Abstract
Background
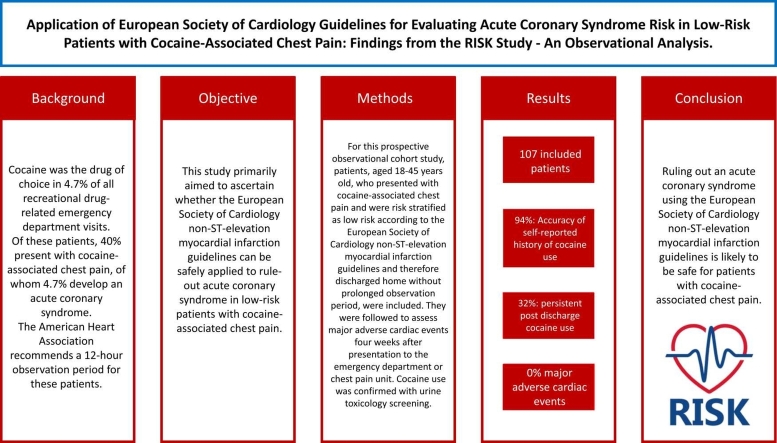
Cocaine was the drug of choice in 4.7 % of all recreational drug-related emergency department visits. Of these patients, 40 % present with cocaine-associated chest pain, of whom 4.7 % develop an acute coronary syndrome. The American Heart Association recommends a 12-hour observation period for these patients.
Objective
This study primarily aimed to ascertain whether the European Society of Cardiology non-ST-elevation myocardial infarction guidelines can be safely applied to rule-out acute coronary syndrome in low-risk patients with cocaine-associated chest pain.
Methods
For this prospective observational cohort study, patients, aged 18–45 years old, who presented with cocaine-associated chest pain and were risk stratified as low risk according to the European Society of Cardiology non-ST-elevation myocardial infarction guidelines and therefore discharged home without prolonged observation period, were included. They were followed to assess major adverse cardiac events four weeks after presentation to the emergency department or chest pain unit. Cocaine use was confirmed with urine toxicology screening.
Results
A total of 107 patients were included and analysed. The accuracy of the self-reported history of recent cocaine use was 94 %. Post-discharge cocaine use persisted among 32 % of patients. None of the included 107 patients died and major adverse cardiac event within four weeks did not occur among 97 patients with available data regarding MACE.
Conclusion
Ruling out an acute coronary syndrome using the European Society of Cardiology non-ST-elevation myocardial infarction guidelines is likely to be safe for patients with cocaine-associated chest pain, however this study was underpowered to reach definitive conclusions.
Keywords: Cocaine-associated chest pain, Acute coronary syndrome, European Society of Cardiology NSTEMI guideline
Graphical Abstract
Highlights
-
•
The ESC NSTEMI guideline is most likely safe to apply in low-irisk patients with CACP for ruling-out ACS.
-
•
No major adverse cardiac events occurred within the post-discharge period of four weeks.
-
•
The reliability of self-reported cocaine use in chest pain patients is high (94 %).
-
•
Many patients (32 %) continue cocaine use after hospital assessment for cocaine-associated chest pain.
1. Introduction
The last-year cocaine use rate in Europe varies between 0.1 % and 4.7 % among young adults, aged 15–34 years old. [1] In 2021, the cocaine lifetime use in the Netherlands was 6.5 %, which was an increase after a relatively stable period between 2016 and 2020. [2] Cocaine causes complications for which medical attention is required. Cocaine was the drug of choice in 4.7 % of all recreational drug-related emergency department visits. [3] Of these patients, 40 % presented with cocaine-associated chest pain (CACP) [4], of whom 4.7 % developed an acute coronary syndrome (ACS). [5] Since the risk of developing ACS is highest in the first hour after cocaine use, two-third of cocaine-associated ACS is diagnosed within three hours after cocaine use. [6], [7] Notably, the American Heart Association issued a scientific statement in 2008 recommending a 12-hour observation period for patients with CACP to exclude the development of ACS. [4]
Since the introduction of high-sensitivity troponin, it has become possible to rapidly and safely rule out ACS in low-risk chest pain patients, as advised by the European Society of Cardiology non-ST-elevation myocardial infarction (NSTEMI) guideline using the 0/3 h protocol, later refined to the 0/1 h protocol. [8] However, there is currently no evidence available to determine whether the European Society of Cardiology NSTEMI guidelines are safe for patients with CACP and no specific recommendations for CACP are formulated, suggesting that CACP patients should be managed accordingly. [8] This contrasts with the American Heart Association scientific statement, which is the most recent guideline on this topic. However, this guideline has not been updated since 2008, and the safety of contemporary algorithms in CACP has not been well-studied. [4]
This study primarily aimed to ascertain whether the European Society of Cardiology non-ST-elevation myocardial infarction guidelines can be safely applied to rule-out acute coronary syndrome in low-risk patients with CACP.
