Summary
Background
The combined vincristine, pegylated liposomal doxorubicin (PLD), and cyclophosphamide (VPC) regimen has never been studied in pediatric patients.
Methods
This open-label, single-center, single-arm phase I study utilizing a “3 + 3” design enrolled children with relapsed/refractory (R/R) solid tumors. Three dose levels of PLD (Duomeisu®) were studied (30, 40, or 50 mg/m2) in combination with cyclophosphamide (1500 mg/m2), mesna (1500 mg/m2), and vincristine (1.5 mg/m2, maximum 2 mg) once every 3 weeks. The primary endpoints included safety, the maximum tolerated dose (MTD) of PLD (Duomeisu®), and the recommended phase 2 dose (RP2D) of PLD (Duomeisu®) for further phase 2 investigation. The secondary endpoints were objective response rate (ORR) and disease control rate (DCR). This study is registered with ClinicalTrials.gov, NCT04213612.
Findings
Between January 7, 2020, and November 18, 2021, 34 patients were eligible and evaluable for toxicity, while 26 patients were evaluable for response. The MTD of PLD (Duomeisu®) was 30 mg/m2. The most common adverse event (AE) was grade 3 or 4 neutropenia (61.8%). The most common grade 1 or 2 non-hematologic AE and cardiotoxicity effects were vomiting (35.3%) and abnormal electrocardiogram T waves (20.6%), respectively. ORR and DCR to VPC regimen after two cycles were 50.0% and 92.3%, respectively. Targeted gene panel sequencing revealed the activation of TP53 mutation may be an adverse prognostic factor.
Interpretation
The VPC regimen showed a promising safety profile and had preliminary efficacy in children with R/R solid tumors. The RP2D for PLD (Duomeisu®) combined with cyclophosphamide and vincristine is 30 mg/m2 once every 3 weeks.
Funding
CSPC Ouyi Pharmaceutical Co., Ltd., Shijiazhuang, the National Key Research and Development Program of China [No. 2022YFC2705005], the National Natural Science Foundation of China [No. 82203303], and the Basic and Applied Basic Research Foundation of Guangdong Province [No. 2021A1515110234].
Keywords: Pediatric oncology, Pegylated liposomal doxorubicin, Relapsed/refractory solid tumor, Cyclophosphamide, Vincristine
Research in context.
Evidence before this study
We searched PubMed, from database inception to May 27, 2024, for papers published in English, using the terms (“PLD” OR “pegylated liposomal doxorubicin” OR “Caelyx”), AND (“cyclophosphamide”), AND (“solid tumor”), AND (“child” OR “pediatric”). We also reviewed references and subsequent citations (identified using Google Scholar). Our search yielded that no studies have been published on the safety and activity of the combination of vincristine, pegylated liposomal doxorubicin (PLD), and cyclophosphamide (VPC) regimens in pediatric patients.
Added value of this study
To our knowledge, this phase I study is the first dose-escalation study to investigate the use of PLD in combination with vincristine and cyclophosphamide in pediatric patients with relapsed/refractory solid tumors. We reported favorable safety profiles and preliminary activity of this regimen. Conducting clinical trials in this rare subset of pediatric patients with limited treatment options is difficult; however, the encouraging results observed in this study suggests that VPC regimen is a promising approach in these patients.
Implications of all the available evidence
Our findings have shown the potential antitumor activity of the VPC regimen in a salvage line setting for pediatric patients with relapsed/refractory solid tumors. If our ongoing expansion study supports these data, VPC regimen may become a new treatment option for salvage-line treatment in these settings. Our study will provide additional evidence supporting the use of PLD (Duomeisu®) in pediatric patients.
Introduction
Doxorubicin is a standard component of therapy for many pediatric malignant solid tumors, such as Wilms tumor, Ewing’s sarcoma, and osteosarcoma. However, cardiotoxicity is a major late toxicity of anthracycline therapy, which may occur years after treatment with cumulative doses and limited dosage escalation.1,2 Cumulative anthracycline dose is an important predictor of cardiomyopathy.3 The cumulative incidence of clinical heart failure in some long-term survivors of pediatric cancer approaches 25.0% by the age of 40 years.4 Doxorubicin-induced heart failure occurs in 3.0–5.0% of patients treated with a cumulative dose of 400 mg/m2 of doxorubicin.5 Furthermore, cardiovascular diseases have become the second leading cause of long-term morbidity and mortality among cancer survivors.6
Thus, new anthracycline preparations, such as pegylated liposomal doxorubicin (PLD, a liposome-encapsulated form of doxorubicin modified with polyethylene glycol), have been developed to maximize the dose intensity of doxorubicin through selective maintenance of a high drug concentration in the tumor tissue.7 Furthermore, PLD reduces the side effects of doxorubicin, including cardiac toxicity and myelosuppression,8 and may improve the prognosis of pediatric patients with malignant solid tumors. PLDs have shown promising efficacy and have been used extensively in adults with solid tumors, such as breast cancer,9 ovarian cancer,10 osteosarcoma,11 and lymphoma.12
PLD exhibits activity equivalent to that of doxorubicin and an improved toxicity profile in soft tissue sarcoma (STS).13 A Pediatric Oncology Group Study determined the pharmacokinetics (PK) of PLD in pediatric relapsed/refractory (R/R) solid tumors and studied its recommended phase 2 dose (RP2D).14 Monotherapy with PLD has been reported to be effective,15 and combined chemotherapy improves the response further. Additionally, the combination of PLD with ifosfamide yields favorable outcomes among adults with STSs.16
Although it is estimated that >80.0% of the children diagnosed with malignant tumors can be cured,17 the survival of pediatric patients with R/R solid tumors remains dismal. Cyclophosphamide and vincristine are effective antitumor drugs for pediatric cancer, applicable in relapse. To our knowledge, the combined vincristine, PLD, and cyclophosphamide (VPC) regimen has not been studied in pediatric patients. Therefore, we conducted a single-center, open-label, phase I trial to evaluate the VPC regimen’s safety and efficacy for treating pediatric patients with R/R solid tumors using PLD.
Methods
Ethics statement
The PLD (Duomeisu®) used in this study (manufactured by CSPC Ouyi Pharmaceutical Co., Ltd., Shijiazhuang, China) is widely used as a bioequivalent to PLD in China.11 The National Medical Products Administration approved the application for evaluating the consistency of PLD (Duomeisu®) and PLD, allowing us to omit studying PLD’s PK in children,14 given the existing quantified PK data of PLD. The study was approved by the Institutional Review Board and Ethics Committee of the Sun Yat-Sen University Cancer Center. Written consent was obtained from each guardian and/or patient.
