Abstract
Seaweed, a valuable marine resource widely cultivated worldwide, can be vulnerable to stress and microbiome alterations, resulting in the decay of seaweeds and substantial economic losses. To investigate the seaweed-microbiome interaction, our study aimed to isolate marine bacteria and fungi that can cause Ice–Ice disease and evaluate their enzymatic characteristics for potential application in bioethanol production from seaweed biomass. Three red seaweed species (Gracilaria edulis, Kappaphycus alvarezii, and Eucheuma cottonii) were obtained for our study and placed in separate culture tanks. Among the 18 isolated marine microbial species, 12 tested positive for agar and carrageenan activity: six exhibited both activities, three displayed only agar activity, and three only carrageenan activity. DNA sequencing of the positive microbes identified ten bacteria and two yeast species. The 3,5-Dinitrosalicylic acid (DNSA) assay results revealed that the identified bacterial Caldibacillus kokeshiiformis strain FJAT-47861 exhibited the highest carrageenase activity (0.76 units/ml), while the yeast Pichia fermentans strain PM79 demonstrated the highest agarase activity (0.52 units/ml). Notably, Pichia fermentans strain PM79 exhibited the highest overall agarase and carrageenase activity, averaging 0.63 units/ml. The average carrageenase activity of all six positive microbes was 1.5 times higher than their agarase activity. These findings suggest that the 12 isolated microbes hold potential for bioethanol production from macroalgae, as their agarase and carrageenase activity indicates their ability to break down seaweed cell wall carbohydrates, causing ice–ice disease. Moreover, these results provide exciting prospects for harnessing the bioconversion capabilities of these microbes, paving the way for sustainable and efficient bioethanol production from seaweed resources.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-024-01205-w.
Keywords: Seaweed-microbiome, Marine bacteria and fungi, Ice–ice disease, Agar and carrageenan activity, Bioconversion capabilities, Bioethanol production
Introduction
Seaweeds' biochemical and phenotypic results are symbiotically connected to the neighboring microbiome. Research into the molecular processes underlying seaweed microbial interactions is still developing. It’s also possible that healthy vegetative development depends on the microbiome [25, 41]. Only a few studies [3, 14, 27, 30] have tried to show that specific bacteria can encourage seaweed growth and can change the biochemical composition of seaweed. Studying the seaweed-associated microbial interaction is essential because it affects the biochemical composition, especially in large-scale production of seaweed, typically grown outdoors and susceptible to changing environmental factors.
When establishing a seaweed farm in aquaculture environments, environmental changes frequently stress the seaweed and alter its associated microbiota [8, 21]. From their larval stage to their adult stage, similar trends are observed in other organisms such as the hispid leaf beetle Octodonta nipae and honey bees. These organisms also experience shifts in their microbiota in response to environmental variations [1, 2, 18]. Abiotic elements like temperature, salinity, nutrient concentrations, and geographic location impact the microbiome associated with the seaweed species [6, 9]. When unfavorable environmental changes occur, it favors opportunistic pathogens. It makes them utilize their motile ability to infest algal host tissue, making the host tissue white and hard. This phenomenon is coined as Ice–Ice disease, leading to declining culture stocks, decreased carrageenan quality, loss of revenue, and employment possibilities for the marginal seaweed producers. An in-depth understanding of the molecular mechanisms implicated in plant-pathogen interactions is imperative for the future management of diseases [42].
Owing to the economic significance of red seaweeds, their cultivation has witnessed a remarkable global surge, escalating from 21,000 tonnes in 1950 to 18.3 million tonnes in 2019. In the same year, red seaweed constituted the majority, comprising 52.6 percent of global seaweed cultivation in tonnage [7]. In the same context, India, with its rich abundance of red seaweeds along its coast, has contributed significantly to this global surge in seaweed cultivation. These red seaweeds are primarily rich in agar (70% agarose and 30% agaropectin) and carrageenan, crucial polysaccharides with high carbohydrate content (56.3–77.2%) for bioethanol production [34]. Third-generation ethanol relies on micro and macroalgae, with macroalgae favored for its greater carbohydrate content [29]. To utilize macroalgae for biofuel, a cell rupture process is necessary to remove intracellular compounds. The released compounds undergo hydrolysis using specific enzymes or concentrated acids, converting complex polysaccharides into fermentable sugars. It is crucial to prioritize saccharification strategies that maximize efficiency and minimize the generation of fermentation-affecting by-products [13, 23, 38].
From an economic perspective, acid hydrolysis is a more cost-effective saccharification method than enzymatic hydrolysis [28]. However, it generates non-sugar by-products that can hinder ethanol production by yeast and bacteria [19]. Additionally, there are challenges in reagent and saccharide recovery. To address these issues, researchers have actively investigated marine algal polysaccharide-degrading enzymes using various organisms for the past decade. Another costlier alternative is pre-treating algal biomass before saccharification when employing purified enzymes in large-scale industrial applications [32]. Therefore, ongoing research focuses on marine microbes that efficiently degrade algal biomass, which is inevitable for biofuel production.
