Cell adhesion plays pivotal roles in the morphogenesis of multicellular organisms. Epithelial cells form several types of cell-to-cell adhesion, including zonula occludens (tight junctions), zonula adhaerens (adherens junctions), and macula adhaerens (desmosomes). Although these adhesion complexes are basically observed only in epithelial cells, cadherins, which are the major cell adhesion molecules of adherens junctions, are expressed in both epithelial and non-epithelial tissues, including neural tissues (Kawauchi, 2012). The cadherin superfamily consists of more than 100 members, but classic cadherins, such as E-cadherin (Cdh1), N-cadherin (Cdh2), and R-cadherin (Cdh4), amount to about 20 members. Classic cadherins are single transmembrane proteins, which basically exhibit homophilic adhesion in an extracellular Ca2+-dependent manner. The cell adhesion activity is also regulated intracellularly. The intracellular domain of classic cadherins binds to β-catenin, which interacts with α-catenin (Figure 1). Because α-catenin binds to an actin-binding protein, vinculin, the catenin complex mediates the interaction between classic cadherins and the actin cytoskeleton, which stabilizes cell-to-cell adhesion (Kawauchi, 2012).
Figure 1.
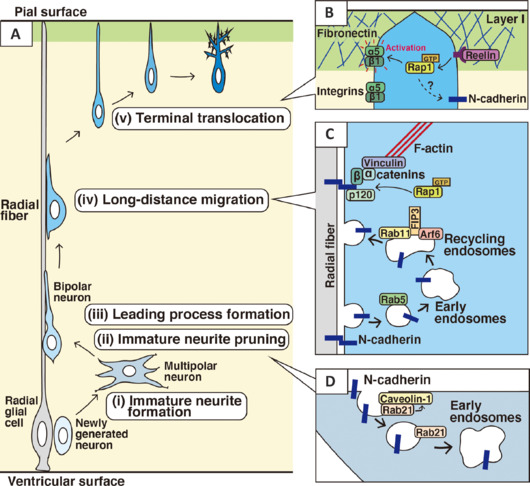
Membrane traffic-mediated regulation of N-cadherin plays critical roles in multiple modes of neuronal migration and morphological changes.
(A) Immature neurons exhibit multiple steps of migration. (i) Radial glial cells are neural progenitors, which directly or indirectly give rise to immature neurons. Newly generated neurons extend immature neurites and exhibit multipolar morphologies. (ii) The multipolar neurons repeatedly extend and retract immature neurites, but subsequently eliminate all immature neurites, besides an axon. (iii) Almost concurrently with the immature neurite pruning, the neurons form a pia-directed thick leading process, resulting in a bipolar morphology. (iv) The bipolar-shaped neurons, called locomoting neurons, undergo long-distance migration along the radial fibers. (v) At the final phase of migration, the neurons undergo terminal translocation. (B) Reelin promotes GDP/GTP exchange reaction of Rap1, which activates α5β1-integrin, a fibronectin receptor, at the front side of the leading process in the outermost layer (layer I) of the cerebral cortex. N-cadherin is also a downstream of Rap1 and may be involved in the terminal translocation. (C) N-cadherin is essential for the attachment of the locomoting neurons to the radial fibers. N-cadherin is internalized and recycled to the forward plasma membrane to promote forward movement along the radial fibers, which is executed by the cooperative and/or sequential activation of Rab5, Rab11, Arf6, and FIP3 (see the main text). Rap1 is required for the stabilization of N-cadherin at the plasma membrane. (D) The immature neurite pruning requires N-cadherin internalization, which is regulated by Rab21 and caveolin-1. Rab21 recruits and may stabilize caveolin-1 at the plasma membrane. The internalized N-cadherin may be transported in part toward the cell front to extend the leading process. Created with Adobe Illustrator. α and β: α- and β-catenins, respectively; α5/β1: α5β1-integrin heterodimer; GTP-Rap1: an activated form of Rap1.
Among classic cadherins, N-cadherin is strongly expressed in neural tissues and has multiple functions in neural development. N-cadherin is reported to regulate neural tube formation, neuronal differentiation, immature neuron migration, neurite extension, dendrite morphogenesis, and AMPA receptor trafficking at the synapses. In this review, we focus solely on the role of N-cadherin in the migration of immature excitatory neurons during the development of the mouse cerebral cortex.
