Introduction: Fluid and positron emission tomography (PET) biomarkers that enable the detection of the hallmark proteins of Alzheimer’s disease (AD) (amyloid and tau) have revolutionized our approach to the diagnosis of AD. The evolution of AD diagnostic criteria to include biological characterization (Alzheimer’s Association Working Group, 2023) provides an appropriate framework to reduce levels of clinico-pathologic mismatch and improve in-vivo diagnostic accuracy. As the therapeutic landscape for neurodegenerative disease evolves, it is increasingly incumbent on clinicians to provide timely, and pathologically precise diagnoses for patients. However, the expensive and invasive nature of these tests limits their scalability.
Blood-based biomarkers (BBM) address many of the practical limitations of cerebrospinal fluid and PET biomarkers as they are cheap, accessible, and repeatable. There has been rapid progress in the field of BBM discovery and validation, to the extent that they are now being included in the latest proposed diagnostic criteria for AD. BBM are likely to have a transformative impact on clinical practice: combinations of plasma amyloid-beta 42/amyloid-beta 40 (Aβ42/Aβ40), p-tau181, and p-tau217 can be used to differentiate AD from other dementias, detect AD pathology in individuals with mild cognitive impairment (MCI) and to predict AD in those with MCI or subjective cognitive impairment (Hansson et al., 2022). The exact role BBM will play in the diagnostic assessment of patients with cognitive concerns remains unclear. Brum et al. (2023) propose a two-step workflow using a combination of p-tau 217, age, and APOE status to inform Amyloid PET requirement in patients with MCI, with only those in the “intermediate risk” profile requiring confirmatory testing. This is estimated to reduce the burden of confirmatory testing by 60%–85%, representing a significant cost-save for healthcare services. In addition, neurofilament (NfL) has shown itself to be a sensitive, albeit non-specific, marker of neuronal injury that may have a role in monitoring response to disease modifying treatments – as has already been the case in neuroinflammatory diseases like Multiple Sclerosis. BBM have the potential to democratize access to accurate biologically based AD diagnoses as they could be used in low resource, and possibly even primary care, settings. However, before BBM are integrated into routine clinical practice there are a number of important caveats to be considered (Figure 1).
Figure 1.
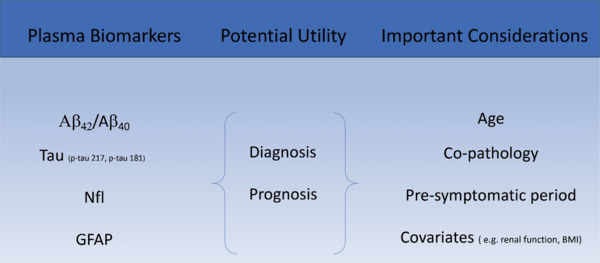
Plasma biomarkers - important clinical considerations.
Created with Microsoft PowerPoint. Aβ: Amyloid-beta; BMI: body mass index; GFAP: glial fibrillary acidic protein; NfL: neurofilament.
Pre-symptomatic period: The earliest pathological changes of AD predate the onset of cognitive symptoms by over two decades. Studies of familial Alzheimer’s disease (FAD), an autosomal dominantly inherited form of AD that has a reasonably predictable age at onset, have enabled characterization of this pre-symptomatic period. Bateman et al. (2012) demonstrated that cerebrospinal fluid Aβ42 levels begin to fall twenty-five years before the expected onset of symptoms, with amyloid deposition on PET scanning beginning at least 15 years before symptom onset. Pre-symptomatic change is also seen in plasma measures in FAD: increases in p-tau181, p-tau217, glial fibrillary acidic protein (GFAP), and NfL begin over a decade prior to estimated symptom onset (O’Connor et al., 2021; Ashton et al., 2022). Sporadic AD also has a pre-symptomatic period. Similar to FAD amyloid deposition begins decades before symptom onset, while there is also evidence of pre-symptomatic change in key plasma biomarkers (Aβ42/40, p-tau181, p-tau217, NfL, and GFAP; Ashton et al., 2022).
