Abstract
The cheese wine pairing is a beloved combination subject to a certain subjectivity due to sensorial, psychological, chemical, and cultural factors. This work represents a first attempt to explore the in vitro interactions between cheese, wine, and saliva to objectively measure the pairing. Two experimental red wines obtained from the same grape cultivar and four different cheeses were studied for their composition. Binding reactions between wine and cheese were carried out in three simulated tasting trials and, after precipitation, the wine phenolic content, Saliva Precipitation Index (SPI), and total proteins were evaluated. The optimal pairing (OP) was calculated considering the decrease in salivary and cheese proteins by wine, defined as the cleansing effect; the decrease in astringency due to the cheese, measured by the SPI, and the coating fat which would remain in mouth after eating a piece of cheese. Based on obtained results, the semi-hard cheese was identified as the best pairing option for the two experimental red wines. The differences in the phenolic content between the two wines were instead not enough to show a significant influence on the OP. The in vitro cheese wine pairing can contribute to understanding of wine tasting but it is only a part of the puzzle. However, this first contribution paves the way for additional studies on the molecular and chemical interactions involved in aroma and textural perception in simulated trials.
Keywords: Saliva, Wine, Cheese, Pairing, SDS-PAGE, Phenolic compounds
Graphical abstract
Highlights
-
•
The cheese wine pairing was objectively measured.
-
•
Binding reactions involving cheese, wine and saliva represented the in vitro tasting.
-
•
An equation for optimal pairing was obtained.
-
•
Cleansing effect and Saliva Precipitation Index represented the key drivers.
1. Introduction
The pairing of cheese and wine has been a culinary tradition enjoyed by connoisseurs and enthusiasts alike for centuries (Fletcher, 2011). In the last years, the appreciation of the pairing of wine and cheese has been the subject of numerous sensory studies with consumers and experts, aimed at finding the ideal pair. Red table wines were considered as a better accompaniment to cheeses than white wines (Bastian et al., 2009), while King and Cliff (2005) found the opposite: cheeses better paired with white wines. Among others, descriptive analysis (Bastian et al., 2010), Temporal Dominance of Sensations (Galmarini et al., 2018), free choice (Durrieu et al., 2023), Just Above Right (King and Cliff, 2005) or Check-All-That-Apply (Moss et al., 2022; Li and He, 2023) methods have been used.
However, the optimal pairing is subjective and can vary widely among individuals (Reed and Knaapila, 2010) and cultures (Durrieu et al., 2023). There are two main approaches for pairing food and drinks: one is based on intellectual and cognitive factors, which could involve understanding cultural, geographical, or theoretical aspects of why certain food and drink pairings are considered good; and the other is based on sensory perceptions (perceptual similarity, contrast, harmony, emergence, and modulation) (Spence, 2020a). Similarly, the approach in food and beverages pairing by experts was described to depend on three perceptual principles: 1) rinsing for maintaining the qualities of each product, 2) masking for suppressing off-flavor in one product, and 3) synergy for enhancing a positive attribute in a product. Moreover, information as the conceptualization and personal preferences are important drivers for the pairing by experts (Eschevins et al., 2019). In addition, the aromatic similarity between two products can lead to a good match, and impact liking (Eschevins et al., 2018). As regard pairing cheese and wine, the combination could be appreciated on a cognitive or intellectual level only if the taster's attention is directed to the qualities that make the pairing noteworthy (Spence, 2020a). This dynamic combination involves a complex relationship of sensory, chemical, and physiological mechanisms that collectively contribute to the overall flavor experience (Spence, 2020b).
In the chemosensory perception, two pivotal factors may contribute to a positive evaluation of the cheese wine pairing: suppression of astringency of red wine and oral cleansing effect (Madrigal-Galan and Heymann, 2006).
Astringency, often associated with red wines due to the presence of tannins, can impart a dry and puckering sensation in the mouth (Gawel, 1998). One of the main mechanisms explaining the astringency is the interaction and precipitation of phenolic compounds, such as tannins, with salivary proteins (Haslam et al., 1988). Certain cheeses, with their rich and varied textures due to the lipidic content, possess the ability to interact with the tannins in red wine, resulting in a suppression or modulation of the astringency perceived by the palate (Dufourc 2021; Saad et al., 2021).
Conversely, the second critical factor in the synergy between cheese and wine revolves around the role of red wine in cleansing the oral cavity from residual sensations left by the cheese (Madrigal-Galan and Heymann, 2006). As cheeses vary widely in terms of fat and protein content, mouth-coating properties, and lingering aftertastes, the cleansing effect of red wine becomes integral to the overall tasting experience influencing both texture and flavor (Galmarini et al., 2017).
During tasting, the interaction between the phenolic compounds in red wine, the proteins and fats in cheese, and saliva in mouth involves a combination of chemical reactions, sensory integration, and individual preferences. Due to the influence of individual variations in taste perception, such as genetic factors (Prescott et al., 2004), cultural preferences (Bromberger and Percival, 2007; Spence, 2020b), and the microbial composition of the olfactory epithelium (Koskinen et al., 2018) on the pairing between cheese and wine, it seems necessary to individuate an objective approach to explore such interactions. While for astringency many in vitro methods using saliva have been developed in the last decades (Gambuti et al., 2006; Rinaldi et al., 2012, 2014; Fleming et al., 2016), an approach to determine the cleansing effect of wine on cheese tasting has never been considered until now. Current work focused on the involvement of wine phenolic compounds, salivary and cheese proteins on the interactions governing the synergy between different types of cheese and red wines. An in vitro simulation of cheese and red wine consumption was carried out determining the precipitation of salivary proteins by wine tannins in presence or not of cheese, and of cheese proteins after interaction with wine and saliva. The astringency of wine was evaluated by the Saliva Precipitation Index (SPI), which is based on the precipitation of salivary proteins after the interaction with wine tannins, and the sodium dodecyl sulphate polyacrylamide gel (SDS–PAGE) electrophoresis of the proteins that have not been reacted. The decrease of selected proteins has been correlated with sensory analysis, as described in Rinaldi et al. (2012). In this way we are able to individuate the cheese wine optimal pairing considering (i) wine phenolic compounds content, (ii) salivary protein binding and (iii) cheese protein composition.
