Abstract
In the current scenario of growing world population, limited cultivable land resources, plant diseases, and pandemics are some of the major factors responsible for declining global food security. Along with meeting the food demand, the maintenance of food quality is also required to ensure healthy consumption and marketing. In agricultural fields, pest infestations and bacterial diseases are common causes of crop damage, leading to massive yield losses. Conventionally, antibiotics and several pesticides have been used to manage and control these plant pathogens. However, the overuse of antibiotics and pesticides has led to the emergence of resistant strains of pathogenic bacteria. The bacteriophages are the natural predators of bacteria and are host-specific in their action. Therefore, the use of bacteriophages for the biocontrol of pathogenic bacteria is serving as a sustainable and green solution in crop protection and production. In this review, we have discussed the important plant pathogens and their impact on plant health and yield loss. Further, we have abridged the role of bacteriophages in the protection of crops from bacterial disease by discussing various greenhouse and field trials. Finally, we have discussed the impact of bacteriophages on the plant microbiome, phage resistance, and legal challenges in the registration and commercial production of bacteriophage-based biopesticides.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-024-01204-x.
Keywords: Bacteriophage, Crop protection, Plant microbiome, Phage resistance
Introduction
The world population is growing continuously and is anticipated to cross 9.6 billion by 2050, but land is limited. Therefore, it is necessary to implement practices that will help attain disease-free, healthy plants and animals while reducing detrimental impact on the environment [1]. Plant diseases result in around US$220 billion loss in the global economy per year, along with a 20–40% loss in crop production [2]. Additionally, geographical location, weather and economic conditions, disease pressure, consumer predilection, and government regulations are critical factors affecting agricultural practices. Since the discovery of antibiotics, they have been used for the control of plant pathogens [3–5] and are also incorporated into animal husbandry [6] for growth promotion and infection control. A recent investigation on antibiotic use in agriculture revealed that 83% of countries have no way of assessing antibiotic use on plants [7]. A report by the Wellcome Trust estimated that more than 100,000 tons of antibiotics are used in the agriculture sector per year [8]. The overuse of antibiotics in agriculture and veterinary applications, apart from their abuse in medicine, has led to the emergence of antimicrobial-resistant (AMR) bacteria [9]. Infections with these resistant strains of the bacteria ultimately increase mortality and morbidity in humans and animals, and now this is also spreading to agricultural soil [10]. The emergence of AMR and the public’s calls for antibiotic-free organic products have led to the eviction of antibiotics from agricultural practices and have prompted the search for other alternatives. Traditional approaches like sowing resistant varieties of crops, hygiene maintenance to restrict the spread of contaminated soil or seed, crop rotation, soil tillage, liming, and irrigation help in controlling or reducing the risk of diseases. Alternatives to antibiotics include biological control measures (use of beneficial microbes like viruses, bacteria, etc.), antimicrobial peptides, and chemical inducers of systemic acquired resistance (SAR) [11, 12]. Among the biological control agents, bacteriophages, which infect and kill bacteria, serve as a beneficial alternative, as they may significantly overcome the awful impact of antibiotic use on agricultural soil and the soil microbiome, with the potential of increasing profit by lowering crop losses.
Bacteriophage Diversity and Biology of Action
Structural Properties and Diversity
Bacteriophages, or bacterial viruses, are biological entities found in various ecological niches ranging from hot and cold deserts, marine and hot springs, water and wastewater, soils, and the human gut. Metagenomic analysis of the phage diversity in the Sahara Desert showed the abundance of phages infecting photosynthetic bacteria like Prochlorococcus and Synechococcus [13]. However, in the extreme cold climate of Antarctica, phage diversity changes from ssDNA phages in the spring to dsDNA phages in the summer [14]. Bacteriophages have also been reported in the extreme hyperthermophilic environment of hot springs at temperatures ranging up to 94 °C [15]. Morphologically, bacteriophages consist of a head and a tail structure, both of which are proteinaceous in nature. The genetic material is present in the form of single- or double-stranded DNA or RNA, which may be circular or linear. Despite bacteriophages being the most abundant entity in the biosphere, the phage genome is only nearly 1% of the bacterial genome. The genetic material is packaged into the capsid, which can be polyhedral, filamentous, or pleomorphic in shape [16]. The size of the capsid depends on the amount of genetic material that is enclosed in the capsid. The phage genome size ranges from ~ 3.3 kb (ssRNA viruses) to 735 kb [17]. The capsid is connected to the tail in the case of tailed bacteriophages, which are the most prevalent among identified bacteriophages. The tail varies in complexity, and has been excellently reviewed by Ackerman [18]. The bacterial virus subcommittee of the International Committee on Taxonomy of Viruses (ICTV) carried out a recent update of the taxonomic classification of phages after including three new families of myoviruses, officially ratifying Ackermannviridae, Chaseviridae, and Herelleviridae; two for the siphoviruses, Demerecviridae, and Drexlerviridae; and one of the podoviruses, Autographiviridae [19].
Phage Life Cycle
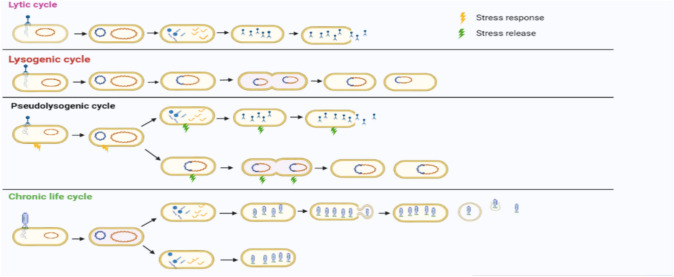
Bacteriophages infect their hosts with the help of proteins in the tail, neck, and head (in the case of tailless bacteriophages) regions. They interact with one or more receptors present on single or multiple hosts with different intensities; this ligand-receptor interaction provides host specificities. Also, the physiological state of the bacteria and the environmental conditions affect the life cycle of the phage. There are four different lifestyles for the bacteriophages (Fig. 1). Among these, the lytic and lysogenic lifecycles are best understood, while the chronic and pseudo-lysogenic lifecycles are alternative and less understood.