Multi-step modes of neuronal migration during cerebral cortical development: Immature excitatory neurons, generated from radial glial cells or secondary progenitors near the ventricle, migrate toward the pial surface. The neuronal migration involves multiple steps associated with various morphological changes (Kawauchi et al., 2010). Newly generated neurons initially exhibit multipolar morphology, extending many immature neurites, of which one is selected as a future axon (Figure 1A-i). The multipolar neurons repeatedly elongate and retract the immature neurites, which subsequently undergo pruning (Shikanai et al., 2018; Figure 1A-ii). Almost simultaneously, the neurons form a thick pia-directed process, called a leading process (Figure 1A-iii). Thus, the multipolar neurons are transformed into bipolar neurons; this is called the multipolar-to-bipolar transition. The bipolar neurons attach to and migrate along the radial fibers, which are very long processes of the radial glial cells that extend from the ventricular side to the pial surface (Figure 1A-iv). This scaffold cell-dependent migration of the bipolar neurons is called the locomotion mode (Kawauchi, 2012). After long-distance migration, the migration mode changes from locomotion to terminal translocation. The terminal translocation is defined as cell soma movement with retraction of the pia-attached leading process (Figure 1A-v). At the end of the terminal translocation, the leading process becomes branched and matures into dendrites.
N-Cadherin in multipolar-to-bipolar transition and early neuronal maturation: After neuronal differentiation, newborn neurons elongate many immature neurites. Knockdown of N-cadherin suppresses the immature neurite extension (Kawauchi et al., 2010), suggesting that N-cadherin is required for immature neurite formation. It is unclear whether homophilic adhesion mediated by N-cadherin is involved in the immature neurite elongation, but a forced increase of N-cadherin surface levels, caused by blocking endocytosis (described below), promotes the attachment of the immature neurites to each other, resulting in “sticky” immature neurites (Shikanai et al., 2023). This suggests that N-cadherins on the immature neurites of neighboring cells exhibit homophilic adhesion, which may contribute to the elongation or stabilization of the immature neurites.
During the multipolar-to-bipolar transition, neurons acquire neuronal polarity, which is accompanied by reorientation of the centrosome and Golgi apparatus (Nishimura et al., 2014). It has been reported that N-cadherin promotes the Golgi reorientation of the multipolar neurons in the cerebral cortex, which is mediated by the stabilization of fibroblast growth factor receptors at least in part (Kon et al., 2019). Furthermore, the knockdown of N-cadherin perturbs the attachment of the bipolar neurons to the radial fibers, indicating that N-cadherin is a major cell adhesion molecule linking the migrating neurons with the radial fibers (Kawauchi et al., 2010). Elongation of the leading process is also suppressed by N-cadherin knockdown. Thus, N-cadherin regulates various steps of the multipolar-to-bipolar transition.
N-Cadherin in locomotion and somal translocation: The multipolar-to-bipolar transition leads to the bipolar locomoting neurons, which migrate along the radial fibers toward the pial surface. As described above, N-Cadherin plays an essential role in the adhesion between the locomoting neurons and the radial fibers (Kawauchi et al., 2010). Interestingly, the attachment to the radial fibers induces axon extension at the opposite side of the neurons from the adhesion site (Xu et al., 2015). In addition to N-cadherin, R-cadherin is also involved in radial fiber-dependent neuronal migration (Martinez-Garay et al., 2016). While N-cadherin-conditional knockout neurons exhibit defects in the cortical neuronal migration, neurons with a double knockout of N-cadherin and R-cadherin show more severe migration defects than the N-cadherin-single knockout neurons, suggesting that both R-cadherin and N-cadherin have roles in neuronal migration in the developing cerebral cortex (Martinez-Garay et al., 2016).
At the final phase of neuronal migration, neurons undergo radial fiber-independent terminal translocation. During the terminal translocation, N-cadherin staining signals at the cell soma are decreased, whereas a leading process, a future dendrite, retains expression of N-cadherin (Kawauchi et al., 2010). While α5β1-integrin, a molecular heterodimer functioning in cell-to-extracellular matrix adhesion, controls the terminal translocation (Sekine et al., 2012; Figure 1B), the involvement of N-cadherin in the terminal translocation is still unclear. During early neurogenesis, however, early-born neurons exhibit somal translocation, which resembles terminal translocation, and this somal translocation requires N-cadherin (Martinez-Garay, 2020; Figure 1B).