2. Methods
2.1. Study design and setting
This multi-centre, prospective, observational cohort study was carried out at three prominent urban hospitals in the Netherlands: OLVG hospital in Amsterdam, Diakonessenhuis in Utrecht and Haaglanden Medical Centre in The Hague. This study was conducted between the third of June 2016 and the first of June 2023, and included patients who presented with CACP to the emergency department or the chest pain unit. The development of major adverse cardiac events (MACE) was assessed with follow up, four weeks after inclusion.
2.2. Population
Patients, aged 18–45 years old, were included if they presented with chest pain, were risk stratified as low-to-intermediate cardiac risk according to the European society of cardiology NSTEMI guideline [8], by either the 0/3 h protocol and/or the 0/1 h protocol, had a positive urine toxicology screening result for cocaine, and were able to be contacted for follow up (through e-mail or telephone). Exclusion criteria were reported chest pain arising from trauma or other explicitly diagnosed non-cardiac causes, unable to participate in follow up, and a lacking ability to communicate in Dutch or English.
2.3. Procedure
All patients aged 18–45 years old presenting with chest pain to either the emergency department or the chest pain unit were queried about their recreational drug use history and subjected to a urine toxicology screening performed using the Triage® TOX Drug Screen, as standard of care. They were subjected to standard of care according to the European Society of Cardiology NSTEMI guideline with standardized high-sensitivity Troponin-T analysis (Roche Diagnostics). [8] Since at the start of the study the 0/3 h algorithm was standard of care, but the 0/1 h protocol was under validation, patients were subjected to both algorithms simultaneously. Medical decisions were based on standard of care and not influenced by the study. Following the exclusion of ACS and ready for discharge, eligible patients were enrolled in the study after informed consent was granted. Assessment of cardiovascular risk factors, recreational drug use history and risk stratification was carried out by the attending physician. [8] Substance use counselling was provided upon discharge.
2.4. Follow-up
After a minimum of four weeks following discharge, patients were approached for a follow-up assessment. During this follow-up, patients were queried about instances of recurring chest pain, with or without seeking attention from a medical specialist, any readmissions to the hospital, and their cocaine use after discharge.
When direct patient follow-up was unattainable, and permission had been granted via informed consent, communication was established with the patient’s general practitioner or designated contact person. In such instances, the general practitioner or contact person was questioned regarding any occurrences of ACS or recurrent chest pain leading to consultation with a medical specialist within the four weeks after discharge. In case patient follow-up was not feasible, despite at least five attempts to reach out, and no recurrent hospital visits had occurred, an exploration of the national mortality database was conducted for the Dutch residents. Foreigners were then lost to follow up. Patients lost to follow-up were not excluded from the study, although they were excluded from statistical analysis.
2.5. Endpoints
The primary aim of this study was to determine if the European society of cardiology NSTEMI guideline can be safely applied to rule out ACS in patients with cocaine-associated chest pain. [8] Therefore, the primary study endpoint is the incidence of major adverse cardiac events four weeks after discharge, as defined by the European society of cardiology. [8] Secondary endpoint was to define the reliability of self-reported cocaine use history by chest pain patients.
