Abstract
Wound is defined as the damage to biological tissues including skin, mucous membranes and organ tissues. The acute wound heals in less than 4 weeks without complications, while a chronic wound takes longer than 6 weeks to heal. Wound healing occurs in 4 phases, namely, coagulation, inflammatory, proliferative and remodeling phases. Triclosan and benzalkonium chloride are commonly used as skin disinfectants in wound healing. However, they cause allergic contact dermatitis and antibiotic resistance. Medicinal plants are widely studied due to the limited availability of wound healing agents. The present review included six commonly available medicinal plants in Malaysia such as Aloe barbadensis Miller, Carica papaya Linn., Centella asiatica Linn., Cymbopogon nardus Linn., Ficus benghalensis Linn. and Hibiscus rosa sinensis Linn. Various search engines and databases were used to obtain the scientific findings, including Google Scholar, ScienceDirect, PubMed Central and Research Gate. The review discussed the possible mechanism of action of medicinal plants and their active constituents in the wound healing process. In addition, their application in nanotechnology and wound dressings was also discussed in detail.
Keywords: Aloe barbadensis Miller, Carica papaya Linn., Centella asiatica Linn., Cymbopogon nardus Linn., Ficus benghalensis Linn., Hibiscus rosa sinensis Linn., Wound healing, Wound repair, Mechanism of action, Angiogenesis
Background
Wound is defined as the damage to biological tissues, including skin, mucous membranes and organ tissues [1]. An acute wound heals in less than 4 weeks without complications and is classified into accidental wounds and surgical wounds [2]. Moreover, surgical wounds are classified into 4 classes. Class I (clean wounds) is primarily closed without infection and inflammation. Additionally, Class I wounds do not enter the respiratory, alimentary, genital or urinary tracts. Class II (clean-contaminated wounds) enters the respiratory, alimentary, genital or urinary tracts under controlled conditions. Class III (contaminated wounds) is fresh, open wounds that arise due to the disruption in sterile techniques or the leakage from the gastrointestinal tract into the wound. Class IV (dirty-infected wounds) arises due to improperly managed traumatic wounds and the growth of microorganisms. Thus, devitalized tissues are observed in Class IV wounds [3].
A chronic wound takes longer than 6 weeks to heal. Examples of chronic wounds are diabetic foot ulcers, venous leg ulcers, arterial leg ulcers, and pressure ulcers. The establishment of chronic wounds is attributed to diabetes, vascular disease, ischemia, and neuropathy [2]. According to the National Health and Morbidity Survey 2019, 1 in 5 adults in Malaysia has diabetes, which is approximately 3.9 million Malaysians are diagnosed with diabetes [4]. Hence, there is also an increase in diabetic-related complications such as diabetic foot ulcers (DFU). Hyperglycemia affects the ability of the immune system to eliminate pathogens, while severe infection causes stress hyperglycemia. This two-way mechanism causes diabetic foot infections (DFI), which further delay wound healing and may lead to limb amputation. Empirical antibiotic treatment is crucial due to the unavailability of culture and sensitivity (C&S) results. However, DFU infected with multidrug-resistant organisms (MDROs) makes antibiotic treatment much more challenging [5]. A study conducted at Hospital Kuala Lumpur (HKL) reported that 41.5% of patients with DFU developed DFI. Based on the study, the prevalence of DFI was associated with ethnicity, blood glucose level, blood pressure and cross-sectional area of DFU. Furthermore, patients with a history of amputation, DFI, fasting blood glucose level of ≥ 7 mmol/l, blood pressure of ≥ 140/90 mmHg and DFU size > 10 cm2 took a longer period for DFU healing [6].
Triclosan and benzalkonium chloride are commonly used as skin disinfectants in wound healing. However, they cause allergic contact dermatitis. Additionally, cells treated with triclosan may show antibiotic resistance to sulfamethoxazole and ampicillin, while cells treated with benzalkonium chloride may show antibiotic resistance to ampicillin, cefotaxime and sulfamethoxazole [7]. On the other hand, hydrogel-based wound dressings are effective in wound repair due to their ability to keep the wounds hydrated, absorb exudates, relieve pain through cooling effect and adhesion-free properties. However, hydrogels have poor mechanical stability [8]. Hence, medicinal plants are widely studied due to the limited availability of wound healing agents.
A review on the phytochemistry and the mechanisms of wound healing of Vietnamese medicinal plants was published by Hua et al. Morinda citrifolia L., Morus alba L., Calophyllum inophyllum L., Momordica charantia L., etc. was shown to promote wound healing [9]. Hosseinkhani et al. reported a review of traditional Persian medicine (Cocos nucifera L., Gentiana lutea L., Teucrium polium L., Punica granatum L., Plantago major L., etc.) for wound healing [10]. Furthermore, B. Kumar et al. reported the potential wound healing effects of some Indian medicinal plants [11]. However, a review of Malaysian medicinal plants with potential wound healing activity has not been conducted before. Hence, this review mainly focused on six commonly available medicinal plants in Malaysia with potential wound healing properties and their application in nanotechnology and wound dressing.
Molecular mechanism in wound healing
Generally, wound healing occurs in 4 phases, namely, coagulation, inflammatory, proliferative and remodeling phases. The initial phase of wound healing involves the activation of platelets for clot formation. The whole process is mediated by platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF) and other growth factors or cytokines released by platelets. Cytokines and growth factors act on different target cells and receptors to show their distinctive functions. For instance, PDGF acts as a mitogen and chemotactic agent for fibroblasts and smooth muscle cells that promote angiogenesis and collagen synthesis, in addition to the stimulation of neutrophils and macrophages [12].
The inflammation phase begins 48 h after injury with neutrophils as the predominant cell type. Then the neutrophil count decreases after 24 to 36 h, followed by the migration of monocytes to the wound site. The maturation of monocytes to macrophages plays a role in the inflammatory and repairing phase of wound healing. For instance, macrophages phagocytize wound debris and bacteria and synthesize inflammatory cytokines such as tumor necrosis factor (TNF), interleukin-6 (IL-6), interleukin-1 (IL-1) and basic fibroblast growth factor (bFGF). TNF-α acts as a mitogen for fibroblasts. IL-1 stimulates the proliferation of inflammatory cells and promotes angiogenesis through endothelial cell replication. bFGF acts as a chemotactic and mitogenic factor for fibroblasts, endothelial cells and other mesenchymal cells to regulate angiogenesis. Moreover, bFGF encourages wound contraction, epithelialization and the production of collagen, fibronectin, and proteoglycans. The activation of macrophages (M1 and M2) is dependent on the types of stimuli. Toll-like receptor (TLR)-4 ligands and interferon-γ (IFN-γ) activate M1, thus triggering pro-inflammatory action at the wound site. In contrast, IL-4 and/or IL-13 activate M2 that release macrophage-derived growth factors, PDGF, α fibroblastic growth factor (αFGF), bFGF, transforming growth factor α (TGFα), transforming growth factor β (TGFβ), etc. Hence, the activation of M2 macrophages is vital throughout the wound healing process [12–14]. Autophage also promotes wound repair through antimicrobial, anti-inflammation and immunomodulatory action [15].
During the inflammatory phase, lymphocytes migrate into the wound and release lymphokines, epidermal growth factor (EGF), bFGF, etc. The migration of T lymphocytes is attributed to IL-1 and is crucial for the regulation of collagenase. Optimization of macrophage-derived growth factors and macrophage-derived angiogenic factors at the end of the inflammatory cycle regulates the distribution of fibroblasts, endothelial cells and keratinocytes at the wound site. Furthermore, macrophages manipulate the composition of extracellular matrix (ECM) by stimulating the proliferation of connective, endothelial and epithelial tissue. This is achieved by the release of degrading enzymes and ECM molecules during the angiogenesis and remodeling phase of wound healing [12]. Besides, the inflammatory phase of wound healing involves 5-lipoxygenase pathway that promotes the release of leukotrienes and inducible nitric oxide synthase that generates nitric oxide [16]. Arachidonic acid acts as a precursor to generate leukotrienes and prostaglandins via lipoxygenase pathway and cyclooxygenase pathway, respectively. While the overproduction of leukotrienes and prostaglandins contributes to inflammation due to their chemoattractive effects on eosinophils, neutrophils and macrophages [17].
The proliferation phase begins after the inflammatory phase, whereby fibroblasts start to migrate, proliferate and produce components of the ECM (glycosaminoglycans, proteoglycans and collagen). Collagen is secreted to the extracellular space as procollagen that subsequently cleaves to tropocollagen. Then, tropocollagen cross-links with other tropocollagen to form hydroxylysine and hydroxyproline-rich collagen filaments. The tensile strength of the healing wounds is directly proportional to the extent of cross-link in the collagen. Besides, endothelial progenitor cells (EPCs) regulate angiogenesis and wound revascularization for effective wound healing. The proliferation and migration of both epidermal cells and epithelial cells contribute to re-epithelialization. Cell differentiation such as epithelial-mesenchymal transition (EMT) mediated by β-2 adrenergic receptors (β2-AR) is important due to its ability to establish basement membrane and promote re-epithelialization of wounds. Thus, it plays an important role in accelerating wound healing, while abnormal regulation of EMT causes hypertrophic scar and tissue fibrosis [12]. Lastly, wound contraction takes place during the remodeling phase.
However, the disruption of any one of the wound healing phases results in the development of chronic wound. Chronic wound is also known as non-healing wound, which is manifested by exudation, re-infection, tissue necrosis, defective re-epithelization, decreased angiogenesis and overproduction of reactive oxygen species (ROS). The progression of chronic wound is attributed to persistent inflammatory phase, MMP production, ECM degradation, cell senescence, infections, drug-resistant microbial biofilms, poor vascularization and the unresponsiveness of dermal and/or epidermal cells to reparative stimuli.
The prolongation of inflammatory phase in chronic wounds is due to impaired immune response during the wound healing process. For instance, the amount of pro-inflammatory macrophages increases, while the anti-inflammatory macrophages decline. In addition, the macrophages fail to eliminate dead neutrophils and release matrix metalloproteinases (MMP), i.e., MMP-2 and MMP-9, which degrade ECM and interfere with the proliferation phase of wound healing. MMPs are also secreted by keratinocytes to cause defective in wound re-epithelialization. Impairment of certain micro-ribonucleic acids (miRNAs) such as miR-34a/c, miR-203, miR-19a/b and miR-20a in keratinocytes interrupts immune functions and causes a delay in wound healing. Moreover, the establishment of a highly inflammatory environment is achieved by the accumulation of inflammatory cytokines, i.e., TNF-α and IL-1β. On the other hand, myeloid cells such as macrophages, neutrophils and monocytes persist in the late stage of inflammation. Chemokine receptor CXCR3 is highly expressed in Type 1 T helper (Th1) cells and the ligand for CXCR3 is observed to be over-expressed in chronic inflammation [18, 19].
Infection causes an inflammatory state at the wound bed by activating neutrophils and pro-inflammatory macrophages. As a result, leads to the accumulation of inflammatory cytokines (TNF-α, IL-6, MMP). The accumulation of inflammatory cells produces ROS that cause damaging effects on ECM and cell membranes and eventually leads to premature cell senescence. Moreover, ROS works together with proinflammatory cytokines to stimulate the production of serine proteinases and MMPs that degrade and inactivate ECM components and growth factors which are necessary for normal cell function [18, 19].
Abnormal expression of vascular cell adhesion molecule 1 (VCAM-1) and interstitial cell adhesion molecule 1 (ICAM-1) by endothelial cells promotes cell extravasation that prolongs the inflammatory phase in wound healing. Furthermore, the proliferation phase is delayed due to the lower density of growth factor receptors and the lower mitogenic potential of some growth factors. Although the active cell proliferation marker, Ki-67 is overexpressed in the chronic wound–derived keratinocytes, it shows impairment in migratory potential. Additionally, these cells promote the activation of A-catenin/c-myc pathway and do not express the differentiation markers, especially keratin 10 and keratin 2 [18, 19].
Experimental models for wound healing
Experimental wound healing models are widely used to study the progression of wound healing. They are divided into in vitro and in vivo models. In vitro wound healing model utilizes cell cultures involving fibroblasts and keratinocytes or a scratch model to investigate for cell viability, proliferation and cytotoxicity [20, 21]. Besides, in vivo model involves laboratory animals with wounds, follows by monitoring of the wound condition over a certain timeframe. Rodents (rats and mice), rabbits and pigs are often used in in vivo models, among these, pig skin is closely related to human skin. However, pigs are prone to infection, costly and difficult to handle and maintain. Hence, the wound healing model is chosen by considering its cost, availability, reproducibility, replicability, ease of handling, similarity to humans and required sample size [20, 22].
Animal models can be subjected to different conditions, namely, incisional wound, excisional wound, burn wound and chronic wound. Incisional wound is created to examine the recovery of surgical wounds, whereas excisional wound is created to investigate wound repair after surgical removal of the skin. Burn wound is created by exposing the skin to hot water (water scalding) or application of direct heat using a hot metal plate. Then, the blister is de-roofed to create an open wound. On the other hand, chronic diabetic wounds can be induced by the administration of alloxan or streptozotocin to promote beta cells destruction in laboratory animals. However, surgical removal of beta cells or the utilization of db/db mice are also commonly practiced. Besides, chorioallantoic membrane (CAM) assay is implemented to study the importance of angiogenesis in wound healing [20, 23].
Excisional wound healing models are vital to study hemorrhage, inflammation, granulation tissue formation, re-epithelialization, angiogenesis and remodeling. In contrast, burn wound healing models are crucial to investigate re-epithelialization, granulation tissue formation, angiogenesis, contraction, scarring and the composition of wound tissues [20].
The wound healing process can be monitored by the following techniques [20]:
Wound healing rate (WHR) is calculated using the equation below:
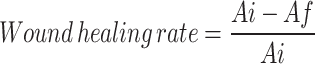 |
where Ai indicates initial wound area and Af indicates final wound area. The level of re-epithelialization at their respective WHR is shown in Table 1.
Table 1.
Level of re-epithelialization [20]
| Wound healing rate (WHR) | Level of re-epithelialization |
|---|---|
| < 0 | An increase in wound area |
| 0 | No re-epithelialization |
| > 0 | A decrease in wound area |
| 1 | Complete re-epithelialization |
-
2)
Visual presentation of the wound over time using a high-resolution camera captures the morphological changes throughout the wound healing process. The utilization of software such as ImageJ eases the measurement of wound diameter as well as the identification and distribution of various tissues.
