As the population ages, the burden of age-related diseases becomes greater. Currently, over 55 million people suffer from dementia worldwide, with Alzheimer’s disease being the most common form. However, it is becoming clearer that underlying vascular pathology such as cerebral small vessel disease (cSVD) may be a more detrimental cause for dementia (Cuadrado-Godia et al., 2018). It is estimated that 10%–30% of the elderly population and 35%–90% of all dementia patients exhibit signs of cSVD. The term cSVD refers to pathology affecting the small vessels of the brain, which can lead to lacunar cerebral infarcts, enlarged perivascular spaces, and cortical hemorrhages (Cuadrado-Godia et al., 2018). CSVD is often associated with cognitive decline, gait problems, and dementia (Cuadrado-Godia et al., 2018). To minimize disease progression, preventive measures, and early interventions, such as risk factor management, are crucial. However, due to its asymptomatic nature, the disease is often silent and diagnosed coincidentally at later stages. Thus, the window of opportunity for prevention and treatment is often missed.
Although several cardiovascular risk factors such as hypertension, diabetes, and smoking are associated with cSVD, the etiopathology of the disease remains largely unknown (Manukjan et al., 2020). We, and others, have proposed a role for cerebral hypoperfusion in cSVD development. In this model, hypertension leads to hypoxia in the brain and endothelial cell dysfunction causing blood–brain barrier (BBB) impairments, inflammation, and white matter lesions (WMLs) (Cuadrado-Godia et al., 2018; Manukjan et al., 2020). Hypertension, brain hypoperfusion, BBB leakages, and WMLs are all common features seen in cSVD patients and might contribute to the development of brain damage and cognitive impairments (Wong et al., 2019). While many studies have demonstrated links between those pathological events, their exact sequence and molecular consequences remain unclear.
The BBB is a dynamic structure that holds center stage in cSVD pathology. Its permeability is increased upon the release of angiogenic factors aimed at stimulating the formation of new vessels (Yuan et al., 2023). While angiogenesis would logically be desired in the context of tissue hypoperfusion, the associated loosening of the BBB is potentially harmful since it may permit infiltration of plasma components, causing neuroinflammation and subsequent neurodegeneration. It is therefore of major importance to identify the molecular and cellular mechanisms promoting increased BBB permeability as this is suggested to initiate the formation of WMLs.
The endothelial cells forming the brain microvessels are specialized cells that differ from peripheral endothelial cells. Brain endothelial cells express tight junction proteins and active transporter mechanisms, and display an absence of fenestrated capillaries and pinocytosis (Yuan et al., 2023). As previously mentioned, endothelial cells interact with surrounding cells to ensure the brain parenchymal homeostasis, but they can also modulate cerebral blood flow by sensing shear stress due to flow and adapting their vessel diameter (Alberts et al., 2002). Astrocytes and microglia secrete factors that are crucial for the formation and maintenance of the BBB, but they also regulate blood flow and contribute to the selective permeability of the BBB. Oligodendrocyte lineage cells, including mature myelinating oligodendrocytes, and the oligodendrocyte precursor cells (OPCs) from which they arise, have bidirectional communication with neighboring cells. For example, neuronal activity is known to influence OPC development and myelination, and myelination may in turn influence neuronal circuit activity (Cuadrado-Godia et al., 2018; Manukjan et al., 2020). Similarly, OPC in the adult brain is not only involved in the generation of new oligodendrocytes, but also respond to neuroligands to regulate OPC fate. They are also involved in formation of neuron-OPC synapses, modulate neuronal signaling by secreting neuromodulators, modulate immune response, and play a crucial role in the development and maintenance of the BBB. In vitro experiments show increased endothelial cell proliferation induced by factors such as transforming growth factor-β and matrix metalloproteases secreted by OPCs in response to inflammatory stress (as reviewed in Manukjan et al., 2020). Additionally, as oxygen diffuses from the vessels into the brain tissue, the parenchymal cells in the deeper and less vascularized brain areas might be the first to be affected by hypoperfusion and hypoxia.
