Summary
In rats, cannulation of the jugular vein and the carotid artery precedes the use of the hyperinsulinemic euglycemic clamp to determine insulin sensitivity in vivo. Here, we present a vascular surgery protocol to allow the infusion of substances via the vein and the collection of blood samples from the artery on the day of the hyperinsulinemic euglycemic clamp. We describe steps for preparing for and performing catheterization surgery. We then detail procedures for clamp preparation and its use.
For complete details on the use and execution of this protocol, please refer to Pereira et al.1,2,3
Subject areas: Health Sciences, Metabolism, Model Organisms
Graphical abstract

Highlights
-
•
Steps for chronic cannulation of the jugular vein and the carotid artery in rats
-
•
Guidance on the hyperinsulinemic euglycemic clamp in rats
-
•
Detailed steps for preparing the surgical area and rat for surgery
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
In rats, cannulation of the jugular vein and the carotid artery precedes the use of the hyperinsulinemic euglycemic clamp to determine insulin sensitivity in vivo. Here, we present a vascular surgery protocol to allow the infusion of substances via the vein and the collection of blood samples from the artery on the day of the hyperinsulinemic euglycemic clamp. We describe steps for preparing for and performing catheterization surgery. We then detail procedures for clamp preparation and its use.
Before you begin
The hyperinsulinemic euglycemic clamp protocol with tracer methodology is used to quantify hepatic and peripheral insulin sensitivity in vivo. We have previously infused treatments into the jugular vein and performed the hyperinsulinemic euglycemic clamp during the last 2 h in rats.1,2,3 In the protocol herein, we do not include intravenous infusion of treatments for simplification. Since chronic catheterization of the jugular vein and carotid artery is required to perform the clamp in free-moving rats, we also describe this vascular surgery in the current manuscript. The jugular vein is used to infuse solutions while the carotid artery is used to obtain blood samples and for the re-infusion of red blood cells. The protocols described herein are sufficiently detailed for researchers to do them independently for the first time.
In the hyperinsulinemic euglycemic clamp, plasma glucose is clamped during intravenous insulin infusion.4,5 This is done by frequently measuring plasma glucose and avoiding insulin-induced glucose lowering via adjusting the rate of an intravenous glucose infusion. In this way, insulin action is assessed in the absence of counterregulatory mechanisms induced by hypoglycemia. Whole-body insulin sensitivity is derived from the amount of glucose infusion required to maintain euglycemia during a constant fixed-dose insulin infusion, as an insulin sensitive individual would require a greater amount of exogenous glucose than an insulin resistant individual to maintain euglycemia. The use of a glucose tracer in the clamp protocol allows quantification of glucose production via the dilution principle, as newly produced endogenous glucose is not labeled and thus dilutes the radioactivity in the plasma glucose pool. Knowing endogenous glucose production, it is possible to calculate its suppression from basal (hepatic insulin sensitivity as glucose is produced mainly by the liver) while peripheral insulin sensitivity (mainly muscle insulin sensitivity) is the stimulation of glucose utilization (clearance of glucose determined by the tracer multiplied by the plasma glucose concentration) from basal glucose utilization (equal to glucose production during the basal period when plasma glucose is stable).
We have recently described how the hyperinsulinemic euglycemic clamp compares to the insulin tolerance test, which is a technique that is commonly used to assess insulin sensitivity in vivo.4 Hepatic and peripheral insulin sensitivity cannot be distinguished with the insulin tolerance test. Furthermore, the insulin dose used in the insulin tolerance test should be such that it minimizes counterregulatory mechanisms caused by hypoglycemia.
Institutional permissions
The Animal Care Committee of the University of Toronto has reviewed and approved the procedures listed below. Similar permission is required from each institution interested in performing such experiments.
Preparation for surgery
Timing: 1–2 h
CRITICAL: The surgery should be sterile.
-
1.Prepare surgical pack, including surgical tools and gauze.
-
a.Sterilize surgical pack in an autoclave.
-
a.
-
2.Prepare catheters (Figures 1A and 1B).
-
a.Cut 20–25 cm of polyethylene (PE50) tubing and make a bevel at one end by cutting at 30°–45°.
-
b.Cut silastic tubing such that there is a bevel at one end (30°–45°) and the total length is 2.5–2.7 cm.
 CRITICAL: The length of silastic tubing depends on the size and strain of the rat.6 In our previous publications, for female Wistar rats, we used ∼2.7 cm total of silastic tubing.
CRITICAL: The length of silastic tubing depends on the size and strain of the rat.6 In our previous publications, for female Wistar rats, we used ∼2.7 cm total of silastic tubing. -
c.Insert PE50 tubing into silastic tubing after dipping silastic tubing in ether to temporarily increase its diameter. Bevels of both types of tubing should align.Note: The purpose of the bevels is to facilitate insertion of catheter into blood vessel.
-
d.The extent of overlap between PE50 tubing and silastic tubing should be ∼1 cm.
-
e.Sterilize catheters with cold sterilant.
-
a.
-
3.Prepare sterile heparinized saline (4 U/mL).
-
a.4 U/mL heparinized saline is prepared, for example, by mixing 2000 U of heparin solution with 500 mL of saline (0.9% NaCl).
-
a.
-
4.
Prepare the analgesic agent in advance (buprenorphine).
-
5.
Set up anesthetic (isoflurane) machine.
-
6.
Turn on heating pad for surgery and heating pad or lamp for recovery.
-
7.
Switch on the bead sterilizer.
-
8.
Obtain clean cages for recovery.
Figure 1.
Preparation of catheters for vascular surgery
(A) A catheter for the jugular vein or carotid artery.
(B) A close-up of the catheter in (A).
