Abstract
Background:
Leishmaniasis is highly prevalent worldwide, and while conventional medicine offers numerous treatment methods for cutaneous leishmaniasis, Iranian traditional medicine suggests various remedies. We aimed to evaluate the efficacy of an herbal combination containing Indigofera argentea leaves, Pistacia atlantica resin, and Salvia hispanica seeds in patients with zoonotic cutaneous leishmaniasis.
Methods:
This study was conducted at the Leishmaniasis Department of Chabahar Health Center in southeastern Iran in 2021. It was a double-blinded randomized clinical trial involving 68 patients enrolled after clinical diagnosis, examination of smear samples using Giemsa staining, and PCR confirmation. The volunteers were randomly divided into intervention and control groups. Both groups received ‘Glucantime ® as the primary medication weekly until complete healing or up to 12 weeks. Glucantime ® was administered intralesionally at a dosage of 0.1 cc on the wound’s margin, with repeat injections at 1 cm intervals along the wound edge when necessary. In addition to the main drug, the intervention group received the herbal product topically twice daily until wound healing or for up to 4 weeks, while the control group received a placebo in the same manner.
Results:
82.35% of patients in the intervention group and 20.58% in the control group achieved complete healing within four weeks. There was a significant difference between the two groups (P < 0.001).
Conclusion:
The herbal product demonstrated effectiveness in treating patients with zoonotic cutaneous leishmaniasis.
Keywords: Leishmaniasis, Indigofera argentea, Pistacia atlantica, Salvia hispanica, Iran
Introduction
In 2021, leishmaniasis remained prevalent in 99 countries (1). In Iran, zoonotic cutaneous leishmaniasis (ZCL) is the predominant form, caused by Leishmania major (2). Treatment of cutaneous leishmaniasis (CL) typically involves both topical and systemic approaches (3), with intralesional injection being one such method (4). Within Iran’s national program, Meglumine antimoniate (Glucantime ®) is frequently employed for CL treatment (2). However, the use of Glucan-time ® can lead to severe side effects and administration limitations (5). Treatment with antifungal agents, miltefosine, amphotericin B and herbal extract such as ZH-E have also been used (6).
On the other hand, Iranian traditional medicine (ITM) has long recommended various remedies (7, 8). The ‘Qarabadin-e-Kabir,’ for instance, prescribes compounds in poultice form for CL treatment (9). Recent studies have investigated the therapeutic potential of medicinal plants in traditional medicine TM for CL (10). Moghadds et al. investigated Iranian native plants on treatment of CL (11). Gharirvand et al. explored the effects of ‘Portulaca oleracea,’ and Parvizi et al. examined the impact of ‘Juniperus excelsa’ on CL (12, 13). A study at Quetta University even extracted anti-leishmanial compounds from ‘Erythrophleum ivorense’ (14). Taran et al. demonstrated the in vivo efficacy of gum obtained from P. atlantica in the experimental treatment of CL (15). Additionally, I. argentea and S. hispanica have exhibited anti-leishmanial effects due to their phenolic, flavonoid, and antioxidant compounds (16, 17).
Each component of the combined herbal product in this study is cited for its wound healing properties in ITM texts (18). Some ITM medications are administered as powders known as ‘Dharoor,’ which are sprinkled on cutaneous lesions (19). The methodology for using this herbal product is derived from folk medicine practices in the Sistan and Baluchistan province. Furthermore, a hydroalcoholic extract of this herbal product was prepared for the analysis of its effective anti-leishmanial substances.
We aimed to assess the impact of a herbal product containing I. argentea leaf, P. atlantica resin, and S. hispanica seeds in patients with ZCL, who were concurrently receiving glucan-time ® treatment.
Materials and Methods
Study Design: This study was conducted as a randomized, double-blinded, controlled clinical trial. The Ethics Committee of Kerman University of Medical Sciences granted approval for the study under the ethical code ‘IR.KMU.REC.1400.488’ on November 9, 2021. The trial was registered with the code IRCT20210905052377N1.
Participants: Volunteers were selected from individuals seeking treatment at the Department of Leishmaniasis at Chabahar Health Center in southeastern Iran during the year 2021. All participants provided informed consent and had the option to withdraw from the study at any time. The confidentiality of patient data was strictly maintained.
Eligibility Criteria: Participants in this study were between the ages of 16 and 70 and had laboratory-confirmed ZCL. According to the Iranian Ministry of Health’s protocol, these patients were indicated for intralesional glucantime ® treatment and met the defined conditions for study participation. Exclusion criteria included the presence of liver or cardiovascular conditions.
