.29 |
ICU intensive care unit, IQR interquartile range, BMI body mass index, SAPS II Simplified Acute Physiology Score version II [29], SOFA Sequential Organ Failure Assessment [30], NMBA neuromuscular blocking agent, RRT renal replacement therapy
aSAPS II values can range from 0 (lowest level of critical illness) to 163 (most severe level of critical illness with 100% predicted mortality). A score of 50 predicts a 46.1% risk of death. The SAPS II was determined 24 h after ICU admission
bSOFA scores can range from 0 (no organ failure) to 24 (most severe level of multi-organ failure). The SOFA sub-score values at ICU admission are reported in eTable 1
cPatients included in the NUTRIREA-3 trial were randomised to early nutrition with either low or standard calorie-protein targets (6 kcal/kg/d and 0·2–0·4 g/kg/d, respectively; and 25 kcal/kg/d and 1·0–1·3 g/kg/d, respectively)
dAnti-microbial treatments included antibiotics, antiviral drugs, and antifungal drugs
eComplications included infections and gastro-intestinal complications acquired during the ICU stay
Resilience, post-traumatic stress disorder symptoms, and quality of life
The median CD-RISC-25 was 69.0 [59.0–78.0]) (Table 2). Of the 382 patients, 203 (53.1%) were resilient, i.e., had CD-RISC-25 scores ≥ 68.
Table 2.
Resilience in the 382 included patients discharged alive from the intensive care unit
| Median [IQR] or n (%) |
|
|---|---|
| CD-RISC-25a, median [IQR] | 69.0 [59.0–78.0] |
| Personal competence, high standards, and tenacity | 24.0 [20.0–27.0] |
| Trust in one’s instincts, tolerance of negative affect, and strengthening effects of stress | 18.0 [15.0–21.0] |
| Positive acceptance of change, and secure relationships | 15.0 [13.0–17.0] |
| Control | 9.0 [7.0–10.0] |
| Spiritual influences | 4.0 [3.0–6.0] |
| Resilienceb, n (%) | |
| Low | 179 (46.9) |
| Normal | 195 (51.0) |
| High | 8 (2.1) |
CD-RISC Connor-Davidson Resilience Scale, IQR interquartile range
aThe CD-RISC-25 has 25 items, with five sub-scales: personal competence, high standards, and tenacity (range, 0–32), trust in one’s instincts, tolerance of negative affect, and strengthening effects of stress (range, 0–28), positive acceptance of change and secure relationships (range, 0–20), control (range, 0–12), and spiritual influences (range, 0–8). Thus, the total CD-RISC-25 score can range from 0 to 100. Higher scores indicate greater resilience [21]
bLow, normal, and high resilience were defined as CD-RISC-25 scores ≤ 67, 68–92, and ≥ 93, respectively [21, 22]
The median total IES-R score was 9.0 [4.0–19.0]) (Table 3). The total score and each of the three sub-scores were significantly lower in resilient than in non-resilient patients. The IES-R score decreased as the CD-RISC-25 score increased (r, − 0.24; 95%CI [− 0.33 to − 0.14]; p < 0.0001; Additional File 3, Figure S1). Comparisons of CD-RISC-25 and IES-R scores obtained at 3 months vs. 12 months revealed no significant differences in the prevalence of resilient patients or in the prevalence of patients with PTSD (Additional File 4, Table S2).The median SF-36 PCS and MCS scores were 43.0 [34.0–51.0]) and 51.0 [40.0–57.0], respectively (Table 4). The MCS, PCS and seven of the eight sub-scores (the exception being role physical) were higher in resilient patients, indicating better quality of life compared to non-resilient patients.
Table 3.