Preparation for the hyperinsulinemic euglycemic clamp
Timing: ∼3 h
-
9.Prepare 0.1% bovine serum albumin (BSA) solution.
-
a.Dissolve 1 g of BSA in 1 L saline, aliquot, and freeze at −20°C. Thaw out on the morning of clamp experiment.
-
b.Alternatively, prepare 5% BSA solution, aliquot, and freeze at −20°C. Thaw out and dilute to 0.1% BSA with saline on the morning of the clamp experiment.
-
a.
-
10.
Prepare tritiated [3-3H] glucose solution.
-
11.Prepare clamp lines (Figure 2).
-
a.Dip 3–4 cm of silastic tubing in ether and a 4-way 20G–22G connector is inserted into one end and a 40–50 cm piece of PE50 tubing is inserted into the other end. Repeat for each line.
-
b.A 4-way connector is used for 3 infusates; one of the lines is attached to the extended jugular catheter.
-
a.
-
12.Prepare heparinized microcentrifuge tubes for collection of blood for tracer assay (“T tubes”).
-
a.Can prepare them up to 1–2 weeks before the clamp. Store closed at 18°C–25°C.
-
a.
-
13.Prepare microcentrifuge tubes for collection of blood for plasma analysis of hormones and metabolites (“G tubes”).
-
a.Prepare them the day before the clamp and keep in fridge until ready to use.
-
a.
-
14.Prepare needles to attach to clamp lines, extended jugular catheter, and extended carotid catheter by breaking off the lancet (sharp tip) of 23G needles using a sterilized serrated hemostat.
-
a.These needles are now blunt-tip needles (they have to be sterilized afterwards if prepared in a non-sterile manner).
-
b.To break off the lancet, close the hemostat around the needle. Next, quickly rotate the hemostat ∼ 30° counterclockwise and then ∼ 30° clockwise. This should be sufficient to break the needle.Note: To maintain sterility, gloves should be sterile.
-
c.Attach each blunt-tip needle to a 1 mL syringe.
 CRITICAL: Ensure that the needle is not occluded after removal of the sharp tip.
CRITICAL: Ensure that the needle is not occluded after removal of the sharp tip.
-
a.
-
15.Prepare sterile heparinized saline (4 U/mL and 2 U/mL).
-
a.2 U/mL heparinized saline is prepared, for example, by mixing 1000 U of heparin solution with 500 mL of saline (0.9% NaCl).
-
b.Heparinized saline should be stored in the fridge but should be at 18°C–25°C (or body temperature) when administered to rats.
-
a.
Note: Sterility is not necessary during the clamp because it is a terminal experiment.
Figure 2.

Clamp lines
Lines for 3 infusates. The 4th line will be connected to the catheter extending from the rat’s jugular vein; a connector made from a 23G needle is also attached to the 4th line.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Heparin solution (various concentrations and sources available) | Sandoz Canada | DIN 023013108 |
| Bovine serum albumin, fatty acid free | Sigma | Cat# A7030 |
| Tritiated [3-3H] glucose | PerkinElmer | Part# NET331C005MC |
| Insulin (Humulin R) | Eli Lilly Canada | DIN 09853766 |
| EDTA | Sigma | Cat# E5134 |
| Aprotinin | Cedarlane Labs | Cat# CLPRO285 |
| Cold sterilant | PREempt CS20 | Cat # CA10836-026 |
| Experimental models: Organisms/strains | ||
| Female Wistar rats | Charles River | Ordered rats that weighed 250–300 g |
| Other | ||
| PE50 tubing | BD Intramedic | Ref# 427411 |
| Silastic tubing for catheters (0.51 mm I.D. x 0.94 mm O.D.) | Liveo laboratory tubing, DuPont | Cat# 508-002 |
| 4-way connector (e.g., 22G) | Instech | Cat# SCX22 |
| Capillary tube for hematocrit | Fisherbrand | Cat# 22-260-943 |
| Gauze (5.1 cm × 5.1 cm; various sources available) (sterilize before use) | Covidien | |
| 23G x 1″ needle | BD | Ref# 305145 |
| 16G x 1½” needle | BD | Ref# 305198 |
| 1 mL syringe | BD | Ref# 309659 |
| 10 mL syringe | BD | Ref# 302995 |
| 20 mL syringe | BD | Ref# 302830 |
| 4-0 wax-coated braided silk suture (non-absorbable; without needle) | Covidien (Sofsilk) | LS-640 |
| 4-0 wax-coated braided silk suture (non-absorbable; with needle) | Covidien (Sofsilk) | VS-881 |
| Scalpel blade (#10) | Almedic | M90-10 |
| Micro-scissors (spring scissors) (sterilize before use) | Fine Science Tools (F.S.T.) | Item# 15003-08 |
| Forceps (sterilize before use) | F.S.T. | Item# 11051-10 |
| Hemostats (serrated; straight, curved) (sterilize before use) | Fisherbrand, F.S.T. | |
| Glucose analyzer | Analox | |
| Infusion pump | Harvard Apparatus | |
| Silastic tubing to cover hemostats (e.g., 1.57 mm I.D. x 2.41 mm O.D.) | DuPont | CA62999-224 |
| Mini, micro centrifuges to obtain plasma and hematocrit | ||
Materials and equipment
Preparation of tritiated [3-3H] glucose solution (Tinf)
Dissolve 5 mL of 5 mCi [3-3H] glucose in tracer diluent to achieve a total volume of 250 mL. The resulting solution is 20 μCi/mL. Store in fridge. This glucose solution does not contain measurable amounts of unlabeled glucose (a minimal amount is used as a “carrier” for the glucose tracer).