Sample Size: The sample size was determined using the formula for calculating the difference in means, with a 5% error rate, 80% power, and a 20% increase in the probability of volume loss. This calculation yielded a final sample size of 68 individuals, who were randomly divided into two groups of 34 each.
Randomization and Blinding: Randomization employed the random block method with a permutation in block size of four. R software was used to prepare a table-categorizing patients into Group A (intervention) and Group B (control). Both groups were indistinguishable to both patients and practitioners. The study results were analyzed by a statistician in a completely blinded manner.
Data Collection: Patient information, including age, gender, time of lesion onset, and lesion location, was recorded.
Interventions and Follow-up: The practitioner examined the CL of the volunteers, and laboratory diagnosis of lesion smears was performed using Giemsa staining. ZCL was confirmed by PCR. Wounds were measured and photographed weekly to assess healing progress. During the first visit, glucantime ® was injected intralesionally at the lesion’s margin as the standard treatment for all patients, with weekly repeat injections. Both the intervention and control groups received medicine containers coded as A or B, and these were used twice a day. The first prescription of the herbal product was made by the doctor for the patients. The patients were taught how to continue the treatment. The patients were asked to stop taking the medicine if any sensitivity, itching, blistering, burning, pain and other abnormal symptoms occur. Call the given phone number and go to the medical center for a visit. To monitor medicine consumption, patients were required to bring their containers to their next visit. The (TM) treatment duration was set at four weeks. If the lesion healed before this time, both the glucantime® and traditional product were discontinued. If the patient did not show signs of healing after four weeks, the TM was stopped, but glucantime® continued until complete healing or up to 12 weeks. At the end of the 4th and 8th weeks, all patients underwent re-examination for the presence of wounds or scars.
Preparation of Traditional Medicine and Placebo: The components of the herbal product, including P. atlantica resins, S. hispanica seeds, I. argentea leaves, were sourced from a local herbal market. Taxonomic identification of the P. atlantica plant was confirmed by Department of pharmacognosy at the faculty of pharmacy, Kerman University of Medical Sciences with the herbarium registration number kf 1136. S. hispanica with the herbarium registration number 9304, and I. argentea with the herbarium registration number 9305 were identified and confirmed in the herbarium of the North Kerman Agriculture and Natural Resources Research and Education Center under the supervision of the Central herbarium of Iran (TARI). Consequently, the herbal formulation components were individually processed into a powder using a mill and passed through a 100-mesh sieve. These powders were combined in equal volume ratios. The final product was exposed to UV rays for disinfection, and antimicrobial tests were conducted in accordance with relevant standards. In this study, the powder was encapsulated in one-gram capsules for blinding purposes. For the intervention group, the powder was removed from the capsule, mixed with 0.6 cc of distilled water, and formed into a medium-consistency paste. Before administration, the wound was cleaned with normal saline, and the appropriate amount of the drug was applied to cover the wound. For the control group, a placebo was prepared using corn flour powder, visually matched to the herbal product, and colored with methylene blue food coloring. The preparation process for the placebo was identical to that of the herbal product.
Safety evaluation
All the components of the product have material safety data sheets (MSDS) and their consumption interaction with glucantime® has not been reported. A registration form for unwanted drug side effects was provided. Practitioners and patients have been taught to report side effects. Since seeds with mucilage, such as S. hispanica, reduce the absorption of some substances in the intestine (20). The possibility of reducing the absorption of glucantime® can be raised. But compared to the control group, the therapeutic effect of the intervention group was significantly higher.
Phytochemical Evaluation
Total Phenol Content Determination: The total phenolic content of the hydroalcoholic herbal extract was determined using the Folin-Ciocalteu reagent assay. Sample of solution was mixed with diluted Folin solution and Na2CO3 solution. The absorbance of the solutions was measured at a wavelength of 765 nm using a spectrophotometer (ELISA). The total phenol content was reported as the equivalent of micrograms of gallic acid per milligram of extract (21).
The colorimetric method was utilized to quantify the total flavonoid content. The sample was diluted with distilled water, and then sodium nitrite solution was added to it, then aluminum chloride and sodium hydroxide were added. The absorbance of the solutions was measured after 15 minutes at a wavelength of 510 nm using a spectrophotometer (ELISA) (22). Due to the presence of indigo coloring matter in the tested product, it was not possible to assess flavonoids in a mixed state using this method. Therefore, the number of flavonoids in the two components, P. atlantica and S. hispanica, was assessed separately.