Evidence shows that OPCs in the deep cortical regions might be one of the first cells to respond to a hypoxic state and secrete signaling molecules as they are most vulnerable to hypoxic stress (Manukjan et al., 2020, 2023). Thus, changes in endothelial barrier function may arise due to cross-talk of the endothelial cells with hypoxia-affected oligodendroglia cells, rather than form direct effects of hypoperfusion on endothelial cells. It has previously been suggested that hypoxia induces the hypoxia inducible factor (HIF) 1/2a mediated release of Wnt7a/b by OPCs leading to an angiogenic response in the endothelial cells (Yuen et al., 2014; Manukjan et al., 2020). However, recent studies show no regulation on the Wnt7a/b genes in OPCs in response to hypoxia or HIF1a stabilization (Kleszka et al., 2020; Zhang et al., 2020, 2021; Allan et al., 2021). Apart from Wnt7a/b, other factors might play a pivotal role in the interaction between OPCs and endothelial cells in cSVD. Hence, we hypothesized that hypoperfusion of the brain leads to local hypoxia affecting OPCs, causing them to secrete factors that promote increased BBB permeability, leading to WML and the development and progression of cSVD. Our findings suggest that the vascular endothelial growth factor A (VEGFA), one of the most important angiogenic factors of the central nervous system, might be one of the first secreted factors underlying cSVD pathology.
VEGFA is a member of the platelet-derived growth factor/VEGF growth factor family and is produced by many cells in response to hypoxia (Abhinand et al., 2016). Endothelial cells express the VEGFR2 receptor and induce angiogenesis by binding VEGFA and activating multiple downstream pathways via intracellular signaling molecules. Activation of this signaling pathway disrupted barrier function by decreasing tight junction proteins Claudin-5 (CLDN5) and Occludin (OCLN) (Argaw et al., 2009), and induced endothelial cell responses such as proliferation and migration, which are necessary for the formation of new vessels (Abhinand et al., 2016). This signaling pathway also mediates cross-talk with other signaling pathways such as the WNT and NOTCH pathways that modulate the angiogenic response (Abhinand et al., 2016). VEGF and its receptors were upregulated at the border of the infarction after 48 hours, accompanied by a strong increase in the number of newly formed vessels in a permanent mouse middle cerebral artery occlusion model of stroke, suggesting a role for VEGF/VEGFR signaling in the formation of new vessels in response to ischemia (Alberts et al., 2002). VEGFA is mainly produced by cells surrounding the endothelial cells, such as the glia, whilst HIF1a stabilization in OPCs regulates angiogenesis in the central nervous system through VEGF-dependent and Wnt-independent signaling (Zhang et al., 2020). OPCs were the only hypoxic glia cells in mice following decreased cerebral blood flow, and expressed increased levels of Vegfa upon hypoxia exposure, via Hif1a and Hif2a signaling.
VEGFA promotes OPC migration in a concentration-dependent manner without impacting the proliferation of these cells (Hayakawa et al., 2011). Thus, VEGFA not only affects the surrounding endothelial cells, but might also impact the myelinating capacity by recruiting OPCs. Similarly, our hypoperfused mice did not show increased OPC densities in hypoxic deep cortical areas (Manukjan et al., 2023). In vitro, exposure of both primary OPCs and an OPC cell line (Oli-neu cells) to hypoxia led to an increase in VEGFA levels, which was mediated by Hif1a and Hif2a. Furthermore, treatment of endothelial bEnd.3 cells with a conditioned medium derived from hypoxic OPCs decreased the mRNA expression of the tight junction proteins CLDN5 and OCLN (Manukjan et al., 2023). The secretome of the hypoxic OPCs is composed of a wide range of factors that can affect the surrounding cells. However, VEGFA might be the most potent inducer of angiogenesis among those. While Vegfa is also expressed by other cells, OPCs might be the first to express this factor and initiate the cascade of pathological processes since they were the only hypoxic glial cells identified following hypoperfusion. We also identified the presence of BBB leakages in hypoperfused mice, without any changes in vascular density.