Tracer diluent
| Reagent | Final concentration | Amount |
|---|---|---|
| D-glucose | 28 mM | 5 g |
| Sodium benzoate | 14 mM | 2 g |
| Saline | N/A | 1 L |
| Total | N/A | 1 L |
Tracer diluent should be kept in the refrigerator (∼ 4°C) for up to 6 months; discard if cloudy.
Preparation of “hot” (i.e., radiolabeled) glucose solution
For each rat, mix 5 mL of 50% dextrose with 3 mL of [3-3H] glucose solution (40 μCi/mL). This is the exogenous glucose solution infused during the clamp to maintain euglycemia. It is called “hot” because in addition to unlabeled glucose (i.e., dextrose), it also contains a glucose tracer ([3-3H] glucose). This allows maintenance of the ratio of radioactive to non-radioactive glucose at the basal level, as otherwise during the clamp this would decrease with exogenous glucose infusion. In other words, by infusing “hot” glucose only the glucose produced by the liver is unlabeled.
Preparation of T tubes
Add 10 U of heparin to clean 1.5 mL microcentrifuge tubes; this amount of heparin is required per 0.2 mL of blood collected during the clamp. Dry these heparinized tubes in oven (37°C).
Preparation of G tubes
Add 10% of blood sample volume of EDTA:Aprotinin (1:1) solution to clean 1.5 mL microcentrifuge tubes. For example, if blood sample collected during the clamp is 0.2 mL, 20 μL of EDTA:Aprotinin solution is added to the microcentrifuge tube.
EDTA solution is prepared by dissolving 2.4 g EDTA in 100 mL double-distilled water. Store in fridge.
Aprotinin is prepared by dissolving it in doubled-distilled water to achieve a final concentration of 10000 KIU/mL. Store in −20°C freezer.
Note: If an experimental protocol involves infusion of a triglyceride emulsion, such as Intralipid in Pereira et al. 2013,1 then a lipase inhibitor like Orlistat (30 μg per ml of blood) should be added to the G tubes containing EDTA:Aprotinin solution. This is necessary in such experimental protocols for accurate measurement of plasma lipid metabolites such as free fatty acids.
Preparation of insulin infusate
Mix 10 μL of insulin (100 U/mL) in X ml of 0.1% BSA, where X = 2 / body weight of rat in kg. For example, if rat weighs 250 g, mix 10 μL of insulin with 8 mL of 0.1% BSA.
Step-by-step method details
Chronic catheterization of jugular vein and carotid artery
Timing: ∼45 min to 1 h
Timing: 5–10 min (for step 1)
Timing: 5–10 min (for step 2)
Timing: ∼30 min (for step 3)
Timing: 5–10 min (for step 4)
Here, we describe the steps necessary to cannulate the right jugular vein and left carotid artery in rats.
-
1.Preparation of surgical area.
-
a.Put on personal protective equipment, including surgical mask, surgical gown, and gloves.
-
i.Spray gloves with disinfectant now and as needed throughout the surgical procedure.
-
ii.Maintain sterility throughout the surgical procedure.
-
i.
-
b.Disinfect surgical area.
-
c.Place disposable underpad over heating pad.
-
d.Open surgical pack.
-
e.Attach 1 mL syringes to 23G needles and remove the needle bevels with a sterilized serrated hemostat.
-
i.Fill each syringe with ∼0.7 mL of heparinized saline (4 U/mL).
-
ii.Attach each blunt-tip needle to a catheter and flush with heparinized saline.
 CRITICAL: Never infuse air bubbles intravenously.
CRITICAL: Never infuse air bubbles intravenously. -
iii.Prepare approximately 4 syringes with blunt-tip needles attached per rat.
-
i.
-
f.Cut pieces of 4-0 suture without needle (6 per rat and each ∼8 cm long).Note: Suture used in all procedures described herein was non-absorbable, but some institutions require the use of absorbable suture.
-
a.
-
2.Preparation of rat.
-
a.Anesthetize the rat with isoflurane in an induction chamber.Note: From this point onward until the end of the surgery, the rat is anesthetized.
-
i.Induction typically involves 5% isoflurane.
-
ii.Maintenance typically requires 2%–3% isoflurane.
-
i.
-
b.Move the rat to the maintenance nose cone.Note: This area (“preparatory station”) is a different, yet adjacent, area to where the surgery per se will take place.
-
c.Apply eye lubricant and check pedal reflex.
-
d.Inject analgesia subcutaneously and fluids if needed.
-
e.Shave the ventral and dorsal side of the neck, where skin incisions will be made.
-
f.Clean the area from excess hair and disinfect it as follows:
-
i.Disinfect the ventral side of the neck that has been previously shaved by applying isopropyl alcohol, followed by betadine. Repeat this a total of 3 times.Note: Initial 2 times are performed at the preparatory station and final time is performed on surgery table.
-
i.
-
g.Place rat on surgical table, with its nose toward the surgeon. Place a 15 mL conical tube or a stack of gauze underneath the rat’s neck.
-
h.Place a sterile drape over the rat, below the skin site that has just been disinfected.
-
i.Ensure rat is anesthetized based on various factors, including pedal reflex and breathing pattern every 5–10 min.
-
a.
-
3.Surgery.
-
a.Make a horizontal incision to the disinfected skin area above the clavicle (Figure 3A).
-
i.Wipe away excess blood with sterile gauze.
-
i.
-
b.Pick up connective tissue with forceps using your non-dominant hand and grab forceps with your dominant hand to blunt dissect (i.e., separate) connective tissue.
-
i.Along the midline, muscle covering the trachea (i.e., sternohyoid muscle) is visible.6
-
ii.To the right of the midline, the right jugular vein appears as a dark red line that is parallel to the midline.
