Abstract
A cDNA encoding the murine homolog of human nectin-1α (also known as poliovirus receptor-related protein 1 [Prr1] and herpesvirus entry protein C [HveC]) was isolated. The protein encoded by this cDNA proved to be 95% identical in sequence to the human protein and to have similar herpesvirus entry activity. Upon expression of the murine cDNA in hamster cells resistant to alphaherpesvirus entry, the cells became susceptible to the entry of herpes simplex virus types 1 and 2 (HSV-1 and -2), pseudorabies virus, and bovine herpesvirus 1. HSV envelope glycoprotein D (gD), a viral ligand for human nectin-1α, is also a ligand for the murine homolog based on evidence that (i) a soluble hybrid protein composed in part of the murine nectin-1 ectodomain bound specifically to purified soluble forms of HSV-1 and HSV-2 gD as demonstrated by enzyme-linked immunosorbent assay, (ii) a soluble hybrid of HSV-1 gD bound to hamster cells expressing murine nectin-1α but not to control cells, and (iii) cells expressing both murine nectin-1α and one of the alphaherpesvirus gDs were resistant to entry of HSV-1, indicative of interference with entry resulting from interactions of cell-associated gD with the entry receptor. Northern blot analysis revealed that nectin-1 is expressed in most of the mouse tissues examined and at high levels in the brain, skin, and kidneys. Immunocytochemical localization demonstrated the presence of nectin-1 in epithelial cells of the mouse vagina and also in neuronal cells of the central nervous system, suggesting an expression pattern relevant to both infection at a portal of entry and spread of infection to the brain.
Members of the alphaherpesvirus subfamily exemplified by herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), bovine herpesvirus 1 (BHV-1) and porcine pseudorabies virus (PRV) have a broad host range in cultured cells. They can also infect animal species other than the natural host and cause symptoms similar to those observed in their natural hosts. For these and other reasons, the mouse has been extensively used as an animal model to study various aspects of alphaherpesvirus pathogenesis including pathology, immune responses, latency, and transneuronal spread of infection. Consequently, there is considerable interest in defining the cell receptors for entry of HSV and other alphaherpesviruses into mouse cells as well as into cells of the natural hosts. The studies described here are part of a larger effort to determine whether evolutionarily related or distinct receptors are used by HSV for entry into mouse and human cells. If related receptors are used and these related receptors have similar expression patterns and functions in mice and humans, this strengthens the case for using mouse models of disease to dissect aspects of pathogenesis that depend on the susceptibility of cells to viral entry.
Human and animal representatives of the alphaherpesvirus subfamily exhibit common requirements for entry into cells (26, 34). The initial interaction of virus with cells involves binding of the virion glycoprotein gC, and in some cases gB, to cell surface glycosaminoglycans, preferentially heparan sulfate. Four envelope glycoproteins, gB, gD, gH, and gL, are required to mediate the fusion between the virion envelope and cell membrane that allows viral penetration. It is believed that interaction between gD and one of its cognate cell surface receptors triggers the process of fusion.
Recently four human cell surface proteins have been identified as gD receptors that mediate the entry into cells of one or more of the alphaherpesviruses (13, 29, 37). One of these proteins is a newly described member of the tumor necrosis factor receptor family, originally designated the herpesvirus entry mediator (HVEM) (29), later designated herpesvirus entry protein A (HveA) (13, 37), and officially named TNFRSF14. The other three proteins are related members of the immunoglobulin (Ig) superfamily, a subfamily including the poliovirus receptor (CD155) (24) and two proteins that are not receptors for poliovirus but were originally designated poliovirus receptor-related protein 1 (Prr1) (22) and poliovirus receptor-related protein 2 (Prr2) (10). CD155 mediates the entry of PRV and BHV-1 but has no activity for HSV strains (13). Prr2 was identified as an entry receptor for strains of HSV-1 that could not use HVEM for entry, was also found to be an entry receptor for HSV-2 and PRV, and was designated HveB (37). Prr1 was found to serve as an entry receptor for HSV-1, HSV-2, PRV, and BHV-1 and was designated HveC (13). Recently both HveB and HveC were shown to be homophilic cell adhesion molecules that localize to sites of cadherin-based cell junctions (20, 35). Based on emerging information about their functions, they were renamed nectin-2 (HveB) and nectin-1 (HveC) (30, 35). In this paper the nectin designations will be used. A newly discovered HSV-1 entry receptor is generated in heparan sulfate by the action of specific glucosaminyl 3-O-sulfotransferases. In the absence of other entry receptors described above, resistant cells can be made susceptible to HSV-1 entry but not to HSV-2, PRV, or BHV-1 entry, by expression of 3-O-sulfotransferase 3, provided that cell surface heparan sulfate is present (31).
Genes encoding CD155 and the nectins are expressed as multiple protein isoforms due to differential mRNA splicing, which yields open reading frames encoding common ectodomains fused to different membrane-spanning regions and cytoplasmic tails or to C-terminal domains lacking a membrane span (6, 10, 18, 22, 24). Two membrane-bound isoforms of human nectin-1 (α and β) and two of human nectin-2 (α and δ) have been described, and all four forms have alphaherpesvirus entry activity, with the specificity for different viruses being determined solely by the ectodomain (6, 13, 21, 37).
Evidence that gD is the ligand for these cell surface receptors comes from findings that (i) mutations in gD can influence receptor usage (29, 37); (ii) recombinant soluble forms of HSV gD, but not gB, gC, or gH-gL, bind to purified soluble forms of HVEM, nectin-1, and 3-O-sulfated heparan sulfate in vitro (5, 19, 31, 39); (iii) soluble hybrid proteins composed of the gD ectodomain fused to the rabbit IgG Fc domain (gD:Fc) bind specifically to cells expressing the appropriate receptor (12, 31); (iv) coexpression of an alphaherpesvirus gD with any of its cognate entry receptors results in interference with entry activity due to interactions of cell-associated gD with the receptor (12, 31, 32); and (v) antibodies specific for HSV gD but not other envelope glycoproteins can block the binding of soluble receptors to HSV-1 virions (19, 38).
We have previously shown that the murine homolog of nectin-2α resembles its human counterpart in mediating the entry of PRV but not of BHV-1, but differs in that the murine form fails to mediate the entry of any HSV strains tested (32). Here we describe the cDNA cloning of murine nectin-1α from FVB/N mice. The murine and human forms of nectin-1α were found to be highly conserved, both in predicted amino acid sequence and in their alphaherpesvirus entry-mediating activities and ability to bind gD. The murine nectin-1α, like its human homolog, mediated the entry of HSV-1 (including various strains that differ in ability to use HVEM or nectin-2 for entry), HSV-2, PRV, and BHV-1. It was shown to bind specifically to soluble forms of HSV gDs and to exhibit sensitivity to interference mediated by several of the alphaherpesvirus gDs. Northern blot analysis revealed expression of murine nectin-1 in many tissues, especially in the brain and skin. Nectin-1 was readily detectable by immunocytochemistry in epithelial cells of the vaginal mucosa and in neurons of the central nervous system, implicating nectin-1 as a prime candidate for a receptor that could mediate the entry of HSV into cells of mucosal surfaces and spread of infection to neurons.