Antioxidant Activity Assay: The antioxidant activity of the extracts was assessed by measuring their ability to reduce DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals. Various concentrations of extracts, along with 1 mL of 0.1 mM DPPH and 1 mL of methanol, were prepared. After 30 minutes at room temperature, the absorbance of the samples was read at a wavelength of 517 nm with DPPH against the blank (23).
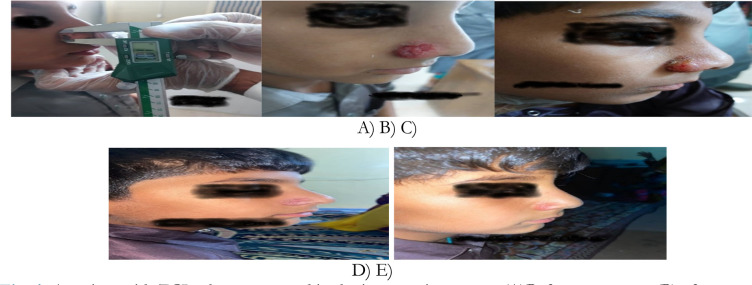
Lesion Assessment: Lesions were measured at the beginning of the study and weekly by the practitioner. Measurements were taken in the directions of the largest and smallest diameters, or perpendicular to each other, using an electronic caliper. The healing rate was then calculated. Imaging of the lesion site was performed for “before, during, and after” comparisons (Fig. 1).
Fig. 1:
A patient with ZCL who was treated in the intervention group. (A)Before treatment, (B) after one week, (C) after two weeks, (D)after three weeks, (E) after four weeks
Response to treatment was categorized as follows:
Completed Cure: Complete reepithelialization of the wound.
Uncompleted Healing: Lack of complete epithelization of the wound.
Preparation of Smear and Diagnosis of Leishmaniasis
Before the intervention, sampling was conducted from the marginal part and the inflamed and swollen tissue of the wound using a scalpel blade. Subsequently, the sample was evenly spread on a slide. After drying the smear on the slide, it was fixed using pure methanol and stained with Giemsa dye diluted with phosphate buffer. Following staining, the slide was rinsed with distilled water and allowed to air dry (24).
Microscopic Examination: The smear was examined under a microscope with a magnification of 100 for the presence of Leishmania amastigote forms (25).
DNA Extraction and PCR: The sample fixed on the slide was utilized for DNA extraction (26). The DNA extraction kit used was the Prep Genomic DNA Isolation Kit from ‘Yekta Tajhiz Azma’ company. This study describes a conventional PCR method based of kinetoplast DNA (kDNA) detection. A pair of oligonucleotide primers, LINR4 (5′-GGGGTTGTGTGTAAAATAGG-3′) of L. major-F and LIN17 (5′-TTTGAACGGGATTTCTG-3′) of L. major-R, were employed. PCR products were analyzed by electrophoresis using a 2% agarose gel. Because this research was done in Chabahar. The only dominant species there in previous studies based on molecular identification was L. major (27). Therefore, L. major strain (MHOM/IR/75/ER) was used as a positive control in this study. with a band size of approximately 650 bp (28).
Statistical Analysis: Statistical analysis was conducted using SPSS version 22 (IBM Corp., Armonk, NY, USA). The normality of variables was assessed using the Kolmogorov-Smirnov and Shapiro-Wilks tests. Relationships between categorized variables and groups were evaluated using the chi-square test and Fisher’s exact test. A repeated measurement test was applied to assess the effect of the product over time. When normality was established, the independent t-test was employed to compare wound diameter and area between the intervention and control groups. If normality was not met, the Mann-Whitney test was used. The significance level was set at (P < 0.005).
Results
Patient Flow Chart: Out of the 128 patients initially screened, 68 individuals met the defined criteria and were included in the study. Specifically, there were 34 patients in-group A (13 men and 21 women) and 34 patients in group B (21 men and 13 women) (Fig. 2).