2.6. Data collection
The following parameters were collected: medical history, demographic characteristics, cardiovascular risk factors, recreational drug use history, vital signs, high-sensitivity troponin-T and urine toxicology screen results, the GRACE 2.0 score, the HEART score and time between last use of cocaine and onset of symptoms.
2.7. Sample size & statistical analysis
Upon the start of the study a power analysis was performed aiming for an upper limit of the confidence interval for the likelihood of MACE of about 2 %, meaning that the incidence of MACE was expected to be around 2 %. It was estimated 300 patients should be included to answer the study question. The primary endpoint is expressed as a percentage of all patients developing MACE, the secondary endpoint is expressed as a percentage of all patients with a compatible history and toxicology result. The descriptive analysis of the collected data was carried out using IBM SPSS Statistics Version 29.0.0.0. This study was approved by the Medical Ethics Commission of the Netherlands with number NL57552.100.16 / R16.029 and the study protocol was published in the Dutch Trial Register with number NL5243.
3. Results
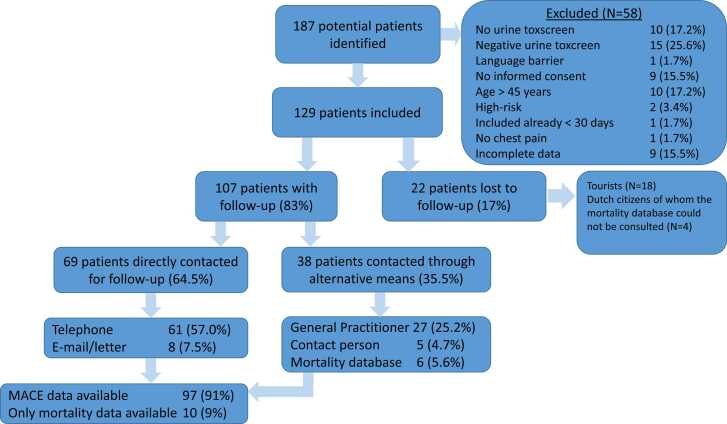
In total, 187 potentials patients were identified over the course of the study period, 58 of those patients did not meet the inclusion criteria (Fig. 1). A total of 129 patients fulfilled the inclusion criteria and provided informed consent (Fig. 1). Among these participants, 69 underwent a comprehensive follow-up through direct communication via telephone, e-mail, or postal correspondence. Additionally, 38 patients received follow-up through their general practitioner, a designated contact person, or consultation of the national mortality database. This resulted in mortality data for 107 patients and MACE data for 97 patients. The loss to follow-up rate was 17 % (n=22). In total, 107 patients were included for analysis. Their demographic and clinical characteristics are elucidated in Table 1. Most patients were male (80.4 %) and active smokers (69.2 %), with a limited number of other cardiovascular risk factors. Among the 107 individuals, 93.5 % acknowledged cocaine use in the last 48 hours.
Fig. 1.
Flowchart of the inclusion process.
Table 1.
Demographics and Characteristics.