-
iii.The right jugular vein is on the rat’s right side, while the left carotid artery is on the rat’s left side.
-
iv.Veins are more superficial than arteries. Figure 3B demonstrates the blood vessels in rodents.
-
i.
-
c.Use forceps and curved hemostats to further remove connective tissue around the jugular vein such that ∼1 cm of the jugular vein is separated from surrounding tissue.Note: Isolation of the jugular vein must be done quickly because it contracts easily.
-
i.While lifting the jugular vein with forceps, slide (but do not drag) 2 pieces of suture underneath it.
-
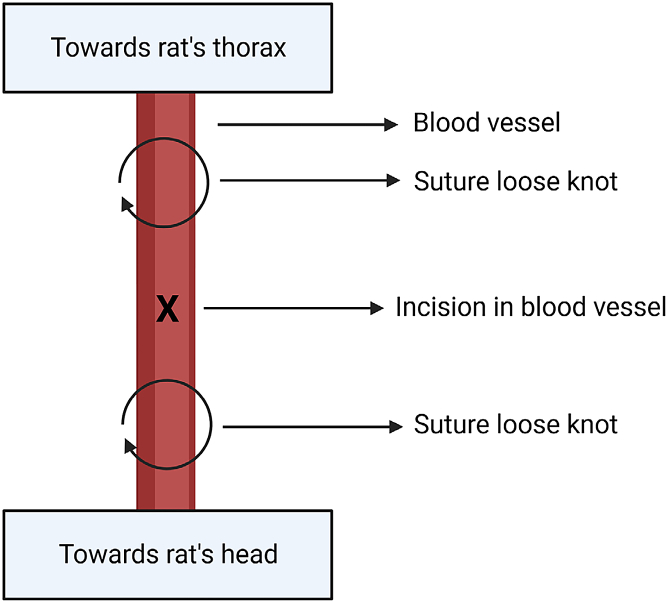
ii.Make two loose knots at the top and bottom of the piece of jugular that has been isolated from surrounding tissue (Figure 4). Do not occlude the jugular vein at this point.
-
iii.Use 2 sterilized hemostats with tips covered by plastic (2–3 cm of silastic tubing) to clasp the ends of each knot. In doing so, create a bit of tension to prevent blood flow by slightly lifting and straightening the jugular vein.
-
iv.Using micro-scissors, make a small hole in the jugular vein at 30°–45°, approximately halfway between the two pieces of suture (Figure 5A).Note: The jugular vein is fragile and can break.
-
v.Use your non-dominant had to pick up the flap of the small hole with forceps and your dominant hand to insert the catheter into the jugular vein.Note: If the researcher cannot see the hole in the jugular vein, they should use magnifying glasses or a dissecting microscope.
-
vi.Release tension in the distal suture as catheter is inserted into the jugular vein.
-
vii.Stop inserting the catheter as soon as the cuff of the catheter (i.e., ∼1 cm of overlap between PE50 and silastic tubing) is completely inserted in the jugular vein.Note: The tip of the catheter should be close to the right atrium.
-
viii.Use a third piece of suture to tie the proximal (toward rat’s head) end of the cuff. Do a single knot, followed by two double knots.
-
ix.Tie the distal (toward rat’s thorax) loose knot using a single knot, followed by two double knots (Figure 4). This knot should be done over the distal end of the cuff.
-
x.Tie the proximal (toward rat’s head) loose knot to cut off blood flow from the head in the jugular vein (Figure 4). Then, make a single knot followed by 2 double knots around the catheter.
-
xi.Check that blood can be withdrawn from the jugular vein and flush the vein.
 CRITICAL: The inability to obtain blood from the vein at this stage usually indicates that the surgical procedure was poor.
CRITICAL: The inability to obtain blood from the vein at this stage usually indicates that the surgical procedure was poor. -
xii.Cut excess suture ends (Figure 5B).
-
i.
-
d.Blunt dissect between muscle layers on the left side of the trachea.
-
i.The point of entry for the curved hemostats is where 3 muscles (sternohyoid, omohyoid, sternomastoid) meet.6
-
ii.Blunt dissect vertically (i.e., parallel to sternohyoid muscle) to avoid damaging the trachea.
-
i.
-
e.The left carotid artery should be visible as a red line, with the vagus nerve appearing as a white line beside it (Figure 5C). To isolate the carotid artery, create a small incision with forceps in the muscle layer covering it.
 CRITICAL: Do not damage the vagus nerve. To avoid damaging the vagus nerve, blunt dissect parallel to it, do not pinch it with forceps, and do not lift it too high.
CRITICAL: Do not damage the vagus nerve. To avoid damaging the vagus nerve, blunt dissect parallel to it, do not pinch it with forceps, and do not lift it too high. -
f.To insert the catheter into the left carotid artery, repeat the same steps as for the right jugular vein in Step #3c i-xii of this section.Note: The tension that needs to be kept to prevent blood flow by slightly lifting and straightening the blood vessel while inserting the catheter is greater for the carotid artery than the jugular vein. This tension is also necessary to avoid excessive blood loss from the carotid artery.Note: The tip of the catheter should be close to the aortic arch.
-
g.Wipe away excess blood, drop ∼ 0.1 mL of heparinized saline to exposed tissue, cover it with sterile gauze and turn the rat over.
-
i.Criss-cross the catheters over the trachea, such that the right jugular vein catheter is externalized via the rat’s left side and the left carotid artery catheter is externalized via the rat’s right side.
-
ii.Criss-crossing the catheters makes it easier to leave more of the catheters inside the rat, which will make it more challenging for the rat to pull out the catheters.
-
i.
-
h.Disinfect the shaved area on the dorsal side with isopropyl alcohol, as in Step #2f i of this section.
