Abstract
The LipiFlow Thermal Pulsation System received its first marketing clearance for the treatment of meibomian gland dysfunction (MGD) 13 years ago. Since then, the evidence evaluating the effectiveness and safety of LipiFlow as a treatment for MGD has grown significantly. The objective of this comprehensive review was to summarize all clinical reports evaluating the effectiveness and safety of LipiFlow over the past 15 years. The literature was systematically reviewed, and 55 unique articles had subjective (patient-reported outcomes) and objective (meibomian gland function, tear production, and ocular staining) outcomes for extraction. Data were collected from 2101 patients and 3521 eyes treated with LipiFlow. Of these, effectiveness was evaluated in 2041 patients and 3401 eyes, and safety was evaluated in 1448 patients and 2443 eyes. Taken together, the studies demonstrate that a single 12-min treatment with LipiFlow safely improves signs and symptoms of MGD and associated evaporative dry eye disease (DED), and the benefits persist up to 3 years in some cases. The findings are corroborated by multiple meta-analyses and consensus guidelines. While some studies showed that daily eyelid hygiene, warm compress, and/or massage had a similar benefit to a single LipiFlow, these treatments were limited by inconvenience, discomfort, and non-compliance. The majority of studies evaluating safety reported no discomfort or pain associated with LipiFlow treatment, which supports the patient acceptability of LipiFlow therapy. All adverse events (AEs) related to LipiFlow were transient, non-vision-threatening, and did not require treatment. No studies reported serious AEs. The data obtained from 55 studies conducted globally overwhelmingly show that LipiFlow is effective and safe for the treatment of MGD and associated evaporative DED. The conclusions are supported by the diversity of the patient populations (geography, race, disease severity, and diagnosis), the large population treated with LipiFlow, the meta-analyses, and that this review analyzed all published clinical studies to date.
Keywords: Dry eye, LipiFlow, Meibomian gland dysfunction, Ocular surface disease, Vectored thermal pulsation system
Key Summary Points
| Data from 2101 patients and 3521 eyes treated with LipiFlow indicate that a single 12-min LipiFlow treatment safely improves signs and symptoms of meibomian gland dysfunction. |
| The benefits of a single LipiFlow persist up to 3 years in some cases. |
| The majority of studies evaluating safety reported no discomfort or pain associated with LipiFlow treatment, which supports the patient acceptability of LipiFlow therapy. |
| While some studies showed that compliant daily eyelid hygiene, warm compress, and/or massage had a similar benefit to a single LipiFlow, these treatments were limited by inconvenience, discomfort, and non-compliance. |
Introduction
Meibomian gland dysfunction (MGD) is the main cause of evaporative dry eye disease, which is the most prevalent type of dry eye disease (DED) globally [1, 2]. DED is a multifactorial condition likely to require multiple treatment interventions; however, diagnosing and treating MGD is a core component of overall DED management, a majority of the time [3]. The International Workshop on MGD defines it as “a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease” [4]. A systematic review that included literature from 16 countries and 26,063 patients concluded that 70.4% of patients with dry eye and no other comorbidities have MGD, and 41.7% of patients across all ages had MGD without ocular symptoms [5].
Over the past 15 years, our understanding of MGD has changed significantly. Historically, MGD was considered predominantly an inflammatory and hyposecretory condition, and was only diagnosed in its latest and most challenging to treat stage [6]. Currently, we understand that the most prevalent etiology of MGD is intraductal meibum stagnation, which slowly reduces meibomian gland function over time. If left undiagnosed and untreated, MGD will advance and ultimately present as the more traditional inflammatory condition. In its earliest stage, non-obvious MGD is only diagnosable by assessing gland function [6] requiring physical manipulation of the eyelids (the look, lift, pull, push examination) [7]. This is because the longer-term sequelae of chronic and untreated obstruction, such as telangiectasia and thickening of the lid margin, anterior displacement of the mucocutaneous junction, and puckering of the gland orifices, are not yet present [6].
As our understanding of MGD has evolved, and so have the treatment options. Traditionally, the treatment for MGD has been a combination of heat and massage placed on the external surface of the ocular adnexa to melt and discharge the meibum that is characteristically solid at body temperature in patients with MGD [8]. This is typically performed with warm compresses and administered at home [3, 4]. More recently, additional treatments have emerged including multiple attempts to standardize and optimize warm compress treatment through the innovation of heated masks of varying technological complexity. In the late 1970s, a study on a small group of patients with meibomitis demonstrated that manually expressing the obstructed contents from the meibomian glands resulted in improved ocular surface health and tear film stability [9]. This finding was later replicated in a group of contact lens wearers with non-obvious MGD where manual expression significantly reduced their symptoms of contact lens discomfort [10]. Despite the knowledge that manual gland expression was efficacious in restoring ocular surface homeostasis, it was not well incorporated into eye care practice for a variety of reasons, including the discomfort associated with manual expression.
The overall goal of MGD treatment is to restore gland function, which is foundational to the formation of a healthy tear film and essential for ocular surface homeostasis [11, 12]. MGD alters the molecular composition of the meibum so that greater than normal body temperature is required for liquefaction of the contents, which is required for the meibum to spread in the tear film [8]. Hence, heating the eyelids increases the flow of meibum lipid. In a breakthrough discovery in 2010, we learned that therapeutic heat (42.5 °C) applied directly to the inner eyelid surface, which allows greater access of heat to the meibomian glands than applying the heat to the external eyelid, rapidly liquifies the gland contents greatly, reducing the amount of pressure necessary to move the contents out of the glands during expression [13]. However, due to the ability of the eyelid vasculature to wick the heat away from the heated ocular adnexa, pressure is best applied simultaneously with the heat. In 2010, a study was published introducing a novel treatment approach for MGD whereby therapeutic heat (42.5 °C) was applied directly to the inner eyelid surface, coupled with simultaneous and directional massage of the glands applied to the external eyelid surface [13]. This breakthrough treatment was later launched as the LipiFlow thermal pulsation treatment in 2012 [14].
Eyelid warming via application of warm towels or commercialized eye masks/patches, thermal pulsation, and intense pulsed light (IPL) therapy are the most commonly used treatments for MGD leveraging heat as a component of the therapy [15]. Other treatments include lid debris debridement, manual gland expression (forcefully squeezing the eyelids between a rigid object such as a glass rod, Q-tip, or metal paddle and finger or rigid object), meibomian gland (MG) probing with an intraductal stainless-steel wire to mechanically open the MG orifices, massage to express the MGs, off-label use of macrolide or tetracycline family antibiotics (topical or oral), lipid-containing eye drops, off-label use of anti-inflammatory eye drops and perfluorohexyloctane eye drops [15–17]. However, treatment of MGD usually begins with eyelid hygiene, warm compresses, and ocular lubricants prior to progressing to the office-based or prescription managements when symptoms do not abate [3, 12].
The LipiFlow Thermal Pulsation System is a vectored thermal pulsation therapy intended for the application of localized heat and pressure therapy in adult patients (18 years or older) with chronic cystic conditions of the eyelids, including MGD (also known as evaporative dry eye or lipid deficiency dry eye) [18]. The system first received marketing clearance in 2011. It consists of a console, reusable cable, and a dome-shaped, single-use sterile device known as the Activator. The Activator includes an eyelid warmer that resembles a large scleral lens that rests lightly on the conjunctiva of the eye and an eye cup that cradles the outer surface of the upper and lower eyelids and applies regulated pressure through an inflatable air bladder (Figs. 1 and 2). The eyelid warmer portion of the Activator is vaulted away from the cornea, creating an air gap to prevent heat from reaching cornea and ocular surface. Thus, the eyelid warmer provides controlled, outward directional (away from the eye) heat to the palpebral conjunctival surfaces only. Over a 12-min period, heat is transferred through the inner surface of the eyelids, warming all of the MGs in the upper and lower eyelids simultaneously. It is important for the heat to be maintained at a high enough temperature to produce liquefaction of the MG contents [8]. Meanwhile, the air bladder in the Activator intermittently inflates to provide controlled massage of the eyelids via a rolling motion. The eyelids are massaged in the direction of the MG orifices to express the liquified contents. For safety considerations, the massaging pressure and heat are not applied to the eyeball. In 2021, the Activator was updated (called Activator Clear) with translucent bladders to enable better positioning. There were no changes to the bladder design or eyelid warmer. A prospective, open-label, multicenter clinical study reported that the investigators agreed or strongly agreed that the translucent components made it easy to access and confidently position the Activator on the patient’s eye, and 100% of the LipiFlow treatments were successful [19].
Fig. 1.
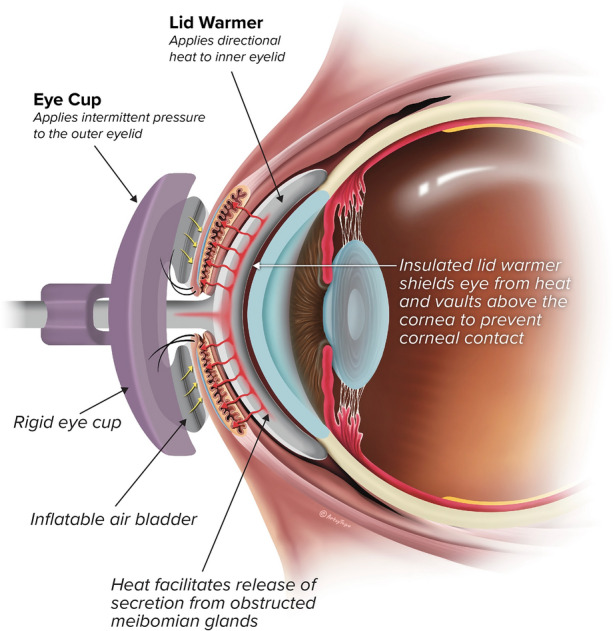
Cross section of Activator placement around the eyelids during use
Fig. 2.
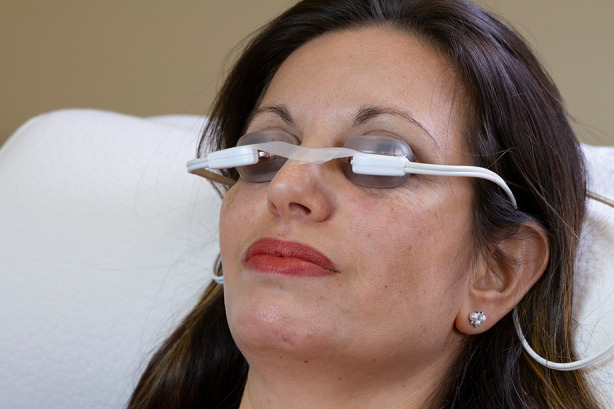
Oblique view of patient being treated with LipiFlow. Consent was obtained from the patient
We last reviewed the LipiFlow literature in 2015 [20]. At that time, six clinical trials and two case reports had been published (between 2011 and 2015). Together those eight studies demonstrated that a single 12-min treatment with LipiFlow significantly increased MG function and improved symptoms. At present, it is 13 years since LipiFlow received its first marketing clearance; accordingly, the evidence evaluating the effectiveness and safety of LipiFlow has grown. The objective of this review was to reflect on the clinical contributions LipiFlow has provided in shaping our understanding of managing ocular surface health by summarizing all clinical reports evaluating the effectiveness and safety of LipiFlow over the past 15 years.
Methods
We systematically examined the literature documenting the outcomes of LipiFlow on MGs and DED. Searches of the PubMed, Cochrane Library, Scopus, Springer Books, and OVID databases were conducted in March 2024 to gather relevant publications on the safety and effectiveness of LipiFlow (Table 1). The structured search approach followed the guidance of Kable et al. [21]. The following search terms were used: “meibomian gland dysfunction,” “LipiFlow treatment,” “MGD,” “vectored thermal pulsation therapy,” “dry eye disease,” and “ocular surface disease.” Included articles met the following criteria: (1) published from January 2010 to March 2024, (2) evaluated LipiFlow or LipiFlow compared to other devices or management strategies, (3) original research studies, (4) meta-analyses, and (5) global consensus guidelines. Excluded articles were (1) published in 2009 or earlier, indicating that a LipiFlow prototype was evaluated; (2) evaluated ocular surface/dry eye devices or other home therapy options without including LipiFlow; (3) evaluated only pharmaceutical drugs; (4) did not pertain to LipiFlow/MGD/dry eye/ocular surface; (5) were conference abstracts/presentations; and (6) were previously published reviews. There were no language restrictions; articles not in English were professionally translated to English. This review is based on previously conducted studies and does not contain any new studies with human participants or animals that were performed by any of the authors.
Table 1.
Search process and number of articles retrieved
| Search engine | Search terms | Number of articles retrieved | Number met inclusion criteria |
|---|---|---|---|
| PubMed | S1, S3 | 47 | 41 |
| PubMed | S1, S4 | 36 | 35 |
| PubMed | S1, S5 | 36 | 33 |
| PubMed | S1, S6 | 29 | 28 |
| PubMed | S2, S3 | 13 | 12 |
| PubMed | S2, S4 | 2 | 1 |
| PubMed | S2, S5 | 13 | 11 |
| PubMed | S2, S6 | 7 | 7 |
| OVID, Scopus, Springer Books, Cochrane Library | S1, S3 | 105 | 68 |
| OVID, Scopus, Springer Books, Cochrane Library | S1, S4 | 85 | 54 |
| OVID, Scopus, Springer Books, Cochrane Library | S1, S5 | 54 | 27 |
| OVID, Scopus, Springer Books, Cochrane Library | S1, S6 | 65 | 36 |
| OVID, Scopus, Springer Books, Cochrane Library | S2, S3 | 26 | 15 |
| OVID, Scopus, Springer Books, Cochrane Library | S2, S4 | 24 | 12 |
| OVID, Scopus, Springer Books, Cochrane Library | S2, S5 | 27 | 12 |
| OVID, Scopus, Springer Books, Cochrane Library | S2, S6 | 25 | 9 |
Search terms abbreviations: S1, LipiFlow treatment; S2, vectored thermal pulsation therapy; S3, meibomian gland dysfunction; S4, MGD; S5, dry eye disease; S6, ocular surface disease
A total of 568 articles were retrieved, and 387 articles met the inclusion criteria. After removing duplicates, 61 unique articles were available for review. Refer to the study flow diagram (Fig. 3) for a summary of the screening and article inclusion. There is no standardization of the outcome measures used to evaluate clinical effectiveness of MGD treatment however it is critical to demonstrate that MG function has improved as a result of any MGD treatment. There are additional measures, often pertaining to accompanying DED that are used to assess the impact of treating MGD in a patient with comorbid DED. We chose to collect subjective and objective data related to the following endpoints because these were most frequently reported in the literature: patient-reported outcomes (Standard Patient Evaluation of Eye Dryness [SPEED] and Ocular Surface Disease Index [OSDI]); MG function (meibomian gland secretion score [MGSS], meibomian glands yielding liquid secretion [MGYLS], and meibomian gland expression [MGE]); tear film quality (tear break-up time [TBUT] and noninvasive tear break-up time [NIKBUT]); ocular staining (corneal and conjunctival); and safety (adverse events [AEs], pain, changes in intraocular pressure [IOP], and changes in visual acuity [VA]).
Fig. 3.
Flow diagram illustrating article screening and study inclusion. Abbreviations: combo combination of a single LipiFlow treatment and additional therapies, DED dry eye disease, inc including, LF LipiFlow, MGD meibomian gland dysfunction, mono single LipiFlow treatment, RCT randomized controlled trial
Results
Clinical studies were located evaluating LipiFlow in the management of dry eye in patients with DED/MGD (N = 45 studies), cataract (N = 7), glaucoma (N = 1), refractive surgery (N = 4), and chalazion (N = 1).
Dry Eye/MGD
Randomized Controlled Trials
A total of 17 randomized controlled trials (RCTs) were identified that evaluated the effectiveness of LipiFlow for treating MGD, and 15 studies included the outcomes of interest. The study design, sample size, last follow-up, effectiveness and safety data, and the main outcomes of all 17 RCTs are summarized in Table 2.
Table 2.