Patients
The inclusion criteria were (a) age between 1 and 18 years; (b) an Eastern Cooperative Oncology Group performance status of 0 or 1; (c) presence of histologically confirmed recurrent or refractory pediatric malignant solid tumors; (d) R/R status after at least first-line treatment; (e) existence of at least one evaluable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.118; (f) estimated life expectancy of ≥6 months; (g) normal hematological values (absolute neutrophil count ≥1000 cells/μL; hemoglobin ≥9.0 g/dL; platelets ≥100,000 platelets/μL); (h) normal liver function (aspartate aminotransferase and alanine aminotransferase levels ≤2.5 × upper limit of normal [ULN], bilirubin level ≤2.5 × ULN); (i) normal renal function (glomerular filtration rate ≥30 mL/min per 1.73 m2; serum creatinine levels ≤1.5 × ULN); and (j) time elapsed from previous therapy was ≥3 weeks for systemic chemotherapy and ≥2 weeks for major surgery or radiation therapy.
Patients were excluded if they had (a) severe cardiovascular diseases; (b) active human immunodeficiency virus, hepatitis B, or hepatitis C infection; (c) persistent clinically significant toxicities caused by previous anticancer therapy; or (d) received cumulative doxorubicin or pirarubicin dose of ≥450 mg/m2 or cumulative epirubicin dose of ≥550 mg/m2.
Study design and treatment
This was an open-label, single-center, single-arm phase Ia dose escalation using a standard “3 + 3” design and phase Ib expansion study. The treatment protocol comprised two courses of the VPC regimen. After completing two courses, the investigators and patients’ guardians decided whether to continue the protocol. The maximum allowed number of courses was 12. The treatment consisted of PLD (Duomeisu®) at an escalated dose and vincristine (1.5 mg/m2, maximum 2 mg), cyclophosphamide (1500 mg/m2), and mesna (1500 mg/m2) at a fixed dose, once every 3 weeks. The PLD (Duomeisu®) dose was initiated at 30 mg/m2 (L1) and escalated to 40 mg/m2 (L2) and 50 mg/m2 (L3). The VPC regimen was administered using a pegylated recombinant human granulocyte colony-stimulating factor (PEG-rhG-CSF) at 100 μg/kg. Both PLD (Duomeisu®) and PEG-rhG-CSF were supplied by CSPC Ouyi Pharmaceutical Co. Ltd. (Shijiazhuang, China). The criteria for terminating treatment included disease progression and completion of the protocol therapy and evaluation period.
The participants were examined for dose-limiting toxicities (DLTs) during the first treatment cycle. The dose of PLD (Duomeisu®) was escalated if none of the first three patients or one of the six patients in the specific cohort experienced DLTs. If DLTs were observed in two or more patients, then dose escalation was halted. Dose-limiting hematologic toxicity was defined as grade 4 neutropenia (<500/mm3) of ≥7 days or grade 4 thrombocytopenia (<25,000/mm3) of ≥7 days, while non-hematologic DLT was defined as ≥ grade 3 adverse events (AEs) or any grade of toxicity resulting in a chemotherapy delay of >2 weeks.19
The maximum tolerated dose (MTD) was defined as the highest dose level at which <33.3% of patients experienced DLTs during cycle 1. The RP2D was at or below the MTD. Once the MTD was defined, 18–24 additional patients were enrolled in phase Ib to further determine the tolerability of the MTD.
The primary endpoints included safety, DLT, MTD of PLD (Duomeisu®) in this combination, and the RP2D of PLD (Duomeisu®). The secondary endpoints were objective response rate (ORR) and disease control rate (DCR). The observation period for toxicity and response assessment spanned the beginning of the VPC regimen until death or end of follow-up.
Safety evaluation
Patient underwent comprehensive baseline physical examinations, covering vital signs, clinical tumor assessments, hematological and serum biochemistry tests, urinalysis, and cardiac dysfunction assessment using a 12-lead electrocardiogram and echocardiogram. Left ventricular ejection fraction, left ventricular systolic (fractional shortening and biplane Simpson’s ejection fraction) were determined through echocardiographic. We also analyzed the levels of serum cardiac biomarkers of myocardial stress (B-type natriuretic peptides [BNP] and N-terminal pro-BNP) and cardiomyocyte injury (high-sensitivity cardiac troponin T). Complete physical examination and 12-lead electrocardiogram were performed at baseline, after two cycles of VPC, and before each subsequent cycle, at off-study, every 3-month follow-up in the first year of off-study, every 6-month follow-up in the second year of off-study, and every 1-year follow-up in the third to fifth years of off-study. Cardiac ultrasonography and assessment of serum cardiac biomarkers were performed at baseline, after two cycles of VPC, at off-study, and when reevaluation was necessary.
The National Cancer Institute Common Toxicity Criteria for Adverse Events version 5.0 were used to evaluate AEs.
Response evaluation
The responses based on the RECIST 1.118 were assessed centrally by an independent radiology review. The ORR was assessed as complete response (CR) or partial response (PR), and the DCR as CR, PR, or stable disease (SD).
Targeted gene panel sequencing
DNA sequencing
We conducted comprehensive genomic profiling using targeted gene panel sequencing on paired samples from 22 patients, including formalin-fixed paraffin-embedded tissue and peripheral blood specimens. DNA libraries were analyzed using Onco PanScan (Genetron Health), which is an 825-gene panel including major tumor-related genes. Somatic insertions and deletions were retrieved using Strelka (https://github.com/Illumina/strelka), and structural variations were determined using GeneFuse version 0.6.1 (https://github.com/OpenGene/GeneFuse). A total of 1000 genomes and variants with population frequency over 0.1% were excluded based on guidelines by the Exome Aggregation Consortium. The other variants were annotated with Oncotator and Vep.
RNA sequencing
A 395-gene RNA panel was analyzed to identify gene fusions at the transcript level. DNA libraries were captured with an Agilent SureSelect V5 system (Agilent), and the captured samples were subjected to Illumina HiSeq X-Ten for paired-end sequencing. Sequencing reads were mapped to a human reference genome (hg19) using Hisat2-2.0.5. Gene fusions were identified using FusionMap.
Statistical methods
Sample size of the phase Ia dose-escalation study was determined using a standard “3 + 3” design; while phase Ib expansion cohort will enroll 18–24 patients, without formal statistical consideration.