-
i.Starting in this disinfected area, close to the midline, insert a 16G needle subcutaneously and tunnel it until the ventral incision is reached (Figure 5D).
-
i.Clamp the catheter with hemostats with plastic covering the tips.Note: PE50 tubing can be damaged if clasped with hemostats that do not have plastic covering their tips.
-
ii.Detach the catheter from the needle and syringe containing heparinized saline.
-
iii.Insert the catheter into the lumen of the needle until it appears on the dorsal side of the rat.
-
iv.Remove the 16G needle.
-
v.Attach the catheter to the needle and syringe containing heparinized saline.
-
vi.Unclamp the catheter.
-
i.
-
j.Repeat Step #3i i-vi in this section for the other side/catheter.
-
k.Check once again that blood can be withdrawn from each catheter and flush both catheters.
 CRITICAL: If there is excessive blood loss during catheterization, this blood volume should be replenished with heparinized saline.
CRITICAL: If there is excessive blood loss during catheterization, this blood volume should be replenished with heparinized saline. -
l.Clamp each catheter on the dorsal side and cut them, leaving ∼2.5 cm of each catheter.
-
i.Close each catheter with a metal pin, release the clamp, and tape both ends of the catheters together.
-
ii.Ensure that enough catheter is left in the rat, so as not to create discomfort or suffocate the rat.
-
i.
-
m.Turn the rat over, remove the gauze, drop ∼0.2 mL of heparinized saline to the exposed tissues and absorb with a new piece of gauze.
-
n.Suture the skin incision using a continuous pattern (use 4-0 suture with needle).
-
i.Wipe away any excess blood.Note: Suture used was non-absorbable, but some institutions require the use of absorbable suture.
-
i.
-
o.Turn off isoflurane and ∼30 s later, once vital signs have returned, turn off the oxygen.
-
p.Clean excess blood from surgical tools, place them in bead sterilizer, and allow tools to cool before using them on the next rat.
-
a.
-
4.Postoperative care.
-
a.Place paper towels on top of bedding.
-
i.Half of the cage should be exposed to a lamp or on top of a heating pad.
-
i.
-
b.Place rat on top of paper towels, lying on its side.
-
c.Put some food pebbles beside rat.
-
d.Once rat is moving around and if it appears healthy, remove the paper towels and place rat where it is normally housed.
 CRITICAL: Rats must be singly housed following the surgery to avoid removal of catheters by cage mates, but additional enrichment such as paper strips and chew toy is usually provided by the animal facility.
CRITICAL: Rats must be singly housed following the surgery to avoid removal of catheters by cage mates, but additional enrichment such as paper strips and chew toy is usually provided by the animal facility. -
e.Apply analgesia as needed and according to the procedures approved by the animal care committee.
-
f.Rats are allowed 4–5 days to recover from this surgery before performing the clamp experiment.
-
a.
Figure 3.
Initial steps of vascular catheterization procedure
(A) Incision above the clavicle.
(B) Arterial and venous blood systems, including left carotid artery and right jugular vein, in rodents. Created with BioRender.com.
Figure 4.
Location of suture and incision in isolated blood vessel
Vessel is either jugular vein or carotid artery. Created with BioRender.com.
Figure 5.
Selected steps of jugular vein and carotid artery catheterization procedure
(A) Isolated right jugular vein with incision.
(B) Completed cannulation of the right jugular vein.
(C) Left carotid artery and vagus nerve are visible, but they are covered by a thin layer of muscle.
(D) Subcutaneous tunneling with 16G needle.
Hyperinsulinemic euglycemic clamp
Timing: ∼5 h 30 min
Timing: ∼30 min (for step 5)
Timing: ∼1 h (for step 6)
Timing: 4 h (2 h for clamp per se, which is preceded by a 2 h infusion of glucose tracer for assessment of basal glucose kinetics) (for step 7)
This section describes how to perform the hyperinsulinemic euglycemic clamp in conscious and unrestrained rats with cannulas in the right jugular vein and left carotid artery.
-
5.Extending catheters from rats on clamp day.Note: Start in the morning.
-
a.Put on personal protective equipment, including lab coat, gloves, and surgical mask.
-
b.Weigh rats, which have been fasted for >5 h.7 Troubleshooting 1.
-
i.In the papers associated with the current protocol, rats were fasted for 12–18 h.
-
ii.Avoid restraining rats when extending catheters to minimize stress. Instead, place a clean towel over their eyes.
-
i.
-
c.Obtain hemostats with tips covered by plastic, 1 mL syringes filled with heparinized saline (4 U/mL) and with attached blunt-tip needles, scissors, and gauze.
-
i.Cut 2 40–50 cm pieces of PE50 tubing and attach a 1-way connector to each.
-
ii.The 1-way connector is made by cutting both metal ends of a 23G needle with serrated hemostats.
-
iii.Flush PE50 tubing with connector with heparinized (4 U/mL) saline.
-
i.
-
d.Remove the tape covering the catheters.
-
e.Clamp one of the catheters and cut off the section containing metal pin.
-
i.Attach PE50 tubing with connector.
-
ii.Check patency. Troubleshooting 2 and 3.
-
iii.Discard ∼0.05 mL of blood from the catheter because this blood may have been sitting in catheter for a few days.
-
iv.Flush catheter with heparinized (4 U/mL) saline.
 CRITICAL: Make note if there is resistance when infusing into the jugular vein because this may affect the rate at which infusates reach the circulation during the clamp. For subsequent rats, improve surgical technique.
