Abstract
A sedentary lifestyle and physical inactivity leads to metabolic syndrome-associated comorbidities involving abdominal obesity, type 2 diabetes, hyperlipidaemia associated Cardiovascular Diseases (CVDs), and Metabolic dysfunction-associated fatty liver disease (MAFLD). In this study, we evaluated the novel hepato/cardio/adipo-protective role of Quercetin via Vitamin D Receptor, and elucidated its underlying mechanisms in reducing lipotoxicity, inflammation and fibrosis in high calorie diet induced metabolic syndrome. Male Swiss albino mice were fed with western diet and sugar water for multiple time intervals. Anti-lipotoxicity, anti-inflammatory, and anti-fibrotic effect of Quercetin was assessed by Oil Red O, H&E and TMS staining at different time points. The lipid profile, mRNA expression of inflammatory markers (TNF- α, IL-1β, IL-6 and MCP-1), fibrotic markers (α-SMA, COL1A1, COL1A2), adiponectin, AdipoR2, and VDR expression levels were measured from RNA pools of adipose, liver and heart tissues. Also, lipid-lowering and anti-steatohepatitic effects of Quercetin was assessed using mouse 3T3-L1 adipocytes, rat H9c2 cardiac cells, and human HepG2 hepatocytes. Our results indicate that, western diet fed mice with Quercetin ameliorated lipid profile and lipotoxicity. Histopathological examination and gene expression data revealed that Quercetin reduced hepatic and cardiac inflammation and fibrosis-associated markers. Interestingly, Quercetin treatment increased the serum levels of adiponectin and mRNA expressions of AdipoR2 and VDR. In-vitro experiments revealed the reduction in lipid accumulation of 3T3-L1 and fatty-acid-treated hepatic and cardiac cells following Quercetin treatment. These findings indicate that Quercetin exhibits a protective role on multiple organs through VDR activation and subsequent Adipo/AdipoR2 signaling in metabolic syndrome associated obesity, hepatic injury, and cardiac dysfunction.
Keywords: Adiponectin, Adiponectin receptor 2, Vitamin D receptor, Quercetin, Obesity, Metabolic associated steatohepatitis, Cardiac fibrosis
1. Introduction
Metabolic syndrome (MS) is a major global pandemic, characterized by obesity, insulin resistance associated type 2 diabetes (T2DM), hypertension, dyslipidemia, with enhanced risk of CVDs and MAFLD [1,2]. Complexity of the syndrome shows the urgent need to explore the molecular mechanism and to discover the suitable diagnostic and prognostic markers for successful therapeutic interventions. Obesity is a major risk factor of MS, it is often associated with sedentary lifestyles, and unhealthy dietary habits, and it can positively contribute towards different metabolism-associated pathophysiological abnormalities [3,4], involving multiple organs like the Adipose tissue, Heart and the Liver (AHL). The molecular cross-talk between these organs during MS-pathophysiology further underlines the risk of CVDs and MAFLD [5]. Therefore, it becomes imperative to understand the cross-talk mechanisms between these three organs during MS-development and progression.
Recently, multiple compounds including flavonoids have been experimentally shown to improve various MS-comorbidities [[6], [7], [8]]. Specifically, Quercetin, a natural dietary plant flavonoid is widely reported for its strong nutraceutical and pharmaceutical activities [9]. However, the role of Quercetin and its mode-of action on the above discussed organs in the context of MS is least understood. Through this study we have elucidated the ameliorating effect of Quercetin on western diet-induced complications of the AHL using in-vitro and in-vivo approaches via novel mechanism by activating Vitamin D Receptor.
Adiponectin (Adipo) is an adipocytokine specific to adipose tissue and is well known for its protective effects against the development and progression of several metabolic diseases [10]. There are compelling evidence to show that this cytokine can bind to specific families of receptors called adiponectin receptor 1 (AdipoR1), present in muscle, and the hypothalamus; adiponectin receptor 2 (AdipoR2), expressed in liver, heart, and other tissues. Binding of adiponectin to its receptors result in activation of AMPK and PPAR-α signaling pathways which results in the inhibition of fatty acid synthase enzymes (SREBP-1c and FASN). This will further induce the expression of fatty acid oxidation genes (CPT1A & ACOX1) [11,12]. Therefore, here we focussed on exploring a new therapeutic facet aimed at increasing adiponectin expression and secretion might be useful in preventing metabolic syndrome-related complications through the activation of the Adipo/AdipoR2 signaling pathway [13]. Due to its multi-organ expression, understanding the cross-regulation involving adiponectin and its receptors will be an interesting approach to mitigate the pathologies of metabolic syndrome associated with different organs such as AHL.
Alternatively, several intervention studies suggest that the Vitamin D Receptor (VDR), a member of the nuclear receptor super family, can regulate the expression of genes involved in metabolic complications in a ligand-dependent manner [14,15]. Some reports suggest that VDR is expressed both in adipose tissue and the liver, and its subsequent activation may result in inhibition of NF-κB and TGF-β signaling pathways thereby mitigating inflammation and fibrosis [16,17]. Our study also sheds the light on the possible role of Quercetin as a potent VDR agonist, resulting in subsequent activation of Adipo/AdipoR2 pathway suggesting its possible application as a novel therapeutic strategy for metabolic diseases, including the advanced complications of obesity such as MAFLD and CVDs.
2. Materials and methods
2.1. Animals
A total of 60 male Swiss albino mice were procured from M/s. Adita Biosys, Tumakuru, which weighed 15–18 g and aged 5–7 weeks and then housed for 7 days before all in vivo studies. All these animals were kept in polypropylene cages at a constant room temperature of 25 ± 3 °C, with a relative humidity of 45–55 % and a 12 h light/12 h dark cycle (artificial photoperiod).
2.2. Study design and intervention
After one week of acclimatization, mice were randomly divided into 3 models based on the time interval that is, 12, 16, and 20 weeks respectively (Fig. 1A). Briefly, each model consisted of 4 groups: The negative control group fed with a chow diet and normal water (CDNW) (n = 5), the control group (WDSW) fed with a western diet (45 % kcal% fat, D12451), and 25 % glucose and fructose solution (n = 5), positive control group (WDSW + Saro (n = 5) fed with western diet +25 % glucose and fructose solution + Saroglitazar (oral administration of 4 mg/kg body weight), test control group (WDSW + Quer) (n = 5) fed with western diet +25 % glucose and fructose solution + Quercetin (oral administration of 100 mg/kg body). The doses of Saroglitazar (positive control) and Quercetin concentration were selected based on a similar previous studies [18,19]. The body weights of each group were recorded once a week. The mice were euthanized by ketamine xylazine inhalation after being fed the experimental diets for up to 20 weeks. The liver, heart, and adipose tissues were immediately excised in pieces and fixed in 10 % buffered formalin for histopathological examinations or frozen in liquid nitrogen and stored at −80 °C for mRNA and protein quantification experiments respectively. Blood samples were collected by retro-orbital route, centrifuged and the serum was stored at −80 °C for further analysis. Quercetin was procured from Sigma-Aldrich (Q4951).
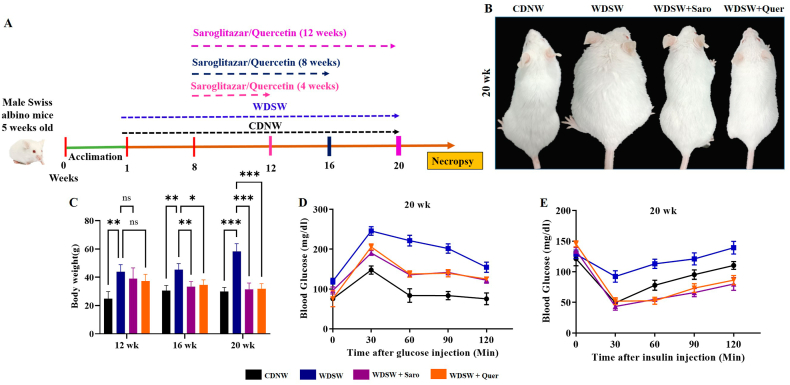
Fig. 1.
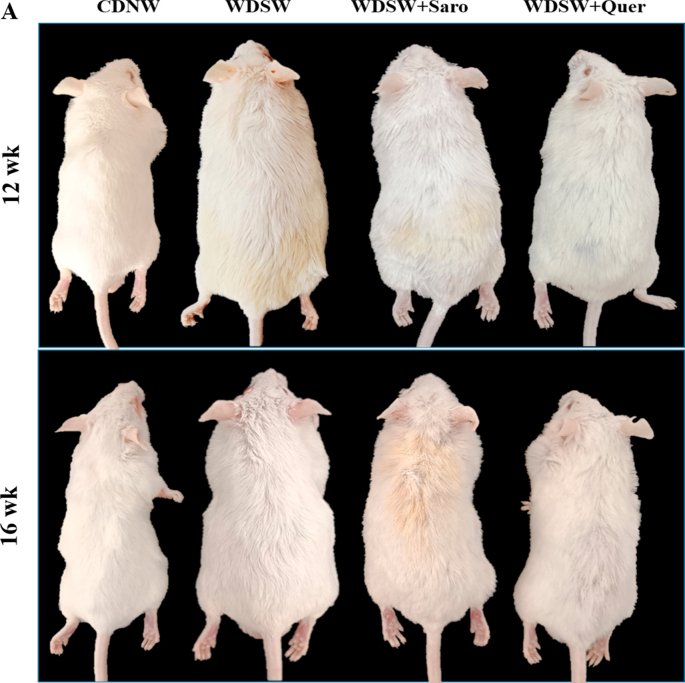
Effect of Quercetin on mice body weight and serum glucose level in WDSW diet induced in Swiss albino mice. (A) Outline representation of experimental design. (B) Photographs represent the changes in body weight of 20 week mice group. (C) Graph represent the mice body weight. (D) Insulin Tolerance Test (ITT). (E) Glucose Tolerance Test (GTT). Data are represented as the mean ± SEM from 5 mice in each group.
2.3. Serum biochemical measurements
Liver enzymes like serum Alanine transaminase (ALT or SGPT), Aspartate aminotransferase (AST or SGOT), Alkaline phosphatase (ALP), and the lipid profile constituents like triglycerides (TG), total cholesterol and high-density lipoprotein (HDL) were determined by using commercially available kits (Agappe diagnostics Ltd, Cochin, Kerala, India).
2.4. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
A glucose tolerance test was performed on overnight fasted mice by measuring the baseline blood glucose level with an Accu-chek Glucometer (Roche, Switzerland). Glucose (2 g/kg body weight) dissolved in phosphate-buffered saline was intra-peritoneally injected into mice. Blood glucose levels were measured after a glucose injection at various time intervals (0, 30, 60, 90, and 120 min). To assess insulin tolerance, mice were kept for fasting up to 5–6 h. The baseline glucose levels were measured similarly to GTT. Intermittent insulin was injected into mice (0.75 U/kg body weight). Blood glucose levels were measured at different time intervals (0, 30, 60, 90, and 120 min). Following 20 weeks of treatment, GTT and ITT were conducted before necropsy.