-
3)
Biophysical investigation: optical coherence tomography (OCT), confocal laser scanning microscopy (CLSM) and diffuse near-infrared spectroscopy (DNIRS).
-
4)
Histopathological evaluation: hematoxylin & eosin (H&E) to quantify white blood cells in the inflammatory phase and examine blood vessels in angiogenesis. H&E also plays a role in assessing fibroblasts and collagen during the remodeling phase. Additionally, trichromes such as Gomori’s trichrome and Masson’s trichrome stains muscle and intercellular fibers, collagen, cytoplasm, keratin and nuclei.
-
5)
Immunohistochemistry and enzyme-linked immunosorbent assay (ELISA) are utilized to identify and quantify surface markers, cytokines, growth factors, antibodies and antigens.
-
6)
Biochemical assay such as hydroxyproline assay is used to investigate collagen synthesis or metabolism; myeloperoxidase assay is used to study the distribution of neutrophils during the inflammatory phase; N-acetylglucosaminidase assay is used to determine the level of activated macrophages.
-
7)
Flow cytometry is used to analyze the characteristics of cells involved in wound healing.
Materials and methods
Various search engines and databases, such as Google Scholar, ScienceDirect, PubMed Central and Research Gate, were used to obtain the scientific findings on six commonly available Malaysian medicinal plants with potential wound healing activity. They are Aloe barbadensis Miller, Carica papaya Linn., Centella asiatica Linn., Cymbopogon nardus Linn., Ficus benghalensis Linn. and Hibiscus rosa sinensis Linn. Some of the terms or keywords used to search for potential publications include Aloe barbadensis Miller, Carica papaya Linn., Centella asiatica Linn., Cymbopogon nardus Linn., Ficus benghalensis Linn., Hibiscus rosa sinensis Linn, wound healing, wound repair, wound dressing, chronic wound, etc.
Aloe barbadensis Miller
Aloe vera (Aloe barbadensis Miller; Family: Asphodelaceae) [24] is well known as lidah buaya in Malay. It has been widely used for the treatment of gingivitis, periodontitis, diabetes, bacterial infections, etc [25]. Aloe vera comprises 3 distinctive layers: (1) Inner clear gel that is composed of ≈ 99% water and the remaining glucomannans, amino acids, lipids, sterols, and vitamins. On a dry basis, aloe vera gel consists of 55% polysaccharides, 17% sugars, 16% minerals, 7% proteins, 4% lipids, and 1% phenolic compounds [26], (2) The middle layer is made up of latex, which is rich in anthraquinones and glycosides, (3) The outer thick layer provides a protective function and synthesizes carbohydrates and proteins [24].
Razia et al. demonstrated that the combination of processed aloe gel (PAG) and aloe vera flower extract (AVF) worked synergistically to promote in vitro wound healing effects. The combination of PAG and AVF (PAG + AVF) increased the cell migration rate and wound healing process after 24 h of treatment in normal human dermal fibroblast (NHDF) cells. However, the wound-healing effect of NHDF cells treated individually with PAG and AVF showed a slower cell migration rate. In addition, PAG + AVF increased the proliferation of NHDF cells by stimulating DNA synthesis. PAG + AVF also enhanced the phosphorylation of protein kinase B (AKT) and extracellular signal-regulated kinase (ERK) as compared to the control and positive control. PAG + AVF promoted angiogenesis by increasing the expression of VEGF and TGF-β. Furthermore, PAG + AVF upregulated the expression of microfibril-associated glycoprotein 4 (MFAP4), type I collagen (COL 1 A), α-smooth muscle (α-SMA), fibrillin and elastin. At the same time, PAG + AVF-treated HaCaT cells showed an increase in cell proliferation and cell migration that promote wound confluence. Anti-inflammatory effects were demonstrated by the decline in inflammatory cytokines such as IL-6 and IL-1β [27].
Aloe vera promotes the synthesis of hyaluronic acid and dermatan sulfate for effective wound healing [28]. Hormozi et al. demonstrated that the expression of bFGF and TGF-β1 in mouse embryonic fibroblast cells was upregulated in a dose-dependent and time-dependent manner [29]. TGF-β1 promotes wound healing by recruiting inflammatory cells and inhibiting ECM degradation. Moreover, TGF-β1 stimulates angiogenesis and fibroblast proliferation. Fibroblasts play a crucial role in the production of collagen and fibronectin, which are essential for the formation of ECM [30]. In contrast, bFGF regulates the replication and migration of epithelial, endothelial and fibroblast cells throughout the wound healing process [31].
Nanofibers formulated with ethylcellulose, hydroxypropyl methylcellulose and 10% aloe vera (EC/HPMC/Alv) improved cell proliferation and adhesion. This is attributed to the presence of aloe vera which increases the hydrophilicity and protein adhesion of nanofibers to NIH-T3T cells. In addition, cells treated with EC/HPMC/Alv did not show cytotoxicity effects regardless of its antibacterial effects against Staphylococcus aureus and Escherichia coli. EC/HPMC/Alv also showed an increase in tensile strength as compared to nanofibers. Hence, EC/HPMC/Alv is effective for rapid wound healing and as an ideal wound dressing [32].
Polylactic-co-glycolic acid (PLGA)-based nanofiber containing aloe vera extract (PLGA-AV) and aloe vera extract alone demonstrated antibacterial effects against Staphylococcus aureus and Staphylococcus epidermidis. PLGA-AV showed an increase in cell proliferation and re-epithelization as compared to control. Furthermore, db/db mice showed a significant improvement in wound closure after 8 days of PLGA-AV treatment [33].
Wound dressing formulated with polyvinyl alcohol, chitosan and aloe vera gel (PVA/CS/AV) improved wound closure by 85.93% after 14 days in adult male Wistar rats. However, simultaneous application of PVA/CS/AV and recombinant human erythropoietin (rhEpo) demonstrated a significantly greater wound healing effect (92.96%) than PVA/CS/AV. Furthermore, wounds treated with PVA/CS/AV did not show any signs of wound inflammation or infection [34].
Nanofibers composed of gelatin, aloe vera and poly (ε-caprolactone) (Gel/AV-PCL) possessed antibacterial activity against Staphylococcus aureus and Escherichia coli. These antibacterial effects are attributed to the inhibition of bacterial protein synthesis by anthraquinone-rich aloe vera. NIH-3T3 fibroblast cells showed higher cell growth after 4 days of treatment with Gel/AV-PCL. Moreover, a significant increase in cell proliferation was observed [35].
Oral administration of aloe vera significantly speeded up recovery of diabetic wounds among Goto-Kakizaki rats. Furthermore, an increase in inflammatory cell infiltration, angiogenesis, epithelialization and ECM deposition was observed. At the same time, the level of TGF-β1 and VEGF was upregulated by aloe vera [36].
Acemannan is recognized as one of the major polysaccharides found in aloe vera gel. Jettanacheawchankit et al. demonstrated that 2 to 16 mg/mL of acemannan significantly stimulated DNA synthesis and subsequently increased gingival fibroblast proliferation. Furthermore, the expression of keratinocyte growth factor-1 (KGF-1), VEGF and COL 1 A was upregulated by acemannan [37]. Gingival fibroblast plays a vital role in wound healing by generating ECM proteins and growth factors. For instance, KGF-1 accelerates re-epithelialization of the wound, VEGF causes angiogenesis, whereas COL 1 A maintains the structure of ECM for cell adhesion and migration [38–40]. Xing et al. reported that acemannan promoted cell proliferation and wound healing through AKT/mTOR signal pathway and upregulation of cyclin D1 [41]. On the contrary, aloe-derived glucomannan binds specifically to β2-integrins (LFA-01 and Mac-1) in fibroblast. This further enhanced the adhesion, migration and proliferation of fibroblast cells. The growth hormone present in aloe (Gibberellins) also stimulates fibroblast proliferation and protein synthesis [42]. Hence, the wound healing effects of aloe vera are mainly contributed to the synergistic effects of acemannan, glucomannan and gibberellins in promoting fibroblast proliferation.
The angiogenic effect of aloe vera gel is mainly attributed to β-sitosterol [43]. Aloesin from aloe vera is involved in the activation of Smad 2, Smad 3 and mitogen-activated protein kinase (MAPK) signaling pathways. As a result, an increase in the expression of TGF-β1, keratinocyte migration, collagen synthesis and angiogenesis were observed. In vivo study demonstrated that aloesin-treated mice showed a significantly higher wound closure rate than vehicle-treated mice. Furthermore, aloesin-treated mice promoted neoepithelium formation and increased the rate of granulation tissue formation, epidermal regeneration and dermal regeneration [44].
Published studies demonstrated that the administration of topical preparation containing aloe vera gel or aloe vera extract is generally well tolerated without any adverse effects or serious side effects [45–50]. However, highly concentrated aloe vera (98%) is reported to cause erythema and desquamation of skin [51]. Guo and Mei reported that topical and oral administration of aloe vera causes skin irritation, urticaria, cramping and diarrhea in patients who are allergic to Liliaceae family. In addition, aloe vera gel causes discomfort, pain and hypersensitive reaction [52]. Although aloe vera is beneficial in promoting wound healing, its complex chemical nature, concentration, composition and individual contaminants should be taken into consideration during the formulation process. Furthermore, encapsulation of aloe vera extract for drug administration provides controlled drug release as compared with direct administration of aloe vera extract [49].
Carica papaya Linn
Papaya (Carica papaya Linn.; Family: Caricaceae) [53] is widely cultivated in tropical countries such as Malaysia with a self-sufficiency ratio (SSR) of 153.1%. A SSR of more than 100% indicates that the supply of papaya is sufficient to meet local demands [54]. The fruit, juice, seed, root, leaves bark and latex of papaya are rich in phytochemicals, vitamins and minerals. Hence, it is well studied from ancient times till now for its medicinal use. Papaya was proven to have antioxidant, anti-hypertensive, anti-inflammatory, anti-helminthic, antimicrobial, hepatoprotective and wound healing effects [55].
C. papaya leaves extract was shown to promote cell proliferation and collagen synthesis throughout the wound healing process. However, its efficacy varies depending on the extraction method and the solvent used in the extraction process [56]. In vivo study demonstrated that C. papaya extract significantly reduced the length of the incised oral wound in mice. Hakim et al. also concluded that the topical application of papaya extract promoted epithelization and fibrillation of the wound [57].
Furthermore, ethanolic and aqueous papaya leaf extract showed antibacterial effects against Coliform bacillus, Staphylococcus epidermidis, Streptococcus viridans and Escherichia coli [58]. According to Femilian et al., ethanolic papaya leaf extract promoted the healing of buccal ulcers in Wistar rats by regulating macrophage activity, angiogenesis and re-epithelization [59].
Gel formulation containing C. papaya L. leaf extract nanoparticles and 1% chitosan increased the number of fibroblasts in the tooth extraction socket of Wistar rats after 7 days. The number of fibroblasts increased proportionally with the concentration of C. papaya L. leaf extract. Among the concentrations tested, 25% of the leaf extract of C. papaya L. showed a lower number of fibroblasts compared to the positive and negative control [60].
According to Rashid et al., an average of 7 papaya dressings were required to promote the growth of healthy pink granulation tissues among patients with deep burn wounds. This is achieved by the enzymatic proteolytic debridement of deep burn wounds by the topical application of papaya. Therefore, it may help reduce the healthcare burden by preventing the risks associated with surgical debridement. Furthermore, the spilt-thickness skin graft was accepted by the papaya-treated pink granulation tissue bed [61].
A case study in Nigeria utilized unripe pawpaw dressing for the treatment of sacral wounds secondary to chemoradiation. As a result, unripe pawpaw dressing promoted the growth of healthy granulation tissue for wound bed preparation. Furthermore, 100% flap survival and consolidation were observed [62]. Xue et al. demonstrated that the hydrogel containing poly (γ-glutamic acid) (γ-PGA), chitooligo-saccharide, and papain promoted wound healing. Additionally, papain inhibited hypertrophic scar formation by regulating fibroblast proliferation and collagen deposition [63].
Simultaneous use of unripe crushed papaya dressing and leeching was effective in the treatment of nonhealing ulcers. This is attributed to the enzymatic debridement effect of unripe papaya. Enzymatic debridement stimulates the growth of healthy granulation tissue. Moreover, leech saliva is having analgesic, anti-inflammatory, antiplatelet, anticoagulant and antimicrobial effects. Therefore, raw papaya and leeching work synergistically to promote wound healing effects [64]. Unripe papaya pulp acts as an enzymatic wound debriding agent due to the presence of heat-resistant proteolytic enzymes such as papain and chymopapain. It promoted the healing of deep burn wounds by producing healthy granulation tissue and loosened eschar for easy removal. Furthermore, papaya is rich in Vitamin C, which stimulates collagen synthesis [65].
Purified papain preparations were reported to cause severe hypersensitivity reactions but not in papaya fruit. Approximately 1% of papain causes anaphylactic reaction in children [66]. However, papaya fruit dressing should be used cautiously for the first time. Due to the safety issue of purified papain, standardization of papaya extract is necessary for the development of papaya dressings. Furthermore, the identification of other active constituents in papaya and their mechanism for wound healing should be further studied [67]. On the other hand, oral consumption of papaya leaf in the form of juice or standardized aqueous extract is widely used to treat dengue and non-dengue-associated thrombocytopenia. It is generally safe for short term treatment but should be use in caution during pregnancy and in patient with liver impairment. Besides, oral consumption of papaya leaf causes mild gastrointestinal disturbances and rash. Herb-drug interaction was reported for papaya leaf with oral hypoglycaemic drugs such as metformin and glimepiride, p-glycoprotein substrates such as digoxin, antibiotics with cation chelating properties such as ciprofloxacin etc [68, 69]. Thus, a detailed and accurate patient medication history should be taken to avoid coadministration of the drugs with papaya leaf extract. Moreover, in vivo study demonstrated that standardized papaya extract causes minimal fibrosis in spleen [70].
Centella asiatica Linn
Centella asiatica Linn. (Family: Apiaceae) [71] is well known as pegaga in Malay. It is widely used in the medical field due to its antimicrobial, anti-inflammatory, anti-cancer, neuroprotective, antioxidant, anti-depressant, antidiabetic, hepatoprotective and wound healing activity [72]. Asiatic acid, madecassic acid, asiaticoside and madecassoside are identified as the active constituents present in C. asiatica [73].