It is imperative to thoroughly investigate potential challenges that could impede seaweed biomass production, particularly in integrated multi-trophic aquaculture (IMTA) or large-scale open seaweed cultivation in ocean environments. Such challenges have far-reaching economic implications for producers; addressing them is paramount. While microorganisms and their intricate interactions within the seaweed life cycle have garnered acknowledgment, a noticeable gap exists in our understanding of their specific roles and impacts. Recognizing their pivotal influence on seaweed cultivation, we embarked on the present study with a clear objective: to isolate marine bacteria and fungi associated with seaweeds and elucidate their contribution to Ice–Ice disease. Additionally, we sought to comprehensively characterize their abilities to degrade agar and carrageenan, assessing their potential application in the burgeoning field of bioethanol production from seaweeds.
Through these investigations, we aim to shed light on the unexplored facets of seaweed-microbial interactions, ultimately contributing to a more comprehensive understanding of seaweed cultivation and its implications for the scientific community and industry stakeholders.
Materials and Methods
Collection and Operational Procedures
Three seaweed species (Kappahucus alvaerezii, Gracilaria edulis, and Eucheuma cottonii) were obtained from the seaweed cultivation site in Mandapam, Ramanathapuram, India (9°16′ N, 79° 07′ E). Transported in sterile plastic bags filled with natural seawater, the seaweeds were washed with autoclaved seawater to remove debris and sand [4]. Each seaweed species was then individually placed in separate glass tanks (Tank 1-Gracilaria edulis, Tank 2-Kappaphycus alvarezii, Tank 3-Ecuheuma cottonii) containing autoclaved seawater for subsequent analysis.
To isolate the epiphytes and endophytes, the thallus of each seaweed species, along with autoclaved seawater, was vortexed for 20 min, creating a homogenous mixture. The resulting suspension was stored at 4 °C for subsequent microbiological analysis. Meanwhile, for growth assessment, the remaining seaweed species in the tanks were provided with essential macro-nutrients (NO3− and PO42−) in the form of sodium Nitrate (NaNO3) and Potassium phosphate (KPO4) salts. Adequate aeration and a 30 ppt salinity were maintained, along with a 12:12 photoperiod [22, 33].
Microbiological Assay
The spread plate technique was employed to isolate epiphytes and endophytes from the three seaweed species [16, 36]. A 100 μl homogenous suspension, obtained after vortexing from each tank, was evenly spread using a sterilized L-shaped glass rod onto Nutrient agar (NA) and potato dextrose agar (PDA) plates for marine bacteria and fungi identification, respectively. Both NA and PDA were prepared using autoclaved distilled water. Incubating the plates at 37(± 0.5)°C for five days, facilitated the growth of microbial colonies. The pH of the Nutrient agar was measured as 7.5 using a LABMAN multiparameter water quality meter.
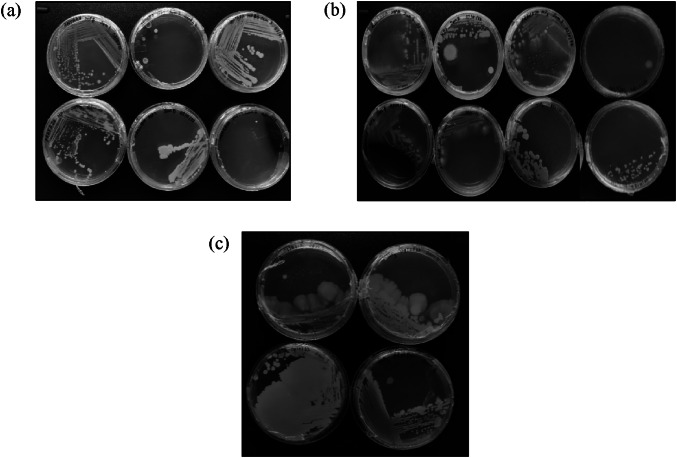
Subsequently, the microbial colonies from master plates collected from Tank 1, Tank 2, and Tank 3, were streaked into separate NA plates to obtain pure cultures through quadrant streaking. A total of eighteen marine microbe isolates were obtained: six from Tank 1 (Fig. 1a), eight from Tank 2 (Fig. 1b), and four from Tank 3 (Fig. 1c).
Fig. 1.
Microbial colonies obtained from the three tanks: a In Tank 1, three motile bacterial colony morphologies with a slightly yellow appearance and three yeast-like colony morphologies were observed. b Tank 2 displayed five bacterial and three yeast-type colony morphologies with motile bacteria and a pale-yellow color. c Tank 3 had two bacterial and two yeast-type colony morphologies with motile ability and a pale-yellow appearance
Qualitative Tests for Agar and Carrageenan Degradation
The ability of the marine microbes to degrade Agar and Carrageenan was qualitatively analyzed using Lugol’s Iodine solution. To determine Agarolytic activity, the microbes were cultured in a media that contained 1.3% nutrient broth and 1.5% agar, and for carrageenolytic activity, the media containing 1.3% nutrient broth and 1.7% kappha carrageenan were used.
