Abstract
Treatment of hepatitis B virus carriers with the nucleoside analog lamivudine suppresses virus replication. However, rather than completely eliminating the virus, long-term treatment often ends in the outgrowth of drug-resistant variants. Using woodchucks chronically infected with woodchuck hepatitis virus (WHV), we investigated the consequences of combining lamivudine treatment with immunotherapy mediated by an adenovirus superinfection. Eight infected woodchucks were treated with lamivudine and four were infected with ∼1013 particles of an adenovirus type 5 vector expressing β-galactosidase. Serum samples and liver biopsies collected following the combination therapy revealed a 10- to 20-fold reduction in DNA replication intermediates in three of four woodchucks at 2 weeks after adenovirus infection. At the same time, covalently closed circular DNA (cccDNA) and viral mRNA levels both declined about two- to threefold in those woodchucks, while mRNA levels for gamma interferon and tumor necrosis factor alpha as well as for the T-cell markers CD4 and CD8 were elevated about twofold. Recovery from adenovirus infection was marked by elevation of sorbitol dehydrogenase, a marker for hepatocyte necrosis, as well as an 8- to 10-fold increase in expression of proliferating cell nuclear antigen, a marker for DNA synthesis, indicating significant hepatocyte turnover. The fact that replicative DNA levels declined more than cccDNA and mRNA levels following adenovirus infection suggests that the former decline either was cytokine induced or reflects instability of replicative DNA in regenerating hepatocytes. Virus titers in all four woodchucks were only transiently suppressed, suggesting that the effect of combination therapy is transient and, at least under the conditions used, does not cure chronic WHV infections.
Hepadnaviruses have a ∼3-kbp relaxed circular DNA genome. Following infection of hepatocytes, this DNA is transported to the nucleus and converted to a covalently closed form (cccDNA) that serves as a transcriptional template. Other steps of virus replication take place in the cytoplasm. Viral DNA is synthesized within nucleocapsids via reverse transcription of a viral RNA known as the pregenome (26). Nucleocapsids containing mature forms of viral DNA are packaged into viral envelopes and secreted from the cell. cccDNA does not replicate (30), but additional copies (up to ∼50 per cell) may be formed from the viral DNA synthesized in the cytoplasm (26). The formation of cccDNA is inhibited by viral envelope proteins (27).
It appears that virus reproduction, and release into the bloodstream, is noncytopathic. Thus, whether the host is transiently or chronically infected depends on the strength of the cellular immune response to infected hepatocytes. Studies of transient hepadnavirus infections in chimpanzees (2, 3, 13, 16), woodchucks (19, 22), and ducks (18) lead to the conclusion that virus can be cleared even after infection of essentially the entire hepatocyte population. The clearance phase appears to be less than 4 weeks in duration. The mechanism(s) of clearance is uncertain.
Experiments with hepatitis B virus (HBV)-transgenic mice support the possibility that hepadnavirus replication intermediates may be cleared by noncytolytic processes (6, 7, 10–12), not just by the destruction of infected hepatocytes. These reports show that loss of viral proteins, DNA replication intermediates, and mRNAs from the liver is induced by cytokines that are elaborated during an inflammatory response in the liver. It is not yet known if cccDNA is eliminated by cytokines, though data from a recent study of the recovery phase of HBV infection of chimpanzees are consistent with such a possibility (13).
In the present study, experiments were carried out to address two issues. First, might cytokines induce a direct, noncytolytic loss of viral nucleic acids during a natural hepadnavirus infection that, in combination with lamivudine therapy, would lead to recovery from a chronic infection? Second, will immunotherapy, possibly through repression of wild-type virus present in the liver, hasten the rebound in virus titers associated with emergence of lamivudine-resistant virus? In particular, we examined the consequences of infection with an unrelated virus on woodchuck hepatitis virus (WHV) in woodchucks chronically infected with WHV. Our results showed that suppression of WHV replication in adenovirus-inoculated woodchucks persisted several months longer than in the woodchucks receiving lamivudine. That is, either directly or indirectly, adenovirus infection enhanced the suppression of WHV that was associated with the lamivudine therapy. Adenovirus infection did not, in this system, enhance the emergence of drug-resistant strains of WHV.
MATERIALS AND METHODS
Woodchucks.
Adult woodchucks (Marmota monax) chronically infected with WHV were acquired from Northeastern Wildlife (South Plymouth, N.Y.) and housed in the Laboratory Animal Facility of the Fox Chase Cancer Center. All experiments carried out with these woodchucks were reviewed and approved by the center's Institutional Animal Care and Use Committee. Lamivudine was orally administered daily to woodchucks in Dyets liquid diet at a dosage of 200 mg per kg of body weight as previously described (21). Where indicated, woodchucks were inoculated intravenously with 5 × 1011 PFU (∼1013 virus particles) of a replication-defective, recombinant adenovirus vector in 200 μl of phosphate-buffered saline (PBS). Control woodchucks received PBS alone. The adenovirus, expressing β-galactosidase under control of a cytomegalovirus immediate-early (CMV IE) promoter (4), was grown in the permissive 293 cell line and purified by isopycnic centrifugation in CsCl density gradients (1). The titer of the purified virus was determined by plaque assay on the same cell line. Serum and liver biopsy samples were collected at the indicated time points, as previously described (19), and stored at −80°C until use.
Nucleic acid analyses.
Viral titers were determined by Southern blot assay for virion DNA. Briefly, 50 μl of woodchuck serum was layered on top of a 10 to 20% sucrose step gradient containing 0.15 M NaCl–20 mM Tris-HCl (pH 7.5). Virus was pelleted by centrifugation for 3 h at 50,000 rpm and 4°C in a Beckman SW60 rotor. The viral pellet was digested in 30 μl of 0.01 M Tris-HCl (pH 7.4)–0.01 M EDTA, 0.2% (wt/vol) sodium dodecyl sulfate (SDS)–pronase (1 mg/ml) for 1 h at 37°C. The mixture was then electrophoresed on a 1.5% agarose gel and subsequently transferred to a nitrocellulose membrane. A 32P-labeled DNA probe representing the complete WHV genome was used for hybridization. Signals were quantified using a Fuji phosphorimager, and virus titers were estimated by comparison to a WHV DNA hybridization standard.