Non-cross-linked asiaticoside-polyvinyl alcohol-sodium alginate nanofibers (AT-PVA-SA-NF) showed greater antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa than cross-linked AT-PVA-SA-NF. The cell migration assay demonstrated that nanofibers containing AT promote wound re-epithelialization, which is vital for the wound healing process. The in vivo study demonstrated that the proliferation phase of the nanofiber-treated group started on day 7 with an increased number of fibroblasts and a reduced number of inflammatory cells. In addition, epithelialization and collagen synthesis were observed on day 7 and 14. The study reported that the maturation and remodeling phases of diabetic wound healing were completed on day 14 in the nanofiber-treated group. Therefore, nanofibers containing asiaticoside are effective in healing diabetic wound [74].
The wound healing rate of normal wounds after 14 days of treatment with Centella asiatica total glycosides nitric oxide gel (CATGNOG) was dose-dependent (medium dose > low dose > high dose), similar to the diabetic wounds. Whereby, the low dose group contains 4% C. asiatica total glycosides, the medium dose group contains 8% C. asiatica total glycosides and the high dose group contains 16% C. asiatica total glycosides [75]. Nitric oxide promotes wound healing by dilating capillaries, improving microcirculation and regulating growth factors and cytokines [76]. In contrast, C. asiatica plays a role in epidermal epithelialization, collagen synthesis and proliferation of fibroblasts [75]. The combination of C. asiatica total glycosides and nitric oxide works synergistically to accelerate wound healing in normal and diabetic wounds.
Asiaticoside-treated human foreskin fibroblasts (HFF-1) demonstrated a lower number of inflammatory cells but a greater distribution of fibroblasts, collagen deposition and angiogenesis as compared to the model group. In addition, HFF-1 showed greater cell migration and proliferation which are vital for the repair of diabetic ulcers. In vivo and in vitro studies demonstrated that asiaticoside acts by increasing the expression of Wnt/β-catenin signaling cascade-linked proteins (Wnt1 and Wnt 4), c-myc, total β-catenin and nuclear β-catenin and increasing Glycogen synthase kinase 3β (GSK3β) phosphorylation level [77]. The role of Wnt/β-catenin signaling cascade in wound healing is demonstrated by the regulation of cell migration, proliferation and differentiation [78].
Somboonwong et al. demonstrated that hexane, ethyl acetate, methanol and aqueous extract of Centella asiatica increased the healing rate of both incision and burn wounds. Thin-layer chromatography revealed that the main active constituents present in hexane, ethyl acetate and methanol extract were β-sitosterol, asiatic acid and asiaticoside and madecassoside respectively [79]. For instance, β-sitosterol promotes angiogenesis, while asiatic acid induces the expression of TNF Alpha-Induced Protein 6 (TNFAIP6) that regulates ECM synthesis and inflammatory cascades in human fibroblasts [80, 81]. In contrast, asiaticoside upregulates the synthesis of type I collagen via TGFbeta receptor I kinase-independent Smad signaling pathway and exerts antioxidant effects [82]. Additionally, extract-treated groups demonstrated a significant increase in tensile strength, which is attributed to the increase in collagen synthesis. Extract-treated groups also showed complete epithelialization and keratinization without fibrinoid necrosis [79].
Asiaticoside, madecassoside, asiatic acid and madecassic acid were examined for their wound healing effects in vitro and in vivo. RT-PCR showed that asiaticoside and madecassoside significantly increased the mRNA levels of type I and type II collagen, while ELISA demonstrated an increase in the levels of procollagen Type I and Type III. The in vitro study showed that collagen synthesis is mainly attributed to the activation of TGF-β/Smad pathway. This is evidenced by the elevated p-Smad 3 levels, increased TGF-β1 and TβRII mRNA levels and decreased Smad 7 expression. Furthermore, madecassoside demonstrated a greater wound healing effect than asiaticoside. In vivo studies showed that both asiaticoside and madecassoside accelerated wound healing. In contrast, asiatic acid and madecassic acid did not show any wound healing effects in vitro and in vivo [83].
Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Klebsiella pneumonia are the most common bacteria that cause wound contamination. Bamboo Fabric Coated Centella asiatica (BFC-Ca) was shown to have antibacterial activity against bacteria. Moreover, in vivo study revealed that BFC-Ca-treated albino Wistar rats showed significant wound healing effects within 17 days of treatment as compared to the untreated group. Despite antibacterial effects, BFC-Ca demonstrated excellent air permeability and swelling properties to provide optimal wound healing conditions [84].
The molecular docking of compounds derived from Centella asiatica with wound healing associated proteins was examined using Schrödinger Release 2020-2. As a result, madecassic acid, madecassoside and asiatic acid were strongly bound to TNF-α (proinflammatory cytokines). In contrast, asiaticoside showed good binding affinity against Nrf2-Keap1, whereby Nrf2-Keap1 regulated reactive oxygen species (ROS) generation in response to oxidative stress. MM-GBSA reported that madecassic acid inhibited the degradation of antioxidants and collagen; asiatic acid inhibited proinflammatory cytokines, while asiaticoside played a role in collagen synthesis. Hence, the binding affinity of madecassic acid, madecassoside, asiatic acid and asiaticoside to the target sites renders their potential as excellent wound healing agents [85]. Madecassoside, terminoloside and isomadecassoside isolated from C. asiatica inhibited lipopolysaccharide-induced nitrite production, thus possessing anti-inflammatory action which may accelerate the wound healing process [86].
Chitosan–aluminum monostearate composite sponge dressing containing asiaticoside was shown to significantly enhance the proliferation of NHDF and normal human epidermal keratinocytes (NHEK). Furthermore, the dressing containing asiaticoside demonstrated dose-dependent angiogenic activity in the chick-chorioallantoic membrane assay. Fibroblast proliferation and angiogenesis work together to promote the healing of chronic wounds [87].
The case report demonstrated that a 15-year-old child and 3 middle-aged women experienced a decline in liver function after taking C. asiatica. The consumption of C. asiatica is reported to be potentially hepatotoxic due to the presence of pentacyclic triterpene derivatives [88, 89]. Thus, the risk of hepatoxicity should be considered especially if patient is taking other hepatotoxic agents such as tetracycline. High doses of C. asiatica may cause headache, mild gastrointestinal disturbances, dizziness and drowsiness [90]. Hence, oral administration of C. asiatica should be further studied to determine its efficacy and safety in promoting wound healing. On the other hand, topical administration of C. asiatica is generally safe at the recommended dose and may cause allergic reactions and burning [91].
Cymbopogon nardus Linn
Citronella grass (Cymbopogon nardus Linn.; Family: Poaceae) [92] is also known as serai wangi in Malay. Hydro-distillation of C. nardus yields approximately 3% essential oil. The main compounds identified in essential oil are citral (38.75%), 2,6-octadienal,3,7-dimethyl- (31.02%), citronellal (6.06%), geranyl acetate (4.28%), geraniol (2.75%) and citronellol (1.89%) [93]. The essential oil of C. nardus showed antioxidant, anti-inflammatory, antiproliferative, and mosquito repellent activity [92, 94, 95].
The diabetic wound heals slowly and provides favorable conditions for the growth of Candida species. The essential oil of C. nardus (EO-CN) inhibited the growth of Candida albicans (MIC: 25 µg/ml), Candida glabrata (MIC: 50 µg/ml) and Candida tropicalis (MIC: 50 µg/ml). Furthermore, EO-CN significantly reduced Candida load at the infected site. EO-CN renders its anti-inflammatory activity by significantly reducing the levels of inflammatory markers (TNF-α and IL-1β). Both the anti-Candida and anti-inflammatory action of EO-CN accelerated diabetic wound healing [93].
In vivo study demonstrated that citronellal promoted the healing of burn wounds, whereby 1% citronellal showed the fastest recovery rate [96]. Citronella grass extract and its gel preparation showed antibacterial effects against Staphylococcus aureus and Escherichia coli. However, further tests need to be conducted to identify their MIC and MBC [97]. Antibacterial effects of citronella oil against Staphylococcus aureus, Staphylococcus epidermidis, Acinetobacter baumannii, Pseudomonas aeruginosa and Streptococcus pyogenes are beneficial to prevent secondary infection of wounds [98]. The antibacterial and antifungal effects of citronella oil are mainly attributed to citronellal [99]. Besides, citronella essential oil and its active compound, geraniol were bactericidal against Staphylococcus aureus through biofilm inhibition [100].
Due to the lipophilic nature of essential oils, they bypass the blood-brain barrier and cause neurological toxicity as shown in essential oils of Salvia officinalis, Thuja plicata, Eucalyptus species, etc. The consumption of lavender oil or tea tree oil causes endocrine disruption. Besides, topical application of essential oils causes skin irritation, photosensitization, sensitization and dermatitis [101]. Moreover, the volatility of essential oils leads to the rapid loss of activity, hence, extended-release systems should be utilized to prolong the duration of contact and minimize toxicity effects arising from the use of essential oils.
Ficus benghalensis Linn
Various parts of the Banyan tree (Ficus benghalensis Linn.; Family: Moraceae) [102] have their own medicinal benefits. For instance, root possesses anti-glycemic, antibacterial, lipid-lowering and immunostimulatory activity in addition to its role in treating dysentery, pimples, diarrhea and hair fall. Stem bark acts as an anti-arthritic, antibacterial, anti-diabetic, anti-inflammatory, analgesic and antifungal agent. The leaves have hypoglycemic, antibacterial and immunostimulatory action, while latex promotes conception [102, 103]. Compounds such as lanostadienylglucosyl cetoleate, bengalensisteroic acid acetate, protodioscin, leucocyanidin, pelargonidin, lupenyl acetate and lanosterol are present in Ficus benghalensis [102].
F. benghalensis extract showed greater cell migration at the wound site as compared to silver nitrate and the control group. However, the percentage of wound closure of F. benghalensis extract (62%) was lower than silver nanoparticles (70.2%) and rifampicin (84%). Besides, F. benghalensis extract showed weaker antibacterial effects than silver nanoparticles and rifampicin. In conclusion, F. benghalensis extract accelerated wound healing by promoting cell migration and inhibiting bacterial growth at the wound site [104].
The excision wound healing model was utilized to examine the wound healing effects of F. benghalensis leaves which were successively extracted using petroleum ether, 95% ethanol and distilled water. The ointment containing 4% petroleum ether extract showed complete wound healing after 21 days, while the ointment containing 4% ethanolic extract and 4% aqueous extract took 25 days and 28 days respectively, for 100% wound closure. The results were comparable to the positive control (nitrofurazone). Hence, F. benghalensis extract is suitable to be used as a wound-healing agent [105].
F. benghalensis extract derived from leaves, roots and fruits demonstrated dose-dependent antibacterial effects against Staphylococcus aureus, Escherichia coli, Pseudomonas protobacteria and Bacillus cereus. This is vital for the prevention of secondary infection at the wound site due to the maintenance of a bacterial-free environment [106]. Silver nanoparticles formulated with aqueous F. benghalensis leaf extract also showed bactericidal activity against Escherichia coli [107]. F. benghalensis bark extract was shown to have anti-inflammatory action by reducing ROS generation, downregulating the expression of TNF-α and upregulating the expression of IL-10. However, the contribution of its anti-inflammatory action toward wound healing requires further justification [108]. F. benghalensis leaves extract showed the greatest wound healing effect in albino Wistar rats as compared to F. microcarpa, F. hispida and F. religiosa. Furthermore, its percentage of wound closure (97.20%) was comparable to Betadine (97.67%) [109].
In vitro wound healing model demonstrated that ethanolic and hydroalcoholic extract of F. benghalensis inhibited hemolysis. However, the extract of F. religiosa showed greater inhibition of hemolysis than F. benghalensis [110]. A high percentage of hemolysis inhibition makes it suitable for topical application due to its excellent blood compatibility [111]. The in vivo wound healing model showed that the ethanolic extract of F. benghalensis took 17 days for complete wound healing, whereas the hydroalcoholic extract of F. benghalensis took 18 days for complete wound healing, similarly to F. religiosa [110].
In silico study demonstrated that lupenyl acetate and lanosterol showed anti-inflammatory action through the inhibition of TNF-α and vascular endothelial growth factor receptor (VEGFR), which helps to accelerate diabetic wound healing [112]. Silver nanoparticles synthesized from F. benghalensis extract stimulate the production of transforming growth factor-alpha (TGF-α) in a dose-dependent manner [113]. The synthesis of TGF-α promotes endothelial proliferation, migration and angiogenesis which helps to accelerate wound healing [114].
Aqueous extract of F. benglensis did not show any signs and symptoms of acute oral toxicity at the dose of 5000 mg/kg in adult albino Wistar strain rats [115]. Khan et al. demonstrated that the LD50 of the aqueous extract of F. benghalensis was 2500 mg/kg in random-bred albino rats [116]. To date, there is no adverse effects or death cases reported with the topical use of F. benghalensis.
Hibiscus rosa sinensis Linn
Chinese hibiscus (Hibiscus rosa sinensis Linn.; Family: Malvaceae) [117] was declared by Malaysia’s first prime minister, Tunku Abdul Rahman as the national flower of Malaysia on 28 July 1960. It is known as Bunga Raya (“celebratory flower”) in Malay. The five petals on the Chinese hibiscus symbolize the five aspects of the National Principles of the Rukun Negara and a symbol of unity [118]. H. rosa sinensis is traditionally used as a cough suppressant, hair growth promoter, diuretic and remedies for dysentery, diarrhea, heart diseases, diabetes, epilepsy, leprosy, etc [119].
The ethanolic extract of Hibiscus rosa sinensis leaves (EEHRL) showed dose-dependent free radical scavenging activity via DPPH, nitric oxide and superoxide radical scavenging assay. Four different experimental animal models were utilized to study the wound healing effects of EEHRL. The percentage of excision wound closure increased significantly after 15 days of EEHRL treatment. The percentage of excision wound closure and the epithelization period of EEHRL was also comparable to 5% povidone-iodine. In addition, EEHRL increased wound breaking strength in incision wounds, which was comparable to 5% povidone iodine. This may be due to an increase in collagen synthesis and thus stabilization of the collagen fibers. In addition, EEHRL promoted the contraction of burn wounds, most probably by stimulating the growth of keratinocytes and fibroblasts. In dead space wounds, EEHRL-treated wounds showed an increase in granulation tissue weight and hydroxyproline content. Increased hydroxyproline concentration leads to faster collagen turnover and thus accelerates wound healing. EEHRL also showed an increase in antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) in granulation tissue, which helps in the removal of oxidative stress that causes the delay in wound healing [120].
