Abstract
In this study, 13 diesel degrading bacteria were isolated from the oil contaminated soils and the promising strains identified as Acinetobacter pittii ED1 and Pseudomonas aeruginosa BN were evaluated for their diesel degrading capabilities. These strains degraded the diesel optimally at 30 °C, pH 7.0 and 1% diesel concentration. Both the strains produced biofilm at 1% diesel concentration indicating their ability to tolerate diesel induced abiotic stress. Gravimetric analysis of the spent medium after 7 days of incubation showed that A. pittii ED1 and P. aeruginosa BN degraded 68.61% and 76% diesel, respectively, while biodegradation reached more than 90% after 21 days. Fourier Transform Infrared (FTIR) analysis of the degraded diesel showed 1636.67 cm−1 (C=C stretch, N–H bond) peak corresponding to alkenes and primary amines, while GC-TOF-MS analysis showed decline in hydrocarbon intensities after 7 days of incubation. The present study revealed that newly isolated A. pittii ED1 and P. aeruginosa BN were able to degrade diesel hydrocarbons (C11–C18, and C19–C24) efficiently and have potential for bioremediation of the oil-contaminated sites.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-024-01317-3.
Keywords: Petroleum, Diesel, Bioremediation, Biodegradation, Gravimetry, Acinetobacter pittii, Pseudomonas aeruginosa
Introduction
Fossil fuels are used universally and their uncontrolled exploitation is responsible for major ecological disturbances occurring due to burning of fossil fuels and oil spillage leading to contamination of soil and water bodies. Thus, besides all other pollutions, petroleum (oil) contamination is more pervasive and common throughout the world. The spillage of petroleum results in the rapid deterioration of environment [1, 2]. The widespread problem of hydrocarbon pollution caused due to petroleum products, and their discharge and accidental oil spillage, particularly in marine environment, is proving to be hazardous. Thus, remediation of these hydrocarbons by natural decontamination process is of utmost importance. Bioremediation is a non-invasive and cost-effective technique for the clean-up of these petroleum hydrocarbons [3–6].
Diesel is a highly demanded and preferred fossil fuel of the transport industry due to its relatively easier availability and lower cost [7]. Composition wise, diesel is a complex mixture of 75% aliphatic hydrocarbons (C10H20–C15H28) and 25% polycyclic aromatic hydrocarbons. Diesel transport is a globally challenging issue and due to oil spillage during transport through sea, marine ecosystems face alarming problems: (i) rise in eutrophication and (ii) decline in primary productivity. For bioremediation, workers have explored diesel degraders isolated from soil and water samples [8–10]. For effective minimization of the load of the dispersed oil spill, bioremediation is one of the effective, eco-friendly and inexpensive technique as compared to other strategies [11, 12]. Myriad microorganisms such as bacteria, fungi, yeast and algae have potential for bioremediation, however, bacteria are considered as one of the predominant and promising hydrocarbon-degrading biological agents [13, 14] which may mineralize organic compound into the carbon dioxide and water [15]. Previously, studies have shown the potential of native bacterial strains obtained from the diesel contaminated soil and water sources [16, 17]. The present study investigated the potential of two newly isolated hydrocarbon degrading bacteria viz. Acinetobacter sp. and Pseudomonas sp. to degrade diesel oil. Soil is the major ecosystem that supports existence and perpetuation of microbial diversity [18]. The objective of the present study was to characterize the diesel hydrocarbon degrading ability of the newly isolated bacterial strains of central India for their effective use in bioaugmentation.