2.5. Tissue histology
Five micro meter-thick sections of liver, adipose tissue, and heart tissues implanted in paraffin were examined for further experiments. The hepatic steatosis, adipose tissue inflammation, and cardiac inflammation were assessed using Haematoxylin and Eosin (H & E) staining. Trichome Masson's staining (TMS) was conducted to assess hepatic and cardiac fibrosis. Oil Red O staining was performed on snap-frozen liver sections to evaluate steatosis and fat accumulation. The sections of adipose and liver tissues were subjected to immunohistochemistry to visualize the expression of VDR (Santa Cruz Biotechnology, USA) marker.
2.6. Cell culture and treatment
3T3-L1 cell line (Passage No-12) was cultured in regular Dulbecco's Modified Eagle's Media (DMEM) with additional supplements like 10 % fetal bovine serum (FBS), 1 % glutamine, and antibiotics (1 % penicillin-streptomycin). 3T3-L1 cells (1.5 × 105) were seeded in 6-well plate and incubated at 37 °C with 5 % CO2 until they reached the 70–80 % of confluence. The wells were separated into six groups consisting of a non-differentiated group (ND), an untreated differentiated group (DMI), differentiated cells treated with positive control (2.5 μM simvastatin), and differentiated cells treated with different concentrations of Quercetin (5 μM, 10 μM and 20 μM) respectively. The schematic representation of treatment pattern to induce differentiation in 3T3-L1 is shown in (Fig. 2A).
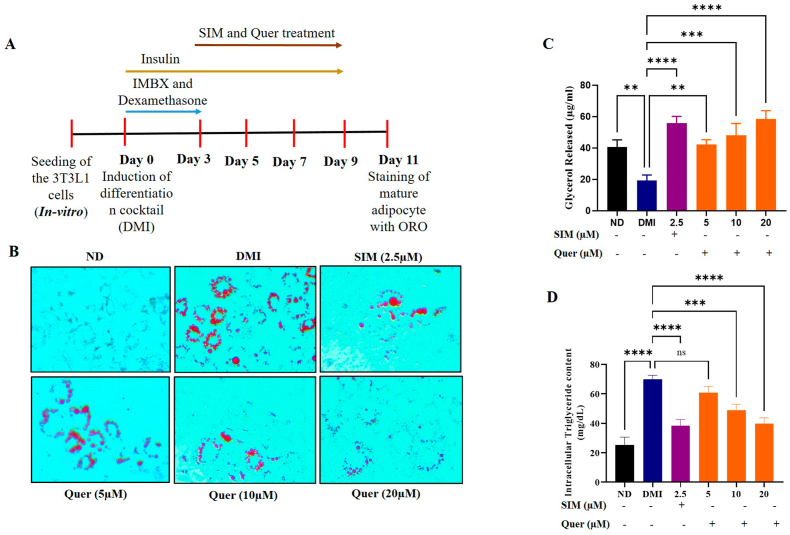
Fig. 2.
Quercetin inhibited differentiation and lipid accumulation in the 3T3-L1 mature adipocytes. (A) Schematic representation of 3T3-L1 differentiation. (B) Oil Red O staining. Images were obtained at 20× magnification and scale bar: 100 μm). (C) Adipolysis assay. (D) Intracellular triglyceride content in 3T3-L1. Data are represented as the mean ± SEM from 5 mice in each group.
2.7. RNA isolation and quantitative RT-PCR
Using the Trizol reagent, total cellular mRNA was isolated from freshly frozen adipose, liver, and heart tissues or cultured cells (HepG2 and H9c2). mRNA was reverse transcribed with a verso cDNA synthesis kit (Thermo Fischer Scientific, USA). Quantitative RT-PCR was performed for the assessment of mRNA expression by using SYBR green kit (Thermo Fischer Scientific, USA). Primers for target mouse genes were purchased from Barcode Biosciences as listed in (Supplementary Table 1). The reactions were run in triplicates using SYBR green gene expression assay. The relative fold-change was normalized with housekeeping beta-actin mRNA using formula 2- ΔΔC.
2.8. Adipogenesis assay
Post-confluency (day 0), the culture medium was replaced with 2 ml of induction medium (1.0 μM Dexamethasone, 0.5 mM Isobutylmethylxanthine (IBMX), and 1.0 μg/ml Insulin) in the control and differentiation groups which contain test compound Quercetin (concentrations ranging from 5 to 20 μM) was incubated for 72 h. The induction medium was replaced with an insulin medium containing test compounds on day 3. Until day 10, the insulin medium and test compounds were replaced every alternative day. On day 12, adipogenesis was assessed using Oil Red O staining following the manufacturer's protocol (Cayman chemical, Adipogenesis Assay kit, no.10006908).
2.9. Adipolysis assay
On day 12, Adipolysis was measured using a glycerol standard as per the manufacturer's instructions (Cayman Chemical, Adipolysis Assay kit no. 10009381).
2.10. Intracellular triglyceride assay (TG)
The intracellular TG content was evaluated as described by Jie Cao et al. [20]. The 3T3-L1 mature adipocytes (day 12) containing 6 wells culture plates were rinsed with PBS buffer. Cell lysis buffer was added to each well and the cells were collected with a scraper into Eppendorf tubes. The cells were disrupted for 30 min using an ultra-sonicator and centrifuged at 13000 g for 15 min at 4 °C. After centrifugation, the supernatant was carefully collected and triglyceride content was measured using a commercial triglyceride assay kit from Agappe diagnostics Ltd, Cochin, Kerala, India.
2.11. Adiponectin-Enzyme Linked Immunosorbent Assay (ELISA)
On the twelfth day, the 3T3-L1 condition media and serum was collected from mice were immediately used for the analysis. The concentration of adiponectin level was measured by using a mouse-specific adiponectin Enzyme Linked Immunosorbent Assay (Cloud-Clone Corp). Each sample was measured in duplicates.
2.12. Mouse adipokine array
Mouse adipokine expression was analysed to check the obesity and associated illnesses such as type 2 diabetes and cardiovascular disease-related proteins. Mature 3T3-L1 cells were cultured and maintained up to 70 % confluency following which Quercetin (20 μM) was treated for a 24 h period. The media was then changed using a serum-free media for a further 24 h period. Supernatants of the cultured cells were collected using which the expression of obesity-associated adipokine markers were assessed using the commercial mouse adipokine array kit according to the manufacturer's protocol (R&D Systems).
2.13. Preparation of stock solutions of fatty acids
The 28.247 mg of oleic acid was dissolved in 1 ml of 70 % ethanol at 70 °C for 30 min, to obtain a final concentration of 100 mM of oleic acid. These solutions were conjugated with 1 % fatty acid-free BSA-containing cell culture medium at 50 °C for 10 min, yielding a working concentration of 250 μM and was stored at −20 °C for future use.
2.14. Induction and evaluation of steatosis
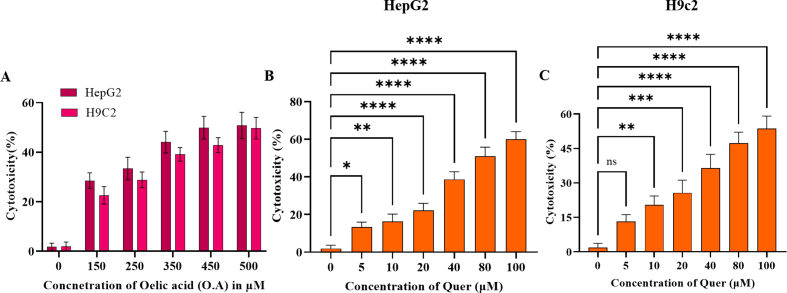
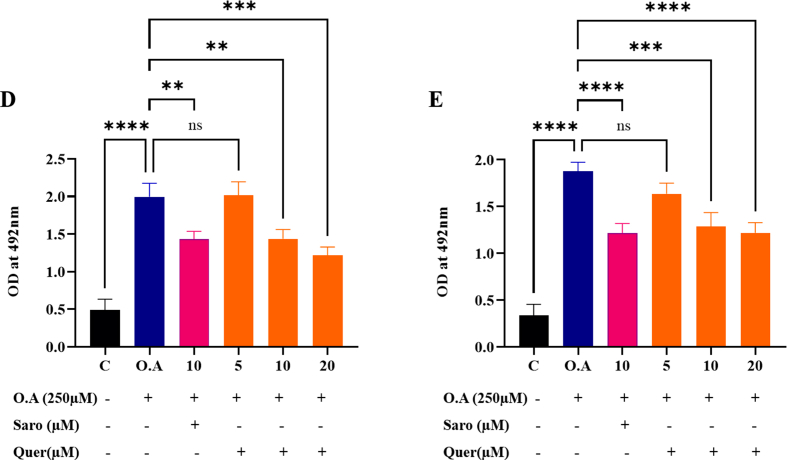
Steatosis was induced in human hepatic cell lines (HepG2) and rat cardiomyocytes (H9c2). The in-vitro study groups included: (1) Control group (cells were exposed to 1 % free fatty acid containing culture media along with 0.1 % ethanol as vehicle control), (2) Model control group (cells were treated with 250 μM Oleic acid), (3) Positive control group (cells were treated with fatty acid along with Saroglitazar (10 μM), (3–5) treatment group (cells were treated with fatty acid along with Quercetin (5, 10 and 20 μM) respectively). The cells were maintained for 24 h. After 24 h of treatment, Oil Red O staining was performed and imaging was done using a Carl Zeiss inverted light microscope. The lipid content was further quantified by measuring the absorbance of the released stain post isopropanol addition at 492 nm.
2.15. Statistical analysis
All the data are represented as the mean ± standard deviation. Statistical significance was determined using one-way or two-way ANOVA with Bonferroni's Multiple Comparison Test using GraphPad prism v.9.5 [*p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001].
3. Results
3.1. Effect of Quercetin on mice body weight and serum glucose level in WDSW diet induced in Swiss albino mice
To assess the therapeutic effect of Quercetin on western diet-induced obesity in-vivo, male Swiss albino mice were fed with both CDNW and WDSW for up to 20 weeks and treated with Quercetin with different time interval as shown in Fig. 1A. After 8 weeks of feeding, the mice were fed with WDSW showed a significant increase in body weight and were significantly obese as compared to the CDNW. The mice treated with Quercetin for 4, 8, and 12 weeks showed a significant reduction in body weight compared to WDSW (Fig. 1B and C). The mouse-body weights at 12 and 16 weeks is represented in Supplementary Fig. 1A. Insulin resistance is one of the key drivers for the development of obesity-associated metabolic comorbidities [21]. Further the administration of Quercetin improved glucose tolerance and insulin sensitivity compared with WDSW (20 weeks) (Fig. 1D and E). Overall, the degree of amelioration was comparable and equivalent to the effects of Saroglitazar which was used as a positive control in the study [19].