Oral administration of ethanolic H. rosa sinensis flower extract demonstrated significantly greater excision wound healing effects as compared to the control group (86% vs. 75%) after 15 days of treatment. Besides, the extract-treated group showed significantly faster epithelialization as compared to the control group (14 days vs. 18 days). Meanwhile, extract-treated incision wounds showed significantly higher tensile strength than the control group. Extract-treated dead space wounds showed a significant increase in granulation tissue weight and hydroxyproline concentration as compared to the control group. Therefore, oral administration of ethanolic H. rosa sinensis flower extract possessed an excellent wound healing effect in excision, incision and dead space wound. Granulation tissues of extract-treated wounds consisted of a higher number of collagen and fibroblast, but a lesser number of inflammatory cells as compared to the control group [121].
Incisional wounds of Sprague Dawley rats demonstrated complete re-epithelialization and remodeling of granulation tissues after 15 days of treatment with aqueous and ethanolic extract of H. rosa sinensis. Among the extracts, the ethanolic extract showed the highest tensile strength on wounded skin. Tensile strength is mainly attributed to collagen deposition at the wound site. Thus, both extracts are having potential as wound-healing agents due to their in vivo efficacy in incision wounds [122].
N-butyl alcohol extract of H. rosa sinensis flowers (NHRS) accelerated excision wound healing by increasing collagen deposition, collagen maturity and fibroblast distribution. Besides, NHRS upregulated the expression of vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGF-β1), which are vital for angiogenesis and ECM deposition respectively. The epidermal epithelialization of NHRS-treated wounds was greater than the recombinant bovine basic fibroblast growth factor (rbFGF). The collagen deposition and ECM deposition of extract-treated wounds are attributed to the activation of macrophages and fibroblasts by NHRS. Extract-treated wounds also possessed anti-inflammatory activity by reducing the number of inflammatory cells [123].
The chicken egg chorioallantoic membrane (CAM) assay revealed that the ethanolic extract of H. rosa sinensis flowers showed concentration-dependent angiogenesis. Angiogenesis promotes wound healing by replenishing the blood supply to the wound site [124]. The aqueous, ethanol and methanol extract of H. rosa sinensis showed antibacterial activity against food poisoning bacteria such as Staphylococcus aureus, Bacillus cereus, Clostridium perfringens, Listeria monocytogenes, Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa [125].
Antibacterial agents prevent secondary infection at the wound site, thus promoting wound healing. A nanocomposite made of zinc oxide nanoparticles containing H. rosa sinensis leaves extract (ZnO-NPs) and cellulose exhibited antibacterial activity against Staphylococcus aureus and Escherichia coli. According to Elemike et al., cellulose did not show antibacterial activity, while the nanocomposite showed greater antibacterial acidity against S. aureus and E. coli as compared to H. rosa sinensis leaves extract and ZnO-NPs. This is due to the synergistic effects brought about by the extract and zinc oxide and the larger surface area of the nanocomposite [126]. Silver nanoparticles synthesized using flower, leaf and bark extracts of H. rosa sinensis possessed antibacterial activity against S. aureus, B. subtilis, P. aeruginosa and E. coli. This is attributed to the large surface area of silver nanoparticles that facilitate the penetration of silver ions into the cell membrane and interrupts the DNA replication of bacteria. Hence, it prevents the multiplication and growth of bacterial cells [127].
The ethanolic extract of H. rosa sinensis flowers (EEHRS) showed significantly greater excision wound contraction than the control (untreated) and the reference standard (0.2% nitrofurazone ointment). Furthermore, complete healing was observed at 18 days and 16 days for 5% EEHRS ointment and 10% EEHRS ointment respectively. The incision wound model demonstrated a significant increase in tensile strength after treatment with 5% and 10% EEHRS ointment as compared to the control group. The increase in tensile strength is attributed to the stimulation of collagen synthesis and maturation by EEHRS. On the other hand, the dead space wound model treated with 5% and 10% EEHRS ointment showed a significant increase in wet granuloma weight, dry granuloma weight and tensile strength as compared to the control group. The biochemical evaluation of wet granulation tissues showed a significantly higher DNA, protein, collagen, hexosamine and uronic acid content on days 4, 8 and 12, as compared to the control group. Among these, hexosamine and uronic acid play a role in the synthesis of extracellular matrix (ECM) [128].
Swiss albino mice treated with green nanofiber mat synthesized from hibiscus leaves mucilage-Pectin-Polyvinyl alcohol (HLM-Pectin-PVA) were shown to have greater collagen and fibroblast growth as compared to the control group. Furthermore, HLM-Pectin-PVA demonstrated faster wound healing (85.71% vs. 60%) and wound contraction (30 mm2 vs. 21 mm2) than the control group after 8 days of treatment. The non-hemolytic nature of HLM-Pectin-PVA makes it suitable as a wound dressing due to its hemocompatibility [129]. An herbal ointment containing H. rosa sinensis flower extract and Curcuma longa rhizomes extract works synergistically to speed up the wound healing process. This is attributed to the anti-inflammatory and antioxidant effects of the active constituents present in the extracts [130].
Acute oral toxicity study in Swiss albino mice demonstrated that the methanolic extract of H. rosa sinensis leaf was relatively safe with LD50 of more than 2000 mg/kg, which is similar to hydroalcoholic extract of Hibiscus rosa sinensis flower in Wistar rats. Kandhare et al. demonstrated that hydroalcoholic extract of H. rosa sinensis leaf was safe up to 5000 mg/kg in Swiss albino mice. Subacute toxicity study of the extract did not show any adverse effects at 400 mg/kg, but hepatotoxicity and renal toxicity were observed at 800 mg/kg of extract [131–133]. Besides, crude H. rosa sinensis flower extract demonstrated anti-fertility effect in male albino rats, which is beneficial for birth control [134]. In vivo study demonstrated that the oral administration of aqueous H. rosa sinensis flower extract increased cardiovascular risk in non-diabetic pregnant Wistar rats. In addition, the extract possessed anti-implantation, abortion and anti-fertility effects which may be harmful to healthy, pregnant women. Hence, H. rosa sinensis flower extract should be avoided during pregnancy [135]. To date, there is no adverse effects or death cases reported with the topical use of H. rosa sinensis.
It can be concluded that wound healing is accelerated by the increase in the rate of cell proliferation, cell migration, angiogenesis, epithelialization, extracellular matrix deposition and collagen synthesis. In addition, the antibacterial, antioxidant and anti-inflammatory actions of Malaysian medicinal plants provide an additional advantage for the recovery of wounds. The wound healing effects may be attributed to the whole medicinal plant extract or the specific compound present in the medicinal plant or the synergistic effects of the medicinal plant/compound to other herbs/compounds. Thus, the study of possible interaction between one medicinal plant towards others is crucial to ensure that the combination is optimal for wound healing. Besides, the interaction between the medicinal plant extract, excipients (e.g.: polymer/dressing) and packaging materials (e.g.: glass/plastic) should also be considered to ensure the stability, quality and safety of the final product. Table 2 summarizes six Malaysian medicinal plants with potential wound healing activity. Currently, wound healing preparations containing Cymbopogon nardus Linn., Ficus benghalensis Linn. and Hibiscus rosa sinensis Linn. are not available in the market. However, wound healing preparations containing Aloe barbadensis Miller, Carica papaya Linn. and Centella asiatica Linn. are available in the market as shown in Table 3.
Table 2.
Malaysian medicinal plants with potential wound healing activity
| Medicinal plant | Active | Study | Model/method used | Mechanism of action | Reference |
|---|---|---|---|---|---|
| ingredient | |||||
| Aloe barbadensis Miller | 50 µg/mL processed aloe gel + 25 µg/mL or 100 µg/mL aloe vera flower extract | In vitro | Cell migration assay, BrdU cell proliferation assay, scratch wound healing assay, qRT-PCR, western blotting, ELISA, MFAP 4 specific siRNA knockdown | Increases cell migration rate | [27] |
| Increases proliferation of NHDF cells | |||||
| Increases the expression of VEGF and TGF-β | |||||
| Reduces inflammatory cytokines (IL-6 and IL-1β) | |||||
| Upregulates the expression of MFAP4, COL 1A, α-SMA, fibrillin and elastin | |||||
| Nanofibers containing 3% aloe vera | In vitro | MTS assay | Promotes the synthesis of hyaluronic acid and dermatan sulfate | [28] | |
| 50, 100 and 150 µg/mL aloe vera gel | In vitro | Mouse embryonic fibroblasts, RT-PCR, ELISA | Increases the expression of bFGF and TGF-β1 | [29] | |
| Nanofibers containing 10% aloe vera powder | In vitro | NIH-3T3 cell viability, SEM technique | Increases cell proliferation and adhesion | [32] | |
| Agar disc diffusion assay | Antibacterial effects against S. aureus and E. coli | ||||
| Nanofibers containing aloe vera extract | In vitro | Agar disc diffusion assay | Antibacterial effects against S. aureus and S. epidermidis | [33] | |
| BalbC/3T3 A31 fibroblast bioactivity assay | Increase in cell proliferation | ||||
| In vivo | Full-thickness wound healing assay in male db/db mice | Significant improvement in wound closure and reepithelization | |||
| Wound dressing containing aloe vera gel | In vivo | Full-thickness excisional wound model in male Wistar rats | Improves the wound closure | [34] | |
| Anti-inflammation | |||||
| Nanofibers containing 5% or 10% aloe vera powder | In vitro | Disc diffusion, broth dilution method | Antibacterial activity against S. aureus and E. coli | [35] | |
| Cell-scaffold interaction, FE-SEM | Increases the cell growth of NIH-3T3 fibroblast cells | ||||
| Colorimetric MTT assay | Increases cell proliferation | ||||
| 30 mg lyophilized aloe vera powder dissolved in 1.5 mL purified water | In vivo | Full-thickness skin wound excision in Goto-Kakizaki rats | Speeds up the recovery of diabetic wounds | [36] | |
| Increases inflammatory cell infiltration, angiogenesis, epithelialization and extracellular matrix deposition | |||||
| Increases TGF-β1 and VEGF | |||||
| Acemannan | In vitro | Human gingival fibroblasts, [3H]-thymidine incorporation assay | Stimulates DNA synthesis | [37] | |
| Increases gingival fibroblast proliferation | |||||
| ELISA | Increase the expression of KGF-1, VEGF and COL 1A | ||||
| Carbopol® containing 0.5%, 1% and 2% acemannan | In vivo | Punch biopsy wounds from the hard palate of male Sprague Dawley rats | Reduces oral wound areas | ||
| Increase re-epithelialization | |||||
| Acemannan | In vitro, in vivo | 35S-methionine incorporation assay, phosphorylation of AKT in acemannan-stimulated fibroblast cells, m7 GTP pull-down assay, full-thickness skin excisional wound model in male BALB/c mice, in vivo block of mTOR signaling by rapamycin | Promotes wound healing through AKT/mTOR signal pathway | [41] | |
| Upregulates cyclin D1 | |||||
| Glucomannan | In vitro | FE-SEM | Binds to β2-integrins (LFA-01 and Mac-1) on fibroblast | [42] | |
| Gibberellins | In vitro | MTS assay | Stimulates fibroblast proliferation and protein synthesis | ||
| β-sitosterol | In vitro | Chorioallantoic membrane assay | Enhances angiogenesis | [43] | |
| Aloesin | In vitro | Western blotting, ELISA | Activation of the Smad 2, Smad 3 and MAPK signaling pathways. Increases the expression of TGF-β1 | [44] | |
| Scratch wound healing assay | Promotes keratinocyte migration | ||||
| Masson’s trichrome staining | Promotes collagen synthesis | ||||
| Endothelial cell tube formation assay | Promotes angiogenesis | ||||
| 0.1% and 0.5% aloesin solution | In vivo | Full-thickness wounds in SKH-1 hairless mice | Promotes the formation of neoepithelium | ||
| Increases the rate of granulation tissue formation, epidermal regeneration and dermal regeneration | |||||
| Carica papaya Linn. | Methanolic leaf extract | In vitro | Scratch assay | Promotes cell proliferation | [56] |
| Sircol collagen assay | Promotes collagen synthesis | ||||
| 25%, 50% and 75% fruit extract | In vivo | Incised wound in Mus musculus | Promotes epithelization and fibrillation of the wound | [57] | |
| Reduces the length of the incised oral wound in mice | |||||
| Ethanolic aqueous leaf extract | In vitro | Disc diffusion, broth dilution method | Antibacterial effects against C. bacillus, S. epidermidis, S. viridans and E. coli | [58] | |
| Ethanolic leaf extract | In vivo | Oral ulcers in Wistar rats | Regulates the activity of macrophages, angiogenesis and re-epithelization | [59] | |
| Papaya paste | Clinical study | Deep burn wound | Promote the growth of healthy granulation tissue | [61] | |
| Enzymatic proteolytic debridement | |||||
| Unripe pawpaw dressing | Case report | Sacral radiation ulcer | Promotes the growth of healthy granulation tissue | [62] | |
| Hydrogel containing 0.4%, 1%, 2% and 4% papain | In vitro | NIH/3T3 fibroblasts activity | Regulates fibroblasts proliferation | [63] | |
| Hydrogel containing 4% papain | In vivo | H&E staining | Inhibits the formation of hypertrophic scar | ||
| Masson staining | Regulates collagen deposition | ||||
| Unripe crushed papaya dressing | Case study | Non-healing chronic ulcer | Enzymatic debridement effect | [64] | |
| Papain, chymopapain, vitamin C | Clinical study | Second-degree and third-degree burns | Promotes the growth of healthy granulation tissue | [65] | |
| Loosens eschar for easy removal | |||||
| Stimulates collagen synthesis | |||||
| Centella asiatica Linn. | Nanofibers containing asiaticoside | In vitro | Agar well disc diffusion method, time kill assay | Antibacterial activity against S. aureus and P. aeruginosa | [74] |
| In vitro scratch assay | Promotes wound re-epithelialization | ||||
| In vivo | Single circular full thickness wound in albino Wistar male rats | Increases fibroblast proliferation | |||
| Reduces inflammatory cells | |||||
| Stimulates epithelialization and collagen synthesis | |||||