Summary of RCTs evaluating LipiFlow to treat MGD
| Author/design/country | Patients (eyes)/age, mean (SD) | Last FU | Study arms | MG function Mean (SD) |
Patient-reported outcomes Mean (SD) |
NIKBUT/TBUT Mean (SD) |
Staining | Overall conclusion and safety | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||
|
Meng 2023 [22] Prospective, randomized, observer-masked China |
N = 50 patients with MGD; LF: N = 25 patients (50 eyes), 58 (12) years; CL: N = 25 patients (50 eyes), 58 (12) years |
3 months |
Single 12-min LF Tx CL: Lid warming BID with EyeGiene Insta-Warmth patch and massage for 2 weeks |
MGSS LF:8.8 (2.2) CL: 9.5 (3.1) MGYLS LF: 3.7 (0.85) CL: 4.1 (1.1) |
MGSS LF:12.8 (3.9)* CL: 10.2 (3.3) MGYLS LF:5.5 (1.2)*† CL: 4.4 (1.2) |
SPEED LF: 7.1 (2.7) CL: 7.2 (2.2) |
SPEED LF: 3.8 (1.5)*† CL: 6.6 (2.8) |
TBUT LF: 2.3 (0.96) CL: 2.7 (1.4) |
TBUT LF: 5.6 (2.2)*† CL: 4.0 (1.9)* |
Corneal LF: 2.8 (1.8) CL: 2.2 (1.3) |
Corneal LF: 1.2 (1.2)* CL: 1.2 (1.2) |
LF Tx can ameliorate subjective systems of MGD and markedly improve MG function for at least 3 months. Safety NR |
|
Wesley 2022 [23] Prospective, randomized, multicenter, assessor-masked, parallel group USA |
N = 236 patients with MGD; LF: 117 patients (234 eyes), 56 (14) years; iLUX: N = 119 (238 eyes), 58 (13) years |
12 months |
Single 12-min LF Tx Single Tx Systane iLux Dry Eye System thermal pulsation Note: LF was the control |
MGSS LF: 6.5 (3.8) iLux: 6.6 (3.7) |
MGSS LF: 23.4 (11.4) iLux: 22.9 (11.3) |
IDEEL-SB LF: 57.2 (14.6) iLux: 55.8 (15.9) |
IDEEL-SB LF: 36.0 (18.1) iLux: 34.4 (16.7) |
NIKBUT LF: 8.0 (3.0) iLux: 8.0 (3.0) |
NIKBUT LF: 10.1 (4.0) iLux: 10.7 (4.6) |
Corneal LF: 1.9 (2.4) iLux: 2.1 (2.9) |
Corneal LF: 0.6 (1.2) iLux: 0.7 (1.5) |
iLux was noninferior to LF AEs None No significant biomicroscopy findings |
|
Holland 2022 [24] Randomized single-masked, multicenter, non-inferiority, post-market USA |
N = 235 patients with DED and MGD, LF:120 patients (240 eyes), 55 (15) years TearCare: 115 patients (230 eyes), 57 (14) years |
1 month |
Single 12-min LF Tx Single 15-min Tx with TearCare |
NR | NR |
OSDI LF:51.7 (15.3) TearCare: 51.7 (14.8) SANDE LF:77.2 (15.8) TearCare: 70.6 (16.8) |
OSDI LF:27.1 (19.0)* TearCare: 22.6 (17.2)*§ SANDE LF:34.0 (28.1)* TearCare: 29.4 (26.6)* |
NR | NR | NR | NR |
LF and the TearCare consistently improved DED symptoms. Safety NR |
|
Li 2021 [25] Prospective, randomized, observer-masked China |
N = 54 patients with MGD (108 eyes), LF:54 eyes, 42 (11) years MiboFlo: 54 eyes, 44 (11) years |
2 months after first Tx |
Single 12-min LF Tx MiboFlo ThermoFlo Tx, three 10-min Tx, each 2 weeks apart. Tx was instantly followed by eyelid compression Note: LF was the control |
MGSS LF: 11.0 (3.8) MiboFlo: 9.2 (3.7) MGYLS LF: 8.4 (3.7) MiboFlo: 8.7 (3.6) |
MGSS LF: 4.8 (2.2)* MiboFlo: 4.8 (2.2)* MGYLS LF: 2.9 (2.8)* MiboFlo: 5.1 (2.3)* |
OSDI LF: 46.1 (17.7) MiboFlo: 44.3 (1.0) |
OSDI LF: 30.6 (19.9)* MiboFlo: 28.7 (18.2)* |
NIKBUT LF: 5.8 (3.0) MiboFlo: 6.4 (3.5) |
NIKBUT LF: 5.9 (2.7) MiboFlo: 6.0 (2.7) |
Corneal LF: 0.38 (0.63) MiboFlo: 0.16 (0.43) |
Corneal LF: 0.18 (0.50) MiboFlo: 0.02 (0.15) |
OSDI, MGYLS, and MGSS improved from BL in both groups, and improvements were maintained at 2 months AEs None No safety-related changes in IOP or VA |
|
Gupta 2022 [26] Randomized, masked, multicenter, controlled USA |
N = 141 patients with DED; LF:72 patients (136 eyes), 52 (15) years; TearCare: 69 patients (134 eyes), 56 (14) years |
1 month |
Single 12-min LF Tx Single 15-min Tx with TearCare plus MGX Note: LF was the control |
MGSS LF:6.29 TearCare: 6.54 MGYLS LF:5.62 TearCare: 5.42 |
MGSS LF:17.38* TearCare: 17.74* MGYLS LF:9.93* TearCare: 9.76* |
OSDI LF:51.1 TearCare: 52.9 SANDE LF:73.2 TearCare: 68.4 |
OSDI LF:27.7* TearCare: 24.2* SANDE LF:33.8* TearCare: 30.1* |
TBUT LF:4.49 TearCare: 4.62 |
TBUT LF:7.08* TearCare: 7.64* |
Corneal LF:2.51 TearCare: 2.51 Conjunct LF:4.85 TearCare: 4.08 |
Corneal LF:1.93 TearCare: 2.25* Conjunct LF:4.07 TearCare: 3.43* |
TearCare was as effective as LF at alleviating signs and symptoms of DED Device-related AEs LF: N = 4 (N = 1 blepharitis, N = 2 foreign body sensation, and N = 1 severe eye dryness) TearCare: N = 3 (N = 1 SPK, N = 1 chalazion, N = 1 blepharitis) |
|
Booranapong 2020 [27] Prospective, observer-masked, randomized, controlled Thailand |
N = 28 patients with moderate MGD (56 eyes), 54 (14) years; LF: 28 eyes; CL: contralateral eye (28 eyes) |
6 months |
Single 12-min LF Tx CL: warm compress for 5 min BID for 3 months |
MGYLS LF: 2.6 (2.8) CL: 2.5 (2.3) |
MGYLS LF: 3.1 (2.1) CL: 2.7 (2.2) |
SPEED LF: 9.5 (4.5) CL: 9.2 (4.4) |
SPEED LF: 7.8 (5.3)* CL: 7.9 (5.8) |
NR | NR | NR | NR |
Both Tx relieved symptoms AEs LF: N = NR (device-related AE of eye discomfort/pain among patients with small eyes, narrow palpebral fissure, or deep-set eyes) |
|
Tauber 2020 [28] Randomized open-label, controlled, multicenter USA |
N = 142 patients with DED and MGD, 55 (15) years LF: 70 patients (140 eyes), age NR; iLUX: N = 71 (142 eyes), age NR |
1 month |
Single 12-min LF Tx Single Tx Systane iLux Dry Eye System thermal pulsation Note: LF was the control in this study |
MGSS LF OD: 6.2 (4.9) LF OS: 6.4 (4.4) iLux OD: 6.0 (3.7) iLux OS: 5.9 (4.1) |
MGSS LF OD: 24.3 (11.2) * LF OS: 23.3 (11.9) * iLux OD: 23.2 (12.1)* iLux OS: 23.8 (11.4)* |
OSDI LF: 50.6 (18.7) iLux: 50.7 (18.6) |
OSDI LF: 22.6 (19.8) * iLux: 19.5 (17.0)* |
TBUT LF OD: 3.9 (2.0) LF OS: 3.8 (2.0) iLux OD: 3.9 (1.9) iLux OS: 3.7 (1.8) |
TBUT LF OD: 6.6 (3.2) * LF OS: 6.5 (3.1) * iLux OD: 6.7 (3.7)* iLux OS: 6.5 (3.6)* |
Corneal LF OD: 2.0 (2.2) LF OS: 2.5 (2.6) iLux OD: 2.1 (2.2) iLux OS: 2.3 (2.2) |
Corneal LF OD: 0.9 (1.6) * LF OS: 1.2 (1.6) * iLux OD: 1.5 (2.4)* iLux OS: 1.4 (2.2)* |
Both Tx had significant improvements in MG function and symptoms Device-related AEs LF: N = 0 iLux: N = 4 patients (N = 2 burning sensations without skin findings and N = 1 petechial hemorrhaging in lower lids, N = 1 transient decrease in BSCVA with findings consistent with exposure keratitis). No other abnormal findings |
|
Tauber 2020 [33] Single-center, 6-week, prospective, randomized, single-masked (investigator) USA |
N = 50 patients with inflammatory MGD; LF: N = 25 patients (25 eyes), 67 (9) years; Liftegrast: N = 25 patients (25 eyes), 64 (8) years |
42 days |
Single 12-min LF Tx plus warm compress/lid compression BID Lifitegrast ophthalmic solution 5% BID plus warm compress/ lid compression BID for 42 days |
MGSS LF: 1.8 (0.55) Lifitegrast: 2.1 (0.49) MG patency LF: 5.4 (2.1) Lifitegrast: 4.8 (2.9) |
MGSS LF: 1.9 (0.81) Lifitegrast: 1.8 (0.97) MG patency LF: 5.3 (2.6) Lifitegrast: 6.1 (2.8) |
VAS Dryness LF: 1.9 (0.83) Lifitegrast: 2.4 (0.70) |
VAS Dryness LF: 1.4 (0.71) Lifitegrast: 1.4 (0.91)† |
NR | NR |
Corneal LF: 0.84 (0.85) Lifitegrast: 1.5 (1.0) |
Corneal LF: 0.96 (0.98) Lifitegrast: 1.0 (1.3)† |
Lifitegrast significantly improved symptoms and signs compared with LF AEs None |
|
Ambaw 2020 [81] Single-center, investigator-masked interventional Singapore |
N = 53 patients, LF: 35 patients (70 eyes); CL: 18 patients (36 eyes) |
3 months |
Single 12-min LF Tx CL: eyelid warming (warm towel, Blephasteam or Eyegiene) 3x/day for 3 months |
NR | NR | NR | NR | NR | NR | NR | NR | Both Tx reduced pro-inflammatory molecules generated by lipoxygenase and oxidative stress |
|
He 2018 [29] Single-blind, prospective, open-label, randomized, controlled China |
N = 50 patients with MGD (100 eyes); LF: N = 25 patients (50 eyes), 40 (12) years; CL: N = 25 patients (50 eyes), 38 (12) years |
3 months |
Single 12-min LF Tx 15-min Tx with warm eye patch (EyeGiene Insta-Warmth) 1x/day for 2 weeks in office |
MGYLS LF: 3.6 (3.8) CL: 4.2 (3.9) |
MGYLS LF: 19.7 (1.0)* CL: 16.2 (10.8) |
OSDI LF: 55.0 (18.0) CL: 47.3 (18.1) |
OSDI LF: 27.5 (18.1)*† CL: 34.2 (19.8)* |
TBUT LF: 6.9 (4.3) CL: 26.3 (6.2) |
TBUT LF: 8.4 (3.7)*† CL: 19.0 (9.9) |
Corneal + Conjunct LF:2.5 (0.8) CL: 2.5 (0.9) |
Corneal + Conjunct LF:2.1 (0.3)*† CL: 2.3 (0.7) |
Both LF and CL were effective at treating MGD. However, LF Tx was more effective and achieved better 3 months long-term efficacy than CL AEs None. No change in IOP |
|
Blackie 2018 [34] Prospective, open-label, randomized, crossover, multicenter USA and Canada |
N = 55 patients, contact lens wearer; LF: N = 29 patients (58 eyes), 40 (13) years CL: N = 26 patients (52 eyes), 44 (16) years; CO: N = 25 patients (50 eyes) in CL who were CO to LF |
3 months; CO: 1 month |
Single 12-min LF Tx plus blinking exercises for 1 month post-Tx to foster healthy blinking habits CL: untreated |
MGSS LF: 8.0 (3.5) CL: 8.2 (4.2) MGYLS LF: 1.9 (1.6) CL: 2.1 (1.7) |
MGSS LF: 20.4 (9.1)† CL: 9.6 (5.7) CO: 22.4 (9.4)† MGYLS LF: 7.1 (3.6)† CL: 2.5 (2.2) CO: 7.9 (3.6)† |
SPEED LF: 14.5 (4.8) CL: 15.3 (4.5) OSDI LF: 39.6 (16.4) CL: 40.8 (20.3) |
SPEED LF: 6.1 (4.6)† CL: 14.5 (5.3) CO: 7.4 (5.0)† OSDI LF: 13.4 (15.5)† CL: 37.5 (23.8) CO: 13.8 (11.2)† |
TBUT LF: 4.8 (2.7) CL: 4.6 (2.0) |
TBUT LF: 6.5 (4.0)† CL: 4.3 (1.7) CO: 5.3 (2.4)† |
Conjunct LF: 3.5 (3.0) CL: 4.3 (3.6) |
Conjunct LF: 3.4 (3.3)† CL: 5.6 (4.5) CO: 3.6 (3.7) |
LF Tx improved signs and symptoms of DED and increased mean comfortable contact wearing time by 4 h, approximately doubling the comfortable wearing time, and was sustained for 3 months AEs None related to LF. N = 11 AEs unrelated LF (N = 5 ocular, N = 6 systemic). Slit-lamp findings not considered AEs were observed immediately after LF: eyelid edema, conjunctival edema, conjunctival hyperemia/injection, petechiae, and SPK and were transient and did not require Tx |
|
Hagen 2018 [30] Prospective, randomized, parallel group, single-masked USA |
N = 28 patients with moderate-to-severe MGD (50 eyes); LF: N = 14 patients (26 eyes), 52 (6) years; Doxy: N = 14 patients (24 eyes), 50 (14) years |
3 months |
Single 12-min LF Tx Oral doxy daily for 3 months (100 mg BID for the first 14 days and 100 mg 1x/day for days 15–90) |
MGYLS LF:4.0 (1.5) Doxy: 4.6 (1.4) |
MGYLS LF: 7.7 (5.5)* Doxy:10.6 (6.0)* |
SPEED LF: 11.0 (3.3) Doxy: 13.4 (4.2) |
SPEED LF: 5.4 (2.2)*† Doxy: 9.4 (5.5)* |
TBUT LF:6.3 (2.0) Doxy: 6.9 (2.6) |
TBUT LF: 8.4 (1.8)* Doxy: 7.6 (2.0) |
Corneal LF: 0.4 (0.5) Doxy: 0.2 (0.4) Conjunct LF: 1.7 (1.9) Doxy: 2.4 (1.9) |
Corneal LF:0.1 (0.3)* Doxy: 0.1 (0.3) Conjunct LF: 0.6 (0.8)* Doxy: 1.1 (1.5)* |
LF was significantly more effective than doxy at treating signs and symptoms of MGD and was a favorable alternative to doxy AEs LF: N = 0 Doxy: N = 2 (stomach upset/intolerance) |
|
Yeo 2016 [82] Singapore |
N = 90 patients, LF: 24 patients (48 eyes) Hot towel: N = 22 patients (44 eyes), EyeGiene: N = 22 patients (44 eyes); Blephasteam: N = 22 patients (44 eyes) |
3 months |
Single 12-min LF Tx Details of other Tx NR |
NR | NR | NR | NR | NR | NR | NR | NR | LF Tx significantly reduced tear evaporation rate |
|
Finis 2014 [31] Prospective, randomized, crossover, observer-masked Germany This is a long-term FU of Finis 2014 [32] |
N = 26 patients with MGD (52 eyes), 50 (22) years; LF: N = 17 patients (34 eyes), 45 (23) years; CL: N = 9 patients (18 eyes) with CL for 3 months prior to LF |
6 months post-LF |
Single 12-min LF Tx CL: Lid warming BID and massage for 3 months |
MGYLS LF: 2.9 (1.6) |
MGYLS LF: 6.4 (4.6)* |
OSDI LF: 42 (19) SPEED LF: 16 (7) CL: 15.9 (6.6) CO: 14.7 (7.7) |
OSDI LF: 33 (21)* SPEED LF: 12 (7)* CL: 14.7 (7.1) CO: 12.6 (6.5) |
TBUT LF: 9.5 (8.7) |
TBUT LF: 10.0 (6.7) |
Corneal + Conjunct LF: 2.0 (2.0) |
Corneal + Conjunct LF: 2.4 (2.3) |
LF reduces subjective symptoms and objective measures of MGD over 6 months but has no effect on atrophy of MG as visualized by meibography. Safety NR |
|
Finis 2014 [32] Prospective, randomized, crossover, observer-masked Germany |
N = 31 patients with MGD; LF: N = 17 patients (34 eyes), 45 (23) years; CL: N = 14 patients, (28 eyes) 50 (19) years After completing the initial study, 9 CL patients (18 eyes) completed a 3-month CO with LF |
3 months |
Single 12-min LF Tx CL: Lid warming BID and massage for 3 months |
MGYLS LF: 2.5 (1.4) CL: 2.1 (1.3) CO: 4.1 (2.5) |
MGYLS LF: 5.5 (3.6)* CL: 4.6 (3.8)* CO:5.8 (3.2) |
OSDI LF: 46.2 (14.8) CL: 40.1 (16.7) CO: 39.7 (26.3) SPEED LF: 16.8 (5.6) CL: 15.9 (6.6) CO: 14.7 (7.7) |
OSDI LF: 34.6 (19.6)* CL: 40.0 (23.4) CO: 32.8 (24.4) SPEED LF: 14.5 (7.2) CL: 14.7 (7.1) CO: 12.6 (6.5) |
NIBUT LF: 7.9 (8.5) CL: 7.7 (6.1) CO: 6.7 (6.1) |
NIBUT LF: 9.9 (7.0) CL: 7.5 (6.1) CO: 7.3 (5.1) |
Corneal + Conjunct LF: 2.6 (2.4) CL:1.9 (2.4) CO: 1.4 (1.4) |
Corneal + Conjunct LF: 3.5 (2.7) CL:1.1 (1.4) CO: 3.0 (2.2) |
Single LF Tx was at least as effective as a 3-month BID lid margin hygiene regimen. Safety NR |
|
Baumann 2014 [35] Single-center, prospective, randomized France |
N = 30 patients with moderate-to-severe DED and MGD; LF: N = 15 patients (30 eyes), 65 (12) years; CL: N = 15 patients (30 eyes), 65 (11) years |
3 months |
Single 12-min LF Tx plus blinking exercises CL: MeiboPatch heat mask for 10 min and massage for 10 min 1x/day for 3 months plus blinking exercises |
MGYLS LF: 3.3 (1.7) CL: 5.7 (3.2) |
MGYLS LF: 8.5 (2.4)* CL: 9.4 (4.3)* |
OSDI LF: 51.0 (20.8) CL: 42.9 (20.3) SPEED LF: 16.5 (4.6) CL: 13.5 (3.1) |
OSDI LF: 27.0 (17.6)* CL: 23.5 (15.3)* SPEED LF: 8.0 (5.3)* CL: 8.9 (5.2)* |
TBUT LF: 4.5 (2.3) CL: 5.2 (2.2) |
TBUT LF: 6.8 (2.5)* CL: 6.9 (2.2)* |
Corneal + conjunct LF: 1.0 (1.1) CL:1.8 (1.2) |
Corneal + conjunct LF: 0.4 (0.8)* CL:0.7 (0.7)* |