Post-traumatic stress disorder symptoms and quality of life in the 382 included patients discharged alive from the intensive care unit
| Overall population N = 382 |
Resilient patientsc N = 203 |
Non-resilient patientsc N = 179 |
p value | |
|---|---|---|---|---|
| Post-traumatic stress disorder symptoms | ||||
| IES-R scorea, median [IQR] | 9.0 [4.0–19.0] | 7.0 [3.0–15.0] | 12.0 [5.0–24.0] | < 0.0001 |
| Intrusion | 4.0 [1.0–9.0] | 3.0 [1.0–8.0] | 5.0 [1.0–10.0] | 0.016 |
| Avoidance | 3.0 [1.0–7.0] | 2.0 [1.0–6.0] | 5.0 [1.0–10.0] | 0.0003 |
| Hyperarousal | 1.0 [0.0–4.0] | 1.0 [0.0–3.0] | 2.0 [0.0–6.0] | < 0.0001 |
| PTSD symptoms, n (%) | ||||
| None | 309 (81.3) | 177 (87.2) | 132 (74.6) | 0.004 |
| Moderate | 29 (7.6) | 13 (6.4) | 16 (9.0) | |
| Severe | 42 (11.1) | 13 (6.4) | 29 (16.4) | |
| Quality of life | ||||
| SF-36b, median [IQR] | ||||
| Bodily pain | 72.0 [41.0–100.0] | 84.0 [51.0–100.0] | 62.0 [41.0–100.0] | 0.006 |
| General health | 57.0 [45.0–72.0] | 67.0 [52.0–80.0] | 52.0 [40.0–62.0] | < 0.0001 |
| Mental health | 72.0 [56.0–84.0] | 76.0 [64.0–88.0] | 64.0 [48.0–80.0] | < 0.0001 |
| Physical functioning | 75.0 [45.0–95.0] | 80.0 [55.0–95.0] | 60.0 [35.0–90.0] | 0.002 |
| Role-emotional | 100.0 [0.0–100.0] | 100.0 [33.0–100.0] | 67.0 [0.0–100.0] | 0.0005 |
| Role-physical | 50.0 [0.0–100.0] | 50.0 [0.0–100.0] | 25.0 [0.0–100.0] | 0.32 |
| Vitality | 55.0 [40.0–70.0] | 60.0 [45.0–75.0] | 45.0 [30.0–60.0] | < 0.0001 |
| Social functioning | 100.0 [63.0–100.0] | 100.0 [63.0–100.0] | 94.0 [50.0–100.0] | 0.001 |
| PCS | 43.0 [34.0–51.0] | 44.0 [34.0–52.0] | 41.0 [33.0–49.0] | 0.026 |
| MCS | 51.0 [40.0–57.0] | 53.0 [45.0–59.0] | 48.0 [35.0–54.0] | < 0.0001 |
IES-R Impact of Event Scale-Revised, IQR interquartile range, PTSD post-traumatic stress disorder, SF-36 Short Form 36, PCS Physical Component Summary, MCS Mental Component Summary
aThe IES-R score can range from 0 to 88 (intrusion sub-score, 0–32; avoidance sub-score, 0–32; hyperarousal sub-score, 0–24). A higher score indicates greater symptom severity [23]. IES-R scores ≥ 33, 24–32, and ≤ 23 indicate severe, moderate and no PTSD, respectively
bSF-36 is a 36-item health-related quality-of-life questionnaire that produces two scores (PCS and MCS) and the eight sub-scores listed in the table, each of which can range from 0 to 100. Higher scores indicate better health-related quality of life
cResilient patients had CD-RISC-25 ≥ 68. Non-resilient patients had CD-RISC-25 ≤ 67
Table 4.
Social support and illness perception in the 382 included patients discharged alive from the intensive care unit
| Overall population N = 382 |
Resilient patientsc N = 203 |
Non-resilient patientsc N = 179 |
p value | |
|---|---|---|---|---|
| MSPSSa, median [IQR] | 75.0 [63.0–83.0] | 79.0 [69.0–84.0] | 71.0 [59.0–79.0] | < 0.0001 |
| Family | 27.0 [23.0–28.0] | 28.0 [25.0–28.0] | 25.0 [20.0–28.0] | < 0.0001 |
| Friends | 28.0 [24.0–28.0] | 25.0 [20.0–28.0] | 22.0 [13.0–28.0] | < 0.0001 |
| Significant other persons | 24.0 [17.0–28.0] | 28.0 [26.0–28.0] | 27.0 [23.0–28.0] | < 0.0001 |
| B-IPQb, median [IQR] | 37.0 [26.0–45.0] | 32.0 [23.0–42.0] | 40.0 [31.0–50.0] | < 0.0001 |
| Cognitive illness representation | 21.1 [15.0–28.0 | 20.0 [12.0–26.0] | 25.0 [18.0–31.0] | < 0.0001 |
| Emotional illness representation | 12.0 [8.0–15.0] | 10.0 [7.0–15.0] | 13.5 [10.0–16.0] | < 0.0001 |
| Illness comprehensibility | 2.0 [0.0–5.0] | 2.0 [0.0–3.0] | 2.0 [1.0–5.0] | 0.009 |
ICU intensive care unit, MSPSS Multidimensional Scale of Perceived Social Support, B-IPQ Brief Illness Perception Questionnaire, IQR interquartile range
aThe total MSPSS score can range from 12 to 84 and each sub-score from 4 to 28. Higher values indicate stronger social support
bThe total B-IPQ score can range from 0 to 80. The sub-scores for cognitive illness representation, emotional illness representation, and illness comprehensibility can range from 0 to 50, 0 to 20, and 0 to 10, respectively. A higher score reflects a more threatening perception of the illness
cResilient patients had CD-RISC-25 ≥ 68. Non-resilient patients had CD-RISC-25 ≤ 67
Factors associated with resilience
The median MSPSS and B-IPQ scores were 75.0 [63.0–83.0] and 37.0 [26.0–45.0], respectively (Table 4). Resilient patients had higher MSPSS score and sub-scores, indicating stronger perceived social support, and lower B-IPQ score and sub-scores, indicating more favourable perceptions of their illness, compared to non-resilient patients. By univariate analysis, resilient and non-resilient patients did not differ regarding baseline characteristics or ICU outcomes (Table 5). Both stronger perceived social support and a more favourable perception of illness were independently associated with resilience (MPSS: OR, 1.027; 95%CI 1.008–1.047; p = 0.005; B-IPQ: OR, 0.973; 95%CI 0.950–0.996; p = 0.02) (Additional File 5, Table S3).