Lytic In the lytic (virulent) life cycle, bacteriophages interact with the bacteria and inject their DNA into the host. After inserting their DNA, the phage hijacks the bacterial machinery and uses it to replicate and translate the necessary information required to produce viral progeny. Then the viral progeny formed within the host produces enzymes such as spannin, holin, and endolysin, which help in the release of viral progeny by breaking the peptidoglycan bond of the cell wall and resulting in host lysis. Except for the filamentous or pleomorphic phages, the lytic life cycle has been used by most of the familiar phages.
Lysogeny In the case of the lysogenic life cycle, a bacteriophage does not hijack the host machinery, instead it integrates its genome within the host chromosome as a prophage, which then multiplies along with the bacterial genome. Therefore, in each successive division, the phage genome is transferred to the successive daughter bacterial cells. These phages are known as temperate phages, and they can switch from the lysogenic to lytic mode of life cycle upon induction by environmental signals (e.g., tailed phages P1 [20], and icosahedral membrane-containing tectivirus GIL01 [21].
Pseudolysogeny Pseudolysogeny is a type of phage-host interaction where the phage genome is maintained for a long period of time in natural environments and remains protected from unfavorable conditions. In this type of interaction, phage genetic material neither integrates within the bacterial chromosome as a prophage nor evokes a lytic life cycle; however, phage nucleic acid resides in the bacterial cell in a non-active form. In addition, the phage genetic material does not replicate in parallel with the bacterial chromosome. Therefore, during bacterial cell division, the phage genome is inherited asymmetrically in one of the daughter cells. Ripp & Miller [22] hypothesized that pseudolysogeny occurs when there is not sufficient energy for the phage to enter a lytic or lysogenic cycle. Pseudolysogeny has been observed in the podovirus P22 [23].
Chronic life cycle A chronic life cycle is an alternative phage infection strategy in which bacterial cells are not lysed when the viral progeny is released. It is of two types: productive and non-productive chronic infection. The productive chronic infection has been mostly observed in the filamentous ds/ssDNA phage and the mycoplasma-infecting dsDNA plasma virus. In the productive phage infection strategy, the phage genome either integrates with the bacterial chromosome or persists in the cytoplasm, and the phage progeny is released through the membrane by budding without bacterial lysis. The best studied examples of a productive phage infection are the Ff phage (infecting Escherichia coli K12) [24, 25]. However, in the non-productive phage infection lifestyle, phage particles are produced in the host cell cytoplasm and are not released into the extracellular environment. This infection strategy is mostly observed in RNA phages. The best studied example of the non-chronic infection lifestyle is the dsRNA phage phi6 infecting Pseudomonas syringae [26, 27]. The exact mechanism of their infection is not known, as it may be due to a change in the host cell membrane receptor or a mutation in the gene encoding endolysin.
Fig. 1.
Different type of life cycles followed by bacteriophages (Illustration is created using Biorender)
Role of Bacteriophages in Soil Ecosystem
In the current literature, there is a gap in understanding of the actual physiochemical interactions between bacteriophages and the soil. The phage capsid, being proteinaceous in nature, contains ionizable groups that may be either positively or negatively charged at different soil pHs. Charged capsid surfaces interact differently with the organic matter and minerals present in the soils, and this interaction contributes a central role in the viral distribution in the soils. Kuzyakov & Mason-Jones [28] speculated that the passive distribution of phages is correlated with water, and their biotic dispersion is associated with bacterial density and diversity. The abundance of the virus-like particles (VLPs) observed by direct microscopy counts in various soils showed their lowest plethora in the hot desert, followed by intermediate abundance in agricultural soil, field soil, and the cold desert, and the highest in forest and wetland soils [29, 30]. Over the past few decades, the role of bacteriophages in aquatic microbiology has been emphasized. They are associated with nearly 16–89% of the microbial mortality in aquatic sediments and are found abundantly not only in wastewater treatment plants but also in the human gastrointestinal tract [31–33]. Hence, phages are pervasive and contribute a major role in the control of microbial populations in all ecosystems. However, data on the viral infection of the soil bacteria are limited due to the problems involved in finding the accurate phage profusion and percentage of lytic infection in the soil. Ecologically, the actual virus-to-bacterium ratio (VBR) is arduous to clarify because the stability of the virion results in high viral richness without proportional infection [34]. In addition, several studies reported that nearly 30–80% of the bacteria isolated from the soil contain inducible prophages [35, 36]. Microbial growth in the soil is promoted by root-released carbon (C) and soil organic matter-released (SOM) nitrogen (N). The bacterivores, mainly protozoa and nematodes, play an important role in directing nutrients through the micro-food web. These bacterivores feed on the microbes and release the mineral N, which promotes plant growth. Trap et al. [37] reported that nearly 16% of the bacterial biomass is reduced by bacterivores, which changes the structure of the microbial community. However, data about the phage-induced mortality of the bacteria is unclear. But the obstacles associated with the movement of bacterivores, such as, they required water to move and nearly 36–41% of the water-filled pore volume, which is accessible to the bacteria but is inaccessible to even the smallest nematodes and protozoa [38]. These implications support the notion that bacteriophages play a crucial role in microbial mortality and nutrient turnover as they can easily gain access to the bacteria in the tiniest water-filled pore. In addition, carbon and energy transfer from bacteria to their predators is nearly 2–4 orders of magnitude less than the bacterial biomass [39, 40], and with increasing soil depth, populations of bacterivores decrease much faster than the bacterial populations [41]. Thus, the high rate of microbial turnover is due to the high rate of phage infections in the soil. Wei et al. [42] reported that in Antarctic desert soil, bacteriophages are widely distributed while bacterivores are sparse; therefore, microbial turnover is due to the presence of bacteriophages.