Regulatory mechanisms of N-cadherin intracellular trafficking in neuronal migration: N-cadherin has multiple roles during cerebral cortical development, and therefore the upstream regulation of N-cadherin localization and cell surface levels is an important issue. Although the mechanisms of spatiotemporal regulation of N-cadherin are still unclear, several key molecules have been identified. Under the control of Reelin, Rap1, a Ras family small GTPase, increases the cell surface level of N-cadherin (Kon et al., 2019; Figure 1B and C). Reelin is a secreted large molecule that has an essential role in the formation of the mammalian-specific six-layered structure of the cerebral cortex and activates both N-cadherin and Integrins, including α5β1-integrin (Figure 1B). Reelin binds to its receptors, apolipoprotein E receptor 2 and very-low-density lipoprotein receptor, and induces tyrosine phosphorylation of Dab1, a cytoplasmic adaptor protein that interacts with the cytoplasmic domain of the Reelin receptors. The phosphorylated Dab1 binds to another adaptor proteins, Crk and CrkL, which recruit C3G, an activator for Rap1 (Sekine et al., 2012; Martinez-Garay, 2020). Expression of Rap1GAP, which suppresses all members of Rap family (Rap1A, Rap1B, Rap2A, Rap2B, and Rap2C), results in perinuclear accumulation of N-cadherin, whereas N-cadherin is mainly localized at the plasma membrane in control cells (Kon et al., 2019). Rap1 is reported to inhibit cadherin endocytosis through the regulation of p120-catenin, resulting in the stabilization of cell surface cadherins (Figure 1C).
On the other hand, N-cadherin endocytosis is also important for proper neuronal migration (Kawauchi et al., 2010, 2012). N-cadherin is essential for the attachment of the locomoting neurons to the radial fibers, and that is why detachment is required for forward movement. The N-cadherin-mediated cell-cell adhesion should be disrupted at the rear of the cell and a new adhesion should be formed at the cell front. It has been reported that endocytic trafficking mediated by Rab family small GTPases plays an important role in the remodeling of the adhesion complex. A Rab family small GTPase, Rab5, activates the endocytic pathway to internalize N-cadherin, and the internalized N-cadherin is transferred to the forward plasma membrane via Rab11-dependent recycling pathways (Kawauchi et al., 2010; Nishimura et al., 2014; Figure 1C). Because a cadherin-mediated adhesion complex plays a role in maintaining actin fibers (F-actin) in the leading process, N-cadherin internalization at the rear of the cell may also lead to the release of the “anchor points of F-actin” that generate the tension derived from the actomyosin-dependent contractile force (Figure 2A). In fact, treatment with calyculin A, an activator of actomyosin-dependent contractility, promotes somal movement towards the pial surface, whereas functional suppression of cadherins blocks the forward movement (Martinez-Garay et al., 2016). From these results, we hypothesize that Rab5-dependent endocytosis of N-cadherin disrupts the anchor points of F-actin and thereby releases the actomyosin-mediated tension, which results in pulling up of the cell soma (Figure 2B). The internalized N-cadherin is transported to the cell front by Rab5- and Rab11-mediated endocytosis-recycling pathways and is utilized for the formation of new attachments at the front (Figure 2C). N-Cadherin-mediated adhesion at the tip of the leading process provides new anchor points of F-actin, which generates another actomyosin contractile force in the leading process (Figure 2A). Such a cooperative regulation of membrane trafficking, cell adhesion dynamics and actomyosin-dependent contractile force is considered to play an essential role in the radial fiber-dependent locomotion mode of neuronal migration.
Figure 2.