Materials and Methods
Isolation and Identification of Diesel Degrading Bacteria
Diesel degrading bacteria (DDB) were isolated from diesel contaminated soil samples collected from two nearby cities, i.e., Jhansi, Uttar Pradesh (78°33′54″ E, 25°26′57″ N) and Bina, Madhya Pradesh (78°08′28″ E, 24°15′42″ N). To screen the bacterial strains capable of efficient diesel hydrocarbon degradation, spread plate method using Bushnell Haas (BH) agar medium was employed [19]. The screening was done using methods as described earlier [20, 21]. Soil samples were serially diluted (10–4, 10–5, and 10–6) and 100 µl of each dilution was spread on to the BH agar medium plates supplemented with 1% diesel. The diesel oil was sterilized by filtering through Millipore filter (cellulose ester, 0.20 µm pore size) and stored in sterile bottles. After 24–48 h of incubation at 30 °C, appearing colonies were aseptically isolated and were screened further. The secondary screening was performed using MS agar medium (0.6 g/L Na2HPO4, 0.2 g/L KH2PO4, 4.0 g/L NaNO3, 1.0 g/L, yeast powder) with diesel as the sole carbon source in place of acenaphthene [22]. Briefly, after the growth of bacterial strains in LB broth, the culture was again streaked on BH agar supplemented with 1% diesel and plates were incubated at 30 °C for up to 48 h. The growth of the hydrocarbon degrading strains on the solid medium was recorded in terms of colony size. The identification of bacterial strains was done by 16S rRNA gene sequencing using automated DNA Sequencer (ABI 3100 genetic analyser) with following primers: AGAGTTTGATCCTGGCTCAG (Forward) and GGTTACCTTGTTACGACTT (Reverse). After DNA sequencing, nucleotide data was subjected to BLAST analysis. The phylogenetic tree was constructed using Neighbour-Joining (NJ) method in MEGA 7 software.
Biofilm Assay
The efficiency of biofilm formation of the diesel degrading bacterial strains in two types of culture media supplemented with 1% diesel (a) Luria Bertani (LB) medium; (b) Mineral Salt (MS) medium [10] was examined. Effect of carbon source and temperature on the biofilm formation by these strains was studied. For this, crystal violet method for the determination of biofilm in 96 well polystyrene plate was used and the optical density (OD) at 600 nm was measured using a multimode reader (Biotek, USA).
Optimization of Growth Parameters
Temperature and pH Optimization
The degradation of diesel hydrocarbon with DDB was studied at different temperature and pH. MS medium containing 1% diesel was inoculated with overnight grown inoculum (1% v/v; OD600 ~0.5) and incubated for seven days at 150 rpm at different temperatures (30–45 °C) and pH (5.0–9.0). Afterwards, the OD was recorded at 600 nm.
Salt Optimization
To investigate the role of salinity on diesel degradation, MS medium was supplemented with diesel oil and varying NaCl concentrations (1–4% v/v). Flasks were inoculated with overnight grown inoculum (1% v/v; OD600 ~0.5) and incubated for 24 h at 150 rpm. Uninoculated MS medium with diesel was used as control and the OD at 600 nm was recorded periodically [23].
Effect of Carbon and Nitrogen Source
Different oils (mineral oil, diesel oil, n-paraffin and aviation fuel) were evaluated as suitable carbon source. MS medium containing 1% (v/v) of different oils was inoculated with overnight grown inoculum (1% v/v; OD600 ~0.5) and incubated for seven days at 150 rpm at 30 °C and the absorbance was recorded at 600 nm. Under similar conditions, the bacterial strains were grown in MS broth containing 1% (v/v) concentration of the organic (tryptone or peptone) or inorganic (KNO3 or NaNO3) nitrogen source. The OD was recorded at 600 nm to determine the growth.
Optimization of Diesel Degradation
MS medium supplemented with various concentrations of diesel oil (1–4%) was used to study the effect of the diesel concentration on degradative ability of the bacterial strain. The inoculum (1% v/v) of the overnight grown bacterial strains (OD 0.5%) was added to MS medium supplemented with different concentrations of diesel. Flasks were incubated for 7 and 14 days at 30 °C at 150 rpm and samples were withdrawn at regular time intervals and the remnant diesel oil was quantified by gravimetric method [24]. The residual amount of biodegraded diesel oil was estimated by the formula as given below [8].