Application of Bacteriophages as Biopesticide in crop Protection and Production
The ideal attributes of a pesticide encompass targeted lethality towards intended pests while sparing non-target species, including soil microbiota and humans. Regrettably, this principle is not invariably upheld in the context of pesticide application. Pesticides, while being efficacious against target organisms, have inadvertent repercussions on the intricate microbial consortia inhabiting the soil. These agents further exhibit protracted residual activity, resulting in their bioaccumulation within the soil matrix and subsequent integration into the trophic chain, thereby instigating deleterious consequences, as indicated by pertinent studies [43, 44]. Numerous scientific reports underscore the detrimental influence of pesticides and chemical fertilizers on the nutritional composition of soil due to their disruption of beneficial microbial communities. For instance, triclopyr, glyphosate, and 2,4-D have been observed to impede the proliferation and metabolic activities of soil bacteria that contribute to nitrogen fixation [45–47]. Conversely, bacteriophages, characterized by their host-specificity, ecological compatibility, and innate occurrence, offer a contrasting approach. Owing to their high specificity to target bacterial pathogens while having a negligible impact on indigenous soil microflora bacteriophages offer a superior and sustainable solution to manage and control bacterial infestations of plants. The use of bacteriophages is not new in plant protection, as the first experimental clue about phage interaction with plant pathogens was provided in 1924 [48]. Kotila and Coons in 1925 observed that exposure to phages inhibited the soft rot disease caused by Pectobacterium spp. on potato tubers and carrot slices [49]. Later, it was observed that cabbage rot caused by Xanthomonas campestris pv. campestris was inhibited by the filtrate obtained from decomposing cabbage. The first phage-mediated field trial was performed by Thomas [50], and the results showed that treatment with phages lowered the extent of Stewart’s wilt disease caused by the phytopathogen Pantoea stewarti from 18% (untreated seeds) to 1.5% (treated seeds). However, the direct exposure of bacteriophages to ultraviolet rays, temperature fluctuations, and the remains of chemical residues used for plant protection pose difficulties in the biocontrol of pathogens in the open environment due to phage inactivation [51]. Therefore, lytic, specific, and stable phage applications are proposed to be used in agriculture [52]. However, Erwinia spp. specific phage Y2 [53], Xanthomonas spp. specific phages—ΦXaacA1, CP2, ΦXac2005-1, ccΦ13, ΦX44 [54], Pseudomonas syringae specific phages—vB_PsyM_KIL1, vB_PsyM_KIL2, vB_PsyM_KIL3, vB_PsyM_KIL4, vB_PsyM_KIL5 [55], and Ralstonia solanacearum specific phage RSL1 [56] have been confirmed for their effectivity in biocontrol. Civerolo and Kiel [57], through their study, concluded that in order to achieve effective biocontrol of X. campestris pv. pruni, a phage must be applied at a higher concentration before or at the early stage of the bacterial infection. Similarly, Zaccardelli et al. [58] treated the X. campestris pv. pruni infected nectarine fruitlets with phage F8 and tested the ability of phage survival under controlled conditions and observed that the disease did not develop in 92% of the treated fruits and the phage population remained high under controlled climatic conditions compared to the uncontrolled orchards. Consecutive studies on phage-mediated control of Xanthomonas spp. causing Asiatic citrus canker (ACC) showed that a phage mixture formulated in skim milk and sugar controlled the disease more effectively in nursery and field trials [59]. It was also reported that early morning and late evening were the best times for phage application, as exposure to UV rays was minimal during these time periods. However, tp reduce the effect of harmful UV rays on bacteriophages, research is underway to prepare stable and effective phage formulations. Born et al. [53] observed that when the mixture of Erwinia amylovora bacteriophage Y2 with one of the protective agents such as Tween 80, natural extracts from red pepper, beetroot, casein, soy peptone, or purified astaxanthin and aromatic amino acids was exposed to UV rays, then all the tested protective agents significantly increased the half-life of the UV-irradiated phage and reduced the negative impact on phage viability. Similar studies were carried out by Eman and Afaf [60]) using the P. syringae phage. The aerosols of a single phage suspension or phage mixture with corn flour and sucrose were evaluated for spray application over the bean leaves before inoculation with bacteria. When used individually, the disease severity was reduced by around 60%, compared to 70% in the case of phage mixtures.
In a study by Obradvic et al. [61] the comparative efficacy of distinct treatments—namely acibenzolar-S-methyl, bacteriophages, and copper-mancozeb—was assessed in the management of tomato bacterial spot disease caused by Xanthomonas spp. It was discerned that the utilization of bacteriophages either solely or in conjunction with acibenzolar-S-methyl yielded superior disease control outcomes in contrast to copper-based pesticides. Furthermore, these bacteriophage-based treatments were conducive to the preservation of the nutritional quality of tomatoes. In a parallel context, the investigation conducted by Lang et al. [62] highlighted the synergistic efficacy of bacteriophages and the plant activator acibenzolar-S-methyl in mitigating leaf blight in onions incited by Xanthomonas spp. This approach exhibited pronounced superiority over copper-based pesticides in terms of disease management and also underscored its potential to circumvent the negative impacts on non-target microorganisms. Similarly, several other attempts are mentioned in Supplementary Table 1, elaborating the control of phytobacteria in adverse environmental conditions that support the idea of using phage-mediated biocontrol as a promising alternative for plant crops (Fig. 2). Along with it, bacteriophages can serve as a biosensor for the specific and rapid detection of pathogenic bacteria [63]. Phage based magnetoelastic biosensors have been developed to detect phytopathogens from the tomato plants and surface of spinach leaves [64].
Fig. 2.
This figure depicts the hypothetical representation of phage-based control of phytopathogens and improvement in plant health (Illustration is created using Biorender)
Effect of Phage Application on the Plant Microbiome
The plant microbiome, or phytomicrobiome, consists of the microorganisms that inhabit the phyllosphere, endosphere, and rhizosphere. Phyllosphere is the microbial habitat above ground plant surfaces, and endosphere is the term used for the microbial habitat within various plant parts. However, the term “rhizosphere” is used for the region of soil directly in contact with the plant’s roots. The plant microbiome confers fitness advantages to the plant host and comprises bacteria, archaea, unicellular and multicellular eukaryotes, and viruses. Among the viruses, bacteriophages are estimated to be much greater in number in the rhizosphere than in the phyllosphere and endosphere due to the direct exposure to ultraviolet rays in these regions. Interaction of bacteriophages with host bacteria alters the plant microbiome as the lytic phages reduce the population of target bacteria, which in turn influences the other organisms that are directly connected with the target bacteria and leads to the co-evolution of both bacteria and bacteriophages [65–67]. The absence of the bacteriophages results in the dominance of one or several strains of the pathogenic bacteria in the niche. Therefore, bacteriophages control and support the diversity of bacterial communities. However, Fierer [39] have argued that phage infection is not a significant factor in controlling bacterial communities. Pratama and Van Elsas, [68] reviewed that soil phages are mostly lysogenic which support the immense plethora of bacterial host. Bacteriophages also act as a vector to spread bacterial genes through horizontal gene transfer from one host to another. This process impacts plant health as it changes the success of bacterial colonization by altering the survival rate of the pathogenic bacteria [67, 69]. Therefore, before using bacteriophage-based biocontrol, bacteriophages must be well characterized and should be selected for strictly lytic life cycle. Phages also contain several auxiliary metabolic genes (AMG) involved in carbon cycling. Hence, they can directly or indirectly influence plant nutrient availability [70, 71].
