Summary
Background
Sézary syndrome is an extremely rare and fatal cutaneous T-cell lymphoma (CTCL). Mogamulizumab, an anti-CCR4 monoclonal antibody, has recently been associated with increased progression-free survival in a randomized clinical trial in CTCL. We aimed to evaluate OS and prognostic factors in Sézary syndrome, including treatment with mogamulizumab, in a real-life setting.
Methods
Data from patients with Sézary (ISCL/EORTC stage IV) and pre-Sézary (stage IIIB) syndrome diagnosed from 2000 to 2020 were obtained from 24 centers in Europe. Age, disease stage, plasma lactate dehydrogenases levels, blood eosinophilia at diagnosis, large-cell transformation and treatment received were analyzed in a multivariable Cox proportional hazard ratio model. This study has been registered in ClinicalTrials (SURPASSe01 study: NCT05206045).
Findings
Three hundred and thirty-nine patients were included (58% men, median age at diagnosis of 70 years, Q1-Q3, 61–79): 33 pre-Sézary (9.7% of 339), 296 Sézary syndrome (87.3%), of whom 10 (2.9%) had large-cell transformation. One hundred and ten patients received mogamulizumab. Median follow-up was 58 months (95% confidence interval [CI], 53–68). OS was 46.5% (95% CI, 40.6%–53.3%) at 5 years. Multivariable analysis showed that age ≥ 80 versus <50 (HR: 4.9, 95% CI, 2.1–11.2, p = 0.001), and large-cell transformation (HR: 2.8, 95% CI, 1.6–5.1, p = 0.001) were independent and significant factors associated with reduced OS. Mogamulizumab treatment was significantly associated with decreased mortality (HR: 0.34, 95% CI, 0.15–0.80, p = 0.013).
Interpretation
Treatment with mogamulizumab was significantly and independently associated with decreased mortality in Sézary syndrome.
Funding
French Society of Dermatology, Swiss National Science Foundation (IZLIZ3_200253/1) and SKINTEGRITY.CH collaborative research program.
Keywords: Cutaneous T-cell lymphoma, Sézary syndrome, Mogamulizumab, Monoclonal antibody, Immunotherapy
Research in context.
Evidence before this study
Sézary syndrome accounts for around 5–10% of cutaneous T-cell lymphomas and is the leukaemic and most aggressive form, with a median overall survival of 1–5 years. In 2018, mogamulizumab was approved for the treatment of subsets of patients with cutaneous T-cell lymphoma, including Sézary syndrome, who received at least one previous systemic therapy on the basis of a randomized, open-label phase 3 study showing superior efficacy on progression-free survival over vorinostat, a histone desacetylase inhibitor. Patients with Sézary syndrome had the best responses to monoclonal antibodies, compared to other cutaneous T-cell lymphoma subtypes, such as mycosis fungoides.
Added value of this study
To our knowledge, this is the first study to report increased overall survival associated with the use of mogamulizumab in Sézary syndrome. We used high-quality data from 24 European reference centers of the French Cutaneous Lymphomas Study Group network. This allowed us to analyze the current treatment landscape of Sézary syndrome, and to study prognostic variables associated with overall survival in this rare and aggressive disease.
Implications of all the available evidence
Our study suggests that the use of mogamulizumab is associated with increased overall survival in patients with Sézary syndrome, independently of other prognostic variables including age, ISCL/EORTC disease stage, plasmatic levels of lactate dehydrogenases, and presence of large-cell transformation. This study is encouraging for countries with access to this innovative drug, and strongly calls for a larger and early access to this immuno-oncology agent for Sézary patients worldwide.
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogeneous group of extranodal non-Hodgkin lymphomas, with approximately 500 new cases diagnosed in France each year.1 Sézary syndrome is a rare subtype, accounting for 5.4% of all cases of cutaneous lymphomas2 characterized by erythroderma, lymphadenopathy, and leukemic involvement.3
Patients with Sézary syndrome commonly have severe pruritus, infections,4 and body disfigurement resulting in altered quality of life.5 Median overall survival (OS) ranges from 1 to 5 years.6 Therapeutic strategies include immunomodulatory treatments such as extracorporeal photopheresis7,8 or interferon alfa,9 which are usually considered as first-line treatments in the American and European guidelines.10,11 Available therapeutic monoclonal antibodies (mAbs) include alemtuzumab (anti-CD52),12,13 lacutamab (anti-KIR3DL2),14 pembrolizumab (anti-PD1)15 and atezolizumab (anti-PDL1)16 which have been studied in CTCL including Sézary syndrome in prospective, open-label phase 1 and 2 studies. Given the rarity of long-lasting remissions in Sézary syndrome, time-to-next treatment (TTNT) has been proposed as a meaningful endpoint in this disease.7, 8, 9 In particular, immunomodulatory strategies such as extracorporeal photopheresis7,8 or interferon alfa9 have been associated with longer TTNT, as compared to chemotherapies. The current American guidelines recommend the use of therapeutic mAbs in Sézary syndrome among other treatments such as extracorporeal photopheresis, methotrexate, bexarotene, peginterferon-alfa-2a, HDAC inhibitors.10 There is limited published real-world data on the association of early use of mAbs with OS in Sézary syndrome. In 2018, mogamulizumab (anti-CCR4 mAb) was approved in Europe and the USA for the treatment of subsets of patients with CTCL, including Sézary syndrome, who received at least one previous systemic therapy on the basis of a randomized, open-label phase 3 study showing superior efficacy on progression-free survival over vorinostat, an histone desacetylase (HDAC) inhibitor.17 Mogamulizumab depletes tumor cells, as well as peripheral regulatory T cells in CTCL, and induces local activation of the antitumor immunity in responders,18 long-term responses and immune-mediated side effects.17, 18, 19, 20, 21, 22 Patients with Sézary syndrome had the best responses to mogamulizumab17 compared to other CTCL subtypes such as mycosis fungoides. Long-term remissions, which are rare in Sézary syndrome, have been observed after treatment with mogamulizumab17,19 as well as allogeneic hematopoietic stem cell transplantation23 suggesting the role of immunomodulation in the induction of long-lasting remissions in this disease. We hypothesized that the use of mogamulizumab was associated with increased overall survival in patients with Sézary syndrome. Here, we took advantage of a large cohort of 339 patients with Sézary syndrome treated at 24 French and Swiss reference centers of the Cutaneous Lymphomas French Study Group network to study the association of immunomodulatory treatments with OS in a real-life setting.