LF Tx is highly effective in treating MGD. The results for 3 months of CL Tx were excellent, but not convenient for the patient Randomized but MGD more severe in LF group at BL Safety NR |
|
Lane 2012 [14] Randomized, controlled, open-label, crossover, multicenter USA |
N = 139 patients with MGD; LF: N = 69 patients (138 eyes), age NR; CL: N = 70 patients (140 eyes), age NR; CO: N = 68 patients (136 eyes) in CL group who were CO to LF |
LF: 1 month; CL: 2 weeks; CO: 2 weeks |
Single 12-min LF Tx CL: Warm compress (iHeat) for 5 min/day for 2 weeks |
MGSS LF: 6.3 (3.5) CL: 5.6 (3.9) MGYLS LF: 0.6 (0.9) CL: 0.4 (0.8) |
MGSS LF: 14.3 (8.7) at 2 weeks*†; 16.7 (8.7) at 1 month* CL: 6.1 (5.6) CO: 11.7 (7.3)† MGYLS LF: 2.0 (2.9) at 2 weeks*†; 2.6 (3.6) at 1 month* CL: 0.5 (1.1) CO: 1.2 (1.9)† |
SPEED LF: 14.3 (4.8) CL: 14.8 (4.8) OSDI LF: 32.0 (20.0) CL: 34.7 (19.6) |
SPEED LF: 8.1 (5.5) at 2 weeks*†; 7.6 (5.8) at 1 month * CL: 11.2 (5.4) CO: 7.9 (5.6)† OSDI LF: 17.3 (17.2) at 2 weeks*†; 16.6 (18.1) at 1 month * CL: 26.9 (18.2) CO: 21.0 (18.3)† |
TBUT LF: 5.5 (2.9) CL: 5.4 (3.5) |
TBUT LF: 6.9 (5.0) at 2 weeks*†; 7.4 (5.5) at 1 month * CL: 5.3 (3.5) CO: 6.3 (4.7)† |
Corneal LF: 2.2 (2.2) Conjunct LF: 1.3 (2.1) |
Corneal LF: 1.5 (1.7) at 1 month* Conjunct LF: 1.6 (2.5) at 1 month* |
LF Tx improved signs and symptoms over 1 month The safety profile of LF Tx reflects a low occurrence of non-serious, transient side effects that resolve quickly and do not require medical treatment AEs LF: N = 4 (N = 3 eyelid pain, N = 1 conjunctival vascular injection) CL: N = 2 (burning) Mean discomfort score during LF Tx was 1.4 (scale 0–10). Slit-lamp findings not considered AEs most frequently were trace to mild conjunct injection, hyperemia, or redness; and trace or mild petechial hemorrhages on the eyelid or conjunct immediately or 1 day post-Tx, which fully resolved by the 2-week visit without Tx. No changes in the intraocular findings except for 1 case of posterior vitreous floaters unrelated to device |
Units of measure are as follows: IDEEL-SB = points, score 0–100; MGSS = points, score 0–45; MGYLS = number of functional glands; NIKBUT = seconds; OSDI = points, score 0–100; SANDE = visual analog scale score, 0–100; SPEED = points, score 0–28; staining = scale score; TBUT = seconds; VAS = scale score
Treatment manufacturers are as follows: Blephasteam (Laboratoires Thea, Clermont-Ferrand, France); EyeGiene® Insta-Warmth™ patch (Eyedetec Medical Inc, Danville, CA); iHeat (manufacturer unknown); Lifitegrast ophthalmic solution 5% (Xiidra; Shire, Lexington, MA); MeiboPatch® heat mask (Butterflies Eyecare, Banbury, UK); MiboFlo ThermoFlo (Mibo Medical, Dallas, TX); Systane iLux Dry Eye System thermal pulsation (Alcon, Ft Worth, Tx); TearCare (Sight Sciences, Menlo Park, CA)
AEs adverse events, BID 2 times per day, BL baseline, CL control, CO crossover group, conjunct conjunctiva, DED dry eye disease, doxy doxycycline, FU follow-up, IDEEL-SB Impact of Dry Eye on Everyday Life—Symptom Bother, IOP intraocular pressure, LF LipiFlow, MG meibomian glands, MGD meibomian gland dysfunction, MGSS meibomian gland secretion score (secretion quality), MGX manual gland expression, MGYLS meibomian glands yielding liquid secretion (glands with secretion capacity), min minutes, NIKBUT noninvasive keratograph break-up time, NR not reported, OD right eye, OS left eye, OSDI ocular surface disease index, pre pretreatment, post post-treatment, SANDE Symptom Assessment in Dry Eye, SD standard deviation, SPEED standard patient evaluation of eye dryness, SPK superficial punctate keratitis, TBUT tear break-up time, Tx treatment, VA visual acuity, VAS visual analog scale
1Data only presented in a figure. We did not extrapolate the data from the figure to avoid presenting estimated data
*P < 0.05 vs. baseline
†P < 0.05 vs. control
§P < 0.05 vs. comparator
Twelve of the 15 RCTs evaluated the effectiveness of a single 12-min LipiFlow treatment in 628 patients (1164 eyes) with MGD [14, 22–32]. In addition, a single 12-min LipiFlow treatment was evaluated when combined with warm compress and eyelid compression (one study treating 25 patients, 25 eyes) [33] or blinking exercises (two studies treating 44 patients, 88 eyes) [34, 35]. Together, the 15 RCTs studies evaluated 697 patients (1277 eyes) treated with LipiFlow.
Seven of the 15 RCTs evaluated the effectiveness of LipiFlow compared with various at-home eyelid warming therapies. Specifically, the effectiveness of LipiFlow was compared to eyelid warming plus massage (48 patients, 96 eyes) in three RCTs [22, 31, 32]; eyelid warming (28 patients, 28 eyes) in one RCT [27]; eyelid warming via a patch/mask (95 patients, 190 eyes) in two RCTs [14, 29]; and eyelid warming via a patch/mask plus massage and blinking exercises (15 patients, 30 eyes) in one RCT [35]. All seven studies concluded that LipiFlow was similar or better than the at-home eyelid warming therapies at improving signs and symptoms of MGD 1–6 months post-treatment. Finis et al. reported that at 6 months, symptom improvement was better in LipiFlow-treated patients with less severe MGD (i.e., less dropout at baseline) [31]. Baumann et al. reported that using a heat mask, massage, and blinking exercises daily for 3 months was as effective as single 12-min LipiFlow treatment plus blinking exercises, but the control treatment was not convenient for the patient [35]. He et al. reported that both treatments were effective at treating MGD, but at 3 months LipiFlow was more effective thereby achieving better long-term effectiveness [29].
Five of the 15 RCTs evaluated the effectiveness of LipiFlow compared with other thermal pulsation treatments in patients with MGD. Specifically, the effectiveness of LipiFlow was compared with iLux (190 patients, 380 eyes) in two RCTs [23, 28], TearCare (115 patients, 230 eyes) in one RCT [24]; TearCare plus manual meibomian gland expression (MGX) (69 patients, 134 eyes) in one RCT [26]; and Mibo ThermoFlo (54 patients, 54 eyes) in one RCT [25]. All five studies used LipiFlow as the control group and concluded that the comparator was similar or non-inferior to LipiFlow 1–12 months post-treatment.
Two of the 15 RCTs evaluated the effectiveness of LipiFlow compared with pharmaceutical treatments for DED in patients with MGD. Specifically, the effectiveness of LipiFlow was compared to Lifitegrast ophthalmic solution 5% twice daily (BID) plus warm compress and eyelid compression (25 patients, 25 eyes) in one RCT [33], and oral doxycycline (14 patients, 24 eyes) in one RCT [30]. The Lifitegrast study concluded that Lifetegrast was better than LipiFlow at improving symptoms of dryness and corneal staining 42 days after treatment [33]. The oral doxycycline study concluded that LipiFlow was better at improving signs and symptoms of MGD 3 months post-treatment [30].
One of the 15 RCTs evaluated contact lens wearers and included an untreated control group (26 patients, 52 eyes), which was later crossed-over to LipiFlow treatment [34]. Blackie et al. concluded that LipiFlow improved signs and symptoms of MGD for 3 months in contact lens wearers and increased the mean comfortable wearing time by 4 h, which approximately doubled the comfortable wearing time.
Together the RCT data demonstrate that a single 12-min LipiFlow treatment was (1) efficacious in improving MG function, patient-reported outcomes, and ocular surface health compared to baseline; (2) was as good or better than alternate therapies; and (3) had a duration of effect up to 12 months [23]. Only one of 15 RCTs concluded that the comparator (Lifitegrast ophthalmic solution 5% BID plus warm compress and eyelid compression) was better than LipiFlow [33].
Prospective, Controlled, Non-randomized Trials
Three prospective, controlled, non-randomized trials were identified that evaluated the effectiveness of LipiFlow for treating MGD (Table 3). These three studies included 82 patients (87 eyes) treated with LipiFlow [36–38]. One study evaluated the effect of LipiFlow plus a dexamethasone intracanalicular insert compared to LipiFlow without the insert (sham insert) and concluded that the addition of a dexamethasone insert provided additional therapeutic benefits at 3 months [36]. The second study evaluated the effect of LipiFlow plus hyaluronic acid eye drops compared to daily massage plus hyaluronic acid eye drops and concluded that both were effective, but the LipiFlow group was more effective at 3 months [37]. The third study compared the effect of LipiFlow with an untreated contralateral eye and concluded that LipiFlow significantly improved MGD compared with baseline and the untreated eye at 3 months [38]. Taken together, the prospective, controlled, non-randomized trials support the effectiveness of LipiFlow in treating MGD, and one study concluded that adding a dexamethasone intracanalicular insert to LipiFlow treatment may improve outcomes [36].
Table 3.
Summary of prospective, masked (non-randomized) studies evaluating LipiFlow to treat MGD
| Author/design/country | Patients (eyes)/age, mean (SD) | Last FU | Study arms | MG function Mean (SD) |
Patient-reported outcomes Mean (SD) |
NIKBUT/TBUT Mean (SD) |
Staining | Overall conclusion and safety | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||
|
Dierker 2022 [36] Single-center, open-label, prospective, non-randomized, subject-masked, sham-controlled USA |
N = 20 patients with MGD, 59 (11) years; LF: 19 eyes, CL: 19 eyes |
3 months |
Single 12-min LF Tx plus Dex intracanalicular insert CL: Single 12-min LF Tx plus sham punctal dilation |
MGSS LF/Dex: 13.3 (8.1) LF/CL: 16.3 (8.0) |
MGSS LF/Dex: 29.8*† (9.9) LF/CL: 27.3 (11.4)* |
NR | NR |
TBUT LF/Dex:3.2 (2.2) LF/CL: 4.1 (3.4) |
TBUT LF/Dex: 6.1 (4.8)*† LF/CL: 4.7 (3.4) |
Corneal + Conjunct LF/Dex: 6.6 (6.3) LF/CL: 5.2 (4.7) |
Corneal + Conjunct LF/Dex: 5.7 (5.9) LF/CL: 6.2 (6.5) |
Dex provides additional therapeutic benefit when combined with LF for improving signs of DED AEs None, IOP and VA remained stable in both groups |
|
Laufenböck 2022 [37] Prospective, non-masked, single-center Austria |
N = 30 patients with DED and MGD, LF: 15 patients (30 eyes), 63 (7) years CL: 15 patients (30,eyes) 61 (6) years |
3 months |
Single 12-min LF Tx plus hyaluronic acid eye drops 3x/day CL: Daily massage plus hyaluronic acid eye drops 3x/day |
MGYLS (yes/no scale)1 LF:1.8 CL: 1.9 |
MGYLS (yes/no scale)1 LF:1.4† CL: 1.75 |
OSDI LF: 22.2 CL: 22.7 |
OSDI LF: 14.2† CL: 18.5 |
NIKBUT LF: 6.8 CL: 6.7 |
NIKBUT LF: 11.2† CL: 8.3 |
NR | NR |
Both Tx improved measures of MGD but more strongly in the LF group. AEs NR. Safety parameters (IOP and VA) were normal in all patients |
|
Zhao 2016 [38] Prospective, examiner-masked, contralateral eye (worst eye treated) China |
N = 29 Chinese patients with MGD (58 eyes), 57 (7) years; LF: 29 patients (29 eyes); CL: 29 patients (29 eyes) |
3 months |
Single 12-min LF Tx CL: untreated |
MGYLS LF: 1.78 (1.8) CL: 2.34 (1.9) |
MGYLS LF: 4.75 (3.1)*† CL: xx2 |
SPEED LF: 11.2 (4.9) CL: xx2 |
SPEED LF: 4.6 (3.4)* CL: xx2 |
TBUT LF: 2.5 (0.8) CL: 2.8 (1.0) |
TBUT LF: 3.5 (1.4)*† CL: xx1 |
Corneal LF: 2.3 (1.8) CL: 1.6 (0.7) |
Corneal LF: 1.3 (1.5)* CL: xx2 |
LF significantly improved objective measures of MGD compared with the contralateral eye. Safety NR |
Units of measure are as follows: MGSS = points, score 0–45; MGYLS = number of functional glands; NIKBUT = seconds; OSDI = points, score 0–100; SPEED = points, score 0–28; staining = scale score; TBUT = seconds
AEs adverse events, CL control, conjunct conjunctival, DED dry eye disease, dex dexamethasone, IOP intraocular pressure, FU follow-up, LF LipiFlow, MG meibomian glands, MGD meibomian gland dysfunction, MGSS meibomian gland secretion score (secretion quality, max 45 points), MGYLS meibomian glands yielding liquid secretion (glands with secretion capacity), min minutes, NIKBUT noninvasive keratograph break-up time, NR not reported, OSDI ocular surface disease index, pre pretreatment, post post-treatment, SD standard deviation, SPEED standard patient evaluation of eye dryness, TBUT tear break-up time, Tx treatment, VA visual acuity
1The only details on this scale provided are “the expression functionality of the meibomian glands using Meibomian Gland Evaluator: Yes/no test, whether meibum can be expressed with the defined pressure on the eyelid”
2Data only presented in a figure. We did not extrapolate the data from the figure to avoid presenting estimated data
*P < 0.05 vs. baseline
†P < 0.05 vs. control
Open-Label Studies
A total of 17 open-label studies were identified that evaluated the effectiveness of LipiFlow for treating MGD, and 16 studies included outcomes of interest (Table 4). Fourteen of the 16 open-label studies evaluated the effectiveness of a single 12-min LipiFlow treatment in 441 patients (814 eyes) with MGD [11, 13, 19, 39–49]. In addition, a single 12-min LipiFlow treatment was evaluated when combined with daily manual eyelid massage and eyelid hygiene (one study treating 24 patients, 24 eyes) [50] or warm compress, massage, and eyelid hygiene (one study treating 32 patients, 64 eyes) [51]. One study treated 14 patients (28 eyes) with LipiFlow bilaterally and as a control the contralateral eye had additional heat and MGX to confirm that all material was removed from the glands [49]. Together, the 16 open-label studies evaluated 497 patients (916 eyes) treated with LipiFlow.