Phage Resistance Mechanisms Adopted by Bacteria
The important mechanisms used by bacteria to acquire phage resistance include blockage of phage adsorption by changing surface receptors, abortive infection, and the CRISPR/Cas system. These mechanisms of phage resistance are needed to be well understood and scrutinized when designing the bacteriophage-based biocontrol strategy to combat plant pathogens. Among these mechanisms, blockage of phage adsorption through mutation in the surface receptor is most common. Nevertheless, the development of such resistance in bacteria occurs at their fitness cost because many of the molecules involved in phage attachment are also involved in bacterial virulence. For example, mutants of X. campestris resistant to bacteriophage L7 had mutations in lipopolysaccharide synthesis (LPS), which also reduced the host virulence [72]. Similarly, a genetic mutation in LPS and flagella formation in P. atrosepticum and Erwinia spp., significantly decreased the effectiveness of φS32 and φAT1 bacteriophages adsorption and reduced their virulence [73, 74]. Abortive infection system is another phage resistance mechanism that interferes with the reproduction of the phage. The abortive infection causes the suicide of the infected cell and is identified in dairy bacteria, but in the case of plant pathogens, an abortive infection system named ToxIN was described in P. atrosepticum. The ToxIN gene leads to the inhibition of infection by several phages as it acts like a Type III protein-RNA toxin-antitoxin (TA) system [75–77]. Another important mechanism of phage resistance is the presence of the clustered regularly interspaced short palindromic repeat (CRISPR) array and CRISPR associated (Cas) proteins in bacteria. CRISPR arrays pick up short stretches of nucleic acid from the infected phages and then transcribe them into small RNAs. These small RNAs then use the Cas protein to target and degrade the complementary viral nucleic acids. In the case of phytopathogens, CRISPR/Cas has been reported in P. atrosepticum, E. amylovora, and X. oryzae [78–80] and provides sequence-specific immunity. The bacteriophage uses various anti-restriction strategies and modifies its ability to bind with the new receptor on the bacterial surface, which is poorly understood. Therefore, for designing bacteriophage-based biocontrol strategies to control plant disease, it must be necessary to understand the resistance mechanisms of bacteria to ensure effectiveness of the strategy. Use of a cocktail of bacteriophages having a broad host range and combination (studied till now majorly with antibiotics and bacteriocins) or sequential action of bacteriophage and chemical control to eliminate the pathogen completely or to decrease the evolution of resistance is also a good approach [81].
Legal Challenges for the Registration and the Production of Commercial Bacteriophage-Based Biopesticides
The increasing incidence of resistant bacteria against antibiotics and copper-based pesticides [82] and increased attention to consumer and environmental safety have propelled the regulatory agencies to pay attention to the use of biopesticides. Biopesticides are composed of living organisms or parts thereof and are thus non-toxic to plants, maintain soil fertility and aren’t hazardous for ecosystem. The biopesticides encompass various types, including bacterial, fungal, viral, and other plant-based or pheromone-based solutions. Biopesticides undergo a careful risk assessment for the safety of food and the environment before their registration and marketing [83]. The Central Insecticides Board and Registration Committee (CIBRC) plays a pivotal role in overseeing the registration process for biopesticides [84]. CIBRC in India has registered around 970 biopesticide products under the Insecticide Act of 1968. Globally many phage based products are being developed commercially for infections of several crops including spinach, tomatoes, peppers, apples, pears, peaches, cherries, almonds, walnut, hazelnut to name a few [85, 86]. In India, the research for developing phage based solutions for agricultural sector is being ramped up as demonstrated by increasing number of phages being isolated and demonstrated for their lytic application against bacterial plant pathogens. Ranjan et al. [87] isolated a siphophage φXOF4 against X. oryzae that was able to lyse all the tested strains of the X. oryzae responsible for causing leaf blight disease (BLB) in rice. Study also observed that seedlings raised from phage treated seeds showed decreased incidence of BLB. A similar study conducted by Barua and Nath [88] observed that phage isolated from chilli showed lytic activity against the several isolates of R. solanacearum. Despite this substantial number of registered biopesticides, and good efficacy of bacteriophage in controlling plant pathogen is noteworthy and bacteriophage-based biopesticides have not been included in this roster due to stringent regulations. In the European Union, the assessment is carried out using directive 91/414/EEC and then repealed and succeeded under regulation number 1107/2009. With such implementation, only one-fourth of the active substances and Plant Protection Products (PPP) are allowed in agriculture [89]. The registration procedure is complex and lengthy, as there are four major authorities involved in this procedure: the Rapporteur Member State, the European Food Safety Agency (EFSA), the Directorate General for Health and Food Safety (DG SANTE), and the Standing Committee on Plants, Animals, Food, and Feed (PAFF Committee). Therefore, in Europe, there are just two companies with registered bacteriophage-based biopesticides. Enviroinvest is a Hungarian company that developed Erwiphage® Plus for the control of fire blight in apples. Similarly, APS Biocontrol Ltd. is a Scottish company that developed bacteriophage containing Biolyse® PB for the control of potato soft rots. However, in the USA, the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) are involved in the regulatory framework for biopesticides. In the USA, OmniLytics, Inc., was the first company to receive EPA registration for their bacteriophage-based biopesticide, AgriPhage, for control of bacterial spots and specks on tomato and pepper caused by X. campestris and P. syringae. Furthermore, recently, this company developed new bacteriophage-based biopesticides for the control of tomato bacterial canker, fire blight of apple and pear, and citrus canker. XylPhi-PD is another bacteriophage-based biopesticide developed by Otsuka Pharmaceutical for the control of X. fastidiosa. As of today, the critical parameters for a suitable phage based product include a strict lytic nature and the lack of any undesirable genes. The promotion of phage-based biopesticides is on rise but it requires a thorough understanding of the bacteriophages used in preparation before release in the market.