2. Materials and methods
2.1. Cheeses
2.1.1. Cheese manufacturing and ripening process
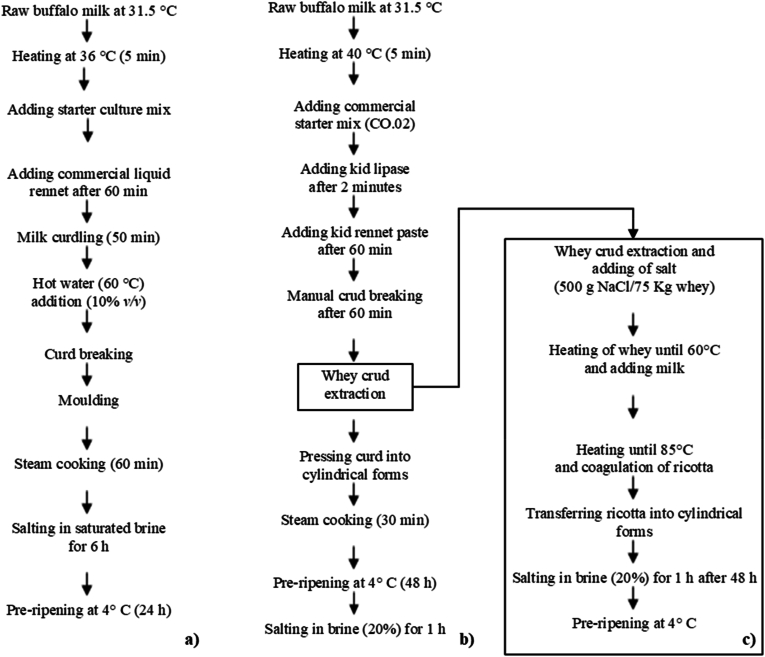
The experiment was carried out by producing in a dairy industry in Southern Italy (Caseificio Bufà, Crotone, KR, Italy). Three typical cheeses at different stages of ripening were selected to provide a different range of cheeses. Buffalo cheese's flow diagrams are shown in Fig. 1. Briefly, raw buffalo milk was heated for 5 min at 40 °C to produce semi-hard (M) and hard (D) cheeses and 36 °C to produce Primo sale (P) cheese. Subsequently, the milk was coagulated using a mixture of commercial starter (Lactococcus lactis spp. cremoris, Lactococcus lactis spp. lactis, Lactococcus lactis spp. lactis biovar. diacetylactis, SACCO SRL, CO, Italy) and a rennet paste (75% chymosin, 25% pepsin; Caglificio Clerici S.p.a., Cadorago, Como, Italy) in 60 min. For the Primo Sale cheese production, the curd was cut into rice grain-sized dimensions, manually pressed into cylindrical, perforated plastic molds, and then steamed at 40 °C for 60 min. Subsequently, the cheeses were submerged in saturated brine for a period of 6 h. In the production of semi-hard and hard cheeses, the resulting curd was sliced into medium-sized grains, the whey was removed, and the curd was pressed into cylindrical shapes before being steamed at 100 °C for 30 min. Subsequently, the cheeses were refrigerated at 4 °C for 48 h, followed by immersion in brine (salt, 20% w/v) for 1 h. For dry ricotta cheese (R) making, the whey collected after the production of buffalo cheeses (hard and semi-hard cheeses) was mixed with 10% of buffalo milk (v/v) preheated to 40 °C. The salt was then added (500 g NaCl/75 kg) and mixture was heated to 85 °C. After coagulation, the cheese was molded into cylindrical forms, soaked in brine (salt, 20% w/v) for 1 h, and then refrigerated at 4 °C for 48 h.
Fig. 1.
Flow diagrams production of Primo Sale (a), hard and semi-hard cheese (b) and dry ricotta cheese (c).
The buffalo cheeses underwent ripening using the Stagionello - European Patented Device and controlled pH—n. EP 2769276B1 at the conditions reported in Table 1. According to Di Paolo et al. (2023), the device utilized for ripening cheeses allow to obtain cheese in fast times thanks to continuous measurements of pH and environmental parameters such as temperature, air flow, and relative humidity (RH). To maintain uniform composition, the cheeses were flipped weekly during the ripening process. After ripening time, cheeses samples were collected and transported to the laboratory using refrigerated boxes for chemical analyses.
Table 1.
Ripening climatic recipes for Primo Sale, hard cheese, semi-hard cheese and dry ricotta cheese.
| Samples |
Ripening Time |
Ripening Steps |
pH |
Air Temperature (°C) |
RH (%) |
Airflow (m/s) |
|---|---|---|---|---|---|---|
| Primo Sale | 6 days | Ripening (72 h) | 5.6 | 10 | 35 | 0 |
| Ripening (3 days) | 5.6 | 10 | 35 | 0 | ||
| Dry ricotta cheese | 7 days | |||||
| Dripping (2 h) | 5.5 | +35 | 60 | 1 | ||
| Stewing (20 h) | 5.5 | +22 | 40 | 1 | ||
| Drying 1 (24 h) | 5.0 | +20 | 45 | 1 | ||
| Drying 2 (24 h) | 5.0 | +20 | 42 | 1 | ||
| Drying 3 (24 h) | 5.0 | +18 | 41 | 1 | ||
| Drying 4 (24 h) | 5.2 | +16 | 40 | 1 | ||
| Drying 5 (24 h) | 5.3 | +14 | 38 | 1 | ||
| Drying 6 (24 h) | 5.4 | +12 | 36 | 1 | ||
| Ripening (24 h) | 5.5 | +8/10 | 35 | 1 | ||
| Semi-hard cheese | 43 days | |||||
| Drying 1 (24 h) | 4.8 | +24 | 75 | 0 | ||
| Drying 2 (24 h) | 5.0 | +23 | 76 | 0 | ||
| Drying 3 (24 h) | 5.0 | +21 | 78 | 0 | ||
| Drying 4 (24 h) | 5.1 | +19 | 80 | 0 | ||
| Drying 5 (24 h) | 5.2 | +17 | 82 | 0 | ||
| Drying 6 (24 h) | 5.3 | +15 | 83 | 0 | ||
| Drying 7 (24 h) | 5.4 | +13 | 84 | 0 | ||
| Drying 8 (24 h) | 5.5 | +12 | 85 | 0 | ||
| Ripening (35 days) | 5.6 | +11 | 75 | 0 | ||
| Hard cheese | 60 days | |||||
| Stewing (1 h) | 5.5 | +26 | 85 | 0 | ||
| Dripping (8 h) | 4.8 | +24 | 80 | 1 | ||
| Drying 1 (24 h) | 5.0 | +22 | 75 | 0 | ||
| Drying 2 (24 h) | 5.0 | +21 | 76 | 0 | ||
| Drying 3 (24 h) | 5.1 | +19 | 78 | 0 | ||
| Drying 4 (24 h) | 5.1 | +17 | 80 | 0 | ||
| Pre- ripening 1 (48 h) | 5.1 | +15 | 82 | 0 | ||
| Pre-ripening 2 (48 h) | 5.2 | +14 | 82 | 0 | ||
| Pre- ripening 3 (48 h) | 5.3 | +13 | 84 | 0 | ||
| Pre- ripening 4 (48 h) | 5.4 | +12 | 85 | 0 | ||
| Ripening (48 days) | 5.5 | +11 | 73 | 1 |
2.1.2. Chemical and instrumental analyses of cheese
The analyses to determinate the chemical composition was performed on grated sampling cheese taken 2 cm from the rind. Moisture (%), salt (% NaCl), and protein (%) contents were evaluated according to the procedures described by AOAC International et al., 2005. The fat content was determinate according to Romano et al. (2011).
2.2. Wines
2.2.1. Vinification process
Two red wines of Southern Italy were used in the study. Wines were produced with Magliocco grapes harvested during 2022 vintage in Calabria region (Italy) by Marrelli Wines Cantina e Vigneti (Crotone, Italy) with a standard industrial process (Wine 2: W2) and an optimized process to obtain a wine richer in phenolic compounds (Wine 1: W1). W2 was obtained by a standard maceration of grapes until the end of fermentation with two punching over per day; W1 was produced with saignée (5%) applied just after the cap raised up and then the maceration of grapes lasted until the end of fermentation. Table 2 showed the basic parameters of wines obtained after centrifugation and 0.45 mm filtration. Microbiological plating showed that wines were not containing yeasts and bacteria after filtration. Wine base parameters were determined by OIV methods of analysis (OIV, 2017).
Table 2.
Base parameters of wines.
| Ethanol (%v/V) | Titratable acidity (g/L) | pH | Glucose + fructose (g/L) | Malic acid (g/L) | Lactic acid (g/L) | Free sulphur dioxide (mg/L) | Total sulphur dioxide (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| Wine 1 | 12.03 ± 0.1 | 4.31 ± 0.02 | 3.96 ± 0.04 | 0.13 ± 0.02 | 0.02 ± 0.01 | 1.7 ± 0.1 | 21.94 ± 1.2 | 36.00 ± 1.22 |
| Wine 2 | 12.11 ± 0.03 | 4.27 ± 0.05 | 3.97 ± 0.03 | 0.10 ± 0.04 | 0.57 ± 0.03 | 1.6 ± 0.1 | 6.53 ± 0.94 | 28.23 ± 1.35 |
2.2.2. Spectrophotometric analyses
Spectrophotometric analysis was conducted using a Jenway 7305 Spectrophotometer. Total anthocyanins, tannins reactive to Bovine Serum Albumine (BSA), sum of polymeric pigments, and iron-reactive phenolic compounds were quantified using the Harbertson–Adams assay (Harbertson et al., 2003).