For extraction of intracellular viral DNAs, liver biopsy samples were disrupted, using a Dounce homogenizer with a loose-fitting pestle, in 1.5 ml of 0.01 M Tris-HCl (pH 7.5)–0.01 M EDTA. Half of the homogenate was used for extraction of replicative intermediate DNA, and half was used for cccDNA preparation. For extraction of replicative intermediates, the homogenate was adjusted to a total volume of 6 ml with 0.025 M Tris-HCl (pH 7.4)–0.01 M EDTA–0.25% (wt/vol) SDS–0.05 M NaCl–pronase (2 mg/ml). After a 1-h incubation at 37°C, nucleic acids were extracted with a 1:1 mixture of phenol-chloroform and collected by ethanol precipitation. For cccDNA extraction, the volume of the remainder of the homogenate was adjusted to 3 ml with 0.01 M Tris-HCl (pH 7.5)–0.01 M EDTA; 200 μl of 10% (wt/vol) SDS was then added, the mixture was briefly vortexed, and 1 ml of 2.5 M KCl was added. The mixture was incubated at room temperature for 20 min, and potassium dodecyl sulfate-protein complexes were then collected by centrifugation at 10,000 rpm for 20 min in a Beckman SS34 rotor at 4°C. The supernatant was subjected to phenol-chloroform extraction, and the DNA was precipitated with ethanol overnight at room temperature. Southern blot analyses were carried out as described above. To assay for WHV replicative intermediate DNAs, 2.5 μg of total cell DNA was loaded; for cccDNA, each lane contained DNA extracted from 5 × 105 cells, as determined by counting, in a hemocytometer, fluorescent nuclei in the initial cell homogenates following staining with ethidium bromide.
RNase protection assay.
Total RNA was extracted from liver as described below. Antisense RNA probes for woodchuck gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), CD4, CD8, and glyceraldehyde-3-phosphate dehydrogenase were prepared as described by Guo et al. (14). Using the Riboprobe Gemini II system (Promega, Madison, Wis.), probe synthesis was carried out in a 20-μl volume of transcription reaction mixture containing 60 μCi of 12 μM [α-32P]UTP, 500 μM each GTP, ATP, and CTP, 10 mM dithiothreitol, 1× transcription optimized buffer, 24 U of RNasin, 20 U of SP6 RNA polymerase, and 1 μg of linearized plasmid DNA template. The mixture was incubated at 39°C for 1 h; 2 U of RNase-free DNase I (RQ1; Promega) was then added, and incubation was continued for 15 min at 37°C. RNA was precipitated by addition of 2.5 volumes of ethanol at −20°C. The RNA pellet was dissolved in 30 μl of gel loading buffer containing 90% formamide, 0.025% xylene cyanol, 0.025% bromophenol blue, 0.025% SDS, and 0.5 mM EDTA, heated 3 min at 95°C, and loaded on a 0.75-mm-thick 5% acrylamide gel containing 8 M urea (15). After electrophoresis, the region containing the full-length probe was excised from the gel and eluted with 400 μl of elution buffer (500 mM ammonium acetate 1 mM EDTA, 0.2% SDS) at 37°C for 3 to 5 h.
For hybridization, an aliquot containing 2 × 105 cpm of each probe was mixed with 10 μg of total liver RNA or yeast RNA, and ammonium acetate was added to a final concentration of 0.5 M. The RNA was precipitated by addition of 2.5 volumes of ethanol at −20°C for 30 min and then collected by centrifugation for 15 min at 4°C. The RNA pellet was dried for 5 min at room temperature and dissolved in 10 μl of hybridization buffer (80% formamide, 100 mM sodium citrate, 300 mM sodium acetate, 1 mM EDTA [pH 6.4]), heated to 95°C for 5 min, and hybridized at 45°C overnight; 100 μl of RNase digestion solution (10 mM Tris-HCl, 0.3 M NaCl, 5 mM EDTA, RNase A [40 ng/ml], RNase T1 [500 U/ml] [pH 7.5]) was then added. After incubation for 30 min at 37°C, 20 μl of a solution containing 3.5% (wt/vol) SDS, tRNA (100 μg/ml), and proteinase K (0.5 mg/ml) was added, followed by incubation at 37°C for 30 min. The remaining RNA was extracted once with an equal volume of phenol-chloroform and precipitated by addition of 2.5 volumes of ethanol. The RNA pellet was dissolved in 10 μl of gel loading buffer and subjected to electrophoresis through a 5% polyacrylamide gel containing 8 M urea (15).
Northern blot assays.
Total RNA was extracted from frozen tissues using Trizol reagent as specified by the manufacturer (Life Technologies, Inc.). Briefly, 10 to 30 mg of liver tissue was homogenized in 1 ml of Trizol reagent; 0.2 ml of chloroform was added to the homogenate, which was then vortexed and centrifuged at 12,000 × g for 15 min at 4°C. RNA was precipitated from the aqueous phase by addition of 0.5 ml of isopropanol, and the RNA was collected by centrifugation at 12,000 × g for 10 min at 4°C. The RNA pellet was washed with 75% ethanol and dissolved in 20 μl of H2O. Ten micrograms of total RNA was treated with glyoxyal-dimethyl sulfoxide solution in 2 mM sodium phosphate (pH 6.8), 1 mM EDTA, 25% dimethyl sulfoxide, and 0.5 M glyoxyal (5). The mixture was incubated at 50°C for 20 min and chilled on ice. The RNA was subjected to electrophoresis into a 1% agarose gel containing 10 mM sodium phosphate (pH 6.8) and 0.01 mM aurintricarboxylic acid. Samples were transferred to a nylon membrane (Hybond-N) in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The membrane was baked at 80°C for 2 h, and the RNA was deglyoxyalated in boiling 20 mM Tris-HCl (pH 8.0). Hybridization was carried out at 42°C using 5′-3′ reagents as specified by the manufacturer (5 Prime→3 Prime, Inc.) The filters were hybridized with a 32P-labeled DNA probe representing the complete WHV genome, and signals were quantified with a Fuji phosphorimager.
Immunohistochemistry.
To assay for β-galactosidase using a specific antibody, frozen liver tissues were first fixed in 2% formaldehyde–PBS for 10 min at room temperature. After a brief rinse with PBS, the tissue sections were further fixed in cold 100% methanol for 10 min at −20°C. After washing with PBS, the samples were washed two times with 0.25% Triton X-100–PBS for 5 min each at room temperature. The following immunostaining was done using Dako LSAB kits as specified by the manufacturer's description (DAKO, Inc., Carpinteria, Calif.). Briefly, the fixed liver section was incubated with 3% H2O2 for 10 min to deplete endogenous peroxidase. After application of the blocking reagent, the tissues were incubated with monoclonal anti-β-galactosidase antibody (Sigma Chemical Co.) at a dilution of 1:500. Biotinylated anti-mouse immunoglobulins and streptavidin-conjugated peroxidase were subsequently applied, and reactions were visualized using the chromogen 3-amino-9-ethylcarbazole. Tissues were counterstained with hematoxylin and mounted in Gel/Mount aqueous mounting medium (Biomeda, Inc., Foster City, Calif.). In an early study (Table 1), β-galactosidase activity was measured directly (32).