3.2. Quercetin inhibited differentiation and lipid accumulation in the 3T3-L1 mature adipocytes
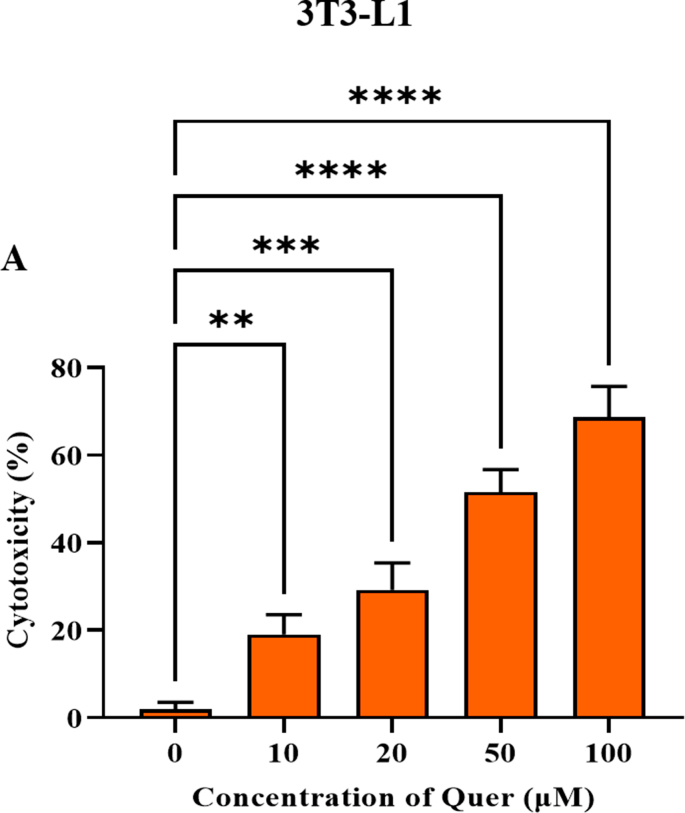
Mouse pre-adipocyte 3T3-L1 cell line was extensively used to study the obesity and as an in-vitro model for adipogenesis-associated experiments [22]. First, the proliferative effect of Quercetin on 3T3-L1 cell lines was measured by WST-1 cell viability assay (Supplementary Fig. 2A). 3T3-L1 pre-adipocytes were treated with various concentrations of Quercetin (0–100 μM) for 24 h. Based on cell viability, we choose three different concentrations of Quercetin for further assays. Interestingly, Quercetin significantly reduced the overall number and size of the accumulated lipid droplets in the differentiated adipocytes (Fig. 2A and B). Surprisingly, the Quercetin treated group with 20 μM concentration showed a similar effect compared with the positive control group involving simvastatin. To further assess the anti-obesity properties of Quercetin, we measured the total amount of released glycerol along with intracellular TAG content in differentiated cells and Quercetin treated cells. We found that there was a dose-dependent increase in the lipolysis and a remarkable decrease in the intracellular TAG content (Fig. 2C and D). Based on these data, we concluded that Quercetin inhibited adipogenesis and elevated lipolysis in differentiated adipocytes.
3.3. Quercetin treatment decreased adipocyte size and alleviated hyperlipidaemia in WDSW diet induced Swiss albino mice
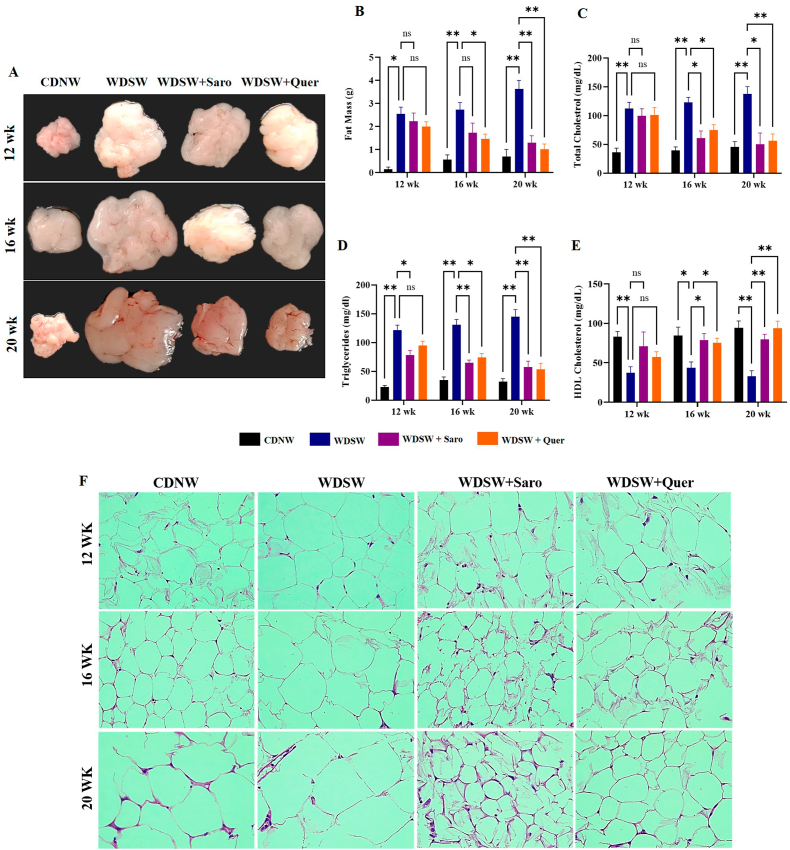
To explore the protective action of Quercetin on high calorie diet-induced obesity, we measured the weight of adipose tissue which were extracted from the mice fed with WDSW. These animals showed a significant increase in fat mass compared to those fed with CDNW. The mice treated with Quercetin showed a better reduction in the fat mass at all treatment periods (4, 8, and 12 weeks) in comparison with the WDSW mice group (Fig. 3A and B). As expected, the WDSW group significantly developed hyperlipidaemia, as an indicator of adipose tissue inflammation. Further, we found a remarkable reduction in the total cholesterol and triglycerides in Quercetin treated mice compared to WDSW fed mice (Fig. 3C and D). Furthermore, we found that Quercetin increased the level of HDL when compared to the WDSW fed mice group (Fig. 3E). The increased adipose tissue mass in obesity condition is due to excess fat accumulation, where hypertrophy (increased cell size) and hyperplasia (increased cell numbers) are the two possible mechanisms that can progress to adipose tissue inflammation [23]. Our histopathological staining showed that WDSW fed mice group had a significant increase in adipocyte size and number when compared to CDNW fed mice. Surprisingly, the treated group showed a gradual reduction in adipocyte cell size and number as compared to WDSW fed mice and almost resembles the CDNW fed mice (Fig. 3F). Taken together, these results demonstrated that, the Quercetin treated mice showed a reduction of lipid accumulation and mitigated adipocyte inflammation in comparison to the WDSW fed group similar to that of the positive control.
Fig. 3.
Quercetin treatment decreased adipocyte size and alleviated hyperlipidaemia in WDSW diet induced Swiss albino mice. (A) Representative morphology of adipose tissue. (B) Adipose tissue weight. (C) Serum cholesterol. (D) Serum triglycerides. (E) Serum High density lipoproteins. (F) H&E staining of adipose tissue. Data are represented as the mean ± SEM from 5 mice in each group.
3.4. Lipid accumulation suppressing and cytoprotective effect of Quercetin on fatty acid exposed HepG2 and H9c2 cell lines
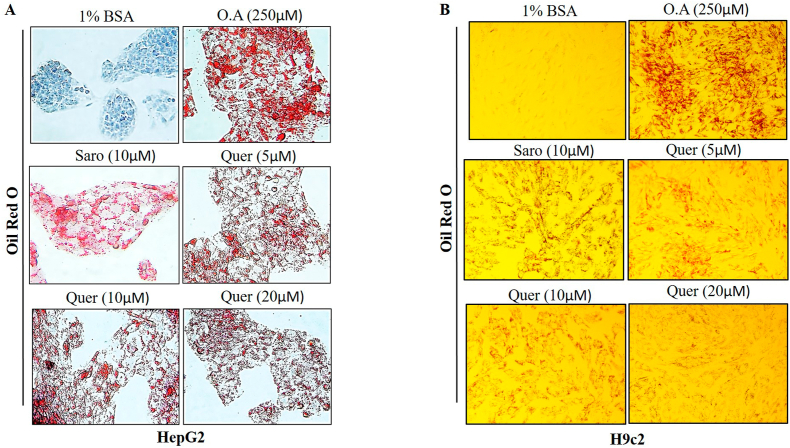
To elucidate the protective effect of Quercetin on fatty acid-induced cellular hepatic steatosis and cardiac lipotoxicity (HepG2 and H9c2 respectively), we induced lipotoxicity by treating cells with free fatty acid (oleic acid 250 μM) concentration which was determined by cell viability (Supplementary Fig. 3A). After 24 h of induction, we observed significant visible lipid droplets. Then, we treated fatty acid-treated cells with dose-dependent concentrations of Quercetin (5, 10, and 20 μM). The cytotoxic effect of Quercetin on HepG2 and H9c2 was optimized before the treatment through WST-1 cell viability assay (Supplementary Figs. 3B and C). After 24 h of Quercetin treatment, we found a gradual decrease in lipid accumulation, a decreased number of total lipid droplets as well as a reduced size of the droplets in dose-dependent concentration of Quercetin compared to the oleic acid treated group (Fig. 4A and B). A semi-quantitative analysis of lipid accumulation was measured in both HepG2 and H9c2 cells (Supplementary Figs. 3D and E). These results concluded that fatty acids-induced steatosis in hepatic and cardiac cells was significantly ameliorated by Quercetin treatment in a dose -dependent manner.
Fig. 4.
Lipid accumulation suppressing and cytoprotective effect of Quercetin on fatty acid exposed HepG2 and H9C2 cell lines. (A–B) Oil Red O staining of steatosis induced by Oleic acid. Images were obtained at 20× magnification and scale bar: 100 μm). (C-D) Lipid content of steatosis induced by oleic acid. Data are represented as the mean ± SEM from 5 mice in each group.