Methods
Study design and patients
Cases of patients included in the present study were retrieved from the database of the French Cutaneous Lymphoma study group. This database currently comprises >20,000 prospectively included cases of cutaneous lymphomas from 42 centers in France, Belgium and Switzerland, over a 25-year time period. To limit the amount of missing data in the present study, the information on prognostic variables and follow-up were retrospectively curated from electronic medical records. Inclusion criteria were: (i) adult patients over 18 years old (ii) Sézary (stage IV) or pre-Sézary (stage IIIB) syndrome diagnosed between 2000 and 2020. Non-inclusion criteria were (i) patient opposition to research, (ii) patient under guardianship or curatorship, unable to express his/her opposition.
The main objective of the study was to describe the prognosis of patients with Sézary syndrome. The primary outcome was 5-year OS. The secondary objective was to study factors associated with OS at 5 years in this population and especially the association of treatments with OS. Since TTNT has recently been proposed as a valuable endpoint in CTCL,7, 8, 9 we performed TTNT analyses as post-hoc analyses.
In this registry-based study, data from patients with Sézary syndrome (defined as B2 blood involvement, IV) or pre-Sézary syndrome (T4 stage with B1 blood involvement, IIIB) according to the International Society of Cutaneous Lymphomas (ISCL)/European Organization for Research and Treatment of Cancer (EORTC) staging guidelines24 were obtained to analyze OS and prognostic factors. Patients were included if diagnosed after the date of computerization of medical records, in order to avoid survivorship bias (i.e. enrolling only patients who survived until the date of computerization of medical records). Patients with loss of follow-up were censored at the time of last visit. Missing data was not replaced.
The data included patient-level and tumor-level characteristics–namely, sex, date of diagnosis, age at diagnosis, ISCL/EORTC TNMB classification and disease stage24 at diagnosis, presence of clinical folliculotropism, presence of histological large-cell transformation at diagnosis or during follow-up, defined by the presence of >25% of large cells on histological examination, blood Sézary cell count (cytological examination) and flow cytometric parameters of B2 blood stage (absolute count of CD4+CD26-and CD4+CD7- T cells/mm3),24,25 plasma levels of lactate dehydrogenase (LDH) and blood eosinophil count at diagnosis, successive treatment lines and disease responses, date of last follow-up, disease status at last-follow-up, date and cause of death, if any. This study has been registered in ClinicalTrials (SURPASSe01 study: NCT05206045).
Ethics statement
This study was approved by the institutional review board of Paris Nord University, Paris, France (CER-2021-96) and the ethics committee of Canton Bern, Switzerland (BASEC-Nr: 2021-01405) and was conducted in accordance with the current version of the Helsinki declaration. Written informed consent was obtained from patients prior to the start of the study.
Statistical analysis
Quantitative variables were described by medians and interquartile ranges, and qualitative variables by numbers and percentages. Time (in months) between the date of diagnosis and the date of death or the last visit, whichever occurred first, was calculated. Survival curves were estimated using Kaplan–Meier estimator. OS is presented as estimate and 95% confidence interval (95% CI) and association of factors with survival in terms of hazard ratio (HR) (95% confidence interval, CI). The primary endpoint was OS at 5-year. We divided the study period into 4 parts: before 2010, 2010–2013, 2014–2017 and since 2018, because mogamulizumab was first used in 2014 as part of the MAVORIC clinical trial17 and approved in 2018.
Associations between OS and age, disease stage, plasma lactate dehydrogenase (LDH) levels, large-cell transformation6 and eosinophilia26 were studied because these factors were previously described as independently associated with OS in advanced-stage CTCL. The treatments received by the patients, and large-cell transformation were treated as time-dependent variables. Exposure to a given treatment was defined as the time period from the start of that treatment until the commencement of the subsequent treatment. Univariable analyses were first carried out using a Cox proportional hazard model. A multivariable analysis including the mentioned factors and the received treatments was subsequently conducted. The multivariable model was stratified on the number of received treatment lines, accounting for changes of the baseline instantaneous risk as the disease progressed. Because most patients treated with mAbs were treated with mogamulizumab (83%) whereas the use of other mAbs was marginal, and because mogamulizumab is the only approved mAb in the treatment of CTCL in Europe and USA, the analysis focused on the use of mogamulizumab. Additionally, we conducted a sensitivity analysis of the 5-year OS on the subset of patients who underwent at least five lines of treatment, who represent a group with a more uniform follow-up duration and disease severity. We assessed the proportional hazards assumptions by analyzing scaled Schoenfeld residuals and testing the slope of time-dependent coefficients for each model covariate. The ‘disease stage’ variable showed non-proportional hazards. We thus stratified our model on this variable, allowing to account for its differential impact on survival across various stages without assuming a constant proportional effect over time. A secondary analysis using TTNT as endpoint was conducted. TTNT was defined as the time from the start of the treatment to initiation of subsequent line of therapy or death, whichever occurred first. Univariable and multivariable analyses, similar to the ones described for OS, were conducted.
In a sensitivity analysis, we addressed missing data for three key variables—disease stage, age category, and LDH increase—using multiple imputation by chained equations.
An alpha risk of 5% was used for all the statistical analyses. All statistical analyses were performed with R software version 4.2.2. Survival analyses and time-dependent covariates modelling have been carried out using the ‘survival’ package version 3.5.7, and missing data imputation relied on the ‘mice’ package version 3.16.0.