While this paper was being written, a report was published on a different allelic form of murine nectin-1α obtained from the C3H strain of mice (25). The isoform of nectin-1 described by these authors was the same as that described here, although it was designated nectin-1δ for unexplained reasons. We have named the molecule described herein nectin-1α, based on a prior designation of the human homolog of this isoform (35) and because it is more closely related to nectin-2α than to nectin-2δ. While alphaherpesvirus entry activities were described for the C3H allele of nectin-1α, attempts to detect interaction with HSV gD failed, leading the authors to speculate that the murine form of nectin-1α might have a different viral ligand from the human form (25). We report here that both the C3H and FVB/N forms of nectin-1α can bind to HSV gD.
(We presented a preliminary report of the isolation and properties of murine nectin-1α at the International Herpesvirus Workshop in 1999 [D. Shukla and P. G. Spear, Molecular cloning of murine HveC: homologous proteins mediate entry of alphaherpesviruses into mouse and human cells, abstr. 5.003].)
MATERIALS AND METHODS
Cells and viruses.
Wild-type CHO-K1 cells (ATCC CCL-61) were obtained from J. Esko (University of California, San Diego, Calif.). The CHO-IEβ8 cell line is a stable CHO-K1 transfectant that maintains a plasmid with the Escherichia coli lacZ gene under control of the HSV-1 ICP-4 promoter; expression of β-galactosidase is not constitutive but is inducible by the tegument transactivator VP16 (29). The CHO cells were passaged in Ham's F12 medium supplemented with 10% fetal bovine serum. Viruses used included HSV-1 strains KOS, mp, HFEM, SC16, Patton, 17, and Ang; HSV-2 strains G and 333; and the HSV-1(KOS) mutant Rid1, selected for resistance to interference mediated by wild-type HSV-1 gD (9). Recombinant viruses expressing β-galactosidase from the viral genome included HSV-1(KOS)tk12 (37), HSV-1(KOS)Rid1/tk12 (37), gH-negative PRV(Kaplan) (provided by T. Mettenleiter, Federal Research Center for Viral Diseases, Insel Riems, Germany), and BHV-1(Cooper)v4a (provided by L. Bello, University of Pennsylvania). The PRV recombinant expresses β-galactosidase under control of the PRV gG promoter from an insert within the gH gene and was propagated and subjected to titer determination on complementing gH-expressing VeroSW78 cells (17). BHV-1(Cooper)v4a, which expresses β-galactosidase under control of the BHV-1 gB promoter from an insert within the viral thymidine kinase gene, was propagated and subjected to titer determination on MDBK cells (27). Except as noted otherwise, virus strains were propagated in HEp-2 cells or Vero cells and subjected to titer determination on Vero cells.
Antibodies and other reagents.
The rabbit antisera raised against human nectin-1 (R165 and R166) and prepared as described previously (19), anti-gD monoclonal antibodies (MAbs) DL6 and DL11 (7, 11), affinity-purified gD-1(306t) and gD-2(306t) (33), and the HSV-1(Patton) gD-expressing plasmid pRE4 (8) were provided by G. Cohen and R. Eisenberg (University of Pennsylvania). The mature forms of HSV-1 gD encoded by HSV-1(Patton) and HSV-1(KOS) are identical. The PRV gD plasmid pCMV-gD (14) was obtained from V. Gerdts and T. Mettenleiter (Federal Research Center for Virus Diseases of Animals, Insel Riems, Germany). The Rid1 gD-expressing plasmid pMW13 was constructed by replacing the appropriate AflII-AccI fragment of the HSV-1(Patton) gD gene in pRE4 with the equivalent fragment of the HSV-1(KOS)Rid1 form of gD present in pHD32 (9). Anti-Myc MAb was obtained from Invitrogen (Carlsbad, Calif.). Bovine serum albumin (BSA) was purchased from Sigma (St. Louis, Mo.). The hybrid proteins gD:Fc and mouse nectin-1:Fc were produced in Peak cells (modified 293 cells) transfected with plasmids pBG64 (gD:Fc) and pCR11 (nectin-1:Fc) and purified from the medium (Dulbecco's minimal essential medium supplemented with 3% fetal bovine serum depleted of IgG) by protein A/G-Sepharose chromatography. The gD:Fc hybrid consists of the entire ectodomain of HSV-1(KOS) gD fused to the Fc region of rabbit IgG and was previously described (12). The nectin-1:Fc hybrid consists of 335 amino acids from the N terminus of the protein encoded by the mouse nectin-1 (FVB/N allele) open reading frame, encompassing almost the entire extracellular domain, fused to the hinge, CH2, and CH3 regions of the rabbit IgG heavy chain. Both hybrid proteins were expressed from inserts cloned into pcDNA3.
Isolation of murine nectin-1α cDNA.
A Rapid Screen day 19 fetal mouse cDNA expression library, prepared from the FVB/N inbred mouse strain (36), was purchased from Origene Technologies, Inc. (Rockville, Md.). Pools and subpools of this library were screened by PCR as recommended by the manufacturer until a single plasmid was identified. The forward PCR primer matched a portion of the sequence of a mouse expressed sequence tag (dbEST no. g1917529): ACTCGCTCTCGGCTTGA. The reverse degenerate primer was based on the consensus sequence (10) for a conserved region found in coding regions for members of the poliovirus receptor-related proteins: AGGTACCAGTTG(T/C)(T/C)ATCA. The plasmid isolated on the basis of homology to these primers was designated pDS101, and the insert was sequenced by the University of Chicago Cancer Center and the Northwestern University Biotechnology sequencing facilities using ABI Prism 377 automated DNA sequencers. Three sets of nucleotide substitutions were introduced into the coding region for the FVB/N allele of nectin-1α in plasmid pDS101 to yield plasmid pDS117, which expresses the C3H allele of nectin-1α. The mutagenesis was performed by using the QuickChange site-directed mutagenesis kit from Stratagene (La Jolla, Calif.). Six oligonucleotide primers (three sense and three antisense, one pair for each mutation) containing the required mutations were designed, and primer extensions were performed using the reagents and the protocol provided by the manufacturer (Stratagene). The entire nectin-1α ORF was sequenced to confirm the nucleotide and amino acid substitutions (see Fig. 1).
FIG. 1.
Amino acid sequence of murine nectin-1α in comparison with that of human nectin-1α. Predicted features of these proteins, which are 95% identical in amino acid sequence, include a signal sequence (dotted underline), a membrane-spanning domain (solid underline) and potential sites for addition of N-linked glycans (boxed residues). The solid diamonds mark the conserved cysteine residues, and the solid circles identify the sequence motif required for binding to the PDZ domain in afadin. The sequence presented here is for murine nectin-1α from FVB/N mice. Amino acid substitutions and a gap in sequence reported for nectin-1α from C3H mice (25) are shown above the FVB/N sequence. The sequence given for human nectin-1α is that reported by Geraghty et al. (13), which differs somewhat from that reported by Lopez et al. (22).