| Characteristics | Outcome (N = 107) |
|---|---|
| Mean age in years (+SD) | 32.4 + 7.7 |
| Male sex – N (%) | 86 (80.4 %) |
| Residency | |
| Dutch citizen | 86 (80.4 %) |
| Tourist | 21 (19.6 %) |
| Risk factors – N (%) | |
| History of cardiovascular disease | 5 (4.7 %) |
| Smoking | 74 (69.2 %) |
| Family history of cardiovascular disease | 18 (16.8 %) |
| Diabetes | 2 (1.9 %) |
| Hypertension | 5 (4.7 %) |
| Hypercholesterolemia | 5 (4.7 %) |
| Other drugs | 26 (24.3 %) |
| No Risk factors | |
| Self-reported Cocaine use – N (%) | 100 (93.5 %) |
| Time between last use and presentation ED/chest pain unit – N (%) | |
| 0–3 h | |
| 4–6 h | 46 (42.9 %) |
| 7–24 h | 17 (15.9 %) |
| >24 h | 26 (24.4 %) |
| Unknown | 16 (14.8 %) |
| 2 (1.9 %) | |
| Route of ingestion – N (%) | |
| Nasal | 68 (63.6 %) |
| Smoking | 3 (2.8 %) |
| Intravenous | 0 (0.0 %) |
| Unknown | 36 (33.6 %) |
| ECG – N (%) | |
| Normal | 81 (75.7 %) |
| Abnormal but not ischaemic | 23 (21.5 %) |
| Ischaemic changes | 3 (2.8 %) |
| Vitals at presentation – N (%) | |
| Systolic blood pressure | |
| <90 mmHG | 1 (0.9 %) |
| 90 – 139 mmHG | 70 (65.4 %) |
| >140 mmHG | 36 (33.6 %) |
| Diastolic blood pressure | |
| <90 mmHG | 69 (64.5 %) |
| >90 mmHG | 38 (35.5 %) |
| Temperature | |
| <36 C | 6 (5.6 %) |
| 36–37.9 C | 81 (75.7 %) |
| >38 C | 5 (4.7 %) |
| Unknown | 15 (14.0 %) |
| Heart rate | |
| <60/min | 3 (2.8 %) |
| 60–100/min | 71 (66.4 %) |
| >100/min | 33 9(0.8 %) |
| Respiratory rate | |
| <12/min | 10 (9.3 %) |
| 12–20/min | 79 (73.8 %) |
| >20/min | 18 (16.8 %) |
| Saturation | |
| <90 % | 3 (2.8 %) |
| 90–94 % | 4 (3.7 %) |
| >95 % | 100 (93.5 %) |
| Troponin – N (%) | |
| 0 h troponin | |
| <5 ng/L | 43 (40.2 %) |
| 5–14 ng/L | 59 (55.1 %) |
| >14 ng/L | 5 (4.7 %) |
| 1 h troponin | |
| <5 ng/L | 24 (22.4 %) |
| 5–14 ng/L | 46 (43.0 %) |
| >14 ng/L | 2 (1.9 %) |
| not measured | 35 (32.7 %) |
| 3 h troponin | |
| <5 ng/L | 15 (14.0 %) |
| 5–14 ng/L | 42 (39.3 %) |
| >14 ng/L | 5 (4.7 %) |
| not measured | 45 (42.1 %) |
| Urine toxicology screen – N (%) | |
| Cocaine | 107 (100 %) |
| Cannabis | 37 (34.6 %) |
| Amphetamine | 11 (10.3 %) |
| Methamphetamine/Ecstasy | 13 (12.1 %) |
| Benzodiazepines | 21 (19.6 %) |
| Opiates | 2 (1.9 %) |
| Methadone | 0 (0.0 %) |
| Phencyclidine | 0 (0.0 %) |
| Barbiturates | 0 (0.0 %) |
| GRACE score – N (%) | |
| Low risk (0−108) | 97 (90.7 %) |
| Intermediate risk (109−139) | 0 (0.0 %) |
| High risk (>140) | 0 (0.0 %) |
| Unknown | 10 (9.3 %) |
| HEART score – N (%) | |
| Low risk (0−3) | 103 (96.3 %) |
| Intermediate risk (3−6) | 3 (2.8 %) |
| High risk (7−10) | 0 (0.0 %) |
| Unknown | 1 (0.9 %) |
Management encompassed benzodiazepines (46 %), aspirin (21 %), and a sole patient received a beta-blocker (Table 2). One patient with cocaine-associated chest pain was admitted for a different diagnosis, a diabetic ketoacidosis, marking a singular instance of hospitalization unrelated to the chest pain and was therefore included in the study. A total of 26 patients (24 %) were scheduled for follow-up appointments.
Table 2.