Table 4.
Summary of open-label studies evaluating LipiFlow to treat MGD
| Author/design/country | Patients (eyes)/age, mean (SD) | Last FU | Study arms | MG function Mean (SD) |
Patient-reported outcomes Mean (SD) |
NIKBUT/TBUT Mean (SD) |
Staining | Overall conclusion and safety | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||
|
Liu 2023 [39] Prospective interventional China |
N = 50 patients with MGD, LF: N = 25 patients (50 eyes), 36 (10) years; CL: N = 25 patients (50 eyes), 35 (6) years |
3 months |
Single 12-min LF Tx CL: warm eyelid with spontaneous steam eyelid masks for 20 min and massage for 15 min, 1x/day for 3 months |
NR |
MGE change from BL LF: − 0.46 (0.58)* CL: − 0.40 (0.49) MGSS change from BL LF: − 5.44 (3.23)* CL: − 3.28 (3.37) |
NR |
OSDI change from BL LF: − 17.4 (20.3)†* CL: -8.8 (13.5) |
NR |
NIKBUT change from BL LF:1.46 (2.18)* CL: 1.07 (2.33) TBUT change from BL LF:1.91 (0.87) CL: 1.49 (1.71) |
NR |
Corneal change from BL LF: − 0.52 (0.84)†* CL: − 0.18 (0.66) |
LF improved MGD and was well tolerated AEs LF: N = 1 patient (slight irritation) CL: N = 10 patients (mild to severe pain/ discomfort). LF was significantly more comfortable than CL. No loss of VA or IOP > 21 mmHg |
|
Novo-Diez 2022 [40] Prospective, single-center, open-label Spain |
N = 21 patients with MGD exposed to adverse environmental humidity (42 eyes), 60 (9) years | 12 months | Single 12-min LF Tx |
MGYLS LF: 10.3 (1.5) |
MGYLS LF: 12.2 (2.2)* |
OSDI LF: 43.0 (23.9) |
OSDI LF: 29.0 (22.2)* |
TBUT LF: 4.2 (4.1) |
TBUT LF: 4.3 (5.2) |
Conjunct LF: 2 (2) |
Conjunct LF: 0 (0)* |
LF improves objective and subjective measures of MGD and the effects are sustained for ≥ 1 year AEs None. No significant changes in VA |
|
Hu 2022 [19] Prospective, open-label, multicenter USA |
N = 44 patients with MGD (88 eyes), 67 (8) years | Post-LF | Single 12-min LF Tx | – | – | – | – | – | – |
Corneal LF: 3.1 (1.9) Conjunct LF: 3.4 (2.3) |
Corneal LF: 3.9 (2.3)* Conjunct LF: 4.3 (2.9)* Note: the differences were considered statistically but not clinically significant |
The LF Activator Clear enables efficient and confident positioning around the eyelids to ensure successful LF Tx when used as indicated AEs None. No safety-related concerns |
|
Fallah 2021 [41] Prospective, open-label USA |
N = 96 patients with MGD (192 eyes), age NR | 3 months | Single 12-min LF Tx | NR | NR |
OSDI LF: 35.7 |
OSDI LF: 30.1* |
NR | NR | NR | NR |
Significant improvement in subjective symptoms, but smaller than reported in previous studies AEs None |
|
Chan 2021 [42] Prospective, observational Hong Kong |
N = 16 patients with MGD (30 eyes), mean age NR | 1 month | Single 12-min LF Tx | NR | NR |
SPEED II LF: 12.9 (1.3) |
SPEED II LF: 8.7 (0.7)* |
NR | NR | NR | NR |
LF Tx was effective in improving both objective and subjective measures of MGD. Safety NR |
|
Li 2020 [43] Prospective, interventional China |
N = 50 patients (50 eyes); N = 25 patients with OMGD (25 eyes), 37 (12) years; N = 25 patients with HMGD (25 eyes), 33 (10) years |
3 months | Single 12-min LF Tx |
MGSS LF/OMGD: xx1 MGE LF/OMGD: xx1 |
MGSS LF/OMGD: xx1* MGE LF/OMGD: xx1* |
SPEED LF/OMDG: 12.0 (2.5) LF/HMGD: 12.1 (2.5) OSDI LF/OMDG: 22.9 (4.4) LF/HMGD: 24.1 (4.8) |
SPEED LF/OMDG: 1.5 (1.3)*† LF/HMGD: 5.2 (2.0)* OSDI LF/OMDG: 1.7 (0.8)*† LF/HMGD: 12.9 (4.1)* |
NIKBUT LF/OMDG: 3.2 (1.5) LF/HMGD: 3.2 (1.4) |
NIKBUT LF/OMDG: 4.4 (1.4)*† LF/HMGD: 3.0 (1.1) |
NR | NR |
LF Tx was effective for OMGD and HMGD. It is more effective for OMGD. Safety NR |
|
Godin 2018 [44] Prospective USA |
N = 13 patients with Sjögren's disease (24 eyes), 62 years | 1 year | Single 12-min LF Tx | NR | NR |
OSDI LF: 40.4 |
OSDI LF: 47.6 |
TBUT LF: 3.8 |
TBUT LF: 7.5* |
Corneal LF: 1.0 Conjunct LF: 1.5 |
Corneal LF: 0.48* Conjunct LF: 0.48* |
LF Tx improved signs of DED and MGD in patients with symptomatic Sjögren's disease on maximum therapy. Safety NR |
|
Zhao 2016 [50] Single-center, controlled, open-label Singapore |
N = 46 patients with MG dropout; LF: N = 24 patients (24 eyes), 56 (13) years; CL: N = 22 patients (22 eyes), 56 (11) years |
3 months |
Single 12-min LF Tx plus daily manual lid massage and lid cleaning for 3 months CL: warm compress with towel 10 min/day BID plus daily manual lid massage and lid cleaning for 3 months |
MGYLS LF: xx1 |
MGYLS LF: xx1* |
SANDE LF: 45.6 (25.2) CL:52.4 (20.4) |
SANDE % change from BL LF: − 30.5 (P < 0.05 vs. BL at 1 month but not 3 months) CL: − 15.9 (P < 0.05 vs. BL at 1 month but not 3 months) |
TBUT LF: 2.4 (1.1) CL: 2.4 (1.3) |
TBUT % change from BL LF: 89.2 (P < 0.05 vs. BL at 1 month but not 3 months) CL:63.0 |
NR | NR |
In Asian patients, LF improved symptoms of MGD, and 1 session of LF was comparable to 3 months of BID lid warming, massage, and hygiene All LF Tx patients had transient eye redness and mild eyelid puffiness for a few min after Tx. No other AEs |
|
Greiner 2016 [45] Prospective, observational, single-center USA |
N = 20 patients with DED and MGD (40 eyes), 61 (11) years | 3 years | Single 12-min LF Tx |
MGSS LF: 4.5 MGYLS LF: 13.7% of glands studied |
MGSS LF: 18.4* MGYLS LF: 53.7% of glands studied |
SPEED LF: 13.4 OSDI LF: 26.0 |
SPEED LF: 9.5 (1.6)* OSDI LF: 22.5 (5.4) (P < 0.05 vs. BL at 1, 9 months, and 1 year but not 3 years) |
TBUT LF: 4.1 (0.4) |
TBUT LF: 4.5 (0.6) (P < 0.05 vs. BL at 1 month and 9 months but not 3 years) |
Corneal LF: 0.4 (0.3) Conjunct LF:0.01 (0.03) |
Corneal LF: 0.9 (0.6) Conjunct LF:0.8 (0.5) |
The study reinforces the potential long-term (3 years) benefits of a single LF Tx. Safety NR |
|
Blackie 2016 [11] Prospective, multicenter, open-label USA |
N = 200 patients with MGD and evaporative dry eye (400 eyes); 56 (15) years; LF: 101 patients (202 eyes); CL: N = 99 patients (198 eyes); CO: N = 93 patients (186 eyes) in CL who were CO to LF |
12 months (9 months for CO group) |
Single 12-min LF Tx CL: warm compress with EyeGiene InstaWarmth and lid hygiene with OCuSOFT Lid Scrub Original 10 min/day BID for 3 months |
MGSS LF: 6.2 (3.7) CL: 6.3 (3.7) |
MGSS LF: 17.3 (9.1)* CL: 11.0 (at 3 months) CO: 18.4 (11.1)* |
OSDI LF: 45.6 (21.2) CL: 51.8 (23.1) |
OSDI LF: 21.6 (21.3)* CL: 31.3 (at 3 months) CO: 24.0 (23.2)* |
NR | NR | NR | NR |
LF Tx has sustained improvement in MG function and symptoms over 12 months AEs LF: N = 10 (N = 3 eye/eyelid discomfort/pain most common, others NR); CL: N = 8 (N = 3 eyelid skin dermatitis most common, others NR). All AEs transient, nonserious, and resolved with no Tx |
|
Satjawatcharaphong 2015 [51] Open-label USA |
N = 32 patients with DED and MGD (64 eyes), 54 (15) years | Mean 52 days (range: 21–84 days) |
Single 12-min LF Tx plus warm compress, massage, and eyelid hygiene were recommended BID until FU |
MGSS LF: 17.3 (12.9) |
MGSS LF: 29.0 (12.6)* |
SPEED LF: 15.7 (5.5) |
SPEED LF: 12.9 (5.7)* |
NIKBUT LF: 6.1 (3.2) |
NIKBUT LF: 7.6 (2.6) |
Corneal LF: 1.8 (3.8) Conjunct LF: 1.4 (2.1) |
Corneal LF: 2.8 (6.6) Conjunct LF: 2.8 (6.6) |
Results identified factors that better select candidates for LF Tx (At BL: men, higher inferior conjunctival staining, greater number of unexpressible glands) AEs NR Bulbar redness after at-home therapy (N = NR) |
|
Greiner 2013 [46] Prospective, single-center, observational, open-label USA |
N = 18 patients with MGD and evaporative DED (36 eyes), 63 (12) years Note: This is a subgroup, long-term FU from Lane 2012 [14] |
12 months | Single 12-min LF Tx |
MGSS LF: 4.0 (3.4) |
MGSS LF: 7.3 (4.6)* |
SPEED 12.9 (3.8) OSDI LF: 22.2 (14.2) |
SPEED 6.3 (5.5)* OSDI LF: 12.4 (14.6)* |
TBUT LF: 4.9 (3.0) |
TBUT LF: 6.0 (4.4) |
NR | NR |
LF Tx-induced improvement is sustained for up to 1 year. Safety NR |
|
Korb 2013 [47] Case report USA |
N = 1 patient (2 eyes) with NOMGD with significant MG dropout and truncation | 7 months | Single 12-min LF Tx |
MGYLS LF OD: 1 LF OS: 1 |
MGYLS LF OD: 4 LF OS: 4 |
SPEED LF OD: 24.0 LF OS: 28.0 |
SPEED LF OD: 6.0 LF OS: 6.0 |
TBUT LF OD: 4 LF OS: 4 |
TBUT LF OD: 7 LF OS: 9 |
NR | NR |
LF Tx was effective in a patient with significant gland dropout and truncation, and glands were restored to functional health for ≥ 7 months AEs None, no discomfort |
|
Greiner 2012 [48] Prospective, open-label USA |
N = 21 patients with MGD and dry eye (42 eyes), 62 years | 9 months | Single 12-min LF Tx |
MGSS LF: 4.4 (4.0) |
MGSS LF: 11.7 (5.9)* |
SPEED LF: 12.9 (4.0) OSDI LF: 23.4 (14.4) |
SPEED LF: 6.2 (4.0)* OSDI LF: 12.4 (15.3)* |
TBUT LF: 9.6 (7.6) |
TBUT LF: 7.1 (5.6)* |
NR | NR |
LF Tx improved signs and symptoms of DED and was maintained for 9 months. Safety NR |
|
Friedland 2011 [49] Multicenter, open-label feasibility USA |
N = 14 patients with moderate-to-severe dry eye and MGD, 54 (10) years; LF: N = 14 eyes; CL: N = 14 eyes |
3 months |
Single 12-min LF Tx CL: Single 12-min LF Tx plus heat and pressure with a handheld device (type NR) with MGX to assess whether all material was removed |
MGSS LF: 3.4 (3.2) CL: 2.5 (2.3) MGYLS LF: 2.9 (2.8) CL: 2.1 (2.4) |
MGSS LF: 9.9 (3.2)* CL: 9.2 (4.3)* MGYLS LF: 9.9 (3.1)* CL: 10.5 (4.9)* |
SPEED LF: 16.2 (5.4) CL: 16.2 (5.4) OSDI LF: 37.0 (23.8) CL: 37.9 (21.4) |
SPEED LF: 7.8 (4.8)* CL: 7.8 (4.8)* OSDI LF: 18.3 (14.0)* CL: 24.5 (26.5)* |
TBUT LF: 5.2 (2.6) CL: 5.8 (3.4) |
TBUT LF: 11.0 (6.3)* CL:11.8 (5.9)* |
Corneal LF: 0.6 (0.8) CL:0.6 (0.7) |
Corneal LF: 0.1 (0.3)* CL: 0.1 (0.3)* |
LF Tx was highly effective at improving signs and symptoms of MGD and DED for 3 months AEs N = 3 (discomfort, chalazion unrelated to Tx, internal hordeola unrelated to Tx) |
|
Korb 2010 [13] Case report USA |
N = 1 patient with NOMGD (2 eyes) | 3 months | Single 12-min LF Tx |
MGYLS LF OD: 0 LF OS: 1 |
MGYLS LF OD: 6 LF OS: 6 |
SPEED LF OD: 20.0 LF OS: 19.0 OSDI LF OD: 70.8 LF OS: 66.7 |
SPEED LF OD: 3.0 LF OS: 4.0 OSDI LF OD: 18.8 LF OS: 14.6 |
TBUT LF OD: 5.2 LF OS: 4.5 |
TBUT LF OD: 10.7 LF OS: 13.8 |
NR | NR |
LF Tx was effective at treating NOMGD for at least 3 months AEs None, no significant discomfort Note: this is the first published report of the use of LF |
Units of measure are as follows: MGSS = points, score 0–45; MGYLS = number of functional glands; NIKBUT = seconds; OSDI = points, score 0–100; SANDE = visual analog scale score, 0–100; SPEED = points, score 0–28; staining = scale score; TBUT = seconds
Notes: Treatment manufacturers are as follows: EyeGiene® InstaWarmth™ patch (Eyedetec Medical Inc, Danville, CA); OCuSOFT® Lid Scrub™ Original (OCuSOFT, Rosenberg, TX)
AEs adverse events, BID 2 times per day, BL baseline, conjunct conjunctival, CL control, CO crossover group, DED dry eye disease, FU follow-up, HMGD hyposecretory meibomian gland disease, IOP intraocular pressure, LF LipiFlow, MGD meibomian gland disease, MGE meibomian gland expressibility score, MGSS meibomian gland secretion score (secretion quality, max 45 points), MGX manual gland expression, MGYLS meibomian glands yielding liquid secretion (glands with secretion capacity), min minutes, NIKBUT noninvasive keratograph break-up time, NOMGD nonobvious obstructive meibomian gland disease, NR not reported, OD right eye, OS left eye, OMGD obstructive meibomian gland disease, OSDI ocular surface disease index, pre pretreatment, post post-treatment, SANDE Symptom Assessment in Dry Eye, SD standard deviation, SPEED standard patient evaluation of eye dryness, TBUT tear break-up time, Tx treatment, VA visual acuity
1Data only presented in a figure. We did not extrapolate the data from the figure to avoid presenting estimated data
*P < 0.05 vs. baseline
†P < 0.05 vs. comparator
Eleven of the open-label studies that evaluated a single 12-min LipiFlow treatment had no control group [13, 19, 40–48]. The 11 studies concluded that LipiFlow was effective at improving the signs and symptoms of MGD 1 month to 3 years post-treatment. One of the studies focused on evaluating the updated LipiFlow Activator Clear and concluded that it enables efficient and confident positioning around the eyelids to ensure successful LipiFlow treatment when used as indicated [19]. One study compared the effectiveness of LipiFlow in patients with hyposecretory MGD (HMGD) or obstructive MGD (OMGD) and concluded that although LipiFlow was effective in both groups, it was more effective in treating OMGD [43]. Two case reports demonstrated the benefits of LipiFlow in patients with nonobvious OMGD [13, 47]. LipiFlow was also shown to be effective in patients with symptomatic Sjögren’s syndrome on maximum therapy [44].