Future Prospects
Since the last decade, phage therapy has shown immense success in human and veterinary applications, leading to a growing number of clinical trials and compassionate uses. However, the knowledge gap in the role of bacteriophages in soil ecosystems has considerably restricted their application in agriculture. In addition, the absence of standard regulatory guidelines has also decelerated the phage application in agriculture. Thus, there is a need to expand the use of phage-mediated biocontrol in agricultural practices. Major areas which require focus include improvement in techniques to obtain copious phage from raw resources which will reduce the time and cost of phage isolation, using polyvalent phages or cocktails to reduce pathogenic bacterial load in soil. Exploring the biodiversity of bacteriophages, bacteria, and other bacterivores in the soil will help in gaining a better understanding of their role in nutrient cycling, and their interactions with other soil organisms like nematodes and protozoa, which impact the overall health of soil ecosystem. Elucidating the role of phages in the transmission of functional genes (such as those responsible for nutrient cycling and pollutant degradation) and antibiotic resistance genes among bacteria will help in understanding their importance in soil carbon sequestration, contaminated soil remediation, and increased crop yield. Lastly, the necessity to generate public awareness about the importance and advantages of using bacteriophages for targeted inactivation of specific bacterial pathogens and their ecological impact on soil systems will strengthen phage-mediated biocontrol in agriculture.
Conclusions and Outlook
Experiments elucidating the efficacy of phage application in mitigating bacterial plant diseases have predominantly been executed within controlled greenhouse environments. However, the predominant agricultural activities occurred in unconfined open settings marked by considerable disparities from the controlled confines of greenhouses. Consequently, outcomes obtained under such controlled settings can be influenced by the distinctive array of environmental factors characterizing open-field conditions. Therefore, there is a need to carry out more field trials to depict a true picture and implement the efficacy of the bacteriophages in agriculture. Bacteriophages, as living entities, hold a distinct advantage of ecological compatibility, thereby harmonizing with ecosystems and contributing to the maintenance of soil microbiota. Furthermore, their amenability to synergistic application with other chemical agents and bactericidal compounds amplifies their potential for integrated biocontrol strategies. Adjunctively, their utility extends to the realm of diagnostics, wherein phage-based methodologies offer the prospect of high-sensitivity detection of plant pathogenic bacteria. The susceptibility of phages to ultraviolet radiation might pose a limitation in their use, so more focus is needed on the preparation of phage formulations with a high level of stability.
Bacteriophages, through direct mechanisms such as cell lysis or alterations in host phenotype post prophage integration, along with indirect influences that alter the plant-bacterial interactions and modulate the dynamic equilibrium within bacterial consortia, possess the capacity to mount ecological modifications in their bacterial hosts. Such multifaceted interactions underscore the need for an enriched comprehension of phage-host interplay, mechanisms underpinning phage resistance, and the exact mechanisms by which phages contribute to overall plant health. By advancing this knowledge frontier, environmentally amicable strategies can be advanced to manage and counteract bacterial infections in agricultural settings.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the support of Council of Scientific and Industrial Research, New Delhi, India; National Agricultural Science Fund, Indian Council of Agricultural Research, New Delhi, India; and National Fellow scheme, Indian Council of Agricultural Research, New Delhi, India.
Author Contributions
Conceptualization: ABJ, TA; Methodology: ABJ; Software: ABJ; Validation: ABJ, MV, TA, and RV; Formal analysis: NV, BCB, RKV, RKS; Resources: TA; Writing—original draft: ABJ and MV; Writing—review and editing: MV, TA, NV and BCB; Visualization, ABJ, MV and PS; Supervision: TA and RKS; Project administration: TA and RV. All authors have agreed to the published version of the manuscript.
Funding
No funding was received to assist with the preparation of the manuscript.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramankutty N, Mehrabi Z, Waha K, et al. Trends in global agricultural land use: implications for environmental health and food security. Annu Rev Plant Biol. 2018;69:789–815. doi: 10.1146/annurev-arplant-042817-040256. [DOI] [PubMed] [Google Scholar]
- 2.FAO (2021) Making agrifood systems more resilient to shocks and stresses. Rome, FAO. 10.4060/cb4476en
- 3.Stockwell VO, Duffy B. Use of antibiotics in plant agriculture. Revue Scientifique Et Technique-Office International Des Epizooties. 2012;31:199–210. doi: 10.20506/rst.31.1.2104. [DOI] [PubMed] [Google Scholar]
- 4.Tancos KA, Villani S, Kuehne S, et al. Prevalence of streptomycin-resistant Erwinia amylovora in New York apple orchards. Plant Dis. 2016;100:802–809. doi: 10.1094/pdis-09-15-0960-re. [DOI] [PubMed] [Google Scholar]
- 5.Miller SA, Ferreira JP, LeJeune JT. Antimicrobial use and resistance in plant agriculture: a one health perspective. Agriculture. 2022;12:289. doi: 10.3390/agriculture12020289. [DOI] [Google Scholar]
- 6.Cheng G, Hao H, Xie S, et al. Antibiotic alternatives: the substitution of antibiotics in animal husbandry. Front Microbiol. 2014;5:217. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Organization for Animal Health . Monitoring global progress on addressing antimicrobial resistance: analysis report of the second round of results of AMR country self-assessment survey 2018. Switzerland: Geneva; 2018. Food and Agriculture Organization. [Google Scholar]