Treatment given at the emergency department/chest pain unit and discharge policy.
| Treatment | N (%) |
|---|---|
| Aspirin | 22 (20.6 %) |
| Benzodiazepine | 49 (45.8 %) |
| Beta-Blocker | 1 (0.9 %) |
| Follow-up appointment | 26 (24.3 %) |
| General practitioner | 9 (8.4 %) |
| Cardiologist | 12 (11.2 %) |
| Another medical specialist | 5 (4.7 %) |
| Discharge medication | 13 (12.1 %) |
| Calcium-antagonist | 1 (0.9 %) |
| Benzodiazepine | 3 (2.8 %) |
| Anti-reflux medication | 4 (3.7 %) |
| Nitro-glycerine | 3 (2.8 %) |
| Aspirin | 2 (1.9 %) |
| Beta-blocker | 1 (0.9 %) |
During the four-week follow-up period (Table 3) post-discharge cocaine use persisted among 32 % of patients whom we were able to contact directly (n = 76). Recurrent chest pain was experienced in 29 % of patients, drawn from the subset of 76 individuals for whom data was attainable. Among 97 patients where follow-up regarding all aspects of MACE was available, no MACE was reported. For 107 patients mortality data was available and no instances of mortality from any cause occurred.
Table 3.
Results of follow-up four weeks after presentation.
| Follow-up results | Patients/total patients (%) |
|---|---|
| Recurrent cocaine use | 24/76 (31.6 %) |
| Recurrent chest pain without presentation to specialist | 22/76 (28.9 %) |
| Recurrent chest pain with presentation to a physician | 17/97 (17.5 %) |
| Re-admittance to hospital | 0/95 (0 %) |
| Non-fatal ACS | 0/97 (0 %) |
| MACE | 0/97 (0 %) |
| Death | 0/107 (0 %) |
4. Discussion
The objective of this study was to assess the safety of applying the European Society of Cardiology NSTEMI guidelines for low-risk cocaine-associated chest pain patients. Within the scope of this study, no patients reported a MACE within four weeks of ruling out ACS.
Cocaine is a risk factor for ACS because it increases myocardial oxygen demand due to its sympathomimetic effects. [9] Simultaneously, cocaine decreases the oxygen supply due to coronary vasoconstriction, induced by alpha-adrenergic stimulation, it has prothrombotic effects and it is associated with premature atherosclerosis when taken chronically. [9] Additionally, the cardiovascular effects increase in combination with tobacco use, which is also by far the most common cardiovascular risk factor in this study population. [10] Beside the higher risk of developing ACS in CACP, the history of presenting symptoms and ECG findings have less predictive value, compared to non-cocaine-associated chest pain patients. [11] These factors make it more challenging to rule out ACS in this specific patient population. Nevertheless, the prevalence of ACS in patients presenting with CACP is 4.7 %, considerably lower compared to the prevalence of ACS in the general population presenting with regular chest pain (10–20 %). [12]
Ruling out ACS in CACP patients with troponin measurement is only investigated in a few studies, but seems to be an accurate and safe predictor. [13], [14], [15], [16] Firstly, Kushman et al. (2000) retrospectively analysed 197 low-risk CACP patients who underwent observation with creatine kinase-myocardial band measurements at 0, 3, 6, and 9 hours and later cardiac troponin I at 0, 3, and 6 hours after presentation. [15] Of the 87 % discharged patients, one patient (1 %) had a MACE within three weeks and died due to cardiogenic shock. [15] Secondly, Weber et al. (2003) prospectively categorized patients with CACP into high-risk and non-high-risk groups. [16] Non-high risk patients (n=302) were enrolled in a 12-hour observation protocol, during which patients underwent measurement of cardiac biomarkers, at 0, 3, 6 and 9 hours after presentation. [16] None of these 302 patients developed ACS. [16] At 30 days follow up, four patients (1.3 %) had developed nonfatal myocardial infarction, all after continuing their cocaine use. [16] Thirdly, Cunningham et al. (2009) conducted a prospective cohort study that followed 219 low-intermediate risk CACP patients where ACS was excluded with negative troponin I at 0, and 9 hours after presentation. [13] None of the included patients had an acute myocardial infarction within one year after discharge. [13] Lastly, a more recent study by Guirgis et al. (2014) advised an eight-hour discharge protocol which consists of serial cardiac biomarker testing, including troponin T, at 0, 2, 4 and 8 hours after presentation. [14] In this study, which involved 101 patients, neither ACS nor fatalities were reported within the subsequent 30-day follow-up duration. [14] These last three studies show that none of the observed patients developed ACS, which is concordant with the results of this study, suggesting that a prolonged observation period is not necessary for CACP patients.