Pre-symptomatic change in key plasma biomarkers means that blood testing could detect AD pathology in asymptomatic individuals. It is paramount that clinicians do not become over reliant on such diagnostic tools and fail to adhere to a systematic work up of a patient’s symptoms as this would bring a risk of overlooking alternative and potentially reversible causes of cognitive impairment. Plasma biomarker positivity needs to be interpreted in the context of the neuropsychological profile, comorbid conditions, medication exposure, and risk factors as there is a risk of misattribution of cognitive symptoms to AD. Additionally, the use of reasonably AD specific plasma biomarkers (Aβ42/40, p-tau181, p-tau217) in clinical practice will bring a risk of inadvertent detection of pre-symptomatic AD – pre-symptomatic screening is not currently indicated as there are no licensed treatments for this stage of AD. It will be critically important that clinicians only perform plasma biomarker testing for AD in the appropriate clinical context and only after appropriate patient counseling.
The predictive value of biomarkers in those with MCI is an important clinical consideration. At a stage where there is active neurodegeneration with relatively mild or subtle symptomatology, accessible biomarkers that can identify the underlying pathology and inform clinical trajectory are invaluable. P-tau181 and p-tau217 have consistently demonstrated accurate prognostic capacity for the progression of cognitive impairment in patients with MCI (Hansson et al., 2022). P-tau 217 in particular, is associated with conventional markers of disease progression such as cognitive change and cortical thickness in AD specific regions (Ashton et al., 2022). Such dynamic biomarkers not only carry significant prognostic potential for patients, families, and clinicians, but may also serve to guide therapeutic intervention in the future.
Influence of age on biomarker positivity: Amyloid biomarker status should be interpreted in the context of an individual’s age as biomarker positivity increases with increasing age: by age 75, over 25% of cognitively normal adults will be amyloid positive, increasing to > 32% by age 80 (Jansen et al., 2015). The high prevalence of amyloid positivity amongst cognitively normal adults aged 75 years and older reduces the positive predictive value of amyloid biomarker status for confirming a diagnosis of AD. There is increasing evidence that certain plasma p-tau species are amyloid response measures (O’Connor et al., 2021), therefore, just as there should be caution when interpreting amyloid biomarker positivity in adults aged 75 years and older, there should also be a case for determining the significance of increased levels plasma p-tau181 and p-tau217 in this age group.
We know from population-based neuropathological studies, that not all individuals with amyloid pathology develop cognitive impairment and that the relationship between pathological change and dementia varies with age: by age 95 people who die with and without dementia show a similar burden of neuropathological change (Savva et al., 2009). The burden of neuritic plaques composed of amyloid, and tau-containing neurofibrillary tangles increases with increasing age at death, irrespective of cognitive status (Savva et al., 2009). In the aging population, we should be especially cognizant of other markers, which have been shown to have a more robust association with dementia irrespective of age. The recently proposed revised criteria for diagnosis and staging of AD, an advancement on the 2018 A/T/N (Aβ, Tau, Neurodegeneration) framework, includes non-AD specific markers of neuronal injury such as atrophy on structural imaging or hypometabolism on FDG-PET, in addition to plasma NfL characterization (Alzheimer’s Association Working Group, 2023). However, similar caution is urged regarding the interpretation of NfL positivity in older adults. As a non-specific marker of neurodegeneration, there is a strong relationship between age and NfL, particularly in the over seventies (Hansson et al., 2022). Employing criteria that are inclusive of all biomarker profiles addresses some of the concerns around age-related AD pathological change, as well as accounting for non-AD and mixed pathologies.