2.3. In vitro interactions
2.3.1. Saliva collection
Saliva was obtained by mixing resting whole mouth saliva samples from different individuals. Saliva collection was performed between 10 and 11 a.m. Participants were asked not to consume any food and beverage for 2 h before saliva collection. Saliva was spontaneously collected from 6 non-smoking volunteers (3 males and 3 females) by expectorating saliva into a pre-weighted ice-cooled tube for 5 min. Volunteers were healthy adults with no history of oral disorders. After the mixing, saliva underwent centrifugation at 10,000 g for 10 min at 4 °C to remove any insoluble components. The resulting supernatant, utilized for analysis, was consistently maintained on ice to prevent enzymatic reactions. Saliva was analyzed for the total protein content by Bradford method. The total protein concentration of saliva was 1764 ± 25 μg/mL. The protein composition was evaluated by the SDS-PAGE as described in Rinaldi et al. (2012). The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
2.3.2. The wine phenolic content: binding reaction 1
From the literature (Repoux et al., 2012) it is shown that after swallowing a piece of cheese which on average weighs 6 g, the cheese residue in the oral cavity is strongly dependent on the fat content. The residue increases in proportion to the fat content of the cheese. In particular, the average residue was 15.19 ± 5.53% for high-fat cheeses and 4.43 ± 3.74% with low-fat cheeses, respectively. For the binding reaction 1 between wine and cheese we have chosen different quantities of cheese based on their fat composition, as shown in Table 3. Ten mL of the experimental wines (W1 and W2) and the four types of cheese (M = semi-hard cheese; P = Primo sale cheese; D = hard cheese; R = dry ricotta) were mixed with 1 mL of saliva for 15 s at 37 °C. Then, the resulting mix was centrifuged at 10,000 g at 4 °C for 10 min to obtain the following experimental samples: W1M - W1P – W1D - W1R; and W2M - W2P – W2D - W2R. Phenolic analyses (total phenolics, BSA-reactive tannins, total anthocyanins, and sum of polymeric pigments) were carried out on the supernatant wines.
Table 3.
The fat content (%), residual fat content (%) and the amount of cheese (g) used for the binding reaction 1.
| % Fat | % Residual fat | Cheese amount (g) | |
|---|---|---|---|
| Primo sale cheese (P) | 17 | 4 | 0.24 |
| Semi-hard cheese (M) | 50 | 15 | 0.90 |
| Hard cheese (D) | 45 | 15 | 0.90 |
| Dry ricotta (R) | 23 | 10 | 0.60 |
2.3.3. Cleansing effect of wine after cheese tasting simulation: binding reaction 2
For the binding reaction 2, 3 mL of saliva interacted with 1 g of cheese (P, D, M, R) at 37 °C for 2 min. The resulting mix was centrifuged at 10,000 g for 10 min at 4 °C to separate the phases, and then the water-soluble saliva fraction (S + P, S + D, S + M, S + R) was carefully collected between the solid phases. For the interaction, 50 μl of water-soluble saliva fraction (S + P, S + D, S + M, S + R) was mixed with 25 μL of diluted wines (1:1 with HPLC grade water). Binding assay 2 was performed in Eppendorfs maintained at 37 °C for 5 min. The mixture was then centrifuged for 10 min at 10,000 g. The resulting supernatant (S + P + W, S + D + W, S + M + W, S + R + W) represented the saliva and cheese proteins that were not precipitated by wine.
2.3.4. The Saliva Precipitation Index (SPI): binding reaction 3
Astringency of red wines was evaluated considering the reactivity of tannins to salivary proteins and was quantified using the SPI method described by Rinaldi et al. (2012). The binding reaction 3 used the same conditions of the binding reaction 2 in presence or not of cheese. Electrophoresis of salivary proteins in the supernatant was conducted using a Mini-PROTEAN Tetra Vertical Electrophoresis System (Bio-Rad, Milano, Italy), as described afterwards. The SPI was calculated based on the density of specific protein bands in saliva before and after the binding reaction 3. These bands were located at approximately 60 kDa (tentatively assigned to α-amylase) and 15 kDa (possibly a basic PRP). The average percentage reduction in band intensity was quantified in terms of gallic acid equivalent (GAE). Gallic acid was chosen due to its correlation with the decrease in protein bands, which reflects the astringency associated with increasing concentrations of tannin solutions measured via the Folin-Ciocalteau assay. Consequently, the SPI, representing the percentage reduction in protein bands, was expressed in gallic acid equivalent as previously described (Rinaldi et al., 2012), and represented an in vitro measure of the astringency of wines.
2.4. Sodium dodecyl sulphate polyacrylamide gel (SDS–PAGE) electrophoresis
The SDS-PAGE analyses were performed on saliva (S), total cheese proteins, saliva interacting with cheese (S + P, S + D, S + M, S + R) and after the interaction with wine W1 (S + P + W1, S + D + W1, S + M + W1, S + R + W1) and W2 (S + P + W2, S + D + W2, S + M + W2, S + R + W2). The total cheese proteins were obtained by mixing 3 mL of HPLC grade water and 1 g of cheese, centrifugation at 10,000 g for 10 min at 4 °C and recovery of the water-soluble phase. The SDS–PAGE electrophoresis of samples was performed on a Mini-PROTEAN Tetra Vertical Electrophoresis System (Bio-Rad, Milano, Italy) using a PowerPac 1000 Bio-Rad power supply set at 150 V/gel for the stacking gel and 180 V/gel for the resolving gel. Samples were mixed with a volume of 2 × electrophoresis sample buffer (0.125 M Tris–HCl, 4% SDS; 20% v/V glycerol, 0.2 M DTT, 0.02% bromophenol blue, pH 6.8) in a 2:1 ration, and then heated at 95 °C for 4 min were analyzed by SDS–PAGE using 30% acrylamide/bisacrylamide (37.5:1) solution. The resolving gels were 12% acrylamide, and stacking gels were 4% acrylamide. The gels were fixed with a mixture of ethanol, acetic acid, and deionized water (40:10:50) for 1 h. After washing in water for 5 min, the gels were stained with Coomassie Brilliant Blue R250 staining solution (Bio-Rad). The destain step was performed by incubation in the destain solution Coomassie Blue R250 (Bio-Rad). Densitometric tracing of gels was performed with a Bio-Rad GS800 densitometer, and electrophoretic data were analyzed by Quantity One analysis software, Version 4.5 (Bio-Rad).
2.5. Optimal pairing (OP)
In equation (1) (Eq. (1)), let:
OP be the optimal pairing score between cheese and wine.
Δcleansing effect be the percentage decrease of total proteins (cheese and saliva) by wine.
ΔSPI be the decrease of astringency by cheese, measured by SPI.
Residual fat be the content in fat (g) that would remain to coating mouth after eating a piece of cheese of 6 g (Repoux et al., 2012).
The OP is a function of these factors:
| OP = [1− (1−Δcleansing effect) × (1−ΔSPI)] × residual fat (g) | (1) |
Eq. (1) assumes that a higher percentage decrease in cheese protein by wine and a higher decrease in astringency (SPI) by cheese in presence of saliva contribute positively to the pairing score. The values of Δcleansing effect and ΔSPI are between 0 and 1, where 0 means no decrease, and 1 means a complete decrease. Similarly, the high value of OP means high paring between cheese and wine.