Role of the funding source
The funders of the study had no role in study design, data collection, analysis, data interpretation, writing of the manuscript or the decision to submit the paper for publication. All authors had access to all study data. All authors agreed to submit for publication.
Results
Patients and baseline clinical characteristics
During the study period, 339 patients met the inclusion criteria and were included in the analysis (58% men, median age at diagnosis of 70 years, Q1-Q3, 61–79): 33 pre-Sézary (stage IIIB–9.7% of 339), 296 Sézary syndrome (stage IV 87.3%), and 10 (2.9%) had Sézary with large-cell transformation at diagnosis (Table 1). Five patients had confirmed visceral involvement at diagnosis. Thirty-five patients presented with clinical folliculotropism at diagnosis. The median number of Sézary cells at diagnosis (measured by cytological examination of peripheral blood smears or flow cytometry) was 2429/mm3 [Q1-Q3: 1242–5407]. Elevated blood LDH at diagnosis were present in 72.3% of patients, and blood eosinophilia (>500/mm3) in 22.6%.
Table 1.
Baseline characteristics of the study population.
| Characteristic | Overall (n = 339) |
|---|---|
| Sex, n (%) | |
| Female | 144 (42.5%) |
| Male | 195 (57.5%) |
| Age at diagnosis (years) | |
| Missing, n | 1 |
| Median (IQR) | 70 (61–79) |
| Diagnosis, n (%) | |
| Pre-Sézary syndrome (stage IIIB) | 33 (9.7%) |
| Sézary syndrome (stage IV) | 296 (87.3%) |
| Sézary with LCT | 10 (2.9%) |
| ISCL/EORTC stage at diagnosis | |
| Missing, n | 3 |
| IIIB, n (%) | 33 (9.8%) |
| IVA1, n (%) | 256 (76.2%) |
| IVA2, n (%) | 42 (12.5%) |
| IVB, n (%) | 5 (1.5%) |
| Sézary cells concentration (/mm3) | |
| Median (IQR) | 2429 (1242, 5406) |
| Plasma LDH increase at diagnosis | |
| Missing, n | 68 |
| No | 75 (27.7%) |
| Yes | 196 (72.3%) |
| Eosinophilia at diagnosis (>500/mm3) | |
| Missing, n | 52 |
| No | 222 (77.4%) |
| Yes | 65 (22.6%) |
| Large cell transformation | |
| Missing, n | 3 |
| No, n (%) | 326 (97%) |
| Yes, n (%) | 10 (3%) |
Abbreviations: IQR: interquartile range, ISCL/EORTC: International Society of Cutaneous Lymphomas/European Organization for Research and Treatment of Cancer, LDH: lactate dehydrogenase.
One hundred thirty-two patients (39.1%) were treated with a mAb at least in one therapeutic line, including 110 patients with mogamulizumab (32.5% of the cohort and 83.3% of patients treated with a mAb). Fourteen (4.1%) patients were treated with alemtuzumab, 22 (6.5%) patients with lacutamab, and 2 (0.6%) patients with cusatuzumab in one therapeutic line (Table 2).
Table 2.
Characteristics of the main treatments received by the patients.
| Treatment | Patient count (%) All lines (n = 339) |
Patient count (%) First line (n = 339) |
|---|---|---|
| Oral retinoid | 211 (62.43%) | 71 (21.01%) |
| Methotrexate | 180 (53.25%) | 113 (33.43%) |
| Phototherapy | 48 (14.20%) | 25 (7.40%) |
| Histone Desacetylase Inhibitor | 44 (13.02%) | 0 (0%) |
| Monochemotherapy | 153 (45.27%) | 21 (6.21%) |
| Polychemotherapy | 63 (18.64%) | 12 (3.55%) |
| Antibody-drug conjugate | 41 (12.13%) | 1 (0.30%) |
| Immunomodulatory treatments | ||
| Interferon-alfa | 95 (28.11%) | 37 (10.95%) |
| Extracorporeal photopheresis | 207 (61.24%) | 103 (30.47%) |
| Allogeneic hematopoietic stem cell transplantation | 17 (5.03%) | 0 (0%) |
| Monoclonal antibodies | ||
| Anti-CD158k (lacutamab) | 22 (6.51%) | 0 (0%) |
| Anti-CD70 (cusatuzumab) | 2 (0.59%) | 0 (0%) |
| Anti-CCR4 (mogamulizumab) | 110 (32.54%) | 3 (0.89%) |
| Anti-CD52 (alemtuzumab) | 14 (4.14%) | 2 (0.59%) |
One hundred and twelve patients (33.1% of 339) received at least one depleting mAb in the first five therapeutic lines: eleven patients were treated with alemtuzumab (3.2%), 95 (28%) patients with mogamulizumab, 13 (3.9%) patients with lacutamab, and 2 (0.6%) patients with cusatuzumab.
Most common first-line treatments, used alone or in combination, included low-dose methotrexate (n = 113, 33.4% of 339), extracorporeal photopheresis (n = 103, 30.5%), oral retinoid (n = 71, 21.0%), subcutaneous interferon alfa (n = 37, 11.0%).
Regarding mogamulizumab, only 3 patients received it as front-line treatment. Twenty-four received it as second line, 30 as third line, 22 as fourth line and 16 as fifth line.