The advice of a nine to twelve hour observation period for low-risk CACP patients given by the scientific statement of the American Heart Association is based on out-dated studies, not using high sensitivity troponin-T. [4], [15], [16], [17] The availability of high-sensitivity troponin-T could lead to shorter observation periods. [13], [14] The present study is the first to investigate the utilization of the European Society of Cardiology guidelines with an observation period as short as two hours in the 0/1 hour protocol for the CACP patients. [8] Applying this guideline shortens discharge times, which is more time and cost effective. [18] A recent Dutch survey, among emergency physicians and cardiologists, underscored that these patients are already being managed in alignment with the European society of cardiology NSTEMI guideline in the Netherlands. [19] The data of this study suggests that this approach is safe.
Additionally, this study showed that the self-reported cocaine use history, has a high reliability in the Dutch population when being explicitly questioned for cocaine use. This is in accordance with a previous retrospective Dutch study [20], but much higher compared to other studies reporting a 50–82 % reliability [16], [21], [22], [23]. This might be explained by the thorough questioning about cocaine use due to the ongoing study.
Regarding treatment, this study and the previous survey [20] show that it is not common Dutch practice to follow the advice stated in the scientific statement of the American Heart Association [4] to administer benzodiazepines and aspirin to CACP patients, since benzodiazepines were only administered in 46 % of patients and aspirin only in 21 % of patients. This is remarkable since the sympathomimetic effect could be well treated with benzodiazepines and the cocaine induced prothrombotic effect with aspirin. This could be due to the absence of advice regarding CACP in the European Society of Cardiology guidelines, and therefore this could be added to future guidelines. [8] Since 32 % of CACP patients persist on using cocaine after discharge, drug counselling is considered very important and could also be added to future guidelines. Recently, a new European Society of Cardiology NSTEMI guideline was published in which specific recommendations regarding CACP were once again not mentioned. [24] Even though this guideline was published after termination of this study, the conclusions and recommendations of this study are still applicable to this new guideline. [24]
It is imperative to acknowledge several limitations inherent to this study. A paramount limitation is the failure to attain the initially intended sample size of 300 patients, culminating in an early termination of the study, despite the seven-year study duration. An expected 3300 annual chest pain patients of whom 12 % would present with CACP in the selected age group suggested it would be feasible to reach the intended sample size. However, owing to factors such as the COVID-19 pandemic's impact, and a notable lack of enthusiasm among this particular patient group to participate in the study, the study was terminated prematurely before achieving the intended sample size. Due to the transition from the 0/3 h algorithm to the 0/1 h algorithm, some patients have missing high-sensitivity troponin values at 1 h or 3 h. This may have led to allocation to a different risk assessment group, although this is deemed unlikely since both algorithms were validated by the European Society of Cardiology. Another limitation is that the number of patients excluded, due to classification as cardiac high risk according to the European Society of Cardiology NSTEMI guideline [8], diagnosed with ACS, admitted despite a low-intermediate risk stratification, unwilling to participate or a missed inclusion, were not recorded. Also, the follow-up within this distinct patient population posed a noteworthy challenge, resulting in a higher-than-expected loss to follow-up rate. If follow-up was not feasible, despite at least five attempts to reach out, the hospital electronic patient database was searched for recurrent hospital visits. If these had not occurred, an exploration of the national mortality database was conducted for the Dutch residents. Foreigners were at that point lost to follow up, and since a considerable portion of the included CACP patients were foreign tourists, this explains the 17 % lost to follow up. This may have resulted in an underestimation of the patients without MACE, since it was not established that these patients did not develop MACE. Therefore, the results from this study need to be interpreted cautiously and further research is necessary to proof that following the European Society of Cardiology NSTEMI guideline for ruling out ACS in low-risk CACP patients is safe. [8]
5. Conclusion
This study investigated the safety of applying the European Society of Cardiology guidelines for ruling out acute coronary syndrome in low-risk cocaine-associated chest pain patients. The results of this study suggest that this is indeed safe, since no major adverse cardiac events occurred among patients discharged within four hours after presentation. Nevertheless, more research is necessary to confirm this conclusion, since this study did not attain the intended sample size. As such, caution is required for drawing conclusions based on this data.