Gas Chromatographic (GC) Analysis
The Gas chromatography (GC) coupled to time-of-flight mass spectrometry (TOF–MS), (Agilent 7890, USA) was used for analysis of crude oil after subjecting to biodegradation [25]. Prior to injecting to GC-FID, diesel samples were dissolved in HPLC grade n-hexane and the samples were analysed using a DB-5 capillary column (30 mm × 0.32 mm internal diameter, 0.1 mm thickness) with Helium as a carrier gas at a constant flow rate of 2 ml/min and injector and detector temperatures as 250 °C and 370 °C, respectively.
FTIR Analysis
To elucidate the molecular mechanism of diesel degradation by the bacterial strains, FTIR spectra of the degraded diesel products [10] was produced using FTIR spectroscope (Alpha II, Bruker Optics, Germany). The spectra were recorded in the mid infrared range of 4000 to 500 cm−1 along with 100 scans for each sample.
Statistical Analysis
All experiments were performed in triplicate and the average values ± SD are reported at significance value p < 0.05.
Results and Discussion
Isolation and Identification of Diesel Degrading Bacteria
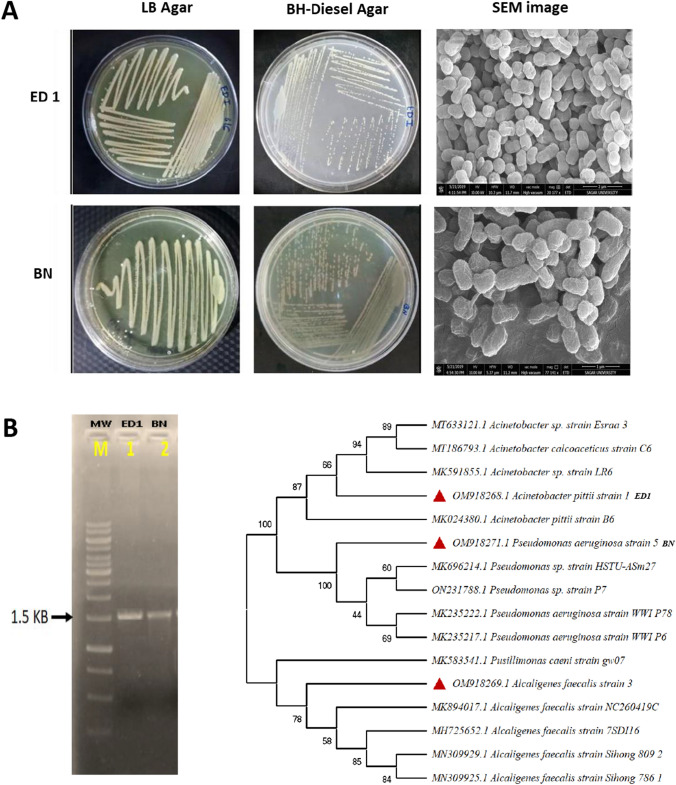
In this study, 13 bacterial strains were isolated from diesel contaminated soils. Identification of the newly isolated promising diesel degrading bacteria was done using 16S rRNA gene sequence analysis (Fig. 1a, b). Based on phylogenetic analysis constructed using Neighbour-Joining (NJ) method by MEGA 7 of 16S rRNA gene sequences, their identity was confirmed as Acinetobacter pittii ED1 and Pseudomonas aeruginosa BN (Suppl Fig. 1). The genome sequence of the newly isolated bacterial strains was deposited in NCBI GenBank platform (OM918268 and OM918271).
Fig. 1.