3.5. Quercetin improved hepatic injury in WDSW fed mice
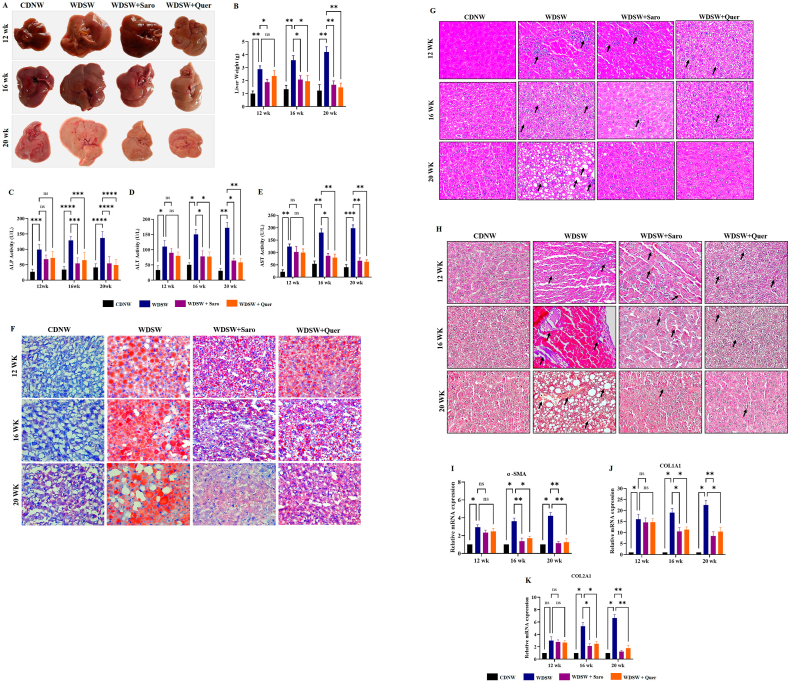
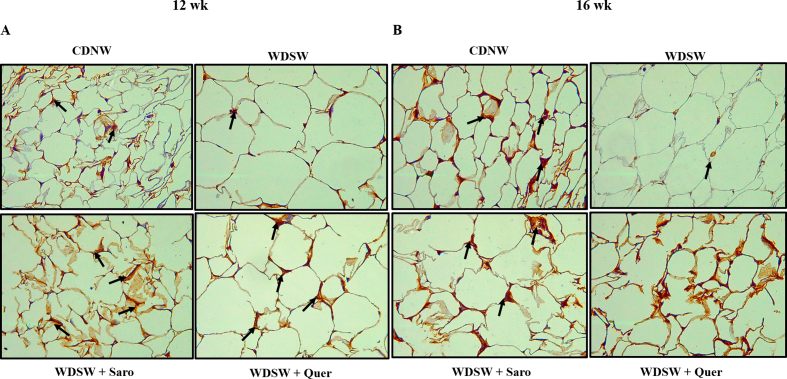
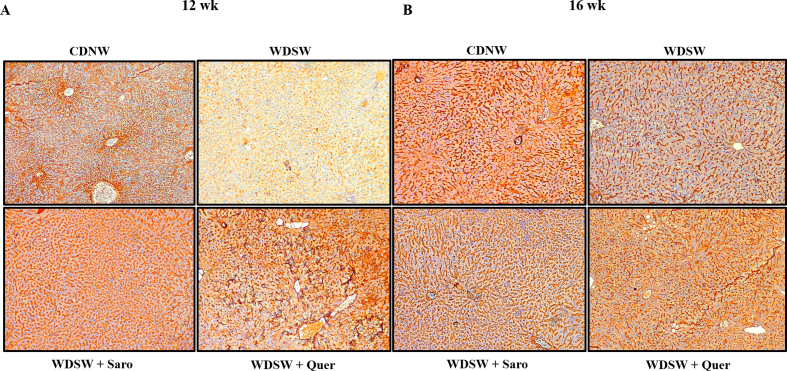
Steatosis is one of the hallmarks of patients with chronic fatty liver diseases. The consumption of a western diet may cause excess accumulation of lipids and fats within the hepatocytes of the liver and lead to MAFLD and advanced metabolic associated steatohepatitis MASH [24]. If untreated in this stage it may further continue to develop cirrhosis and final-stage liver disease like Hepatocellular Carcinoma (HCC). Therefore, preventing MASH is the need of the hour. Mice fed with the WDSW group showed an increase in liver weight as well as morphological changes compared to CDNW. Quercetin treatment gradually decreased the liver weight and 12 weeks of Quercetin treatment in mice almost reversed the MASH and these livers resembled CDNW fed mice livers in terms of their overall weight and morphology (Fig. 5A and B). Elevated levels of serum liver enzymes are the primary indication of liver injury. To determine the degree of liver injury, we measured the liver enzymes such as ALT, AST, and ALP by using commercially available kits. As expected, we found an elevation of liver enzymes in mice that were fed with WDSW. Quercetin treatment gradually decreased the level of liver enzymes unanimously at all treatment intervals of 4, 8, and 12 weeks respectively (Fig. 5C–E). Excess fat accumulation in the liver may trigger the increased expression of various inflammatory and fibrosis markers which may play a prominent contribution in the development of hepatocyte ballooning and fibrosis in liver tissue. To determine hepatocyte ballooning, we performed H&E and Oil Red O staining where we observed the accumulation of lipid droplets and hepatocyte ballooning in WDSW diet fed mice liver tissue when compared to the CDNW mice group. Here also, Quercetin treatment significantly reduced the lipid accumulation in both the size and number of lipid droplets and improvised hepatocyte ballooning when compared to WDSW fed mice (Fig. 5F and G). MTS fibrosis staining also yielded a similar array of results. A higher degree of fibrosis was observed in 20 weeks of WDSW fed mice against the CDNW mice group. Quercetin treatment gradually reduced the degree of fibrosis in all the three treatment groups as compared to WDSW fed mice (Fig. 5H). Further, we investigated the mRNA expression of molecular markers of fibrosis like collagen type 1, collagen type 2, and alpha-SMA in the liver. We could find that Quercetin treatment resulted in notable reduction of the expression of fibrosis markers when compared to WDSW fed mice (Fig. 5I–K). All these findings strongly support that, Quercetin could alleviate WDSW-induced obesity and its associated hepatic damage, inflammation, and fibrosis.
Fig. 5.
Quercetin improved hepatic injury in WDSW fed mice. (A) Morphology images of the liver. (B) Liver weight. (C) ALP. (D) AST. (E) ALT. (F) Oil Red O staining of liver tissue. (G) H&E staining, (H) TMS staining, (I–K) Relative mRNA expression of fibrosis target genes in liver tissue. For all images, magnification: 40× and Scale bar: 50 μm. Data are represented as the mean ± SEM from 5 mice in each group.
3.6. Quercetin ameliorates cardiac inflammation and fibrosis in WDSW fed mice
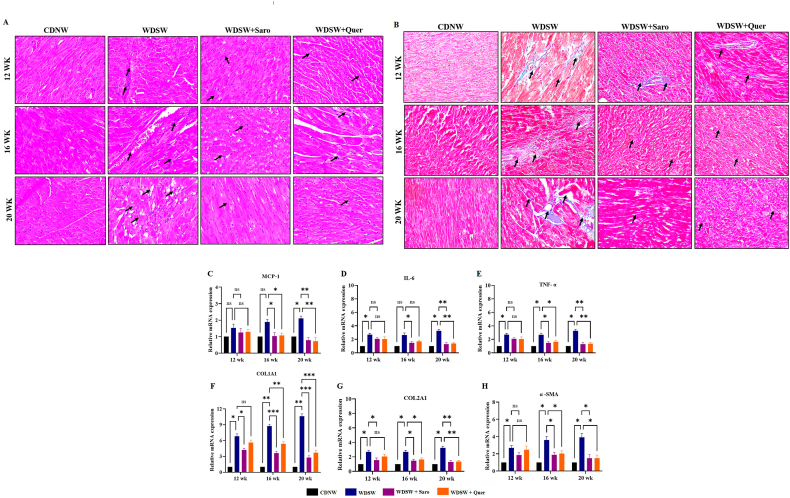
Studies have shown that obesity is a main contributor to the development and advancement of cardiovascular diseases and cardiac dysfunction [25,26]. To determine the effect of prolonged consumption of a western diet on cardiac physiology, we performed H&E and TMS staining to assess the level of cardiac inflammation and fibrosis-associated injury. As expected, WDSW fed mice showed a higher degree of cardiac inflammation and fibrosis which are the key drivers of cardiovascular diseases. Our histopathology results revealed that Quercetin ameliorated cardiac inflammation and fibrosis compared to the WDSW fed mice group (Fig. 6A and B). We also measured the inflammatory markers mRNA expression in cardiac tissue, we found that Quercetin significantly reduced the levels of inflammatory markers (MCP-1, IL-6, and TNF-α mRNAs) (Fig. 6C–E). We then measured the expression of fibrosis specific genes such as collagen type 1, collagen type 2, and alpha-SMA. Quercetin treated groups showed a gradual decrease in the expression of fibrosis markers for up to 12 weeks (Fig. 6F–H). Our results concluded that Quercetin potentially exhibited a cardioprotective role in WDSW induced obesity-associated cardiac injury.
Fig. 6.
Quercetin ameliorates cardiac inflammation and fibrosis in WDSW diet fed mice. (A) H& E staining, (B) TMS staining, Relative mRNA expression of inflammatory markers in heart tissue. (C) MCP-1. (D) IL-6. (E) TNF-α relative mRNA expression of fibrosis target genes in heart tissue. (F) COL1A1. (G) COLA2A1. (H) α-SMA. For all images, magnification: 40× and Scale bar: 50 μm. Data are represented as the mean ± SEM from 5 mice in each group.