Primary endpoint: 5-year overall survival
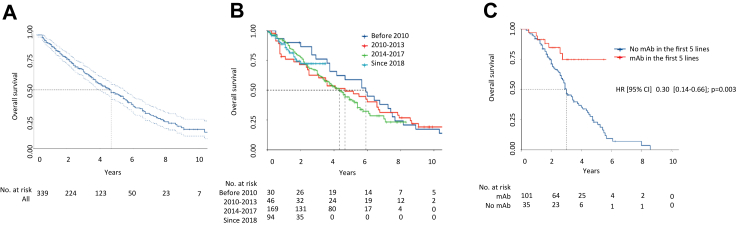
A total of 185 deaths were observed during the study period. The median follow-up was 58 months (95% CI: 53–68) and the median overall survival was 56 months (95% CI, 47–65). OS was 46.5% (95% CI, 40.6–53.3) at 5 years and 16.4% (10.8%–24.9%) at 10 years (Fig. 1a).
Fig. 1.
Overall survival in the whole cohort of 339 patients, according to the time period and to the use of monoclonal antibodies in the first five lines of treatments. A. Overall survival in 339 patients. Overall survival was estimated from the date of diagnosis by the Kaplan–Meier method (dashed lines, 95% confidence interval). B. Overall survival in 339 patients according to the period of diagnosis. No significant difference was observed in terms of overall survival according to the time period. C. Overall survival according to the use, or not, of a therapeutic monoclonal antibody in the first 5 lines of treatments, analyzed in the subgroup of patients who had received at least 5 successive lines of treatments. The median number of consecutive treatment lines received by the patients was 4 (range, 0 to 14).
Seventy-six patients died of progressive CTCL (41% of 185).
Causes of death included infection in 46 cases (24.9%). Two patients died of a complication of allogeneic hematopoietic stem cell transplantation (pulmonary graft-versus-host disease and hepatocellular insufficiency). Two patients died of a second cancer, five of cardiovascular disease and 46 from equivocal causes.
We first analyzed OS as a function of date of diagnosis, dividing our study group into 4 time periods: before 2010, 2010–2013, 2014–2017 and since 2018. There was no significant difference in overall survival according to the time period of inclusion (respectively 71 months (95% CI: 50; 90); 56 (30; 81), 52 (44; 63) and not reached, p = 0.70) (Fig. 1b).
Secondary endpoints
Univariable analysis on overall survival
Treatments
The median number of consecutive treatment lines received by the patients was 4 (range, 0 to 14).
Treatment with mAb in the first 5 lines of treatments (analysed in the subgroup of patients who had received at least 5 successive lines of treatments) was significantly associated with higher OS compared to patients who had not received any mAb in the first 5 lines of treatments (median OS, 36 months versus not reached, HR = 0.30 (95% CI, 0.14–0.66, p = 0.003, Fig. 1c).
In the whole cohort of 339 patients, treatment with mogamulizumab was significantly associated with OS in univariable analysis (median OS, 92 months versus 55 months, HR = 0.60, 95% CI 0.36–1.0, p = 0.049, Fig. 2a and Table 3).
Fig. 2.
Overall survival according to the treatments received by the patients. A. Overall survival according to the use, or not, of mogamulizumab at any time point during the course of the disease. B. Overall survival according to the use, or not, of extracorporeal photopheresis at any time point during the course of the disease. C. Overall survival according to the use, or not, of interferon alfa at any time point during the course of the disease. Treatments were analyzed as time-dependent variables and numbers at risk not indicated for this reason. Hazard ratios, 95% confidence intervals and p-values are from univariable Cox analysis.
Table 3.
Uni and multivariable analysis on overall survival in the whole cohort of 339 patients.
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p.value | adjusted HR | 95% CI | p.value | |
| No mogamulizumab treatment | 1 | 1 | ||||
| Treated with mogamulizumab | 0.60 | 0.36; 1.00 | 0.049 | 0.34 | 0.15; 0.80 | 0.013 |
| Diagnosis age (years): <50 | 1 | 1 | ||||
| Diagnosis age (years): 50–59 | 1.41 | 0.72; 2.77 | 0.316 | 0.85 | 0.34; 2.16 | 0.70 |
| Diagnosis age (years): 60–69 | 1.67 | 0.92; 3.06 | 0.094 | 1.38 | 0.59; 3.21 | 0.50 |
| Diagnosis age (years): 70–79 | 2.37 | 1.31; 4.28 | 0.004 | 2.47 | 1.09; 5.58 | 0.03 |
| Diagnosis age (years): ≥ 80 | 4.38 | 2.37; 8.07 | <0.0001 | 4.88 | 2.13; 11.19 | 0.001 |
| No eosinophilia | 1 | 1 | ||||
| Eosinophilia | 1.46 | 1.02; 2.10 | 0.041 | 1.59 | 0.96; 2.64 | 0.07 |
| No LDH increase | 1 | 1 | ||||
| LDH increase | 1.35 | 0.91; 1.99 | 0.133 | 1.07 | 0.65; 1.75 | 0.80 |
| No large cell transformation | 1 | 1 | ||||
| Large cell transformation | 4.12 | 2.91; 5.85 | <0.0001 | 2.83 | 1.58; 5.06 | 0.001 |
Abbreviations: HR: hazard ratio, CI: confidence interval, LDH: lactate dehydrogenase.
HRs are estimated using Cox proportional hazard regressions. Multivariable HRs are obtained using models stratified on the number of received lines and that include age, disease stage, plasma lactate dehydrogenase (LDH) levels, large-cell transformation (time-dependent covariable) and eosinophilia as covariables.
All other treatments were analyzed as time-dependent variables for their association with OS in univariable analysis. The only other treatments associated with longer OS were extracorporeal photopheresis (median OS, 66 months versus 44, HR = 0.68, 95% CI 0.50–0.92, p = 0.012, Fig. 2b) and interferon alfa (median OS, 68 months versus 52, HR = 0.68, 95% CI 0.43–0.89, p = 0.009, Fig. 2c).
Other prognostic variables
Age >80 years (versus <50, HR = 4.4, 95% CI 2.4–8.1, p = 0.001, Table 3), eosinophilia (>500/mm3, HR = 1.5, 95% CI, 1.0–2.1, p = 0.041), and large-cell transformation (HR = 4.1, 95% CI, 2.9–5.9, p = 0.001) were significantly associated with shorter OS in univariable analysis.