Entry assays.
Entry assays were based on quantitation of β-galactosidase expressed from the viral genome or by CHO-IEβ8 cells in which β-galactosidase expression is inducible by HSV infection (29). CHO-K1 cells were transiently transfected in six-well dishes, using Lipofectamine (Gibco-BRL) with the nectin-1α-expressing plasmids (pDS101 or pDS117) or control plasmid (pcDNA3). At 24 h after transfection and at least 16 h prior to infection, the cells were replated in 96-well tissue culture dishes (2 × 104 to 4 × 104 cells/well). The cells were washed and exposed for 6 h at 37°C to virus in 50 μl of phosphate-buffered saline (PBS) containing glucose and 1% calf serum (PBS-G-CS) before being solubilized in 100 μl of PBS containing 0.5% NP-40 and the β-galactosidase substrate o-nitrophenyl-β-d-glucopyranoside (ONPG) (3 mg/ml). The enzymatic activity was monitored by spectrometry at several time points after the addition of ONPG to define the interval over which the generation of product was linear with time (Dynatech enzyme-linked immunosorbent assay [ELISA] reader or Spectromax 250 ELISA reader).
ELISA.
Purified soluble gD(306t) from HSV-1(KOS), gD(306t) from HSV-2(333), or BSA in PBS (100 ng/well) was bound to microtiter plates overnight at 4°C. The plates were washed with 0.1% Tween 20 in PBS (PBS-Tween) and incubated in PBS plus 5% milk and 0.2% Tween 20 (blocking solution) for 30 min at room temperature (RT). The plates were washed with PBS-Tween, incubated with various concentrations of purified soluble murine nectin-1:Fc in blocking solution for 2 h at RT, washed again with PBS-Tween, incubated in blocking solution for 30 min at RT, and finally incubated for 30 min at RT with horseradish peroxidase-conjugated anti-rabbit Fc antibody diluted 1:1,000 in blocking solution. Peroxidase activity was quantitated using 3,3′,5,5′-tetramethyl benzidine (Sigma, St. Louis, Mo.) in 50 mM phosphate-citrate buffer as the substrate, and the product was detected at 370 nm using a Spectromax 250 ELISA reader (or at 410 nm after adding stop solution).
Detection of gD receptors on cells.
CHO-K1 cells were transiently transfected with either pDS101, pDS117, or a control plasmid and plated in 96-well plates as described above. After blocking for 30 min with PBS containing 3% BSA, the cells were incubated with purified gD:Fc or nonimmune rabbit IgG (1 μg/ml) in 1% BSA–PBS for 45 min at room temperature. The cells were then washed and fixed with 2% formaldehyde–0.2% glutaraldehyde in PBS. They were then incubated sequentially with biotin-conjugated goat anti-rabbit IgG antibody (Sigma) and Amdex streptavidin-horseradish peroxidase (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). The peroxidase activity was quantitated as described above.
gD interference assay.
CHO-K1 cells were cotransfected, using Lipofectamine (Gibco-BRL), with a nectin-1α expression plasmid (pDS101 or pDS117) and gD expression plasmids (pRE4 for HSV-1 wild-type gD, pMW13 for HSV-1 mutant Rid1 gD, and pCMV-gD for PRV gD) or control plasmid (pcDNA3), in a 1:4 (nectin-1α to gD or control) ratio. After 24 h, the transfected cells were replated in 96-well plates and, 24 h later, exposed to various doses of β-galactosidase-expressing HSV-1(KOS). At 6 h later, the cells were rinsed and solubilized and β-galactosidase activity was quantitated as described above (see “Entry assays”).
Northern blot analysis.
A mouse (Swiss Webster) multiple-tissue Northern blot membrane was purchased from Origene Technologies. It contained 2 μg of poly(A)+ RNAs from mouse brain, heart, kidney, lung, testis, and skin. The membranes were prehybridized at 65°C for 1 h in 5 ml of ExpressHyb solution (Clontech). Hybridization was then performed for 18 h at 65°C with a nectin-1 probe at a concentration of 106 cpm/ml in 10 ml of ExpressHyb solution. The probe was the 766-bp internal EcoRI fragment from the nectin-1α insert in pDS101, labeled to a specific activity of approximately 2 × 109 cpm/ml using random primers and [32P]dCTP. After hybridization, the membrane was washed twice for 5 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate at RT and then for 30 min in 0.1× SSC–0.1% sodium dodecyl sulfate at 50°C. The hybridized probe was detected by exposure to X-ray film.
Immunohistochemistry.
Female BALB/c mice, 12 weeks of age and purchased from Jackson Laboratories, were anesthetized with pentobarbital and sacrificed by perfusion first with PBS and then with 4% paraformaldehyde–0.01% glutaraldehyde fixative in PBS, according to an Animal Care and Use Committee-approved protocol. The brains and vaginas were removed, fixed by a standard procedure, and embedded in paraffin. Paraffin sections were deparaffinized, and endogenous peroxidase activity was ablated by incubation in 2% hydrogen peroxide in methanol for 20 min. The sections were heated in an oven to 70°C in 0.1 M citrate buffer (pH 6.0) for 20 min and then blocked with diluted goat serum (1:50) overnight at 4°C. A 1:1 mixture of two rabbit antisera specific for human nectin-1 (R165 and R166), a 1:1 mixture of preimmune sera from the rabbits, and a Vectastain Elite ABC kit (Vector Laboratories) were used for immunohistochemistry. The prepared sections were incubated with preimmune or immune serum at a dilution of 1:50. After 1 h, the sections were washed in PBS and incubated with biotinylated goat anti-rabbit IgG for 30 min. After further washes with PBS, the sections were incubated with an avidin-biotinylated horseradish peroxidase complex for 1 h. The sections were washed in PBS and developed by the addition of Vector VIP substrate for peroxidase (Vector Laboratories) as specified by the manufacturer.
Nucleotide sequence accession number.
The sequence for murine nectin-1α from FVB/N mice has been deposited in the GenBank database under accession no. AF270977.
RESULTS
Sequence of murine nectin-1α.