Table 5.
Univariate analysis of demographic characteristics in resilient and non-resilient patients
| Resilient patientsa N = 203 |
Non-resilient patientsa N = 179 |
p value | |
|---|---|---|---|
| Age, years, mean ± SD | 60.9 ± 12.9 | 62.0 ± 11.9 | 0.63 |
| Males, n (%) | 137 (67.5) | 120 (67.0) | 0.92 |
| Marital status, n (%) | 0.97 | ||
| Single | 34 (18.9) | 30 (17.9) | |
| Married or living with a partner | 113 (62.8) | 107 (63.7) | |
| Divorced, separated, or widowed | 33 (18.3) | 31 (18.5) | |
| Employment status, n (%) | 0.46 | ||
| Employed | 89 (49.4) | 76 (45.5) | |
| Retired | 91 (50.6) | 91 (54.5) | |
| Children, n, median [IQR] | 2 [0; 3] | 2 [1; 3] | 0.57 |
| History of psychiatric disorder | 13 (21.0) | 4 (11.4) | 0.23 |
| McCabe score, n (%) | 0.87 | ||
| 0 (no fatal underlying disease) | 164 (80.8) | 148 (82.7) | |
| 1 (death expected within 5 years) | 33 (16.3) | 27 (15.1) | |
| 2 (death expected within 1 year) | 6 (3.0) | 4 (2.2) | |
| Pre-existing illness at ICU admission, n (%) | 114 (56.7) | 108 (60.7) | 0.43 |
| Chronic renal failure | 12 (6.0) | 9 (5.1) | 0.70 |
| Liver disease | 13 (6.5) | 15 (8.4) | 0.47 |
| Cardiovascular disease | 27 (13.5) | 28 (15.7) | 0.54 |
| Chronic respiratory failure | 12 (6.0) | 13 (7.3) | 0.59 |
| Neurologic disease | 18 (9.0) | 16 (9.0) | 0.99 |
| Cancer or immune deficiency | 43 (21.5) | 42 (23.6) | 0.63 |
| Oesophageal, gastric, or duodenal ulcer | 7 (3.5) | 12 (6.7) | 0.15 |
| Diabetes mellitus | 40 (19.9) | 33 (18.5) | 0.74 |
| Weight, kg, median [IQR] | 82.5 [71.0; 94.0] | 76.0 [65.0; 90.7] | 0.009 |
| BMI, kg/m2, median [IQR] | 28.1 [24.7; 33.1] | 26.8 [23.3; 31.2] | 0.02 |
| SAPS IIb, median [IQR] | 53.0 [43.0; 70.0] | 56.0 [44.0; 68.0] | 0.43 |
| SOFA scorec, median [IQR] | 10.0 [8.0; 12.0] | 10.0 [8.0; 12.0] | 0.85 |
| Medical diagnosis at admission, n (%) | 161 (79.3) | 140 (78.2) | 0.79 |
| Acute illness at ICU admission, n (%) | 0.57 | ||
| Cardiac arrest | 34 (16.7) | 25 (14.0) | |
| Acute heart failure | 36 (17.7) | 29 (16.2) | |
| Acute central nervous system failure | 9 (4.4) | 16 (8.9) | |
| Acute respiratory failure | 84 (41.4) | 73 (40.8) | |
| Trauma | 5 (2.5) | 3 (1.7) | |
| Miscellaneous | 35 (17.2) | 33 (18.4) | |
| Cause of shock, n (%) | 0.81 | ||
| Cardiac | 47 (23.2) | 37 (20.7) | |
| Sepsis | 109 (53.7) | 97 (54.2) | |
| Other | 47 (23.1) | 45 (25.1) | |
| Ongoing treatments at inclusion, n (%) | |||
| Randomised in the Low Group of NUTRIREA-3d | 97 (47.8) | 79 (44.1) | 0.47 |
| Prone position | 14 (6.9) | 7 (3.9) | 0.20 |
| Sedative agents | 187 (92.1) | 161 (89.9) | 0.46 |
| NMBA | 78 (38.4) | 61 (34.1) | 0.38 |
| Insulin | 76 (37.4) | 71 (39.7) | 0.65 |
| Anti-microbial treatmente | 177 (87.2) | 149 (83.2) | 0.28 |