Quantification of Agarase and Carrageenase Enzyme
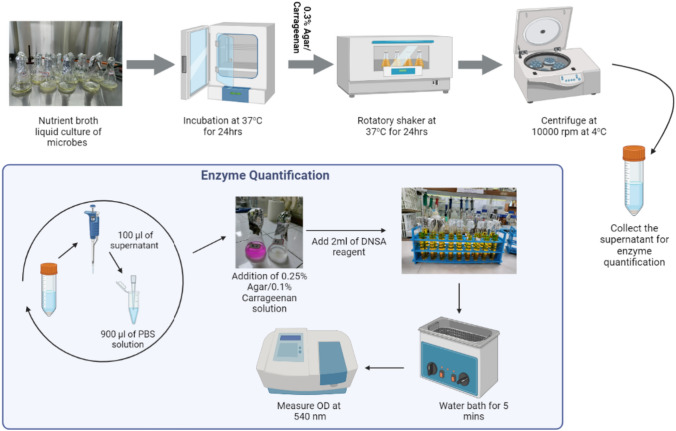
For the quantitative assessment of agarase and carrageenase enzymes produced by marine microbes, the 3,5-dinitro salicylic acid (DNSA) method, as recommended by Miller [26] was employed. This method measures the quantity of reducing sugars released due to agarase and carrageenase enzymes. Optical density measurements were taken using a UV–Vis spectrophotometer at 540 nm (Fig. 2). The standard curve was established using d-galactose as a reference.
Fig. 2.
Figure explaining the quantification of Agarase and Carrageenase by DNSA assay
DNA Isolation, PCR Amplification, Gel Purification, Sanger Sequencing, and Phylogenetic Analysis
DNA isolation was performed using the CTAB method for bacterial and yeast samples. After isolation, the DNA was purified using a DNA column and followed by quantification through agarose gel electrophoresis. PCR reactions were conducted using a DNA template, primers, and Taq polymerase under specific conditions. The PCR conditions involved an initial denaturation step at 95 °C for 2 min and a final denaturation step at 95 °C for 30 s.
The annealing step was carried out at 50 °C for 30 s, followed by an elongation step at 72 °C for 1 min. This denaturation, annealing, and elongation cycle was reiterated for 30 cycles. Subsequently, a final elongation step was performed at 72 °C for 10 min, and the samples were held at 4 °C for further analysis. Gel purification was employed to extract the desired DNA band, and Sanger sequencing PCR was carried out for further analysis. Post-sequencing, PCR purification was performed using ethanol, and the samples were air-dried before being treated with HiDiFormamide. Phylogenetic analysis was then conducted utilizing the neighbor-joining method with 1000 bootstrap replicates via MEGA software version 5.0 to identify species and their close relatives.
Results
Growth Assessment of Seaweeds
After five days, all seaweed species in the tanks exhibited symptoms of Ice–Ice disease, losing their pigments and turning pale white, indicating seaweed degradation (Figs. 3 and 4). This preliminary observation strongly suggests that the epiphytes and endophytes associated with the seaweed can degrade agar and carrageenan, the major polysaccharides found in red seaweeds.
Fig. 3.

Pale white appearance of K.alvarezii thallus
Fig. 4.

Pigment discoloration of E.cottonii thallus
Qualitative test for Agar and Carrageenan Degradation
The presence of clear zones around the surface of the microbial colony indicates agarolytic activity (Fig. 5) and carrageenolytic activity (Fig. 6). Of the 18 species, six tested positive for Agar and Carrageenan (MP1 Sam 3, MP2 Sam 2, MP2 Sam 3, MP2 Sam 4, MP3 Sam 1, MP3 Sam 4) three tested positive for Agar only (MP1 Sam 5, MP1 Sam 6, MP2 Sam 6), and three tested positive for carrageenan only (MP2 Sam 5, MP2 Sam 7, MP2 Sam 8).
Fig. 5.
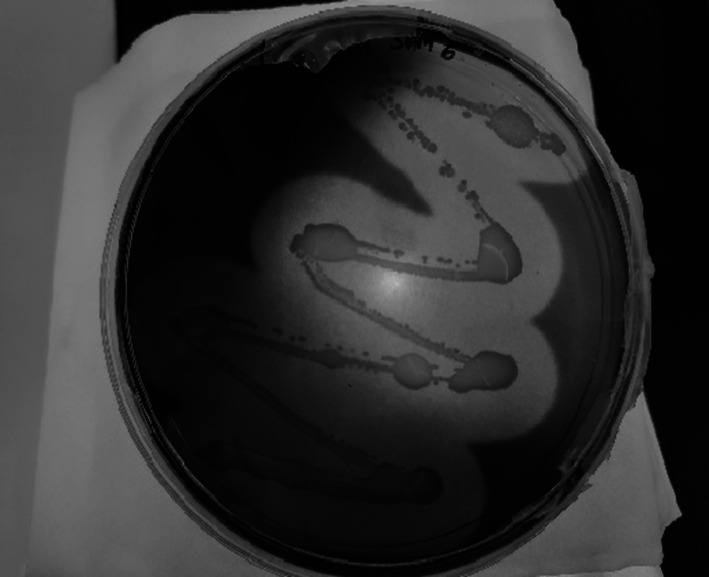
Presence of clear zone indicates the agarolytic activity of the microbial sample MP1 Sam 6
Fig. 6.