In cSVD patients, VEGFA was elevated in the plasma and correlated positively with an increase in BBB leakage rate in the normal appearing white matter seen in these patients. No significant correlation was observed in the control group, or in WML size and BBB leakage rate in WMLs. Hence, our results suggest that VEGFA might be one of the first contributors in the development of WMLs by inducing angiogenesis and increasing vascular permeability, thus paving the way for the inflammatory responses mediating white matter damage in normal appearing white matter. Although peripheral sampling does not indicate the source of VEGFA, these correlations highlight VEGFA as a promising biomarker for the early identification of patients at risk for cSVD. To support our OPC-derived VEGFA hypothesis, future studies should focus on replicating our findings in a large cSVD cohort with blood plasma VEGFA protein levels as primary outcome measure, and additional post-mortem brain tissue analysis such as VEGFA levels in normal appearing white matter and white matter lesions, and histological identification of OPCs in these areas.
VEGFA administration has been shown to have promising effects on stroke recovery, such as reducing lesion size, decreasing infarct size, promoting angiogenesis, and improving cognitive function. However, the effectiveness of VEGFA modulation in improving clinical outcomes in humans is yet to be proven. Moreover, it is essential to acknowledge the dual nature of modulating VEGFA signaling (le Noble et al., 2023). While beneficial angiogenic properties may facilitate compensatory vascular network growth in the brain and reduce hypoxia, there is a risk of increased BBB permeability contributing to inflammation and tissue damage. Another potentially adverse aspect of stimulating angiogenesis by systemic interventions is the risk of promoting the vascularization of any malignancies that may be present in the patient. A solution would be local delivery to the brain or specific modulation of brain VEGF receptors. The use of VEGFA isoforms, which elicit different biological effects, might be one strategy. Timing of VEGFA administration is also critical. Angiogenesis induction in the early silent stages of cSVD may help prevent ischemic injury, while post-stroke stimulation of new vessel formation and increased BBB permeability might aggravate disease progression. Thus, the therapeutic potential of VEGFA might be a double-edged sword and must be considered carefully.
In conclusion, we would like to propose the following mechanism as one of the pathways leading to the development of WMLs in cSVD (Figure 1). Chronic cardiovascular risk factors such as hypertension may induce cerebral hypoperfusion and local hypoxia in deep, poorly vascularized cerebral structures. As a result, oxygen-vulnerable OPCs secrete VEGFA in an attempt to increase vascularization and hence oxygen supply. This causes an increase in BBB permeability, permitting a neuroinflammatory reaction. Ultimately, the repetition and/or spatial accumulation of these events can lead to cerebral tissue damage and the development of WMLs, causing clinical cSVD symptoms. The detection of VEGFA (alone and/or in combination with other biomarkers) in fluid material (plasma, cerebrospinal fluid) may bring new insights for the early identification of patients at risk for the development of cSVD.
Figure 1.
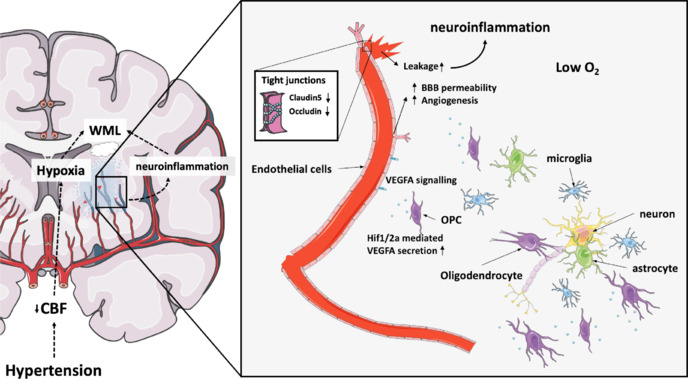
Schematic overview of proposed mechanism as one of the pathways leading to the development of WMLs in cSVD.