| Dialysis | 12 (5.9) | 18 (10.1) | 0.13 |
| Outcomes | |||
| RRT during the ICU stay, n (%) | 36 (17.7) | 40 (22.3) | 0.26 |
| At least one complicationf during the ICU stay, n (%) | 24 (11.8) | 26 (14.5) | 0.43 |
| Duration of mechanical ventilation, days, median [IQR] | 5.0 [2.0; 10.0] | 6.0 [3.0; 12.0] | 0.32 |
| ICU length of stay, days, median [IQR] | 9.0 [5.0; 15.0] | 10.0 [6.0; 16.0] | 0.44 |
| Hospital length of stay, days, median [IQR] | 19.0 [12.0; 31.0] | 22.0 [13.0; 35.0] | 0.31 |
| MSPSS score | 79.0 [69.0; 84.0] | 71.0 [59.0; 79.0] | < 0.0001 |
| B-IPQ score | 32.0 [23.0; 42.0] | 40.0 [31.0; 50.0] | < 0.0001 |
ICU intensive care unit, BMI body mass index, SAPS II Simplified Acute Physiology Score version II, SOFA Sequential Organ Failure Assessment, NMBA neuromuscular blocking agent, RRT renal replacement therapy, MSPSS Multidimensional Scale of Perceived Social Support, B-IPQ Brief Illness Perception Questionnaire
aResilient patients were defined as having a CD-RISC-25 score ≥ 68 and non-resilient patients as having a CD-RISC-25 score ≤ 67
bSAPS II values can range from 0 (lowest level of critical illness) to 163 (most severe level of critical illness with 100% predicted mortality). A score of 50 predicts a 46.1% risk of death. The SAPS II was determined 24 h after ICU admission
cSOFA scores can range from 0 (no organ failure) to 24 (most severe level of multi-organ failure). The SOFA sub-score values at ICU admission are reported in eTable 1
dPatients included in the NUTRIREA-3 trial were randomised to early nutrition with either low or standard calorie-protein targets (6 kcal/kg/d and 0·2–0·4 g/kg/d, respectively; and 25 kcal/kg/d and 1·0–1·3 g/kg/d, respectively)
eAnti-microbial treatments included antibiotics, antiviral drugs, and antifungal drugs
fComplications included infections and gastro-intestinal complications acquired during the ICU stay
Discussion
In this large prospective study, half the survivors of severe critical illness had normal to high resilience. These resilient patients were less affected by PTSD symptoms and had higher health-related quality of life scores than did non-resilient patients. Resilience was associated neither with the characteristics of the acute illness nor with sex, age, or any other baseline variables. In contrast, stronger perceived social support and a more favourable perception of the illness were independently associated with resilience. Although causal interferences cannot strongly be assessed with our study design, these findings suggest interventions for increasing resilience with the goal of decreasing trauma-induced adverse responses and for improving quality of life in survivors of critical illness.