- 8.O’Neill J. Tackling drug-resistant infections globally: final report and recommendations–the review on antimicrobial resistance wellcome trust. London, UK: HM Government; 2016. [Google Scholar]
- 9.Sundin GW, Wang N. Antibiotic resistance in plant-pathogenic bacteria. Annu Rev Phytopathol. 2018;56:161–180. doi: 10.1146/annurev-phyto-080417-045946. [DOI] [PubMed] [Google Scholar]
- 10.Popowska M, Rzeczycka M, Miernik A, et al. Influence of soil use on prevalence of tetracycline, streptomycin, and erythromycin resistance and associated resistance genes. Antimicrob Agents Chemother. 2012;56:1434–1443. doi: 10.1128/AAC.05766-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien PA. Biological control of plant diseases. Australas Plant Pathol. 2017;46:293–304. doi: 10.1007/s13313-017-0481-4. [DOI] [Google Scholar]
- 12.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 13.Fancello L, Trape S, Robert C, et al. Viruses in the desert: a metagenomic survey of viral communities in four perennial ponds of the Mauritanian Sahara. The ISME J. 2013;7:359–369. doi: 10.1038/ismej.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Bueno A, Tamames J, Velázquez D, et al. High diversity of the viral community from an Antarctic lake. Sci. 2009;326:858–861. doi: 10.1126/science.1179287. [DOI] [PubMed] [Google Scholar]
- 15.Xiang X, Chen L, Luo Y, et al. Sulfolobus tengchongensis spindle shaped virus SSTSV1: virus–host interactions and genomic features. J Virol. 2005;79:8677–8686. doi: 10.1128/JVI.79.14.8677-8686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White HE, Orlova EV (2019) Bacteriophages: their structural organisation and function. Renos S (ed) Bacteriophages - Perspectives and Future.Birkbeck, University of London
- 17.Al-Shayeb B, Sachdeva R, Chen LX, et al. Clades of huge phages from across Earth’s ecosystems. Nature. 2020;578:425–431. doi: 10.1038/s41586-020-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackermann HW. Phage classification and characterization. Methods Mol Biol. 2009;501:127–140. doi: 10.1007/978-1-60327-164-6_13. [DOI] [PubMed] [Google Scholar]
- 19.Turner D, Kropinski AM, Adriaenssens EM. A roadmap for genome-based phage taxonomy. Viruses. 2021;13:506. doi: 10.3390/v13030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Łobocka MB, Rose DJ, Plunkett G, III, et al. Genome of bacteriophage P1. J Bacteriol. 2004;186:7032–7068. doi: 10.1128/JB.186.21.7032-7068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verheust C, Jensen G, Mahillon J. pGIL01, a linear tectiviral plasmid prophage originating from Bacillus thuringiensis serovar israelensis. Microbiology. 2003;149:2083–2092. doi: 10.1099/mic.0.26307-0. [DOI] [PubMed] [Google Scholar]
- 22.Ripp S, Miller RV. The role of pseudolysogeny in bacteriophage-host interactions in a natural freshwater environment. Microbiology. 1997;143:2065–2070. doi: 10.1099/00221287-143-6-2065. [DOI] [PubMed] [Google Scholar]
- 23.Cenens W, Makumi A, Mebrhatu MT, et al. Phage-host interactions during pseudolysogeny: lessons from the Pid/dgo interaction. Bacteriophage. 2013;3:e25029. doi: 10.4161/bact.25029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maniloff J, Das J, Christensen JR. Viruses of mycoplasmas and spiroplasmas. Adv Virus Res. 1977;21:343–380. doi: 10.1016/s0065-3527. [DOI] [PubMed] [Google Scholar]
- 25.Rakonjac J. Filamentous bacteriophages: biology and applications. eLS. 2012;3:1–15. doi: 10.1002/9780470015902.a0029482. [DOI] [Google Scholar]
- 26.Onodera S, Olkkonen VM, Gottlieb P, et al. Construction of a transducing virus from double-stranded RNA bacteriophage phi6: establishment of carrier states in host cells. J Virol. 1992;66:190–196. doi: 10.1128/JVI.66.1.190-196.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pourcel C, Midoux C, Vergnaud G, et al. A carrier state is established in Pseudomonas aeruginosa by phage LeviOr01, a newly isolated ssRNAlevivirus. J Gen Virol. 2017;98:2181–2189. doi: 10.1099/jgv.0.000883. [DOI] [PubMed] [Google Scholar]
- 28.Kuzyakov Y, Mason-Jones K. Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol Biochem. 2018;127:305–317. doi: 10.1016/j.soilbio.2018.09.032. [DOI] [Google Scholar]
- 29.Swanson MM, Fraser G, Daniell TJ, et al. Viruses in soils: morphological diversity and abundance in the rhizosphere. Ann Appl Biol. 2009;155:51–60. doi: 10.1111/j.1744-7348.2009.00319.x. [DOI] [Google Scholar]
- 30.Williamson KE, Fuhrmann JJ, Wommack KE. Viruses in soil ecosystems: an unknown quantity within an unexplored territory. Annu Rev Virol. 2017;4:201–219. doi: 10.1146/annurev-virology-101416-041639. [DOI] [PubMed] [Google Scholar]
- 31.Hewson I, Vargo GA, Fuhrman JA. Bacterial diversity in shallow oligotrophic marine benthos and overlying waters: effects of virus infection, containment, and nutrient enrichment. Microb Ecol. 2003;46:322–336. doi: 10.1007/s00248-002-1067-3. [DOI] [PubMed] [Google Scholar]
- 32.Santiago-Rodriguez TM, Ly M, Daigneault MC, et al. Chemostat culture systems support diverse bacteriophage communities from human feces. Microbiome. 2015;3:1–6. doi: 10.1186/s40168-015-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Łusiak-Szelachowska M, Weber-Dąbrowska B, Jończyk-Matysiak E, et al. Bacteriophages in the gastrointestinal tract and their implications. Gut Pathog. 2017;9:1–5. doi: 10.1186/s13099-017-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emerson JB, Roux S, Brum JR, et al. Host-linked soil viral ecology along a permafrost thaw gradient. Nat Microbiol. 2018;3:870–880. doi: 10.1038/s41564-018-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson KE, Radosevich M, Smith DW, et al. Incidence of lysogeny within temperate and extreme soil environments. Environ Microbiol. 2007;9:2563–2574. doi: 10.1111/j.1462-2920.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh D, Roy K, Williamson KE, et al. Prevalence of lysogeny among soil bacteria and presence of 16S rRNA and trzN genes in viral-community DNA. Appl Environ Microbiol. 2008;74:495–502. doi: 10.1128/AEM.01435-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trap J, Bonkowski M, Plassard C, et al. Ecological importance of soil bacterivores for ecosystem functions. Plant Soil. 2016;398:1–24. doi: 10.1007/s11104-015-2671-6. [DOI] [Google Scholar]