Four of the 16 open-label studies compared LipiFlow to a control treatment (warm compress, eyelid hygiene, massage, and/or eyelid pressure) and concluded that effectiveness of LipiFlow was similar or better than the at-home therapies [11, 39, 49, 50]. In particular, Zhao et al. concluded that one session of LipiFlow was comparable to 3 months of eyelid warming, massage, and hygiene BID [50].
The objective of one open-label study was to identify patient characteristics that would help navigate when to recommend LipiFlow treatment (i.e. who would have the most robust outcomes) [51]. The study concluded that men, patients with greater inferior conjunctival staining at baseline, and patients with a greater number of unexpressible lower eyelid MGs at baseline receive the most benefit from LipiFlow treatment. A limitation of this study is that only 32 patients were included.
Five open-label studies evaluated the duration of effect 1–3 years post-treatment [11, 40, 44–46], and the benefits were shown to last up to 3 years [45].
Retrospective Studies
Eight retrospective studies were identified that evaluated the effectiveness of LipiFlow for treating MGD, and included 367 LipiFlow-treated patients (646 eyes) (Table 5). Four of the eight retrospective studies evaluated the effectiveness of a single 12-min LipiFlow treatment in 235 patients (435 eyes) with MGD and concluded that LipiFlow improved signs and symptoms of MDG/DED 2–12 months post-treatment [52–55]. Four of the eight retrospective studies evaluated LipiFlow treatment combined with at-home therapies in 132 patients (211 eyes), and all concluded that LipiFlow was effective 1–12 months post-treatment [56–59]. Epitropoulos et al. compared the effect of LipiFlow in patients with and without Sjögren’s syndrome and concluded that while the treatment was effective for both groups, it was more effective in patients without Sjögren’s syndrome [54]. Hura et al. reported that LipiFlow treatment may restore acini activity [52]. Arita et al. compared LipiFlow with other treatments and recommended LipiFlow for mild to moderate MG loss and IPL for severe MG loss [58]. Gibbons et al. concluded that patients with signs of lower tear production, higher corneal and conjunctival staining scores, and higher osmolarity tend to respond better to LipiFlow [59].
Table 5.
Summary of retrospective studies evaluating LipiFlow to treat MGD
| Author/design/country | Patients (eyes)/age, mean (SD) | Last FU | Study arms | MG function, mean (SD) | Patient-reported outcomes Mean (SD) |
NITBUT/TBUT, mean (SD) | Staining | Overall conclusion and safety | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||
|
Chung 2022 [56] Retrospective South Korea |
N = 23 patients with refractory MGD (23 eyes), 42 (13) years | 12 months | 12-min LF Tx plus IPL plus MGX all 3 times (once every 3 weeks); followed by IPL plus MGX monthly through 18 weeks; followed by MGX, monthly through 12 months |
MGE Grade Score BL: 2.6 (0.6) Note: graded 0–3 |
MGE Grade Score 1.2 (0.6)* |
OSDI BL: 49.0 (14.9) |
OSDI 15.5 (8.7)* |
NIBUT BL: 3.2 (1.2) |
NIBUT 8.0 (1.9)* |
NR | NR |
LF combination Tx was effective and safe for treating refractory MGD with prolonged effects maintained with monthly MGX AEs N = 3 (gritty sensation with mild discharge for 1 day). No sign changes in VA |
|
Kim 2020 [57] Retrospective South Korea |
N = 30 patients with obstructive MGD (60 eyes); LF: 15 patients (30 eyes); 40 (7) years CL: 15 patients (30 eyes), 39 (11) years |
6 months |
12-min LF Tx 3 times (1x/month for 3 months) CL: Single 12-min LF Tx followed by MGX 3 times (1x/month for 3 months) All eyes had artificial tears containing 0.1% hyaluronic acid (Haylene) 3 × or 4x/day for 6 months |
NR | NR |
OSDI LF: 43.6 (10.4) CL: 51.3 (15.5) |
OSDI LF: 33.3 (13.2)* CL: 13.1 (6.9)*† |
NIBUT LF: NR1 CL: 3.2 (1.2) |
NIBUT LF: NR1 CL: 8.5 (1.5)*† |
NR | NR |
Both LF only and MGX after LF were clinically effective for obstructive MGD. Efficacy and persistence were greater in the MGX plus LF group AEs None |
|
Hura 2020 [52] Retrospective, single masked USA |
N = 43 patients with DED and MGD (70 eyes); LF: N = 30 patients (48 eyes), age NR; CL: N = 13 patients (22 eyes), age NR |
12 months |
Single 12-min LF Tx CL: untreated but LF recommended |
MGSS LF: 5 CL: 7 |
MGSS LF: 12*† CL: 6 |
SPEED LF: 15 CL:12 |
SPEED LF: 10 CL: 9 |
TBUT LF: 3 CL: 4 |
TBUT LF: 7*† CL: 3 |
Corneal LF: 0.44 CL: 0.39 Note: score 1 = presence and 0 = absence |
Corneal LF: 0 31*† CL: 0.67 |
Compared with CL, MG structure may increase post-LF Tx suggesting restored acini activity. Safety NR |
|
Arita 2021 [58] Retrospective, randomized Japan |
N = 165 patients with MGD (165 eyes); LF: 30 patients/eyes, 63 (14) years; CL 1: 30 patients/eyes, 59 (19) years; CL 2: 30 patients/eyes, 59 (15) years; CL 3: 38 patients/eyes, 60 (16) years; CL 4: 37 patients/eyes, 61 (18) years |
1 month |
Single 12-min LF Tx plus warm compress and lid hygiene BID for 1 month CL1: warm compress and lid hygiene BID for 3 months CL 2: Artia MG compressor every 3 weeks for 4 times plus warm compress and lid hygiene BID for 3 months CL 3:Azithromycin eyedrops plus warm compress and lid hygiene BID for 2 weeks CL 4: IPL (M22) every 3 weeks for 4 sessions plus warm compress and lid hygiene BID for 3 months |
MGE Grade Score LF: 2.3 (0.4) CL 1: 2.3 (0.5) CL 2: 2.4 (0.5) CL 3: 2.3 (0.4) CL 4: 2.6 (0.5) Note: graded 0–3 |
MGE Grade Score LF: 1.7 (1.0)* CL 1: 1.8 (0.9)* CL 2: 1.6 (0.9)* CL 3: 1.2 (0.8)* CL 4: 0.1 (0.4)* |
SPEED LF:11.3 (3) CL 1: 12.2 (3.9) CL 2: 12.5 (4.2) CL 3: 12.6 (4) CL 4: 13.4 (3.2) |
SPEED LF: 8.7 (4.2)* CL 1: 9.0 (3.6)* CL 2: 8.9 (4.9)* CL 3: 6.2 (4.5)* CL 4: 3.7 (2.8)* |
TBUT LF: 3.3 (0.7) CL 1: 3.1 (1.2) CL 2: 3.0 (1.1) CL 3: 3.2 (1.0) CL 4: 3.1 (1.2) |
TBUT LF: 3.1 (1.7)* CL 1: 3.4 (1.3)* CL 2: 3.9 (0.6)* CL 3: 5.8 (2.8) CL 4: 6.7 (2.4)* |
Corneal + Conjunct LF: 0.9 (0.6) CL 1: 0.8 (0.6) CL 2: 0.8 (0.8) CL 3: 1.0 (1.2) CL 4: 1.0 (1.1) Note: Score 0–9 |
Cornea + Conjunct LF: 0.8 (0.6)* CL 1: 0.6 (0.6)* CL 2: 0.5 (0.7)* CL 3: 0.4 (1.0)* CL 4: 0.1 (0.3)* |
When MG loss is early and mild or moderate, several Tx options are available. When MG loss is severe, IPL is recommended. Safety NR |
|
Kim 2017 [53] Retrospective chart review USA |
N = 98 patients with MGD and DED (189 eyes), 60 years | Mean 77 days | Single 12-min LF Tx | NR | NR |
OSDI LF: 50.5 (25.1) |
OSDI LF: 41.4 (26.4)* |
TBUT LF: 4.5 (1.4) |
TBUT LF: 8.5 (1.7)* |
NR | NR |
LF Tx improves subjective and objective measures of DED. Safety NR |
|
Gibbons 2017 [59] Retrospective interventional case series USA |
N = 49 patients with symptomatic MGD (98 eyes); N = 32 patients who responded to Tx, 63 (15) years; N = 17 patients with no response to Tx, 62 (16) years |
3–4 months | Single 12-min LF Tx followed by 10 min heat (gel mask), lid hygiene, and MGX every 4–6 weeks for 3 times |
MGE Grade Score LF responded to Tx: 5.9 (1.9) LF no response to Tx: 5.6 (1.9) Note: grading not defined |
MGE Grade Score NR |
OSDI LF responded to Tx: 54.0 (24.3) LF no response to Tx: 57.6 (19.9) |
OSDI NR |
TBUT LF responded to Tx: 4.1 (3.0) LF no response to Tx: 4.6 (3.5) |
TBUT NR |
Corneal LF responded to Tx: 4.4 (5.0) LF no response to Tx: 0.4 (1.1) Conjunct LF responded to Tx: 3.1 (2.4) LF no response to Tx: 1.5 (1.8) |
Corneal NR† Conjunct NR† |
Patients with signs of lower tear production, higher corneal and conjunct staining scores, and higher osmolarity tend to respond better to LF AEs None |
|
Epitropoulos 2017 [54] Retrospective USA |
N = 59 patients with MGD (102 eyes), 62 (14) years; LF SS+: N = 23 patients (43 eyes); LF SS-: N = 36 patients (59 eyes) |
2 months | Single 12-min LF Tx |
MGSS LF SS +: 2.1 LF SS-: 2.4 |
MGSS LF SS+: 13.0* LF SS-: 15.9*† |
SPEED LF SS+: 17.9 LF SS-: 15.9 |
SPEED LF SS+: 12.5* LF SS-: 10.1* |
TBUT LF SS+: 3.7 LF SS-: 3.8 |
TBUT LF SS+: 9.6* LF SS-: 8.3* |
NR | NR |
LF Tx in patients SS+ and with MGD had significant improvement in signs and symptoms of dry eye. Improvement in MGS scores were > in SS- patients. Safety NR |
|
Liang 2015 [55] Retrospective case series China |
N = 48 patients with MGD (96 eyes) | 3 months | Single 12-min LF Tx |
MGSS LF: 6.2 (2.5) MGYLS LF: 2.9 (1.1) |
MGSS LF: 12.7 (3.4)* MGYLS LF: 5.2 (2.1)* |
OSDI LF: 45.4 (19.3) |
OSDI LF: 25.7 (14.1)* |
TBUT LF: 4.7 (2.3) |
TBUT LF: 9.9 (3.0)* |
Corneal + Conjunct LF: 2.4 (2.2) |
Corneal + Conjunct LF: 2.1 (1.1) |
LF is an effective and safe Tx for MGD AEs None No discomfort /pain, tearing, or irritation 1 day post Tx. No sign changes in VA or IOP |
Units of measure are as follows: MGSS = points, score 0–45; NIKBUT = seconds; OSDI = points, score 0–100; SPEED = points, score 0–28; staining = scale score; TBUT = seconds
Treatment manufacturers are as follows: Artia MG compressor (Katena Products/Corza Medical, Parsippany, NJ); Azithromycin eyedrops (Azimychin, Senju Pharmaceutical Co, Kobe, Japan); Haylene Eye Drop (Binex Pharmaceutical Corp, Incheon, Korea); M22 (Lumenis, Yokneam, Israel)
AEs adverse events, BID 2 times per day, BL baseline, CL control, conjunct conjunctival, FU follow-up, IPL intense pulsed light, LF LipiFlow, MG meibomian glands, MGD meibomian gland dysfunction, MGE meibomian gland expression, MGSS meibomian gland secretion score (secretion quality), MGX practitioner-administered manual meibomian gland expression, MGYLS meibomian glands yielding liquid secretion (glands with secretion capacity), min minutes, NIKBUT noninvasive keratograph break-up time, NR not reported, OSDI ocular surface disease index, SD standard deviation, sign significant, SPEED standard patient evaluation of eye dryness, SS ± Sjögren’s syndrome positive or negative, TBUT tear break-up time, Tx treatment, VA visual acuity
1Data only presented in a figure. We did not extrapolate the data from the figure to avoid presenting estimated data
*P < 0.05 vs. baseline
†P < 0.05 vs. comparator
Safety in MGD Populations
A total of ten RCTs evaluated the safety of LipiFlow when treating MGD, and the data are presented in Table 2. Of these, seven RCTs (359 LipiFlow-treated patients, 637 eyes) reported that there were no LipiFlow-related AEs or other safety concerns [23, 25, 28–30, 33, 34], and three RCTs (237 LipiFlow-treated patients, 438 eyes) reported LipiFlow-related safety events [14, 26, 27]. Namely, of 209 patients (410 eyes) treated with LipiFlow there were eight device-related AEs (eyelid pain [N = 3], conjunctival vascular injection [N = 1], blepharitis [N = 1], foreign body sensation [N = 2], and severe eye dryness [N = 1]) [14, 26]. Booranapong et al. reported that LipiFlow-related AEs of eye discomfort/pain only occurred among patients with small eyes, narrow palpebral fissure, or deep-set eyes (study evaluated 28 patients/eyes, and the number with AEs was not reported) [27]. Taken together, the RCT studies yield an AE rate of 0.76% (8 AEs in 1047 eyes, excluding Booranapong because the number of AEs was not reported). Slit-lamp findings not considered AEs were most frequently trace to mild conjunctival injection, edema, hyperemia, or redness; eyelid edema; petechial hemorrhages on the eyelid or conjunctiva; and superficial punctate keratitis (SPK) immediately or 1-day post-treatment [14, 34]. All slit-lamp findings resolved rapidly and without treatment. There were no reports of significant safety-related changes in IOP or VA. Lane et al. and Blackie et al. concluded that the safety profile of LipiFlow treatment reflects a low occurrence of non-serious, transient side effects that resolve quickly and do not require medical treatment [14, 34]. It should be noted that the control groups also had adverse safety events [14, 26, 28, 30].
Safety was reported in two prospective, controlled (non-randomized) studies (Table 3). One study with 20 LipiFlow-treated patients (19 eyes) reported that there were no AEs, the other study did not report AEs, and both studies reported no significant safety-related changes in IOP or VA (49 patients, 48 eyes) [36, 37].
Nine open-label studies, which included 420 LipiFlow-treated patients (816 eyes), evaluated the safety of LipiFlow when treating MGD, and the data are presented in Table 4. Of these, five open-label studies (163 LipiFlow-treated patients, 326 eyes) reported that there were no LipiFlow-related AEs or other safety concerns [13, 19, 40, 41, 47], and four open-label studies (257 LipiFlow-treated patients, 490 eyes) reported LipiFlow-related safety events [11, 39, 49, 50]. Namely, of 420 patients (816 eyes) treated with LipiFlow, there were 60 device-related AEs (irritation [N = 1], redness [N = 24], eyelid puffiness [N = 24], discomfort/pain [N = 4], and “other” [N = 7]) [11, 39, 49, 50]. Taken together, the open-label studies yield an AE rate of 7.35% (60 AEs in 816 eyes). Zhao et al. reported that all LipiFlow-treated patients (N = 24, 24 eyes) had eye redness and mild eyelid puffiness after treatment, which resolved after a few minutes (no additional information was reported) [50]. Blackie et al. also reported that all AEs were nonserious, transient, and resolved without treatment [11]. There were no reports of significant safety-related changes in IOP or VA. It is noteworthy that the control groups in the open-label studies also had adverse safety events [11, 39, 51]. Liu et al. reported that ten of 25 patients treated with a warm mask and massage had AEs of mild to severe pain/discomfort; while none of the LipiFlow-treated patients reported pain/discomfort. The authors concluded that LipiFlow was significantly more comfortable than the at-home treatment [39].