All the statistical analyses were performed using GraphPad Prism 8.0 Software (San Diego, CA, USA). Descriptive measures for continuous variables were range, and for categorical variables were frequencies (percentage [%]). ORR and DCR were calculated with 95% confidence intervals (CI) by the Clopper-Pearson method. Fisher exact test was used to compare the between-group differences. Any participant who experienced hematologic and non-hematologic DLTs during the first 21 days after receiving the initial dose of VPC was considered evaluable for toxicity. Progression-free survival (PFS) was defined as the time from the enrollment to the occurrence of disease progression or death from any cause or time of last follow-up if no event had occurred. PFS and 95% CI values were calculated using the Kaplan–Meier method and compared using the two-sided log-rank test for between-group differences. Two-sided P values < 0.05 were considered statistically significant. This study is registered with ClinicalTrials.gov, NCT04213612.
Role of the funding source
CSPC Ouyi Pharmaceutical Co., Ltd., provided the study drug for research, but otherwise had no role in study design, data collection, analysis, or interpretation. The study was also funded by the sponsor, YZZ and SYL, who involved in study design, data collection, data analysis, writing the report, and data interpretation. All authors had full access to all the data in the study and accepted responsibility to submit for publication.
Results
Patient characteristics
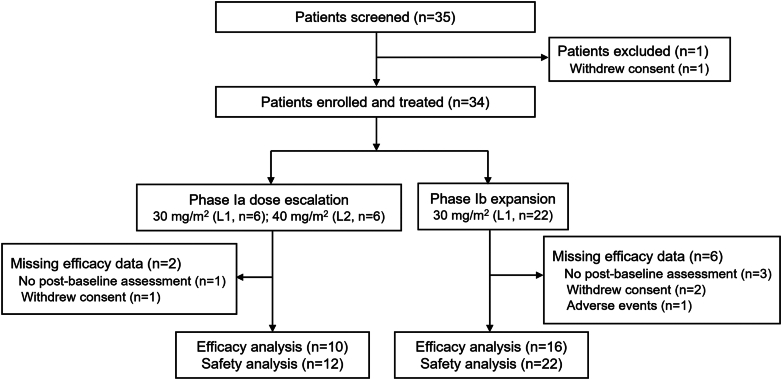
Between January 7, 2020, and November 18, 2021, 35 participants were screened. Among them, 34 patients enrolled were eligible and evaluable for toxicity (Ia, n = 12; Ib, n = 22), while 26 (76.5%) were evaluable for response (Ia, n = 10; Ib, n = 16; Fig. 1). The patient characteristics are summarized in Table 1. The median age of the patients at enrollment was 6 (range, 1–18) years. The major diseases were rhabdomyosarcoma (70.6%) and Ewing’s sarcoma (11.8%). At the data cutoff date (November 9, 2023), the median follow-up was 24.2 (range, 6.7–32.2) months. Approximately 41.2% of them received more than three lines of chemotherapy regimens. Moreover, 12 patients were treated for a first relapse, 5 for a second relapse, and 1 for a fourth relapse. Twenty-four and five patients were treated with pirarubicin or doxorubicin in previous chemotherapy, respectively; the median cumulative doses of pirarubicin and doxorubicin were 200.0 (range, 50.0–360.0) mg/m2 and 250.0 (range, 200.0–300.0) mg/m2, respectively.
Fig. 1.
Trial profile.
Table 1.
Baseline characteristics.
| Characteristics | Total (n = 34) |
|---|---|
| Median age, years (range) | 6 (1–18) |
| Sex | |
| Female | 15 (44.1) |
| Male | 19 (55.9) |
| Reasons for enrollment | |
| Relapse | 18 (52.9) |
| Progressive disease | 6 (17.6) |
| Refractory | 10 (29.4) |
| Diagnosis | |
| Rhabdomyosarcoma | 24 (70.6) |
| Ewing’s sarcoma | 4 (11.8) |
| Wilms tumor | 2 (5.9) |
| Osteosarcoma | 1 (2.9) |
| Embryonal sarcoma | 1 (2.9) |
| Epithelioid sarcoma | 1 (2.9) |
| Malignant rhabdoid tumor of soft tissues | 1 (2.9) |
| Previous chemotherapy | 34 (100) |
| Median lines (range) | 2 (1–5) |
| 1 | 14 (41.2) |
| 2 | 6 (17.6) |
| ≥3 | 14 (41.2) |
| Median cycles (range) | 10 (2–25) |
| Prior anthracycline-based chemotherapy | 29 (85.3) |
| Median cycles (range) | 4 (1–7) |
| Prior cyclophosphamide-based chemotherapy | 31 (91.2) |
| Median cycles (range) | 4 (1–21) |
| Median cumulative dose of cyclophosphamide, g/m2 (range) | 9.6 (1.5–33.4) |
| Prior VCR + THP + CTX regimen | 21 (61.8) |
| Median cycles (range) | 4 (1–7) |
| Prior pirarubicin | 24 (70.6) |
| Median cumulative dose, mg/m2 (range) | 200 (50–360) |
| Prior doxorubicin | 5 (14.7) |
| Median cumulative dose, mg/m2 (range) | 250 (200–300) |
VCR, vincristine; THP, pirarubicin; CTX, cyclophosphamide.
Data are n (%), unless otherwise specified.
Dose escalation
The escalation scheme and DLTs were listed in Supplementary Table S1. No DLT occurred at the first level (L1, 30 mg/m2), while two (33.3%) of the six patients had DLTs at the second level (L2, 40 mg/m2). Both DLTs involved grade 4 neutropenia persisting for ≥7 days. Therefore, three more patients were enrolled in L1 (30 mg/m2) to confirm the MTD, and no DLT occurred. The MTD of PLD (Duomeisu®) was 30 mg/m2. Subsequently, 22 patients were enrolled to confirm the safety of the VPC regimen in phase Ib.
Safety
The dominant toxicity of the VPC regimen was bone marrow suppression. The most common grade 3 or 4 AEs were neutropenia (61.8%), febrile neutropenia (20.6%), and anemia (20.6%) (Table 2). No grade 3 or 4 non-hematologic AE was observed. The most common grade 1 or 2 non-hematologic AEs were vomiting (35.3%), hypertriglyceridemia (26.5%), and alopecia (17.6%). Hand-foot syndrome (HFS) was observed in one patient. No treatment-related deaths occurred. Among 34 patients, the median cumulative dose was 60.0 (range, 30.0–270.0) mg/m2 for PLD (Duomeisu®) and 3.0 (range, 1.5–13.5) g/m2 for cyclophosphamide after enrollment.