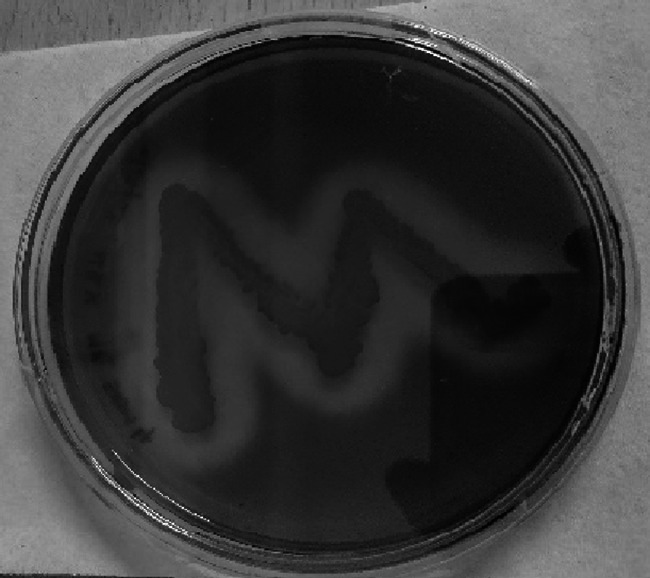
Presence of clear zone indicates the carragenolytic activity of the microbial sample MP2 Sam 4
Quantification of Agarase and Carrageenase Enzyme
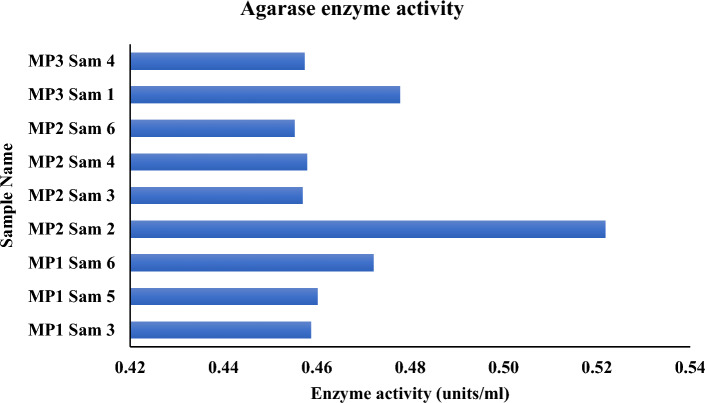
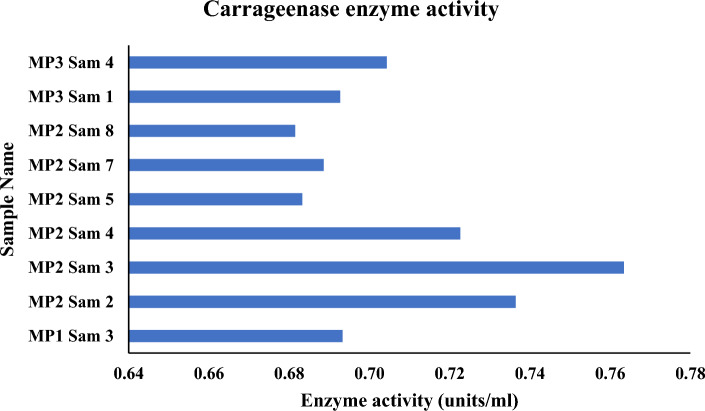
One unit of enzymatic activity is defined as the quantity of enzyme that releases1μmol of reducing sugar per minute under this condition. Specifically, the MP2 Sam 2 sample has 0.52 units/ml of Agarase enzymatic activity, which is the highest among the nine microbial samples that tested positive for Agarolytic assay. The overall average agarase enzymatic activity of all the microbes is 0.47 units/ml (Fig. 7). Of all the nine microbial samples that tested positive for carrageenolytic assay, the sample MP2 Sam 3 exhibits the maximum amount of carrageenase enzymatic activity of about 0.76 units/ml. All the microbes' average carrageenase enzymatic activity is 0.71 units/ml (Fig. 8). For the six microbial samples that tested positive for agar and carrageenan assay, the sample MP2 Sam 2 possesses an average of 0.63 units/ml of agarase and carrageenase enzymatic activity, the highest among all. All six microbes' average carrageenase enzymatic activity is 1.5 times higher than their agarase enzymatic activity (Fig. 9).