CRITICAL: Make note if there is resistance when infusing into the jugular vein because this may affect the rate at which infusates reach the circulation during the clamp. For subsequent rats, improve surgical technique. CRITICAL: When checking patency or obtaining blood samples at any point, do not pull back too fast and this may cause the blood vessel to collapse onto the tip of the catheter, thereby occluding blood flow.
CRITICAL: When checking patency or obtaining blood samples at any point, do not pull back too fast and this may cause the blood vessel to collapse onto the tip of the catheter, thereby occluding blood flow.
-
i.
-
f.Repeat previous step (e) for the other catheter.
-
g.Tape connectors to PE50 tubing.
-
h.Cut a 1 mL syringe in half and use one half to hold the catheters in place at the back of the rat’s head. Tape the syringe to the extended catheters.
-
i.Wait 30 min–1 h for rats to calm down before obtaining basal blood samples.Note: To minimize stress to the rats on the clamp day, rats should be sufficiently handled by researchers beforehand. Traffic and noise should also be minimized during the clamp.
-
j.From here onwards, monitor rats so that they do not bite their extended catheters.
-
a.
-
6.Preparation on clamp day.
-
a.Prepare heparinized saline (2 U/mL).
-
b.Calibrate Analox glucose analyzer.
-
c.Remove bevels from 23G needles that will be attached to syringes containing infusates.
-
d.Load tritiated glucose tracer onto syringe and attach blunt-tip 23G needle.
-
e.Prepare exogeneous glucose solution containing tritiated glucose. Load it onto syringe and attach blunt-tip 23G needle.
-
i.This infusate is known as “hot” glucose solution and it is used to avoid major changes in specific activity during the clamp, which would result in erroneous calculations of glucose kinetics.8
-
i.
-
f.Prepare insulin infusate by adding insulin to 0.1% BSA. Load it onto syringe and attach blunt-tip 23G needle.
-
g.Place syringes containing infusates on pumps, attach clamp lines to infusate-containing syringes, and clamp (with hemostats with tips covered by silastic) the lines.
-
i.Attach a connector made from a 23G needle to the piece of tubing from the clamp lines that will be attached to the jugular vein.
-
i.
-
h.Flush the clamp lines by setting the pump at 999 μL/min, one infusate at a time.
-
i.Start with the infusate that will be the last to be infused into the rat and work backwards (i.e., “hot” glucose solution, then insulin, and finally glucose tracer solution (Tinf)).
-
ii.Unclamp the tubing attached to the syringe of the infusate that is being flushed, while ensuring that the rest of the tubing attached to the other infusate-containing syringes is clamped.
-
i.
-
a.
-
7.Hyperinsulinemic (5 mU kg−1 min−1) euglycemic clamp.
-
a.Obtain a basal blood sample.
-
i.To obtain a blood sample during the clamp experiment, start with 0.1–0.2 mL of heparinized saline in a syringe (4 U/mL) and withdraw ∼0.3–0.4 mL of blood from the carotid artery into this syringe.
-
ii.Clamp extended carotid catheter, remove syringe, and place syringe aside.
-
iii.Use a new syringe to obtain the blood sample or simply let blood drop from extended carotid catheter into a microcentrifuge tube.
 CRITICAL: Never let blood sit in catheter for >1–2 min because blood will clot or the probability that sampling from the carotid will stop increases.
CRITICAL: Never let blood sit in catheter for >1–2 min because blood will clot or the probability that sampling from the carotid will stop increases. -
iv.After obtaining each blood sample, re-infuse the blood obtained in step#7a i of this section, then flush the line with ∼0.3 mL of heparinized (4 U/mL) saline (just enough to leave a clear solution in the carotid catheter).
-
v.Leave ∼0.1 mL of heparinized saline in the syringe for the next sample.
-
vi.Clamp the extended carotid catheter close to the syringe until the next sample.
-
i.
-
b.Measure plasma glucose concentration and transfer plasma to clean microcentrifuge tubes for subsequent assays. Troubleshooting 4.
-
c.Measure hematocrit.
-
i.To obtain the hematocrit, start by placing a few drops of blood in a hematocrit glass capillary tube and then sealing one end of the tube.
-
ii.Centrifuge the glass capillary tube containing blood at ∼ 3,500 × g for ∼ 4 min.
-
iii.Use a ruler to measure the length of the capillary tube containing packed red blood cells (numerator) and the length of the capillary tube containing both packed red blood cells and plasma (denominator).
-
iv.Divide this numerator by the denominator and multiply by 100 to calculate the hematocrit, which is expressed as a percentage.
-
i.
-
d.Mix red blood cells from centrifuged blood samples (∼ 2,000 × g for 30 s–1 min) with heparinized (4 U/mL) saline in 1:1 ratio (e.g., start off with 0.3 mL heparinized saline and re-infuse when total volume reaches 0.6 mL) and re-infuse into rat.
-
e.Attach the connector of the clamp lines to the extended jugular catheter.
-
f.Start tracer (Tinf) infusion (t = 0 min).
-
i.Give bolus (400 μL over 1 min) using an infusion pump, then set rate at 7.5 μL/min until the end of the clamp.
-
i.
-
g.At t = 90–120 min, the basal period is established.
-
i.This is a time of physiological and tracer equilibrium.
-
i.
-
h.Blood is collected to measure plasma glucose concentration and obtain plasma for assays.
-
i.Obtain ∼ 40–50 μL of blood for plasma glucose measurements at t = 90, 100, 110, and 120 min, and every 5 min afterwards until the end of the clamp.
-
ii.Collect 0.2 mL of blood for tracer assay at t = 90, 100, 110, and 120 min.
-
iii.Collect 0.2 mL or 0.3 mL of blood for hormone/metabolite assays at t = 100 min and t = 120 min.
-
i.