Identification of the diesel oil degrading bacterial strains, a Growth response and scanning electron micrograph (SEM) image of Acinetobacter pittii Strain ED1 and Pseudomonas aeruginosa Strain BN; b 16S rRNA gene sequence-based phylogenetic tree showing relationship with previously described closest relatives (LB Luria Bertani, BH Bushnell Haas)
Optimization of Growth Parameters in Diesel Enriched Medium
The effect of temperature on the growth of A. pittii ED1 and P. aeruginosa BN is depicted in Fig. 2a. Based on the results, it was concluded that optimal growth temperature and pH were 30 °C and 7.0, respectively, for both the diesel degrading bacteria (Fig. 2b). In a recent study that explores marine bacteria for oil degradation, Bacillus licheniformis produced lipase (72 U/ml) on medium containing 1% crude oil as the sole carbon and energy source [26]. P. aeruginosa BN showed better growth than A. pittii ED1 at alkaline pH. It was observed that 1% NaCl was optimal for the growth of A. pittii ED1, as further increase in the salinity reduced the growth significantly (Fig. 2c). However, for P. aeruginosa BN, although 1% NaCl supported good growth, but the strain could also tolerate 3% NaCl (Suppl Fig. 2c). Increase in the salinity (1 mM to 5 M) promoted the growth of Acinetobacter baumannii in MS medium containing 4% diesel oil and further increase in diesel concentration led to cessation of the bacterial growth, possibly due to hydrocarbon stress [27]. However, higher concentrations of NaCl (5%) delayed the onset of mineralization of both 14C-hexadecane and 14C-phenanthrene [28]. Both the bacterial strains showed no growth on the other test hydrocarbons excepting diesel (Fig. 2d). In a similar study, Hong et al. [29] revealed that over period of 13 days in a soil-slurry phase, P. aeruginosa IU5 degraded about 60% of the applied diesel. In the present study, it was observed that the increase in NaCl concentration beyond 1% (w/v) led to the increase in lag time and decreased the rate and extent of mineralization of aliphatic and aromatic substrates. Low concentrations of salt (≤ 1% NaCl) slightly stimulated diesel mineralization in some cases [30]. The result of bacterial growth in various diesel concentrations is represented in Fig. 2e. A. pittii ED1 and P. aeruginosa BN showed optimal growth at 1% diesel concentration. The present study is in agreement with the concentration dependent effect of diesel on the bacterial growth. Similarly, Luo et al. [31] have concluded that optimum diesel concentration was 2%, wherein Pseudomonas sp. showed highest biodegradation 14.19% and 11.97%, while Acinetobacter sp. degraded only 8.2% and 6.09%, respectively, for diesel (4% v/v) and burnt engine oil (8% v/v) after 7 days of incubation. According to the study, all of the strains grew at a slower pace as diesel oil concentration in the medium increased. Diesel is needed as a carbon source but at certain concentrations, diesel can be toxic to microorganisms due to the solvent effect of diesel which destroys bacterial cell membrane [32]. With regard to protein source, A. pittii ED1 showed slightly better growth in tryptone supplemented medium than peptone (Fig. 2f) whereas, the growth of P. aeruginosa BN was better in medium supplemented with inorganic nitrogen (Suppl Fig. 2). A comparative account of diesel and petroleum hydrocarbon degrading ability of various Acinetobacter and Pseudomonas spp. from recent literature is presented in Table 1. Accordingly, the strains reported in the present study appear to be efficient diesel degraders.
Fig. 2.
Growth pattern or growth response of A. pittii strain ED1 at different a temperature, b pH, c NaCl concentration, d carbon sources, e diesel concentration, f nitrogen sources
Table 1.
Comparative account of the diesel and petroleum hydrocarbon degrading Acinetobacter and Pseudomonas spp.