Impact of covariates and comorbidities on biomarker levels: Large and diverse population-based studies addressing potential confounding factors are required before the wide-scale implementation of plasma biomarkers. Aside from age, a number of factors have been linked to altered plasma levels of commonly used biomarkers. The recent work of Binette et al. (2023), has demonstrated the influence of body mass index and renal function on plasma biomarkers such as NfL, GFAP and to a lesser extent p-tau181 and p-tau217. Elevated body mass index was associated with reduced concentration of plasma biomarkers, a consequence attributed to higher circulating blood volume. Elevated creatinine was associated with higher levels of plasma biomarkers, likely due to lower blood filtration rates. However, the clinical relevance of these factors remains unclear, adjusting for such confounders at a group level had a minor influence on the prediction of dementia. Head trauma can lead to transient elevations in p-tau and NfL, while sustained elevations in NfL are also seen in other neurodegenerative conditions such as ALS (Alzheimer’s Association Working Group, 2023). A number of other potential confounding variables such as cardiovascular disease and medication effects have been suggested, and further studies examining these factors in diverse populations are needed. The influence of non-medical confounders is also of relevance, such as ethnicity, education, and socioeconomic status and there are conflicting reports of lower p-tau levels in Black or Latin American populations (Hansson et al., 2022). The presence of such potential confounding factors further emphasizes the need for clinical judgment in the interpretation of BBMs.
Influence of co-pathology: There is increasing awareness of the influence of co-pathology on the clinical phenotype and trajectory of cognitive decline in AD. Testing for amyloid and tau in isolation provides an incomplete picture of the neuropathological processes at play. The development of a reliable in vivo biomarker for alpha-synuclein pathology has enabled estimation of the frequency of alpha-synuclein co-pathology in AD; rates of up to 36% in those with AD-MCI increasing to 63% for those with AD dementia (Quadalti et al., 2023). The presence of alpha-syunclein co-pathology impacts on prognosis - it is associated with a faster rate of cognitive decline, and poorer performance on measures of global cognition and memory than those with isolated AD pathology (Quadalti et al., 2023). If we fail to account for co-pathology in the clinical assessment of our patients, we stand to miss important prognostic and treatment-related considerations. We must be vigilant for classical clinical features of co-existent synucleinopathy such as REM sleep behavior disorder, anosmia, dysautonomia, fluctuating cognition, hallucinations, and parkinsonism. However, we must also take into account the trajectory of cognitive decline, and look for features of co-pathology in those progressing more rapidly. Lewy body pathology has also been shown to have independent effects on attention/executive and visuospatial function (Quadalti et al., 2023), and identifying these as leading cognitive symptoms may alert clinicians to the presence of underlying co-pathology. This further serves to remind us that biomarkers do not remove the need for an individualized and multifaceted approach to the assessment of cognitive complaints in all patients.
The co-existence of Lewy body pathology in those with AD is of heightened significance in the era of pathologically targeted disease modifying therapies. Although it remains to be seen what role BBMs for AD will play in the selection of patients for treatment, comprehensive clinical assessment will remain a critical component of this process. A profound limitation of anti-amyloid clinical trials has been the failure to account for Lewy body co-pathology, due to the lack of available in-vivo biomarkers for alpha-synuclein. Emerging data suggests that such patients have an expectedly poorer clinical response to anti-amyloid therapies, with continued clinical progression despite amyloid reduction on PET (VandeVrede et al., 2023). Furthermore, these treatments carry a risk profile far beyond the current symptomatic treatments for AD, in particular, the risk of the potentially severe side effect of amyloid-related imaging abnormalities is not trivial. Additional concerns include infusion-related reactions, and impact on quality of life with frequent hospital visits for infusions and monitoring. Selecting patients for treatment with anti-amyloid therapies who may be less likely to benefit due to the additional burden of Lewy body pathology risks these safety concerns outweighing the balance of treatment effect.