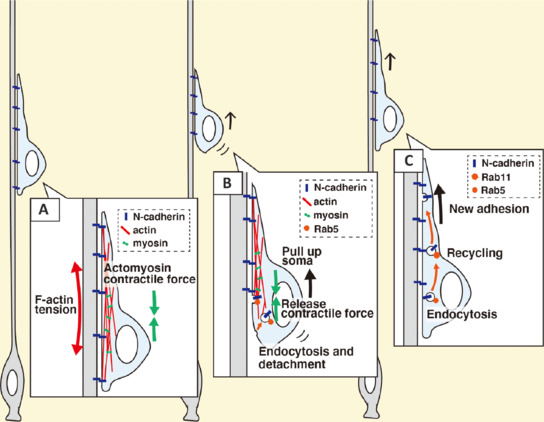
A new hypothesis for the locomotion mode of neuronal migration that is mediated by cooperative regulation of cell adhesion, endocytic membrane trafficking, actin cytoskeletal organization and actomyosin-mediated contractile force.
(A) N-Cadherin serves as anchor points for actin fibers (F-actin), which generate a tension derived from the actomyosin contractile force. (B) Detachment of the F-actin-anchors by N-cadherin endocytosis releases the actomyosin contractile force, causing the neuronal cell soma to be pulled up. (C) The internalized N-cadherin is transported to the forward plasma membrane to form new attachment points to the radial fibers. Created with Adobe Illustrator.
Rab7, Rab18, and Rab23 also control cell surface and/or total protein levels of N-cadherin and cortical neuronal migration (Kawauchi et al., 2010; Martinez-Garay, 2020). In contrast, N-cadherin protein level is not changed in Rab35-knockout cortical neurons, which normally migrate toward the pial surface (Maejima et al., 2023). Thus, many, but not all, Rab family proteins are involved in the regulation of N-cadherin dynamics and cortical neuronal migration.
Not only Rab family but also Arf family of small GTPases are involved in regulation of the intracellular trafficking. Arf4 and Arf6 are reported to regulate cortical neuronal migration through the regulation of N-cadherin (Hara et al., 2023). FIP3, a dual effector of Arf6 and Rab11, is also required for the neuronal migration. Knockdown of Arf6 or FIP3 causes abnormal perinuclear accumulation of N-cadherin in migrating neurons, suggesting that Arf6 and FIP3 control neuronal migration through the regulation of N-cadherin trafficking (Figure 1C). Interestingly, the migration defects of FIP3-knockdown neurons can be restored by the expression of wild type FIP3, but not mutant FIP3 lacking the binding site for Arf6 or Rab11. These results suggest that FIP3 functions as an effector of both Arf6 and Rab11 in cortical neuronal migration.
The immature neurite pruning in multipolar neurons also requires N-cadherin endocytosis, but the underlying molecular mechanisms are different (Figure 1D). Endocytosis is classified into several types, including clathrin- and caveolin-mediated. Although Rab5 is thought to be a key regulator of both clathrin- and caveolin-mediated endocytosis, a recent study indicates that Rab5 prefers to control clathrin-mediated, but not caveolin-mediated, endocytosis (Shikanai et al., 2023). In contrast, Rab21 is required for caveolin-mediated endocytosis. Rab21 recruits caveolin-1 at the plasma membrane, which prevents the lysosomal degradation of caveolin-1 (Shikanai et al., 2023; Figure 1D). Both Rab5 and Rab21 are involved in N-cadherin endocytic trafficking, but knockdown of Rab5 suppresses the radial fiber-dependent locomotion mode of the neuronal migration and the extension of immature neurites, whereas Rab21 knockdown causes defects in immature neurite pruning of the multipolar neurons (Figure 1D). These results also support the idea that Rab5 and Rab21 differentially regulate N-cadherin trafficking and cortical development. Interestingly, knockdown of caveolin-1 displays quite similar phenotypes to Rab21 knockdown (Shikanai et al., 2018, 2023). Thus, unlike Rab5, Rab21 controls immature neurite pruning through the regulation of caveolin-mediated endocytic pathways.
Conclusion: During cerebral cortical development, immature neurons exhibit multi-step modes of migration. Among them, the locomotion mode of neuronal migration covers most of the migration route. This migration mode is unique, because it depends on cell-to-cell adhesion, but not cell-to-extracellular matrix adhesion, which is referred to as scaffold cell-dependent migration (Kawauchi, 2012). Therefore, the radial fiber-dependent migration requires a cell-to-cell adhesion molecule, N-cadherin. Interestingly, N-cadherin is also required for radial fiber-independent modes of neuronal migration. N-Cadherin dynamics are regulated by not only cytoskeletal rearrangements, but also membrane trafficking, which is mainly regulated by Rab family small GTPases. The Rab family consists of about 60 members in mammalian cells, and membrane trafficking pathways are very complicated, so it is likely that many unidentified Rab proteins and membrane trafficking pathways are involved in cerebral cortical development. Research on membrane trafficking-mediated regulation of cell adhesion dynamics and its roles in neuronal migration has only just begun, and extensive studies will be needed to uncover in detail the regulatory mechanisms of N-cadherin in brain development.