A plasmid containing an insert with homology to human nectin-1α was isolated from a mouse cDNA library as described in Materials and Methods. Nucleotide sequencing of the insert [1,889 nucleotides devoid of poly(A)] revealed an open reading frame 95% identical in derived amino acid sequence (Fig. 1) to that of human nectin-1α. Both the mouse and human forms are predicted to have cleavable signal peptides of 25 amino acids, ectodomains of 329 amino acids, and membrane-spanning regions of 24 amino acids. The C-terminal tail of the mouse form is predicted to be 138 amino acids compared with 139 amino acids for the human form. The ectodomains of both mouse and human nectin-1α consist of three Ig-like regions, with the one at the N terminus resembling a V domain and the other two resembling C domains. The mouse form of nectin-1α has seven potential sites for the addition of N-linked glycans. All seven of these sites are conserved in the human form, which has an additional potential site. Both the mouse and human forms of nectin-1α have the C-terminal motif (E/A-X-Y-V), which identifies a binding site for proteins with PDZ domains (15). Shown above the sequence line in Fig. 1 are two amino acid substitutions and one single-amino-acid gap observed in the sequence of another form (C3H) of murine nectin-1α, recently reported as the sequence of nectin-1δ (25). The cDNA studied here was isolated from the FVB/N inbred strain of mice.
Alphaherpesvirus entry mediated by murine nectin-1α.
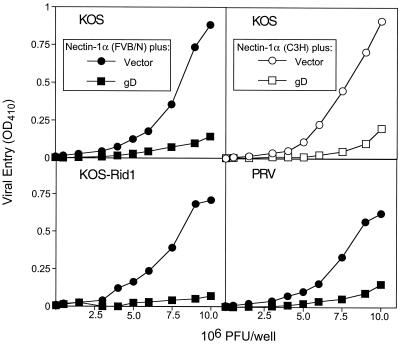
Both CHO-K1 cells and CHO-IEβ8 cells are highly resistant to the entry of HSV-1, PRV, and BHV-1 and are partially resistant to the entry of HSV-2. These cells were transfected with a plasmid expressing mouse nectin-1α (FVB/N allele) or with a control plasmid and then challenged with virus to determine whether nectin-1α conferred susceptibility to alphaherpesvirus entry. In one type of experiment, transfected CHO-IEβ8 cells were exposed to serial dilutions of a variety of HSV-1 and HSV-2 strains. At 6 h after the addition of virus, the cells were solubilized for quantitation of β-galactosidase activity. Expression of this enzyme signals the entry of virus into the cells because expression depends on activation, by VP16 of input virus, of a cell-associated lacZ gene under the control of an immediate-early HSV-1 promoter (29). Figure 2 shows the results obtained at a single input dose of virus within the linear range of the dose-response curve for each virus. It is evident that expression of mouse nectin-1α confers susceptibility to, or enhances, entry of all the virus strains tested. The HSV-1 strains differ in specificity for other entry receptors. For example, strains KOS-Rid1 and Ang can use human nectin-2 but not human HVEM for entry whereas the converse is true for all the other HSV-1 strains shown.
FIG. 2.
Entry of various HSV strains mediated by nectin-1α (FVB/N form). CHO-IEβ8 cells were transfected with nectin-1α-expressing pDS101 (hatched bars) or control vector pcDNA3 (open bars), replated in 96-well plates, and then inoculated with serial dilutions of the viruses indicated. After 6 h, the cells were permeabilized and incubated with ONPG substrate for quantitation of β-galactosidase activity. Entry of virus was signaled by induction of β-galactosidase expression from the lacZ gene in the CHO-IEβ8 cells. The values shown (means of triplicate determinations) represent the amount of reaction product detected spectrophotometrically (optical density at 410 nm [OD410]) at a single input dose of 106 PFU/well in the linear range of the dose-response curve for each virus. In this and other figures, each value shown is the mean of three or more determinations.
In another type of experiment, CHO-K1 cells were transfected with a plasmid expressing mouse nectin-1α (FVB/N allele) or control plasmid and then challenged with various dilutions of viruses carrying the lacZ gene as a reporter of viral entry. Figure 3 shows representative results obtained with β-galactosidase-expressing strains of PRV and BHV-1. The cells expressing nectin-1α were highly susceptible to the entry of both viruses, whereas the cells transfected with control plasmid were as resistant as untransfected cells. Results similar to those shown in Fig. 3 were obtained for β-galactosidase-expressing strains of HSV-1(KOS) and HSV-1(KOS)Rid1.
FIG. 3.
Entry of PRV and BHV-1 mediated by nectin-1α (FVB/N form). CHO-K1 cells were transfected with nectin-1α-expressing plasmid pDS101 or control plasmid pcDNA3, replated in 96-well plates, and then inoculated with serial dilutions of β-galactosidase-expressing PRV or BHV-1. After 6 h, the cells were permeabilized and incubated with ONPG substrate for quantitation of β-galactosidase activity expressed from the input viral genome. The activity was measured as an optical density at 410 nm (OD410) as described in the text.
These results are indistinguishable from those reported previously for human nectin-1α (HveC) (13). Similar results were also obtained with the other known human isoform, nectin-1β, which has the same ectodomain as nectin-1α but differs in the membrane span and cytoplasmic tail (6). Similar qualitative results were also obtained with nectin-1α from the C3H mouse strain (25).
Binding of gD to murine nectin-1α.
Three kinds of experiments were done to determine whether mouse nectin-1α binds to the gDs of HSV and other alphaherpesviruses, as was previously shown for human nectin-1α. First, purified nectin-1 was tested by ELISA for the ability to bind to purified gD. Soluble ectodomains of HSV-1 gD and HSV-2 gD, produced from baculovirus vectors in insect cells, were purified and bound to ELISA plates. Then a soluble hybrid protein, consisting of the mouse nectin-1 ectodomain (FVB/N allele) fused to rabbit Fc and produced in human cells, was incubated with the gD-coated or control BSA-coated wells. Figure 4 shows that nectin-1:Fc bound to the wells coated with gD-1(306t) or gD-2(306t) but not to control wells. The amount of binding detected was dependent on the amount of nectin-1:Fc added.
FIG. 4.
Binding of nectin-1:Fc to gD detected by ELISA. Plates (96 wells) were coated with gD-1(306t) (top) or gD-2(306t) (bottom) or BSA and incubated with the concentrations of nectin-1:Fc indicated. The nectin-1:Fc bound to wells was detected with horseradish peroxidase-conjugated anti-rabbit Fc antibody and substrate. The reaction product was detected spectrophotometrically (optical density at 410 nm [OD410]).
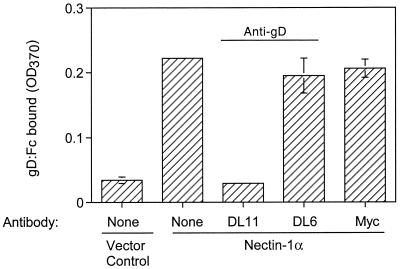
Second, a purified soluble form of HSV-1 gD (gD:Fc) was tested for the ability to bind to cells expressing mouse nectin-1α. The sensitivity of this binding to anti-gD (MAbs) was also tested. CHO-K1 cells were transfected with a plasmid expressing mouse nectin-1α (FVB/N allele) or with control plasmid. The cells were then incubated with gD:Fc, and binding of the hybrid protein was quantitated using a detection system for rabbit IgG. The results presented in Fig. 5 demonstrate that significantly more gD:Fc bound to cells expressing nectin-1α than to control cells and that one of the anti-gD MAbs completely blocked the specific binding whereas the other did not. A control antibody (anti-myc) also did not block the binding. The anti-gD MAb that blocked binding (DL11) was previously shown to block the binding of human nectin-1α to gD in HSV-1 virions, whereas the other antibody (DL6) was unable to inhibit this binding (19, 38). Thus, the mouse and human forms of nectin-1α bind to the same region of gD or to overlapping regions whose accessibility to the receptor is altered by prior binding of the DL11 MAb but not the DL6 MAb.