| SN | Bacterial strain | Source | Substrate | Conditions | Degradation | References |
|---|---|---|---|---|---|---|
| 1 | Acinetobacter haemolyticus MJ01 | Diesel oil contaminated site | Diesel oil | 30 °C, 7.0 pH, 7 days | 97.7% | [33] |
| 2 | A. pittii ABC | Oily sludge sediments | Crude oil | 10 days | 99.9% | [34] |
| 3 | A. calcoaceticus CA16 | Canola roots | Diesel oil | 28 °C, 6.0 pH, 28days | 82—92% | [35] |
| 4 | A. baumannii | Petroleum waste dumping site | Diesel oil | 30 °C, 7.0 pH, 21days | 61.43% | [36] |
| 5 | A. junii WCO-9 | Oil-contaminated soil | p-nitrophenyl decanoate, edible oils | 30 °C, 6.0 pH | – | [37] |
| 6 | A. pittii Strain ED 1 | Diesel oil contaminated site | Diesel oil | 30 °C, 7.0 pH,21 days | 98.3% | This study |
| 7 | Pseudomonas sp. WD23 | Petroleum refinery effluent | Petroleum | 100 RPM,7.5 pH, 28 days | 88.1% | [38] |
| 8 | P. aeruginosa AKS1 | Assam refinery | Hydrocarbon | 1% crude oil | Half-life 18.09 days | [39] |
| 9 | P. nitroreduens S8, P. monteilii S17 | Polyethylene tetraphthalate waste | Polyethylene terephthalate | 6 days | 94.5% | [40] |
| 10 | P. aeruginosa MFO69166 | Crude oil-contaminated site | Crude oil | 21 days | 87% | [41] |
| 11 | P. aeruginosa PP4 | Automobile oil contaminated soil | Hydrocarbon | 4.0 and 7.0 pH | 68% and 78% | [42] |
| 12 | P. aeruginosa | Refinery site | Dibenzothiophene in diesel | 30 °C | 70% | [17] |
| 13 | P. aeruginosa Strain BN | Diesel oil contaminated site | Diesel oil | 30 °C, 7.0 pH, 21 days | 96.5% | This study |
Biofilm Formation by Diesel Degrading Bacteria
Both the bacterial strains produced biofilm upon exposure to diesel based environmental stress. Significant differences in the biofilm formation were observed with the variation in the parameters. LB medium promoted biofilm formation at both temperatures 30 °C and 40 °C for both the bacterial strains (Fig. 3a) than the MS medium. P. aeruginosa BN produced more biofilm when inoculated in LB medium compared to MS medium with 1% diesel. However, MS medium with 1% diesel resulted in better biofilm formation at both the temperature for A. pittii ED1 as compared to P. aeruginosa BN. This indicated that A. pittii ED1 is more robust in tolerating diesel induced abiotic stress than P. aeruginosa BN.
Fig. 3.
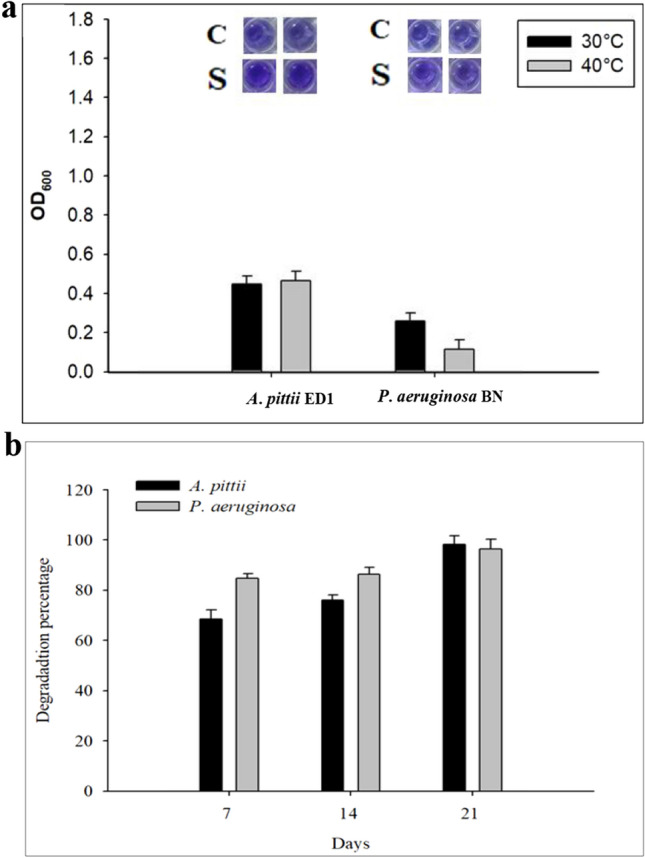
Biofilm formation and diesel degradation by A. pitti ED1 and P. aeruginosa BN. a The efficiency of biofilm formation of the diesel degrading bacterial strains in two types of culture media supplemented with 1% diesel a Luria Bertani (LB) medium; b Mineral Salt (MS) medium. b Degradation of diesel by bacterial strains as determined using gravimetric method after 7 days (68.6; 77.3%), 14 days (76.0; 84.71%) and 21 days (98.3; 96.5%) of incubation by A. pitti ED1 and P. aeruginosa BN, respectively
Gravimetric Analysis of Diesel Degradation