Fig. 7.
Agarase enzyme activity of the positive tested microbes in agar assay
Fig. 8.
Carrageenase enzyme activity of the positive tested microbes in carrageenan assay
Fig. 9.
Agarase and Carrageenase enzyme activity of the microbes that tested positive for both agar and carrageenan assay
Molecular Identification of Bacteria and Yeast
The data obtained from Sanger sequencing is then analyzed using the National Center for Biotechnology Information (NCBI) BLAST server to identify the microbial species. The detailed species-wise information is provided in Tables 1 and 2.
Table 1.
Bacterial identification using 16 s rRNA sequencing
| sample name | Molecular identification | Percentage identity (%) | Gene bank accession no. |
|---|---|---|---|
| MP1 Sam 3 | Bacillus kokeshiiformis strain SM24 | 98.54 | MT356179.1 |
| MP1 Sam 5 | Nitratireductor kimnyeongensis strain E14 | 99.59 | MH746061.1 |
| MP1 Sam 6 | Brevibacillus agri strain 13 | 99.15 | FJ715821.1 |
| MP2 Sam 3 | Caldibacillus kokeshiiformis strain FJAT-47861 | 98.52 | MG651263.1 |
| MP2 Sam 4 | Lentibacillus salarius strain BH139 | 98.30 | NR_043081.1 |
| MP2 Sam 5 | Bacillus thermolactis strain SM10 | 98.16 | MT356165.1 |
| MP2 Sam 6 | Micrococcus endophyticus strain HCDB6 | 99.09 | ON509665.1 |
| MP2 Sam 8 | Caldibacillus thermolactis strain NWFY-11-4 | 99.22 | OL656828.1 |
| MP3 Sam 1 | Oceanobacillus caeni strain LNPC16 | 97.75 | OM319758.1 |
| MP3 Sam 4 | Bacillus thuringiensis strain SH26 | 96.43 | MW694938.1 |
Table 2.
Yeast identification using Internal Transcribed Spacer (ITS) rRNA sequencing
| Sample name | Molecular identification | Percentage identity (%) | Gene bank accession no. |
|---|---|---|---|
| MP2 Sam 2 | Pichia fermentans strain PM79 | 96.79 | OP696694.1 |
| MP2 Sam 7 | Geotrichum candidum | 97.39 | MK559408.1 |
The supplementary files, Figs. 1a–j and 2 a–b, provide individual phylogenetic trees illustrating the evolutionary relationships of all identified bacterial and yeast species, respectively. Additionally, Fig. 10 displays the phylogenetic tree encompassing all identified bacterial species from our study and their inter-relationships. Notably, Bacillus thermolactis and Caldibacillus thermolactis, Nitratireductor kimnyeongensis, and Micrococcus endophyticus, as well as Lentibacillus salarius and Oceanobacillus caeni, exhibit similar sequences, clustering together in specific clades. Furthermore, Bacillus thermolactis, caldibacillus thermolactis, Caldibacillus kokeshiiformis, Bacillus kokeshiiformis, and Brevibacillus agri demonstrate close evolutionary relationships. Regarding branch length, it is indicative of the evolutionary distance between the given taxa and other clades, thereby revealing that Bacillus thermolactis and Caldibacillus thermolactis are less related to Bacillus thuringensis species (Fig. 10).
Fig. 10.

Phylogenetic tree of all the identified bacterial isolates and their inter-relations
Discussion
This study significantly advances our understanding of microbial strains associated with red seaweeds. Notably, we have isolated four novel microbial strains: Caldibacillus kokeshiiformis strain FJAT-47861, Lentibacillus salarius strain BH139, Micrococcus endophyticus strain HCDB6, and Caldibacillus thermolactis strain NWFY-11-4, from red seaweeds. While earlier studies [5, 10, 15, 37, 40] have documented the isolation of Brevibacillus sp., Bacillus thermolactis, Oceanobacillus sp., and Bacillus thuringiensis, our research presents a novel suite of these bacterial strains, encompassing Brevibacillus agri strain 13, Bacillus thermolactis strain SM10, Oceanobacillus caeni strain LNPC16, and Bacillus thuringiensis strain SH26.
Brevibacillus agri strain 13, Micrococcus endophyticus strain HCDB6, Bacillus thermolactis strain SM10, and Caldibacillus thermolactis strain NWFY-11-4, producing agarase and carrageenase enzymes, respectively, offer potential for novel enzymatic hydrolysis and enzymatic degradation methods to extract and purify agar and carrageenan from seaweeds. These methods not only hold the potential for improving the yield of agar and carrageenan but also contribute to environmentally friendly and specific extraction processes, distinct from traditional chemical methods. Additionally, applying these enzymes offers promising avenues for sustainable resource utilization.