3.7. Quercetin intake suppresses NF-κB activity in adipose tissue of WDSW diet induced Swiss albino mice
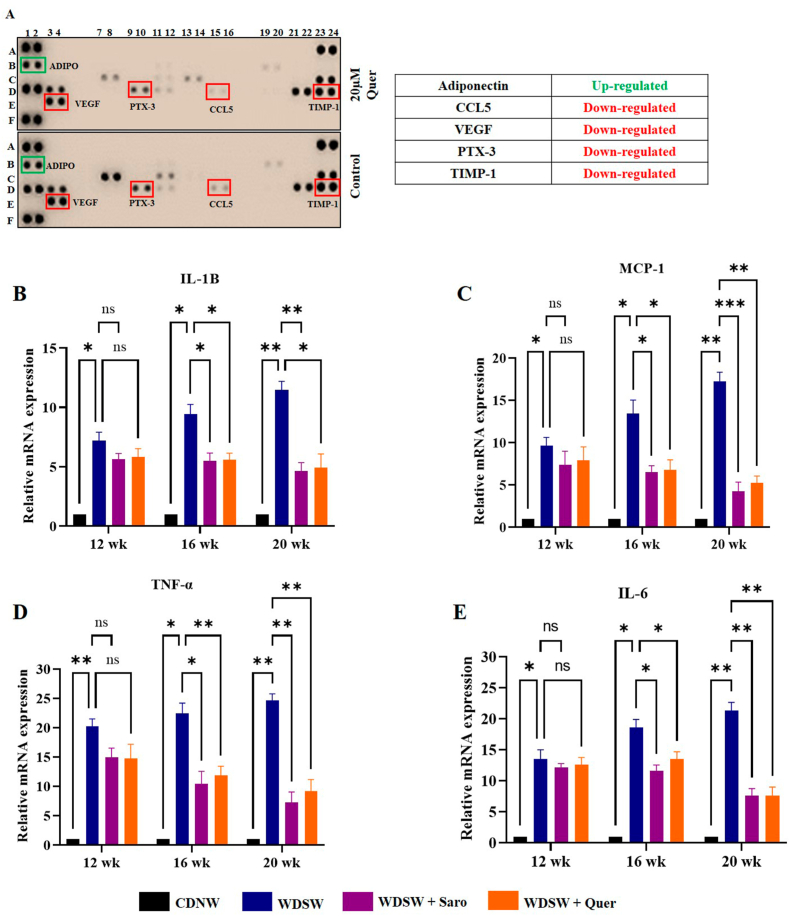
Obesity is associated with excess fat deposition in adipose tissue which stimulates the secretion of various inflammatory mediators which are the key drivers to a pro-inflammatory state and oxidative stress [27]. Analysing the expression profile of obesity-related proteins is essential for understanding the roles these molecules play in obesity and associated illnesses such as diabetes and cardiovascular disease. To determine the effect of Quercetin inhibition on adipokines production, we first identified the differentially secreted adipokines in the cell culture supernatants from 3T3-L1 mature adipocytes using an adipokine array, surprisingly, we found that, 5 adipokines that were down-regulated in Quercetin treated mature adipocyte cells compared to untreated mature adipocytes. Quercetin (25 μM) treatment decreased the secretion of various adipokines and cytokines like RANTES, VEGF, PTX3, and TIMP-1 in mature 3T3-L1 adipocytes (Fig. 7A). Additionally, we observed the upregulation of adiponectin, which is an adipocytokine that may have an adipocyte protective role. The RANTES, VEGF, PTX3, and TIMP-1 are very well known for their pivotal role in differentiation, angiogenesis, and inflammation [28]. Interestingly, these factors are the target genes of NF-κB, which is a master regulator of various inflammatory signaling pathways [29]. Although several studies have explored the effect of obesity on inflammatory mediators, surprisingly few studies have directly compared the activation of NF-κB itself in obese individuals compared with lean controls [30,31]. To support this data, we measured inflammatory markers like TNF-α, IL-6, IL-1β, and MCP-1 which are the direct target genes of NF-κB. Quercetin treatment gradually decreased the inflammation-related mRNA expression when compared to the WDSW fed mice group (Fig. 7B–E).
Fig. 7.
Quercetin intake suppresses NF-κB activity in adipose tissue of WDSW diet induced Swiss albino mice. (A) Adipokine array (B) Relative mRNA expression of inflammatory markers in adipose tissue. (G) IL-1β. (H) MCP-1. (I) TNF-α. (J) IL-6.
3.8. Quercetin regulates MAFLD and cardiac lipotoxicity through Adiponectin/AdipoR2 signaling pathway activation
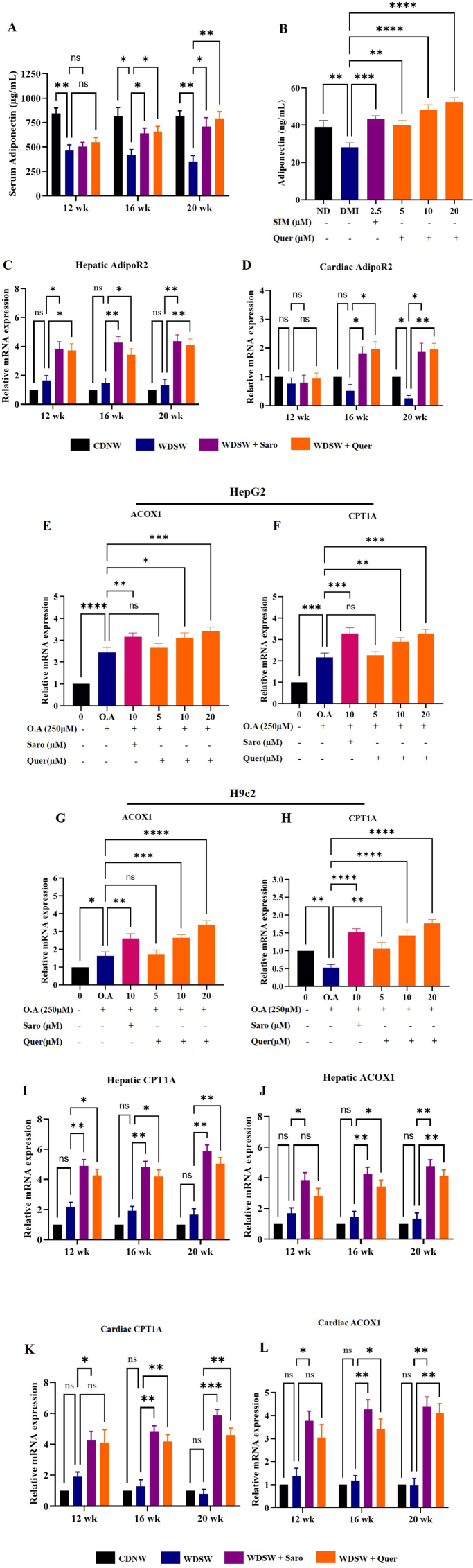
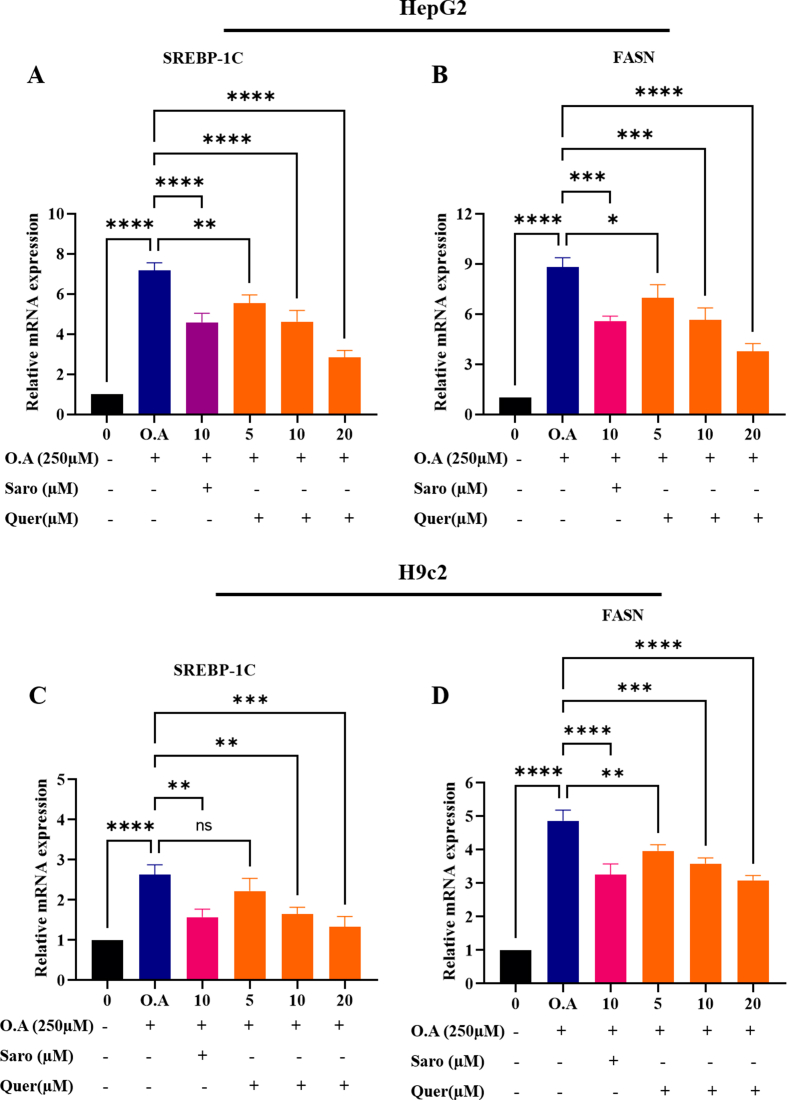
Since inflammation serves as a key hallmark in the pathogenesis and progression of steatohepatitis and cardiac cell damage, fibrosis in WDSW fed mice. Thus, modulation and induction of anti-inflammatory responses may serve as a potential strategy to treat obesity-associated steatohepatitis and cardiac lipotoxicity. Since previous studies have shown that adiponectin an adipokine secreted from adipose tissue acts as an anti-inflammatory molecule, we performed ELISA to analyse the levels of adiponectin in both cell culture supernatants and in mice serum. Our results showed that adiponectin concentration was significantly decreased in differentiated or mature adipocytes in comparison with the non-differentiated 3T3-L1 pre-adipocytes. Quercetin treatments at of 5, 10, and 20 μM concentrations exhibited a remarkable increase in the adiponectin concentration compared to differentiated mature adipocytes. Similar results were also found in our in-vivo study, where Quercetin treated mice showed a gradual increase in the concentration of adiponectin in 8 and 12 weeks compared to WDSW fed mice, but there were no significant changes in 4 weeks (Fig. 8A and B). As, adiponectin exerts its effect by binding to its receptors (AdipoR1 and AdopoR2) and play a major role in the lipid homeostasis and glucose metabolism, we measured the AdipoR2 mRNA expression which is mostly expressed in the liver, and the heart. Interestingly, hepatic and cardiac tissues also showed increased expression of AdipoR2 mRNA upon Quercetin treatment as compared to WDSW fed mice (Fig. 8C and D). Some studies suggest that AdipoR2 activates PPAR-α which belongs to the peroxisome proliferator-activated receptor family and it plays a major role in glucose and lipid homeostasis [32,33]. The PPAR-α activation aids in the inhibition of fatty acid synthesis transcription factor and enzymes (SREBP1-c and FASN) and enhances the β-oxidation of fatty acid. Therefore, we measured the mRNA expression of PPAR-α target genes (CPT1A and ACOX1), Quercetin treated group effectively increased the expression of CPT1A and ACOX1 in dose dependent manner in both HepG2 and H9c2 oleic acid treated cells (Fig. 8E–H). Meanwhile, we also observed Quercetin treated mice showed a significant increase in the mRNA of PPAR-α target genes in 16 and 20 weeks treatment groups as compared with the WDSW mice group (Fig. 8I-L). To determine the effect of Quercetin on fatty acid synthesis enzymes, we measured the mRNA expression of SREBP1-c and FASN in-vitro. We observed that Quercetin treated group effectively decreased the expression of fatty acid synthesis enzymes in a dose-dependent manner in both HepG2 and H9c2 oleic acid treated cells (Supplementary Figs. 4A and D). Collectively, these data demonstrated that Quercetin significantly increased adiponectin and its receptor expression exerting a hepato/cardioprotective effect through activation of PPARα/γ target genes thereby modulating the inflammatory pathways.