Four retrospective studies reported on the safety of LipiFlow treatment in 150 patients (277 eyes), and the data are presented in Table 5. Three of the four retrospective studies, which included 127 LipiFlow-treated patients (254 eyes), reported that there were no LipiFlow-related AEs or other safety concerns [55, 57, 59]. One study with 23 LipiFlow-treated patients (23 eyes) reported three AEs of gritty sensation with mild discharge[56]. Taken together, the retrospective studies yield an AE rate of 1.08% (three AEs in 277 eyes).
Cataract
Cataract surgery can cause or worsen MGD and is associated with loss of MG and deterioration of gland morphology [60–62]. In addition, DED in general can cause imprecision of biometry and keratometry readings resulting in suboptimal refractive results [63]. Accordingly, seven studies evaluated the effect of LipiFlow on 280 patients (271 eyes) undergoing cataract surgery, and the studies are described in Table 6. Three studies evaluated the effect of pre-operative LipiFlow treatment on intraocular lens (IOL) selection. One study of 31 patients (29 eyes) with MGD concluded that LipiFlow improved tear film parameters but had negligible effect on spherical IOL selection [63]. In contrast, two studies of 29 patients with MGD (36 eyes) concluded that LipiFlow treatment stabilized the tear film and improved the accuracy of the IOL power calculations especially the keratometry readings [64, 65]. Three studies evaluated 160 patients (156 eyes) with cataract and MGD, and one study evaluated 60 patients (60 eyes) with cataract and optional MGD; all four studies concluded that preoperative LipiFlow treatment prevented postoperative MGD related DED and improved postoperative ocular surface health [66–69]. Matossian et al. reported that LipiFlow treatment either before or after cataract surgery with implantation of an extended depth of focus IOL had beneficial effects on postoperative VA, halos, and signs and symptoms of MGD [67].
Table 6.
Summary of studies evaluating LipiFlow in patients undergoing cataract surgery or with treated glaucoma
| Author/design/country | Patients (eyes)/age, mean (SD) | Last FU after LF Tx | Study arms | MG function Mean (SD) |
Patient-reported symptoms Mean (SD) |
TBUT Mean (SD) |
Staining | Overall conclusion and safety | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||
| Cataract | ||||||||||||
|
Schlatter 2023 [63] Prospective, randomized, controlled, investigator-masked |
N = 31 patients with MGD and cataract (62 eyes), 44 (16) years; LF: N = 29 eyes; CL: N = 29 eyes |
3 months preop |
Single 12-min LF Tx before cataract surgery CL: untreated |
MGSS LF: 9.5 (5.2) CL: 9.9 (4.9) |
MGSS LF: 14.4 (9.1)* CL: 13.6 (10.5) |
NR | NR |
TBUT LF: 5.6 (2.7) CL: 4.9 (2.1) |
TBUT LF: 8.8 (4.4)* CL: 7.8 (4.4)* |
NR | NR |
Tear film parameters improved in LF eyes, but effect on spherical IOL selection was negligible AEs N = 1 (stye unrelated to LF) |
|
Mencucci 2023 [66] Prospective, unmasked, randomized controlled Italy |
N = 46 patients with cataract and mild/moderate MGD (46 eyes), LF: N = 23 patients (23 eyes), 73 (8) years CL: N = 23 patients (23 eyes), 75 (8) years |
1 month (pre-cataract surgery) 2 months (= 1 month post-cataract surgery) |
Single 12-min LF Tx CL: warm compress, massage BID for 1 month |
MGYLS LF: 7.3 (0.8) CL: 7.7 (0.6) MGSS LF: 0.65 (0.77) CL: 0.33 (0.56) |
MGYLS 1 month (preop): LF: 7.8 (0.51)* CL: 7.8 (0.4) 2 months (postop): LF: 7.9 (0.3)*† CL: 7.5 (0.7) MGSS 1 month (preop): LF: 0.21 (0.52)* CL: 0.21 (0.41) 2 months (postop): LF: 0.13 (0.34)*† CL: 0.54 (0.60) |
SPEED LF: 6.1 (2.8) CL: 5.8 (1.5) |
SPEED 1 month (preop): LF: 3.9 (2.2)*† CL: 5.1 (1.5) 2 months (postop): LF: 4.0 (1.8)*† CL: 6.0 (1.2) |
NIKBUT LF: 5.2 (1.3) CL: 5.7 (1.2) |
NIKBUT 1 month (preop): LF: 6.2 (1.7)* CL: 6.0 (1.2) 2 months (postop): LF: 6.3 (1.9)*† CL: 5.1 (1.5) |
Corneal LF: 0.26 (0.44) CL: 0.13 (0.33) |
Corneal 1 month (preop): LF: 0.13 (0.33) CL: 0.13 (0.33) 2 months (postop): LF: 0.13 (0.34) CL: 0.13 (0.33) |
Preop LF Tx prevented postcataract surgery DED in patients with mild-to-moderate MGD. LF-treated patients had a better ocular surface status than CL AEs None No pain or discomfort |
|
Matossian 2023 [67] Prospective, randomized, open-label, crossover, multicenter USA |
N = 121 patients with mild-to-moderate MGD and cataract; LF: N = 117 eyes, 65 (8) years; CL: N = 115 eyes, 65 (8) years |
3 months (3 months post-cataract surgery) CO: 1 month (4 months post-cataract surgery) |
Single 12-min LF Tx CL: untreated |
NR |
MGSS: Change from BL to 3 months (postop): LF: 7.3 (9.3)† CL: 4.7 (10.1) MGYLS Change from BL to 1 month postop: LF: 1.6 (3.1) CL: 1.1 (3.3) CO MGSS: Change from 3 to 4 months postop: LF: 4.1 (11.0)† |
NR |
SPEED: Change from BL to 3 months: LF: -2.1 (5.3) CL:-1.5 (5.6) CO SPEED: Change from 3 to 4 months postop: LF: − 1.2 (5.6) |
NR |
TBUT Change from BL to 1 month postop: LF: 0.69 (4.6) CL: 0.06 (3.7) |
NR |
Corneal Change from BL to 1 month postop: LF: − 0.57 (2.3)† CL: 0.20 (3.2) Conjunct Change from BL to 1 month postop: LF: − 1.2 (3.8)† CL: 0.45 (4.0) |
Presurgical LF Tx of patients implanted with range-of-vision IOLs improved MG function and postop ocular surface health. LF Tx reduced postop halos and improved VA AEs None related to LF, all related to cataract surgery |
|
Park 2021 [69] Prospective, randomized controlled study Korea |
N = 124 patients with cataract (MGD was not a requirement) (124 eyes); LF: N = 60 patients (60 eyes), 64 (9) years CL: N = 48 patients (48 eyes), 65 (12) years |
3 months postop |
Single 12-min LF Tx CL: untreated |
MGSS LF: 1.0 (0.9) CL: 0.9 (0.8) MGYLS LF: 6.0 (2.1) CL:6.6 (2.1) |
MGSS LF: 0.9 (0.9)*† CL: 1.7 (0.8)* MGYLS LF: 7.1 (1.7)*† CL: 5.6 (2.6)* |
OSDI LF: 37.9 (20.2) CL: 34.3 (20.1) |
OSDI LF: 22.3 (16.5)*† CL: 29.8 (20.8) |
TBUT LF: 3.5 (1.5) CL: 3.7 (1.5) |
TBUT LF: 4.4 (1.8)*† CL: 3.6 (1.6) |
Corneal LF:0.77 (0.90) CL: 0.68 (0.92) |
Corneal LF: 0.46 (0.56)* CL: 0.62 (0.56) |
LF Tx conducted prior to cataract surgery may be safe and effective for relieving MGD and dry eye induced by surgery AEs None. No pain at insertion, treatment, or removal |
|
Zhao Y 2021 [68] Prospective, examiner-masked controlled, contralateral eye China |
N = 32 patients with MGD and cataract (64 eyes); N = 16 patients undergoing cataract surgery, LF: N = 16 eyes, CL: N = 16 eyes, 62 (10) years; N = 16 patients no surgery (control), LF: N = 16 eyes, CL: N = 16 eyes, 62 (2) years In both groups LF in 1 eye and untreated contralateral eye |
3 months |
Single 12-min LF Tx CL: untreated |
MGYLS LF/surgery: 2.1 (1.8) CL/surgery: 1.9 (1.8) LF/non-surgery: 1.8 (1.8) CL/non-surgery: 2.6 (2.5) |
MGYLS median change from BL: LF/surgery: 4.0* CL/surgery: − 2.0 LF/non-surgery: 3.0* CL/non-surgery: 0.0 (P < 0.05 vs. CL/surgery) |
SPEED xx1 |
SPEED xx1 |
TBUT LF/surgery: 5.0 (2.3) CL/surgery: 4.9 (2.6) LF/non-surgery: 2.5 (0.6) CL/non-surgery: 2.8 (1.1) |
TBUT median change from BL: LF/surgery: − 2.5 CL/surgery: − 2.0 LF/non-surgery: 1.0 (P < 0.05 vs. LF/surgery) CL/non-surgery: 0.0 (P < 0.05 vs. CL/surgery) |
Corneal LF/surgery: 3.1 (0.4) CL/surgery: 3.2 (0.5) LF/non-surgery: 2.0 (0.5) CL/non-surgery: 1.9 (0.8) |
Corneal xx1 |
LF Tx before cataract surgery is effective in diminishing the blockage of MG and preventing a decline of TBUT after cataract surgery. Safety NR |
|
Szabelska 2023 [64] Open-label case series Poland |
N = 6 patients with MGD and cataract (11 eyes), 67 years | 6 weeks | Single 12-min LF Tx 6 weeks pre-cataract surgery | NR | NR |
OSDI LF: 31.1 |
OSDI LF: 22.7 |
TBUT LF: 9.1 |
TBUT LF: 13.9 |
NR | NR |
Stabilization of visual system parameters after LF improved accuracy of IOL power recommendation. Safety NR |
|
Matossian 2020 [65] Single-center, pilot, retrospective USA |
N = 23 patients with MGD and cataract (25 eyes), 73 (8) years | 6 weeks | Single 12-min LF Tx 6 weeks pre- cataract surgery | NR | NR | NR | NR | NR | NR | NR | NR |
LF Tx resulted in less residual refractive astigmatism compared with pre-LF keratometry readings, which altered astigmatism management/ IOL selection. Safety NR |
| Glaucoma | ||||||||||||
|
Kasetsuwan 2020 [70] Prospective, randomized, observer-blind Thailand |
N = 60 patients with well-controlled glaucoma and MGD; LF: N = 26 patients (26 eyes), 67 (11) years; CL: N = 22 patients (22 eyes), 70 (8) years |
6 months |
Single 12-min LF Tx plus lid hygiene CL: lid hygiene: warm compress, massage, baby shampoo, BID for 6 months |
MGSS LF: 21.5 (5.8) CL: 22.5 (5.9) |
MGSS change from BL: LF: 4.7* CL: 3.0* |
OSDI LF: 23.7 (11.8) CL: 26.8 (18.2) |
OSDI change from BL: LF: − 7.8* CL: -11.8* |
TBUT LF: 5.4 (3.7) CL: 6.0 (5.4) |
TBUT Change from BL: LF: − 0.30 CL:-0.58 |
Corneal LF: 1.3 (0.8) CL: 1.0 (0.6) |
Corneal Change from BL: LF: 0 CL: 0 |
Adding LF Tx to lid hygiene significantly improved MG function and symptoms at 6 months AEs LF: N = 7 patients (N = 1 difficulty inserting the device, N = 5 mild discomfort immediately after Tx, N = 1 mild conjunctival injection CL: N = NR discomfort during massage. No IOP elevation or uncontrolled IOP that required additional antiglaucoma Tx |
Units of measure are as follows: MGSS = points, score 0–45; MGYLS = number of functional glands; NIKBUT = seconds; OSDI = points, score 0–100; SANDE = visual analog scale score, 0–100; SPEED = points, score 0–28; staining = scale score; TBUT = seconds; VAS = scale score
AEs adverse events, BID 2 times per day, BL baseline, CL control, CO crossover group, conjunct conjunctival, D diopters, DED dry eye disease, FU follow-up, IOL intraocular lens, LF LipiFlow, MG meibomian glands, MGSS number of expressible meibomian glands, MGYLS meibomian glands secretion score, min minutes, NIKBUT noninvasive keratograph break-up time, NR not reported, OSDI ocular surface disease index, pre pretreatment, post post-treatment, SD standard deviation, SPEED standard patient evaluation of eye dryness, TBUT tear break-up time, Tx treatment, VA visual acuity
*P ≤ 0.05 vs. baseline
†P ≤ 0.05 vs. control
1Data only presented in a figure. We did not extrapolate the data from the figure to avoid presenting estimated data
Safety in Cataract Studies
Four studies evaluated the safety of LipiFlow when treating MGD in patients with cataract (235 patients, 229 eyes), and the data are presented in Table 6. There were no AEs, pain, or safety concerns related to LipiFlow treatment [63, 66, 67, 69].
Glaucoma
Many patients being treated for glaucoma have MGD, which may be attributed to polypharmacy management [70]. Only one glaucoma study was identified, and it evaluated the effect of LipiFlow on patients with well-controlled glaucoma and MGD (Table 6). The study compared LipiFlow plus eyelid hygiene (26 patients, 26 eyes) to warm compress, massage, and eyelid hygiene (22 patients, 22 eyes) [70]. Both groups similarly improved MG function and symptoms at 6 months. According to the authors, this is the first study to evaluate LipiFlow in patients using long-term anti-glaucoma medications. LipiFlow was safe to use in this population; treatment did not elevate IOP or cause the need for additional antiglaucoma treatment. There were seven AEs (difficulty inserting the device [N = 1], mild discomfort immediately after treatment [N = 5], and mild conjunctival injection [N = 1].
Refractive Surgery
Refractive surgery can cause DED; therefore, it is important to identify MGD as part of the preoperative work-up [71]. Three studies and one case report evaluated the effect of LipiFlow on 91 patients (177 eyes) pre- or post-refractive surgery (Table 7). One study in 32 patients (64 eyes) treated with LipiFlow preoperatively concluded that LipiFlow prior to refractive surgery can help lessen the symptoms of dry eyes after refractive surgery and may reduce the patient’s dependence on ocular surface lubrication after refractive surgery [71]. Two studies evaluated the effect of postoperative LipiFlow in 58 patients (111 eyes) post-LASIK or photorefractive keratectomy (PRK) and concluded that LipiFlow improved signs and symptoms of MGD 3 months post-treatment [72, 73]. A case report demonstrated that LipiFlow was an effective therapy 4 years post-LASIK in a patient with MGD and severe LASIK-induced dry eye [73]. Safety was not evaluated in any of the studies.
Table 7.