Multivariable analysis on overall survival
Treatment with mogamulizumab, age, plasma lactate dehydrogenase (LDH) levels, large-cell transformation and eosinophilia were used as covariables because the latter clinical variables were previously described as independently associated with OS in advanced-stage CTCL.
Mogamulizumab treatment was an independent factor associated with an increased OS in multivariable analysis (HR: 0.34, 95% CI, 0.15–0.80, p = 0.013). Age and the presence of large-cell transformation remained significantly associated with OS in multivariable analysis.
The summary of the univariable and multivariable survival analyses are shown in Table 3.
Extracorporeal photophoresis remained significantly associated with OS in multivariable analysis (HR: 0.68, 95% CI 0.50–0.92, p = 0.012), but not interferon alpha (HR: 0.89, 95% CI 0.49–1.64, p = 0.72).
Time-to-next treatment analysis
TTNT analyses were performed on all treatment types and summarized in Table 4. Treatments with mogamulizumab, extracorporeal photopheresis, and allogeneic hematopoietic stem cell transplantation were associated with significantly higher TTNT in multivariable analysis, thus confirming the potential benefit associated with the use of immunomodulatory strategies in Sézary syndrome. As previously described (9), mono- and polychemotherapy were associated with a trend towards shorter TTNT in the multivariable analysis (respectively adjusted p = 0.008 and p = 0.055).
Table 4.
Summary of time to next treatment analyses.
| Variable | Median TTNT (months) [95% CI] | Univariable HR [95% CI] | p.value | Multivariable HR [95% CI] | p.value |
|---|---|---|---|---|---|
| Not treated with ECP | 6 [5; 7] | ||||
| Treated with ECP | 17 [13; 20] | 0.77 [0.66; 0.88] | 0.001 | 0.78 [0.64; 0.95] | 0.013 |
| Not treated with MTX | 6 [5; 8] | ||||
| Treated with MTX | 13 [10; 17] | 0.78 [0.68; 0.90] | 0.001 | 0.94 [0.77; 1.15] | 0.54 |
| Not treated with allo HSCT | 8 [7; 9] | ||||
| Treated with allo HSCT | 41 [36; NA] | 0.33 [0.16; 0.71] | 0.004 | 0.26 [0.10; 0.66] | 0.005 |
| Not treated with interferon | 7 [6; 9] | ||||
| Treated with interferon | 13 [8; 20] | 1.21 [1.03; 1.41] | 0.02 | 1.42 [1.12; 1.79] | 0.004 |
| Not treated with moga | 7.5 [7; 9] | ||||
| Treated with moga | 24 [19; 28] | 0.87 [0.68; 1.10] | 0.24 | 0.51 [0.35; 0.73] | 0.001 |
| Not treated with monochemotherapy | 8 [7; 10] | ||||
| Treated with monochemotherapy | 7 [5.5; 10] | 2.23 [1.91; 2.60] | <0.0001 | 1.41 [1.09; 1.81] | 0.008 |
| Not treated with polychemotherapy | 8 [7; 10] | ||||
| Treated with polychemotherapy | 6 [3; 10] | 2.51 [2.02; 3.13] | <0.0001 | 1.44 [0.99; 2.08] | 0.055 |
Abbreviations: ECP, extracorporeal photopheresis, MTX, low dose methotrexate, moga, mogamulizumab, CI, confidence interval, HR, hazard ratio.
HRs are estimated using Cox proportional hazard regressions. Multivariable HRs are obtained using models stratified on the number of received lines and that include age, disease stage, plasma lactate dehydrogenase (LDH) levels, large-cell transformation (time-dependent covariable) and eosinophilia as covariables.
A sensitivity analysis, addressing missing data for three key variables–disease stage, age, and LDH increase—using multiple imputation by chained equations, performed on OS and TTNT, provided the same conclusions (Supplemental Tables S1 and S2).
Discussion
To our knowledge, the present study is the largest to evaluate survival and prognostic factors specifically in Sézary syndrome.
Large international studies have identified stage IV, age >60 years, large-cell transformation, and increased LDH as independent prognostic variables in CTCL in general6 but have not focused on Sézary syndrome patients and association of the use of specific treatments with OS. Immunomodulatory strategies such as extracorporeal photopheresis7,8 or interferon alfa9 have been associated with longer TTNT, as compared to chemotherapies and our study confirmed the benefit of immune modulation in Sézary syndrome: the only treatments associated with a significantly longer OS in our study were mogamulizumab, extracorporeal photopheresis and interferon alfa. Interestingly, the effect of mogamulizumab on OS was mainly visible after a few years (Fig. 2a), suggesting long-term effects possibly associated with the local activation of the immune responses, as previously described in responders.18 Additionally, TTNT analyses in the present study showed significantly higher TTNT associated with the use of mogamulizumab, allogeneic HSCT and extracorporeal photopheresis, also strengthening the hypothesis of a long-term benefit induced by immunomodulatory strategies in this disease.23
This study assessed the 5-year OS of a large multicenter European cohort (n = 339) over a 20-year period. The results confirm the poor survival of these patients with a median survival of 56 months. A 2015 international study involving 29 centers and including all advanced-stage CTCL patients found similar data with a median OS of 47 months in patients with stage IVA disease6 and identified stage, age, large-cell transformation and elevated LDH levels as independent adverse prognostic factors of OS. Large-cell transformation is an identified poor prognostic factor in Sézary syndrome27 whereas the absence of CD30 expression in transformed mycosis fungoides,28 as well as other biological factors such as CAF-1/p60 overexpression29 have been associated with shorter overall survival. There was no significant difference in terms of OS according to the study period, suggesting that differences in OS associated with the different specific treatments of Sézary syndrome were not simply reflecting improved supportive care over time (anti-infectious drugs, earlier diagnosis, for example).