2.6. Statistical analysis
Analysis of variance (ANOVA) was carried out on three replicates, and the Tukey's test was applied to discriminate amongst the means of data at the 95% confidence level (p < 0.05). ANCOVA analysis considering the phenolic variables (total phenolics, BSA-reactive tannins, total anthocyanins, and sum of polymeric pigments), and the typology of wine (Wine) was performed on OP. All analyses were performed using the XLSTAT software (by Lumivero, 2023).
3. Results and discussion
In this study, in order to simulate the processes happening in mouth during the tasting of cheese and wine, three in vitro interactions were carried out to study the influence of cheese on i) wine phenolic compounds, and ii) SPI, and iii) the influence of wine on cheese proteins residual in the mouth after tasting. Given the complexity of two matrices, the characterization of cheese and wines under study was firstly reported.
3.1. Cheese composition
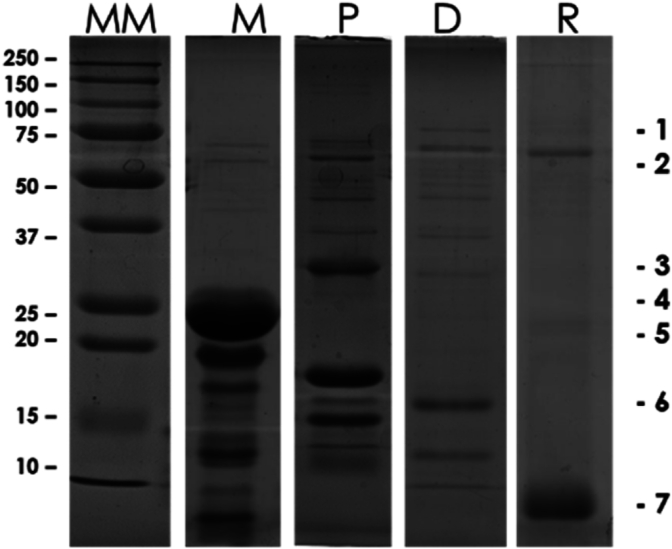
The physicochemical, organoleptic, and nutritional attributes of dairy products are primarily influenced by milk proteins. Manufacturing processes induce changes in protein structure through unfolding, denaturation, aggregation, and glycation potentially altering the nutritional properties, reactivity, and functionality of milk proteins in specific dairy products, such as cheese (Borad et al., 2017; Guinee, 2016; Verhoeckx et al., 2015). The selected cheeses (M = semi hard cheese; P = Primo sale cheese; D = hard cheese; R = dry ricotta) were produced with different methods as shown in Material and Methods section 2.1.1, and had different compositions as shown in Table 4. The M had the highest content in fat (50%) but a similar protein content as D (25%). The P showed the highest content in salt (4%), while the R showed the highest moisture content (50%), indicating a hard type of ricotta cheese. The different proteic pattern, representing the water-soluble fraction of cheese, is shown in Fig. 2.
Table 4.
Composition of cheeses.
| Parameter | Semi-hard cheese (M) | Primo sale cheese (P) | Hard cheese (D) | Dry ricotta (R) |
|---|---|---|---|---|
| Fat (%) | 50.3 ± 1.0a | 17.3 ± 1.6d | 44.9 ± 2.4b | 23.0 ± 1.2c |
| Protein (%) | 25.8 ± 0.6a | 13.1 ± 0.4b | 25.5 ± 2.5a | 13.9 ± 0.3b |
| Moisture (%) | 18.6 ± 0.3c | 42.4 ± 0.7b | 17.6 ± 0.8c | 50.6 ± 1.6a |
| Salt (%) | 1.0 ± 0.2d | 4.3 ± 0.3a | 1.7 ± 0.2c | 2.9 ± 0.3b |
The composition is expressed as percentage (w/w). Data represent the mean ± standard deviation of three independent experiments; values within a row with different superscript letters are significantly different.
Fig. 2.
SDS-PAGE of the total cheese proteins (M = semi hard cheese; P = Primo sale cheese; D = hard cheese; R = dry ricotta). MM: molecular marker (kDa).
The diversity in these bands and density can depend on milk or whey composition (milk containing a mix of caseins and whey proteins), production method, and ripening period. From a comparison with bibliography (Gaiaschi et al., 2000; Salvatore et al., 2014), the bands 1–7 may correspond to bovine serum albumin (1); immunoglobulin heavy chain (2); αS1-casein (3); αS2-casein (4); β-casein (5); β-lactoglobulin (6); α-lactalbumin (7), respectively. The casein family proteins are mainly present in M, P, and less in D, which are obtained from buffalo milk while in R, prepared from whey, the main protein is represented by α-lactalbumin. In particular, the β-casein, among the others, is remarkably abundant in M.
3.2. Phenolic composition of wines
Although the wines exhibited similar fundamental characteristics (Table 3), W1 demonstrated a greater abundance of phenolic compounds attributable to the specific winemaking protocol applied (Fig. 3). The beneficial impact of saignèe, a technique involving the concentration of fermentation solids (skins and seeds) by drawing fresh juice before alcoholic fermentation commences, on phenolic compounds in red wines has been previously documented (Casassa et al., 2016). In our samples, this effect notably influenced the extraction of anthocyanins during winemaking and the formation of polymeric pigments. This phenomenon may be linked not only to enhanced anthocyanin extraction but also to increased levels of phenolics, which act as reactants in condensation reactions, resulting in the formation of polymers that stabilize the color of wine (He et al., 2012; Forino et al., 2020).
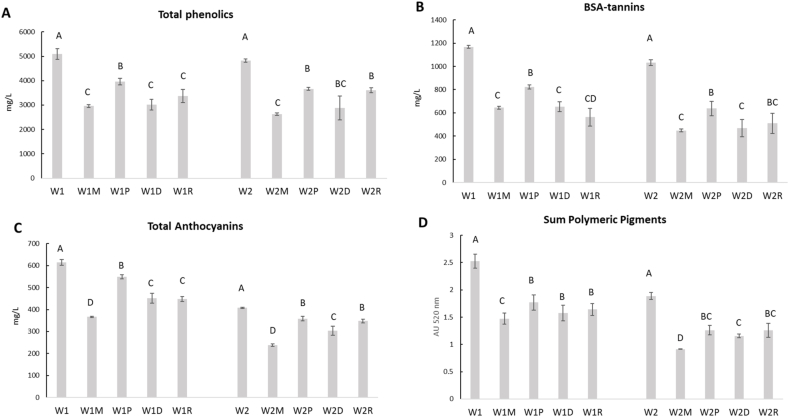
Fig. 3.
Concentration of total phenolics (A), BSA-reactive tannins (B), total anthocyanins (C), and sum of polymeric pigments (D) in wines before (W1 and W2), and after the binding reaction 1 with saliva and cheese (M = semi-hard cheese; P = Primo sale cheese; D = hard cheese; R = dry ricotta). Error bars represent standard deviation over three replications.
Furthermore, the winemaking treatments exerted a discernible impact on the tannin concentration of the final wines, consistent with Casassa et al. (2016), who noted a moderate but significant rise in tannin levels associated with saignèe treatment during Cabernet Sauvignon winemaking. Even total phenolics are significantly higher in W1 than W2 (Fig. 3). Total phenolics, more accurately termed as iron-reactive phenolics, encompass all phenolic compounds containing vicinal dihydroxyl groups, including tannins, flavan-3-ols, and flavonols. However, monohydroxylated phenols and anthocyanins are excluded from this measurement because the reagent utilized to quantify total phenolics, ferric chloride, cannot form colored ligands, thereby precluding the quantification of monohydroxylated phenols and anthocyanins (Harbertson and Spayd, 2006).