TABLE 1.
Woodchuck liver is susceptible to infection by a human adenovirusa
| Woodchuck | Time (days) | Infected hepatocytes (%) | Total DNA (copies/hepatocyte) | cccDNA (copies/hepatocyte) |
|---|---|---|---|---|
| 320 | 3 | 80 | 450 | 12.1 |
| 31 | <1 | 50 | 1.2 | |
| 321 | 3 | 70 | 370 | 17.3 |
| 31 | <1 | 190 | 4.1 |
WHV carrier woodchucks were infected with an adenovirus vector that directs expresison of β-galactosidase from a CMV IE promoter. Liver biopsies were taken at 3 and 31 days postinfection. The percentage of adenovirus-infected hepatocytes was estimated by assaying for β-galactosidase activity on frozen sections as described by Yang et al. (32). Copy numbers for replicating viral DNA and cccDNA were determined as described in Materials and Methods and are based on the assumption that the liver is ca. 70% hepatocytes and that the DNA content of a diploid woodchuck cell is 5 pg.
Immunoperoxidase assays for detection of WHV core antigen and proliferating cell nuclear antigen (PCNA) were carried out on acetic acid-ethanol-fixed and paraffin-embedded tissues as previously described (21). Woodchuck CD3 was detected using a rabbit polyclonal antibody raised against human CD3 epsilon chain (DAKO). Sections were counterstained with hematoxylin, dehydrated in ethanol, and mounted in Permount.
Serum SDH.
Sorbitol dehydrogenase (SDH) assays on woodchuck serum samples stored at −80°C were carried out by AniLytics, Inc., Gaithersburg, Md. Results are reported as international units per liter. The normal SDH level for uninfected woodchucks is generally below 20 IU/liter (17).
RESULTS
Adenovirus infection suppresses WHV expression in chronically infected woodchucks.
Infection of livers of HBV transgenic mice with a replication-defective adenovirus vector leads to a transient loss of HBV proteins and replicating HBV DNAs. This process is apparently induced by the cytokines produced during the inflammatory response to the adenovirus infection (7) and is referred to as a bystander effect. A preliminary study was carried out to determine if this same phenomenon would occur following adenovirus infection of a host that had a chronic hepadnavirus infection. Two woodchucks chronically infected with WHV were inoculated i.v. with an adenovirus vector that expressed β-galactosidase from a CMV IE promoter, and assays were carried out to determine if the vector infected the liver and altered WHV expression. As summarized in Table 1, β-galactosidase activity was readily detected in hepatocytes at 3 days postinoculation. Examination of liver tissue sections suggested that up to 80% of the hepatocytes may have been infected by the adenovirus vector. β-Galactosidase activity was no longer detectable at 31 days.
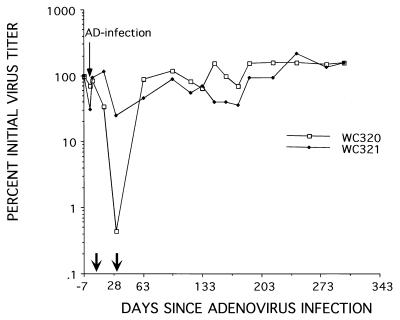
Adenovirus infection and the subsequent host response thereto were associated with a partial loss of replicative intermediate and WHV cccDNAs from the liver (Table 1). In woodchuck 320, viral DNA levels dropped approximately 10-fold, compared with a 2- to 4-fold reduction observed in woodchuck 321. Consistent with these observations, a transient suppression in viremia was detected in woodchuck 320 in a serum sample taken at the same time as the 31-day liver biopsy (Fig. 1). However, there was no long-term effect on WHV production, as revealed by assays for virus in serum samples collected over the next 315 days. Thus, suppression of WHV replication was short term and coincided with the time for recovery from the adenovirus infection.
FIG. 1.
Adenovirus infection causes a transient suppression of WHV viremia. Chronically infected woodchucks 320 and 321 were inoculated i.v. with ∼5 × 1011 PFU of the adenovirus vector. WHV titers in serum were determined by Southern blot assays for viral DNA. All titers are normalized to the titer at the time of adenovirus infection (ca. 109 per ml of serum). Arrows along the abscissa show the timing of liver biopsies, described further in the footnote to Table 1.
These observations are consistent with results obtained with HBV-infected chimpanzees that were challenged with hepatitis A or D virus (28, 29). These animals also exhibited a temporary suppression of HBV replication that ceased following resolution of the superinfection. The mechanism contributing to the loss of cccDNA and replicative intermediates from the liver was not identified in this and previous studies. The losses of cccDNA and replicative intermediates detected at 31 days (Table 1) might be explained by cytotoxic T-lymphocyte killing of the adenovirus-infected hepatocytes and/or by a bystander effect as described for HBV-transgenic mice infected with adenovirus (7). Moreover, because no other inhibitor was present, it was possible that a rebound in one or both types of WHV DNA might have been under way when the 31-day sample was collected. To further explore the possible role of bystander effects on intracellular WHV DNAs following adenovirus infection, as well as their implications for antiviral therapy, we examined the consequences of adenovirus infection in woodchucks receiving lamivudine.
Adenovirus infection of woodchucks receiving lamivudine leads to a differential loss of WHV DNA replication intermediates.
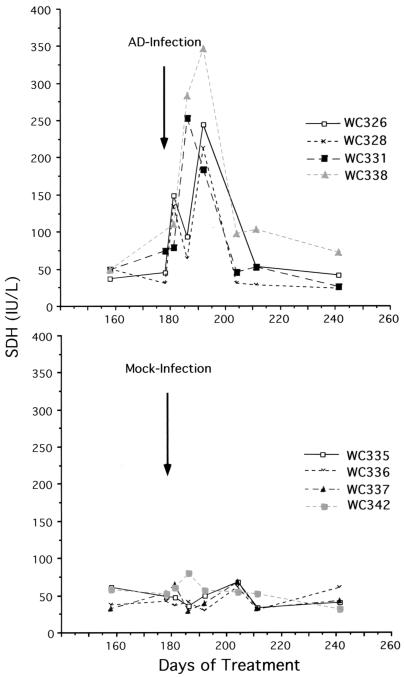
We previously reported that lamivudine administration to woodchucks chronically infected with WHV leads to a gradual decline in viremia and a partial reduction of DNA replication intermediates in the liver (21). In the present study, we treated woodchucks with lamivudine for 6 months to depress virus replication and then infected them with the adenovirus vector described above. Infection was followed by a transient elevation of SDH levels in the serum, an indicator of hepatocyte necrosis (17) (Fig. 2). This elevation was detected as early as 3 days postinfection, when nearly all or a major fraction of hepatocytes expressed the adenovirus-encoded β-galactosidase, and increased during the clearance phase of the virus until 26 days postinfection, when it was no longer evident. By the latter time point, β-galactosidase expression was no longer detected in the livers of three of the four woodchucks examined (Table 2).