Fig. 2:
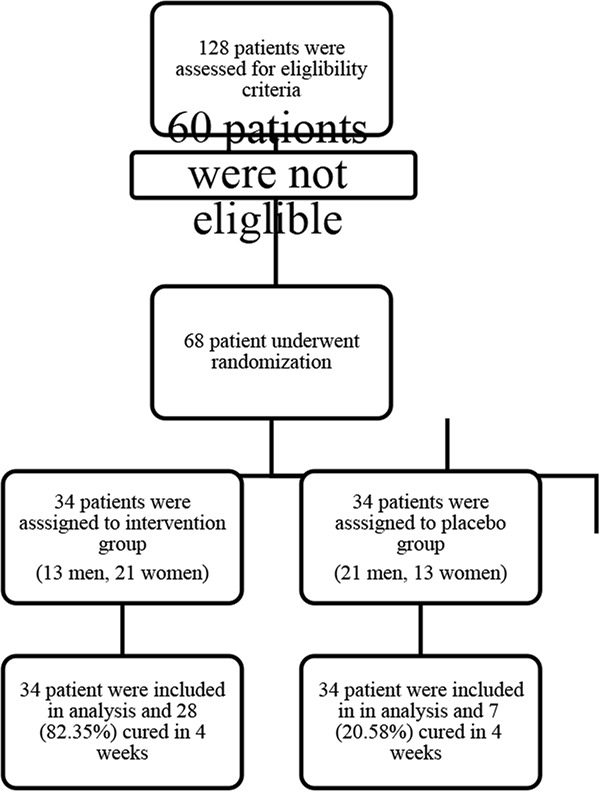
Consort flow diagram of clinical trial.
The basic demographic data for both groups are presented in Table 1.
Table 1:
Demographic information table of the participants
| Variable | Intervention | Control | P-value | Test |
|---|---|---|---|---|
| Sex | ||||
| Male | 13(38.2%) | 21(61.8%) | 0.052 | Chi-square |
| Female | 21(61.8%) | 13(38.2%) | ||
| Average age group/yr | ||||
| (10–20) | 28(82.35 %) | 12(35.29%) | ||
| (20–30) | 3(8.82%) | 6(17.64%) | ||
| (30–40) | 0 | 3(8.82%) | <0.001 | Fishers exact test |
| (40–50) | 0 | 3(8.82%) | ||
| (50–60) | 1(2.94%) | 3(8.82%) | ||
| (60–70) | 2 | 7(20.58%) | ||
| Education | ||||
| Illiterate | 16(47.1%) | 13(38.2%) | ||
| Elementary | 6(17.6%) | 8(23.5%) | ||
| Middle | 10(29.4%) | 8(23.5%) | 0.199 | Fishers exact test |
| Diploma | 2(5.9%) | 5(11.8%) | ||
| Location of the lesion | ||||
| Head and neck | 20(58.82%) | 5(14.7%) | ||
| Trunk | 1(2.94%) | 1(2.94%) | ||
| Upper limb | 11(32.35%) | 18(52.94%) | <0.001 | Chi-square |
| Lower limb | 2(5.88%) | 10(41.29%) | ||
| Average time from onset of lesion to referral (Month) | 1.74±2.47 | 2.20±2.35 | 0.808 | Independent T-test |
| Kind of leision | ||||
| Open lesion | 34(100%) | 34 (100%) | ||
| Closed lesion | 0 | 0 | ||
| Number of lesions in each patient | ||||
| One lesion | ||||
| Two or more lesions | 34 (100%) | 34 (100%) | ||
| 0 | 0 |
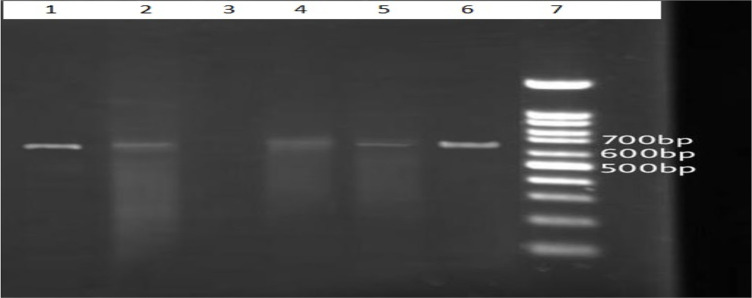
PCR analysis (Fig. 3) revealed that all patients in both groups were infected with L. major.