- 38.Neher DA. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu Rev Phytopathol. 2010;48:371–394. doi: 10.1146/annurev-phyto-073009-114439. [DOI] [PubMed] [Google Scholar]
- 39.Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol. 2017;15:579–590. doi: 10.1038/nrmicro.2017.87. [DOI] [PubMed] [Google Scholar]
- 40.Pausch J, Kramer S, Scharroba A, et al. Small but active–pool size does not matter for carbon incorporation in below-ground food webs. Funct Ecol. 2016;30:479–489. doi: 10.1111/1365-2435.12512. [DOI] [Google Scholar]
- 41.Potapov AM, Goncharov AA, Semenina EE, et al. Arthropods in the subsoil: abundance and vertical distribution as related to soil organic matter, microbial biomass and plant roots. Euro J Soil Biol. 2017;82:88–97. doi: 10.1016/j.ejsobi.2017.09.001. [DOI] [Google Scholar]
- 42.Wei ST, Higgins CM, Adriaenssens EM, et al. Genetic signatures indicate widespread antibiotic resistance and phage infection in microbial communities of the McMurdo Dry valleys, East Antarctica. Polar Biol. 2015;38:919–925. doi: 10.1007/s00300-015-1649-4. [DOI] [Google Scholar]
- 43.Suresh Babu G, Farooq M, Ray RS, et al. DDT and HCH residues in Basmati rice (Oryza sativa) cultivated in Dehradun (India) Water Air Soil Pollut. 2003;144:149–157. doi: 10.1023/A:1022929502510. [DOI] [Google Scholar]
- 44.Nakata H, Kawazoe M, Arizono K, et al. Organochlorine pesticides and polychlorinated biphenyl residues in foodstuffs and human tissues from China: status of contamination, historical trend, and human dietary exposure. Arch Environ Contam Toxiicol. 2002;43:0473–0480. doi: 10.1007/s00244-002-1254-8. [DOI] [PubMed] [Google Scholar]
- 45.Santos A, Flores M. Effects of glyphosate on nitrogen fixation of free-living heterotrophic bacteria. Lett Appl Microbiol. 1995;20:349–352. doi: 10.1111/j.1472-765X.1995.tb01318.x. [DOI] [Google Scholar]
- 46.Fabra A, Duffard R, Evangelista DDA. Toxicity of 2,4-dichlorophenoxyacetic acid in pure culture. Bull Environ Contam Toxicol. 1997;59:645–652. doi: 10.1007/s001289900528. [DOI] [PubMed] [Google Scholar]
- 47.Pell M, Stenberg B, Torstensson L. Potential denitrification and nitrification tests for evaluation of pesticide effects in soil. Ambio. 1998;27:24–28. [Google Scholar]
- 48.Mallmann WL, Hemstreet CA. Isolation of an inhibitory substance from plants. Agric Res. 1924;28:599–602. [Google Scholar]
- 49.Kotila J, Coons G. Investigations on the Black Leg disease of Potato. Mich Agri Exp Stat Techl Bull. 1925;67:3–29. [Google Scholar]
- 50.Thomas RC. A bacteriophage in relation to Stewart’s disease of corn. Phytopathology. 1935;25:371–372. [Google Scholar]
- 51.Fernández L, Gutiérrez D, Rodríguez A, et al. Application of bacteriophages in the agro-food sector: a long way toward approval. Front Cell Infect Microbiol. 2018;8:296. doi: 10.3389/fcimb.2018.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kazi M, Annapure US. Bacteriophage biocontrol of foodborne pathogens. J Food Sci Technol. 2016;53:1355–1362. doi: 10.1007/s13197-015-1996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Born Y, Bosshard L, Duffy B, et al. Protection of Erwinia amylovora bacteriophage Y2 from UV-induced damage by natural compounds. Bacteriophage. 2015;5:e1074330. doi: 10.1080/21597081.2015.1074330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balogh B, Canteros BI, Stall RE, et al. Control of citrus canker and citrus bacterial spot with bacteriophages. Plant Dis. 2008;92:1048–1052. doi: 10.1094/PDIS-92-7-1048. [DOI] [PubMed] [Google Scholar]
- 55.Rombouts S, Volckaert A, Venneman S. Characterization of novel bacteriophages for biocontrol of bacterial blight in leek caused by Pseudomonas syringae Pv. Porri. Front Microbiol. 2016;15:279. doi: 10.3389/fmicb.2016.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujiwara A, Fujisawa M, Hamasaki R, et al. Biocontrol of Ralstonia solanacearum by treatment with lytic bacteriophages. Appl Environ Microbiol. 2011;77:4155–4162. doi: 10.1128/AEM.02847-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Civerolo EL, Keil HL. Inhibition of bacterial spot of peach foliage by Xanthomonas pruni bacteriophage. Phytopathology. 1969;59:1966–1967. [Google Scholar]
- 58.Zaccardelli M, Saccardi A, Gambin E, et al. Xanthomonas campestris Pv. Pruni bacteriophages on peach trees and their potential use for biological control. Phytopathol Mediterr. 1992;31:133–140. [Google Scholar]
- 59.Ibrahim YE, Saleh AA, Al-Saleh MA. Management of asiatic citrus canker under field conditions in Saudi Arabia using bacteriophages and acibenzolar-S-methyl. Plant Dis. 2017;101:761–765. doi: 10.1094/PDIS-08-16-1213-RE. [DOI] [PubMed] [Google Scholar]
- 60.Eman OH, Afaf ZAE. Biocontrol of halo blight of bean caused by Pseudomonas Phaseolicola. Int J Virol. 2014;10:235–242. doi: 10.3923/ijv.2014.235.242. [DOI] [Google Scholar]
- 61.Obradovic A, Jones JB, Momol MT, et al. Management of tomato bacterial spot in the field by foliar applications of bacteriophages and SAR inducers. Plant Dis. 2004;88:736–740. doi: 10.1094/PDIS.2004.88.7.736. [DOI] [PubMed] [Google Scholar]
- 62.Lang JM, Gent DH, Schwartz HF. Management of Xanthomonas leaf blight of onion with bacteriophages and a plant activator. Plant Dis. 2007;91:871–878. doi: 10.1094/pdis-91-7-0871. [DOI] [PubMed] [Google Scholar]
- 63.Farooq U, Yang Q, Ullah MW, et al. Bacterial biosensing: recent advances in phage-based bioassays and biosensors. Biosens Bioelectron. 2018;118:204–216. doi: 10.1016/j.bios.2018.07.058. [DOI] [PubMed] [Google Scholar]