Critical illness is a traumatic event that can be followed by PTSD symptoms and quality-of-life impairments. Previous studies in non-critically ill patients have shown that interventions to improve resilience shortly after trauma exposure or in patients with PTSD may be useful to limit PTSD symptoms and improve quality of life [12]. A pilot randomised clinical trial showed that a brief six-session resilience-building programme started just before ICU discharge in survivors of critical neurological injury was feasible and associated with reduced symptoms of anxiety, depression, and PTSD three months later compared to minimally enhanced standard care in the patients and their informal caregivers [17]. This finding is important, as it supports the ability of resilience-building to prevent subsequent mental distress. The median IES-R score and proportion of patients with PTSD symptoms in our cohort are in agreement with earlier reports [1]. Importantly, PTSD symptoms were twice as common in the non-resilient group as in the resilient group, in keeping with a study in trauma patients [16]. Resilient patients also had higher quality of life scores as compared to non-resilient patients. Moreover, given that 47% of patients had low resilience, these findings suggest that improving resilience may be associated with a reduction in PTSD and improved quality of life in survivors of critical illness.
Resilience was not associated neither with the characteristics of the acute illness nor with the baseline features of the patients. This finding is consistent with a previous study in trauma patients [16]. In contrast the independent associations with social support and illness perception suggest avenues for intervention. The effect size seems small in our study, but others have also reported associations linking mental health, social support and resilience [16, 31]. Moreover perception of illness was associated with psychological well-being and improved coping [32–34]. A highly positive perception of illness was associated with greater treatment adherence and improved recovery and mental health [35, 36]. Illness perception is probably amenable to modification via psychological support [37]. Programmes have been developed in settings other than critical illness to increase the perception of control over the disease [38]. In a randomised controlled trial in patients with heart failure, an educational programme was effective in improving illness perception and was also associated with better results on measures of quality of life and self-care [39]. Improving illness perception resulted in better outcomes of patients with chronic low back pain in another randomised controlled trial [40]. Illness perception seems readily amenable to change via simple interventions, which thus deserve to be investigated in survivors of critical illness [41]. In the ICU, research is needed to determine whether assessing illness perception may help intensivists to apply communication techniques likely to improve their patients’ understanding of their illness and experience in the ICU. Finally, our study suggests that interventions focused on promoting resilience may be more effective than targeting specific disorders in ICU survivors. Qualitative studies showed that patients experienced difficulties in accessing the appropriate care for post-ICU syndrome, obtaining information about their post-resuscitation symptoms, and understanding what they had experienced in the ICU [42, 43]. Our study showing high levels of PTSD symptoms supports the development of post-ICU follow-up visits and the routine provision of psychologist support after ICU discharge. Assessment of social support and illness perception may allow multidisciplinary interventions aimed at improving resilience, thereby alleviating PTSD symptoms and improving quality of life [44].
Our study has several limitations. First, that 31% of patients eligible for the study could not be interviewed may have introduced selection bias. However, we evidenced no significant differences between these patients and those who were interviewed. Moreover, to our knowledge, RESIREA is the largest study to date of resilience after critical illness. Second, logistical issues prevented us from performing two interviews, three months and one year after ICU discharge, in all patients. Also, we had no data on resilience or PTSD symptoms before the critical illness. We were consequently unable to assess possible changes in these two variables over time in individual patients. However, data collected at 3 and at 12 months did not differ significantly regarding the proportions of resilient patients or of patients with PTSD symptoms. These findings align with a scoping review showing no clinically significant changes in mental-health symptoms over the first year after ICU discharge, also with no pre-illness data [18]. Using both 3-month and 12-month data in our study was therefore legitimate and cannot have affected the results, their interpretation, or our conclusion. Importantly, the 53.1% proportion of resilient patients was within the previously reported range (28–67%) in critical-care settings [18]. Third, all 41 participating ICUs were in France. This may limit the general applicability of our findings. Nonetheless, the strengths of our study include the prospective design, multicentre recruitment providing a large sample size, and use of the CD-RISC-25 to assess resilience. This tool has well-validated psychometric properties [18, 21]. Also, the data were collected by psychologists trained in telephone interviewing and in administration of the five questionnaires used for the study. Questionnaires data were missing for less than 2% of patients. These facts support the reliability and general applicability of our data. Fourth, the observational design of the study precludes definitive conclusions about causal relationships between resilience and PTSD in our cohort. Measurements at baseline then repeatedly during follow-up would be ideal to assess causality. Nonetheless, our findings generate strong hypotheses for designing interventional trials aimed at enhancing resilience and thereby possibly mitigating PTSD and improving quality of life. Last, we focussed on patients who survived an episode of severe critical illness requiring at least invasive mechanical ventilation and vasoactive drugs. We thus studied a uniform population of patients at high risk for long-term mental-health disturbances and, therefore, likely to benefit the most from strategies designed to improve resilience.