Fig. 8.
Quercetin regulates MAFLD and cardiac lipotoxicity through Adiponectin/AdipoR2 signalling pathway activation. (A) Adiponectin level in 3T3-L1 condition media. (B) Serum adiponectin level. (C) Relative hepatic AdipoR2 mRNA expression in liver tissue. (E) Relative cardiac AdipoR2 mRNA expression in heart tissues. Relative mRNA expression of PPAR-α target genes. (E–F) HepG2, (G-H) H9c2, (I-J) Hepatic (K-L) Cardiac. Data are represented as the mean ± SEM from 5 mice in each group.
3.9. Quercetin treatment leads to Adiponectin/AdipoR2 activation via VDR
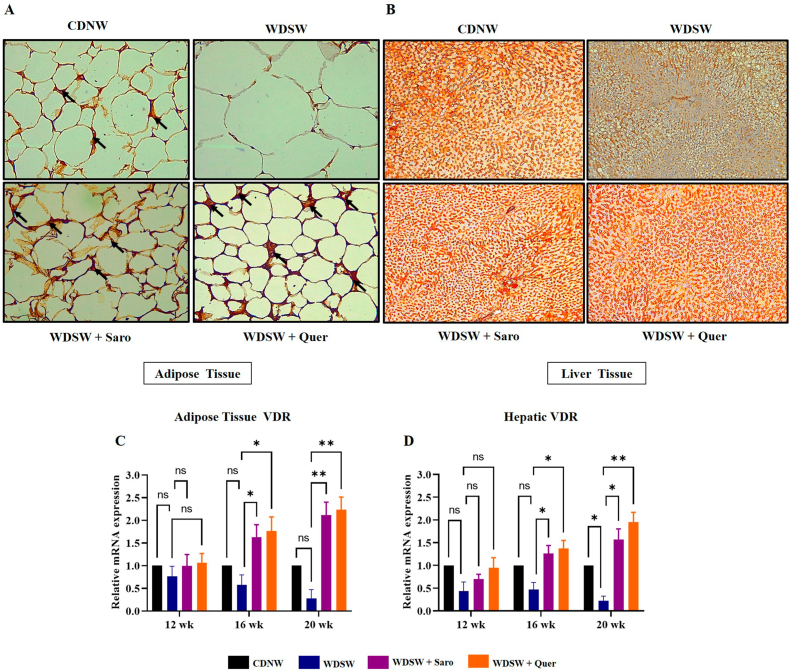
Recently, targeting the Adipo/AdipoR2 signalling pathway represents an attractive therapeutic approach for obesity-associated MAFLD and cardiac lipotoxicity which is involved in the modulation of glucose and lipid homeostasis. Recent studies have speculated that VDR activation in adipose tissue may lead to the inhibition of NF-κB transcriptional activity in obesity [34]. Meanwhile, hepatic activation of VDR could improve the hepatic fibrosis through the inhibition of the TGF-β signaling pathway [35]. Therefore, it will be interesting to understand the mechanism and effect of Quercetin on lipid homeostasis through Adipo/AdipoR2 activation, Based on these facts, we speculated that VDR-mediated inhibition of obesity-associated MAFLD and cardiac injury by Quercetin could be attributed to the activation of Adipo/AdipoR2 signalling pathway. Interestingly immunohistochemistry results showed that Quercetin effectively enhances the expression of VDR in both adipose and liver tissue at 20 weeks (Fig. 9A and B) whereas the expression of VDR was almost nil in the mice fed with WDSW for 20 weeks. The adipose and hepatic expression of VDR at 12 and 16 weeks of time interval are mentioned in Supplementary Figs. 5A–D. We also measured the mRNA expression of VDR in both adipose tissue and liver (Fig. 9C and D) which also showed a significant increase in the expression of VDR in dose dependent manner. All these data suggests that Quercetin improved lipid homeostasis through VDR activation and subsequent downstream Adipo/AdipoR2 signalling.
Fig. 9.
Quercetin treatment leads to Adiponectin/AdipoR2 activation via VDR. (A) VDR expression in adipose tissue at 20 weeks. (B) VDR expression in liver tissue at 20 weeks. Relative mRNA expression of VDR gene. (C) Adipose tissue. (B) Liver. For all images, magnification: 40× and Scale bar: 50 μm. Data are represented as the mean ± SEM from 5 mice in each group.
4. Discussion
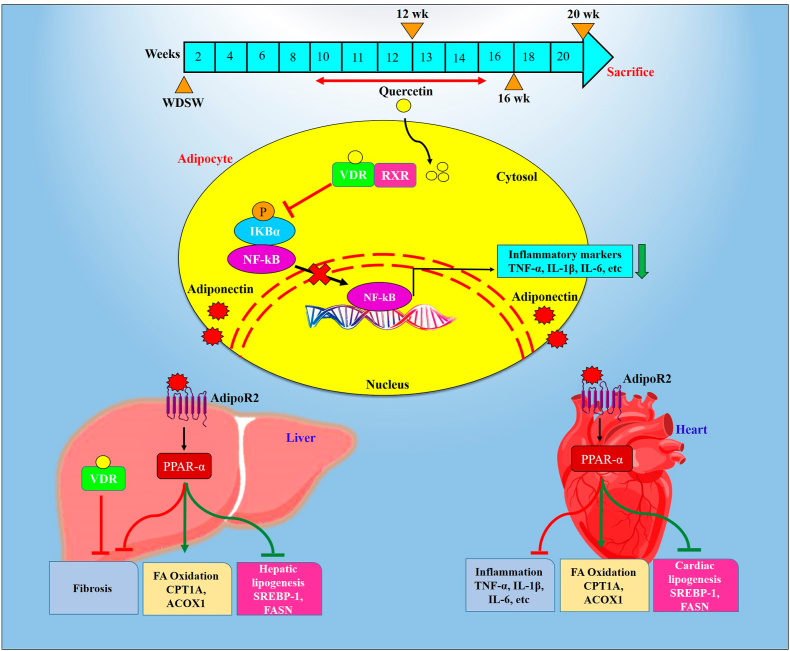
Through our extensive study, we elucidated the novel role of Quercetin and demonstrated its therapeutic role on metabolic syndrome associated obesity, cardiac lipotoxicity, and steatohepatitis. MS burden has risen due to sedentary lifestyles and consumption of excess high-calorie/processed diet resulting in a spectrum of diseases including obesity, T2D, CVDs, MAFLD, and dyslipidaemia [36]. Switching to proper dietary habits and adopting appropriate life-style is critical in preventing MS. In this study, using cell lines and mouse models, we explored the beneficial effects of Quercetin and its mechanistic role in the suppression and prevention of MS, and its associated pathologies like obesity, MAFLD, and cardiac injury (Fig. 10).
Fig. 10.
Schematic representation depicting the cross-talk mechanisms involved in western diet induced metabolic syndrome and therapeutic effect of Quercetin. Western diet induced obesity associated MAFLD and cardiac fibrosis finally causes metabolic syndrome. Quercetin treatment activates VDR in adipose tissue and aid in the inhibition of the activity of NF-κB. Quercetin treatment with WDSW diet up to 20 weeks ameliorated obesity associated MAFLD and cardiac fibrosis by inhibiting the lipid accumulation, inflammation and fibrosis through Adipo/AdipoR2 signalling via VDR activation. This provides experimental evidences that Quercetin has multifaceted potential as a therapeutic drug to treat metabolic syndrome.
Excess fat accumulation in AHL during MS increases the morbidity and mortality through complications, like T2D, hypertension, insulin resistance, MAFLD and CVDs. Some studies strongly believe that excess accumulation and storage of fat in the abdominal region is one of the primary hits for the development and long term progression of chronic metabolic syndrome [37,38]. In our study, obese mice treated with Quercetin at different time periods, showed significant reductions in animals body weight and fat mass. A significant reduction was also observed in terms of the blood glucose level upon Quercetin administration in these animals. The 3T3-L1 murine pre-adipocyte cell line is a well-known model for in-vitro obesity-related studies. Our in-vitro data using 3T3-L1 also demonstrated that Quercetin treatment reduced lipid accumulation as lipid droplets in a dose-dependent manner. Further, Quercetin exhibited a lipolytic effect in 3T3-L1 cells and a significant increase in free glycerol was observed in the treated group suggesting an enhanced lipolysis. Furthermore, intracellular triglyceride levels was reduced, supporting the claim of Quercetin-mediated lipolysis. All these results suggest the effect of Quercetin in mitigating obesity which is comparable to that of simvastatin (a well-known lipid-lowering drug) which was used as a positive control in our study. Dyslipidaemia characterized by elevated triglycerides and cholesterol are of paramount significance in causing MS associated complications like CVDs. We found that Quercetin improved the lipid profile levels. Quercetin supplemented WDSW mice had higher levels of high-density lipoprotein (HDL), which is known to have a cardioprotective effect. Several studies have shown that increased adipose tissue mass directly contributes to the onset of inflammation at an early stage resulting in of the progression of MS-associated complications [26,37]. Our H&E tissue staining results from the treatment group showed a significant reduction in the adipocyte cell size and numbers, suggesting a possible protective effect against western diet induced inflammation. All these results supplements to the possible effects of Quercetin as an anti-obesity and anti-inflammatory drug.
Lipotoxicity plays a pivotal task in the pathogenesis of MAFLD and cardiac dysfunction which acts as a contributor to MS [39,40]. Lasting effects of lipotoxicity results in the damage of the liver and heart through steatohepatitis and cardiac fibrosis. The exorbitant uptake of free fatty acids like sodium palmitate and oleic acid induces damage to hepatocytes and cardiac cells through the activation of inflammatory response [41,42]. Through our in-vitro study we provide an empirical proof that Quercetin can reduce the lipid accumulation in free fatty acid-induced steatotic HepG2 (liver) and H9c2 (cardiac) cells suggesting that Quercetin can mitigate lipid-induced cellular damage.