FIG. 2.
Adenovirus inoculation was followed by transient elevation in the serum of the liver enzyme SDH. Adenovirus inoculation was carried out at the indicated time after initiation of lamivudine therapy. Mock-infected animals received PBS rather than adenovirus.
TABLE 2.
Loss of adenovirus from the livera
| Woodchuck | 3 days, positive hepatocytes (%) | 15 days
|
26 days, positive hepatocytes (%) | 6 mo
|
||||
|---|---|---|---|---|---|---|---|---|
| Positive hepatocytes (%) | Nucleic acid level
|
Positive hepatocytes (%) | Nucleic acid level
|
|||||
| cccDNA | mRNA | cccDNA | mRNA | |||||
| 326 | 100 | 50 | 0.35 | 0.49 | 1 | 0 | 0.03 | 0.06 |
| 328 | 100 | 30 | 0.29 | 0.5 | 0 | 0 | 0.08 | 0.15 |
| 331 | 20 | 1 | 0.38 | 0.42 | 0 | 0 | 0.86 | 1.06 |
| 338 | 80 | 90 | 0.77 | 0.68 | 30 | <0.1b | 0.60 | 0.74 |
Expression of β-galactosidase in hepatocytes was determined using frozen liver sections as described in Materials and Methods, and the percentage of positive hepatocytes was determined. The loss of cccDNA was calculated from the results in Fig. 4; the decline in WHV mRNAs was calculated from the results in Fig. 7. The results of the WHV nucleic acid analyses were normalized by dividing by the amount of nucleic acid present 1 month before adenovirus infection; thus, the amount present in an individual woodchuck 1 month before preinfection has a value of 1.
Scattered β-galactosidase-positive hepatocytes.
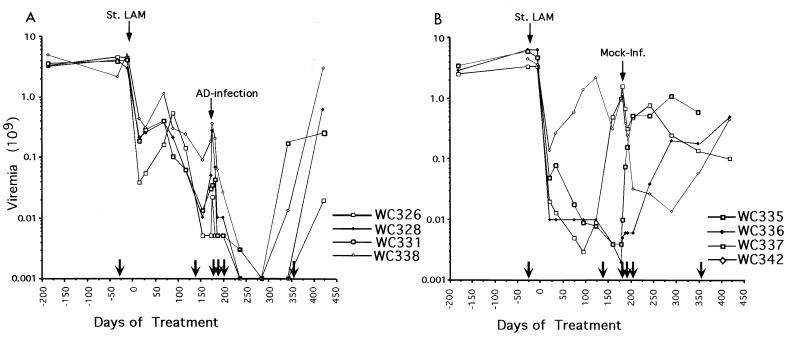
We also observed a decline of WHV titers that persisted for at least 2 to 3 months (Fig. 3). Mock infection was not associated with an elevation in SDH levels or decline in virus replication (Fig. 2 and 3). Eventually, virus titers rose in all of the woodchucks despite the maintenance of lamivudine therapy (Fig. 3). A statistical evaluation of the viremia data is presented in the legend to Fig. 3.
FIG. 3.
Adenovirus infection leads to a prolonged suppression of WHV viremia. (A) Infected woodchucks. Adenovirus was inoculated at the indicated time after initiation of lamivudine therapy (St. LAM). WHV titers in the serum were determined by Southern blot assays for viral DNA. (B) Woodchucks inoculated with PBS rather than adenovirus. Lamivudine administration was continued until the end of the study. Arrows along the abscissa show the timing of liver biopsies, described further in the legend to Fig. 4 and Table 2, footnote a. To test that the response to the adenovirus was statistically significant, the woodchuck viremia data for the adenovirus- and mock-infected woodchucks were evaluated separately, before and after infection. We first used the data collected from 1 week before initiation of lamivudine therapy through to the time of infection. Second-degree polynomials (20) were fitted to the data from each set of animals, and the sum of squared errors (ss) committed for each group was determined: ss(treated) and ss(mock), and the sum ss(treated) +ss (mock). We next fitted the data for all eight animals by a single second-order curve and determined the sum of squared errors [ss(both)] using just one rather than two curves. The ratio ss(both)/[ss(treated) + ss(mock)] is necessarily greater than 1.0. To assess the significance of this ratio, we permuted the data for the eight woodchucks so that the treated group was not the original but one of 69 other permutions of eight animals. The above ratio was determined for all 69 permutions. Under the null hypothesis that the two groups are identical, the true ratio (the one determined by the actual data) is uniformly distributed, in rank order, among the 70 values obtained. It was the 15th largest. Hence, the null hypothesis is accepted at the 30% level, using a two-sided test. The same procedure was repeated on the posttreatment data, except that a one-sided test was used to determine the probability that the responses to mock infection and adenovirus infection were identical. This time the ratio obtained from the original data was the largest of the set of 70. Thus, the null hypothesis that the treatment response was the same for both groups (P = 1/70 = 0.0143) was rejected.