Table 2.
Safety profile in 34 safety population.
| Grade 1 or 2 n (%) | Grade 3 n (%) | Grade 4 n (%) | |
|---|---|---|---|
| Hematologic | |||
| Anemia | 17 (50.0) | 7 (20.6) | 0 |
| Neutropenia | 5 (14.7) | 7 (20.6) | 14 (41.2) |
| Thrombocytopenia | 5 (14.7) | 2 (5.9) | 3 (8.8) |
| Febrile neutropenia | 0 | 4 (11.8) | 3 (8.8) |
| Non-hematologic | |||
| Vomiting | 12 (35.3) | 0 | 0 |
| Hypertriglyceridemia | 9 (26.5) | 0 | 0 |
| Alopecia | 6 (17.6) | – | – |
| Diarrhea | 4 (11.8) | 0 | 0 |
| Anorexia | 3 (8.8) | 0 | 0 |
| Constipation | 2 (5.9) | 0 | 0 |
| Rash | 2 (5.9) | 0 | 0 |
| Cough | 2 (5.9) | 0 | 0 |
| Hand-foot syndrome | 1 (2.9) | 0 | 0 |
| Oral mucositis | 1 (2.9) | 0 | 0 |
| Cystatin C increased | 1 (2.9) | 0 | 0 |
| Abdominal pain | 1 (2.9) | 0 | 0 |
| Alanine aminotransferase increased | 1 (2.9) | 0 | 0 |
| γ glutamyltransferase increased | 1 (2.9) | 0 | 0 |
| Epistaxis | 1 (2.9) | 0 | 0 |
| Fever | 1 (2.9) | 0 | 0 |
| Anorexia | 1 (2.9) | 0 | 0 |
| Rhinorrhea | 1 (2.9) | 0 | 0 |
| Creatinine increased | 1 (2.9) | 0 | 0 |
| Urea increased | 1 (2.9) | 0 | 0 |
| Weight loss | 1 (2.9) | 0 | 0 |
| Cardiotoxicity | |||
| Abnormal electrocardiogram T wave | 7 (20.6) | – | – |
| Aspartate aminotransferase increased | 4 (11.8) | 0 | 0 |
| Blood lactate dehydrogenase increased | 4 (11.8) | 0 | 0 |
| Blood creatine phosphokinase increased | 3 (8.8) | 0 | 0 |
| Sinus tachycardia | 3 (8.8) | 0 | 0 |
| Accelerated atrial heart rate | 1 (2.9) | 0 | 0 |
| Sinus bradycardia | 1 (2.9) | 0 | 0 |
| Lactic dehydrogenase increased | 1 (2.9) | 0 | 0 |
Data are n (%).
Cardiotoxicity
The most common grade 1 or 2 cardiotoxicities were the presence of abnormal electrocardiogram T waves (20.6%), aspartate aminotransferase increased (11.8%), blood lactate dehydrogenase increased (11.8%), blood creatine phosphokinase increased (8.8%), and sinus tachycardia (8.8%) (Table 2). Grade 3 or 4 cardiotoxicities were not observed. No patient experienced symptoms of congestive heart failure (CHF) or was diagnosed with CHF.
Efficacy
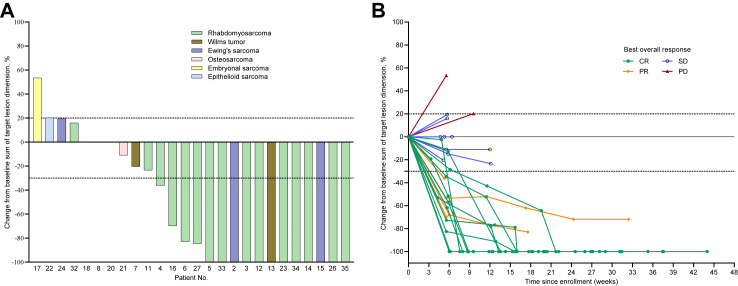
The total ORR and DCR after two cycles were 50.0% and 92.3%, respectively (Supplementary Table S2), and the best responses were ORR and DCR of 61.5% and 92.3%, respectively (Fig. 2). At the data cutoff point, 12 patients achieved CR, including 9 with rhabdomyosarcoma, 2 with Ewing’s sarcoma, and 1 with Wilms tumor (Fig. 2). After 2-cycle VPC, the ORR was 66.7% for phase Ia (L1), 25.0% for phase Ia (L2), and 50.0% for phase Ib study.
Fig. 2.
Clinical activity of the VPC regimen. (A) Waterfall plot for the best percentage change in target lesion size (n = 26). (B) Spider plot showing individual patients and the time frame (n = 26). Tumor size is measured centrally as the sum of the longest diameters of the target lesions according to the RECIST 1.1 by independent radiology review for both (A) and (B). Only patients with at least one evaluable post-baseline target lesion scan (A and B) are included. Plots of the best percentage changes in the sum of the longest diameters of the target lesions are shown. Three patients had 0% change since baseline. Dashed lines at 30% decrease and 20% increase represent the cut-offs for identifying PR and PD, respectively, according to the RECIST 1.1. Abbreviations: VPC, vincristine, pegylated liposomal doxorubicin, and cyclophosphamide; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, disease progression.
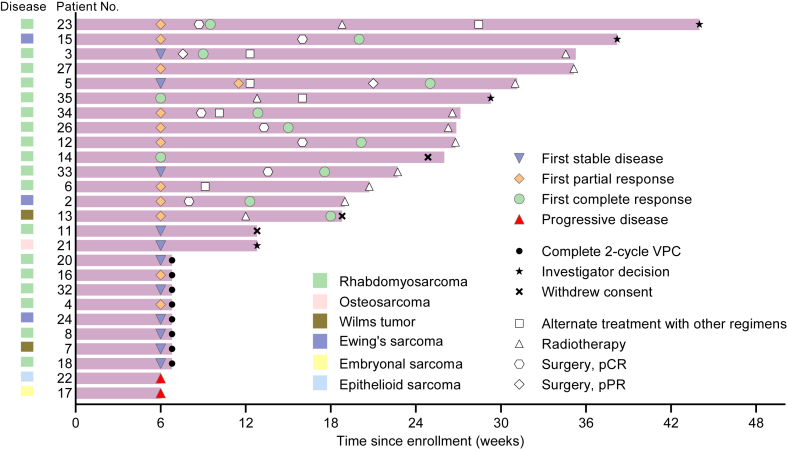
Four patients had a confirmed PR, all with rhabdomyosarcomas. Before enrollment, 18 patients with evaluable efficacy data were treated with the combination of vincristine, pirarubicin, and cyclophosphamide. The best response of these patients was CR in ten, PR in two, SD in four, and disease progression (PD) in two patients, indicating the VPC regimen’s efficacy even after treatment with combination of vincristine, pirarubicin, and cyclophosphamide. Seven patients responding to the VPC regimen (PR = 6, SD = 1) underwent surgery, achieving pathological complete response. Nine rhabdomyosarcoma patients who achieved CR had the response duration of >22 weeks (Fig. 3). Additionally, patient 23 with rhabdomyosarcoma treated with the VPC regimen and other alternative chemotherapy regimens achieved CR, with a response duration of 45 weeks. Two of the three patients with Ewing’s sarcoma had CR. Both patients with Wilms tumor had a response, including one CR and one SD (the tumor shrank by 20.0%).