Obesity is a major risk factor for MAFLD. Liver is one of the primary target of lipid-mediated organ damage. Excess triglyceride accumulation as lipid droplets within hepatocytes can result in hepatocyte injury, and fibrosis. Our study revealed that WDSW diet caused hepatomegaly and damaged the microstructure of the liver, which improved upon Quercetin treatment. Further, the histopathological staining results demonstrated that Quercetin significantly reduced the lipid accumulation, hepatic ballooning, inflammation, and fibrosis which was caused by high calorie western diet consumption. Furthermore, gene expression results depicted that Quercetin reduced the expression of pro-fibrogenic target genes (COL1A1, COL2A1, and α-SMA). Also, Quercetin treatment improved the liver functions which is evident through reduced levels of liver enzymes like ALP, AST, and ALT. Collectively, these findings strongly suggested that Quercetin has a potent hepatoprotective effect through effective regulation of lipid accumulation, inflammation, and fibrosis induced by the WDSW diet.
Multiple studies have demonstrated that excess fat storage promotes the risk of developing CVDs [43,44]. As per our histopathological findings, Quercetin treated mice exhibited reduced cardiac inflammation and fibrosis caused by WDSW. Quercetin treatment experimentally could reduce the expressions of inflammatory markers (TNF-α, IL-6 and MCP-1), and fibrosis markers (COL1A1, COL2A1, and α-SMA) respectively, supporting our claim of cardioprotective action of Quercetin on WDSW induced cardiac inflammation and fibrosis.
NF-κB transcription factor is a master regulator of genes involved in inflammation [45,46]. In adipokine array, we could find a panel of NF-κB target cytokines [29], RANTEES, VEGF, PTX3, and TIMP-1 as down regulated genes upon Quercetin treatment. Other inflammatory markers like TNF-α, IL-1β, IL-6, and MCP-1 expressions were also observed to be decreased in the treatment group. This projects Quercetin as a potential anti-inflammatory regiment for improving inflammatory responses in MS.
Adiponectin (Adipo), an adipokine secreted by adipocytes or fat cells is well-known for regulating glucose and lipid metabolism [47]. Previous studies have shown that adiponectin knockout mice on a high-calorie diet developed hepatic lipid accumulation and that adiponectin supplementation reversed the effect [48]. Existing literature also suggest that adiponectin preserves the normal physiology of the heart by reducing inflammation and fibrosis [49,50]. It is well studied and reported that adiponectin has an anti-inflammatory and anti-fibrogenic effect but there are no reports on how Quercetin induces adiponectin and its receptor expression. With this background, we hypothesized that Quercetin can improve adiponectin levels and reported that an increase in the serum levels of adiponectin and in the supernatants of treated 3T3-L1 adipocytes. Quercetin exposure to 3T3-L1 cells increased adiponectin secretion and inhibited adipogenesis in dose dose-dependent manner. In support of our observations and claims, an earlier study also showed an impaired adiponectin signalling mechanism with decreased expression of adiponectin receptors in obese individuals [51]. Adiponectin binding to AdipoR2 exerts its function in regulating energy homeostasis and anti-inflammatory response. Other studies also suggest that upregulation of adiponectin and its receptor activate PPAR-α which is involved in the inhibition of fatty acid synthesis enzymes, and lipogenesis and enhances beta-oxidation [33,52]. In our study, we reported that Quercetin treatment increased the mRNA expression of AdipoR2 in the liver as well as in the heart tissues. Gene expression profiling confirmed that Quercetin treatment significantly upregulated the mRNA expression of PPAR-α target genes (CPT1A and ACOX1) and down regulated the mRNA expression of SREBP1-c and FASN both in the liver and in the heart. All these findings further strongly support our claim that Quercetin exerts its anti-obesity, cardioprotective, and hepatoprotective effect via increased adiponectin secretions from adipocytes which are released into the blood stream making it available for AdipoR2 activation in the liver and the heart.
Studies have shown that Vitamin D receptor (VDR) play a pivotal role in reducing inflammation and fibrosis [35,53]. VDR is predominantly expressed in the adipose tissue, liver, and other tissues where its activation is expected to prevent obesity-associated inflammation and fibrosis. According to a recent study, activation of VDR in adipose tissue inhibits the NF-κB activation [54]. Another study reported that activation of hepatic VDR inhibits TGF-β which aids in the suppression of hepatic fibrosis [55]. These findings suggest that discovering a potential VDR agonist could be extremely beneficial for the inhibition of MS. We investigated the efficacy of Quercetin as a VDR agonist in preventing and reversing the symptoms of metabolic complications in our study. One of our recent docking studies revealed that Quercetin effectively binds to VDR which was involved in the inhibition of hepatic inflammation and fibrosis induced by breast cancer [56]. It is known that obesity and metabolic syndrome increases the risk of breast cancer [57]. Therefore, based on our previous study, we were interested in exploring the action of Quercetin on VDR in the inhibition of MS. Surprisingly, our immunohistochemistry and gene expression validation data revealed that Quercetin significantly increased the expression of VDR in adipose tissue and liver, whose expression was found to be almost nil in WDSW fed mice making our study the first to elucidate that Quercetin can act as a VDR agonist and can modify the adipokine levels by improving adiponectin and AdipoR2 expressions.
5. Conclusion
According to our knowledge and from extensive recent literature studies, this is the first study that elucidates the roles of Quercetin in reducing adipogenesis, cardiac fibrosis, and steatohepatitis and other metabolic complications through Adipo/AdipoR2 signalling via VDR activation. Our study further offers novel dimensions towards therapeutic strategies to prevent metabolic syndrome by enhancing constant crosstalk between the adipose tissue, the heart, and the liver at a larger picture. We believe that our findings have translational importance and aids better understanding of the multifaceted therapeutic role of Quercetin in the mitigation of metabolic syndrome.
Funding
This work was partially supported by Ramalingaswami Re-entry fellowship, Department of Biotechnology (DBT), Govt. of India to PKS and DBT-BUILDER scheme to JSS AHER with sanction number: BT/INF/22/SP43045/2021. NGS acknowledges ICMR (No.3/1/2/282/2021-Nut) for awarding Senior Research Fellowship.
Ethical approval
The Institutional Animal Ethics Committee (JSSAHER/CPT/IAEC/012/2020), JSS Medical College, JSS AHER, Mysore, approved the animal experimental procedure and all experiments were conducted according to the guidelines of the Committee for Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
CRediT authorship contribution statement
Nirmala G. Sannappa Gowda: Writing – original draft, Methodology, Data curation. Varsha D. Shiragannavar: Writing – review & editing, Formal analysis, Data curation. Shreyas H. Karunakara: Writing – review & editing, Formal analysis. Ravindra P. Veeranna: Writing – review & editing, Investigation, Formal analysis. Deepak Suvarna: Writing – review & editing, Formal analysis. Divya P. Kumar: Writing – review & editing, Investigation, Formal analysis. Prasanna K. Santhekadur: Writing – review & editing, Supervision, Project administration, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors gratefully acknowledge the JSS Medical College and JSS AHER for their constant support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2024.101754.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
Data availability
Data will be made available on request.
References
- 1.Noubiap J.J., Nansseu J.R., Lontchi-Yimagou E., Nkeck J.R., Nyaga U.F., Ngouo A.T., et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: a systematic review and modelling analysis. Lancet Child Adolesc. Health. 2022;6(3):158–170. doi: 10.1016/S2352-4642(21)00374-6. [DOI] [PubMed] [Google Scholar]
- 2.ochlani Y., Pothineni N.V., Kovelamudi S., Mehta J.L. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017;11(8):215–225. doi: 10.1177/1753944717711379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santhekadur P.K., Kumar D.P., Seneshaw M., Mirshahi F., Sanyal A.J. The multifaceted role of natriuretic peptides in metabolic syndrome. Biomed. Pharmacother. 2017;92:826–835. doi: 10.1016/j.biopha.2017.05.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fruh S.M. Obesity: risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017;29:S3–S14. doi: 10.1002/2327-6924.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han T.S., Lean M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016;5 doi: 10.1177/2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiragannavar V.D., Sannappa Gowda N.G., Puttahanumantharayappa L.D., Karunakara S.H., Bhat S., Prasad S.K., Kumar D.P., Santhekadur P.K. The ameliorating effect of withaferin A on high-fat diet-induced non-alcoholic fatty liver disease by acting as an LXR/FXR dual receptor activator. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1135952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivas A.N., Suresh D., Suvarna D., Pathak P., Giri S., SumanSatish S., Chidambaram S.B., Kumar D.P. Unraveling the potential role of tecomella undulata in experimental NASH. Int. J. Mol. Sci. 2023;24(4):3244. doi: 10.3390/ijms24043244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batiha G.E., Beshbishy A.M., Ikram M., Mulla Z.S., El-Hack M.E.A., Taha A.E., Algammal A.M., Elewa Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods. 2020;9(3):374. doi: 10.3390/foods9030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NeriNuma I.A., Cazarin C.B.B., Ruiz A.L.T.G., Paulino B.N., Molina G., Pastore G.M. Targeting flavonoids on modulation of metabolic syndrome. J. Funct.Foods. 2020;73 doi: 10.1016/j.jff.2020.104132. [DOI] [Google Scholar]
- 10.Ghadge A.A., Khaire A.A., Adiponectin A.A. Kuvalekar. A potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018;39:151–158. doi: 10.1016/j.cytogfr.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Son Y.J., Jung D.S., Shin J.M., et al. Heracleum dissectum Ledeb. Ethanol extract attenuates metabolic syndrome symptoms in high-fat diet-induced obese mice by activating adiponectin/AMPK signaling. J. Funct.Foods. 2021;84 doi: 10.1016/j.jff.2021.104581. [DOI] [Google Scholar]