Conclusions
Among survivors of severe critical illness, those with normal or high resilience were less affected by PTSD symptoms and had higher health-related quality-of-life scores. Greater resilience was independently associated with stronger social support and a more favourable perception of the illness but not with the characteristics of the acute illness or the baseline variables. Attention to social support and illness perception may help to strengthen resilience with the goal of improving the psychological outcomes of ICU survivors. `
Supplementary Information
Acknowledgements
We are indebted to A. Wolfe, MD, for assistance in preparing and reviewing the manuscript; Carine Coffre and Frédérique Musset for managing the database; and Diane Maugars and Manon Rouaud for coordinating the study. We are grateful to the medical staff, nurses, and research nurses at the 41 participating centres for their valuable contribution to the successful conduct of the study.
Abbreviations
- PTSD
Post-traumatic stress disorder
- ICU
Intensive Care Unit
- CD-RISC-25
Connor-Davidson Resilience Scale
- IES-R
Impact of Event-Revised scale
- SF-36
Short Form-36
- PCS
Physical component summary
- MCS
Mental component summary
- MSPSS
Multidimensional Scale of Perceived Social Support
- B-IPQ
Brief Illness Perception Questionnaire
Author contributions
AM, AL, JR, JBL and ALG designed the study. AM, JR, GP, JPM, LA, PA, JB, NVB, HNB, DC, LC, CC, MD, AD, JD, LMD, OG, SG, YH, SJ, FL, BM, JM, OM, VM, EM, MAN, SN, GP, JPQ, AR, JPR, FS, MS, BS, FT, DT, NTR, FT, PT, IV, CV, JBL, and AL approved the study design, coordinated individual sites, participated in the inclusion of study participants, and collected the data. JR and ALG directly accessed and verified all the data reported in the manuscript. ALG performed the statistical analysis. AM wrote the first draft of the manuscript with input from JR and AL. All authors had full access to the study data, revised the manuscript for important intellectual content, and read and approved the final version before submission. All authors accept responsibility for submitting the final manuscript for publication.
Funding
The RESIREA study and the NUTRIREA-3 study were supported by the Nantes University Hospital. The NUTRIREA-3 study was funded by a 2017 Programme Hospitalier de Recherche Clinique National grant from the French Ministry of Health (#PHRC-17-0213).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
RESIREA was a planned study conducted in parallel with the randomised controlled multicentre NUTRIREA-3 trial, which was approved by the competent ethics committee (Comité de Protection des Personnes Sud-Méditerranée 2, #2018-A00424-51). The RESIREA study protocol was approved by the ethics committee of the French Intensive Care Society (CE SRLF 18–19). Specific informed consent for inclusion in the RESIREA study was obtained from the patients, or from their next of kin in patients unable to consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests related to this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jean Reignier, Email: jean.reignier@chu-nantes.fr.
Alexandra Laurent, Email: alexandra.laurent@u-bourgogne.fr.
References
- 1.Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis*. Crit Care Med. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 2.Boer KR, Mahler CW, Unlu C, Lamme B, Vroom MB, Sprangers MA, et al. Long-term prevalence of post-traumatic stress disorder symptoms in patients after secondary peritonitis. Crit Care. 2007;11(1):R30. doi: 10.1186/cc5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuthbertson BH, Hull A, Strachan M, Scott J. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensiv Care Med. 2004;30(3):450–455. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- 4.Svenningsen H, Langhorn L, Ågård AS, Dreyer P. Post-ICU symptoms, consequences, and follow-up: an integrative review: review post-ICU. Nurs Crit Care. 2017;22(4):212–220. doi: 10.1111/nicc.12165. [DOI] [PubMed] [Google Scholar]
- 5.Yehuda R. 011002 Post-Traumatic Stress Disorder. N Engl J Med. [DOI] [PubMed]