Future directions: Future directions of BBM include proteomic and metabonomic BBM, an expanding field that enables deep profiling of brain tissue and related biofluids. A number of proteomic BBM that reflect disease activity and pathology have been discovered using an endophenotype approach. Biomarkers of neuroprotection and neurorepair such as clusterin, which has demonstrated association with hippocampal atrophy and clinical progression (Baird et al., 2015) would be particularly valuable as they may open up new therapeutic avenues. While further work is needed before such BBM enter the clinical arena, the field of “omics” research offers bespoke pathogenic, diagnostic, and prognostic information and could facilitate a personalized medicine approach in AD.
Conclusion: The use of BBMs in clinical practice is fast approaching and when used appropriately, are likely to shorten the time to diagnosis and expedite access to disease-modifying therapies. However, prospective work determining the optimal combination of biomarkers and the clinical robustness of such biomarkers in large and diverse populations remains outstanding. The intended use of BBMs needs to be carefully evaluated prior to any large-scale implementation and their role in the diagnostic workup of patients with cognitive complaints will have to be clearly defined. Additionally, clinicians and policy makers will need to be cognizant that BBM will not obviate the need for critical thinking and holistic clinical assessment.
Footnotes
C-Editors: Zhao M, Sun Y, Qiu Y; T-Editor: Jia Y
References
- Ashton NJ, et al. Differential roles of Abeta42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat Med. 2022;28:2555–2562. doi: 10.1038/s41591-022-02074-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird AL, Westwood S, Lovestone S. Blood-based proteomic biomarkers of Alzheimer’s disease pathology. Front Neurol. 2015;6:236. doi: 10.3389/fneur.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum WS, Cullen NC, Janelidze S, Ashton NJ, Zimmer ER, Therriault J, Benedet AL, Rahmouni N, Tissot C, Stevenson J, Servaes S, Triana-Baltzer G, Kolb HC, Palmqvist S, Stomrud E, Rosa-Neto P, Blennow K, Hansson O. A two-step workflow based on plasma p-tau217 to screen for amyloid β positivity with further confirmatory testing only in uncertain cases. Nature Ageing. 2023;3:1079–1090. doi: 10.1038/s43587-023-00471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group AsAW Revised criteria for diagnosis and staging of Alzheimer’s disease. Alzheimer’s Associations International Conference. Amsterdam 2023 [Google Scholar]
- Hansson O, Edelmayer RM, Boxer AL, Carrillo MC, Mielke MM, Rabinovici GD, Salloway S, Sperling R, Zetterberg H, Teunissen CE. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. 2022;18:2669–2686. doi: 10.1002/alz.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor A, et al. Plasma phospho-tau181 in presymptomatic and symptomatic familial Alzheimer’s disease: a longitudinal cohort study. Mol Psychiatry. 2021;26:5967–5976. doi: 10.1038/s41380-020-0838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichet Binette A, Janelidze S, Cullen N, Dage JL, Bateman RJ, Zetterberg H, Blennow K, Stomrud E, Mattsson-Carlgren N, Hansson O. Confounding factors of Alzheimer’s disease plasma biomarkers and their impact on clinical performance. Alzheimers Dement. 2023;19:1403–1414. doi: 10.1002/alz.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadalti C, Palmqvist S, Hall S, Rossi M, Mammana A, Janelidze S, Dellavalle S, Mattsson-Carlgren N, Baiardi S, Stomrud E, Hansson O, Parchi P. Clinical effects of Lewy body pathology in cognitively impaired individuals. Nat Med. 2023;29:1964–1970. doi: 10.1038/s41591-023-02449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, Medical Research Council Cognitive F, Ageing S. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- VandeVrede L, La Joie R, Horiki S, Mundada NS, Koestler M, Hwang JH, Ljubenkov PA, Rojas JC, Rabinovici GD, Boxer AL, Seeley WW. Co-pathology may impact outcomes of amyloid-targeting treatments: clinicopathological results from two patients treated with aducanumab. Acta Neuropathol. 2023;146:777–781. doi: 10.1007/s00401-023-02631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