Treatment with mogamulizumab was associated with increased OS in multivariable analysis. Our results are in agreement with the recent results of the MAVORIC study, which excluded patients with large-cell transformation, and found an increase in progression-free survival in the mogamulizumab group compared to the vorinostat group with an HR of 0.53, (95% CI: 0.41, 0.69) and suggested increased OS in a post-hoc analysis adjusted for cross-over and relying on a small number of patients.30 A study including 16 Sézary syndrome and 5 mycosis fungoides patients treated with mogamulizumab, also showed the benefit of mogamulizumab in real life in the treatment of advanced CTCL with a median progression-free survival of 22 months.31
In our study, a significant number of patients died of disease progression (76 patients or 41%) and 25% of sepsis; this was probably underestimated since some patients died from an equivocal cause. Sepsis remained a leading cause of death in our cohort, as previously described4,32 and this provides an additional argument supporting the use of immunomodulatory treatments, and not chemotherapies, in this disease.
The main limitation is the retrospective nature of this study and heterogeneity of the treatments received as a direct consequence of the multicentre retrospective setting of this study, which is usual in this disease since most patients receive several consecutive lines of treatments. The sequential treatments are tailored to each case in this disease and, guided by decisions in tumor boards, further add to the variability of therapeutic approaches. It is important to note that the availability of treatment options varied throughout the study period due to factors such as drug approvals and market withdrawals. While this could introduce bias, it also serves to reflect the authentic context of our investigation. Additionally, this variability aligns with the real-world conditions of advanced-stage CTCL care and enhances the external validity of our findings. We strived to limit the amount of missing data in this largest multicentre study ever published in this particularly rare disease. In our study, the number of complete cases, i.e. cases without any missing information, on the prognostic covariables in this disease (LDH levels, large-cell transformation, age and disease stage) was 249 (73% of 339 patients).
The strengths of our study are the multicenter setting, the use of international diagnostic and response criteria, and the size of the cohort (n = 339). It constitutes one of the largest published international series of patients with Sézary syndrome. Although relatively large compared to existing studies in the field of this extremely rare disease, our cohort was smaller than expected (700 initially expected patients), due to the fact that we enrolled only patients whose diagnosis date was posterior to the digitalization of medical records to avoid major survivorship bias. Among the subset of patients diagnosed before digitalization, only those who survived until digitalization are included in our cohort. Additionally, the protocol was written and collection of data began at the time of the 2018 diagnostic recommendations.3 Consequently, some patients diagnosed as Sézary with B2 criteria were later reclassified as Mycosis Fungoides without B2 phase and excluded from the analysis. We included only patients with B2 criteria according to the latest ISCL/USCLC/EORTC staging guidelines.24 Our intention was to ensure homogeneity of the study group, enable a more accurate and clinically relevant classification of patients, and eventually improve the validity of our results in the context of the existing staging guidelines. Nonetheless, this cohort represents one of the largest prospective international cohort of patients with Sézary syndrome. No study had yet studied the impact of mAbs and in particular mogamulizumab on OS in a large cohort of Sézary syndrome patients. This study is not without limitations. The findings are derived from an observational design, which is inherently prone to confounding bias. We attempted to mitigate this both at the data collection step (only including patients diagnosed after medical records digitization), and in the statistical analysis (using a multivariate, stratified, time-dependent Cox regression model). We reported the significance testing of various exposure factors, leading to type I error rate inflation, that is an increased likelihood of erroneously identifying results as statistically significant. Given these considerations, the results should be interpreted as exploratory.
The significantly and independently increased OS associated with mogamulizumab treatment at any time point, in a real-life setting, is encouraging for patients with Sézary syndrome. Further molecular studies and deep learning approaches, together with large-scale cohorts like this one or the Cutaneous Lymphoma International Consortium database33 may help to better identify the best treatment for each patient at each time point during the course of the disease.
Contributors
A. Bozonnat participated in data curation, formal analysis, writing of the original draft and review & editing.
A. Serret-Larmande performed formal analysis and contributed to the methodology, writing of the original draft, review & editing.
C. Montlahuc and M. Resche-Rigon contributed to the methodology, review & editing.
A. de Masson supervised the study, participated in formal analysis, writing of the original draft and review & editing.
M. Beylot-Barry, O. Dereure, M. d Incan, G. Quereux, E. Guenova, M. Perier-Muzet, S. Dalle, F. Grange, M. Viguier, C. Ram-Wolff, L. Feldmeyer, H. Beltraminelli, N. Bonnet, F. Amatore, E. Maubec, N. Franck, L. Machet, F. Chasset, F. Brunet-Possenti, J.D. Bouaziz, M. Battistella, M. Donzel, A. Pham-Ledard, C. Bejar, H. Moins-Teisserenc, S. Mourah, P. Saiag E. Hainaut, C. Michel, G. Bens, H. Adamski, F. Aubin, S. Boulinguez, P. Joly, B. Tedbirt, I. Templier, L. Troin, H. Montaudié, S. Ingen-Housz-Oro, S. Faiz, L. Mortier, G. Dobos, M. Bagot, participated in data collection, manuscript review & editing.
All authors contributed to manuscript review, approved the final draft for submission, and are accountable for accuracy and integrity of any part of the work. All authors had full access to all data in the study and agreed to submit for publication.
Data sharing statement
According to the French General Data Protection regulation at the time of publication, all proposals requesting access to anonymised individual participant data from the SURPASSe study will need to specify how the data will be used and for what purpose. Requests for access to data should be addressed to Adèle de Masson at adele.demasson@aphp.fr.
Declaration of interests
AdM declares nonfinancial support from Kyowa Kirin and Recordati Rare Diseases; fees from Takeda, Almirall and Recordati Rare Diseases, and research funding, outside the scope of this study, from Kyowa Kirin, Innate Pharma, Almirall and Takeda.