The identification of species with bioremediation potential, such as Pichia, Geotrichum, Caldibacillus, Lentibacillus, Micrococcus, and Bacillus, holds significance due to their demonstrated ability to remove organic pollutants, hydrocarbons, and polycyclic aromatic hydrocarbons (PAHs) from the environment [11, 12, 20, 24, 39]. This suggests their potential application in developing environmentally friendly solutions for remediating contaminated marine habitats. Additionally, Halophytic and thermophilic bacterial species, including Bacillus thermolactis strain SM10, Caldibacillus thermolactis strain NWFY-11-4, and Oceanobacillus caeni strain LNPC16, along with the acid-tolerant and thermotolerant Geotrichum candidum yeast offer an alternative approach to traditional biofuel production methods, particularly in bioethanol production from macroalgae. Furthermore, these newly discovered bacterial and yeast strains hold promise for applications in the food and bioethanol industries [17, 31, 35].
This study enriches our understanding of microbial diversity in seaweeds and underscores the diverse potential applications of the isolated strains across various industries. Further research is warranted to fully harness the capabilities of these strains and explore new horizons in biotechnological applications.
Conclusions
This investigation highlights the potential of the 12 marine microbes isolated from three distinct red seaweed species for bioethanol production from macroalgae. DNA sequencing of the positive microbes identified ten bacteria and two yeast species. The 3,5-Dinitrosalicylic acid (DNSA) assay results revealed that the identified bacterial Caldibacillus kokeshiiformis strain FJAT-47861 exhibited the highest carrageenase activity (0.76 units/ml). In comparison, the yeast Pichia fermentans strain PM79 demonstrated the highest agarase activity (0.52 units/ml). Notably, six of these species displayed positive enzymatic activity for agar and carrageenan degradation, with Pichia fermentans strain PM79 exhibited promising results, notably higher carrageenase activity. These findings suggest that the 12 isolated microbes hold potential for bioethanol production from seaweed, as their agarase and carrageenase activity indicates their ability to break down seaweed cell wall carbohydrates, causing ice–ice disease. To further explore the efficiency of these microbes in converting seaweed biomass into bioethanol, future studies may encompass several vital areas: evaluating the efficiency of extracting polysaccharides from seaweed biomass and their subsequent hydrolysis into fermentable sugars, quantifying the yield of fermentable sugars obtained from the hydrolysis process, assessing the efficiency of the fermentation process itself, calculating the overall yield of ethanol produced from seaweed biomass, and investigating the ability of these microbes to biodegrade hydrocarbons, which may have environmental applications. In summary, our research underscores the suitability of isolated marine microbes for bioethanol production from seaweed biomass. It suggests promising directions for further enhancing the efficiency and viability of bioethanol production using these microbial candidates.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the seaweed farmers from Rameshwaram, India and specifically Brahma Kamal Research LLP, India for standardizing the seaweed cultivation in the field and providing seaweed samples for this study.
Author Contributions
PS conceptualized and ideated this research under the mentorship of AC and GDB, PS performed the formal analysis and experiments. KKJ and AC assisted in the lab investigations. GDB, PKS, and VN arranged the funding and resources. PS wrote the full original manuscript draft, and GDB did the final edit. All authors read and approved the final manuscript.
Funding
The authors gratefully acknowledge the Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India (SRG/2022/002212) for their financial assistance.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
No ethical approval is required in this type of research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ali H, Muhammad A, Islam SU, Islam W, Hou Y. A novel bacterial symbiont association in the hispid beetle, Octodonta nipae (Coleoptera: Chrysomelidae), their dynamics and phylogeny. Microb Pathog. 2018;118:378–386. doi: 10.1016/j.micpath.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Ali H, Muhammad A, Sanda NB, Huang Y, Hou Y. Pyrosequencing uncovers a shift in bacterial communities across life stages of Octodonta nipae (Coleoptera: Chrysomelidae) Front Microbiol. 2019 doi: 10.3389/fmicb.2019.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsufyani T, Alsufyani T, Califano G, Deicke M, Grueneberg J, Grueneberg J, Weiss A, Weiss A, Engelen AH, Kwantes M, Mohr JF, Mohr JF, Ulrich JF, Wichard T, Wichard T. Macroalgal-bacterial interactions: identification and role of thallusin in morphogenesis of the seaweed Ulva (Chlorophyta) J Exp Bot. 2020;71(11):3340–3349. doi: 10.1093/jxb/eraa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UshaKiran B, Treasa M, Kaladharan P. Phycocolloid contents in certain economically important seaweeds of Kerala coast, India. J Mar Biol Assoc India. 2021;63:05–11. doi: 10.6024/jmbai.2021.63.2.2269-01. [DOI] [Google Scholar]