-
i.At t = 120 min, infusion of insulin starts (i.e., the hyperinsulinemic euglycemic clamp starts). Infuse insulin solution at 10 μL/min.
-
j.Measure plasma glucose concentrations every 5 min until the end of the clamp. Troubleshooting 5.
-
i.Infusion of exogenous glucose should start when plasma glucose decreases by ∼5 mg/dL, at a rate of ∼ 15 μL/min for an insulin sensitive rat in our previously published studies.
-
ii.Even if plasma glucose concentration has not decreased by t = 130 min (for example, because the rat is insulin resistant), it is suggested that exogenous glucose infusion be started at a rate of ∼10 μL/min at this time.
-
i.
-
k.During the clamp experiment (starting at t = 90 min), use 2 U/mL heparinized saline instead of 4 U/mL heparinized saline to avoid infusing too much heparin.
-
l.Mix red blood cells from centrifuged blood samples with heparinized (2 U/mL) saline in 1:1 ratio.
-
m.Adjust rate of exogenous glucose infusion such as to maintain plasma glucose concentration ± 10 mg/dL compared to the average plasma glucose concentration during the basal period.Note: A drop in blood glucose concentration > 20 mg/dL, especially if concentration of plasma glucose is low during the basal period, may trigger a counterregulatory response. The parameter of glucose kinetics that will be most affected in this scenario is endogenous glucose production (EGP) during the clamp and consequently, quantification of hepatic insulin sensitivity may not be reliable.
-
n.The clamp period, which is a steady state period, occurs at 210–240 min.
-
i.Collect 0.2 mL of blood for tracer assay at t = 210, 220, 230, and 240 min.
-
ii.Collect 0.2 mL or 0.3 mL of blood for hormone/metabolite assays at t = 220 min and t = 240 min.
-
i.
-
o.At the time of the last blood sample, measure hematocrit.
 CRITICAL: Hematocrit should not decrease by more than 10%, comparing the beginning to the end of the clamp experiment.
CRITICAL: Hematocrit should not decrease by more than 10%, comparing the beginning to the end of the clamp experiment. -
p.Humanely euthanize rats using methods approved by the animal care committee and collect tissues if necessary.
-
a.
Expected outcomes
The primary outcome of this protocol is glucose kinetics.1,3 Specifically, the rate of exogenous glucose infusion (Ginf) during the clamp steady state (last 30 min when insulin action has reached its plateau) is obtained on the day of the clamp. Ginf is a measure of whole-body insulin sensitivity. After doing a tracer assay, the rate of glucose utilization (=rate of disappearance, Rd) is calculated from the total radioactive glucose infused divided by the radioactive glucose concentration (glucose clearance) multiplied by the plasma glucose. Because both during the basal period and during the clamp period plasma glucose does not change, Rd is equal to the rate of appearance (Ra). During the basal state, Ginf is 0 and Ra corresponds to EGP. During the steady state of the clamp, Ra is Ginf plus EGP. Stimulation of Rd during the clamp period, expressed as clamp Rd (i.e., insulin-stimulated state) - basal Rd (Rd difference) or percent Rd difference of basal Rd, indicates peripheral insulin sensitivity since glucose after insulin stimulation is mainly taken up by muscle (although hepatic glucose uptake is also included in Rd), while suppression of EGP during the clamp, expressed as basal EGP – clamp EGP (EGP difference) or percent EGP difference of basal EGP represents hepatic insulin sensitivity (with a minor kidney component after an overnight fast in rats9). The glucose kinetics data in a control group of one of our published papers1 are in Figures 6A–6C. A schematic representation of the hyperinsulinemic euglycemic clamp is shown in Figure 6D.
Figure 6.
Example of glucose kinetics results from the hyperinsulinemic euglycemic clamp and overview of the hyperinsulinemic euglycemic clamp
(A) Glucose infusion rate during the clamp, (B) Endogenous glucose production during basal and clamp periods, and (C) Glucose utilization during basal and clamp periods. These glucose kinetics results are for a control group from our published paper,1 and results are shown as individual data points and mean ± SEM. Figures in (A), (B), and (C) are reprinted with Journal of Endocrinology permission from Pereira et al., 2013.1
(D) Scheme of the hyperinsulinemic euglycemic clamp. Created with BioRender.com.
Limitations
Good surgical technique is very important to the success of this protocol. Moreover, avoiding >10 mg/dL decreases in plasma glucose concentration during the hyperinsulinemic euglycemic clamp will avoid a counterregulatory response that increases EGP, thereby creating a confounding variable for glucose kinetics.
It is important to note that the current protocol is specific for rats. If performing a hyperinsulinemic euglycemic clamp in mice, the lower blood volume and smaller blood vessels make differences in the surgical procedure and clamp per se between the two species necessary.4 For example, in mice, the carotid artery is often not cannulated and red blood cells are often obtained from a “donor”. Detailed descriptions of the hyperinsulinemic euglycemic clamp protocol in mice have been published.10,11
Troubleshooting
Problem 1
On the morning of the clamp experiment, you notice that the rat pulled out the jugular vein or carotid artery catheter (related to Step 5b in “hyperinsulinemic euglycemic clamp”).
Potential solution
Humanely euthanize the rat and for subsequent rats:
-
•
Ensure catheters are externalized close to the midline at the back of the neck.
-
•
Do not leave long pieces of catheter at the back of the neck.
Problem 2
Either the jugular vein or carotid artery catheter is not patent (related to Step 5e ii in “hyperinsulinemic euglycemic clamp”).
Potential solution
-
•
The position of the rat is preventing flow of fluid through the catheter. Gently nudge the rat to move and check patency again. This is especially an issue in heavy and obese rodents.
-
•
The silastic portion of the catheter should be increased by ∼2 mm at a time.