Fig. 3.
Duration of treatment. Twelve of twenty-six patients achieved a confirmed CR. Abbreviations: VPC, vincristine, pegylated liposomal doxorubicin, and cyclophosphamide; pCR, pathological complete response; pPR, pathological partial response.
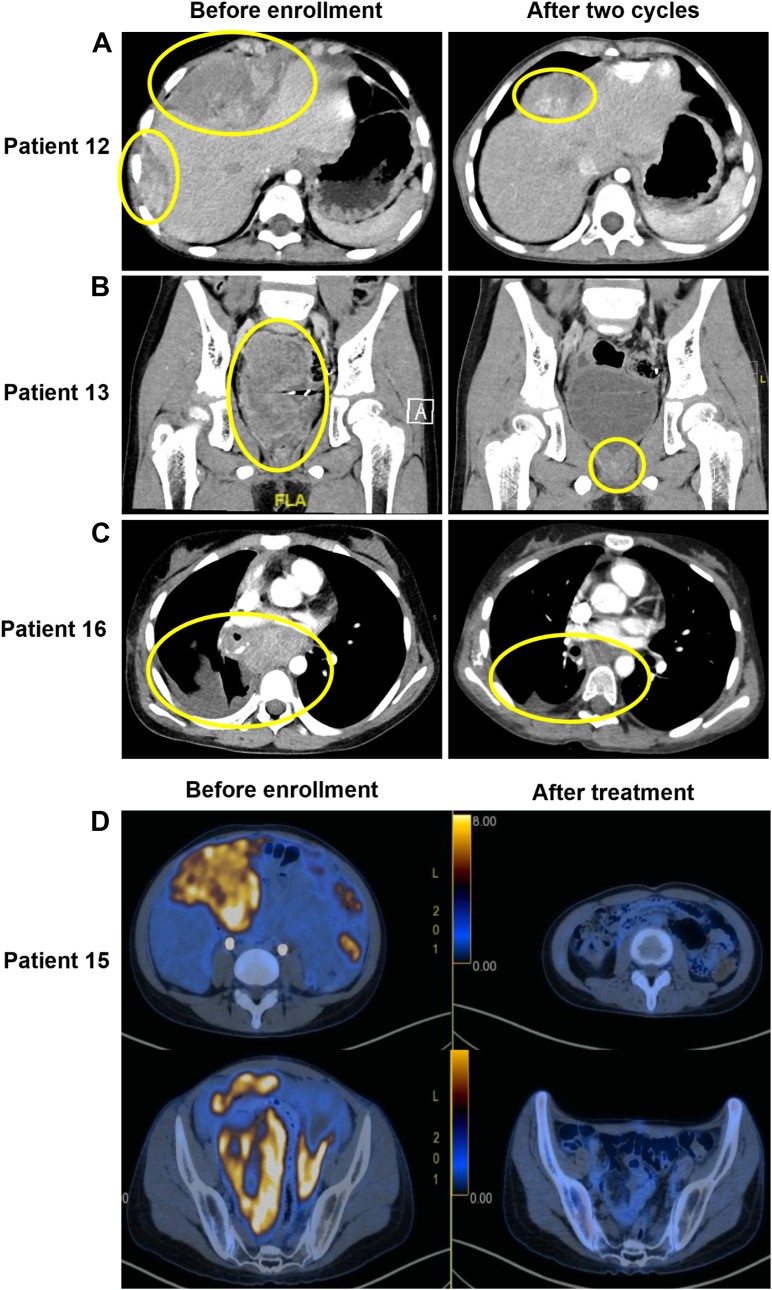
Radiologic responses in four patients who achieved PR after 2 cycles were selected to present in Fig. 4. We observed a remarkable decrease in tumor size in patients 12, 13, 16, and 15. Pathological types in these four patients were embryonal rhabdomyosarcoma, Wilms tumor, alveolar rhabdomyosarcoma, and Ewing’s sarcoma, respectively.
Fig. 4.
Radiologic response in four patients. Panel A shows a CT scan of bulky disease in the abdominal cavity in Patient 12, diagnosed as embryonal rhabdomyosarcoma before and after two cycles of the VPC regimen. The maximum lesion diameter decreased from 84 mm to 55 mm (a decrease of 34.5%). Panel B shows a CT scan of bulky disease in the vesicorectal pouch in Patient 13, diagnosed as a Wilms tumor before and after two cycles of the VPC regimen. The maximum lesion diameter decreased from 80 mm to 14 mm (a decrease of 82.5%). Panel C shows a CT scan of bulky disease in enlarged lymph nodes in the mediastinum and hilum of the lungs in Patient 16, diagnosed as alveolar rhabdomyosarcoma before and after two cycles of VPC regimen. The maximum lesion diameter decreased from 53 mm to 16 mm (a decrease of 69.8%). Panel D shows an 18F-FDG-PET/CT scan of bulky disease in abdominal and pelvic cavities in Patient 15, diagnosed as Ewing’s sarcoma before enrollment and after treatment. After two cycles of the VPC regimen, the maximum lesion diameter decreased from 95 mm to 26 mm (a decrease of 72.6%). The patient underwent surgery following five VPC regimen cycles, achieving pathologic complete response. After another four cycles of the VPC regimen, the 18F-FDG PET/CT scan showed CR. Abbreviations: CT, computed tomographic; VPC, vincristine, pegylated liposomal doxorubicin, and cyclophosphamide; CR, complete response; 18F-FDG PET/CT, 18F-Fluorodeoxyglucose positron emission tomography/computed tomography.