- 6.Bienvenu OJ, Friedman LA, Colantuoni E, Dinglas VD, Sepulveda KA, Mendez-Tellez P, et al. Psychiatric symptoms after acute respiratory distress syndrome: a 5-year longitudinal study. Intensiv Care Med. 2018;44(1):38–47. doi: 10.1007/s00134-017-5009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, Yehuda R. Resilience definitions, theory, and challenges: interdisciplinary perspectives. Eur J Psychotraumatol. 2014;5(1):25338. doi: 10.3402/ejpt.v5.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayordomo T, Viguer P, Sales A, Satorres E, Meléndez JC. Resilience and coping as predictors of well-being in adults. J Psychol. 2016;150(7):809–821. doi: 10.1080/00223980.2016.1203276. [DOI] [PubMed] [Google Scholar]
- 9.Ye ZJ, Qiu HZ, Li PF, Liang MZ, Zhu YF, Zeng Z, et al. Predicting changes in quality of life and emotional distress in Chinese patients with lung, gastric, and colon-rectal cancer diagnoses: the role of psychological resilience: predicting changes in emotional distress and quality of life. Psychooncology. 2017;26(6):829–835. doi: 10.1002/pon.4237. [DOI] [PubMed] [Google Scholar]
- 10.Ye ZJ, Liang MZ, Qiu HZ, Liu ML, Hu GY, Zhu YF, et al. Effect of a multidiscipline mentor-based program, Be Resilient to Breast Cancer (BRBC), on female breast cancer survivors in mainland China—a randomized, controlled, theoretically-derived intervention trial. Breast Cancer Res Treat. 2016;158(3):509–522. doi: 10.1007/s10549-016-3881-1. [DOI] [PubMed] [Google Scholar]
- 11.Sihvola S, Kuosmanen L, Kvist T. Resilience and related factors in colorectal cancer patients: a systematic review. Eur J Oncol Nurs. 2022;56:102079. doi: 10.1016/j.ejon.2021.102079. [DOI] [PubMed] [Google Scholar]
- 12.Horn SR, Charney DS, Feder A. Understanding resilience: new approaches for preventing and treating PTSD. Exp Neurol. 2016;284:119–132. doi: 10.1016/j.expneurol.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Macía P, Barranco M, Gorbeña S, Iraurgi I. Expression of resilience, coping and quality of life in people with cancer. Frey R, editor. PLoS ONE. 2020;15(7):e0236572. [DOI] [PMC free article] [PubMed]
- 14.Chiu HC, Lin CY, Kuo YL, Hou WL, Shu BC. Resilience among women with breast cancer surviving longer than five years: the relationship with illness perception and body image. Eur J Oncol Nurs. 2023;62:102254. doi: 10.1016/j.ejon.2022.102254. [DOI] [PubMed] [Google Scholar]
- 15.Stewart DE, Yuen T. A systematic review of resilience in the physically Ill. Psychosomatics. 2011;52(3):199–209. doi: 10.1016/j.psym.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 16.Nehra D, Herrera-Escobar JP, Al Rafai SS, Havens J, Askari R, Nitzschke S, et al. Resilience and long-term outcomes after trauma: an opportunity for early intervention? J Trauma Acute Care Surg. 2019;87(4):782–789. doi: 10.1097/TA.0000000000002442. [DOI] [PubMed] [Google Scholar]
- 17.Vranceanu AM, Bannon S, Mace R, Lester E, Meyers E, Gates M, et al. Feasibility and efficacy of a resiliency intervention for the prevention of chronic emotional distress among survivor-caregiver dyads admitted to the neuroscience intensive care unit: a randomized clinical trial. JAMA Netw Open. 2020;3(10):e2020807. doi: 10.1001/jamanetworkopen.2020.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pauley E, Walsh TS. Resilience in survivors of critical illness: a scoping review of the published literature in relation to definitions, prevalence, and relationship to clinical outcomes. J Intensiv Care Soc. 2022;23(3):345–358. doi: 10.1177/17511437211034701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reignier J, Le Gouge A, Lascarrou JB, Annane D, Argaud L, Hourmant Y, et al. Impact of early low-calorie low-protein versus standard-calorie standard-protein feeding on outcomes of ventilated adults with shock: design and conduct of a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3) BMJ Open. 2021;11(5):e045041. doi: 10.1136/bmjopen-2020-045041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reignier J, Plantefeve G, Mira JP, Argaud L, Asfar P, Aissaoui N, et al. Low versus standard calorie and protein feeding in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3). Lancet Respir Med. 2023;S2213260023000929. [DOI] [PubMed]
- 21.Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depress Anxiety. 2003;18(2):76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 22.Maley JH, Brewster I, Mayoral I, Siruckova R, Adams S, McGraw KA, et al. Resilience in survivors of critical illness in the context of the survivors’ experience and recovery. Ann Am Thorac Soc. 2016;13(8):1351–1360. doi: 10.1513/AnnalsATS.201511-782OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rash CJ, Coffey SF, Baschnagel JS, Drobes DJ, Saladin ME. Psychometric properties of the IES-R in traumatized substance dependent individuals with and without PTSD. Addict Behav. 2008;33(8):1039–1047. doi: 10.1016/j.addbeh.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creamer M, Bell R, Failla S. Psychometric properties of the impact of event scale—revised. Behav Res Ther. 2003;41(12):1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Chrispin PS, Scotton H, Rogers J, Lloyd D, Ridley SA. Short Form 36 in the intensive care unit: assessment of acceptability, reliability and validity of the questionnaire. Anaesthesia. 1997;52(1):15–23. doi: 10.1111/j.1365-2044.1997.015-az014.x. [DOI] [PubMed] [Google Scholar]