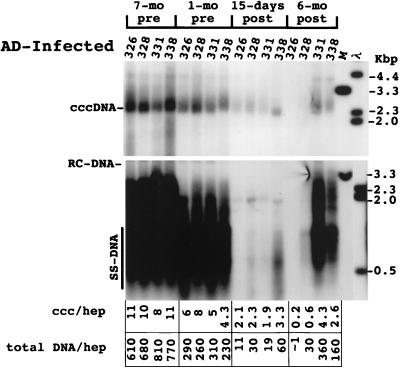
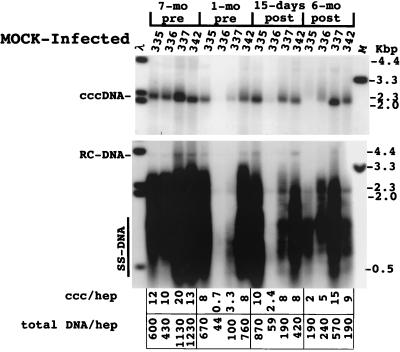
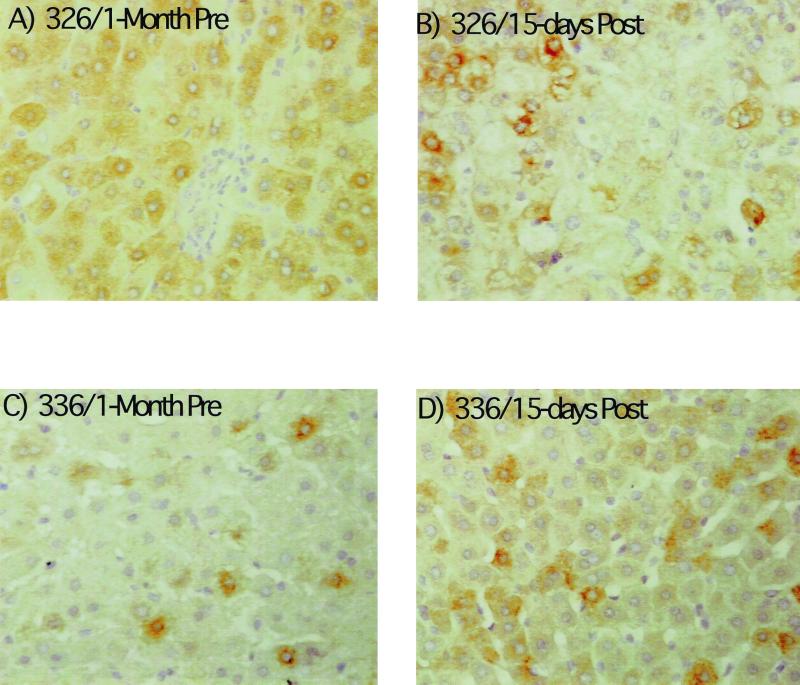
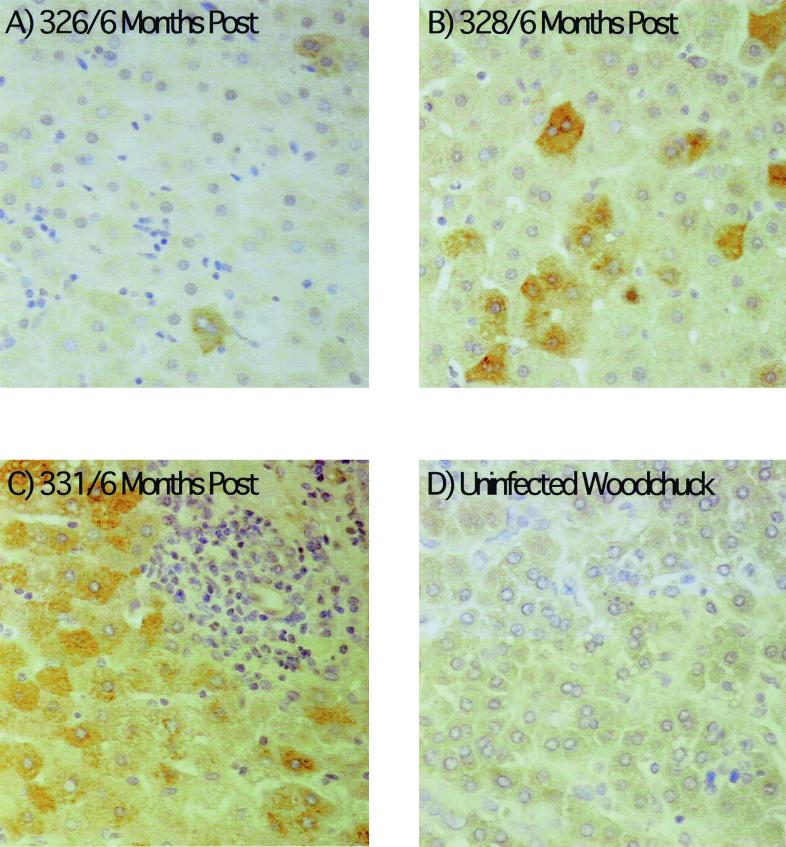
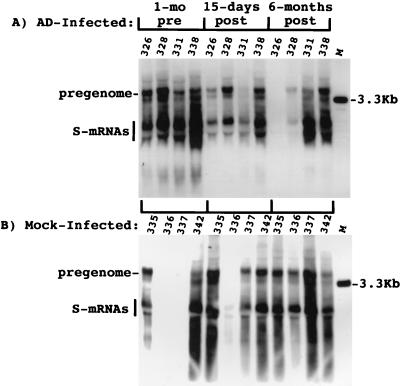
The effect of adenovirus infection on WHV replication intermediates in the liver was also determined. As shown in Fig. 4, a two- to threefold decline in cccDNA was observed at 15 days postinoculation. The decline in replicating DNA in each case exceeded that of cccDNA by 2.5- to 9-fold. There was also a decline in the amount of core antigen in hepatocytes in woodchuck 326 that was revealed by an immunoperoxidase assay for this viral protein. In particular, there was a decrease in the fraction of hepatocytes with strong anticore staining (Fig. 5). A similar though less pronounced decline was noted with woodchuck 328 (not shown). A decline was not noted in any of the other woodchucks during this time span, as illustrated for woodchuck 336, in which viremia was still strongly suppressed by lamivudine administration. This reduction was still evident in woodchucks 326 and 328 6 months later (Fig. 6). As found following HBV-transgenic mouse experiments (7), the decline in viral mRNAs following adenovirus infection did not correlate in amount with the decline in replicating WHV DNAs (Fig. 7). Overall, changes in mRNAs were correlated with changes in cccDNA levels (Table 2). No decline in cccDNA, replicating DNAs, and core antigen staining was observed in the mock-infected woodchucks over the same time period (Fig. 4 and 5). The reason that woodchucks 336 and 337 displayed a much greater suppression of virus replication before and during the period of the mock infection is unknown; however, it is important to note that the mock infection did not lower the level of cccDNA, mRNA, or viral DNA replication intermediates in any woodchuck.
FIG. 4.
Analysis of WHV DNA in livers of adenovirus (AD)-infected woodchucks. Total DNA and cccDNA were extracted and subjected to Southern blotting following electrophoresis in 1.5% agarose gels. The filters were hybridized with a 32P-labeled probe representing the complete viral genome. In the upper panel, cccDNA collected from 5 × 105 liver cells, as determined by nuclear counts, was loaded into each lane of the gel; 2.5 μg of total liver DNA was loaded in each lane in the lower panel. cccDNA (ccc) and total DNA copy numbers (equivalents of 3.3 kbp of double-strand WHV DNA) were quantified using a Fuji phosphorimager to detect radioactivity bound to the filters. Copy numbers were calculated assuming that the liver is comprised of 70% hepatocytes (hep). The infiltration of lymphocytes did not appear to cause a major alteration in this estimation (≤2-fold [Fig. 9A]). RC-DNA, relaxed circular DNA; SS-DNA, single-stranded DNA.
FIG. 5.
Adenovirus infection produced a decline in viral core antigen in the liver. Immunoperoxidase staining of fixed liver tissue for detection of viral core (nucleocapsid) antigen was carried out as described in Materials and Methods. Two biopsies are illustrated, one collected 1 month before adenovirus or mock infection the other collected 15 days after. The major effect noticed for woodchuck 326 was a decline in the number of hepatocytes with a strong staining reaction. A lesser decline was observed with woodchuck 328 (not shown). No appreciable decline was observed in woodchucks 331 and 338 or in any of the mock-infected controls (for example, 336 shown here). Magnification, ×200.