- 5.Bai MD, Chen CY, Lu WC, Wan HP, Ho SH, Chang JS. Enhancing the oil extraction efficiency of Chlorella vulgaris with cell-disruptive pretreatment using active extracellular substances from Bacillus thuringiensis ITRI-G1. Biochem Eng J. 2015;101:185–190. doi: 10.1016/j.bej.2015.05.020. [DOI] [Google Scholar]
- 6.Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S. Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J. 2011;5(4):590–600. doi: 10.1038/ismej.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai J, Lovatelli A, Aguilar-Manjarrez J, Cornish L, Dabbadie L, Desrochers A, Diffey S, Garrido Gamarro E, Geehan J, Hurtado A, Lucente D, Mair G, Miao W, Potin P, Przybyla C, Reantaso M, Roubach R, Tauati M, Yuan X (2021) Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. FAO Fisheries and Aquaculture Circular No. 1229. Rome, FAO. 10.4060/cb5670en
- 8.Califano G, Kwantes M, Abreu MH, Costa R, Wichard T. Cultivating the macroalgal holobiont: effects of integrated multi-trophic aquaculture on the microbiome of Ulva rigida (Chlorophyta) Front Mar Sci. 2020 doi: 10.3389/fmars.2020.00052. [DOI] [Google Scholar]
- 9.Campbell AH, Marzinelli EM, Gelber J, Steinberg PD. Spatial variability of microbial assemblages associated with a dominant habitat-forming seaweed. Front Microbiol. 2015 doi: 10.3389/fmicb.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coorevits A, Logan NA, Dinsdale AE, Halket G, Scheldeman P, Heyndrickx M, Schumann P, van Landschoot A, de Vos P. Bacillus thermolactis sp. nov., isolated from dairy farms, and emended description of Bacillus thermoamylovorans. Int J Syst Evol Microbiol. 2011;61(8):1954–1961. doi: 10.1099/ijs.0.024240-0. [DOI] [PubMed] [Google Scholar]
- 11.Das N, Chandran P. Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int. 2011;2011:1–13. doi: 10.4061/2011/941810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcıa IG, Pena PJ, Venceslada JB, Martın AM, Santos MM, Gomez ER. Removal of phenol compounds from olive mill wastewater using Phanerochaete chrysosporium, Aspergillus niger, Aspergillus terreus and Geotrichum candidum. Process Biochem. 2000;35:751–758. doi: 10.1016/S0032-9592(99)00135-1. [DOI] [Google Scholar]
- 13.Gerken HG, Donohoe B, Knoshaug EP. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta. 2013;237(1):239–253. doi: 10.1007/s00425-012-1765-0. [DOI] [PubMed] [Google Scholar]
- 14.Ghaderiardakani F, Coates JC, Wichard T. Bacteria-induced morphogenesis of Ulva intestinalis and Ulva mutabilis (Chlorophyta): a contribution to the lottery theory. FEMS Microbiol Ecol. 2017 doi: 10.1093/FEMSEC/FIX094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta RS, Patel S, Saini N, Chen S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the subtilis and cereus clades of species. Int J Syst Evol Microbiol. 2020;70(11):5753–5798. doi: 10.1099/ijsem.0.004475. [DOI] [PubMed] [Google Scholar]
- 16.Ismail A, Ktari L, Ahmed M, Bolhuis H, Boudabbous A, Stal LJ, Cretoiu MS, El Bour M. Antimicrobial activities of bacteria associated with the brown alga Padina pavonica. Front Microbiol. 2016 doi: 10.3389/fmicb.2016.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamilari E, Stanton C, Reen FJ, Ross RP. Uncovering the biotechnological importance of Geotrichum candidum. Foods. 2023;12:1124. doi: 10.3390/foods12061124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan KA, Al-Ghamdi AA, Ghramh HA, Ansari MJ, Ali H, Alamri SA, Al-Kahtani SN, Adgaba N, Qasim M, Hafeez M. Structural diversity and functional variability of gut microbial communities associated with honey bees. Microb Pathog. 2020;138:103793. doi: 10.1016/j.micpath.2019.103793. [DOI] [PubMed] [Google Scholar]
- 19.Klinke HB, Thomsen AB, Ahring BK. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol. 2004;66:10–26. doi: 10.1007/s00253-004-1642-2. [DOI] [PubMed] [Google Scholar]