From an enological perspective, a comparison between W1 and W2 unequivocally demonstrates the utility of saignèe in producing wines suitable for extended aging, primarily due to the heightened transfer of phenolic compounds from grape skins and seeds.
3.3. Effect of cheese on wine phenolic compounds and SPI after the in vitro interaction
One of the basics for the cheese wine pairing is the ability of cheese to decrease the astringency sensation of wine elicited by phenolic compounds such as tannins. The more tannic are the wines the less the interaction with cheese. From the binding reaction 1 the experimental wines (W1 and W2) and the four types of cheese (M = semi-hard cheese; P = Primo sale cheese; D = hard cheese; R = dry ricotta) were mixed with saliva, and after precipitation the following experimental tests were obtained: W1M - W1P – W1D - W1R; and W2M - W2P – W2D - W2R. The levels of total phenolics (A), BSA-reactive tannins (B), total anthocyanins (C), and sum of polymeric pigments (D) measured in the supernatant were shown in Fig. 3.
The wines W1 and W2 were produced with different vinification protocols to obtain wines with different phenolic content, which is mainly related to the total anthocyanins (600 vs 400 mg/L). After the binding reaction 1 with cheeses in presence of saliva, similarly to what happens in mouth, the best cheeses able to reduce in a significant manner the W1 total phenolics were M and D (42% and 41%), while for W2 it was only the M (45%). As regard the BSA-reactive tannins, the R showed a decrease of 52% for W1, although there were no differences with D and M, which on turn showed a decrease of 57% and 55% for W2, respectively. The cheeses similarly interacted with the phenolic compounds responsible for the color of wine, such as the total anthocyanins and the sum of polymeric pigments, and in both cases the higher decrease was due to the M cheese accounting for 40% and 42% for W1, and 42% and 52% for W2, respectively. No differences in the interaction between the anthocyanins and tannins with the cheeses were denoted. As cheese is composed of milk proteins (primarily caseins) and fat, the affinity with phenolic compounds is mainly due to hydrophobic, ionic, and covalent interactions, and hydrogen bonding (Chanphai et al., 2018). Molecular studies pointed out that strong hydrophobic bindings were the driven forces for the molecular interactions between milk proteins and phenolic compounds (Han et al., 2019). It is not surprising that the M cheese showed the highest binding capacity because of its high content in caseins than the other cheeses (Fig. 2). Phenolic compounds can bind milk proteins with a different order of stability, being β-casein>α-casein>β-lactoglobulin and alter the protein secondary structure with an increase in β-sheet and α-helix for β-lactoglobulin and reduces α-helix and β-sheet structures in α- and β-caseins, leading to a partial protein structural destabilization (Chanphai et al., 2018). On the other hand, the interaction of the anthocyanins with β-casein resulted in changes in the conformation and secondary structure, denoting a high stability of the formed complex (Wei et al., 2018). In addition, phenolic compounds also showed a high capability to bind lipid molecules of cheese by hydrophobic and/or cation–π associations. However, the extent of association may vary widely across different cheese varieties depending on the phospholipid content (Rashidinejad et al., 2017).
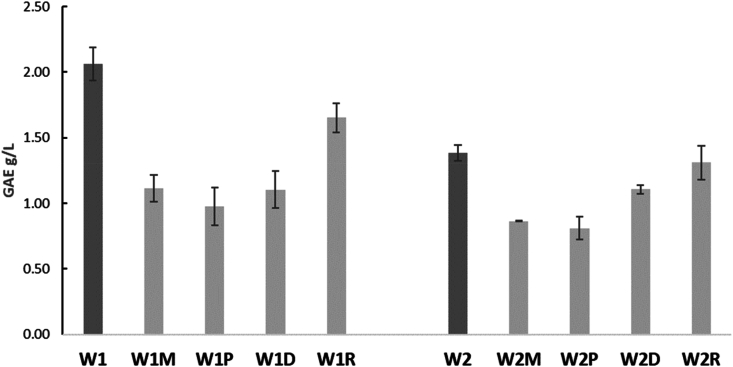
Regarding the quantity of residual tannins, for the W1 the order of pairing with cheeses is R = D = M > P, while for the W2 it is M = D ≥ P = M. This consideration derives from the fact that the greater the quantity of tannins in the supernatant, the lower the ability of the wine to interact with the cheeses. The result is that the wine can be perceived as more astringent. However, the astringency of wine can be objectively measured by the Saliva Precipitation Index (SPI), a method based on the SDS-PAGE of salivary proteins before and after the interaction with wine, being the protein decrease correlated with the astringency sensation (Gambuti et al., 2006; Rinaldi et al., 2012). The decrease in astringency by cheese was measured by the SPI of the wine before and after the binding reaction 3 with cheeses, as shown in Fig. 4.
Fig. 4.
The Saliva Precipitation Index (SPI) of wines before (W1 and W2) and after the binding reaction 3 (W1M, W1P, W1D, W1R; W2M, W2P, W2D, W2R) with cheeses (M = semi-hard cheese; P = Primo sale cheese; D = Hard cheese; R = dry ricotta) expressed in g/L of gallic acid equivalent (GAE). Error bars represent standard deviation over three replications.
A high SPI decrease, which means a high reduction of astringency, was obtained by the P and M cheese in both wines. The dry ricotta (R) didn't show much influence on SPI. The glycoprotein casein in milk is relatively rich in proline residues when compared to the whey proteins (Lemieux and Simard, 1994), explaining the difference in the interaction with wine polyphenols between M, P and D from R. For the W1 no differences between P, M, and D were denoted, with a decrease of about 50%. For the W2, the decrease in the potential astringency was around 40% for both M and P. However, M and P cheeses showed a different composition: semi-hard cheese is rich in protein and fat, while Primo sale cheese showed a high salt content, respectively (Table 4). Cheese proteins can bind to polyphenols and cause a reduction in the perceived astringency similarly to what happen during Parmigiano cheese and beer pairing (Donadini et al., 2013). Besides, the interaction of polyphenols with lipids can influence the astringency (Reis et al., 2020). Tannis strongly interact with lipids and can precipitate them, indicating that eating during drinking may diminish the availability of tannins for saliva proteins and hence modulate the astringency feeling (Furlan et al., 2015). However, as salts, and then ionic strength, can affect protein-tannin interactions (Brandão et al., 2020), the high content of salts in P may also cause a high precipitation of salivary proteins by this cheese. These results are quite in accordance with that from phenolic compounds analysis, but it needs to also consider the effect of the wine on the removal of cheese protein in mouth, that is the cleansing effect (Madrigal-Galan and Heymann, 2006).