| Gel containing 8% C. asiatica total glycosides | In vivo | Diabetic cutaneous ulcers in Sprague Dawley rats | Promotes epidermal epithelialization | [75] | |
| Promotes collagen synthesis | |||||
| Stimulates fibroblast proliferation | |||||
| Fabric coated C. asiatica extract | In vitro | Fabric diffusion method | Antibacterial activity against E. coli, S. aureus, B. subtilis and K. pneumonia | [84] | |
| In vivo | Wound on dorsal side of male albino Wistar rats | Significant wound healing effects | |||
| Dressing containing 0.012% asiaticoside | In vitro | Cytotoxicity study | Enhance the proliferation of NHDF and NHEK | [87] | |
| Chick-chorioallantoic membrane assay | Promotes angiogenesis | ||||
| Asiaticoside | In vitro, in vivo | H&E staining | Reduces the number of inflammatory cells | [77] | |
| Increases fibroblast distribution | |||||
| Enhance angiogenesis | |||||
| Masson staining | Increases collagen deposition | ||||
| CCK8 assay, transwell assay, flow cytometry | Stimulates cell migration and proliferation | ||||
| Diabetic rats with full-thickness wounds, western blotting | Increases the expression of Wnt/β-catenin signaling cascade-linked proteins (Wnt1 and Wnt 4), c-myc, total β-catenin and nuclear β-catenin | ||||
| Increases GSK3β phosphorylation level | |||||
| 10% hexane, ethyl acetate, methanol and aqueous extract | In vivo | Male Sprague Dawley rats with incision and burn wound | Increases the healing rate of incision and burn wounds | [79] | |
| Increases in tensile strength by collagen synthesis | |||||
| Complete epithelialization and keratinization | |||||
| β-sitosterol | In vitro | Chick embryo chorioallantoic membrane assay | Promotes angiogenesis | [81] | |
| Asiatic acid | In vitro | cDNA microarrays, RT-PCR | Induces the expression of TNFAIP6 | [80] | |
| Asiaticoside | In vitro | Smad immunoprecipitation and blotting, western blotting | Upregulates the synthesis of COL 1A via TGF-β receptor I kinase-independent Smad signaling pathway | [82] | |
| Asiaticoside | In vitro, in vivo | RT-PCR, ELISA, western blotting, full-thickness burn injury in male ICR mice | Increases the mRNA levels of type I and type II collagen | [83] | |
| Increase in procollagen type I and type III levels | |||||
| Activation of TGF-β/Smad pathway | |||||
| Accelerates wound healing | |||||
| Madecassoside | In vitro, in vivo | RT-PCR, ELISA, western blotting, full-thickness burn injury in male ICR mice | Increases the mRNA levels of type I and type II collagen | ||
| Increase in procollagen type I and type III levels | |||||
| Activation of TGF-β/Smad pathway | |||||
| Accelerates wound healing | |||||
| Madecassic acid | In silico | Glide module, grid-based docking protocol, glide extra precision (XP) docking protocol, Qik-Prop, Prime/MM-GBSA module | Strongly bound to TNF-α | [85] | |
| Inhibits the degradation of antioxidants and collagen | |||||
| Madecassoside | In silico | Glide module, grid-based docking protocol, glide extra precision (XP) docking protocol, Qik-Prop, Prime/MM-GBSA module | Strongly bound to TNF-α | ||
| Asiatic acid | In silico | Glide module, grid-based docking protocol, glide extra precision (XP) docking protocol, Qik-Prop, Prime/MM-GBSA module | Strongly bound to TNF-α | ||
| Inhibits proinflammatory cytokines | |||||
| Asiaticoside | In silico | Glide module, grid-based docking protocol, glide extra precision (XP) docking protocol, Qik-Prop, Prime/MM-GBSA module | Good binding affinity against Nrf2-Keap1 | ||
| Promotes collagen synthesis | |||||
| Madecassoside, terminoloside, isomadecassoside | In vitro | Colorimetric nitrite assay | Inhibits lipopolysaccharide-induced nitrite production | [86] | |
| Cymbopogon nardus Linn. | Ethanolic extract of leaves and stem | In vitro | Disc diffusion method, macro dilution test | Antibacterial effects against S. aureus and E. coli | [97] |
| Antiseptic gel containing 1% citronella grass extract | In vitro | Disc diffusion method, macro dilution test | Antibacterial effects against S. aureus and E. coli | ||
| Essential oil | In vitro | Agar well diffusion method, broth macro dilution method | Inhibits the growth of C. albicans, C. glabrata and C. tropicalis | [93] | |
| 25 µg essential oil in 100 mL olive oil | In vivo | Fungal infected diabetic wound in Swiss albino mice | Effectively eradicates C. albicans colonization and accelerates wound closure | ||
| Reduces the levels of inflammatory markers (TNF-α and IL-1β) | |||||
| Essential oil | In vitro | Broth microdilution method | Antibacterial effects against S. aureus, S. epidermidis, A. baumannii, P. aeruginosa and S. pyogenes | [98] | |
| Essential oil | In vitro | Disc diffusion method, broth microdilution method | Bactericidal against S. aureus | [100] | |
| Geraniol | In vitro | Disc diffusion method, broth microdilution method | Bactericidal against S. aureus | ||
| 0.25%, 0.5% and 1% citronellal ointment | In vivo | Burn wound in Mus musculus | Promotes the healing of burn wounds | [96] | |
| Citronellal | In vitro | Disc diffusion method, serial dilution method | Antibacterial and antifungal effects | [99] | |
| Ficus benghalensis Linn. | Aqueous bark extract | In vitro | Cell scratch assay | Promotes cell migration at wound site | [104] |
| Kirby-Bauer disk diffusion method | Antibacterial effects | ||||
| Ointment containing 4% petroleum ether, ethanolic and aqueous leaf extract | In vivo | Excision wound healing model in male adult albino rats | Promotes wound healing and wound closure | [105] | |
| Ethanolic extract | In vitro | Disc diffusion method | Antibacterial effects against S. aureus, E. coli, P. protobacteria and B. cereus | [106] | |
| Methanolic bark extract | In vitro | Nitroblue tetrazolium assay, nitric oxide assay, lipid peroxidation assay | Anti-inflammatory action | [108] | |
| PCR | Downregulates the expression of TNF-α expression and upregulates the expression of IL-10 | ||||
| 10% aqueous leaf extract ointment | In vivo | Excision wound model in albino Wistar rats | Greatest wound healing effects among the Ficus species | [109] | |
| Comparable percentage of wound closure with Betadine | |||||
| Ethanolic and hydroalcoholic extract | In vitro | Red blood cells membrane stabilization | Inhibits hemolysis | [110] | |
| Gel containing 10% ethanolic/ hydroalcoholic extract | In vivo | Excision wound model in rats | Complete wound healing after 17–18 days | ||
| Nanoparticles synthesized from extract | In vitro | ELISA | Stimulates the production of TGF-α | [113] | |
| Nanoparticles containing aqueous leaf extract | In vitro | Microdilution method | Bactericidal activity against E. coli | [107] | |
| Lupenyl acetate, lanosterol | In silico | Discovery Studio software | Inhibits TNF-α and VEGFR | [112] | |
| Hibiscus rosa sinensis Linn. | Ethanolic leaves extract | In vitro | DPPH, nitric oxide, superoxide radical scavenging activity | Free radical scavenging activity | [120] |
| 10% ethanolic leaves extract ointment | In vivo | Excision wound model in Swiss albino mice | Significantly increase the percentage of excision wound closure | ||
| Incision wound model in Swiss albino mice | Increases wound breaking strength in incision wounds by increasing collagen synthesis | ||||
| Burn wound model in Swiss albino mice | Stimulates the growth of keratinocytes and fibroblasts | ||||
| 200 mg/kg ethanolic leaves extract | Dead space wound model in Swiss albino mice | Increases granulation tissue weight and hydroxyproline content of dead space wound | |||
| In vivo antioxidant assay | Increase in antioxidant enzymes (SOD, CAT) in granulation tissue | ||||
| 120 mg/kg ethanolic flower extract | In vivo | Masson’s trichrome staining | Stimulates the synthesis of collagen and fibroblast | [121] | |
| Reduces the number of inflammatory cells in granulation tissue | |||||
| Excision wound model in Sprague Dawley rats | Significantly enhance excision wound healing effects | ||||
| Increases the rate of epithelialization in excision wound | |||||
| Incision wound model in Sprague Dawley rats | Increases the tensile strength in incision wound | ||||
| Dead space wound model in Sprague Dawley rats | Significantly increase the granulation tissue weight and hydroxyproline concentration in dead space wounds | ||||
| Aqueous and ethanolic extract | In vivo | Incision wound model in Sprague Dawley rats | Promotes re-epithelialization and remodeling of granulation tissues in incision wounds | [122] | |
| Increases the tensile strength in wounded skin | |||||
| 80, 160 and 320 mg/mL N-butyl alcohol flower extract | In vivo | Excision wound model in Sprague Dawley rats, H&E staining, Masson’s trichrome staining, immunohistochemistry | Increases the collagen deposition, collagen maturity and fibroblast distribution | [123] | |
| Promotes epidermal epithelialization of wounds | |||||
| Upregulates the expression of VEGF and TGF-β1 | |||||
| Anti-inflammatory effects | |||||
| Ethanolic flower extract | In vivo | Chicken egg chorioallantoic membrane assay | Promotes angiogenesis | [124] | |
| Aqueous, ethanol and methanol extract | In vitro | Agar diffusion assay, micro-dilution method | Antibacterial activity against S. aureus, B. cereus, C. perfringens, L. monocytogenes, E. coli, S. typhi and P. aeruginosa | [125] | |
| Nanocomposite containing leaves extract | In vitro | Agar well diffusion method | Antibacterial effects against S. aureus and E. coli | [126] | |
| Silver nanoparticles synthesized using flowers, leaves and bark extract | In vitro | Not specified | Antibacterial activity against S. aureus, B. subtilis, P. aeruginosa and E. coli | [127] | |
| Ointment containing 5% and 10% ethanolic flower extract | In vivo | Excision wound model in Wistar albino rats | Significantly improves excision wound contraction | [128] | |
| Complete wound healing at 18 days (5% ointment) and 16 days (10% ointment) | |||||
| Incision wound model in Wistar albino rats | Increase in tensile strength of incision wounds | ||||
| Dead space wound model in Wistar albino rats | Significantly increases wet granuloma weight, dry granuloma weight and tensile strength of dead space wound | ||||
| Increases DNA, protein, collagen, hexosamine and uronic acid content of wet granulation tissue | |||||
| Nanofibers synthesized from 1% leaves mucilage | In vivo | Incision wound model in Swiss albino mice | Promotes collagen and fibroblast growth | [129] | |
| Excision wound model in Swiss albino mice | Faster wound healing and wound contraction | ||||
| Hemo-compatibility analysis | Non-hemolytic | ||||
| Ointment containing ethanolic flower extract | In vivo | Excision wound model in Sprague Dawley rats | Anti-inflammatory and antioxidant effects | [130] |
Table 3.
Wound healing preparations available in the market
| Product name | Ingredient | Manufacturer | Reference |
|---|---|---|---|
| Lucas’ Papaw Ointment | Fermented C. papaya fresh fruit, 39 mg/g | Lucas’ Papaw Remedies© | [136] |
| Real Paw Paw Ointment | Fermented pawpaw, 80 mg/g | Real Paw Paw | [137] |
| Théra Wise Natural Wound Care Ointment | Aloe extract | Derma Wise Skin Care Ltd. | [138] |
| Beaphar Wound Ointment | Aloe vera | Beaphar UK Ltd. | [139] |
| I’m Wounded! Natural Wound Cream | Aloe vera | Holistic Healer & Wellness | [140] |
| Elements Wellness Wound Healing Cream | Aloe vera | Elements Wellness Pvt. Ltd. | [141] |
| WoundKreme™ Organic Healing Ointment | Aloe vera | Feuilleorganix Sdn. Bhd. | [142] |
| Melcura™ HoneyPlus Honey-Based Wound Ointment | Aloe vera | Medika SA Trading | [143] |
| GoHeal Antiseptic Skin Ointment | Aloe vera | Naturaline Nutraceuticals Pvt. Ltd. | [144] |
| Hurix’s Aloe Vera Plus Cream | Aloe vera | J.B. Pharmacy Group Sdn. Bhd. | [145] |
| Scar and Wound Healing Cream | Aloe vera | Natural Scents Aromatherapy | [146] |
|
Nemoderm® Wound Healing Cream |
Aloe vera juice | FAME Pharmaceuticals Industry Co., Ltd. | [147] |
| Centella Cream | C. asiatica | Abhaibhubejhr Thai Herbal Medicine Museum | [148] |
| Madecare Ointment |
C. asiatica quantitative extract, 1 g (400 mg as asiaticoside) |
Firson Co., Ltd | [149] |
| FUYUAN Centella asiatica Cream Ointment | C. asiatica, 2.5% | Hainan Poly Pharm. Co., Ltd. | [150] |
Limitations and strengths
The present review focused on only six commonly available Malaysian medicinal plants with potential wound healing properties. However, many medicinal plants were not included in this review or are still in the discovery phase. Moreover, the source, method of cultivation, processing, extraction and storage of the medicinal plants are not standardized. This led to the variation in phytochemicals content which may affect the efficacy and safety of the end products. Hence, the standardization of medicinal plant extract is crucial to provide quality assurance. Additionally, the active constituents possessing wound healing properties in some medicinal plants have not yet been identified. Clinical trials should be conducted on medicinal plants to demonstrate their clinical efficacy and long-term safety after administration. Furthermore, the maximum and minimum concentrations of medicinal plant extract to be used should be identified to avoid treatment failure and toxicity. Researchers are encouraged to disclose the concentration of extracts or compounds upon publication of the original research paper to ensure the reproducibility and replicability of the experiment. In this review, some of the cellular and molecular aspects of medicinal plants in wound healing are briefly discussed. More research should be conducted in the future to reveal their exact biologically active cellular and molecular mechanisms. Thus, this review acts as a stepping stone to discover the wound healing mechanism of medicinal plants with potential wound healing activity in detail. In silico modeling should be utilized in the future to discover the pharmacologic effects or mechanisms of active constituents or herbal extracts in the wound healing process. The research on the pharmacokinetics and bioavailability of medicinal plants and their respective compounds is still lacking, thus further research should be conducted to determine the interaction of the body with the medicinal plants and their respective compounds. Pharmacokinetics and bioavailability are crucial to ensure that appropriate amounts of medicinal plants or active constituents are delivered to the target sites for wound healing effects and to minimize toxicity at the same time. The present review is the first review of Malaysian medicinal plants with wound healing properties. The present review also included the in vitro and in vivo wound healing effects of the plant extracts, the active constituents and the formulations containing plant extract.