- 12.Iwabu M., Okada-Iwabu M., Yamauchi T., Kadowaki T. Adiponectin/AdipoR Research and its implications for lifestyle-related diseases. Front. Cardiovasc. Med. 2019;6:116. doi: 10.3389/fcvm.2019.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., Zhang D., Vatner D.F., Goedeke L., Hirabara S.M., Zhang Y., Perry R.J., Shulman G.I. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc. Natl. Acad. Sci. U. S. A. 2020;117(51):32584–32593. doi: 10.1073/pnas.1922169117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin T., Lu W., Gong X., Zhou J., Wu F. Association of vitamin D receptor polymorphisms with metabolic syndrome-related components: a cross-sectional study. J. Clin. Lab. Anal. 2021;35(7) doi: 10.1002/jcla.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fronczek M., Osadnik T., Banach M. Impact of vitamin D receptor polymorphisms in selected metabolic disorders. Curr. Opin. Clin. Nutr. Metab. Care. 2023;26(4):1316–1322. doi: 10.1097/MCO.0000000000000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nimitphong H., Guo W., Holick M.F., Fried S.K., Lee M.J. Vitamin D inhibits adipokine production and inflammatory signaling through the vitamin D receptor in human adipocytes. Obesity. 2021;29(3):562–568. doi: 10.1002/oby.23109. [DOI] [PubMed] [Google Scholar]
- 17.Lu W., Li X., Liu N., Zhang Y., Li Y., Pan Y., Yang J., Liu Z., Kong J. Vitamin D alleviates liver fibrosis by inhibiting histidine-rich calcium binding protein (HRC) Chem. Biol. Interact. 2021;25:109334–109355. doi: 10.1016/j.cbi.2020.109355. [DOI] [PubMed] [Google Scholar]
- 18.Kumar D.P., Caffrey R., Marioneaux J., Santhekadur P.K., Bhat M., Alonso C., Koduru S.V., Philip B., Jain M.R., Giri S.R., Bedossa P., Sanyal A.J. The PPAR α/γ agonist saroglitazar improves insulin resistance and steatohepatitis in a diet induced animal model of nonalcoholic fatty liver disease. Sci. Rep. 2020;10(1):9330. doi: 10.1038/s41598-020-66458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sikder K., Kesh S.B., Das N., Manna K., Dey S. The high antioxidative power of Quercetin (aglycone flavonoid) and its glycone (rutin) avert high cholesterol diet induced hepatotoxicity and inflammation in Swiss albino mice. Food Funct. 2014;5:1294–1303. doi: 10.1039/c3fo60526d. [DOI] [PubMed] [Google Scholar]
- 20.Cao J., Dai D.L., Yao L., Yu H.H., Ning B., Zhang Q., et al. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol. Cell. Biochem. 2012;364:15–29. doi: 10.1007/s11010-011-1211-9. [DOI] [PubMed] [Google Scholar]
- 21.Tong Y., Xu S., Huang L., Chen C. Obesity and insulin resistance: pathophysiology and treatment. Drug Discov. Today. 2022;27(3):822–830. doi: 10.1016/j.drudis.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Cave E., Crowther N.J. The use of 3T3-L1 murine preadipocytes as a model of adipogenesis. Methods Mol. Biol. 2019;19:263–272. doi: 10.1007/978-1-4939-8994-2_25. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz A., Birk R. Adipose tissue hyperplasia and hypertrophy in common and syndromic obesity-the case of BBS obesity. Nutrients. 2023;15(15):3445. doi: 10.3390/nu15153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velázquez K.T., Enos R.T., Bader J.E., Sougiannis A.T., Carson M.S., Chatzistamou I., et al. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 2019;11:619–637. doi: 10.4254/wjh.v11.i8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediat. Inflamm. 2010 doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feriani A., Bizzarri M., Tir M., Aldawood N., Alobaid H., Allagui M.S., et al. High-fat diet-induced aggravation of cardiovascular impairment in permethrin-treated Wistar rats. Ecotoxicol. Environ. Saf. 2021;222 doi: 10.1016/j.ecoenv.2021.112461. [DOI] [PubMed] [Google Scholar]
- 27.Rohm T.V., Meier D.T., Olefsky J.M., Donath M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiragannavar V.D., Sannappa Gowda N.G., Kumar D.P., Mirshahi F., Santhekadur P.K. Withaferin A acts as a novel regulator of liver X receptor-α in HCC. Front. Oncol. 2021;10 doi: 10.3389/fonc.2020.628506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh S., Hayden M.S. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 30.Carlsen H., Haugen F., Zadelaar S., Kleemann R., Kooistra T., Drevon C.A., Blomhoff R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009;4(3):215–222. doi: 10.1007/s12263-009-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harte A.L., Tripathi G., Piya M.K., Barber T.M., Clapham J.C., Al-Daghri N., et al. NFκB as a potent regulator of inflammation in human adipose tissue, influenced by depot, adiposity, T2DM status, and TNFα. Obesity. 2013;21(11):2322–2330. doi: 10.1002/oby.20336. [DOI] [PubMed] [Google Scholar]
- 32.Souza-Mello V. Peroxisome proliferator-activated receptors as targets to treat non-alcoholic fatty liver disease. World J. Hepatol. 2015;7(8):1012–1019. doi: 10.4254/wjh.v7.i8.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y., Park C.W. Mechanisms of adiponectin action: implication of adiponectin receptor agonism in diabetic kidney disease. Int. J. Mol. Sci. 2019;20(7):1782. doi: 10.3390/ijms20071782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang R., Wang M., Wang M., Zhang L., Ding Y., Tang Z., et al. Vitamin D level and vitamin D receptor genetic variation were involved in the risk of non-alcoholic fatty liver disease: a case-control study. Front. Endocrinol. 2021;6 doi: 10.3389/fendo.2021.648844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonas M.I., Kuryłowicz A., Bartoszewicz Z., Lisik W., Jonas M., Kozniewski K., Puzianowska-Kuznicka M. Vitamin D receptor gene expression in adipose tissue of obese individuals is regulated by miRNA and correlates with the pro-inflammatory cytokine level. Int. J. Mol. Sci. 2019;20(21):5272. doi: 10.3390/ijms20215272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Després J.P. Abdominal obesity: the most prevalent cause of the metabolic syndrome and related cardiometabolic risk. Eur. Heart J. Suppl. 2006;8:B4–B12. doi: 10.1093/eurheartj/sul002. [DOI] [Google Scholar]
- 37.Zhang W., Lu J., Wang Y., Sun P., Gao T., Xu N., Zhang Y., Xie W. Canagliflozin attenuates lipotoxicity in cardiomyocytes by inhibiting inflammation and ferroptosis through activating AMPK pathway. Int. J. Mol. Sci. 2023;4(1):858. doi: 10.3390/ijms24010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Branković M., Jovanović I., Dukić M., Radonjić T., Oprić S., Klašnja S., et al. Lipotoxicity as the leading cause of non-alcoholic steatohepatitis. Int. J. Mol. Sci. 2022;23(9):5146. doi: 10.3390/ijms23095146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montgomery M.k., De Nardo W., Watt M.J. Impact of lipotoxicity on tissue "cross talk" and metabolic regulation. Physiology. 2019;34(2):134–149. doi: 10.1152/physiol.00037.2018. [DOI] [PubMed] [Google Scholar]
- 40.Luo T., Jiang S., Zhou B., Song Q., Du J., Liu P., et al. Protective effect of isoorientin on oleic acid-induced oxidative damage and steatosis in rat liver cells. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.818159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa Y., Imajo K., Honda Y., Kessoku T., Tomeno W., Kato S., et al. Palmitate-induced lipotoxicity is crucial for the pathogenesis of nonalcoholic fatty liver disease in cooperation with gut-derived endotoxin. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-29735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhandari U., Kumar V., Khanna N., Panda B.P. The effect of high-fat diet-induced obesity on cardiovascular toxicity in Wistar albino rats. Hum. Exp. Toxicol. 2011;30:1313–1321. doi: 10.1177/0960327110389499. [DOI] [PubMed] [Google Scholar]
- 43.Wali J.A., Jarzebska N., Raubenheimer D., Simpson S.J., Rodionov R.N., O'Sullivan J.F. Cardio-metabolic effects of high-fat diets and their underlying mechanisms-A narrative review. Nutrients. 2020;12:1505. doi: 10.3390/nu12051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shatoor A.S., Al Humayed S. Astaxanthin Ameliorates high-fat diet-induced cardiac damage and fibrosis by upregulating and activating SIRT1. Saudi J. Biol. Sci. 2021;28(12):7012–7021. doi: 10.1016/j.sjbs.2021.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao M., Cheng Y., Wang X., Cui X., Cheng X., Fu Q., et al. Hydrogen sulfide attenuates high-fat diet-induced obesity: involvement of mTOR/IKK/NF-κB signaling pathway. Mol. Neurobiol. 2022;59(11):6903–6917. doi: 10.1007/s12035-022-03004-0. [DOI] [PubMed] [Google Scholar]
- 46.Carlsen H., Haugen F., Zadelaar S., Kleemann R., Kooistra T., Drevon C.A., Blomhoff R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009;4(3):215–222. doi: 10.1007/s12263-009-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afzal S., Sattar M.A., Johns E.J., Eseyin O.A., Attiq A. Antioxidant potential of adiponectin and full PPAR-γ agonist in correcting streptozotocin-induced vascular abnormality in spontaneously hypertensive rats. PPAR Res. 2021 doi: 10.1155/2021/6661181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asano T., Watanabe K., Kubota N., Gunji T., Omata M., Kadowaki T., et al. Adiponectin knockout mice on high caloriediet develop fibrosing steatohepatitis. J. Gastroenterol. Hepatol. 2009;24:1669–1676. doi: 10.1111/j.1440-1746.2009.06039.x. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto Y. Adiponectin provides cardiovascular protection in metabolic syndrome. Cardiol. Res. Pract. 2011 doi: 10.4061/2011/313179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Botta A., Liu Y., Wannaiampikul S., Tungtrongchitr R., Dadson K., Park T.S., et al. An adiponectin-S1P axis protects against lipid induced insulin resistance and cardiomyocyte cell death via reduction of oxidative stress. Nutr. Metab. 2019;16:14. doi: 10.1186/s12986-019-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onodera T., Ghazvini Zadeh E., Xu P., Gordillo R., Guo Z., Joffin N., et al. PEGylated AdipoRon derivatives improve glucose and lipid metabolism under insulinopenic and high-fat diet conditions. J. Lipid Res. 2021;62 doi: 10.1016/j.jlr.2021.100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun S., Xu M., Zhuang P., Chen G., Dong K., Dong R., et al. Effect and mechanism of vitamin D activation disorder on liver fibrosis in biliary atresia. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-99158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jonas M.I., Kuryłowicz A., Bartoszewicz Z., Lisik W., Jonas M., Kozniewski K., Puzianowska-Kuznicka M. Vitamin D receptor gene expression in adipose tissue of obese individuals is regulated by miRNA and correlates with the pro-inflammatory cytokine level. Int. J. Mol. Sci. 2019;20(21):5272. doi: 10.3390/ijms20215272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gong J., Gong H., Liu Y., Tao X., Zhang H. Calcipotriol attenuates liver fibrosis through the inhibition of vitamin D receptor-mediated NF-κB signaling pathway. Bioengineered. 2022;13(2):2658–2672. doi: 10.1080/21655979.2021.2024385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sannappa Gowda N.G., Shiragannavar V.D., Puttahanumantharayappa L.D., Shivakumar A.T., Dallavalasa S., Basavaraju C.G., et al. Quercetin activates vitamin D receptor and ameliorates breast cancer induced hepatic inflammation and fibrosis. Front. Nutr. 2023;10 doi: 10.3389/fnut.2023.1158633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osaki Y., Taniguchi S., Tahara A., Okamoto M., Kishimoto T. Metabolic syndrome and incidence of liver and breast cancers in Japan. Cancer Epidemiol. 2012;36:141–147. doi: 10.1016/j.canep.2011.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.