Fig. 3:
Results of the electrophoresis of the products of the PCR–based amplification of DNA extracted from the stained smears. The 7 lanes contain a molecular-weight “ladder” (lane 7), the products from reference strains of L.major (lane 1)650 bp, a negative control (lane 3), and test samples identified L. major (lanes 2,4–6)
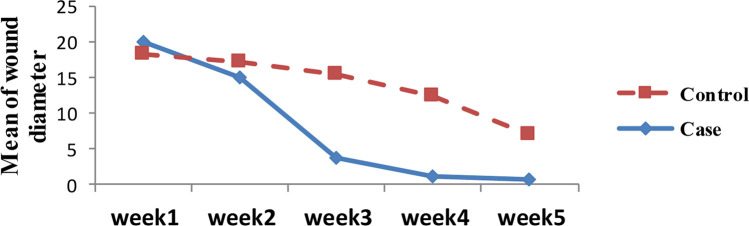
In the intervention group, 28 out of 34 patients (82.35%) achieved complete cures within 4 weeks. In contrast, only 7 out of 34 patients (20.58%) in the control group completed treatment in the same timeframe (p < 0.001). No patients in either group experienced treatment failure. Additionally, the intervention group had a significantly smaller average wound diameter during treatment (Fig. 4).
Fig. 4:
The diagram of patients’ healing process compared to the size of the lesion diameter
The treatment status of patients in both groups is detailed in Table 2.
Table 2:
Treatment results in both intervention and control groups
| Variable | Intervention | Control | p-value | TEST |
|---|---|---|---|---|
| Comparison of the number of people treated in 4 weeks | 28(82.35%) | 7(20.58%) | <0.001 | |
|
| ||||
| Comparison average consumption of glucantim or healing per week | 1.02±2.62 | 5.15±0.95 | <0.001 | Mann-Whitney |
|
| ||||
| Comparison healing in parts of body per week | ||||
| head & neck | 2.5±0.88 | 5±0.71 | <0.001 | Mann–Whitney |
| upper limb | 2.63±1.28 | 4.78±1.01 | <0.001 | |
| lower limb | 3.5±0.7 | 5.8±0.63 | 0.001 | |
|
| ||||
| Scar comparison in week8 | ||||
| No scar | 6 | 1 | chi-score | |
| color change | 17 | 6 | <0.001 | |
| Scarred | 11 | 27 | ||
|
| ||||
| Comparison of lesion | Total P<0.001 | |||
| Average diameter (mm) | ||||
| Week1 | 18.87±9.45 | 18.51±9.68 | 0.875 | Repeated measurments |
| Week2 | 13.93±10.08 | 17.52±9.60 | 0.137 | |
| Week3 | 3.84±5.74 | 15.84±9.33 | <0.001 | |
| Week4 | 1.09±3.35 | 13.13±9.54 | <0.001 | |
At the 8-week mark, it was observed that the control group had more scars than the intervention group. The product was examined for active metabolites. From a 1-gram sample, 178.58 mg of hydroalcoholic extract (20:80) was obtained, and the total phenol content was determined to be 37.4 ± 2.38 μg based on gallic acid per 1 mg of dry extract. Furthermore, the sample yielded an IC50 of 1558.16 μg/ml in the DPPH free radical inhibition antioxidant test. Utilizing the hydroalcoholic extraction method (20:80), 0.472 mg of extract was obtained from 1 gram of resin, containing 28.13 ± 4 μg of flavonoid compounds per 1 mg of dry extract. Similarly, when extracting 1 gram of S. hispanica powder using the same method, 0.053% extract was obtained, containing 70.06 ± 3.94 μg of flavonoids per 1 mg of dry extract.
None of the patients showed any complications that led to the discontinuation of the drug. Not all patients had any burning or itching complications compared to placebo. Some patients with induration in around of open wounds in the intervention and control groups experienced opening of subcutaneous wounds after subcutaneous injection of glucantim in the first week, which was similar in both groups. There was no obstacle to stopping the treatment.
Discussion
While this herbal product has been traditionally used in folk medicine in the southeast of Iran for treating ZCL, there have been limited studies exploring the therapeutic effects of each component of the product independently in both in vitro and in vivo conditions for ZCL treatment.
Pistacia atlantica : In the ‘Makhzhan-Al-Advia,’ the effects of P. atlantica resin have been proposed as a treatment for chronic wounds and bites (18). Research on the effects of P. atlantica essential oil on L. major in vitro and in vivo conditions demonstrated its effectiveness, comparable to glucantime ®, in inhibiting the diameter of wounds caused by L.major. Flow cytometry results also showed that P.atlantica essential oil induced 10% apoptosis in treated promastigotes. Moreover, it was found to be effective in eliminating L. major amastigotes in macrophages and culture media (29). Analysis of resin compounds confirmed the presence of phenol metabolites, flavonoids, antioxidants, and other anti-leishmanial compounds (30).