- 64.Ali Q, Ahmar S, Sohail MA, et al. Research advances and applications of biosensing technology for the diagnosis of pathogens in sustainable agriculture. Environ Sci Pollut Res. 2021;28:9002–9019. doi: 10.1007/s11356-021-12419-6. [DOI] [PubMed] [Google Scholar]
- 65.Buttimer C, McAuliffe O, Ross RP, et al. Bacteriophages and bacterial plant diseases. Front Microbiol. 2017;8:34. doi: 10.3389/fmicb.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morella NM, Gomez AL, Wang G. The impact of bacteriophages on phyllosphere bacterial abundance and composition. MolEcol. 2018;27:2025–2038. doi: 10.1111/mec.14542. [DOI] [PubMed] [Google Scholar]
- 67.Koskella B, Taylor TB. Multifaceted impacts of bacteriophages in the plant microbiome. Annu Rev Phytopathol. 2018;56:80. doi: 10.1146/annurev-phyto-080417-045858. [DOI] [PubMed] [Google Scholar]
- 68.Pratama AA, Van Elsas JD. The ‘neglected’soil-virome potential role and impact. Trends Microbiol. 2018;26:649–662. doi: 10.1016/j.tim.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Varani AM, Monteiro-Vitorello CB, Nakaya HI, et al. The role of prophage in plant-pathogenic bacteria. Annu Rev Phytopathol. 2013;51:429–451. doi: 10.1146/annurev-phyto-081211-173010. [DOI] [PubMed] [Google Scholar]
- 70.Trubl G, et al. Soil viruses are underexplored players in ecosystem carbon processing. Am Soc Microbiol. 2018;3:e00076–e00018. doi: 10.1128/mSystems.00076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Emerson JB. Soil viruses: a new hope. MSystems. 2019;4:e00120–e00119. doi: 10.1128/mSystems.00120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hung CH, Wu HC, Tseng YH. Mutation in the Xanthomona Scampestris xanA gene required for synthesis of xanthan and lipopolysaccharide drastically reduces the efficiency of bacteriophage ΦL7 adsorption. Biochem Biophys Res Commun. 2002;291:338–343. doi: 10.1006/bbrc.2002.6440. [DOI] [PubMed] [Google Scholar]
- 73.Evans TJ, Ind A, Komitopoulou E, et al. Phage-selected lipopolysaccharide mutants of Pectobacterium atrosepticum exhibit different impacts on virulence. J Appl Microbiol. 2010;109:505–514. doi: 10.1111/j.1365-2672.2010.04669.x. [DOI] [PubMed] [Google Scholar]
- 74.Evans TJ, Trauner A, Komitopoulou E, et al. Exploitation of a new flagellatropic phage of Erwinia for positive selection of bacterial mutants attenuated in plant virulence: towards phage therapy. J Appl Microbiol. 2010;108:676–685. doi: 10.1111/j.1365-2672.2009.04462.x. [DOI] [PubMed] [Google Scholar]
- 75.Fineran PC, Blower TR, Foulds IJ, et al. The phage abortive infection system, ToxIN, functions as a protein–RNA toxin–antitoxin pair. Proc Natl Acad Sci. 2009;106:894–899. doi: 10.1073/pnas.080883210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blower TR, Fineran PC, Johnson MJ, et al. Mutagenesis and functional characterization of the RNA and protein components of the toxIN abortive infection and toxin-antitoxin locus of Erwinia. J Bact. 2009;191:6029–6039. doi: 10.1128/JB.00720-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blower TR, Pei XY, Short FL, et al. A processed noncoding RNA regulates an altruistic bacterial antiviral system. Nat Struct Mol Biol. 2011;18:185–190. doi: 10.1038/nsmb.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Przybilski R, Richter C, Gristwood T, et al. Csy4 is responsible for CRISPR RNA processing in Pectobacterium atrosepticum. RNA Biol. 2011;8:517–528. doi: 10.4161/rna.8.3.15190. [DOI] [PubMed] [Google Scholar]
- 79.Rezzonico F, Smits TH, Duffy B. Diversity, evolution, and functionality of clustered regularly interspaced short palindromic repeat (CRISPR) regions in the fire blight pathogen Erwinia amylovora. Appl Environ Microbiol. 2011;77:3819–3829. doi: 10.1128/AEM.00177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Semenova E, Nagornykh M, Pyatnitskiy M, et al. Analysis of CRISPR system function in plant pathogen Xanthomona soryzae. FEMS Microbiol Lett. 2009;296:110–116. doi: 10.1111/j.1574-6968.2009.01626.x. [DOI] [PubMed] [Google Scholar]
- 81.Stachler E, Kull A, Julian TR. Bacteriophage treatment before chemical disinfection can enhance removal of plastic-surface-associated Pseudomonas aeruginosa. Appl Environ Microbiol. 2021;87:e00980–e00921. doi: 10.1128/AEM.00980-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gutiérrez-Barranquero JA, de Vicente A. Recruitment and rearrangement of three different genetic determinants into a conjugative plasmid increase copper resistance in Pseudomonas syringae. Appl Environ Microbiol. 2013;79:1028–1033. doi: 10.1128/AEM.02644-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stefani E. Biosafety and agro-environmental concerns related to the use of bacterial biocontrol agents in crop protection. J Plant Pathol. 2016;98:31–32. [Google Scholar]
- 84.Chakraborty N, Mitra R, Pal S, et al. Biopesticide consumption in India: insights into the current trends. Agriculture. 2023;13:557. doi: 10.3390/agriculture13030557. [DOI] [Google Scholar]
- 85.Dy RL, Rigano LA, Fineran PC. Phage-based biocontrol strategies and their application in agriculture and aquaculture. Biochem Soc Trans. 2018;46:1605–1613. doi: 10.1042/BST20180178. [DOI] [PubMed] [Google Scholar]
- 86.Ahmed T, Li B. Phage–plant interactions: a way forward toward sustainable agriculture. Viruses. 2023;15:329. doi: 10.3390/v15020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ranjani P, Gowthami Y, Gnanamanickam SS, et al. Bacteriophages: a new weapon for the control of bacterial blight disease in rice caused by Xanthomonas oryzae. Microbiol Biotechnol Lett. 2018;46:346–359. doi: 10.4014/mbl.1807.07009. [DOI] [Google Scholar]
- 88.Barua P, Nath PD. Isolation of bacteriophages infecting Ralstonia solanacearum causing bacterial wilt disease in Naga Chilli (Capsicum chinense Jacq.) Int J Curr Microbiol Appl Sci. 2019;8:927–937. doi: 10.20546/ijcmas.2019.802.106. [DOI] [Google Scholar]
- 89.European Commission Regulation (EC) no 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council directives 79/117/EEC and 91/414/EEC. Off J Eur Union. 2009;52:1–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.