FIG. 6.
Viral replication may still be suppressed 6 months after adenovirus infection. Immunoperoxidase assays for viral core antigen in liver sections were carried as described in Materials and Methods. As illustrated, the majority of hepatocytes in two woodchucks (326 and 328) still expressed little or no core antigen 6 months after adenovirus infection. At the end of the study, the majority were positive in all of the woodchucks.
FIG. 7.
Adenovirus (AD) infection induced a slight decline in viral mRNAs. RNA was extracted from liver biopsy specimens, and 10 μg was subjected to Northern blot analysis. Filters were hybridized with a 32P-labeled DNA probe representing the entire viral genome. The results were quantified with a Fuji image analyzer and are summarized in Table 2. mRNA signals for woodchucks 336 and 337 were low but detectable at 1 month before mock infection.
Adenovirus infection induced liver inflammation and hepatocyte destruction.
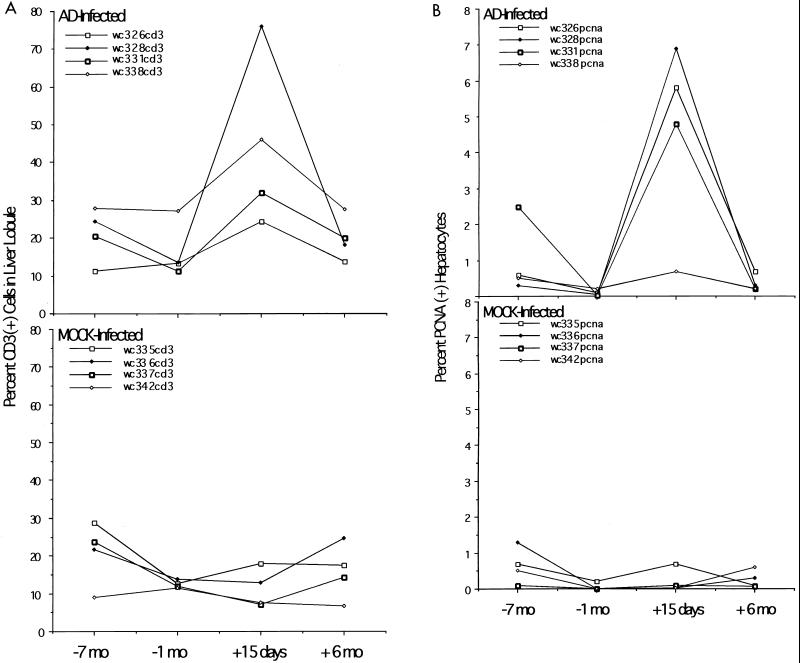
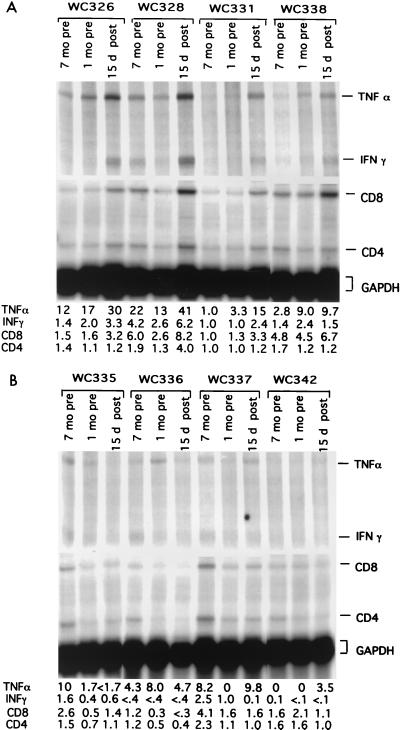
Evidence that the decline of WHV replication intermediates correlated with an inflammatory response to adenovirus infection was provided by assaying for infiltration of CD3-positive leukocytes into the liver (Fig. 8A) and increased expression of various mRNAs associated with the immune response (Fig. 9). As shown, there was an increase of specific markers of inflammation (CD8, IFN-γ, and TNF-α mRNAs; CD3-positive cells) in three of the four adenovirus-infected woodchucks (Fig. 8A and Fig. 9A). These three woodchucks also showed the greatest elevation in PCNA-positive hepatocytes (Fig. 8B), probably reflecting cell division following enhanced cell killing. The least elevation in the PCNA staining index was seen in the fourth adenovirus-infected woodchuck, 338, which also showed the smallest loss of virus replication intermediates and cccDNA (Fig. 4).
FIG. 8.
Adenovirus (AD) infection is associated with infiltration of CD3+ leukocytes and an elevation in the fraction of hepatocytes with PCNA-positive nuclei. CD3+ leukocytes (A) and PCNA-positive hepatocytes (B) were determined as described previously (14) and in Materials and Methods. The percent CD3+ cells in the lobule is the intralobular count of CD3+ cells divided by the number of hepatocytes times 100.
FIG. 9.
Adenovirus infection induced hepatic inflammation and expression of inflammatory cytokines. 32P-labeled probes for woodchuck cytokine mRNAs were produced, and RNase protection assays were carried out as described previously (14) and in Materials and Methods. Following gel electrophoresis, radioactive signals were quantified using a Fuji image analyzer. Relative signal intensities are shown at the bottom. (A) Adenovirus infected; (B) mock infected. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Drug-resistant variants of WHV and response to infection.
Treatment of chronically infected woodchucks with either lamivudine or L-FMAU ultimately leads to the outgrowth of variants that are drug resistant, due to mutations in the active site of the viral DNA polymerase. Among these, the type I variant (Table 3) generally appears earliest and may sometimes be replaced by other, presumably more fit, variants (21, 33, 34). In the present study, this mutant was prevalent in the sera of five of eight woodchucks at the time of adenovirus or mock infection, including three of four that received the adenovirus vector (Fig. 3). Nonetheless, virus titers in all four dropped below the level of detection of the assay (106 virions per ml) after infection with the adenovirus vector (Table 3; Fig. 3). A corresponding decline was not observed in the control animals. Thus, the presence of drug-resistant virus in the serum does not preclude a temporary adenovirus-induced reduction of virus replication. In addition, the observation that viremia was suppressed in the adenovirus-infected woodchucks for several months suggests that the bystander effect on WHV does not enhance the spread of lamivudine-resistant WHV.
TABLE 3.