FIG. 5.
Binding of gD:Fc to CHO cells expressing nectin-1α (FVB/N form). CHO-K1 cells transfected with nectin-1α-expressing plasmid (pDS101) or control plasmid (pcDNA3) were replated in 96-well plates and exposed to purified HSV-1 gD:Fc that had been preincubated with anti-gD MAbs (or control anti-Myc MAb) or buffer alone. After 30 min, the cells were washed, fixed with formaldehyde-glutaraldehyde, and incubated with biotinylated anti-rabbit Fc antibody followed by streptavidin-conjugated horseradish peroxidase. Peroxidase activity was assayed as a measure of gD:Fc binding to the cell surface. The results shown are means of three determinations with standard deviations indicated. OD370, optical density at 370 nm.
Because interaction of gD with the C3H form of nectin-1α could not be detected in a previous study (25), we compared the ability of gD:Fc to bind to the C3H and FVB/N forms of nectin-1α. Mutations were engineered into the plasmid expressing the FVB/N form to generate a new plasmid capable of expressing the C3H form. CHO-K1 cells were transfected with plasmids expressing either the C3H or FVB/N form of mouse nectin-1α or with control plasmid. The cells were then incubated with gD:Fc or nonimmune rabbit IgG as a control, in the presence or absence of an anti-gD antibody, and binding was quantitated using a detection system for the Fc region of rabbit IgG. The results presented in Fig. 6 demonstrate that equivalent amounts of gD:Fc bound specifically to cells expressing either allelic form of nectin-1α and that this binding was blocked by the D11 anti-gD antibody.
FIG. 6.
Binding of gD:Fc to CHO cells expressing either the FVB/N or C3H form of nectin-1α. CHO-K1 cells transfected with plasmids expressing either the FVB/N (pDS101) or the C3H (pDS117) form of nectin-1α or with control plasmid were replated in 96-well plates and exposed to purified HSV-1 gD:Fc or to nonimmune rabbit IgG that had been preincubated with the anti-gD MAb D11 or buffer alone. The cells were then processed and binding was quantitated as described in the legend to Fig. 5.
Third, CHO-K1 cells were cotransfected with nectin-1α and one of several forms of alphaherpesvirus gD to determine whether the various forms of gD could interfere with the use of mouse nectin-1α for viral entry. Figure 7 shows that the cells expressing (i) either the FVB/N or C3H form of nectin-1α and HSV-1(KOS) gD or (ii) the FVB/N form of nectin-1α and any one of the gDs [HSV-1(KOS), HSV-1(KOS)Rid1, or PRV] were significantly more resistant to the entry of HSV-1(KOS) than were the cells expressing nectin-1α alone. These results are indistinguishable from those previously obtained in similar interference experiments using human nectin-1α and the same forms of gD (12). As also shown in this previous study, coexpression of gD with the receptor does not alter receptor expression or translocation to the cell surface but does make the receptor inaccessible to gD, as determined by quantitation of gD:Fc binding. The results presented in Fig. 7 are consistent with the ability of mouse nectin-1α to mediate the entry of both wild-type and mutant HSV-1 strains and PRV and indicate that the mouse protein is a receptor for all three forms of gD (and probably also for BHV-1 gD).
FIG. 7.
Effect of coexpression of alphaherpesvirus gDs with nectin-1α on entry of HSV-1. CHO-K1 cells were cotransfected with a nectin-1α-expressing plasmid (solid symbols for the FVB/N form and open symbols for the C3H form) and gD-expressing plasmids (pRE4 for KOS-gD, pMW13 for Rid1-gD, pCMV-gD for PRV-gD) or pcDNA3 as a control, in a 1:4 ratio. The transfected cells were then exposed to β-galactosidase-expressing HSV-1(KOS) at the doses indicated. At 6 h later, the cells were permeabilized and assayed for β-galactosidase activity as described in the legends to Fig. 2 and 3.
Taken together, the results presented in Fig. 4 to 7 demonstrate that HSV gD engages in specific physical and functional interactions with mouse nectin-1α, interactions that are demonstrable with both soluble and membrane-bound forms of each protein and with two different allelic forms of nectin-1α. The results presented in Fig. 7 predict the existence of physical as well as functional interactions of nectin-1α with each of the alphaherpesvirus gDs encoded by viruses that can use this cell surface protein as an entry receptor. In a previous study (25), similar experiments performed with the C3H form of mouse nectin-1α failed to demonstrate binding to HSV-1 gD despite evidence that expression of this allele could make cells susceptible to HSV-1 entry. We have no explanation for the discrepancy in results but can only suggest that technical difficulties in the previous study may have prevented detection of the specific gD-receptor interactions shown here for the C3H allele.
Expression of murine nectin-1α in mouse tissues and cells.
Northern blot analyses were performed using polyadenylated RNAs extracted from various tissues of the mouse and a probe restricted to the ectodomain of nectin-1α. The probe would very probably detect mRNAs encoding not only nectin-1α but also other isoforms, should they be expressed in the mouse. The results shown in Fig. 8 demonstrated that RNAs homologous to nectin-1 were extracted from all the tissues tested except heart. The hybridization signal was faint for testis and indicated the presence of a 4.8-kb RNA related to nectin-1α. The signal was stronger in other samples and identified an RNA species of about 6.0 kb. Kidney, brain, and skin had abundant amounts of this RNA. These results are complementary to those published previously for mouse nectin-1 expression. Northern blot analyses using nectin-1-specific probes revealed the presence of homologous RNA in liver as well as brain and kidney (25, 30). A previous study of human nectin-1 expression by Northern blot analysis demonstrated the presence of two RNA species (3.5 and 5.9 kb) (6). The 5.9-kb species was abundant in brain and spinal cord. Expression in skin was not assessed.
FIG. 8.
Northern blot analysis of nectin-1α expression in mouse tissues. A mouse poly(A)+ RNA blot membrane was hybridized with a 32P-labeled probe encompassing the ectodomain of nectin-1α. Each lane contains 2 μg of poly(A)+ RNA isolated from the organs indicated.