Note: Excessively long catheters have adverse effects on animal health.
-
•
Use more heparinized saline during the surgery, but do not exceed limits set by your institution’s animal care committee.
-
•
Flush the catheters with heparinized saline (4 U/mL) at least once between the day of surgery and the day of catheter extension (i.e., clamp day).
Problem 3
Rat has a stroke at time of catheter extension or during the clamp (related to Step 5e ii in “hyperinsulinemic euglycemic clamp”).
Potential solution
-
⋅
Use more heparinized saline during the surgery, but do not exceed limits set by your institution’s animal care committee.
-
⋅
Flush the catheters with heparinized saline (4 U/mL) at least once between the day of surgery and the day of catheter extension (i.e., clamp day).
-
⋅
Dilute red blood cells in heparinized saline quickly after obtaining them.
-
⋅
Double-check that no air bubbles are present in catheters extending from the jugular vein or carotid artery.
Problem 4
Plasma is hemolyzed (related to Step 7b in “hyperinsulinemic euglycemic clamp”).
Potential solution
Dispense the blood from the syringe to the microcentrifuge tube slowly.
Problem 5
Sampling from carotid artery stops during clamp (related to Step 7j in “hyperinsulinemic euglycemic clamp”).
Potential solution
-
•
Infuse extra heparinized saline.
-
•
Check if there is a blockage in connector of carotid artery. Disconnect the carotid line at the connector and let a bit of blood (and hopefully blood clot) flow out of carotid catheter. Re-connect it.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Adria Giacca (adria.giacca@utoronto.ca).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Sandra Pereira (sandra.pereira@camh.ca).
Materials availability
There are no newly generated materials associated with this protocol.
Data and code availability
Datasets were not generated in this protocol. However, we present glucose kinetics data from one experimental group from a study that was previously published by us1 in Figure 6.
Acknowledgments
A.G. is supported by Canadian Institutes of Health Research (CIHR), the Heart and Stroke Foundation of Canada, and a Drucker Family Innovation Fund from Banting and Best Diabetes Centre (BBDC). M.K.H. is supported by grants from the Drucker Family Innovation Fund, BBDC, CIHR, and PSI Foundation. She also has support from an Academic Scholars Award from the Department of Psychiatry, University of Toronto, and is the Kelly and Michael Meighen Chair in Psychosis Prevention. S.M.A. is supported in part by an Academic Scholars Award from the Department of Psychiatry, University of Toronto, and the Centre for Addiction and Mental Health (CAMH) Discovery Fund. The graphical abstract and some of the figures (specified in the figure legends) were created using BioRender.com.
Author contributions
A.G. designed the experiments and S.P. performed the experiments. S.P. wrote the protocol. All authors edited and approved the manuscript.
Declaration of interests
M.K.H. received consultant fees from Alkermes, Inc. S.M.A. has served as a consultant for HLS Therapeutics and Boehringer Ingelheim Canada.
Contributor Information
Sandra Pereira, Email: sandra.pereira@camh.ca.
Adria Giacca, Email: adria.giacca@utoronto.ca.
References
- 1.Pereira S., Yu W.Q., Frigolet M.E., Beaudry J.L., Shpilberg Y., Park E., Dirlea C., Nyomba B.L.G., Riddell M.C., Fantus I.G., Giacca A. Duration of rise in free fatty acids determines salicylate's effect on hepatic insulin sensitivity. J. Endocrinol. 2013;217:31–43. doi: 10.1530/joe-12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira S., Park E., Mori Y., Haber C.A., Han P., Uchida T., Stavar L., Oprescu A.I., Koulajian K., Ivovic A., et al. FFA-induced hepatic insulin resistance in vivo is mediated by PKCδ, NADPH oxidase, and oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2014;307:E34–E46. doi: 10.1152/ajpendo.00436.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira S., Moore J., Li J.X., Yu W.Q., Ghanim H., Vlavcheski F., Joseph Y.D., Dandona P., Volchuk A., Cummins C.L., et al. 4-Phenylbutyric acid improves free fatty acid-induced hepatic insulin resistance in vivo. Endocr. Connect. 2021;10:861–872. doi: 10.1530/ec-21-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn M.K., Giacca A., Pereira S. In vivo techniques for assessment of insulin sensitivity and glucose metabolism. J. Endocrinol. 2024;260 doi: 10.1530/joe-23-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira S., Giacca A. In: Insulin Resistance and Cancer: Epidemiology, Cellular and Molecular Mechanisms and Clinical Implications. Fantus I.G., editor. Springer; 2011. Insulin and the physiology of carbohydrate metabolism; pp. 1–51. [Google Scholar]
- 6.Waynforth H.B., Flecknell P.A. Second Edition. Academic Press Ltd.; 1992. Experimental and Surgical Technique in the Rat. [Google Scholar]
- 7.Lam T.K.T., Gutierrez-Juarez R., Pocai A., Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- 8.Finegood D.T., Bergman R.N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36:914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- 9.Perry R.J., Zhang X.M., Zhang D., Kumashiro N., Camporez J.P.G., Cline G.W., Rothman D.L., Shulman G.I. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat. Med. 2014;20:759–763. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayala J.E., Samuel V.T., Morton G.J., Obici S., Croniger C.M., Shulman G.I., Wasserman D.H., McGuinness O.P. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3:525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J.K. In: Type 2 Diabetes: Methods and Protocols. Stocker C., editor. Humana Press; 2009. Hyperinsulinemic–Euglycemic Clamp to Assess Insulin Sensitivity In Vivo; pp. 221–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets were not generated in this protocol. However, we present glucose kinetics data from one experimental group from a study that was previously published by us1 in Figure 6.