- 27.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. J Pers Assess. 1988;52(1):30–41. doi: 10.1207/s15327752jpa5201_2. [DOI] [PubMed] [Google Scholar]
- 28.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Le Gall JR. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 30.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensiv Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 31.Eakman AM, Schelly C, Henry KL. Protective and vulnerability factors contributing to resilience in post-9/11 veterans with service-related injuries in postsecondary education. Am J Occup Ther. 2016;70(1):7001260010p1–10. [DOI] [PubMed]
- 32.Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cogn Ther Res. 1992;16(2):143–163. doi: 10.1007/BF01173486. [DOI] [Google Scholar]
- 33.Leventhal H, Phillips LA, Burns E. The Common-Sense Model of Self-Regulation (CSM): a dynamic framework for understanding illness self-management. J Behav Med. 2016;39(6):935–946. doi: 10.1007/s10865-016-9782-2. [DOI] [PubMed] [Google Scholar]
- 34.Lazarus RS, Folkman S. The concept of coping. New York, NY, US: Columbia University Press; 1991. 189 p. (Stress and coping: An anthology, 3rd ed).
- 35.Hagger MS, Orbell S. Illness representations and emotion in people with abnormal screening results. Psychol Health. 2006;21(2):183–209. doi: 10.1080/14768320500223339. [DOI] [PubMed] [Google Scholar]
- 36.Aujla N, Walker M, Vedhara K, Sprigg N. The relationship between patients’ illness beliefs and recovery after stroke. Psychol Health Med. 2019;24(5):551–558. doi: 10.1080/13548506.2018.1557712. [DOI] [PubMed] [Google Scholar]
- 37.Keogh KM, White P, Smith SM, McGilloway S, O’Dowd T, Gibney J. Changing illness perceptions in patients with poorly controlled type 2 diabetes, a randomised controlled trial of a family-based intervention: protocol and pilot study. BMC Fam Pract. 2007;8(1):36. doi: 10.1186/1471-2296-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skinner TC, Khunti K, Carey ME, Dallosso H, Heller S, Davies MJ. Stability and predictive utility, over 3 years, of the illness beliefs of individuals recently diagnosed with Type 2 diabetes mellitus. Diabet Med. 2014;31(10):1260–1263. doi: 10.1111/dme.12484. [DOI] [PubMed] [Google Scholar]
- 39.Sadeghi Akbari A, Cheraghi MA, Kazemnejad A, Nomali M, Zakerimoghadam M. Effect of illness perception correction—based educational program on quality of life and self- care in patients with heart failure: a randomized controlled trial. J Caring Sci. 2019;8(2):89–93. doi: 10.15171/jcs.2019.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siemonsma PC, Stuive I, Roorda LD, Vollebregt JA, Walker MF, Lankhorst GJ, et al. Cognitive treatment of illness perceptions in patients with chronic low back pain: a randomized controlled trial. Phys Ther. 2013;93(4):435–448. doi: 10.2522/ptj.20110150. [DOI] [PubMed] [Google Scholar]
- 41.Dempster M, Howell D, McCorry NK. Illness perceptions and coping in physical health conditions: A meta-analysis. J Psychosom Res. 2015;79(6):506–513. doi: 10.1016/j.jpsychores.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Brown SM, Bose S, Banner-Goodspeed V, Beesley SJ, Dinglas VD, Hopkins RO, et al. Approaches to addressing post-intensive care syndrome among intensive care unit survivors. A narrative review. Ann Am Thorac Soc. 2019;16(8):947–956. doi: 10.1513/AnnalsATS.201812-913FR. [DOI] [PubMed] [Google Scholar]
- 43.Rousseau AF, Prescott HC, Brett SJ, Weiss B, Azoulay E, Creteur J, et al. Long-term outcomes after critical illness: recent insights. Crit Care. 2021;25(1):108. doi: 10.1186/s13054-021-03535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ungar M, Theron L. Resilience and mental health: how multisystemic processes contribute to positive outcomes. Lancet Psychiatry. 2020;7(5):441–448. doi: 10.1016/S2215-0366(19)30434-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.