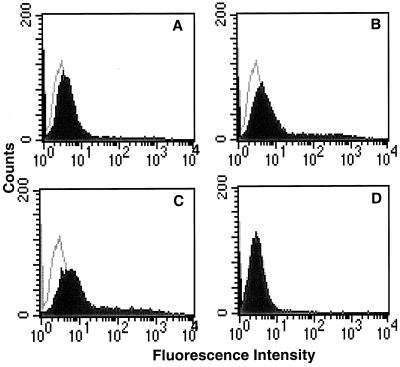
Immunocytochemical experiments were done to identify cells that expressed nectin-1α in mouse brain and mucosal epithelium. Rabbit antisera raised against human nectin-1α were found to be suitable for this purpose. Binding of the antibodies to the mouse form of nectin-1α was demonstrated by flow cytometry. CHO-K1 cells were transfected with a plasmid expressing mouse nectin-1α (FVB/N allele) or with the empty plasmid vector and then incubated with antiserum R165, antiserum R166, a mixture of the two, or preimmune control serum. Enhanced binding of antibodies to the nectin-1α-expressing cells was evident, in comparison with the control cells, for each antiserum and the mixture but not for the preimmune control serum (Fig. 9). Thus, antigenic determinants, as well as the primary amino acid sequence, are conserved in the mouse and human forms of nectin-1α.
FIG. 9.
Antibodies against human nectin-1α cross-react with mouse nectin-1α. CHO-K1 cells transfected with nectin-1α-expressing plasmid (pDS101) (A to C) or control plasmid (pcDNA3) (D) were incubated with anti-nectin-1α antiserum (solid profile) or preimmune serum (open profile), followed by a fluoresceinated secondary antibody, and analyzed by flow cytometry. (A) R165 antiserum or preimmune serum; (B) R166 antiserum or preimmune serum; (C and D) 1:1 mixture of R165 and R166 antisera or 1:1 mixture of preimmune sera.
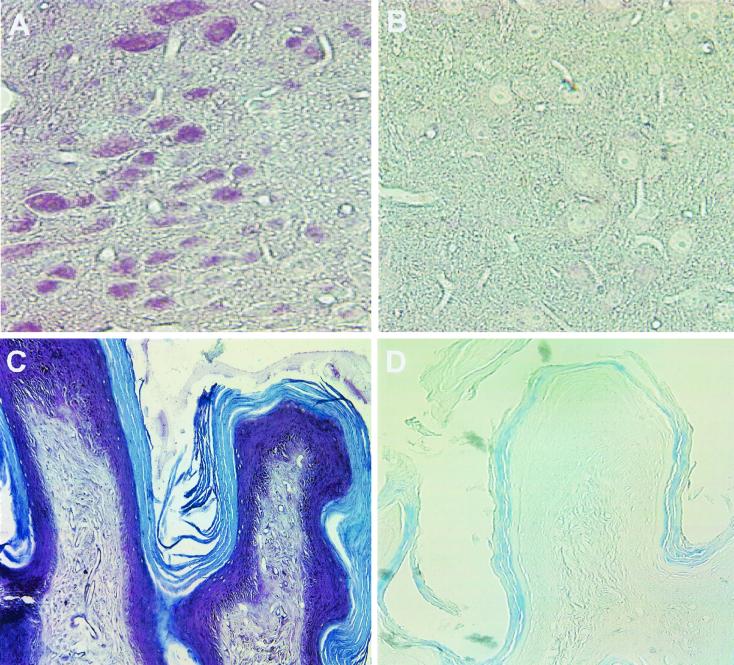
Paraformaldehyde-fixed tissue sections were prepared from the brains and vaginas of BALB/c mice for incubation with a mixture of the two antisera (Fig. 10A and C) or with preimmune control serum (Fig. 10B and D). In the vagina, the entire epithelial layer was heavily stained by reaction with the antisera but not with the control serum (Fig. 10C and D, respectively). In the brain, many neurons were stained specifically with the antisera but not with the control serum (Fig. 10A and B, respectively). The brain section shown is from the hypothalamus, but neurons in other parts of the brain were also stained. A detailed study of the entire brain will be required to determine whether all neurons contain nectin-1α-related antigen and whether this antigen is restricted to neurons.
FIG. 10.
Immunohistochemical analysis of nectin-1α expression in mouse tissues. Paraformaldehyde-fixed sections from the brains (A and B) and vaginas (C and D) of BALB/c mice were stained with a mixture of anti-nectin-1α antisera R165 and R166 (A and C) or preimmune serum (B and D). Binding of antibodies was detected by using a Vectastain Elite ABC kit and Vector VIP substrate (purple) for peroxidase. The tissue sections were counterstained with methyl green.
These results demonstrate that nectin-1α is expressed in cells that are known to be susceptible to HSV entry in vivo. It seems likely that this cell surface protein could be used by either HSV-1 or HSV-2 for entry into vaginal epithelial cells and neurons, although nectin-1α need not be the only receptor used.
DISCUSSION
The mouse is used extensively as an experimental animal to study various aspects of HSV pathogenesis and to test vaccines and antiviral drugs. The extent to which the mouse provides a faithful model for investigating human HSV disease depends in part on similarities and differences in the host proteins and other factors required by the virus for infection at the portal of entry, spread to distant sites, establishment of latency, and reactivation from latency, as well as host factors that determine immune responses to the virus. Mouse models of disease could be extremely useful in defining aspects of HSV pathogenesis that depend on cellular expression of viral receptors, i.e., on innate susceptibility or resistance of specific cells to viral entry. To assess the usefulness of mice for this purpose, it is important to determine (i) the extent to which entry of HSV into mouse and human cells relies on the cell surface expression of homologous receptors (cell surface proteins and sites in heparan sulfate), (ii) the similarities and differences in the specificity of homologous mouse and human receptors for different HSV strains, and (iii) the similarities and differences in the patterns of expression and functions of the homologous mouse and human receptors.
The results presented here demonstrate that mouse nectin-1α is indistinguishable from human nectin-1α with respect to alphaherpesvirus entry activity and ability to serve as a receptor for HSV gD, at least on the basis of comparisons performed to date. These include demonstrations that (i) entry of multiple strains of HSV-1 and HSV-2 and single strains of PRV and BHV-1 can be mediated by mouse (Fig. 2 and 3) (25) and human (6, 13) nectin-1, (ii) HSV-1 and HSV-2 gDs bind to both mouse (Fig. 4 to 6) and human (5, 19) nectin-1α, (iii) this binding can be inhibited by anti-gD antibodies (Fig. 5 and 6) that also block binding to human nectin-1α (19, 38), and (iv) several forms of alphaherpesvirus gD interfere with entry via mouse (Fig. 7) or human (12) nectin-1α.
Mouse and human nectin-1 probably also have similar patterns of expression in mouse and human tissues. Northern blot analyses gave similar results with respect to expression in brain tissue (Fig. 8) (6, 25, 30). Expression in skin was examined only in this study. Nectin-1 mRNAs were detected by reverse transcription-PCR in human cell lines of neuronal origin and in primary human foreskin keratinocytes (13), and immunocytochemistry revealed that nectin-1 is expressed as a protein in mouse brain neuronal cells and vaginal epithelial cells (Fig. 10).