Salvia hispanica : Recent findings suggest therapeutic effects of S. hispanica not only on skin wounds caused by diabetes in animals but also additional antimicrobial and anti-inflammatory effects. S. hispanica seeds are rich in omega fatty acids, including alpha-linolenic acid (31). Analysis of S. hispanica has revealed the presence of effective anti-leishmanial substances, as well as antioxidant and anti-cytotoxic properties (32).
Indigofera argentea (Indigo): Indigo, extracted from the I. argentea leaf, has been used traditionally to treat insect bites and has shown anti-inflammatory properties (16). In result of previous study, have indicated the anti-leishmanial activity of these metabolites in different extracts (33). Indigo has also demonstrated effective anti-leishmanial properties (34).
The curative effects of CL are attributed to the various types of metabolites present in this herbal product, including phenols, terpenoids, and flavonoid compounds (35–37). Phenolic compounds and flavonoids are believed to release lysate dehydrogenase by macrophages, contributing to their anti-leishmanial activity. Additionally, antioxidants and anti-inflammatory agents can accelerate the healing process of chronic wounds such as cutaneous leishmaniasis. Antioxidants play a vital role in suppressing oxidative processes during the early stages of wound healing (38). These antioxidant mechanisms gradually detoxify free radicals and oxidative agents, helping cells return to redox homeostasis (39).
Paromomycin ointment, thermotherapy, cryotherapy, photodynamic therapy, and intralesional injection with pentavalent antimoniate compounds are used in the local treatment of skin lesions. A meta-analysis of 14 studies involving 279 patients treated with topical paromomycin found that clinical cure rates were significantly higher compared to placebo; in cutaneous leishmaniasis, its efficacy was similar to that of intralesional pentavalent antimony. Thermotherapy, cryotherapy, and photodynamic therapy have also shown efficacy rates of approximately 73%, 67%, and 37%, respectively (40). Miltefosine is the first oral drug for the treatment of zoonotic cutaneous leishmaniasis caused by L. major. Compared to meglumine antimoniate, it is as effective for the treatment of CL caused by L. major in Iran (41)
In Iran’s national program, glucantime ® is commonly used for skin lesion treatment. However, it can have adverse effects, including fever, arthralgia, myalgia, nausea, vomiting, and various clinical signs. In a study of 19 patients (21%) who received intramuscular glucantime ®, cases of fever [3], arthralgia and myalgia [4], nausea and vomiting [2], erythema nodosum [1], acute renal failure [3], including 1 fatal case, hepatic cytolysis [1], skin rash [4), and injection site inflammation [1] were reported (42). In our study, the comparison of the remaining scars in the eighth week in the two intervention and control groups showed that there are more scars in the control group. All patients of the intervention group cured completely during the fifth week, and all patients of the control group cured completely during the seventh week. From the third week, the average wound diameter in the intervention group decreased significantly, while the average decrease in wound diameter in the control group accelerated from the fifth week onwards.
Limitations of the Study
This study has certain limitations. Firstly, it did not explore the effect of different concentrations of the traditional product on ulcers, which could provide valuable insights into dose-response relationships. Secondly, children under 16 years of age were excluded from this study due to ethical considerations, so the effectiveness of this product in pediatric patients remains unexamined. Additionally, we did not evaluate the serum levels of the active ingredients in this product to estimate its skin absorption rate.
Conclusion
This study demonstrates the significant effectiveness of the herbal product in treating ZCL. Given the safety, affordability, and accessibility of this product, in contrast to the potential side effects and risks associated with glucantime, we propose that this combination could serve as an adjuvant, alternative, or even monotherapy for ZCL. However, it is essential to acknowledge that further research is warranted in this field to corroborate these findings and expand our understanding of its potential applications.
Ethical Considerations
We would like to affirm that all ethical considerations, including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, and others, have been scrupulously observed by the authors throughout the entire research process.
Acknowledgements
This paper was part of Ph.D. thesis with a grant No 00/10/60/218, supported by School of Traditional Iranian Medicine kerman University of Medical Sciences.
Footnotes
Conflict of Interest
Non-declared.