Biomarker analysis
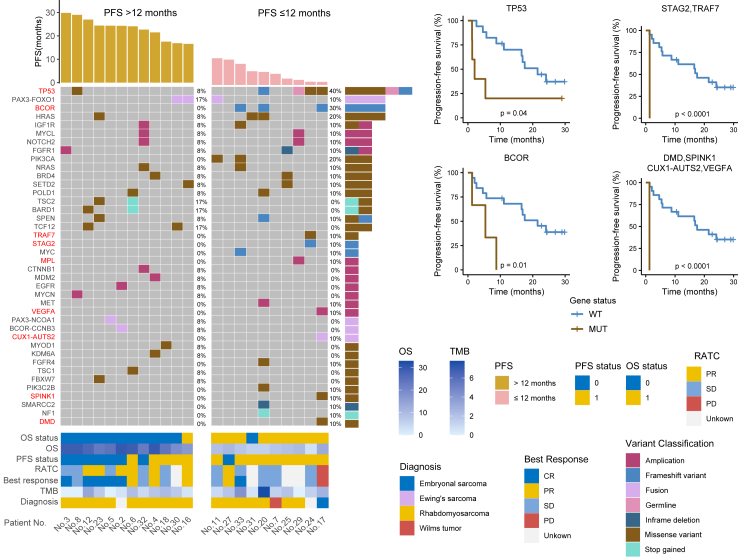
Genomic analysis was also performed to identify potential molecular determinants of treatment response in pediatric patients with solid tumors. In this study, we conducted targeted gene panel sequencing for 22 patients to comprehensively characterize the mutational landscape of pediatric R/R solid tumors. Targeted gene panel sequencing revealed TP53 as the most frequently altered gene, occurring in five (22.7%) patients, including three with missense mutations. PAX3-FOXO1, BOCR, and HRAS were altered in three patients (13.6%) (Fig. 5). The altered genes were significantly enriched in the RTK-RAS, P53, Notch, and PI3K pathways (Supplementary Fig. S1).
Fig. 5.
Biomarker results based on PFS. Genomic events based on the timing of progression following treatment (PFS > 12 vs. ≤12 months). Kaplan–Meier estimates of PFS in 22 patients by specific somatic mutations. Frameshift variant of STAG2 and missense variant of TRAF7 occurred in the same sample from the same patient, which generated the same survival curve. Similarly, the missense variant of DMD, missense variant of SPINK1, CUX1-AUTS2 fusion, and VEGFA amplification occurred in the same sample from the same patient, which generated the same survival curve. Genes significantly associated with PFS were highlighted in red. Presented P values were log-rank, and two-sided P values < 0.05 were considered statistically significant. Abbreviations: PFS, progression-free survival; RATC, response after two cycles; OS, overall survival; TMB, Tumor Mutation Burden; CR, complete response; PR, partial response; SD, stable disease; PD, disease progression.
All patients with targeted gene panel sequencing included in these analyses had >12 months of follow-up. The median PFS for this trial was 16.9 months (95% CI, 1.3–29.8). The gene alteration rates of TP53, BCOR, STAG2, TRAF7, DMD, and SPINK1 mutations, VEGFA and MPL amplification, and CUX1-AUTS2 fusion were higher in the PFS ≤12 months group than in the PFS > 12 months group. To summarize, both the mutation of TP53 (P = 0.04) and the frameshift variant of BCOR (P = 0.01) indicated poor PFS (Fig. 5). Missense variant mutation of STAG2 also indicated poor PFS (P < 0.0001), and the same trend was observed for the frameshift variant mutation of TRAF7.
DMD somatic mutation indicated poor PFS (P˂0.0001), similarly observed for SPINK1 and VEGFA mutations and CUX1-AUTS2 fusion. Patient 17, diagnosed as embryonal sarcoma, exhibited TP53 mutation, BCOR frameshift variant, VEGFA amplification, CUX1-AUTS2 fusion, SPINK1 and DMD missense variant, progressing after two VPC regimen cycles. Compared to the PFS > 12 months group, the PFS ≤ 12 months group showed more mutated genes in the CPF pathway, highlighting its activation as a poor prognostic indicator (Supplementary Fig. S1).
Discussion
Our study is the first to assess the safety and MTD of PLD combined with cyclophosphamide and vincristine in pediatric patients with R/R solid tumors. We selected this regimen based on its established efficacy. The RP2D of PLD (Duomeisu®) was 30 mg/m2, alongside cyclophosphamide at 1500 mg/m2, mesna at 1500 mg/m2, and vincristine at 1.5 mg/m2 (maximum 2 mg) once every 3 weeks. This dose has been recommended as the starting dose for adults.16,20
PLD monotherapy is well tolerated in pediatric and adult patients.14,21 In two studies, the MTD of PLD administered every 4 weeks to pediatric patients was 60 mg/m2,14 and in another clinical study, the RP2D of PLD was 50 mg/m2 every 4 weeks for Japanese adult patients.22 One study demonstrated PLD’s tolerability as a single agent at 50 mg/m2 monthly in pediatric patients, with three of eight patients showing an objective response to advanced sarcomas.15 Another study reported no objective response in 15 adult patients with advanced STSs treated solely with PLD,21 suggesting further investigation of PLD in combination with agents, such as ifosfamide, is warranted. The recommended doses for future studies are 30 mg/m2 PLD on day 1 and 3000 mg/m2 intravenous ifosfamide on days 1–3 every 3 weeks for previously untreated adult patients with metastatic or advanced STSs.16 The combination of temsirolimus with PLD (30 mg/m2 every 4 weeks) is safe and well tolerated in recurrent and refractory bone and soft tissue sarcomas.20 We designed the combined regimen to improve the efficacy of PLD. In this dose-finding phase I study, the feasibility of using PLD (Duomeisu®) combined with cyclophosphamide and vincristine in pediatric solid tumors was evaluated to test the possibility of administering these three drugs at therapeutic doses. This study demonstrated that the VPC regimen is safe for pediatric patients, allowing cyclophosphamide and vincristine to be administered at a dose similar to that used when each is administered alone. Moreover, despite our patient population being heavily pretreated, we observed a good response to the VPC regimen. The ORR and DCR of response to the VPC regimen after two cycles highlighted the promising antitumor activity of this combination. Our findings were consistent with those of previous studies on PLD and bolus or metronomic cyclophosphamide for patients with metastatic breast cancer.23 They were also consistent with the results of another study that demonstrated the safe and effective use of PLD with cyclophosphamide in patients with metastatic breast cancer previously treated with anthracycline.9
The VPC regimen exhibited a manageable toxicity profile. Specifically, no unexpected toxicity was observed. Myelosuppression was reported as the most common AE. Given the support of PEG-rhG-CSF, the most common grade 3 or 4 AEs were hematologic toxicities similar to those of other combination chemotherapy regimens.19 With respect to safety, grade 3–4 febrile neutropenia (20.6% vs. 58.0%) in this study was lower than that observed on treatment with regular vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide induction (VDC plus IE regimen) for newly diagnosed Ewing’s sarcoma.24 The incidence of grade 3–4 anemia (20.6% vs. 62.0%) and thrombocytopenia (14.7% vs. 56.0%) was also lower, whereas that of grade 3–4 neutropenia (61.8% vs. 55.0%) was higher than that observed with regular VDC plus IE.24 However, the toxicity of ifosfamide and etoposide cannot be excluded.