The authors thank Dr. Richard Steele (UK) for the English proofreading of the manuscript. We apologize to all authors for papers omitted due to space limitations.
The authors’ research group was funded by JSPS KAKENHI Grant Numbers JP26290015 and JP21H02655 (to TK) from Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT).
Footnotes
C-Editors: Zhao M, Sun Y, Qiu Y; T-Editor: Jia Y
References
- Hara Y, Katsuyama T, Fukaya M, Sugawara T, Shiroshima T, Sadakata T, Osumi N, Sakagami H. ADP ribosylation factor 4 (Arf4) regulates radial migration through N-cadherin trafficking during cerebral cortical development. eNeuro. 2023;10 doi: 10.1523/ENEURO.0125-23.2023. ENEURO.0125-0123.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo K, Nakajima K, Nabeshima Y, Hoshino M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Kawauchi T. Cell adhesion and its endocytic regulation in cell migration during neural development and cancer metastasis. Int J Mol Sci. 2012;13:4564–4590. doi: 10.3390/ijms13044564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon E, Calvo-Jimenez E, Cossard A, Na Y, Cooper JA, Jossin Y. N-cadherin-regulated FGFR ubiquitination and degradation control mammalian neocortical projection neuron migration. Elife. 2019;8:e47673. doi: 10.7554/eLife.47673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima I, Hara T, Tsukamoto S, Koizumi H, Kawauchi T, Akuzawa T, Hirai R, Kobayashi H, Isobe I, Emoto K, Kosako H, Sato K. RAB35 is required for murine hippocampal development and functions by regulating neuronal cell distribution. Commun Biol. 2023;6:440. doi: 10.1038/s42003-023-04826-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garay I, Gil-Sanz C, Franco SJ, Espinosa A, Molnar Z, Mueller U. Cadherin 2/4 signaling via PTP1B and catenins is crucial for nucleokinesis during radial neuronal migration in the neocortex. Development. 2016;143:2121–2134. doi: 10.1242/dev.132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garay I. Molecular mechanisms of cadherin function during cortical migration. Front Cell Dev Biol. 2020;8:588152. doi: 10.3389/fcell.2020.588152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura YV, Shikanai M, Hoshino M, Ohshima T, Nabeshima Y, Mizutani K, Nagata K, Nakajima K, Kawauchi T. Cdk5 and its substrates, Dcx and p27kip1, regulate cytoplasmic dilation formation and nuclear elongation in migrating neurons. Development. 2014;141:3540–3550. doi: 10.1242/dev.111294. [DOI] [PubMed] [Google Scholar]
- Sekine K, Kawauchi T, Kubo K, Honda T, Herz J, Hattori M, Kinashi T, Nakajima K. Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin alpha5beta1. Neuron. 2012;76:353–369. doi: 10.1016/j.neuron.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai M, Nishimura YV, Sakurai M, Nabeshima YI, Yuzaki M, Kawauchi T. Caveolin-1 promotes early neuronal maturation via caveolae-independent trafficking of N-cadherin and L1. iScience. 2018;7:53–67. doi: 10.1016/j.isci.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai M, Ito S, Nishimura YV, Akagawa R, Fukuda M, Yuzaki M, Nabeshima YI, Kawauchi T. Rab21 regulates caveolin-1-mediated endocytic trafficking to promote immature neurite pruning. EMBO Rep. 2023;24:e54701. doi: 10.15252/embr.202254701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Funahashi Y, Watanabe T, Takano T, Nakamuta S, Namba T, Kaibuchi K. Radial glial cell-neuron interaction directs axon formation at the opposite side of the neuron from the contact site. J Neurosci. 2015;35:14517–14532. doi: 10.1523/JNEUROSCI.1266-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