References
- 1.Ruiz-Postigo JA, Jain S, Madjou S, et al. Global leishmaniasis surveillance: 2021, assessing the impact of the COVID-19 pandemic. Weekly Epidemiological Record. 2022. [Google Scholar]
- 2.Shirzadi MR. Guideline to the care of cutaneous leishmaniasis in Iran. Ministry of Health, Disease Management Center. Tehran; 2012. [Google Scholar]
- 3.Goldman L, Schafer AI. Goldman-Cecil Medicine E-Book. Elsevier Health Sciences; 2019. [Google Scholar]
- 4.Loscalzo J, Fauci AS, Kasper DL, et al. Harrison’s Principles of Internal Medicine. McGraw Hill LLC; 2022. [Google Scholar]
- 5.Dinulos JGH. Habif’Clinical Dermatology E-Book. Elsevier Health Sciences; 2019. [Google Scholar]
- 6.Firooz A, Mortazavi H, Khamesipour A, et al. Old world cutaneous leishmaniasis in Iran: clinical variants and treatments. J Dermatolog Treat. 2021; 32(7):673–83. [DOI] [PubMed] [Google Scholar]
- 7.Avicenna H. Qanoon Dar Teb. University of Tehran medical science. Tehran; 1978. [Google Scholar]
- 8.Maleki M, Yousefi M, Bazzaz SMM, et al. An overview of skin lesions adapted to Cutaneous Leishmaniasis in Persian Medicine. Electronic Physician. 2017; 9(11):5854–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aqili-Khorasani MH. Qarabadin-e-Kabir. Research Institute for Islamic and Complementary Medicine.Tehran; 2007. [Google Scholar]
- 10.Mohseni N, Sajjadi SE, Eskandarian AA, et al. Natural Anti-Leishmaniasis Compounds in Traditional Iranian Medicine. Journal of Islamic and Iranian Traditional Medicine. 2012; 3(1):41–50. [Google Scholar]
- 11.Moghaddas E, Khamesipour A, Mohebali M, et al. Iranian Native Plants on Treatment of Cutaneous Leishmaniosis: A Narrative Review. Iran J Parasitol. 2017; 12(3):312–22. [PMC free article] [PubMed] [Google Scholar]
- 12.Gharirvand-Eskandari E, Doudi M. The Study of Composition and Anti-leishmanial Effect of Portulaca Oleracea Aerial Organs Hydroalcoholic Extract on Leishmania. major (Mrho/Ir/75/Er) and A Clinical Isolate In Vitro. Journal of Rafsanjan University of Medical Sciences. 2016; 15(5):425–38. [Google Scholar]
- 13.Parvizi MM, Handjani F, Moein M, et al. Efficacy of cryotherapy plus topical Juniperus excelsa M. Bieb cream versus cryotherapy plus placebo in the treatment of Old World cutaneous leishmaniasis: A triple-blind randomized controlled clinical trial. PLoS Negl Trop Dis. 2017; 11(10):e0005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armah FA, Amponsah IK, Mensah AY, et al. Leishmanicidal activity of the root bark of Erythrophleum Ivorense (Fabaceae) and identification of some of its compounds. J Ethnopharmacol. 2018; 211:207–16. [DOI] [PubMed] [Google Scholar]
- 15.Taran M, Mohebali M, Esmaeli J. In vivo efficacy of gum obtained Pistacia atlantica in experimental treatment of cutaneous leishmaniasis. Iran J Public Health. 2010; 39(1):36–41. [PMC free article] [PubMed] [Google Scholar]
- 16.Javed F, Jabeen Q, Aslam N, et al. Pharmacological evaluation of analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of Ieishmania argentea Burm. f. J Ehnopharmacol. 2020; 259:112966. [DOI] [PubMed] [Google Scholar]
- 17.Mhaidi I, El Kacem S, Ait Kbaich M, et al. Molecular identification of Leishmania infection in the most relevant sand fly species and in patient skin samples from a cutaneous leishmaniasis focus, in Morocco. PLoS Negl Trop Dis. 2018; 12(3):e0006315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aghili- Khorasani MH. Makhzan-al-advia. Tehran University of Medical Sciences. Tehran; 2009. [Google Scholar]
- 19.Afsharypuor S, Shams-Ardekani MR, Mosadegh M, et al. Iranian traditional pharmacy and pharmaceutical dosage forms. choogan. Tehran; 2017. [Google Scholar]
- 20.Tamargo A, Martin D, Navarro del Hierro J, et al. Intake of soluble fibre from chia seed reduces bioaccessibility of lipids, cholesterol and glucose in the dynamic gastrointestinal model simgi®. Food Res Int. 2020; 137:109364. [DOI] [PubMed] [Google Scholar]
- 21.Raeiszadeh M, Pardakhty A, Sharififar F, et al. Phytoniosome: a Novel Drug Delivery for Myrtle Extract. Iran J Pharm Res. 2018; 17(3):804–17. [PMC free article] [PubMed] [Google Scholar]