3.4. Effect of wine on protein decrease after the in vitro interaction: cleansing effect
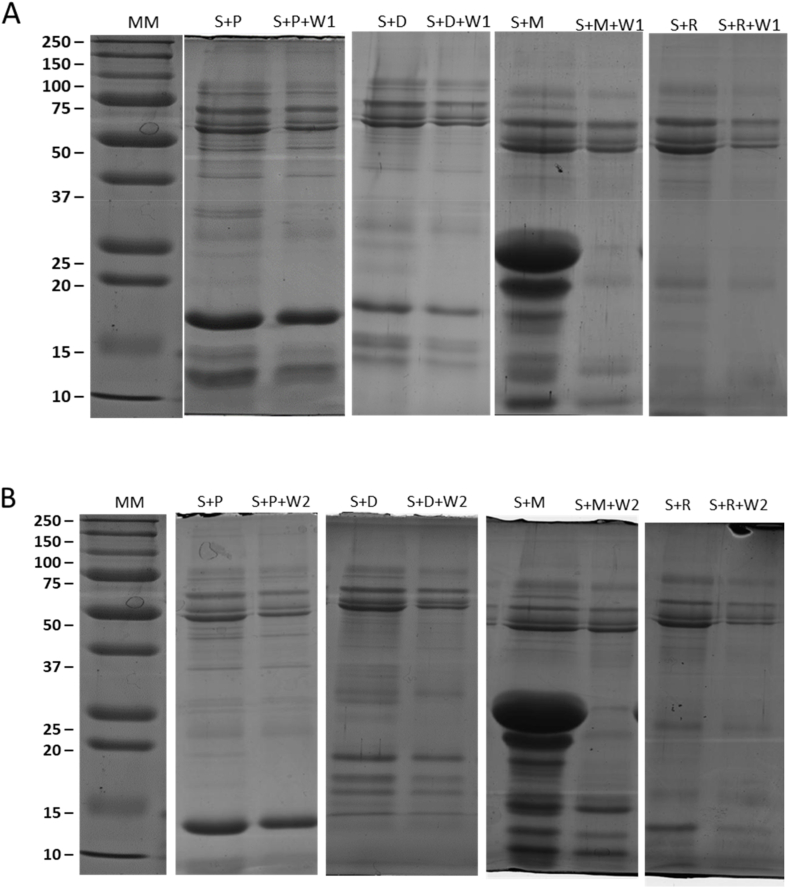
Fig. 5 showed the SDS-PAGE electrophoretic pattern of salivary proteins (S) interacting with cheeses before (S + P, S + D, S + M, S + R) and after (S + P + W1, S + D + W1, S + M + W1, S + R + W1) the in vitro interaction with wine W1 (Fig. 5A) and W2 (Fig. 5B). Band density was carried out by densitometric analysis. The % protein decrease resulted from the binding reaction 2 between salivary and cheese proteins was measured before and after wine interaction. The higher the decrease in the density of protein bands by wine in presence of saliva, the higher is the cleansing effect of the wine on cheese. However, as cheese is generally abundant in proteins and fats, it is possible that the latter will compete with salivary proteins in binding polyphenols (Haslam et al., 1988). According to the % decrease of proteins, the cleansing effect by the W1 is in the order: M (71%) > R (30%) ≥ D (26%) > P (17%), while for the W2 the order is R (73%) > M (51%) > D (16%) ≥ P (10%).
Fig. 5.
The SDS-PAGE electrophoretic pattern of salivary proteins (S) interacting with cheeses (P, D, M, R), before (S + P, S + D, S + M, S + R) and after (S + P + W1, S + D + W1, S + M + W1, S + R + W1) the binding reaction 2 with wine W1 (Fig. 5A) and W2 (Fig. 5B). MM: molecular marker (kDa).
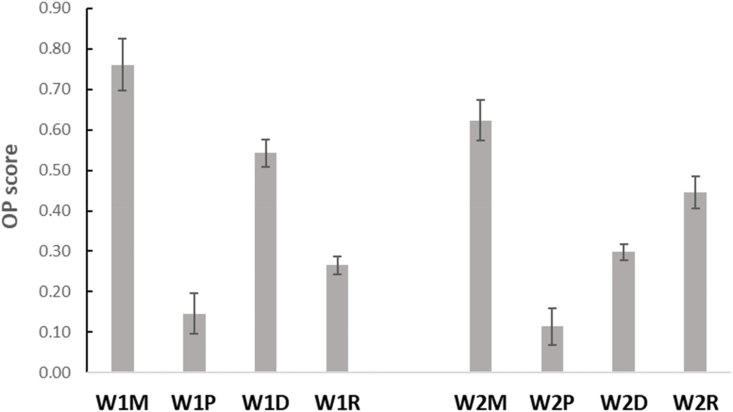
Considering both the effect of cheese on reducing the astringency (% SPI decrease) in the binding reaction 3 and the effect of wine on reducing the cheese protein in mouth (cleansing effect) in the binding reaction 2, the optimal paring (OP) can be calculated from these factors according to equation (1) (Eq. (1)), taking into consideration also the residual fat that would remain in mouth after eating a piece of cheese. Then, the cheese wine OP was calculated (Fig. 6). The highest value of OP means highest paring between cheese and wine, being the value comprised between 0 and 1, where 0 means no pairing, and 1 means the complete pairing.
Fig. 6.
Cheese wine optimal pairing (OP) between wines (W1 and W2) and cheeses (M = semi-hard cheese; P = Primo sale cheese; D = hard cheese; R = dry ricotta). Error bars represent standard deviation over three replications.
According to the in vitro tasting, for both wines the cheese wine OP is the semi-hard cheese (M), with a score of 0.76 for W1 and 0.62 for W2, respectively. However, the order of pairing is different according to the wine: W1 = M > D > R > P, and W2 = M > R > D > P. The M cheese also contributed to better remove the BSA-reactive tannins from the wines, showing a similar trend to the calculated values. For this reason, an ANCOVA analysis was performed to understand if the phenolic variables (total phenolics, BSA-reactive tannins, total anthocyanins, and sum of polymeric pigments), and the typology of wine (Wine) has an influence on the OP and should be included in the equation. Table 5 showed the occurrence of the sources in explaining the variable optimal pairing.
Table 5.
ANCOVA analysis on optimal pairing.
| Source | DF | Sum of squares | Mean squares | F | Pr > F | p-values |
|---|---|---|---|---|---|---|
| Total phenolics | 1.000 | 0.049 | 0.049 | 1.136 | 0.398 | >0.1 |
| BSA-reactive tannins | 1.000 | 0.002 | 0.002 | 0.048 | 0.846 | >0.1 |
| Total anthocyanins | 1.000 | 0.006 | 0.006 | 0.147 | 0.738 | >0.1 |
| Sun of polymeric pigments | 1.000 | 0.021 | 0.021 | 0.491 | 0.556 | >0.1 |
| Wine | 1.000 | 0.064 | 0.064 | 1.493 | 0.346 | >0.1 |
Among the explanatory variables, based on the Type III sum of squares, variable Wine is the most influential in explaining the variability of the OP. However, the level of significance is higher than p = 0.05. Probably the differences in the phenolic content between the two wines are not enough to invoke a marked influence on the pairing with cheese. Products such as wine of diverse varietal origin and beer, indeed, appeared to behave differently in cheese pairings (Donadini et al., 2013), so our future works will apply the OP on a wide variety of cheeses and wines or beverages of different typologies and comparing it with the sensory experience. In addition, we will consider the effect that food and beverage odors and their combination may have on salivary protein composition, as observed after bread olfactory stimulation (Carreira et al., 2020).
4. Conclusions
This work focused on the ability of different cheeses and two wines to interact with saliva to objectively evaluate the cheese wine pairing. The in vitro interactions were based on the precipitation of salivary proteins by wine in presence of cheese (decrease of SPI), and on the total saliva and cheese protein by wine (cleansing effect) as it happens in mouth during tasting. These key factors are at the basis for the calculation of the optimal pairing, which also consider the cheese fat that would remain in mouth after eating a piece of cheese. The OP values indicated that the semi-hard cheese represented the optimal pairing with the two experimental red wines. The phenolic content of wines after the interaction with wine and saliva also suggested that the binding with the semi-hard cheese was the ideal combination. However, the differences in the phenolic content between the two wines were not enough to show a significant influence on the OP.