6. Lessons learned
-
•
The European Society of Cardiology guidelines for ruling out acute coronary syndrome in low-risk cocaine-associated chest pain patients appears to be safe
-
•
The reliability of self-reported cocaine use history upon cocaine-associated chest pain patients is high in the Dutch population
-
•
Many patients continue cocaine use after hospital assessment for cocaine-associated chest pain, suggesting drug counselling is important
-
•
Follow-up has proven to be challenging within this patient population (patients with cocaine-associated chest pain), partly because of the high rate of tourists in this particular population.
Author statement
FG, EF, and RR conceived the study and designed the trial. FG, LH, DL and RR, supervised the trial. All contributors performed data collection and JE performed statistical analyses. FG, LH and JE drafted the manuscript, and all authors contributed substantially to its revision. FG takes responsibility for the paper as a whole.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank all the physicians and nurses from the participating hospitals of OLVG, Diakonessenhuis and Haaglanden Medical Centre for their contribution in recruiting patients for the study. Also, we would like to acknowledge Nanda Gubbels, emergency physician, for her work on developing the study protocol. We thank the 'Stichting Wetenschap', OLVG hospital, Amsterdam, the Netherlands (WO15.110) for partly funding this study.
Handling Editor: Prof. L.H. Lash
Data Availability
Data will be made available on request.
References
- 1.European Monitoring Centre for Drugs and Drug Addiction (2023), European Drug Report 2023: Trends and Developments, 〈https://www.emcdda.europa.eu/publications/european-drug-report/2023_en〉 2023.
- 2.Trimbos-Instituut. Gebruik: algemene bevolking. Nationale Drug Monitor. 2021 [cited 2023 Aug 3]. Available from: 〈http://www.nationaledrugmonitor.nl/cocaine-gebruik-algemene-bevolking/〉.
- 3.Substance Abuse and Mental Health Services Administration. Preliminary Findings from Drug-Related Emergency Department Visits, 2021; Drug Abuse Warning Network (HHS Publication No. PEP22-07-03-001). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2022. retrieved from: 〈https://www.samhsa.gov/data/〉.
- 4.McCord J., Jneid H., Hollander J.E., de Lemos J.A., Cercek B., Hsue P., et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117(14):1897–1907. doi: 10.1161/CIRCULATIONAHA.107.188950. [DOI] [PubMed] [Google Scholar]
- 5.Wang J., Patel P.S., Andhavarapu S., Bzihlyanskaya V., Friedman E., Jeyaraju M., et al. Prevalence of myocardial infarction among patients with chest pain and cocaine use: a systematic review and meta-analysis. Am. J. Emerg. Med. 2021;50:428–436. doi: 10.1016/j.ajem.2021.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Hollander J.E., Hoffman R.S. Cocaine-induced myocardial infarction: an analysis and review of the literature. J. Emerg. Med. 1992;10(2):169–177. doi: 10.1016/0736-4679(92)90212-c. [DOI] [PubMed] [Google Scholar]
- 7.Mittleman M.A., Mintzer D., Maclure M., Tofler G.H., Sherwood J.B., Muller J.E. Triggering of myocardial infarction by cocaine. Circulation. 1999;99(21):2737–2741. doi: 10.1161/01.cir.99.21.2737. [DOI] [PubMed] [Google Scholar]
- 8.Collet J.P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 9.Lucyk S.N. Acute cardiovascular toxicity of cocaine. Can. J. Cardiol. 2022;38(9):1384–1394. doi: 10.1016/j.cjca.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Moliterno D.J., Willard J.E., Lange R.A., Negus B.H., Boehrer J.D., Glamann D.B., et al. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N. Engl. J. Med. 1994;330(7):454–459. doi: 10.1056/NEJM199402173300702. [DOI] [PubMed] [Google Scholar]