- 22.Amini F, Amini-Khoei H, Haratizadeh S, et al. Hydroalcoholic extract of Passiflora incarnata improves the autistic-like behavior and neuronal damage in a valproic acid-induced rat model of autism. J Tradit Complement Med. 2023; 13(4):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrabani M, Raeiszadeh M, Najafipour H, et al. Evaluation of the Cytotoxicity, Antibacterial, Antioxidant, and Anti-inflammatory Effects of Different Extracts of Punica granatum var. pleniflora. Journal of Kerman University of Medical Sciences. 2020; 27(5):414–25. [Google Scholar]
- 24.El-Hajj R, Bou-Youness H, Lachaud L, et al. EAPB0503: An Imiquimod analog with potent in vitro activity against cutaneous leishmaniasis caused by Leishmania major and Leishmania tropica. PLoS Negle Trop Dis. 2018; 12(11):e0006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO . Basic laboratory methods in medical parasitology. World Health Organization; 1991. https://apps.who.int/iris/handle/10665/40793 [Google Scholar]
- 26.Khademvatan S, Neisi N, Maraghi S, et al. Diagnosis and identification of Leishmania spp. from Giemsa-stained slides, by real-time PCR and melting curve analysis in south-west of Iran. Ann Trop Med Parasitol. 2011; 105(8):559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soleimanpoor H, Dabirzadeh M, Fooladi B. Identification of Species Causing Cutaneous Leishmaniasis by PCR in Chahbahar, Iran. Medical Laboratory Journal. 2016; 10(2):46–51. [Google Scholar]
- 28.Hanada K. Introduction and perspectives of DNA electrophoresis. Springer. 2020. [DOI] [PubMed] [Google Scholar]
- 29.Hesami D, Ghaffarifar F, Dalimi Asl A, et al. A study on the effects of Pistacia atlantica Desf. essential oil on Leishmania major in vitro and in vivo. Iranian Journal of Medicinal and Aromatic Plants Research. 2019; 35(1):44–53. [Google Scholar]
- 30.Mahjoub F, Rezayat KA, Yousefi M, et al. Pistaci atlantica Desf. A review of its traditional uses, phytochemicals and pharmacology. J Med Life. 2018; 11(3):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zargari A. Iranian medicinal plants. University of Tehran Publishing Institute Tehran; 2014. [Google Scholar]
- 32.Many JN, Sarasvathi V. Analysis of Chia Seed–Physiochemical and Proximate Analysis. Research Journal of Recent Sciences. 2016; 227:2502. [Google Scholar]
- 33.Shen Y, Zheng L, Jin J, et al. Phytochemical and biological characteristics of mexican chia seed oil. Molecules. 2018; 23(12):3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atta E, Al-faifi T, El-Shabasy A. Chemotaxonomic and morphological classification of six Indigofera species in Jazan region, KSA. Journal of Saudi Chemical Society. 2022; 26(3):101476. [Google Scholar]
- 35.Isah MB, Tajuddeen N, Umar MI, et al. Chapter 7 - Terpenoids as Emerging Therapeutic Agents: Cellular Targets and Mechanisms of Action against Protozoan Parasites. Elsevier; (2018). [Google Scholar]
- 36.Monzote L, Córdova WHP, García M, et al. In-vitro and In-vivo Activities of Phenolic Compounds A gainst Cutaneous Leishmaniasis. Records of Natural Products. 2016; 10(3):269–276. [Google Scholar]
- 37.Tasdemir D, Kaiser M, Brun R, et al. Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, In Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies. Antimicrob Agents Chemother. 2006; 50(4):1352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzmaurice SD, Sivamani RK, Isseroff RR. Antioxidant Therapies for Wound Healing: A Clinical Guide to Currently Commercially Available Products. Skin Pharmacol Physiol. 2011; 24(3):113–26. [DOI] [PubMed] [Google Scholar]
- 39.Roy S, Khanna S, Nallu K, et al. Dermal wound healing is subject to redox control. Mol Ther. 2006; 13(1):211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett JE, Dolin R, Blaser MJ. Mandell, douglas, and bennett’s principles and practice of infectious diseases E-book. Elsevier health sciences; 2019. [Google Scholar]
- 41.Mohebali M, Fotouhi A, Hooshmand B, et al. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007; 103(1):33–40. [DOI] [PubMed] [Google Scholar]
- 42.Masmoudi A, Maalej N, Mseddi M, et al. Glucantime injection: benefit versus toxicin. Med Mal Infect. 2005; 35(1):42–5. [DOI] [PubMed] [Google Scholar]