One study showed that in a multivariable model, an increase in the doxorubicin dose by 10 mg/m2 is associated with heart failure.25 Although most patients (85.3%) in this study were previously treated with conventional anthracycline, none developed grade 3 or 4 cardiotoxicity. All cardiotoxicities in this study were asymptomatic, potentially indicating the promising cardiac safety of our VPC comprising the PLD regimen. Besides, several long-term follow-up studies of PLD demonstrated the low incidence and severity of cardiotoxicities in either adult or pediatric patients,26,27 further supporting the long-term cardiac safety of PLD. As such, we reason that the PLD regimen may serve as a treatment option for patients too frail or vulnerable to receive conventional doxorubicin, with encouraging cardiac safety. However, it should be noted that the late-onset cardiotoxicity of anthracyclines appeared ≥1 year after treatment cessation; thus, considering limited follow-up in our study, the long-term cardiac safety of our VPC combination in pediatric patients should be further monitored and confirmed over a prolonged follow-up.
Although HFS was observed in only one patient in our study, a previous study reported an incidence rate of up to 80.0% in patients who received a high dose of PLD.28 Thus, it is necessary to increase the sample size to further observe HFS occurrence. To fill in the gaps in the limited clinical trials of new drugs in pediatric patients in China and determine the long-term cardiotoxicity of the VPC regimen, a phase II prospective clinical study of this combination is ongoing at our center.
In our cohort, apart from one patient with Wilms tumor, four patients with rhabdomyosarcoma had TP53 mutation. Rhabdomyosarcoma patients with TP53 mutation had a worse PFS compared to patients without this mutation, aligning with existing literature that reported TP53 mutations were associated with worse outcomes in both FOXO1 fusion-negative and FOXO1 fusion-positive rhabdomyosarcoma cases.29 Although the embryonal rhabdomyosarcoma patient 20 responded to the VPC regimen with a best response of SD, the PFS was short. This outcome may be attributed to TP53, BCOR, HRAS, POLD1, PIK3C2B, and NF1 mutations. The embryonal rhabdomyosarcoma patient 33 had BCOR-NRAS co-mutation, which is in accordance with the report that significant interactions included BCOR with NRAS in FOXO1 fusion-negative rhabdomyosarcoma was observed.29 The Ewing’s sarcoma patient 24, harboring TP53 and STAG2 mutations, did not benefit from the VPC regimen, showing short PFS and OS. This is because TP53 and STAG2 mutations are often concurrent and associated with poor outcomes in Ewing sarcoma.30
The main strength of this study was the development of a novel regimen to treat pediatric patients with R/R solid tumors. The findings of this phase I study raise an important question on the optimal use of the VPC regimen. Larger data on PLD (Duomeisu®) at RP2D are needed to evaluate its optimal use in appropriate disease types. Although the VPC regimen showed preliminary efficacy, the limitation of the small sample size of patients treated in each diagnostic subgroup cannot be ignored.
In conclusion, the recommended PLD (Duomeisu®) dose for future studies is 30 mg/m2 in combination with 1500 mg/m2 cyclophosphamide and mesna, and 1.5 mg/m2 (≤2 mg) vincristine daily for 21 days. This regimen, supported by PEG-rhG-CSF, showed acceptable toxicity. PLD (Duomeisu®) along with and may synergize with cyclophosphamide and vincristine enhancing their efficacy in children with R/R solid tumor. A phase II study on the VPC regimen is underway.
Contributors
All authors had full access to all the data in the study and accepted responsibility to submit for publication. YZZ, XFS and SYL designed the study. SYL interpreted the data and wrote the report. YZZ, JW, JTH and FFS revised the manuscript for important intellectual content. YG contributed to the statistical analysis. SYL, JW, JTH, FFS, JZ, YQ, RQC, and ZJZ collected data. HL evaluated the response. All authors reviewed the manuscript and approved the final version for submission. YZZ and XFS contributed equally to the work. SYL, JW, and JTH contributed equally to the work. YZZ, SYL, JW, and JTH accessed and verified the data.
Data sharing statement
Data are available from the corresponding authors upon reasonable request and with the permission of Sun Yat-sen University Cancer Centre.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
We appreciate Editage for the English language editing. The investigators are grateful to all the patients and their guardians for their support of this trial. We also express gratitude Professor. Ye Cao from Department of Clinical Research of Sun Yat-sen University Cancer Center for study design assisting. The abstract of this manuscript was submitted and selected for presentation as part of a poster discussion session at the 2022 ASCO annual meeting (Temporary Abstract Submission ID: 377450).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102701.
Contributor Information
Xiaofei Sun, Email: sunxf@sysucc.org.cn.
Yizhuo Zhang, Email: zhangyzh@sysucc.org.cn.