MB declares consultant fees from Innate Pharma, Kyowa Kirin, Takeda, BMS, Sanofi, Quantum Genomics, and research funding from Kyowa Kirin and Takeda, outside the scope of this study.
SM declares consultant fees outside the scope of this study, from Pierre Fabre, Sanofi, Novartis and Biocartis, and has received research funding from BMS, Novartis and Roche.
NF declares having received nonfinancial support from Kyowa Kirin.
PS received a research grant from Pierre Fabre, fees unrelated to this manuscript from Bristol-Myers Squibb, MSD, Merck-Serono, Pfizer, Roche-Genentech, Pierre Fabre, and Novartis; received nonfinancial support from Bristol-Myers Squibb, MSD, Roche-Genentech, Pierre Fabre, and Novartis outside of the scope of this study.
MBB declares consultant fees from Kyowa Kirin, Takeda and Recordati and research funding from Kyowa Kirin and Almirall outside the scope of this study.
HMT declares consultant fees outside from the scope of this study from Innate Pharma, and has received research funding, outside the scope of this study, from Kyowa Kirin.
SIHO consultant fees outside the scope of this study from Takeda and Recordati.
GD declares consultant fees outside of the scope of this study from Kyowa Kirin and Recordati.
EG declares consultant fees and/or grant support from Mallinckrodt, Helsinn, Takeda, Novartis and Kyowa Kirin unrelated to this work.
FG declares consultant fees from Recordati and Kyowa Kirin, outside from the scope of this study.
GQ declares consultant fees from Takeda, Recordati and Kyowa Kirin, outside the scope of this study.
CM declares nonfinancial support from MSD, Pfizer, Novartis, Bristol-Myers Squibb, Pierre Fabre, Leo Pharma, Sanofi Aventis, Jannsen Cilag outside of the scope of this study.
SB declares having received nonfinancial support from Kyowa Kirin and Recordati.
MBag declares consulting fees from Kyowa Kirin, Takeda, Recordati.
HB declares consultant fees from Kyowa Kirin.
EH declares consultant fees outside the scope of this study from Bristol-Myers Squibb, Takeda, Sanofi, Jannsen Cilag, Blueprint Medicines, AbbVie and nonfinancial support from Kyowa Kirin, MSD, UCB Pharma, Novartis, Almirall, Pierre Fabre.
The other authors declare that they have no conflict of interest.
Acknowledgements
This study was funded by the French Society of Dermatology, by the Swiss National Science Foundation (IZLIZ3_200253/1) and by the SKINTEGRITY.CH collaborative research program. We thank all the patients who participated in this study, and their families and caregivers. We also thank all the investigators for their contributions to the administration and execution of the study, as well as Marion Dubois and Razika Guizem for their excellent administrative support. The sponsor had no role in the study design, data collection, data analysis, interpretation of data, writing of the report, or the decision to submit the paper for publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102679.
Appendix A. Supplementary data
References
- 1.Dobos G., Pohrt A., Ram-Wolff C., et al. Epidemiology of cutaneous T-cell lymphomas: a systematic review and meta-analysis of 16,953 patients. Cancers. 2020;12 doi: 10.3390/cancers12102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobos G., de Masson A., Ram-Wolff C., et al. Epidemiological changes in cutaneous lymphomas: an analysis of 8593 patients from the French Cutaneous Lymphoma Registry. Br J Dermatol. 2020;184:1059. doi: 10.1111/bjd.19644. published online Oct 31. [DOI] [PubMed] [Google Scholar]
- 3.Willemze R., Cerroni L., Kempf W., et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703–1714. doi: 10.1182/blood-2018-11-881268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaizot R., Ouattara E., Fauconneau A., Beylot-Barry M., Pham-Ledard A. Infectious events and associated risk factors in mycosis fungoides/Sézary syndrome: a retrospective cohort study. Br J Dermatol. 2018;179:1322–1328. doi: 10.1111/bjd.17073. [DOI] [PubMed] [Google Scholar]
- 5.Ottevanger R., van Beugen S., Evers A.W.M., Willemze R., Vermeer M.H., Quint K.D. Quality of life in patients with Mycosis Fungoides and Sézary Syndrome: a systematic review of the literature. J Eur Acad Dermatol Venereol. 2021;35:2377–2387. doi: 10.1111/jdv.17570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarisbrick J.J., Prince H.M., Vermeer M.H., et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766–3773. doi: 10.1200/JCO.2015.61.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao C., McCormack C., van der Weyden C., et al. Prolonged survival with the early use of a novel extracorporeal photopheresis regimen in patients with Sézary syndrome. Blood. 2019;134:1346–1350. doi: 10.1182/blood.2019000765. [DOI] [PubMed] [Google Scholar]
- 8.Campbell B.A., Dobos G., Haider Z., et al. International study of SS shows superiority of combination therapy & heterogeneity of treatment strategies. Blood Adv. 2023;7 doi: 10.1182/bloodadvances.2023011041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes C.F.M., Khot A., McCormack C., et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sézary syndrome: a comparative study of systemic therapy. Blood. 2015;125:71–81. doi: 10.1182/blood-2014-07-588236. [DOI] [PubMed] [Google Scholar]
- 10.Mehta-Shah N., Horwitz S.M., Ansell S., et al. NCCN guidelines insights: primary cutaneous lymphomas, version 2.2020: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2020;18:522–536. doi: 10.6004/jnccn.2020.0022. [DOI] [PubMed] [Google Scholar]