Polymerase variants arising during lamivudine therapya
| Woodchuck | Titer (virions/ml) at indicated time (days) from start of lamivudine treatment
|
|||
|---|---|---|---|---|
| −28 | 178 | 347 | 423 | |
| AD infected | ||||
| 326 | 4.4 × 109 WT | 5 × 106 WT/I | <1 × 106 WT | 2 × 107 WT |
| 328 | 4 × 109 WT | 5 × 107 WT/I | <1 × 106 WT/I | ? |
| 331 | 3.7 × 109 WT | 3 × 107 WT/I | 1.7 × 108 I/? | 6 × 108 I/? |
| 338 | 2.1 × 109 WT | 1.8 × 108 WT | 1.3 × 107 WT/I | 3 × 108 WT/I |
| Mock infected | ||||
| 335 | 3.4 × 109 WT | 1 × 109 WT/I | 1.4 × 108 WT/I | 1 × 108 WT/I/II |
| 336 | 6.4 × 109 WT | 2 × 106 WT/II | 1.8 × 108 II | 5 × 108 II |
| 337 | 6 × 109 WT | 4 × 106 WT/I | 6 × 108 WT/I/II | ND |
| 342 | 4.5 × 109 WT | 1 × 109 WT | 6 × 107 WT/I | 5 × 108 WT/I |
| B domainC domain |
| Wild type543-LAHPFIMGFR KLPMGVGLSP FLLAQFTSAL ASMVRRNFPH CVVFAYMDDL VLGARTSEHL-602 |
| Type I---------- –--------- ---T------ ---------- ---------- ---------- |
| Type II---------- ---------- --MV------ ---------- ---------- ---------- |
Genotypes of virus in serum samples were estimated by direct sequencing of PCR products. The second sample (178 days) was collected at the time of adenovirus infection. Variants representing less than 10% of the total will not be detected. Identification of the type II variant is tentative, as the observed sequence could have been produced by a mixture of mutants with the single amino acids changes. WT, wild type; I, type I variant; II, type II variant.
DISCUSSION
This study was carried out to test whether a combination therapy with lamivudine and adenovirus could trigger recovery from a chronic hepadnavirus infection. Our results showed that this is not the case. Although adenovirus infection was accompanied by a significant reduction of viral replication in livers of the chronically infected animals, rebound of viremia occurred in all cases examined so far.
However, combination therapy was effective in suppressing viral replication for approximately 3 months, compared to monotherapy with lamivudine. Indeed, in two of the woodchucks (326 and 328) virus replication had not fully recovered even 6 months after infection (Table 3; Fig. 4 and 6). The reason for this prolonged effect of adenovirus infection is unclear, as expression of β-galactosidase had ceased within a month. One possible explanation for this sustained response is that virus replication was temporarily suppressed by activation of the host immune response to WHV. Another possibility is that the adenovirus vector persisted in the liver long after β-galactosidase expression became undetectable. This possibility is at least consistent with the observation that adenovirus DNA was still detectable in the liver by PCR 6 months postinfection (data not shown). Thus, the prolonged suppression of WHV might have been due to an enhanced inflammatory reaction in response to other vector-encoded proteins. As noted in studies of transgenic mice, this indirect mode of suppression of the hepadnavirus need not be accompanied by any major increase in hepatocyte destruction (Fig. 2) (7, 10).
A major unresolved question concerns the mechanisms responsible for the natural recovery from hepadnavirus infections, which lead to permanent suppression of viral replication, not just the transient response observed in this study. We previously observed that recovery from hepadnavirus infections was accompanied by a significant influx of lymphocytes into the liver and by increased expression of IFNs as well as TNF-α (14, 19). In this respect, there was no obvious difference from the present observations. Likewise, recovery from transient WHV infections is accompanied by an increased rate of hepatocyte death. Again, within the limitations of the time points available, the response to the adenovirus infection did not differ in an obvious way from the response during the recovery phase of a transient WHV infection. Thus, our results suggest that non-antigen-specific mechanisms (for example, cytokine-mediated suppression of hepadnaviruses) may not be sufficient to cure an infection.
One issue of particular interest was whether or not adenovirus infection of chronically infected woodchucks would produce a loss of cccDNA beyond that which could be attributed to cytolysis of adenovirus-infected hepatocytes. Compared to cccDNA, there was a greater loss of DNA replication intermediates, consistent with the idea that replicating DNAs are depleted noncytolytically. Moreover, the average depletion of cccDNA that was detected (ca. 70%) in the four woodchucks receiving combination therapy was small, especially in view of the 10-fold elevation in the PCNA staining index in these samples (Fig. 7B). This high staining index suggests that a large number of hepatocytes may have been destroyed by the immune response, perhaps sufficient to explain most of the decline in cccDNA. Alternatively, we cannot rule out differences in the stability between cccDNA and core particle-associated DNA in regenerating hepatocytes. Thus, with this caveat, our data are consistent with the observation, in studies using primary hepatocyte cultures, that IFN-α and -γ do not induce loss of viral cccDNA (24, 25). However, our results do not rule out the likelihood that noncytolytic mechanisms play a major role in the recovery from hepadnavirus infections (7, 10, 12, 31).
It should be noted that the WHV production remained suppressed even though at least one drug-resistant variant (type I) was prevalent in the sera of three of four woodchucks at the time of the adenovirus infection (Table 3). This raises a question about the significance of detection of this variant early in therapy. In previous studies, this variant was detected as a major species in the sera of some woodchucks at times when virus titers were still declining (33, 34). This was surprising, since the type I variant appears to be lamivudine resistant and can be the major species in the serum when virus titers again increase during drug treatment. One possibility that needs to be considered is that the variant results from an error in polymerase II transcription, so that the virus is prevalent in serum even when a mutant cccDNA template is not prevalent in the liver. In summary, evidence was obtained for the clearance of WHV DNA replication intermediates during the immune response to a second virus infection in the presence of lamivudine. This clearance was not associated with a more rapid emergence of drug-resistant WHV.
The conclusion that both cytolytic and noncytolytic mechanisms were responsible for the loss of viral DNA is based on the assumption that cytolysis would produce equal losses of replicating and viral cccDNAs. However, the exact quantitative contribution of such mechanisms will not be firmly established until methods are available to evaluate cumulative cell death during the resolution of an infection.
ACKNOWLEDGMENTS
We are grateful to Glenn Rall, John Taylor, and Jesse Summers for helpful suggestions during this work and for critical reading of the manuscript, to A. Cywinski and the staff of the DNA Sequencing Facility of the Fox Chase Cancer Center for sequence determinations, and to M. Einenkel for help in preparation of the manuscript. Oligonucleotides were synthesized in the institutional DNA Synthesis Facility under the direction of T. Yeung.
This work was supported by grants from the National Institutes of Health and by an appropriation from the Commonwealth of Pennsylvania.