- 11.Finkel J.B., Marhefka G.D. Rethinking cocaine-associated chest pain and acute coronary syndromes. Mayo Clin. Proc. 2011;86(12):1198–1207. doi: 10.4065/mcp.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang A.M., Fischman D.L., Hollander J.E. Evaluation of chest pain and acute coronary syndromes. Cardiol. Clin. 2018;36(1):1–12. doi: 10.1016/j.ccl.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham R., Walton M.A., Weber J.E., O'Broin S., Tripathi S.P., Maio R.F., et al. One-year medical outcomes and emergency department recidivism after emergency department observation for cocaine-associated chest pain. Ann. Emerg. Med. 2009;53(3):310–320. doi: 10.1016/j.annemergmed.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guirgis F.W., Gray-Eurom K., Mayfield T.L., Imbt D.M., Kalynych C.J., Kraemer D.F., et al. Impact of an abbreviated cardiac enzyme protocol to aid rapid discharge of patients with cocaine-associated chest pain in the clinical decision unit. West. J. Emerg. Med. 2014;15(2):180–183. doi: 10.5811/westjem.2013.11.19232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushman S.O., Storrow A.B., Liu T., Gibler W.B. Cocaine-associated chest pain in a chest pain center. Am. J. Cardiol. 2000;85(3):394–396. doi: 10.1016/s0002-9149(99)00755-9. a10. [DOI] [PubMed] [Google Scholar]
- 16.Weber J.E., Shofer F.S., Larkin G.L., Kalaria A.S., Hollander J.E. Validation of a brief observation period for patients with cocaine-associated chest pain. New Engl. J. Med. 2003;348(6):510–517. doi: 10.1056/NEJMoa022206. [DOI] [PubMed] [Google Scholar]
- 17.Hollander J.E., Hoffman R.S., Burstein J.L., Shih R.D., Thode H.C., Jr. Cocaine-associated myocardial infarction. Mortality and complications. Cocaine-Associated Myocardial Infarction Study Group. Arch. Intern Med. 1995;155(10):1081–1086. [PubMed] [Google Scholar]
- 18.Ljung L., Lindahl B., Eggers K.M., Frick M., Linder R., Löfmark H.B., et al. A rule-out strategy based on high-sensitivity troponin and HEART score reduces hospital admissions. Ann. Emerg. Med. 2019;73(5):491–499. doi: 10.1016/j.annemergmed.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Gresnigt F.M.J., Gubbels N.P., Riezebos R.K. The current practice for cocaine-associated chest pain in the Netherlands. Toxicol. Rep. 2021;8:23–27. doi: 10.1016/j.toxrep.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gresnigt F., Janszen A., Franssen E.J.F., Lange D., Riezebos R.K. Reliability of self-reported recreational drug use in young chest pain patients, a retrospective study. Eur. J. Emerg. Med. 2022;29(4):307–308. doi: 10.1097/MEJ.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 21.Lee M.O., Vivier P.M., Diercks D.B. Is the self-report of recent cocaine or methamphetamine use reliable in illicit stimulant drug users who present to the Emergency Department with chest pain? J. Emerg. Med. 2009;37(2):237–241. doi: 10.1016/j.jemermed.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Liakoni E., Yates C., Dines A.M., Dargan P.I., Heyerdahl F., Hovda K.E., et al. Acute recreational drug toxicity: comparison of self-reports and results of immunoassay and additional analytical methods in a multicenter European case series. Med. (Baltim. ) 2018;97(5) doi: 10.1097/MD.0000000000009784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monte A.A., Heard K.J., Hoppe J.A., Vasiliou V., Gonzalez F.J. The accuracy of self-reported drug ingestion histories in emergency department patients. J. Clin. Pharm. 2015;55(1):33–38. doi: 10.1002/jcph.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrne R.A., Rossello X., Coughlan J.J., Barbato E., Berry C., Chieffo A., et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023;44(38):3720–3826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.