Summary of studies evaluating LipiFlow in patients undergoing refractive surgery
| Author/design/country | Patients (eyes)/mean age (SD) | Last FU | Study arms | MG function Mean (SD) |
Patient-reported outcomes Mean (SD) |
NITBUT/TBUT Mean (SD) |
Staining | Overall conclusion and safety | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||
|
Shetty 2023 [71] Prospective interventional India |
N = 59 patients with mild-to-moderate evaporative DED and/or MGD undergoing refractive surgery, LF pre-LASIK: N = 32 patients (64 eyes), 28 (5) years; LF post-LASIK: N = 27 patients (52 eyes), 27 (5) years |
3 months |
Single 12-min LF Tx pre-LASIK CL: Single 12-min LF Tx 3 months post-LASIK |
NR | NR |
OSDI LF pre-LASIK group preop: 18.0 (2.2) LF post-LASIK group preop: 4.5 (0.9) |
OSDI LF pre-LASIK group postop: 9.5 (1.6)* LF post-LASIK group pre-LF postop: 35.2 (2.3)* LF post-LASIK group post-LF & postop: 17.4 (2.6)*† |
TBUT LF pre-LASIK group preop: 6.9 (0.2) LF post-LASIK group preop: 8.0 (0.1) |
TBUT LF pre-LASIK group postop: 8.3 (0.2)* LF post-LASIK group pre-LF postop: 7.0 (1.5)* LF post-LASIK group post-LF & postop: 9.4 (0.2)*† |
NR | NR |
LF Tx prior to refractive surgery can help lessen the clinical effects of dry eyes after refractive surgery. Safety NR |
|
Schallhorn 2017 [72] Retrospective United Kingdom |
N = 57 patients with dry eye post-LASIK or PRK (109 eyes), 49 years | Various: FU reported under each outcome |
Single 12-min LF Tx Note: LF Tx mean 40.5 months after LASIK/PRK |
MGD Grade Score NR Note: graded 0–4 |
MGD Grade Score LF: Change from BL: − 0.69* Note: BL is pre-LF but post-LASIK/PRK |
SPEED II LF: 17.5 |
SPEED II LF: 10.2* Note: FU mean 25 days |
TBUT LF: 3.7 Note: value pre-LF but post-LASIK/PRK |
TBUT LF: 5.75* Note: FU mean 89 days |
Corneal NR |
Corneal LF: change from BL -0.74* Note: BL is pre-LF but post-LASIK/PRK |
LF Tx may have utility in managing dry eye symptoms following laser vision correction. Safety NR |
|
Petzold 2016 [73] Case report Germany |
N = 1 patient (2 eyes) 4 years post-LASIK with severe dry eye | 18 months | Single 12-min LF Tx |
MGYLS LF OD: 8 LF OS: 10 |
MGYLS LF OD: 12 LF OS: 13 |
SPEED LF: 20 |
SPEED LF: 12 |
TBUT LF OD: < 5 LF OS: < 5 |
TBUT LF OD: 8 LF OS: 14 |
Corneal LF OD: 2–3 LF OS: 1 |
Corneal LF OD: 0 LF OS: 0 |
LF Tx successfully treated a case of post-LASIK dry eye that was refractory to conventional treatment. Safety NR |
Units of measure are as follows: MGYLS = number of functional glands; OSDI = points, score 0–100; SPEED = points, score 0–28; staining = scale score; TBUT = seconds
AEs adverse events, BL baseline, CL control, DED dry eye disease, FU follow-up, LASIK laser-assisted in situ keratomileusis, LF LipiFlow, MG meibomian glands, MGD meibomian gland dysfunction, MGYLS meibomian glands yielding liquid secretion (glands with secretion capacity), min minutes, NR not reported, OD right eye, OS left eye, OSDI ocular surface disease index, post post-treatment, postop postoperation, pre pretreatment, preop preoperation, PRK photorefractive keratectomy, SD standard deviation, SPEED standard patient evaluation of eye dryness, TBUT tear break-up time, Tx treatment
1Data only presented in a figure. We did not extrapolate the data from the figure to avoid presenting estimated data
*P < 0.05 vs. baseline
†P < 0.05 vs. LipiFlow post-LASIK group, pre-LipiFlow
Chalazion
One case report was identified that used LipiFlow as part of the treatment of chalazion with associated hyperopia. LipiFlow treatment was performed 1 month after chalazion incision and drainage (note: LipiFlow is contraindicated within 3 months of ocular surgery). After LipiFlow treatment, corneal epithelial thickness and topographic changes normalized, and VA improved toward emmetropia [74].
Discussion
The present study is the most comprehensive clinical analysis of LipiFlow in patients with MGD to date. The scientific literature obtained from 2010 (first clinical study published) through March 2024, includes 2101 patients and 3521 eyes treated with LipiFlow. Of these, effectiveness was evaluated in 2041 patients and 3401 eyes, and safety was evaluated in 1448 patients and 2443 eyes. Taken together the studies demonstrate that a single 12-min treatment with LipiFlow safely improves both subjective (patient-reported outcomes) and objective (MG function, tear production, and ocular staining) measures of MGD, and the benefits persist up to 3 years. This conclusion is supported by meta-analyses. One meta-analysis included 10 RCTs (401 LipiFlow-treated patients) published through January 2021 [75]. The analysis concluded that LipiFlow treatment can improve subjective and objective measures of MGD without an increase in AEs. Another meta-analysis included four RCTs (199 LipiFlow-treated patients) published between 2012 and 2016, and concluded that LipiFlow treatment was more efficacious than at-home treatments [76]. Corroborating with the study conclusion, members of the Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II reviewed the literature of dry eye therapies, and using the modified American Academy of Ophthalmology (AAO) Preferred Practices guidelines they developed consensus guidelines [3]. The staged management and treatment recommendations for DED with MGD instruct the physician to consider treatment with LipiFlow if first-line treatments are inadequate. Finally, a recent meta-analysis applying Cochrane methodology included 13 clinical trials and concluded that a single in-office LipiFlow treatment for overall DED was not inferior to compliant, daily, ongoing use of other DED therapies (for the signs and symptoms of overall DED) [77].
In addition, the data extracted during this review demonstrate that LipiFlow treatment may be a safe and effective preoperative intervention for alleviating and preventing the MGD and dry eye associated with ocular surgery. Many patients who have MGD prior to refractive surgery continue to have it post-refractive surgery [72]. Also, there is an increased incidence of MG dysfunction or evaporative dry eye post-refractive or cataract surgery [61, 72]. To this end, the American Society of Cataract and Refractive Surgery (ASCRS) Cornea Clinical Committee published a consensus-based practical diagnostic ocular surface disease algorithm. The algorithm includes treatment of MGD before cataract and refractive surgery and mentions the use of LipiFlow as a viable treatment option [7]. LipiFlow is recommended not only for patients with preoperative MGD but also for those without baseline MGD [67, 69].
It is important to note that while some studies showed that eyelid hygiene, warm compress, and massage had a similar benefit to LipiFlow [27, 32, 35, 49, 50, 58], these treatments were needed 2–3 times per day for several months to be comparable to a single 12-min treatment with LipiFlow. Patients report that eyelid hygiene BID is inconvenient and eyelid massage is uncomfortable [70]. Also, compliance declines over time [51], and patients report that they forget to do it [70]. Lifitegrast ophthalmic solution 5% plus warm compress and eyelid compression BID was shown to be similarly effective or better than LipiFlow for the treatment of DED; however, the treatment required twice daily administration for 42 days compared with a single treatment with LipiFlow [33]. The advantage of LipiFlow treatment is the long duration of improvement in signs and symptoms of MGD after a single in-office visit without the complications of repeated intervention use as well as compliance and overall patient experience [11, 40, 44–46, 48]. MGD is a chronic disease, so ideally treatment would be effective for at least 3 months to avoid pain and discomfort associated with frequent manual eyelid massage [39]. Depending upon the patient’s lifestyle and symptom severity, and due to the chronic nature of MGD, LipiFlow retreatment is likely to be necessary. One study evaluated the sustained improvement of a single LipiFlow treatment, and the participants self-assessed the adequacy of symptom relief 3–12 months post-treatment [11]. Additional dry eye treatment was requested by 3.8% (7/186) of patients at 6 months, and another 8.6% (16/186) of patients requested dry eye treatment at 12 months.
Aside from compliance issues, it is also difficult for patients to get the warm compress into the therapeutic temperature range for a duration long enough to liquify the meibum without causing thermal damage. Lipid delivery increases as eyelid temperature increases from 25 to 45 °C [8]. LipiFlow can effectively elevate the inner surface temperatures to achieve the targeted therapeutic threshold for melting obstructed meibum [8, 78]. A study was conducted that evaluated the temperature of the palpebral conjunctiva following four methods of heat therapy, each used according to the manufacturers’ instructions; namely, Bruder mask (Bruder Healthcare, Alpharetta, GA, USA), Blephasteam (Théa, Clermont-Ferrand, France), MiBoFlo (Mibo Medical, Dallas, TX, USA), and LipiFlow in a single patient with MGD. It has been shown that effective liquefaction of diseased MG requires > 40 °C of heat [8]. The study concluded that only LipiFlow was able to increase the temperature of the palpebral conjunctiva to > 40 °C [78]. Raising the temperature increases the risk of discomfort, thermal injury to the eyelid skin, and vulnerability of corneal tissue deformation [78, 79]. Despite these risks, the absence of device-related AEs in 20 studies (904 patients, 1465 eyes) confirms the low-risk safety profile of the LipiFlow. The majority of studies evaluating safety reported no discomfort or pain associated with LipiFlow treatment, which supports the patient acceptability of LipiFlow therapy. In contrast, the effectiveness of manual expression is limited by the pain induced by the forces required to express the gland [80]. According to Booranapong et al., eye discomfort/pain during LipiFlow treatment may occur primarily among patients with small eyes, narrow palpebral fissure, or deep-set eyes [27]. All AEs related to LipiFlow were transient and did not require treatment [11, 14, 34]. No studies reported serious AEs. No study reported any safety event that was unanticipated per the Instructions for Use [18]. This safety profile was additionally validated in a recent review [77].
This literature evaluation has some limitations. The results of RCTs, especially double-masked studies, are more reliable than those of other experimental designs. However, it is not possible to mask the patient due to the physical sensation of heat and directional massage, so studies were observer-masked only. A placebo effect may have confounded improvements in subjective symptoms. Nonetheless, patients consider improvement in subjective symptoms the most important measure, and the studies show improvements in patient-reported outcomes. The newer studies evaluating LipiFlow are RCTs, while the older studies are mostly open-label. Older open-label studies can have a placebo effect particularly because LipiFlow was a novel treatment. The aims of older studies were to determine the effectiveness and safety of LipiFlow treatment and also to understand how to select patients for treatment based on baseline characteristics to improve chances of success [51]. Retrospective studies have potential biases such as a lack of rigorous and impartial clinical evaluation at the follow-up visit, and lack of a control group, masking, and randomization.
There are many options to treat MGD. Accordingly, LipiFlow effectiveness and safety were compared to 14 different treatments (plus numerous combinations) and was shown repeatedly to be an effective and safe treatment option for MGD. Further, LipiFlow is the standard by which other devices that provide heat and massage aspire for noninferiority or superiority [23–26, 28]. This supports the industry acceptance of the effectiveness and safety of LipiFlow.
While everyone is at risk for MGD, there are some populations that are contraindicated according to the product labelling. LipiFlow is contraindicated in patients who within the prior 3 months have had ocular surgery, ocular injury, ocular herpes, eyelid herpes, active ocular inflammation, or history of chronic recurrent ocular inflammation (e.g., retinitis, macular inflammation, choroiditis, uveitis, iritis, scleritis, episcleritis, and keratitis); have an active ocular infection; have eyelid abnormalities that affect lid function; or have an ocular surface abnormality that may compromise corneal integrity (e.g., prior chemical burn, recurrent corneal erosion, corneal epithelial defect, Grade 3 corneal fluorescein staining, or map dot fingerprint dystrophy) [18]. Patients who are not suitable for LipiFlow will need to use alternative treatments. Another barrier to use of LipiFlow is lack of patient access since not all offices have the device. It should be noted that in the past, when LipiFlow was the only device of its kind, the cost of LipiFlow was a barrier to the patient. However, current LipiFlow treatment costs are competitive with other single-use in-office modalities with similar mechanisms of action {Mukamal, 2020 #613}. Also, the current out-of-pocket cost of a single in-office LipiFlow treatment can be significantly less than the total yearly cost of daily multi-use modalities (including over-the-counter and prescription eye drops) that are prescribed to treat MGD/DED {Nau, 2024 #612}. The lack of evaluation of off-label use of combination therapies for ocular surface disease is a gap in the current knowledge. The majority of research evaluates single therapies; however, in the real-world, patients have multiple conditions that need treatment simultaneously.
Conclusions
Despite the limitations inherent to each individual study, the conclusions of this comprehensive literature review are supported by the diversity of the patient populations (geography, race, disease severity, and diagnosis), the large population treated with LipiFlow (2101 patients and 3521 eyes), the meta-analyses, and that this review analyzed all published clinical studies to date. The data obtained from 55 unique clinical studies conducted globally overwhelmingly show that LipiFlow is effective and safe for the treatment of DED associated with MGD. This comprehensive review provides the totality of usable clinical evidence and should be updated every 5 years to re-evaluate the effectiveness and safety of LipiFlow.
Author Contributions
Caroline A. Blackie and David Murakami delivered the study concept and design overview; David Murakami performed the literature search and initial literature screening; Heather S. Oliff performed the data extraction and analysis; Heather S. Oliff drafted the article; and Eric Donnenfeld, Caroline A. Blackie, and David Murakami critically revised the work. All authors read and approved the final manuscript.
Funding
This study was sponsored by Johnson & Johnson Surgical Vision Inc., Irvine, CA, USA. Publication costs, including the journal’s Rapid Service Fee, was funded by Johnson & Johnson Surgical Vision.
Data Availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of Interest
Heather S. Oliff declares that she has no competing interests. Caroline A. Blackie and David Murakami are employees of Johnson & Johnson Surgical Vision. Eric Donnenfeld is a consultant to Johnson & Johnson Surgical Vision.
Ethical Approval
This review is based on previously conducted studies and does not contain any new studies with human participants or animals that were performed by any of the authors. Consent for publication was obtained from the individual presented in Fig. 2.
References
- 1.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):93-107. [DOI] [PubMed]
- 2.Nichols KK. The international workshop on meibomian gland dysfunction: introduction. Invest Ophthalmol Vis Sci. 2011;52(4):1917–1921. doi: 10.1167/iovs.10-6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackie CA, Folly E, Ruppenkamp J, Holy C. Prevalence of meibomian gland dysfunction—a systematic review and analysis of published evidence. IOVS. 2019;60(9):2736. [Google Scholar]
- 6.Blackie CA, Korb DR, Knop E, Bedi R, Knop N, Holland EJ. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010;29(12):1333–1345. doi: 10.1097/ICO.0b013e3181d4f366. [DOI] [PubMed] [Google Scholar]
- 7.Starr CE, Gupta PK, Farid M, Beckman KA, Chan CC, Yeu E, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669–684. doi: 10.1016/j.jcrs.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Borchman D, Foulks GN, Yappert MC, Bell J, Wells E, Neravetla S, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(6):3805–3817. doi: 10.1167/iovs.10-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCulley JP, Sciallis GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977;84(6):788–793. doi: 10.1016/0002-9394(77)90497-4. [DOI] [PubMed] [Google Scholar]
- 10.Henriquez AS, Korb DR. Meibomian glands and contact lens wear. Br J Ophthalmol. 1981;65(2):108–111. doi: 10.1136/bjo.65.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackie CA, Coleman CA, Holland EJ. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol. 2016;10:1385–1396. doi: 10.2147/OPTH.S109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12(3):1397–1418. doi: 10.1007/s40123-023-00669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korb DR, Blackie CA. Restoration of meibomian gland functionality with novel thermodynamic treatment device—a case report. Cornea. 2010;29(8):930–933. doi: 10.1097/ICO.0b013e3181ca36d6. [DOI] [PubMed] [Google Scholar]
- 14.Lane SS, DuBiner HB, Epstein RJ, Ernest PH, Greiner JV, Hardten DR, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396–404. doi: 10.1097/ICO.0b013e318239aaea. [DOI] [PubMed] [Google Scholar]
- 15.Lam PY, Shih KC, Fong PY, Chan TCY, Ng AL, Jhanji V, et al. A review on evidence-based treatments for meibomian gland dysfunction. Eye Contact Lens. 2020;46(1):3–16. doi: 10.1097/ICL.0000000000000680. [DOI] [PubMed] [Google Scholar]
- 16.Kawahara A. Treatment of Dry Eye Disease (DED) in Asia: strategies for short tear film breakup time-type DED. Pharmaceutics. 2023;15(11):2591. doi: 10.3390/pharmaceutics15112591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villani E, Marelli L, Dellavalle A, Serafino M, Nucci P. Latest evidences on meibomian gland dysfunction diagnosis and management. Ocul Surf. 2020;18(4):871–892. doi: 10.1016/j.jtos.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 18.LipiFlow Thermal Pulsation System Instructions for Use. Johnson & Johnson Vision Care; 2021.