REFERENCES
- 1.Alan R, Wilson J. Adenoviral vectors. In: Dracapoli N, Haines J L, Korf B R, Moir D T, Morton C C, Seidman C E, Seidman J G, Smith D R, Boyle A, editors. Current protocols in human genetics. II. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 12.4.1–12.4.18. [Google Scholar]
- 2.Barker L F, Chisari F V, McGrath P P, Dalgard D W, Kirschstein R L, Almeida J D, Edgington T S, Sharp D G, Peterson M R. Transmission of type B viral hepatitis to chimpanzees. J Infect Dis. 1973;127:648–652. doi: 10.1093/infdis/127.6.648. [DOI] [PubMed] [Google Scholar]
- 3.Berquist K R, Peterson J M, Murphy B L, Ebert J W, Maynard J E, Purcell R H. Hepatitis B antigens in serum and liver of chimpanzees acutely infected with hepatitis B virus. Infect Immun. 1975;12:602–605. doi: 10.1128/iai.12.3.602-605.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boshart M F, Weber F, Jahn G, Dorsch-Hasler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael G G, McMaster G K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65:380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh V J, Guidotti L, Chisari F V. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236–3243. doi: 10.1128/jvi.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanaugh V J, Guidotti L G, Chisari F V. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in transgenic mice. J Virol. 1998;72:2630–2637. doi: 10.1128/jvi.72.4.2630-2637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirmule N, Moscioni A D, Qian Y, Qian R, Chen Y, Wilson J M. Fas-Fas ligand interactions play a major role in effector functions of cytotoxic T lymphocytes after adenovirus vector-mediated gene transfer. Hum Gene Ther. 1999;10:259–269. doi: 10.1089/10430349950019048. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti L G, Borrow P, Brown A, McClary H, Koch R, Chisari F V. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J Exp Med. 1999;189:1555–1564. doi: 10.1084/jem.189.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidotti L G, Borrow P, Hobbs M V, Matzke B, Gresser I, Oldstone M B, Chisari F V. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci USA. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidotti L G, Guilhot S, Chisari F V. Interleukin-2 and alpha/beta interferon down-regulate hepatitis B virus gene expression in vivo by tumor necrosis factor-dependent and -independent pathways. J Virol. 1994;68:1265–1270. doi: 10.1128/jvi.68.3.1265-1270.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 13.Guidotti L G, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 14.Guo J-T, Zhou H, Liu C, Aldrich C, Saputelli J, Whitaker T, Barrasa M I, Mason W S, Seeger C. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J Virol. 2000;74:1495–1505. doi: 10.1128/jvi.74.3.1495-1505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobbs M V, Weigle W O, Noonan D J, Torbett B E, McEvilly R J, Koch R J, Cardenas G J, Ernst D N. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- 16.Hoofnagle J H, Michalak T, Nowoslawski A, Gerety R J, Barker L F. Immunofluorescence microscopy in experimentally induced, type B hepatitis in the chimpanzee. Gastroenterology. 1978;74:182–187. [PubMed] [Google Scholar]
- 17.Hornbuckle W E, Graham E S, Roth L, Baldwin B H, Wickenden C, Tennant B C. Laboratory assessment of hepatic injury in the woodchuck (Marmota monax) Lab Anim Sci. 1985;35:376–381. [PubMed] [Google Scholar]
- 18.Jilbert A R, Wu T T, England J M, Hall P M, Carp N Z, O'Connell A P, Mason W S. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992;66:1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kajino K, Jilbert A R, Saputelli J, Aldrich C E, Cullen J, Mason W S. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J Virol. 1994;68:5792–5803. doi: 10.1128/jvi.68.9.5792-5803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendall M G, Stuart A. The advanced theory of statistics. 2nd ed. Vol. 2. New York, N.Y: Hafner Publishing Company; 1967. [Google Scholar]
- 21.Mason W S, Cullen J, Moraleda G, Saputelli J, Aldrich C E, Miller D S, Tennant B, Frick L, Averett D, Condreay L D, Jilbert A R. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32. doi: 10.1006/viro.1998.9150. [DOI] [PubMed] [Google Scholar]
- 22.Ponzetto A, Cote P J, Ford E C, Purcell R H, Gerin J L. Core antigen and antibody in woodchucks after infection with woodchuck hepatitis virus. J Virol. 1984;52:70–76. doi: 10.1128/jvi.52.1.70-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponzetto A, Cote P J, Popper H, Hoyer B H, London W T, Ford E C, Bonino F, Purcell R H, Gerin J L. Transmission of the hepatitis B virus-associated delta agent to the eastern woodchuck. Proc Natl Acad Sci USA. 1984;81:2208–2212. doi: 10.1073/pnas.81.7.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz U, Chisari F V. Recombinant duck interferon gamma inhibits duck hepatitis B virus replication in primary hepatocytes. J Virol. 1999;73:3162–3168. doi: 10.1128/jvi.73.4.3162-3168.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz U, Summers J, Staeheli P, Chisari F V. Elimination of duck hepatitis B virus RNA-containing capsids in duck interferon-alpha-treated hepatocytes. J Virol. 1999;73:5459–5465. doi: 10.1128/jvi.73.7.5459-5465.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Summers J, Mason W S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 27.Summers J, Smith P M, Huang M J, Yu M S. Morphogenetic and regulatory effects of mutations in the envelope proteins of an avian hepadnavirus. J Virol. 1991;65:1310–1317. doi: 10.1128/jvi.65.3.1310-1317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tassopoulos N, Papaevangelou G, Roumeliotou-Karayannis A, Kalafatas P, Engle R, Gerin J, Purcell R H. Double infections with hepatitis A and B viruses. Liver. 1985;5:348–353. doi: 10.1111/j.1600-0676.1985.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsiquaye K N, Harrison T J, Portmann B, Hu S, Zuckerman A J. Acute hepatitis A infection in hepatitis B chimpanzee carriers. Hepatology. 1984;4:504–509. doi: 10.1002/hep.1840040325. [DOI] [PubMed] [Google Scholar]
- 30.Tuttleman J S, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 31.Wieland S F, Guidotti L G, Chisari F V. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J Virol. 2000;74:4165–4173. doi: 10.1128/jvi.74.9.4165-4173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou T, Saputelli J, Aldrich C E, Deslauriers M, Condreay L D, Mason W S. Emergence of drug-resistant populations of woodchucks hepatitis virus in woodchucks treated with the antiviral nucleoside, lamivudine. Antimicrob Agents Chemother. 1999;43:1947–1954. doi: 10.1128/aac.43.8.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu, Y., T. Yamamoto, J. Cullen, J. Saputelli, C. E. Aldrich, D. S. Miller, S. Litwin, P. A. Furman, A. R. Jilbert, and W. S. Mason. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J. Virol., in press. [DOI] [PMC free article] [PubMed]