The functions of mouse and human nectin-1α are also likely to be similar, given the high degree of sequence identity and information summarized below. Both nectin-1 (human) and nectin-2 (mouse and human) mediate cell adhesion through homophilic interactions of their ectodomains (1, 20, 35). A third related member of the nectin family, mouse nectin-3, can cause cell adhesion by engaging in heterophilic interactions with either nectin-1 or nectin-2 (30). It is not yet known whether nectin-3 has viral entry activity.
Table 1 lists the known members of the nectin family, all of which have similar Ig-like ectodomains (VCC) and some isoforms of which have PDZ-binding domains as indicated. Several of the latter forms, both mouse and human, localize to the sites of cadherin-based adherens junctions in epithelial cells (28, 30, 35). This localization is dependent on interaction of the nectin with the PDZ domain of afadin, which is a component of the cadherin-based adherens junctions and binds to actin filaments (2, 23, 35). As described above, results presented here and elsewhere for human and mouse nectin-1 indicate that expression is most abundant in the brain, liver, kidney, and skin. Nectin-2, on the other hand, is expressed at relatively high levels in many tissues, whereas nectin-3 is expressed most abundantly in the testis, with some isoforms also being highly expressed in the liver and kidney (10, 30, 32). Nectin-2 has been deleted in mice. Interestingly, the only overt phenotype that resulted was male infertility due to the production of spermatozoa with aberrations in nuclear and cytoskeletal morphology and mitochondrial localization (3). Taken together, the available data on nectins indicate that the PDZ-binding isoforms could influence cytoskeletal organization through interactions with actin-binding proteins and could participate in the formation or stability of specialized cell junctions in appropriate cell types. It remains to be determined whether any of the nectins is localized at neuronal synapses.
TABLE 1.
Nectin family members and alphaherpesvirus entry activitya
| Nameb | PDZ-binding domainc | Identified in:
|
Shown to mediate entry of:
|
|||||
|---|---|---|---|---|---|---|---|---|
| HSV-1
|
HSV-2 | PRV | BHV-1 | |||||
| Mouse | Human | WTd | Ridd | |||||
| Nectin-1α | Yes | Yes | Yes | M-Yese | M-Yes | M-Yes | M-Yes | M-Yes |
| H-Yes | H-Yes | H-Yes | H-Yes | H-Yes | ||||
| Nectin-1β | No | —f | Yes | H-Yes | H-Yes | H-Yes | H-NTg | H-Yes |
| Nectin-2α | Yes | Yes | Yes | M-No | M-No | M-No | M-Yes | M-No |
| H-No | H-Yes | H-Yes | H-Yes | H-No | ||||
| Nectin-2δ | Yes | Yes | Yes | M-NT | M-NT | M-NT | M-NT | M-NT |
| H-No | H-Yes | H-NT | H-NT | H-NT | ||||
| Nectin-3α | Yes | Yes | — | H-NT | H-NT | H-NT | H-NT | H-NT |
| Nectin-3β | Yes | Yes | — | H-NT | H-NT | H-NT | H-NT | H-NT |
| Nectin-3γ | No | Yes | — | H-NT | H-NT | H-NT | H-NT | H-NT |
Results on alphaherpesvirus entry activity are presented in this paper or were published elsewhere (6, 13, 21, 25, 32, 37).
The nomenclature for members of this family was described by Takahashi et al. (35) and Satoh-Horikawa et al. (30).
WT denotes wild-type strains of HSV-1, while Rid denotes strains with amino acid substitutions at position 25 or 27 in gD, which confer resistance to interference mediated by wild-type gD (4, 9, 12).
M-Yes or M-No indicates whether the mouse form of the indicated nectin can mediate entry of the virus indicated; H-Yes or H-No indicates the same for the human form of nectin.
—, this form of nectin has not yet been identified or isolated.
M-NT or H-NT indicates that the mouse or human form of nectin, respectively, has not yet been tested for viral entry activity.
If nectin-1α is the principal gD receptor for entry of HSV-1 or HSV-2 into key cell types targeted by HSV-1 or HSV-2, aspects of pathogenesis determined by viral entry are likely to be similar in mice and humans. Other gD receptors for HSV entry must also be considered, however. HVEM, a member of the tumor necrosis factor receptor family, is not very highly conserved in primary sequence (45% identity between the mouse and human forms), but both the mouse and human forms are able to mediate the entry of HSV-1 and HSV-2 strains. The only difference is that the mouse form of HVEM can mediate the entry of Rid strains of HSV-1 (D. Shukla and P. G. Spear, unpublished data) whereas the human form cannot (29). HVEM has been studied mostly in cells of lymphoid origin but is widely expressed in human and mouse tissues (16, 29).
Enzymes that can generate gD-binding sites in heparan sulfate (mouse and human 3-O-sulfotransferases, isoform 3) are highly conserved. In the absence of any known protein receptors, these enzymes can render cells susceptible to HSV-1 but not HSV-2 (31). It remains to be determined whether the HSV-1 gD receptors generated by 3-O-sulfotransferase-3 are similarly expressed on mouse and human tissues. The only HSV entry receptor that is active in its human form but not in its mouse form is nectin-2 (Table 1). Human nectin-2 is a receptor for PRV and HSV-1 Rid mutants and is also a weak receptor for HSV-2 strains (21, 37), but the mouse form is without detectable entry activity except for PRV (32). Thus, mice and humans will differ in any aspects of pathogenesis that depend on viral entry via nectin-2.
Identification of cell surface molecules that are principally responsible for the entry of HSV into specific cells of organized tissues in the intact mouse or human will require several complementary approaches. The use of mutant mice, of viral mutants with altered receptor preferences, or of antibodies and other agents capable of blocking entry via specific receptors should be helpful in determining which receptors are most important at the portal of entry and perhaps for spread to distant sites. Epithelial cells are likely to express most or all of the identified HSV gD receptors. Analysis of mRNA expression by reverse transcription-PCR revealed that cultured primary human foreskin keratinocytes expressed HVEM, nectin-1, and nectin-2 (13, 37). If all three are also expressed in keratinocytes in vivo and if most forms of nectin-1 and nectin-2 are inaccessible to virus because of their localization at cell junctions, then HVEM might serve as the principal receptor for viral entry into the epithelium whereas the nectins could play a key role in cell-to-cell spread within the epithelium and to neurons of the peripheral and central nervous systems.
The results presented here demonstrate that mouse nectin-1α is indistinguishable from human nectin-1α in entry activity for HSV-1, HSV-2, PRV, and BHV-1 and in its interactions with viral gD, at least with HSV gD. The abundant levels of expression of nectin-1 in epithelial cells of the vaginal mucosa and in neurons make this protein a prime candidate for an entry receptor that allows the entry of HSV-1 or HSV-2 into the epithelium and/or that permits the spread of infection within the epithelium and to cells of the nervous system. Nectin-1 could be the receptor that allows transneuronal passage of HSV (and PRV) in the rodent nervous system.