Conclusions
The identification of medicinal plants with wound healing potential is crucial due to the limited availability of wound healing agents and the risk of allergic contact dermatitis and antibiotic resistance with the use of current skin disinfectants such as triclosan and benzalkonium chloride. This review summarizes that Aloe barbadensis Miller, Carica papaya Linn., Centella asiatica Linn., Cymbopogon nardus Linn., Ficus benghalensis Linn. and Hibiscus rosa sinensis Linn. demonstrated wound healing properties through various in vitro and in vivo studies. However, clinical trials should also be conducted to ensure the efficacy, quality and safety of medicinal plants as wound healing agents. Moreover, further research is required to assess the short-term and long-term safety/toxicity of medicinal plants. The safest route of administration of the medicinal plant extract for wound healing should be selected by considering their ease of administration, patient compliance, pharmacokinetics, toxicity and use in pregnancy.
Acknowledgements
The authors would like to express their gratitude to the Ministry of Higher Education Malaysia for funding this research project through Fundamental Research Grant Scheme (FRGS) with project code: FRGS/1/2021/SKK0/UCSI/03/1.
Abbreviations
- α-SMA
α-smooth muscle
- bFGF
Basic fibroblast growth factor
- CAM
Chorioallantoic membrane
- CCK8
Cell Counting Kit8
- CLSM
Confocal laser scanning microscopy
- COL 1A
Type I collagen
- DPPH
1,1-diphenyl-2- picrylhydrazyl
- DNIRS
Diffuse near-infrared spectroscopy
- ELISA
Enzyme-linked immunosorbent assay
- FE-SEM
Field emission scanning electron microscopy
- GSK3β
Glycogen synthase kinase 3β
- H&E
Hematoxylin and eosin
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- KGF-1
Keratinocyte growth factor-1
- LFA-01
Lymphocyte function-associated antigen 1
- Mac-1
Macrophage-1 antigen
- MAPK
Mitogen-activated protein kinase
- MFAP4
Microfibril-associated glycoprotein 4
- MTS assay
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2 H-tetrazolium) assay
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2 H-tetrazolium bromide
- NHDF
Normal human dermal fibroblast
- NHEK
Normal human epidermal keratinocyte
- NIH-3T3
Mouse embryonic fibroblast cell line
- OCT
Optical coherence tomography
- qRT-PCR
Quantitative real time-PCR
- RT-PCR
Reverse transcription polymerase chain reaction
- SEM
Scanning electron microscope
- siRNA
Small interfering RNA
- TNFAIP6
TNF Alpha Induced Protein 6
- TGF-α
Transforming growth factor-α
- TGF-β
Transforming growth factor-β
- TGF-β1
Transforming growth factor-β1
- TNF-α
Tumor necrosis factor-α
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
- WHR
Wound healing rate
- A. baumannii
Acinetobacter baumannii
- B. cereus
Bacillus cereus
- B. subtilis
Bacillus subtilis
- C. albicans
Candida albicans
- C. glabrata
Candida glabrata
- C. tropicalis
Candida tropicalis
- C. perfringens
Clostridium perfringens
- C. bacillus
Coliform bacillus
- E. coli
Escherichia coli
- K. pneumonia
Klebsiella pneumonia
- L. monocytogenes
Listeria monocytogenes
- P. aeruginosa
Pseudomonas aeruginosa
- P. protobacteria
Pseudomonas protobacteria
- S. aureus
Staphylococcus aureus
- S. epidermidis
Staphylococcus epidermidis
- S. pyogenes
Streptococcus pyogenes
- S. typhi
Salmonella typhi
- S. viridans
Streptococcus viridans
Author contributions
The original draft was prepared by PL. CW and MR contributed to conceptualization and design of the study. YC wrote sections of the manuscript. MY, LF and VL were involved in manuscript reviewing and editing. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The study was funded by the Malaysian Ministry of Higher Education through the Fundamental Research Grant Scheme with project code: FRGS/1/2021/SKK0/UCSI/03/1.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christophe Wiart, Email: christophewiart@ums.edu.my.
Puay Luan Tan, Email: 1001645248@ucsiuniversity.edu.my.
Mogana Rajagopal, Email: mogana@ucsiuniversity.edu.my.
References
- 1.Herman TF, Bordoni B. StatPearls. Treasure Island. Florida: StatPearls Publishing; 2022. Wound classification. [PubMed] [Google Scholar]
- 2.Shedoeva A, Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evidence-Based Complement Altern Med. 2019;2019:2684108. doi: 10.1155/2019/2684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saleh K, Sönnergren HH. Control and treatment of infected wounds. In: Ågren MS, editor. Wound Healing Biomaterials. Sawston, United Kingdom: Woodhead Publishing; 2016. pp. 107–15. [Google Scholar]
- 4.Institute for Public Health. National Health & Morbidity Survey (NHMS). 2019: Non-communicable diseases, healthcare demand and healthcare literacy - Key findings. National Institutes of Health, Ministry of Health Malaysia. 2020. https://iptk.moh.gov.my/images/technical_report/2020/4_Infographic_Booklet_NHMS_2019_-_English.pdf. Accessed 25 May 2022.
- 5.Liu X, Ren Q, Zhai Y, Kong Y, Chen D, Chang B. Risk factors for multidrug-resistant organisms infection in diabetic foot ulcer. Infect Drug Resist. 2022;15:1627–35. doi: 10.2147/IDR.S359157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kee KK, Nair HKR, Yuen NP. Risk factor analysis on the healing time and infection rate of diabetic foot ulcers in a referral wound care clinic. J Wound Care. 2019;28:S4–13. doi: 10.12968/jowc.2019.28.Sup1.S4. [DOI] [PubMed] [Google Scholar]
- 7.Lachenmeier DW. Chapter 21 - antiseptic drugs and disinfectants. In: Ray SD, editor. Side effects of drugs Annual. Amsterdam, Netherlands: Elsevier; 2020. pp. 247–52. [Google Scholar]
- 8.Koehler J, Brandl FP, Goepferich AM. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur Polymer J. 2018;100:1–11. doi: 10.1016/j.eurpolymj.2017.12.046. [DOI] [Google Scholar]
- 9.Hua OH, Tran QTT, Trinh D-TT, Nguyen V-D, Duong DPN, Nguyen TT. A review of traditional uses, phytochemistry and pharmacological properties of some Vietnamese wound-healing medicinal plants. Nat Prod Commun. 2022;17:1–11. [Google Scholar]
- 10.Hosseinkhani A, Falahatzadeh M, Raoofi E, Zarshenas MM. An evidence-based review on wound healing herbal remedies from reports of traditional persian medicine. J Evidence-Based Complement Altern Med. 2017;22:334–43. doi: 10.1177/2156587216654773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing - exploring medicinal plants of India. J Ethnopharmacol. 2007;114:103–13. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Qing C. The molecular biology in wound healing & non-healing wound. Chin J Traumatol. 2017;20:189–93. doi: 10.1016/j.cjtee.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delavary BM, van der Veer WM, van Egmond M, Niessen FB, Beelen RHJ. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–62. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Mills C. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32:463–88. doi: 10.1615/CritRevImmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 15.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–35. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mogana R, Teng-Jin K, Wiart C. Anti-inflammatory, anticholinesterase, and antioxidant potential of scopoletin isolated from Canarium patentinervium Miq. (Burseraceae Kunth) Evidence-based Complement Altern Med. 2013;2013:734824. doi: 10.1155/2013/734824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mogana R, Teng-Jin K, Wiart C. The medicinal timber Canarium Patentinervium Miq. (Burseraceae Kunth.) Is an anti-inflammatory bioresource of dual inhibitors of cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) ISRN Biotechnol. 2013;2013:986361. doi: 10.5402/2013/986361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: Biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25:304–14. doi: 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of acute and chronic wound healing. Biomolecules. 2021;11:700. doi: 10.3390/biom11050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masson-Meyers DS, Andrade TAM, Caetano GF, Guimaraes FR, Leite MN, Leite SN, et al. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101:21–37. doi: 10.1111/iep.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelm KP, Wilhelm D, Bielfeldt S. Models of wound healing: an emphasis on clinical studies. Skin Res Technol. 2017;23:3–12. doi: 10.1111/srt.12317. [DOI] [PubMed] [Google Scholar]
- 22.Grada A, Mervis J, Falanga V. Research techniques made simple: animal models of wound healing. J Invest Dermatology. 2018;138:2095–105. doi: 10.1016/j.jid.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Grambow E, Sorg H, Sorg CGG, Strüder D. Experimental models to study skin wound healing with a focus on angiogenesis. Med Sci. 2021;9:55. doi: 10.3390/medsci9030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatology. 2008;53:163–6. doi: 10.4103/0019-5154.44785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP. Pharmacological update properties of Aloe vera and its major active constituents. Molecules. 2020;25:1324. doi: 10.3390/molecules25061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahlawat KS, Khatkar BS. Processing, food applications and safety of Aloe vera products: a review. J Food Sci Technol. 2011;48:525–33. doi: 10.1007/s13197-011-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razia S, Park H, Shin E, Shim K-S, Cho E, Kang MC, et al. Synergistic effect of Aloe vera flower and aloe gel on cutaneous wound healing targeting MFAP4 and its associated signaling pathway: In-vitro study. J Ethnopharmacol. 2022;290:115096. doi: 10.1016/j.jep.2022.115096. [DOI] [PubMed] [Google Scholar]
- 28.Ezhilarasu H, Ramalingam R, Dhand C, Lakshminarayanan R, Sadiq A, Gandhimathi C, et al. Biocompatible Aloe vera and tetracycline hydrochloride loaded hybrid nanofibrous scaffolds for skin tissue engineering. Int J Mol Sci. 2019;20:5174. doi: 10.3390/ijms20205174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hormozi M, Assaei R, Boroujeni MB. The effect of Aloe vera on the expression of wound healing factors (TGFβ1 and bFGF) in mouse embryonic fibroblast cell: in vitro study. Biomed Pharmacother. 2017;88:610–6. doi: 10.1016/j.biopha.2017.01.095. [DOI] [PubMed] [Google Scholar]
- 30.Faler BJ, Macsata RA, Plummer D, Mishra L, Sidawy AN. Transforming growth factor-β and wound healing. Perspect Vasc Surg Endovasc Ther. 2006;18:55–62. doi: 10.1177/153100350601800123. [DOI] [PubMed] [Google Scholar]
- 31.Hom DB, Unger GM, Pernell KJ, Manivel JC. Improving surgical wound healing with basic fibroblast growth factor after radiation. Laryngoscope. 2005;115:412–22. doi: 10.1097/01.mlg.0000157852.01402.12. [DOI] [PubMed] [Google Scholar]
- 32.Mohebian Z, Tajmohammadi I, Yavari Maroufi L, Ramezani S, Ghorbani M. A novel Aloe vera-loaded ethylcellulose/hydroxypropyl methylcellulose nanofibrous mat designed for wound healing application. J Polym Environ. 2022;30:867–77. doi: 10.1007/s10924-021-02240-0. [DOI] [Google Scholar]
- 33.Garcia-Orue I, Gainza G, Gutierrez FB, Aguirre JJ, Evora C, Pedraz JL, et al. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int J Pharm. 2017;523:556–66. doi: 10.1016/j.ijpharm.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Naseri-Nosar M, Farzamfar S, Salehi M, Vaez A, Tajerian R, Azami M. Erythropoietin/Aloe vera-releasing wet-electrospun polyvinyl alcohol/chitosan sponge-like wound dressing: in vitro and in vivo studies. J Bioactive Compatible Polym. 2018;33:269–81. doi: 10.1177/0883911517731793. [DOI] [Google Scholar]
- 35.Baghersad S, Bahrami SH, Mohammadi MR, Mojtahedi MRM, Milan PB. Development of biodegradable electrospun gelatin/aloe-vera/poly(εcaprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Mater Sci Engineering: C. 2018;93:367–79. doi: 10.1016/j.msec.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Atiba A, Ueno H, Uzuka Y. The effect of Aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J Vet Med Sci. 2011;73:583–9. doi: 10.1292/jvms.10-0438. [DOI] [PubMed] [Google Scholar]
- 37.Jettanacheawchankit S, Sasithanasate S, Sangvanich P, Banlunara W, Thunyakitpisal P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type I collagen; and wound healing. J Pharmacol Sci. 2009;109:525–31. doi: 10.1254/jphs.08204FP. [DOI] [PubMed] [Google Scholar]
- 38.Pierce GF, Yanagihara D, Klopchin K, Danilenko DM, Hsu E, Kenney WC, et al. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994;179:831–40. doi: 10.1084/jem.179.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 40.Aukhil I. Biology of wound healing. Periodontol 2000. 2000;22:44–50. doi: 10.1034/j.1600-0757.2000.2220104.x. [DOI] [PubMed] [Google Scholar]
- 41.Xing W, Guo W, Zou C-H, Fu T-T, Li X-Y, Zhu M, et al. Acemannan accelerates cell proliferation and skin wound healing through AKT/mTOR signaling pathway. J Dermatol Sci. 2015;79:101–9. doi: 10.1016/j.jdermsci.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Suganya S, Venugopal J, Agnes Mary S, Ramakrishna S, Lakshmi BS, Giri Dev VR. Aloe vera incorporated biomimetic nanofibrous scaffold: a regenerative approach for skin tissue engineering. Iran Polym J. 2014;23:237–48. doi: 10.1007/s13726-013-0219-2. [DOI] [Google Scholar]
- 43.Moon E-J, Lee YM, Lee O-H, Lee M-J, Lee S-K, Chung M-H, et al. A novel angiogenic factor derived from Aloe vera gel: β-sitosterol, a plant sterol. Angiogenesis. 1999;3:117–23. doi: 10.1023/A:1009058232389. [DOI] [PubMed] [Google Scholar]
- 44.Wahedi HM, Jeong M, Chae JK, Do SG, Yoon H, Kim SY. Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and smad signaling pathways in vitro and in vivo. Phytomedicine. 2017;28:19–26. doi: 10.1016/j.phymed.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Eshghi F, Hosseinimehr SJ, Rahmani N, Khademloo M, Norozi MS, Hojati O. Effects of Aloe vera cream on posthemorrhoidectomy pain and wound healing: results of a randomized, blind, placebo-control study. J Altern Complement Med. 2010;16:647–50. doi: 10.1089/acm.2009.0428. [DOI] [PubMed] [Google Scholar]
- 46.Panahi Y, Sharif MR, Sharif A, Beiraghdar F, Zahiri Z, Amirchoopani G et al. A randomized comparative trial on the therapeutic efficacy of topical Aloe vera and Calendula officinalis on diaper dermatitis in children. The Scientific World Journal. 2012;2012:810234. [DOI] [PMC free article] [PubMed]
- 47.Miroddi M, Navarra M, Calapai F, Mancari F, Giofrè SV, Gangemi S, et al. Review of clinical pharmacology of Aloe vera L. in the treatment of psoriasis. Phytother Res. 2015;29:648–55. doi: 10.1002/ptr.5316. [DOI] [PubMed] [Google Scholar]
- 48.Anjum S, Gupta A, Sharma D, Gautam D, Bhan S, Sharma A, et al. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater Sci Engineering: C. 2016;64:157–66. doi: 10.1016/j.msec.2016.03.069. [DOI] [PubMed] [Google Scholar]
- 49.Ghayempour S, Montazer M, Mahmoudi Rad M. Encapsulation of Aloe vera extract into natural tragacanth gum as a novel green wound healing product. Int J Biol Macromol. 2016;93:344–9. doi: 10.1016/j.ijbiomac.2016.08.076. [DOI] [PubMed] [Google Scholar]
- 50.Akhdar M, Abedini R, Tavakolpour S, Gholibeigian Z, Azizpour A. A randomized, double-blind, placebo-controlled trial of a commercial Aloe vera gel for mitigation of phototherapy side-effects in vitiligo patients. J Herb Med. 2021;28:100442. doi: 10.1016/j.hermed.2021.100442. [DOI] [Google Scholar]
- 51.Coelho FH, Salvadori G, Rados PV, Magnusson A, Danilevicz CK, Meurer L et al. Topical Aloe vera (Aloe barbadensis Miller) extract does not accelerate the oral wound healing in rats. Phytother Res. 2015;29. [DOI] [PubMed]
- 52.Guo X, Mei N. Aloe vera: a review of toxicity and adverse clinical effects. J Environ Sci Health Part C Environ Carcinog Ecotoxicol Reviews. 2016;34:77–96. doi: 10.1080/10590501.2016.1166826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sekeli R, Hamid MH, Razak RA, Wee CY, Ong-Abdullah J. Malaysian Carica papaya L. var. Eksotika: current research strategies fronting challenges. Front Plant Sci. 2018;9:1380. doi: 10.3389/fpls.2018.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahidin MU. Supply and utilization accounts selected agricultural commodities, Malaysia 2015–2019. Department of Statistics, Malaysia. 2020. https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=164&bul_id=OTM1TDMzS1IvYm5mU1JiU1Fwekt3UT09&menu_id=Z0VTZGU1UHBUT1VJMFlpaXRRR0xpdz09. Accessed 7 May 2022.