Appendix A. Supplementary data
References
- 1.Lenihan D.J., Cardinale D.M. Late cardiac effects of cancer treatment. J Clin Oncol. 2012;30(30):3657–3664. doi: 10.1200/JCO.2012.45.2938. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz S.E., Scully R.E., Lipsitz S.R., et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11(10):950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipshultz S.E., Adams M.J., Colan S.D., et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128(17):1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 4.Chow E.J., Chen Y., Kremer L.C., et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33(5):394–402. doi: 10.1200/JCO.2014.56.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wouters K.A., Kremer L.C., Miller T.L., Herman E.H., Lipshultz S.E. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131(5):561–578. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller K.D., Siegel R.L., Lin C.C., et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 7.Sekiya N., Imamura A. [Doxil--pegylated liposomal doxorubicin] Gan To Kagaku Ryoho. 2008;35(8):1439–1443. [PubMed] [Google Scholar]
- 8.O'Brien M.E., Wigler N., Inbar M., et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 9.Trudeau M.E., Clemons M.J., Provencher L., et al. Phase II multicenter trial of anthracycline rechallenge with pegylated liposomal doxorubicin plus cyclophosphamide for first-line therapy of metastatic breast cancer previously treated with adjuvant anthracyclines. J Clin Oncol. 2009;27(35):5906–5910. doi: 10.1200/JCO.2009.22.7504. [DOI] [PubMed] [Google Scholar]
- 10.Hamanishi J., Takeshima N., Katsumata N., et al. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: open-label, randomized trial in Japan (NINJA) J Clin Oncol. 2021;39(33):3671–3681. doi: 10.1200/JCO.21.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen X.Z., Pan Q.Z., Xu B.S., et al. Phase I study of pegylated liposomal doxorubicin and cisplatin in patients with advanced osteosarcoma. Cancer Chemother Pharmacol. 2022;89(2):209–215. doi: 10.1007/s00280-021-04371-6. [DOI] [PubMed] [Google Scholar]
- 12.Levine A.M., Noy A., Lee J.Y., et al. Pegylated liposomal doxorubicin, rituximab, cyclophosphamide, vincristine, and prednisone in AIDS-related lymphoma: AIDS Malignancy Consortium Study 047. J Clin Oncol. 2013;31(1):58–64. doi: 10.1200/JCO.2012.42.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Judson I., Radford J.A., Harris M., et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2001;37(7):870–877. doi: 10.1016/s0959-8049(01)00050-8. [DOI] [PubMed] [Google Scholar]
- 14.Marina N.M., Cochrane D., Harney E., et al. Dose escalation and pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in children with solid tumors: a pediatric oncology group study. Clin Cancer Res. 2002;8(2):413–418. [PubMed] [Google Scholar]
- 15.Munoz A., Maldonado M., Pardo N., Fernandez J.M., Vela E., Cubells J. Pegylated liposomal doxorubicin hydrochloride (PLD) for advanced sarcomas in children: preliminary results. Pediatr Blood Cancer. 2004;43(2):152–155. doi: 10.1002/pbc.20029. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen O.S., Reichardt P., Christensen T.B., et al. Phase 1 European Organisation for Research and Treatment of Cancer study determining safety of pegylated liposomal doxorubicin (Caelyx) in combination with ifosfamide in previously untreated adult patients with advanced or metastatic soft tissue sarcomas. Eur J Cancer. 2006;42(14):2303–2309. doi: 10.1016/j.ejca.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Adamson P.C. Improving the outcome for children with cancer: development of targeted new agents. CA Cancer J Clin. 2015;65(3):212–220. doi: 10.3322/caac.21273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Bautista F., Paoletti X., Rubino J., et al. Phase I or II study of ribociclib in combination with topotecan-temozolomide or everolimus in children with advanced malignancies: arms A and B of the AcSe-ESMART trial. J Clin Oncol. 2021;39(32):3546–3560. doi: 10.1200/JCO.21.01152. [DOI] [PubMed] [Google Scholar]
- 20.Trucco M.M., Meyer C.F., Thornton K.A., et al. A phase II study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcomas. Clin Sarcoma Res. 2018;8:21. doi: 10.1186/s13569-018-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chidiac T., Budd G.T., Pelley R., et al. Phase II trial of liposomal doxorubicin (Doxil) in advanced soft tissue sarcomas. Invest New Drugs. 2000;18(3):253–259. doi: 10.1023/a:1006429907449. [DOI] [PubMed] [Google Scholar]
- 22.Fujisaka Y., Horiike A., Shimizu T., Yamamoto N., Yamada Y., Tamura T. Phase 1 clinical study of pegylated liposomal doxorubicin (JNS002) in Japanese patients with solid tumors. Jpn J Clin Oncol. 2006;36(12):768–774. doi: 10.1093/jjco/hyl109. [DOI] [PubMed] [Google Scholar]
- 23.Rau K.M., Lin Y.C., Chen Y.Y., et al. Pegylated liposomal doxorubicin (Lipo-Dox(R)) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: an open-label, multi-center, non-comparative phase II study. BMC Cancer. 2015;15:423. doi: 10.1186/s12885-015-1433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan B., Kirton L., Marec-Berard P., et al. Comparison of two chemotherapy regimens in patients with newly diagnosed Ewing sarcoma (EE2012): an open-label, randomised, phase 3 trial. Lancet. 2022;400(10362):1513–1521. doi: 10.1016/S0140-6736(22)01790-1. [DOI] [PubMed] [Google Scholar]
- 25.Heemelaar J.C., Speetjens F.M., Al Jaff A.A.M., et al. Impact of age at diagnosis on cardiotoxicity in high-grade osteosarcoma and ewing sarcoma patients. JACC CardioOncol. 2023;5(1):117–127. doi: 10.1016/j.jaccao.2022.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khallaf S.M., Roshdy J., Ibrahim A. Pegylated liposomal doxorubicin in patients with metastatic triple-negative breast cancer: 8-year experience of a single center. J Egypt Natl Cancer Inst. 2020;32(1):20. doi: 10.1186/s43046-020-00034-4. [DOI] [PubMed] [Google Scholar]
- 27.Jaffray M., Buchbinder N., Lutun A., Schneider P., Piquenot J.M., Vannier J.P. Salvage therapy with gemcitabine, vinorelbine, and pegylated liposomal doxorubicin for relapsed or refractory pediatric Hodgkin lymphoma. Results of a retrospective series of four children. Ann Hematol. 2015;94(8):1401–1406. doi: 10.1007/s00277-015-2362-7. [DOI] [PubMed] [Google Scholar]
- 28.Nikolaou V., Syrigos K., Saif M.W. Incidence and implications of chemotherapy related hand-foot syndrome. Expert Opin Drug Saf. 2016;15(12):1625–1633. doi: 10.1080/14740338.2016.1238067. [DOI] [PubMed] [Google Scholar]
- 29.Shern J.F., Selfe J., Izquierdo E., et al. Genomic classification and clinical outcome in rhabdomyosarcoma: a report from an international consortium. J Clin Oncol. 2021;39(26):2859–2871. doi: 10.1200/JCO.20.03060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tirode F., Surdez D., Ma X., et al. Genomic landscape of Ewing sarcoma defines an aggressive subtype with co-association of STAG2 and TP53 mutations. Cancer Discov. 2014;4(11):1342–1353. doi: 10.1158/2159-8290.CD-14-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.