- 19.Hu JG, Dang VT, Chang DH, Goldberg DF, McKinnon C, Makedonsky K, et al. Performance of a translucent activator for LipiFlow Vectored Thermal Pulse (VTP) treatment of meibomian gland dysfunction. Clin Ophthalmol. 2022;16:963–971. doi: 10.2147/OPTH.S354738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackie CA, Carlson AN, Korb DR. Treatment for meibomian gland dysfunction and dry eye symptoms with a single-dose vectored thermal pulsation: a review. Curr Opin Ophthalmol. 2015;26(4):306–313. doi: 10.1097/ICU.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 21.Kable AK, Pich J, Maslin-Prothero SE. A structured approach to documenting a search strategy for publication: a 12 step guideline for authors. Nurse Educ Today. 2012;32(8):878–886. doi: 10.1016/j.nedt.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Meng Z, Chu X, Zhang C, Liu H, Yang R, Huang Y, et al. Efficacy and safety evaluation of a single thermal pulsation system treatment (LipiFlow®) on meibomian gland dysfunction: a randomized controlled clinical trial. Int Ophthalmol. 2023;43(4):1175–1184. doi: 10.1007/s10792-022-02516-x. [DOI] [PubMed] [Google Scholar]
- 23.Wesley G, Bickle K, Downing J, Fisher B, Greene B, Heinrich C, et al. Comparison of two thermal pulsation systems in the treatment of meibomian gland dysfunction: a randomized, multicenter study. Optom Vis Sci. 2022;99(4):323–332. doi: 10.1097/OPX.0000000000001892. [DOI] [PubMed] [Google Scholar]
- 24.Holland EJ, Loh J, Bloomenstein M, Thompson V, Wirta D, Dhamdhere K. A comparison of TearCare and LipiFlow systems in reducing dry eye disease symptoms associated with meibomian gland disease. Clin Ophthalmol. 2022;16:2861–2871. doi: 10.2147/OPTH.S368319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Yang K, Wang J, Li S, Zhu L, Feng J, et al. Effect of a novel thermostatic device on meibomian gland dysfunction: a randomized controlled trial in Chinese patients. Ophthalmol Ther. 2022;11(1):261–270. doi: 10.1007/s40123-021-00431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta PK, Holland EJ, Hovanesian J, Loh J, Jackson MA, Karpecki PM, et al. TearCare for the treatment of meibomian gland dysfunction in adult patients with dry eye disease: a masked randomized controlled trial. Cornea. 2022;41(4):417–426. doi: 10.1097/ICO.0000000000002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booranapong W, Prabhasawat P, Chotikavanich S, T S, Naranunn P, Thaweerattanasilp W, et al. Comparison of an automated thermodynamic treatment system (LipiFlow) and warm compresses for the treatment of moderate severity of meibomian gland dysfunction. Siriraj Med J. 2020;72(1):79–86. doi: 10.33192/Smj.2020.11. [DOI] [Google Scholar]
- 28.Tauber J, Owen J, Bloomenstein M, Hovanesian J, Bullimore MA. Comparison of the iLUX and the LipiFlow for the treatment of meibomian gland dysfunction and symptoms: a randomized clinical trial. Clin Ophthalmol. 2020;14:405–418. doi: 10.2147/OPTH.S234008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J-ZZM. Comparative study between the meibomian pulsation system and the warm compress treatment for MGD. Guoji Yanke Zazhi. 2018;18(7):1324–1328. [Google Scholar]
- 30.Hagen KB, Bedi R, Blackie CA, Christenson-Akagi KJ. Comparison of a single-dose vectored thermal pulsation procedure with a 3-month course of daily oral doxycycline for moderate-to-severe meibomian gland dysfunction. Clin Ophthalmol. 2018;12:161–168. doi: 10.2147/OPTH.S150433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finis D, Konig C, Hayajneh J, Borrelli M, Schrader S, Geerling G. Six-month effects of a thermodynamic treatment for MGD and implications of meibomian gland atrophy. Cornea. 2014;33(12):1265–1270. doi: 10.1097/ICO.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 32.Finis D, Hayajneh J, Konig C, Borrelli M, Schrader S, Geerling G. Evaluation of an automated thermodynamic treatment (LipiFlow®) system for meibomian gland dysfunction: a prospective, randomized, observer-masked trial. Ocul Surf. 2014;12(2):146–154. doi: 10.1016/j.jtos.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Tauber J. A 6-week, prospective, randomized, single-masked study of Lifitegrast ophthalmic solution 5% versus thermal pulsation procedure for treatment of inflammatory meibomian gland dysfunction. Cornea. 2020;39(4):403–407. doi: 10.1097/ICO.0000000000002235. [DOI] [PubMed] [Google Scholar]
- 34.Blackie CA, Coleman CA, Nichols KK, Jones L, Chen PQ, Melton R, et al. A single vectored thermal pulsation treatment for meibomian gland dysfunction increases mean comfortable contact lens wearing time by approximately 4 hours per day. Clin Ophthalmol. 2018;12:169–183. doi: 10.2147/OPTH.S153297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumann A, Cochener B. Meibomian gland dysfunction: a comparative study of modern treatments. J Fr Ophtalmol. 2014;37(4):303–312. doi: 10.1016/j.jfo.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Dierker DS, Hauswirth SG. Thermal pulsation with or without dexamethasone intracanalicular insert for meibomian gland dysfunction: a prospective, masked trial. Clin Ophthalmol. 2022;16:1477–1485. doi: 10.2147/OPTH.S359719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laufenbock C. Thermal pulsation system (LipiFlow®) for treatment of meibomian gland dysfunction (MGD) from the perspective of an ophthalmologist in private practice. Ophthalmologie. 2022;119(6):605–610. doi: 10.1007/s00347-021-01541-y. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Xie J, Li J, Fu Y, Lin X, Wang S, et al. Evaluation of monocular treatment for meibomian gland dysfunction with an automated thermodynamic system in elderly Chinese patients: a contralateral eye study. J Ophthalmol. 2016;2016:9640643. doi: 10.1155/2016/9640643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W, Lin T, Gong L. Meibomian gland dysfunction patients benefit in ocular parameters and tear chemokines after thermal pulsation treatment. Int J Med Sci. 2023;20(1):11–22. doi: 10.7150/ijms.76603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novo-Diez A, Lopez-Miguel A, Fernandez I, Blanco-Vazquez M, Valencia-Sandonis C, Enriquez-de-Salamanca A, et al. Effect of a single vectored thermal pulsation treatment of meibomian gland dysfunction patients under controlled environmental conditions. Sci Rep. 2022;12(1):16761. doi: 10.1038/s41598-022-20994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fallah S, Loer CJ. Effects of vectored thermal pulsation on objective tear film measures. Cornea. 2021;40(12):1594–1599. doi: 10.1097/ICO.0000000000002714. [DOI] [PubMed] [Google Scholar]
- 42.Chan AYY, Chuang JC, Wong VWY. Evaluation of meibomian gland dysfunction among ophthalmic healthcare workers. Clin Ophthalmol. 2021;15:1201–1206. doi: 10.2147/OPTH.S299338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B, Fu H, Liu T, Xu M. Comparison of the therapeutic effect of Meibomian Thermal Pulsation LipiFlow® on obstructive and hyposecretory meibomian gland dysfunction patients. Int Ophthalmol. 2020;40(12):3469–3479. doi: 10.1007/s10792-020-01533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godin MR, Stinnett SS, Gupta PK. Outcomes of thermal pulsation treatment for dry eye syndrome in patients with Sjögren's disease. Cornea. 2018;37(9):1155–1158. doi: 10.1097/ICO.0000000000001621. [DOI] [PubMed] [Google Scholar]
- 45.Greiner JV. Long-term (3 year) effects of a single thermal pulsation system treatment on meibomian gland function and dry eye symptoms. Eye Contact Lens. 2016;42(2):99–107. doi: 10.1097/ICL.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 46.Greiner JV. Long-term (12-month) improvement in meibomian gland function and reduced dry eye symptoms with a single thermal pulsation treatment. Clin Exp Ophthalmol. 2013;41(6):524–530. doi: 10.1111/ceo.12033. [DOI] [PubMed] [Google Scholar]
- 47.Korb DR, Blackie CA. Case report: a successful LipiFlow treatment of a single case of meibomian gland dysfunction and dropout. Eye Contact Lens. 2013;39(3):e1–e3. doi: 10.1097/ICL.0b013e31824ccbda. [DOI] [PubMed] [Google Scholar]
- 48.Greiner JV. A single LipiFlow® Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res. 2012;37(4):272–278. doi: 10.3109/02713683.2011.631721. [DOI] [PubMed] [Google Scholar]
- 49.Friedland BR, Fleming CP, Blackie CA, Korb DR. A novel thermodynamic treatment for meibomian gland dysfunction. Curr Eye Res. 2011;36(2):79–87. doi: 10.3109/02713683.2010.509529. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Veerappan A, Yeo S, Rooney DM, Acharya RU, Tan JH, et al. Clinical trial of thermal pulsation (LipiFlow) in meibomian gland dysfunction with preteatment meibography. Eye Contact Lens. 2016;42(6):339–346. doi: 10.1097/ICL.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satjawatcharaphong P, Ge S, Lin MC. Clinical outcomes associated with thermal pulsation system treatment. Optom Vis Sci. 2015;92(9):e334–e341. doi: 10.1097/OPX.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 52.Hura AS, Epitropoulos AT, Czyz CN, Rosenberg ED. Visible meibomian gland structure increases after vectored thermal pulsation treatment in dry eye disease patients with meibomian gland dysfunction. Clin Ophthalmol. 2020;14:4287–4296. doi: 10.2147/OPTH.S282081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim MJ, Stinnett SS, Gupta PK. Effect of thermal pulsation treatment on tear film parameters in dry eye disease patients. Clin Ophthalmol. 2017;11:883–886. doi: 10.2147/OPTH.S136203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epitropoulos AT, Goslin K, Bedi R, Blackie CA. Meibomian gland dysfunction patients with novel Sjögren's syndrome biomarkers benefit significantly from a single vectored thermal pulsation procedure: a retrospective analysis. Clin Ophthalmol. 2017;11:701–706. doi: 10.2147/OPTH.S119926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang Q, Liu H, Guo Y, Cui R, Li B, Wang N, et al. Clinical evaluation of a thermodynamic treatment system for meibomian gland dysfunction. Zhonghua Yan Ke Za Zhi. 2015;51(12):924–931. [PubMed] [Google Scholar]
- 56.Chung HS, Rhim JW, Park JH. Combination treatment with intense pulsed light, thermal pulsation (LipiFlow), and meibomian gland expression for refractory meibomian gland dysfunction. Int Ophthalmol. 2022;42(11):3311–3319. doi: 10.1007/s10792-022-02330-5. [DOI] [PubMed] [Google Scholar]
- 57.Kim HJ, Park JH. Clinical efficacy of immediate manual meibomian gland expression after thermal pulsation (LipiFlow) for obstructive meibomian gland dysfunction: comparison with thermal pulsation. Cornea. 2020;39(8):975–979. doi: 10.1097/ICO.0000000000002328. [DOI] [PubMed] [Google Scholar]
- 58.Arita R, Fukuoka S, Kawashima M. Proposed algorithm for management of meibomian gland dysfunction based on noninvasive meibography. J Clin Med. 2020;10(1):65. doi: 10.3390/jcm10010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibbons A, Waren D, Yesilirmak N, Davis K, Valenzuela F, Murillo JC, et al. Ocular surface parameters predicting patient satisfaction after a single vectored thermal pulsation procedure for management of symptomatic meibomian gland dysfunction. Cornea. 2017;36(6):679–683. doi: 10.1097/ICO.0000000000001196. [DOI] [PubMed] [Google Scholar]
- 60.Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802–812. doi: 10.1016/j.jtos.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Miura M, Inomata T, Nakamura M, Sung J, Nagino K, Midorikawa-Inomata A, et al. Prevalence and characteristics of dry eye disease after cataract surgery: a systematic review and meta-analysis. Ophthalmol Ther. 2022;11(4):1309–1332. doi: 10.1007/s40123-022-00513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang P, Qian S, Xu Z, Huang F, Zhao Y, Li Z, et al. Meibomian gland morphology changes after cataract surgery: a contra-lateral eye study. Front Med (Lausanne) 2021;8:766393. doi: 10.3389/fmed.2021.766393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schlatter A, Palkovits S, Ruiss M, Fisus AD, Hirnschall N, Schmidl D, et al. Repeatability of biometry in patients with meibomian gland dysfunction before and after vectored thermal pulsation therapy: a randomized, controlled trial. Acta Ophthalmol. 2023 doi: 10.1111/aos.15711. [DOI] [PubMed] [Google Scholar]
- 64.Szabelska P, Golebiewska J, Rozycki R. Impact of thermal pulsation system therapy on pre-operative intraocular lens calculations before cataract surgery in patients with meibomian gland disfunction. Medicina (Kaunas) 2023;59(4):658. doi: 10.3390/medicina59040658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matossian C. Impact of thermal pulsation treatment on astigmatism management and outcomes in meibomian gland dysfunction patients undergoing cataract surgery. Clin Ophthalmol. 2020;14:2283–2289. doi: 10.2147/OPTH.S263046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mencucci R, Mercuri S, Cennamo M, Morelli A, Favuzza E. Efficacy of vector thermal pulsation treatment in reducing postcataract surgery dry eye disease in patients affected by meibomian gland dysfunction. J Cataract Refract Surg. 2023;49(4):423–429. doi: 10.1097/j.jcrs.0000000000001124. [DOI] [PubMed] [Google Scholar]
- 67.Matossian C, Chang DH, Whitman J, Clinch TE, Hu J, Ji L, et al. Preoperative treatment of meibomian gland dysfunction with a vectored thermal pulsation system prior to extended depth of focus IOL implantation. Ophthalmol Ther. 2023;12(5):2427–2439. doi: 10.1007/s40123-023-00740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y, Li J, Xue K, Xie J, Xie G, Gu S, et al. Preoperative management of MGD with vectored thermal pulsation before cataract surgery: a prospective, controlled clinical trial. Semin Ophthalmol. 2021;36(1–2):2–8. doi: 10.1080/08820538.2021.1881567. [DOI] [PubMed] [Google Scholar]
- 69.Park J, Yoo YS, Shin K, Han G, Arita R, Lim DH, et al. Effects of LipiFlow treatment prior to cataract surgery: a prospective, randomized, controlled study. Am J Ophthalmol. 2021;230:264–275. doi: 10.1016/j.ajo.2021.04.031. [DOI] [PubMed] [Google Scholar]
- 70.Kasetsuwan N, Suwajanakorn D, Tantipat C, Reinprayoon U. The efficacy between conventional lid hygiene and additional thermal pulsatile system in meibomian gland dysfunction patients treated with long-term anti-glaucoma medications in a randomized controlled trial. Clin Ophthalmol. 2020;14:2891–2902. doi: 10.2147/OPTH.S259692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shetty R, Khamar P, Nair AP, Pandian PR, Vaidya TA, Trivedi D, et al. Assessing clinical and molecular outcomes of prophylactic thermal pulsation therapy on ocular surface health following refractive surgery. Indian J Ophthalmol. 2023;71(4):1508–1516. doi: 10.4103/IJO.IJO_3361_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schallhorn CS, Schallhorn JM, Hannan S, Schallhorn SC. Effectiveness of an eyelid thermal pulsation procedure to treat recalcitrant dry eye symptoms after laser vision correction. J Refract Surg. 2017;33(1):30–36. doi: 10.3928/1081597X-20161006-05. [DOI] [PubMed] [Google Scholar]
- 73.Petzold G, Bedi R, Blackie CA. Management of dry-eye syndrome after laser in situ keratomileusis with a vectored thermal pulsation system. JCRS Online Case Reports. 2016;4:34–37. doi: 10.1016/j.jcro.2016.02.005. [DOI] [Google Scholar]
- 74.Kalas T, Gunn D. Corneal epithelial remodeling as a cause of chalazion-induced hypermetropia. Cornea. 2022;41(6):785–788. doi: 10.1097/ICO.0000000000002899. [DOI] [PubMed] [Google Scholar]
- 75.Hu J, Zhu S, Liu X. Efficacy and safety of a vectored thermal pulsation system (LipiFlow®) in the treatment of meibomian gland dysfunction: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2022;260(1):25–39. doi: 10.1007/s00417-021-05363-1. [DOI] [PubMed] [Google Scholar]
- 76.Pang SP, Chen YT, Tam KW, Lin IC, Loh EW. Efficacy of vectored thermal pulsation and warm compress treatments in meibomian gland dysfunction: a meta-analysis of randomized controlled trials. Cornea. 2019;38(6):690–697. doi: 10.1097/ICO.0000000000001907. [DOI] [PubMed] [Google Scholar]
- 77.Pucker AD, Yim TW, Rueff E, Ngo W, Tichenor AA, Conto JE. LipiFlow for the treatment of dry eye disease. Cochrane Database Syst Rev. 2024;2(2):CD015448. doi: 10.1002/14651858.CD015448.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kenrick CJ, Alloo SS. The limitation of applying heat to the external lid surface: a case of recalcitrant meibomian gland dysfunction. Case Rep Ophthalmol. 2017;8(1):7–12. doi: 10.1159/000455087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McMonnies CW, Korb DR, Blackie CA. The role of heat in rubbing and massage-related corneal deformation. Cont Lens Anterior Eye. 2012;35(4):148–154. doi: 10.1016/j.clae.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Korb DR, Blackie CA. Meibomian gland therapeutic expression: quantifying the applied pressure and the limitation of resulting pain. Eye Contact Lens. 2011;37(5):298–301. doi: 10.1097/ICL.0b013e31821bc7c5. [DOI] [PubMed] [Google Scholar]
- 81.Ambaw YA, Fuchs D, Raida M, Mazengia NT, Torta F, Wheelock CE, et al. Changes of tear lipid mediators after eyelid warming or thermopulsation treatment for meibomian gland dysfunction. Prostaglandins Other Lipid Mediat. 2020;151:106474. doi: 10.1016/j.prostaglandins.2020.106474. [DOI] [PubMed] [Google Scholar]
- 82.Yeo S, Tan JH, Acharya UR, Sudarshan VK, Tong L. Longitudinal changes in tear evaporation rates after eyelid warming therapies in meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2016;57(4):1974–1981. doi: 10.1167/iovs.16-19088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.