This in vitro simulation of tasting involved evaluating how cheese and wine interact when paired together. However, the analysis of the proteins and phenolic compounds after the binding reactions represented only one side of the chemical interactions in food and wine pairing. The study of the aroma and texture of both wine and cheese should also be considered from a sensory point of view. This will pave the way for more in-depth research, which should also consider genetic differences in populations with increased or decreased sensitivity to certain attributes, the familiarity with cheese and wine matching, and the correlation between olfactory function and the microbial composition of the olfactory epithelium. This can contribute to understanding of wine tasting by engaging multiple senses, exploring flavor interactions and texture dynamics.
Funding
This work was financially supported by the co-financing of PON I&C 2014-2020 - CAPSULE [grant number F/200016/01-03/X45].
Ethical statement
No human ethics committee or formal documentation process is available but, for collecting saliva, appropriate protocols for protecting the rights and privacy of all participants were utilized during the execution of the research. The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
CRediT authorship contribution statement
Alessandra Rinaldi: Conceptualization, Experimental work, Data curation, Writing – original draft. Giovanna Bifulco: Experimental work, Data curation, Investigation. Alessandra Luciano: Investigation, Experimental work, Data curation. Luigi Picariello: Experimental work, Data curation. Luigi Moio: Conceptualization, Supervision. Raffaele Marrone: Investigation, Experimental work, Data curation. Giuseppe Campanile: Conceptualization, Funding acquisition, Supervision. Angelita Gambuti: Conceptualization, Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- AOAC International . In: Official Methods of Analysis. 18th ed. Horwitz W., Latimer G., editors. AOAC; Gaithersburg, MD, USA: 2005. ISBN 0935584773. [Google Scholar]
- Bastian S.E.P., Payne C.M., Perrenoud B., Joscelyne V.L., Johnson T.E. Comparisons between Australian consumers' and industry experts' perceptions of ideal wine and cheese combinations. Aust. J. Grape Wine Res. 2009;15(2):175–184. doi: 10.1111/j.1755-0238.2008.00043.x. [DOI] [Google Scholar]
- Bastian S.E., Collins C., Johnson T.E. Understanding consumer preferences for Shiraz wine and Cheddar cheese pairings. Food Qual. Prefer. 2010;21(7):668–678. doi: 10.1016/j.foodqual.2010.02.002. [DOI] [Google Scholar]
- Borad S.G., Kumar A., Singh A.K. Effect of processing on nutritive values of milk protein. Crit. Rev. Food Sci. Nutr. 2017;57(17):3690–3702. doi: 10.1080/10408398.2016.1160361. [DOI] [PubMed] [Google Scholar]
- Brandão E., Silva M.S., Garcia-Estevez I., Williams P., Mateus N., Doco T., de Freitas V., Soares S. Inhibition mechanisms of wine polysaccharides on salivary protein precipitation. J. Agric. Food Chem. 2020;68(10):2955–2963. doi: 10.1021/acs.jafc.9b06184. [DOI] [PubMed] [Google Scholar]
- Bromberger B., Percival F. Culture shock: principles for successful wine-and-cheese pairing. World of Fine Wine. 2007;16:139–144. [Google Scholar]
- Carreira L., Midori Castelo P., Simões C., Capela e Silva F., Viegas C., Lamy E. Changes in salivary proteome in response to bread odour. Nutrients. 2020;12(4):1002. doi: 10.3390/nu12041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casassa L.F., Larsen R.C., Harbertson J.F. Effects of vineyard and winemaking practices impacting berry size on evolution of phenolics during winemaking. Am. J. Enol. Vitic. 2016;67(3):257–268. doi: 10.5344/ajev.2016.15105. [DOI] [Google Scholar]
- Chanphai P., Bourassa P., Kanakis C.D., Tarantilis P.A., Polissiou M.G., Tajmir-Riahi H.A. Review on the loading efficacy of dietary tea polyphenols with milk proteins. Food Hydrocolloids. 2018;77:322–328. doi: 10.1016/j.foodhyd.2017.10.008. [DOI] [Google Scholar]
- Di Paolo M., Vuoso V., Ambrosio R.L., Balestrieri A., Bifulco G., Anastasio A., Marrone R. Role of feeding and novel ripening system to enhance the quality and production sustainability of curd buffalo cheeses. Foods. 2023;12(4):704. doi: 10.3390/foods12040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadini G., Fumi M.D., Lambri M. A preliminary study investigating consumer preference for cheese and beer pairings. Food Qual. Prefer. 2013;30(2):217–228. doi: 10.1016/j.foodqual.2013.05.012. [DOI] [Google Scholar]
- Dufourc E.J. Wine tannins, saliva proteins and membrane lipids. Biochim. Biophys. Acta Biomembr. 2021;1863(10) doi: 10.1016/j.bbamem.2021.183670. [DOI] [PubMed] [Google Scholar]
- Durrieu F., Lick E., Lorey T., Stöckl A.F. The impact of country and wine culture on ideal pairings of French white wine and cheese. Int. J. Gastron. Food Sci. 2023;32 doi: 10.1016/j.ijgfs.2023.100735. [DOI] [Google Scholar]
- Eschevins A., Giboreau A., Allard T., Dacremont C. The role of aromatic similarity in food and beverage pairing. Food Qual. Prefer. 2018;65:18–27. doi: 10.1016/j.foodqual.2017.12.005. [DOI] [Google Scholar]
- Eschevins A., Giboreau A., Julien P., Dacremont C. From expert knowledge and sensory science to a general model of food and beverage pairing with wine and beer. Int. J. Gastron. Food Sci. 2019;17 doi: 10.1016/j.ijgfs.2019.100144. [DOI] [Google Scholar]
- Fleming E.E., Ziegler G.R., Hayes J.E. Salivary protein levels as a predictor of perceived astringency in model systems and solid foods. Physiol. Behav. 2016;163:56–63. doi: 10.1016/j.physbeh.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. 2011. Cheese & Wine: A Guide to Selecting, Pairing, and Enjoying. Chronicle Books. [Google Scholar]
- Forino M., Picariello L., Lopatriello A., Moio L., Gambuti A. New insights into the chemical bases of wine color evolution and stability: the key role of acetaldehyde. Eur. Food Res. Technol. 2020;246(4):733–743. doi: 10.1007/s00217-020-03442-x. [DOI] [Google Scholar]
- Furlan A.L., Castets A., Nallet F., Pianet I., Grelard A., Dufourc E.J., Géan J. Red wine tannins fluidify and precipitate lipid liposomes and bicelles. A role for lipids in wine tasting? Langmuir. 2015;30(19):5518–5526. doi: 10.1021/la5005006. [DOI] [PubMed] [Google Scholar]
- Gaiaschi A., Beretta B., Poiesi C., Conti A., Giuffrida M.G., Galli C.L., Restani P. Proteolysis of αs-casein as a marker of Grana Padano cheese ripening. J. Dairy Sci. 2000;83(12):2733–2739. doi: 10.3168/jds.S0022-0302(00)75167-8. [DOI] [PubMed] [Google Scholar]
- Galmarini M.V., Dufau L., Loiseau A.L., Visalli M., Schlich P. Wine and cheese: two products or one association? A new method for assessing wine-cheese pairing. Beverages. 2018;4(1):13. doi: 10.3390/beverages4010013. [DOI] [Google Scholar]