ACKNOWLEDGMENTS
We thank G. H. Cohen, R. J. Eisenberg, T. C. Mettenleiter, V. Gerdts, and L. Bello for materials used in this study and N. Susmarski, M. L. Parish, A. Fridberg, and J. D. Prasad for excellent technical assistance.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases to the Midwest Sexually Transmitted Diseases Cooperative Research Center (AI 31494). D.S. was supported by National Research Service Award F32 AI 09951. C.L.R. was supported by a fellowship from the American Social Health Association.
REFERENCES
- 1.Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235:374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- 2.Asakura T, Nakanishi H, Sakisaka T, Takahashi K, Mandai K, Nishimura M, Sasaki T, Takai Y. Similar and differential behaviour between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells. 1999;4:573–581. doi: 10.1046/j.1365-2443.1999.00283.x. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard M J, Dong Y, McDermott B M, Jr, Lam D-H, Brown K R, Shelanski M, Bellve A R, Racaniello V R. Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell-cell adherens junctions. Mol Cell Biol. 2000;20:2865–2873. doi: 10.1128/mcb.20.8.2865-2873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume G, Qi S, Avitabile E, Foa-Tomasi L, Brandimarti R, Roizman B. Glycoprotein D of herpes simplex virus encodes a domain which precludes penetration of cells expressing the glycoprotein by superinfecting herpes simplex virus. J Virol. 1990;64:6070–6079. doi: 10.1128/jvi.64.12.6070-6079.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocchi F, Lopez M, Menotti L, Aoubala M, Dubreuil P, Campadelli-Fiume G. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc Natl Acad Sci USA. 1998;95:15700–15705. doi: 10.1073/pnas.95.26.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen G H, Isola V J, Kuhns J, Berman P W, Eisenberg R J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986;60:157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen G H, Wilcox W C, Sodora D L, Long D, Levin J Z, Eisenberg R J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol. 1988;62:1932–1940. doi: 10.1128/jvi.62.6.1932-1940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean H J, Terhune S, Shieh M-T, Susmarski N, Spear P G. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell type-dependent alterations in infectivity. Virology. 1994;199:67–80. doi: 10.1006/viro.1994.1098. [DOI] [PubMed] [Google Scholar]
- 10.Eberlé F, Dubreuil P, Mattei M G, Devilard E, Lopez M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995;159:267–272. doi: 10.1016/0378-1119(95)00180-e. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg R J, Long D, Ponce de Leon M, Matthews J T, Spear P G, Gibson M G, Lasky L A, Berman P, Golub E, Cohen G H. Localization of epitopes of gD of herpes simplex virus type 1 glycoprotein D. J Virol. 1985;53:634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geraghty R J, Jogger C R, Spear P G. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology. 2000;268:147–158. doi: 10.1006/viro.1999.0157. [DOI] [PubMed] [Google Scholar]
- 13.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 14.Gerdts V, Jons A, Makoschey B, Visser N, Mettenleiter T C. Protection of pigs against Aujeszky's disease by DNA vaccination. J Gen Virol. 1997;78:2139–2146. doi: 10.1099/0022-1317-78-9-2139. [DOI] [PubMed] [Google Scholar]
- 15.Hata Y, Butz S, Südhof T C. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu H, Solovyev I, Colombero A, Elliott R, Kelley M, Boyle W J. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5*. J Biol Chem. 1997;272:13471–13474. doi: 10.1074/jbc.272.21.13471. [DOI] [PubMed] [Google Scholar]
- 17.Klupp B G, Baumeister J, Karger A, Visser N, Mettenleiter T C. Identification and characterization of a novel structural glycoprotein in pseudorabies virus, gL. J Virol. 1994;68:3868–3878. doi: 10.1128/jvi.68.6.3868-3878.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koike S, Horie H, Ise I, Okitsu A, Yoshida M, Iizuka N, Takeuchi K, Takegami T, Nomoto A. The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J. 1990;9:3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krummenacher C, Nicola A V, Whitbeck J C, Lou H, Hou W, Lambris J D, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpes virus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood. 1998;92:4602–4611. [PubMed] [Google Scholar]
- 21.Lopez M, Cocchi F, Menotti L, Avitabile E, Dubreuil P, Campadelli-Fiume G. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J Virol. 2000;74:1267–1274. doi: 10.1128/jvi.74.3.1267-1274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez M, Eberlé F, Mattei M G, Gabert J, Birg F, Bardin F, Maroc C, Dubreuil P. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene. 1995;155:261–265. doi: 10.1016/0378-1119(94)00842-g. [DOI] [PubMed] [Google Scholar]
- 23.Mandai K, Nakanishi H, Satoh A, Takahashi K, Satoh K, Nishioka H, Mizoguchi A, Takai Y. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J Cell Biol. 1999;144:1001–1017. doi: 10.1083/jcb.144.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 25.Menotti L, Lopez M, Avitabile E, Stefan A, Cocchi F, Adelaide J, Lecocq E, Dubreuil P, Campadelli-Fiume G. The murine homolog of human nectin1δ serves as a species nonspecific mediator for entry of human and animal αherpesviruses in a pathway independent of a detectable binding to gD. Proc Natl Acad Sci USA. 2000;97:4867–4872. doi: 10.1073/pnas.97.9.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mettenleiter T C. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet Res. 2000;31:99–115. doi: 10.1051/vetres:2000110. [DOI] [PubMed] [Google Scholar]
- 27.Miller J M, Whetstone C A, Bello L J, Lawrence W C, Whitbeck J C. Abortion in heifers inoculated with a thymidine kinase-negative recombinant of bovine herpesvirus 1. Am J Vet Res. 1995;56:870–874. [PubMed] [Google Scholar]
- 28.Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cisdimerization or transinteraction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 30.Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- 31.Shukla D, Liu J, Blaiklock P, Shworak N W, Bai X, Esko J D, Cohen G H, Eisenberg R J, Rosenberg R D, Spear P G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 32.Shukla D, Rowe C L, Dong Y, Racaniello V R, Spear P G. The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. J Virol. 1999;73:4493–4497. doi: 10.1128/jvi.73.5.4493-4497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sisk W P, Bradley J D, Leipold R J, Stoltzfus A M, Ponce de Leon M, Hilf M, Peng C, Cohen G H, Eisenberg R J. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J Virol. 1994;68:766–775. doi: 10.1128/jvi.68.2.766-775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 35.Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taketo M, Schroeder A C, Mobraaten L E, Gunning K B, Hanten G, Fox R R, Roderick T H, Stewart C L, Lilly F, Hansen C T, Overbeek P A. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci USA. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 38.Whitbeck J C, Muggeridge M I, Rux A H, Hou W, Krummenacher C, Lou H, van Geelen A, Eisenberg R J, Cohen G H. The major neutralizing antigenic site on herpes simplex virus glycoprotein D overlaps a receptor-binding domain. J Virol. 1999;73:9879–9890. doi: 10.1128/jvi.73.12.9879-9890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]