- 55.Vij T, Prashar Y. A review on medicinal properties of Carica papaya Linn. Asian Pac J Trop Disease. 2015;5:1–6. doi: 10.1016/S2222-1808(14)60617-4. [DOI] [Google Scholar]
- 56.Soib HH, Ismail HF, Husin F, Abu Bakar MH, Yaakob H, Sarmidi MR. Bioassay-guided different extraction techniques of Carica papaya (Linn.) Leaves on in vitro wound-healing activities. Molecules. 2020;25:517. doi: 10.3390/molecules25030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hakim RF, Fakhrurrazi Dinni. Effect of Carica papaya extract toward incised wound healing process in mice (Mus musculus) clinically and histologically. Evidence-Based Complement Altern Med. 2019;2019:8306519. doi: 10.1155/2019/8306519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Airaodion AI, Ekenjoku JA, Akaninyene IU, Megwas AU. Antibacterial potential of ethanolic and aqueous extracts of Carica papaya leaves. Asian J Biochem Genet Mol Biology. 2020;3:33–8. doi: 10.9734/ajbgmb/2020/v3i330088. [DOI] [Google Scholar]
- 59.Femilian A, Agustina D, Subagyo G. The effect of papaya leaf extract (Carica papaya L) on healing process of buccal traumatic ulcer in Wistar rats. Majalah Kedokteran Gigi Indonesia. 2019;5:15–22. doi: 10.22146/majkedgiind.37026. [DOI] [Google Scholar]
- 60.Sim M, Nauli HT, Khanh DP, Novelya, Nicoline FF. The effectiveness of the combination of nanoparticles from Carica papaya L. leaf and chitosan 1% against fibroblasts in the tooth socket of Wistar rats. Biomedical J Indonesia. 2021;7:388–94. doi: 10.32539/bji.v7i2.378. [DOI] [Google Scholar]
- 61.Rashid S, Khan H, Iqbal Y. Role of topical papaya application in debridement of deep burn wounds. J Rawalpindi Med Coll. 2021;25:171–4. doi: 10.37939/jrmc.v25i2.1385. [DOI] [Google Scholar]
- 62.Nwankwo EU, Maduba CC, Modekwe VI, Nnadozie UU. The use of unripe pawpaw for wound bed preparation following radiation-induced sacral ulcer: a case report and review of literature. Niger J Med. 2021;30:339–41. doi: 10.4103/NJM.NJM_209_20. [DOI] [Google Scholar]
- 63.Xue Y, Qi C, Dong Y, Zhang L, Liu X, Liu Y, et al. Poly (γ-glutamic acid)/chitooligo-saccharide/papain hydrogel prevents hypertrophic scar during skin wound healing. J Biomedical Mater Res Part B Appl Biomaterials. 2021;109:1724–34. doi: 10.1002/jbm.b.34830. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad M, Nasir A. Management of non-healing ulcer by the use of crushed raw papaya along with leeching: a case study. J Drug Delivery Ther. 2021;11:1–4. doi: 10.22270/jddt.v11i5.4978. [DOI] [Google Scholar]
- 65.Jayarajan RC, Narayanan Pv, Adenwalla HS. Papaya pulp for enzymatic wound debridement in burns. Indian J Burns. 2016;24:24–8. doi: 10.4103/0971-653X.195533. [DOI] [Google Scholar]
- 66.Starley IF, Mohammed P, Schneider G, Bickler SW. The treatment of paediatric burns using topical papaya. Burns. 1999;25:636–9. doi: 10.1016/S0305-4179(99)00056-X. [DOI] [PubMed] [Google Scholar]
- 67.Murthy MB, Murthy BK, Bhave S. Comparison of safety and efficacy of papaya dressing with hydrogen peroxide solution on wound bed preparation in patients with wound gape. Indian J Pharmacol. 2012;44:784–7. doi: 10.4103/0253-7613.103302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nafiu AB, Alli-Oluwafuyi AM, Haleemat A, Olalekan IS, Rahman MT. Chapter 3.32 - papaya (Carica papaya L., Pawpaw) In: Nabavi SM, Silva AS, editors. Nonvitamin and Nonmineral Nutritional supplements. Massachusetts, United States: Academic; 2019. pp. 335–59. [Google Scholar]
- 69.Lim XY, Chan JSW, Japri N, Lee JC, Tan TYC. Carica papaya L. leaf: a systematic scoping review on biological safety and herb-drug interactions. Evidence-based Complement Altern Med. 2021;2021:5511221. doi: 10.1155/2021/5511221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anjum V, Arora P, Ansari SH, Najmi AK, Ahmad S. Antithrombocytopenic and immunomodulatory potential of metabolically characterized aqueous extract of Carica papaya leaves. Pharm Biol. 2017;55:2043–56. doi: 10.1080/13880209.2017.1346690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bylka W, Znajdek-Awizeń P, Studzińska-Sroka E, Brzezińska M. Centella asiatica in cosmetology. Adv Dermatology Allergology/Postępy Dermatologii i Alergologii. 2013;30:46–9. doi: 10.5114/pdia.2013.33378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prakash V, Jaiswal N, Srivastava M. A review on medicinal properties of Centella asiatica. Asian J Pharm Clin Res. 2017;10:69–74. doi: 10.22159/ajpcr.2017.v10i10.20760. [DOI] [Google Scholar]
- 73.Hashim P, Sidek H, Helan MHM, Sabery A, Palanisamy UD, Ilham M. Triterpene composition and bioactivities of Centella asiatica. Molecules. 2011;16:1310–22. doi: 10.3390/molecules16021310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anand S, Rajinikanth PS, Arya DK, Pandey P, Gupta RK, Sankhwar R, et al. Multifunctional biomimetic nanofibrous scaffold loaded with asiaticoside for rapid diabetic wound healing. Pharmaceutics. 2022;14:273. doi: 10.3390/pharmaceutics14020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Zhang D, Deng J, Liu Y, Li W, Nie X. Preparation and safety evaluation of Centella asiatica total glycosides nitric oxide gel and its therapeutic effect on diabetic cutaneous ulcers. Evidence-Based Complement Altern Med. 2022;2022:1419146. doi: 10.1155/2022/1419146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide: Biology Chem. 2002;7:1–10. doi: 10.1016/S1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 77.Li J, Zhang H, Tan Q. Asiaticoside expedites recovery of diabetic ulcers through activation of Wnt1/β-catenin signaling cascade. Res Square. 2022;:1–27.
- 78.Shi Y, Shu B, Yang R, Xu Y, Xing B, Liu J, et al. Wnt and notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Therapy. 2015;6:120. doi: 10.1186/s13287-015-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Somboonwong J, Kankaisre M, Tantisira B, Tantisira MH. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: an experimental animal study. BMC Complement Med Ther. 2012;12:103. doi: 10.1186/1472-6882-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coldren CD, Hashim P, Ali JMohd, Oh S-K, Sinskey AJ, Rha CK. Gene expression changes in the human fibroblast induced by Centella asiatica triterpenoids. Planta Med. 2003;69:725–32. doi: 10.1055/s-2003-42791. [DOI] [PubMed] [Google Scholar]
- 81.Choi S, Kim K-W, Choi J-S, Han S-T, Park Y-I, Lee S-K, et al. Angiogenic activity of β-sitosterol in the ischaemia/reperfusion-damaged brain of Mongolian gerbil. Planta Med. 2002;68:330–5. doi: 10.1055/s-2002-26750. [DOI] [PubMed] [Google Scholar]
- 82.Lee J, Jung E, Kim Y, Park J, Park J, Hong S, et al. Asiaticoside induces human collagen I synthesis through TGFβ receptor I kinase (TβRI kinase)-independent smad signaling. Planta Med. 2006;72:324–8. doi: 10.1055/s-2005-916227. [DOI] [PubMed] [Google Scholar]
- 83.Wu F, Bian D, Xia Y, Gong Z, Tan Q, Chen J, et al. Identification of major active ingredients responsible for burn wound healing of Centella asiatica herbs. Evidence-Based Complement Altern Med. 2012;2012:848093. doi: 10.1155/2012/848093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Syed Zameer Ahmed S, Balu N, Khader SZA, Mahboob MR, Lakshmanan SO, Vetrivel M. Fabrication and evaluation of bamboo fabric coated with extracts of Curcuma longa, Centella asiatica and Azadirachta indica as a wound dressing material. Adv Traditional Med. 2021;21:83–95. doi: 10.1007/s13596-020-00503-0. [DOI] [Google Scholar]
- 85.Khotimah H, Setiawan A, Rita CI, Mardiyah M, Ali A, Sukatman MP et al. In silico studies of natural compounds of Centella asiatica as anti-aging and wound healing agents. In: AIP Conference Proceedings. Maryland, United States: American Institute of Physics Publishing; 2021. p. 030031.