- 20.Kong X, Dong R, King T, Chen F, Li H. Biodegradation potential of Bacillus sp. PAH-2 on PAHs for oil-contaminated seawater. Molecules. 2022 doi: 10.3390/molecules27030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin G, Sun F, Wang C, Zhang L, Zhang X. Assessment of the effect of Enteromorpha prolifera on bacterial community structures in aquaculture environment. PLoS ONE. 2017 doi: 10.1371/journal.pone.0179792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Zhao J, Jiang P. Easy removal of epiphytic bacteria on Ulva (Ulvophyceae, Chlorophyta) by vortex with silica sands. Microorganisms. 2022 doi: 10.3390/microorganisms10020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LuizaAstolfi A, Rempel A, Cavanhi VAF, Alves M, Deamici KM, Colla LM, Costa JAV. Simultaneous saccharification and fermentation of Spirulina sp. and corn starch for the production of bioethanol and obtaining biopeptides with high antioxidant activity. Bioresour Technol. 2020 doi: 10.1016/j.biortech.2019.122698. [DOI] [PubMed] [Google Scholar]
- 24.Mandree P, Masika W, Naicker J, Moonsamy G, Ramchuran S, Lalloo R. Bioremediation of polycyclic aromatic hydrocarbons from industry contaminated soil using indigenous Bacillus spp. Processes. 2021 doi: 10.3390/pr9091606. [DOI] [Google Scholar]
- 25.Matsuo Y, Imagawa H, Nishizawa M, Shizuri Y. Isolation of an algal morphogenesis inducer from a marine bacterium. Science. 2005;307(5715):1598. doi: 10.1126/science.1105486. [DOI] [PubMed] [Google Scholar]
- 26.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. J Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 27.Nakanishi K, Nishijima M, Nishimura M, Kuwano K, Saga N. Note bacteria that induce morphogenesis in Ulva pertusa (Chlorophyta) grown under axenic conditions. J Phycol. 1996;32:479–482. doi: 10.1111/j.0022-3646.1996.00479.x. [DOI] [Google Scholar]
- 28.Offei F, Mensah M, Thygesen A, Kemausuor F. Seaweed bioethanol production: a process selection review on hydrolysis and fermentation. Fermentation. 2018 doi: 10.3390/fermentation4040099. [DOI] [Google Scholar]
- 29.Özçimen D, Inan B. Biofuels—status and perspective. InTech; 2015. An overview of bioethanol production from algae. [Google Scholar]
- 30.Polikovsky M, Califano G, Dunger N, Wichard T, Golberg A. Engineering bacteria-seaweed symbioses for modulating the photosynthate content of Ulva (Chlorophyta): significant for the feedstock of bioethanol production. Algal Res. 2020;49:101945. doi: 10.1016/J.ALGAL.2020.101945. [DOI] [Google Scholar]
- 31.Promon SK, Kamal W, Rahman SS, Hossain MM, Choudhury N. Ethanol production using vegetable peels medium and the effective role of cellulolytic bacterial (Bacillus subtilis) pre-treatment. F1000Research. 2018 doi: 10.12688/f1000research.13952.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabelo SC, Filho RMI, Costa AC. Lime pretreatment of sugarcane bagasse for bioethanol production. Appl Biochem Biotechnol. 2009;153(1–3):139–150. doi: 10.1007/s12010-008-8433-7. [DOI] [PubMed] [Google Scholar]
- 33.Redmond S, Green L, Yarish C, Kim J, Neefus C (2014) New England seaweed culture handbook nursery systems. http://seagrant.uconn.edu
- 34.Salehi B, Sharifi-Rad J, Seca AML, Pinto DCGA, Michalak I, Trincone A, Mishra AP, Nigam M, Zam W, Martins N. Current trends on seaweeds: looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules. 2019 doi: 10.3390/molecules24224182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selim KA, El-Ghwas DE, Easa SM, Abdelwahab Hassan MI. Bioethanol a microbial biofuel metabolite; new insights of yeasts metabolic engineering. Fermentation. 2018 doi: 10.3390/fermentation4010016. [DOI] [Google Scholar]
- 36.Setyati WA, Susanto AB, Pamungkas DBP, Makrima DB, Senduk JL. Isolation and identification of seaweed-associated bacteria and their antibacterial activity against skin disease agents. Trends Sci. 2023 doi: 10.48048/tis.2023.6517. [DOI] [Google Scholar]
- 37.Subramanian M, Maruthamuthu M. Draft genome sequences of two Bacillus spp. and an Oceanobacillus sp. strain isolated from marine macroalgae. Microbiol Resour Announc. 2019 doi: 10.1128/mra.01417-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudhakar MP, Kumar BR, Mathimani T, Arunkumar K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J Clean Prod. 2019;228:1320–1333. doi: 10.1016/j.jclepro.2019.04.287. [DOI] [Google Scholar]
- 39.Sultana OF, Lee S, Seo H, Mahmud HA, Kim S, Seo A, Kim M, Song HY. Biodegradation and removal of PAHs by Bacillus velezensis isolated from fermented food. J Microbiol Biotechnol. 2021;31(7):999–1010. doi: 10.4014/jmb.2104.04023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tahiluddin AB, Nuñal SN, Luhan MRJ, Santander-De Leon SMS. Vibrio and heterotrophic marine bacteria composition and abundance in nutrient-enriched Kappaphycus striatus. Philipp J Sci. 2021;150(6):1751–1763. doi: 10.56899/150.6b.12. [DOI] [Google Scholar]
- 41.Wichard T, Charrier B, Mineur F, Bothwell JH, de Clerck O, Coates JC. The green seaweed Ulva: a model system to study morphogenesis. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaynab M, Fatima M, Sharif Y, Zafar MH, Ali H, Khan KA. Microbial pathogenesis. Academic Press; 2019. Role of primary metabolites in plant defense against pathogens. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.