Chronic cardiovascular risk factors such as hypertension can lead to reduced blood flow and oxygen levels in poorly vascularized areas of the brain. Consequently, oxygen-sensitive oligodendrocyte precursor cells release VEGFA, mediated by Hif1/2a signaling, which triggers angiogenesis in an attempt to boost vascularization and improve oxygen supply. This process triggers increased BBB permeability by reducing tight junction proteins such as Claudin-5 and Occludin, which can lead to BBB leakages and neuroinflammation. Over time, the recurrence or accumulation of these events can result in brain tissue damage and the formation of white matter lesions, leading to clinical symptoms of cerebral small vessel disease (cSVD). Adapted from Manukjan et al. (2020). BBB: Blood–brain barrier; CBF: cerebral blood flow; Hif1/2a: hypoxia inducible factor 1/2a; O2: oxygen; OPC: oligodendrocyte precursor cell; VEGFA: vascular endothelial growth factor A; WML: white matter lesion.
Footnotes
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal. 2016;10:347–354. doi: 10.1007/s12079-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th. New York: Garland Science; 2002. Blood vessels and endothelial cells. [Google Scholar]
- Allan KC, Hu LR, Scavuzzo MA, Morton AR, Gevorgyan AS, Cohn EF, Clayton BLL, Bederman IR, Hung S, Bartels CF, Madhavan M, Tesar PJ. Non-canonical targets of HIF1a impair oligodendrocyte progenitor cell function. Cell Stem Cell. 2021;28:257–272. doi: 10.1016/j.stem.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado-Godia E, Dwivedi P, Sharma S, Ois Santiago A, Roquer Gonzalez J, Balcells M, Laird J, Turk M, Suri HS, Nicolaides A, Saba L, Khanna NN, Suri JS. Cerebral small vessel disease: a review focusing on pathophysiology, biomarkers, and machine learning strategies. J Stroke. 2018;20:302–320. doi: 10.5853/jos.2017.02922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD D, Som AT, Lee BJ, Guo S, Lo EH, Arai K. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neuroscience. 2011;31:10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleszka K, Leu T, Quinting T, Jastrow H, Pechlivanis S, Fandrey J, Schreiber T. Hypoxia-inducible factor-2α is crucial for proper brain development. Sci Rep. 2020;10:19146. doi: 10.1038/s41598-020-75838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Noble FAC, Mourad JJ, Levy BI, Struijker-Boudier HAJ. VEGF (vascular endothelial growth factor) inhibition and hypertension: does microvascular rarefaction play a role? Hypertension. 2023;80:901–911. doi: 10.1161/HYPERTENSIONAHA.122.19427. [DOI] [PubMed] [Google Scholar]
- Manukjan N, Ahmed Z, Fulton D, Blankesteijn WM, Foulquier S. A systematic review of WNT signaling in endothelial cell oligodendrocyte interactions: potential relevance to cerebral small vessel disease. Cells. 2020;9:1545. doi: 10.3390/cells9061545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manukjan N, Majcher D, Leenders P, Caiment F, van Herwijnen M, Smeets HJ, Suidgeest E, van der Weerd L, Vanmierlo T, Jansen JFA, Backes WH, van Oostenbrugge R J, Staals J, Fulton D, Ahmed Z, Blankesteijn WM, Foulquier S. Hypoxic oligodendrocyte precursor cell-derived VEGFA is associated with blood-brain barrier impairment. Acta Neuropathol Commun. 2023;11:128. doi: 10.1186/s40478-023-01627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SM, Jansen JFA, Zhang CE, Hoff EI, Staals J, van Oostenbrugge RJ, Backes WH. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology. 2019;92:e1669–1677. doi: 10.1212/WNL.0000000000007263. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Sun J, Dong Q, Cui M. Blood–brain barrier endothelial cells in neurodegenerative diseases: signals from the “barrier”. Front Neurosci. 2023;17:1047778. doi: 10.3389/fnins.2023.1047778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SPJ, Zahed H, Maltepe E, Rowitch DH. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158:383–396. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kim B, Zhu X, Gui X, Wang Y, Lan Z, Prabhu P, Fond K, Wang A, Guo F. Glial type specific regulation of CNS angiogenesis by HIFα-activated different signaling pathways. Nat Commun. 2020;11:2027. doi: 10.1038/s41467-020-15656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang Y, Xu J, Kim B, Deng W, Guo F. HIFα regulates developmental myelination independent of autocrine Wnt signaling. J Neurosci. 2021;41:251–268. doi: 10.1523/JNEUROSCI.0731-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