- 86.Chianese G, Masi F, Cicia D, Ciceri D, Arpini S, Falzoni M, et al. Isomadecassoside, a new ursane-type triterpene glycoside from Centella asiatica leaves, reduces nitrite levels in LPS-stimulated macrophages. Biomolecules. 2021;11:494. doi: 10.3390/biom11040494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phaechamud T, Yodkhum K, Charoenteeraboon J, Tabata Y. Chitosan-aluminum monostearate composite sponge dressing containing asiaticoside for wound healing and angiogenesis promotion in chronic wound. Mater Sci Engineering: C. 2015;50:210–25. doi: 10.1016/j.msec.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 88.Jorge OA, Jorge AD. Hepatotoxicity associated with the ingestion of Centella asiatica. Rev Esp Enferm Dig. 2005;97:115–24. doi: 10.4321/S1130-01082005000200006. [DOI] [PubMed] [Google Scholar]
- 89.Dantuluri S, North-Lewis P, Karthik SV. Gotu Kola induced hepatotoxicity in a child - need for caution with alternative remedies. Dig Liver Disease. 2011;43:500. doi: 10.1016/j.dld.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 90.Gohil KJ, Patel JA, Gajjar AK. Pharmacological review on Centella asiatica: a potential herbal cure - all. Indian J Pharm Sci. 2010;72:546–56. doi: 10.4103/0250-474X.78519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bylka W, Znajdek-Awizeń P, Studzińska-Sroka E, Dańczak-Pazdrowska A, Brzezińska M. Centella asiatica in dermatology: an overview. Phytother Res. 2014;28:1117–24. doi: 10.1002/ptr.5110. [DOI] [PubMed] [Google Scholar]
- 92.Arpiwi NL, Muksin IK, Kartini NL. Essential oil from Cymbopogon nardus and repellant activity against Aedes aegypti. Biodiversitas. 2020;21:3873–8. [Google Scholar]
- 93.Kandimalla R, Kalita S, Choudhury B, Dash S, Kalita K, Kotoky J. Chemical composition and anti-candidiasis mediated wound healing property of Cymbopogon nardus essential oil on chronic diabetic wounds. Front Pharmacol. 2016;7:198. doi: 10.3389/fphar.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bayala B, Coulibaly AY, Djigma FW, Nagalo BM, Baron S, Figueredo G, et al. Chemical composition, antioxidant, anti-inflammatory and antiproliferative activities of the essential oil of Cymbopogon nardus, a plant used in traditional medicine. Biomol Concepts. 2020;11:86–96. doi: 10.1515/bmc-2020-0007. [DOI] [PubMed] [Google Scholar]
- 95.Solomon B, Gebre-Mariam T, Asres K. Mosquito repellent actions of the essential oils of Cymbopogon citratus, Cymbopogon nardus and Eucalyptus citriodora: evaluation and formulation studies. J Essent Oil Bearing Plants. 2012;15:766–73. doi: 10.1080/0972060X.2012.10644118. [DOI] [Google Scholar]
- 96.Anwar Y, Ningtiyas NA, Simanjuntak P. Isolation of citronellal from Cymbopogon nardus (L) Rendle and its activity test as a burn healing in mice. Curr Res Biosci Biotechnol. 2020;2:105–8. [Google Scholar]
- 97.Sagala RJ, Kambira PFA, Gunawan U, Pollin G. IAI special edition: the potential of citronella grass to inhibit growth of Escherichia coli and Staphylococcus aureus bacteria. Pharm Educ. 2022;22:218–24. doi: 10.46542/pe.2022.222.218224. [DOI] [Google Scholar]
- 98.Sounouvou HT, Toukourou H, Catteau L, Toukourou F, Evrard B, van Bambeke F, et al. Antimicrobial potentials of essential oils extracted from west African aromatic plants on common skin infections. Sci Afr. 2021;11:e00706. [Google Scholar]
- 99.Mangalagiri NP, Panditi SK, Jeevigunta NLL. Antimicrobial activity of essential plant oils and their major components. Heliyon. 2021;7:e06835. doi: 10.1016/j.heliyon.2021.e06835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pontes EKU, Melo HM, Nogueira JWA, Firmino NCS, de Carvalho MG, Catunda Júnior FEA, et al. Antibiofilm activity of the essential oil of citronella (Cymbopogon nardus) and its major component, geraniol, on the bacterial biofilms of Staphylococcus aureus. Food Sci Biotechnol. 2019;28:633–9. doi: 10.1007/s10068-018-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vostinaru O, Codruta Heghes S, Filip L. Safety profile of essential oils. In: de Oliveira MS, Silva S, Da Costa WA, editors. Essential oils - bioactive compounds, New perspectives and Applications. London, United Kingdom: IntechOpen; 2020. pp. 41–50. [Google Scholar]
- 102.Murugesu S, Selamat J, Perumal V. Phytochemistry, pharmacological properties, and recent applications of Ficus benghalensis and Ficus religiosa. Plants. 2021;10:2749. doi: 10.3390/plants10122749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahirwar SK, Singh A, Singh PK, Bijauliya RK, Pateriya K. An updated review of pharmacological studies on Ficus benghalensis Linn. Int J Pharmacognosy. 2018;5:546–62. [Google Scholar]
- 104.Lu H, Liu S, Zhang Y. Development of novel green synthesized silver nanoparticles with superior antibacterial and wound healing properties in nursing care after rectal surgery. J Inorg Organomet Polym Mater. 2022;32:773–80. doi: 10.1007/s10904-022-02223-1. [DOI] [Google Scholar]
- 105.Imran M, Sharma JN, Kamal M, Asif M. Standardization and wound-healing activity of petroleum, ethanolic and aqueous extracts of Ficus benghalensis leaves. Pharm Chem J. 2021;54:1057–62. doi: 10.1007/s11094-021-02319-x. [DOI] [Google Scholar]
- 106.Afzal T, Ali Q, Malik A. Phenolic compounds proliferation by HPLC: to find out antibacterial activities in Ficus benghalensis plant extract. Int J Bot Stud. 2020;5:140–4. [Google Scholar]
- 107.Saxena A, Tripathi RM, Zafar F, Singh P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater Lett. 2012;67:91–4. doi: 10.1016/j.matlet.2011.09.038. [DOI] [Google Scholar]
- 108.Sabi EM, Mujamammi AH, Sumaily KM, Dahman LS, bin, Althafar ZM. Anti-inflammatory and anti-hyperuricemic effect of Ficus benghalensis bark extract in raw 246.7 cell line. Indian J Pharm Educ Res. 2022;56:520–8. doi: 10.5530/ijper.56.2.74. [DOI] [Google Scholar]
- 109.Mothilal K, Akila C, Mahender K, Chaitanya Kumar K, Ravi D. Comparison of the Ficus for the wound healing activity in various species. Int J Res Phytochemistry Pharmacol. 2020;10:67–70. doi: 10.26452/ijrpp.v10i4.1381. [DOI] [Google Scholar]
- 110.Raisagar A, Kaur CD, Sawarkar HA, Kumar L, Raisagar A, Karmakar A, et al. Comparative study of wound-healing effect of bark extracts of Ficus religiosa & Ficus benghalensis by mice model. J Pharmacognosy Phytochemistry. 2019;8:1815–21. [Google Scholar]
- 111.Wang T, Yang L, Wang G, Han L, Chen K, Liu P, et al. Biocompatibility, hemostatic properties, and wound healing evaluation of tilapia skin collagen sponges. J Bioactive Compatible Polym. 2021;36:44–58. doi: 10.1177/0883911520981705. [DOI] [Google Scholar]
- 112.Yueniwati Y, Syaban MFR, Erwan NE, Putra GFA, Krisnayana AD. Molecular docking analysis of Ficus religiosa active compound with anti-inflammatory activity by targeting tumour necrosis factor alpha and vascular endothelial growth factor receptor in diabetic wound healing. Open Access Macedonian J Med Sci. 2021;9 A:1031–6. doi: 10.3889/oamjms.2021.7068. [DOI] [Google Scholar]
- 113.Hinaz N, Priya VV, Gayathri R. In vitro tissue repairing properties of green synthesized silver nanoparticles from Ficus benghalensis. Drug Invention Today. 2019;11:1432–4. [Google Scholar]
- 114.Honnegowda TM, Kumar P, Udupa EGP, Kumar S, Kumar U, Rao P. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesthetic Res. 2015;2:243–9. doi: 10.4103/2347-9264.165438. [DOI] [Google Scholar]
- 115.Patel M, Patel P, Patel M. Aqueous extract of Ficus bengalensis Linn. Bark for inflammatory bowel disease. J Young Pharmacists. 2010;2:130–6. doi: 10.4103/0975-1483.63149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khan T, Tatke P, Gabhe SY. Immunological studies on the aerial roots of the Indian Banyan. Indian J Pharm Sci. 2008;70:287–91. doi: 10.4103/0250-474X.42970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Upadhyay S, Upadhyay P. Hibiscus rosa-sinensis: pharmacological review. Int J Res Pharm Biomedical Sci. 2011;2:1449–50. [Google Scholar]
- 118.The Malaysian Administrative Modernisation and Management Planning Unit. MyGOV - Malaysia Information | National Flower. 2016. https://www.malaysia.gov.my/portal/content/139. Accessed 22 May 2022.
- 119.Sivaraman CM, Saju F. Medicinal value of Hibiscus rosa sinensis : a review. Int J Pharmacognosy Chem. 2021;2:1–11. doi: 10.46796/ijpc.vi.128. [DOI] [Google Scholar]
- 120.Mondal S, Ghosh D, Sagar N, Ganapaty S. Evaluation of antioxidant, toxicological and wound healing properties of Hibiscus rosa-sinensis L. (Malvaceae) ethanolic leaves extract on different experimental animal models. Indian J Pharm Educ Res. 2016;50:620–37. doi: 10.5530/ijper.50.4.15. [DOI] [Google Scholar]
- 121.Shivananda Nayak B, Sivachandra Raju S, Orette FA, Chalapathi Rao AV. Effects of Hibiscus rosa sinensis L (Malvaceae) on wound healing activity: a preclinical study in a Sprague Dawley rat. Int J Low Extremity Wounds. 2007;6:76–81. doi: 10.1177/1534734607302840. [DOI] [PubMed] [Google Scholar]
- 122.Ali AA, Jusoh NH, Saridin N, Wahab MSA, Zohdi RM. Evaluation of Hibiscus rosa-sinensis leaves extracts as wound healing promoter on rats. In: 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES). 2014. pp. 352–5.
- 123.Shen H-M, Chen C, Jiang J-Y, Zheng Y-L, Cai W-F, Wang B, et al. The N-butyl alcohol extract from Hibiscus rosa-sinensis L. flowers enhances healing potential on rat excisional wounds. J Ethnopharmacol. 2017;198:291–301. doi: 10.1016/j.jep.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 124.Sharma S, Kota K, Pandya ND. A study of angiogenic activity of Hibiscus rosa-sinensis Linn. Using chick chorioallantoic membrane model. Natl J Physiol Pharm Pharmacol. 2021;11:1080–4. doi: 10.5455/njppp.2021.11.03094202102052021. [DOI] [Google Scholar]
- 125.Karnwal A. Vitro antibacterial activity of Hibiscus rosa sinensis, Chrysanthemum indicum, and Calendula officinalis flower extracts against gram negative and gram positive food poisoning bacteria. Adv Traditional Med. 2022;22:607–19. doi: 10.1007/s13596-021-00562-x. [DOI] [Google Scholar]
- 126.Elemike EE, Onwudiwe DC, Mbonu JI. Facile synthesis of cellulose–ZnO-hybrid nanocomposite using Hibiscus rosa-sinensis leaf extract and their antibacterial activities. Appl Nanosci. 2021;11:1349–58. doi: 10.1007/s13204-021-01774-y. [DOI] [Google Scholar]
- 127.Periasamy S, Jegadeesan U, Sundaramoorthi K, Rajeswari T, Tokala VNB, Bhattacharya S, et al. Comparative analysis of synthesis and characterization of silver nanoparticles extracted using leaf, flower, and bark of Hibiscus rosasinensis and examine its antimicrobicidal activity. J Nanomaterials. 2022;2022:8123854. doi: 10.1155/2022/8123854. [DOI] [Google Scholar]
- 128.Bhaskar A, Nithya V. Evaluation of the wound-healing activity of Hibiscus rosa sinensis L (Malvaceae) in Wistar albino rats. Indian J Pharmacol. 2012;44:694–8. doi: 10.4103/0253-7613.103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sen S, Bal T, Rajora AD. Green nanofiber mat from HLM–PVA–Pectin (Hibiscus leaves mucilage–polyvinyl alcohol–pectin) polymeric blend using electrospinning technique as a novel material in wound-healing process. Appl Nanosci. 2022;12:237–50. doi: 10.1007/s13204-021-02295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mustaffa F, Parasuraman S, Sahgal G. Wound healing activity of herbal ointment containing the extracts of Hibiscus rosa-sinensis flowers and Curcuma longa rhizomes. Free Radicals Antioxid. 2020;10:86–8. doi: 10.5530/fra.2020.2.15. [DOI] [Google Scholar]
- 131.Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Gosavi TP, Badole SL, et al. Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. Leaves in experimental colitis in rats. Asian Pac J Trop Biomed. 2012;2:337–44. doi: 10.1016/S2221-1691(12)60053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nath P, Yadav AK. Acute and sub-acute oral toxicity assessment of the methanolic extract from leaves of Hibiscus rosa-sinensis L. in mice. J Intercultural Ethnopharmacol. 2015;4:70–3. doi: 10.5455/jice.20141028021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pethe M, Yelwatkar S, Manchalwar S, Gujar V. Evaluation of biological effects of hydroalcoholic extract of Hibiscus rosa sinensis flowers on alloxan induced diabetes in rats. Drug Res. 2017;67:485–92. doi: 10.1055/s-0043-109434. [DOI] [PubMed] [Google Scholar]
- 134.Jana TK, Das S, Ray A, Mandal D, Giri JS, Bhattacharya J. Study of the effects of Hibiscus-rosa-Sinensis flower extract on the spermatogenesis of male albino rats. J Physiol Pharmacol Adv. 2013;3:167–71. doi: 10.5455/jppa.20130616115400. [DOI] [Google Scholar]
- 135.Afiune LAF, Leal-Silva T, Sinzato YK, Moraes-Souza RQ, Soares TS, Campos KE, et al. Beneficial effects of Hibiscus rosa-sinensis L. flower aqueous extract in pregnant rats with diabetes. PLoS ONE. 2017;12:e0179785. doi: 10.1371/journal.pone.0179785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lucas’. May Papaw Remedies©. Lucas’ Papaw Remedies©: Ingredients. https://lucaspapaw.com.au/pages/our-ingredients. Accessed 22 2024.
- 137.Real Paw Paw. Real Paw Paw ointment: The ingredients. https://realpawpaw.com.my/. Accessed 22 May 2024.
- 138.Théra Wise. Natural wound care ointment (SHO natural skin healing ointment). https://therawise.com/products/naturalwoundskinhealing. Accessed 23 May 2024.
- 139.Beaphar. Beaphar wound ointment for dogs, cats, small animals & birds 30ml. https://www.beaphar.co.uk/product/13764-beaphar-wound-ointment/. Accessed 23 May 2024.
- 140.Holistic H, Wellness. I’m wounded! Natural wound cream. https://holistichealeronline.com/im-wounded-natural-wound-care-cream/. Accessed 23 May 2024.
- 141.Amazon. Elements wound healing cream 25gms. https://www.amazon.in/Elements-Wound-Healing-Cream-25Gms/dp/B00T00MJA0. Accessed 23 May 2024.
- 142.WoundKreme™. WoundKreme™ organic healing ointment: All natural ingredients. https://woundkreme.com/about-us/#list. Accessed 23 May 2024.
- 143.Melcura™. Melcura™ HoneyPlus. https://www.melcura.co.za/products/melcura-plus. Accessed 23 May 2024.
- 144.Naturaline. GoHeal antiseptic skin ointment for diabetic wounds, burns, non-healing wounds, bedsores, cuts and bruises – 30gms. https://naturaline.in/product/goheal_single_pack/. Accessed 23 May 2024.
- 145.Hurix’s. Hurix’s Aloe vera plus cream. https://www.hurixs.com.my/onlinestore/en/for-skin/142-205-hurix-s-krim-luka-aloe-vera-plus.html. Accessed 23 May 2024.
- 146.Natural Scents Aromatherapy. Scar and wound healing cream. https://www.naturalscents.com.au/shop-now/scar-and-wound-healing-cream. Accessed 23 May 2024.
- 147.FAME Pharmaceuticals Industry. Nemoderm® wound healing cream. https://famepharma.com/nemoderm/. Accessed 23 May 2024.
- 148.Abhaiherb.net. Centella cream. https://www.abhaiherb.net/en/product/55/. Accessed 23 May 2024.
- 149.Firson Co. Ltd. Non-medicinal products: Madecare ointment (Centella quantitative extract). https://www.firson.co.kr/eng/sub_products/prod_view.php?p_idx=12&pg=1&block=1&cate=0004_. Accessed 23 May 2024.
- 150.Yami. FUYUAN Centella asiatica cream ointment 2.5%. https://www.yamibuy.com/en/p/fever-warming-patch-baby-patch-female-aunt-pain-patch-10-patches-bag-wormwood-warm-patch/3030053171?scene=item_category.item&bu_type=category_tree&module_name=category_main&index=1599&content=Skin%20Treatments&track=category-1599-30-3030053171&pg=2. Accessed 23 May 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