- 11.Trautinger F., Eder J., Assaf C., et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome – update 2017. Eur J Cancer. 2017;77:57–74. doi: 10.1016/j.ejca.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Lundin J., Hagberg H., Repp R., et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101:4267–4272. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- 13.de Masson A., Guitera P., Brice P., et al. Long-term efficacy and safety of alemtuzumab in advanced primary cutaneous T-cell lymphomas. Br J Dermatol. 2014;170:720–724. doi: 10.1111/bjd.12690. [DOI] [PubMed] [Google Scholar]
- 14.Bagot M., Porcu P., Marie-Cardine A., et al. IPH4102, a first-in-class anti-KIR3DL2 monoclonal antibody, in patients with relapsed or refractory cutaneous T-cell lymphoma: an international, first-in-human, open-label, phase 1 trial. Lancet Oncol. 2019;20:1160–1170. doi: 10.1016/S1470-2045(19)30320-1. [DOI] [PubMed] [Google Scholar]
- 15.Khodadoust M.S., Rook A.H., Porcu P., et al. Pembrolizumab in relapsed and refractory mycosis fungoides and sézary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38:20–28. doi: 10.1200/JCO.19.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadler R., Romero P.O., Bagot M., et al. Phase II trial of atezolizumab (anti-PD-L1) in the treatment of stage IIb-IVB mycosis fungoides/Sézary syndrome patients relapsed/refractory after a previous systemic treatment (PARCT) Eur J Cancer. 2021;156(Suppl 1):S22–S23. doi: 10.1016/S0959-8049(21)00668-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y.H., Bagot M., Pinter-Brown L., et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19:1192–1204. doi: 10.1016/S1470-2045(18)30379-6. [DOI] [PubMed] [Google Scholar]
- 18.de Masson A., Darbord D., Dobos G., et al. Macrophage-derived CXCL9 and CXCL11, T-cell skin homing, and disease control in mogamulizumab-treated CTCL patients. Blood. 2022;139:1820–1832. doi: 10.1182/blood.2021013341. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet P., Battistella M., Roelens M., et al. Association of autoimmunity and long-term complete remission in patients with Sézary syndrome treated with mogamulizumab. Br J Dermatol. 2019;180:419–420. doi: 10.1111/bjd.17320. [DOI] [PubMed] [Google Scholar]
- 20.Algarni A.S., Ram-Wolff C., Bagot M., De Masson A. Mogamulizumab-induced vitiligo in patients with Sézary syndrome: three cases. Eur J Dermatol. 2021;31:213–216. doi: 10.1684/ejd.2021.4002. [DOI] [PubMed] [Google Scholar]
- 21.Roelens M., de Masson A., Andrillon A., et al. Mogamulizumab induces long-term immune restoration and reshapes tumour heterogeneity in Sézary syndrome. Br J Dermatol. 2022;186:1010–1025. doi: 10.1111/bjd.21018. [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Ram-Wolff C., Dobos G., et al. Head and neck granulomatous rash associated with mogamulizumab mimicking mycosis fungoides. Br J Dermatol. 2022;187:129–131. doi: 10.1111/bjd.21030. [DOI] [PubMed] [Google Scholar]
- 23.de Masson A., Beylot-Barry M., Ram-Wolff C., et al. Allogeneic transplantation in advanced cutaneous T-cell lymphomas (CUTALLO): a propensity score matched controlled prospective study. Lancet. 2023;S0140-6736(23):329–X. doi: 10.1016/S0140-6736(23)00329-X. [DOI] [PubMed] [Google Scholar]
- 24.Olsen E.A., Whittaker S., Willemze R., et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood. 2021;140 doi: 10.1182/blood.2021012057. blood.2021012057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarisbrick J.J., Hodak E., Bagot M., et al. Blood classification and blood response criteria in mycosis fungoides and Sézary syndrome using flow cytometry: recommendations from the EORTC cutaneous lymphoma task force. Eur J Cancer. 2018;93:47–56. doi: 10.1016/j.ejca.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 26.Tancrède-Bohin E., Ionescu M.A., de La Salmonière P., et al. Prognostic value of blood eosinophilia in primary cutaneous T-cell lymphomas. Arch Dermatol. 2004;140:1057–1061. doi: 10.1001/archderm.140.9.1057. [DOI] [PubMed] [Google Scholar]
- 27.Bontoux C., de Masson A., Thonnart N., et al. Large-cell transformation is an independent poor prognostic factor in Sézary syndrome: analysis of 117 cases. Br J Dermatol. 2022 doi: 10.1111/bjd.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Travaglino A., Russo D., Varricchio S., et al. Prognostic significance of CD30 in transformed mycosis fungoides. Am J Clin Pathol. 2021;156:350–355. doi: 10.1093/ajcp/aqaa261. [DOI] [PubMed] [Google Scholar]
- 29.Mascolo M., Travaglino A., Varricchio S., et al. Role of chromatin assembly factor-1/p60 and poly [ADP-ribose] polymerase 1 in mycosis fungoides. Virchows Arch. 2021;478:961–968. doi: 10.1007/s00428-020-02952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkins N., Muszbek N., Evans R., Dequen-O’Byrne P., Jones T., McNamara L. Adjusting for treatment crossover in the MAVORIC trial: survival in advanced mycosis fungoides and Sézary syndrome. J Comp Eff Res. 2022;11 doi: 10.2217/cer-2022-0070. cer-2022-0070. [DOI] [PubMed] [Google Scholar]
- 31.Jouandet M., Nakouri I., Nadin L., et al. Impact of mogamulizumab in real-life advanced cutaneous T-cell lymphomas: a multicentric retrospective cohort study. Cancers. 2022;14:1659. doi: 10.3390/cancers14071659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarisbrick J.J. Infections in mycosis fungoides and Sézary syndrome are a frequent cause of morbidity and contribute to mortality. What can be done? Br J Dermatol. 2018;179:1243–1244. doi: 10.1111/bjd.17194. [DOI] [PubMed] [Google Scholar]
- 33.Quaglino P., Maule M., Prince H.M., et al. Global patterns of care in advanced stage mycosis fungoides/Sezary syndrome: a multicenter retrospective follow-up study from the Cutaneous Lymphoma International Consortium. Ann Oncol. 2017;28:2517–2525. doi: 10.1093/annonc/mdx352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.