- Galmarini M.V., Loiseau A.L., Debreyer D., Visalli M., Schlich P. Use of multi‐intake temporal dominance of sensations (TDS) to evaluate the influence of wine on cheese perception. J. Food Sci. 2017;82(11):2669–2678. doi: 10.1111/1750-3841.13932. [DOI] [PubMed] [Google Scholar]
- Gambuti A., Rinaldi A., Pessina R., Moio L. Evaluation of aglianico grape skin and seed polyphenol astringency by SDS–PAGE electrophoresis of salivary proteins after the binding reaction. Food Chem. 2006;97(4):614–620. doi: 10.1016/j.foodchem.2005.05.038. [DOI] [Google Scholar]
- Gawel R. Red wine astringency: a review. Aust. J. Grape Wine Res. 1998;4(2):74–95. doi: 10.1111/j.1755-0238.1998.tb00137.x. [DOI] [Google Scholar]
- Guinee T.P. Protein in cheese and cheese products: structure-function relationships. Adv. Dairy Chem. Vol. 1B: Proteins Appl. Aspects. 2016:347–415. doi: 10.1007/978-1-4939-2800-2_14. [DOI] [Google Scholar]
- Han J., Chang Y., Britten M., St-Gelais D., Champagne C.P., Fustier P., Lacroix M. Interactions of phenolic compounds with milk proteins. Eur. Food Res. Technol. 2019;245:1881–1888. doi: 10.1007/s00217-019-03293-1. [DOI] [Google Scholar]
- Harbertson J.F., Picciotto E.A., Adams D.O. Measurement of polymeric pigments in grape berry extract sand wines using a protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003;54(4):301–306. doi: 10.5344/ajev.2003.54.4.301. [DOI] [Google Scholar]
- Harbertson J.F., Spayd S. Measuring phenolics in the winery. Am. J. Enol. Vitic. 2006;57(3):280–288. doi: 10.5344/ajev.2006.57.3.280. [DOI] [Google Scholar]
- Haslam E., Lilley T.H., Butler L.G. Natural astringency in foodstuffs—a molecular interpretation. Crit. Rev. Food Sci. Nutr. 1988;27(1):1–40. doi: 10.1080/10408398809527476. [DOI] [PubMed] [Google Scholar]
- He F., Liang N.N., Mu L., Pan Q.H., Wang J., Reeves M.J., Duan C.Q. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules. 2012;17(2):1483–1519. doi: 10.3390/molecules17021483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M., Cliff M. Evaluation of ideal wine and cheese pairs using a deviation‐from‐ideal scale with food and wine experts. J. Food Qual. 2005;28(3):245–256. doi: 10.1111/j.1745-4557.2005.00033.x. [DOI] [Google Scholar]
- Koskinen K., Reichert J.L., Hoier S., Schachenreiter J., Duller S., Moissl-Eichinger C., Schöpf V. The nasal microbiome mirrors and potentially shapes olfactory function. Sci. Rep. 2018;8:1296. doi: 10.1038/s41598-018-19438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L., Simard R.E. Astringency, a textural defect in dairy products. Lait. 1994;74(3):217–240. doi: 10.1051/lait:1994319. [DOI] [Google Scholar]
- Li R., He W. Chinese red wine and cheese pairings: A preliminary study of consumer perception using check‐all‐that‐apply and hedonic tests. J. Sensory Stud. 2023 doi: 10.1111/joss.12867. [DOI] [Google Scholar]
- Madrigal-Galan B., Heymann H. Sensory effects of consuming cheese prior to evaluating red wine flavor. Am. J. Enol. Vitic. 2006;57(1):12–22. doi: 10.5344/ajev.2006.57.1.12. [DOI] [Google Scholar]
- Moss R., Barker S., McSweeney M.B. Using check‐all‐that‐apply to evaluate wine and food pairings: an investigation with white wines. J. Sensory Stud. 2022;37(1) doi: 10.1111/joss.12720. [DOI] [Google Scholar]
- OIV . International Organisation of Vine and Wine; Paris, France: 2017. Compendium of International Methods of Wine and Must Analysis.https://www.oiv.int/public/medias/7907/oiv-vol1-compendium-of-international-methods-of-analysis.pdf [Google Scholar]
- Prescott J., Soo J., Campbell H., Roberts C. Responses of PROP taster groups to variations in sensory qualities within foods and beverages. Physiol. Behav. 2004;82(2–3):459–469. doi: 10.1016/j.physbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Rashidinejad A., Birch E.J., Hindmarsh J., Everett D.W. Molecular interactions between green tea catechins and cheese fat studied by solid-state nuclear magnetic resonance spectroscopy. Food Chem. 2017;215:228–234. doi: 10.1016/j.foodchem.2016.07.179. [DOI] [PubMed] [Google Scholar]
- Reed D.R., Knaapila A. Genetics of taste and smell: poisons and pleasures. Progr. Mol. Biol. Transl. Sci. 2010;94:213–240. doi: 10.1016/B978-0-12-375003-7.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A., Soares S., Sousa C.F., Dias R., Gameiro P., Soares S., de Freitas V. Interaction of polyphenols with model membranes: putative implications to mouthfeel perception. Biochim. Biophys. Acta Biomembr. 2020;1862(2) doi: 10.1016/j.bbamem.2019.183133. [DOI] [PubMed] [Google Scholar]
- Repoux M., Septier C., Palicki O., Guichard E., Feron G., Labouré H. Solid cheese consumption: quantification of oral coating. Arch. Oral Biol. 2012;57(1):81–86. doi: 10.1016/j.archoralbio.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Rinaldi A., Gambuti A., Moio L. Application of the SPI (Saliva Precipitation Index) to the evaluation of red wine astringency. Food Chem. 2012;135(4):2498–2504. doi: 10.1016/j.foodchem.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Rinaldi A., Iturmendi N., Gambuti A., Jourdes M., Teissedre P.L., Moio L. Chip electrophoresis as a novel approach to measure the polyphenols reactivity toward human saliva. Electrophoresis. 2014;35(11):1735–1741. doi: 10.1002/elps.201300622. [DOI] [PubMed] [Google Scholar]
- Romano R., Giordano A., Chianese L., Addeo F., Musso S.S. Triacylglycerols, fatty acids and conjugated linoleic acids in Italian Mozzarella di Bufala Campana cheese. J. Food Compos. Anal. 2011;24(2):244–249. doi: 10.1016/j.jfca.2010.10.004. [DOI] [Google Scholar]
- Saad A., Bousquet J., Fernandez-Castro N., Loquet A., Géan J. New insights into wine taste: impact of dietary lipids on sensory perceptions of grape tannins. J. Agric. Food Chem. 2021;69(10):3165–3174. doi: 10.1021/acs.jafc.0c06589. [DOI] [PubMed] [Google Scholar]
- Salvatore E., Pes M., Falchi G., Pagnozzi D., Furesi S., Fiori M., et al. Effect of whey concentration on protein recovery in fresh ovine ricotta cheese. J. Dairy Sci. 2014;97(8):4686–4694. doi: 10.3168/jds.2013-7762. [DOI] [PubMed] [Google Scholar]
- Spence C. Food and beverage flavour pairing: a critical review of the literature. Food Res. Int. 2020;133 doi: 10.1016/j.foodres.2020.109124. [DOI] [PubMed] [Google Scholar]
- Spence C. Multisensory flavour perception: blending, mixing, fusion, and pairing within and between the senses. Foods. 2020;9(4):407. doi: 10.3390/foods9040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeckx K.C., Vissers Y.M., Baumert J.L., Faludi R., Feys M., Flanagan S., Herouet-Guicheney C., Holzhauser T., Shimojo R., van der Bolt N., Wichers H., Kimber I. Food processing and allergenicity. Food Chem. Toxicol. 2015;80:223–240. doi: 10.1016/j.fct.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Wei J., Xu D., Yang J., Zhang X., Mu T., Wang Q. Analysis of the interaction mechanism of anthocyanins (Aronia melanocarpa elliot) with β-casein. Food Hydrocolloids. 2018;84:276–281. doi: 10.1016/j.foodhyd.2018.06.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.