Gravimetric analysis of the spent medium after 7 days of incubation showed that A. pittii ED1 and P. aeruginosa BN degraded 68.61% and 76% diesel, while it reached more than 90% after 21 days (Fig. 3b). Ljesevic et al. [43] reported diesel degradation up to 77.4% based on gravimetric analysis after 30 days by Oerskovia sp., while Acinetobacter sp. showed 8.2% and 6.09% of reported percentage of diesel and engine oil, respectively after 7 days incubation. Diesel degradation by Raultella planticola M01 and Acinetobacter calcoaceticus M1B after addition of surfactants reached upto 92% and 38% after 14 days of incubation [44] after14 days (92%) of the process. Glucose addition influences the microbial enumeration so that degradation also increases. P. aeruginosa and P. putida bacterial strains were successfully employed for desulfurization of synthetic and real diesel [17].
Gas Chromatographic Analysis of the Degradation Products
Time dependent analysis of diesel biodegradation by newly isolated bacterial strains showed complete degradation of diesel hydrocarbons in 14 days by A. pittii ED1 and P. aeruginosa BN. Precisely, GC chromatograph of A. pittii ED1 degradation products appearing at 4.69, 6.09, 7.04, 7.45, 13.52, 14.56, 15.56, 16.50, 17.41, 18.28, 19.12 and 19.92 retention time (RT) after 7 days of incubation. In case of P. aeruginosa BN, degradation products appeared at 4.69, 6.07, 14.56, 15.56, 16.50, 17.41, 18.28, 19.12 5.99, 14.22, 14.82 and 19.92 RT after 7 days of incubation (Suppl Fig. 3). However, after 14 days absence of any peak in gas chromatogram indicated total degradation of the hydrocarbons. Similar GC analysis of diesel degradation using Gordonia amicalis HS-11 [45] showed complete degradation after 16 days, while Staphylococcus epidermidis EVR4 [46] strain also potentially degraded diesel with 96% efficacy and degradation products appearing at 4.36 to 22.44 RT.
FTIR Analysis of the Degraded Diesel
The FTIR analysis of the degraded diesel revealed presence of C–O stretch (724 cm−1, 1374.13 cm−1), C=O aromatic stretch (1457.08 cm−1), C=C stretch (1591 cm−1, 1636 cm−1), C-H stretch (2856.97 cm−1), di-substituted benzene (2092 cm−1, 2919 cm−1), N–H stretch (3343 cm−1, 3704 cm−1and 3783 cm−1) (Suppl Fig. 4). The spectrum has the various CH stretch bands usually exhibited by hydrocarbons near 4000 cm−1. FTIR analysis of the diesel degraded by Acinetobacter venetianus peaks at 2956.87 cm−1, 2924.09 cm−1, 2854.65 cm−1, 1629.85 cm−1, 1467.83 cm−1, and 1091.71 cm−1 corresponding to the CH, OH, and NH stretch of alkanes, alcohols, and amines [47]. Similar results for control and biodegraded diesel oil were reported [48] and large number of bands of FTIR of degraded diesel by immobilized A. venetianus, ranged between at 1300–1500 cm−1, concluded diesel oil was degraded and new bands were formed.
Conclusion
Biodegradation of diesel based on the potential of native bacteria is an important bioremediation approach. Isolated bacterial strains had ability to utilize diesel as the sole carbon source and showed more than 90% diesel degradation after 21 days. Qualitative and quantitative techniques were used for the determination of residual hydrocarbons of diesel. Gravimetric method applied to the quantitative evaluation determined 68.61% and 84.71% degradation of diesel oil in 7 days, while sophisticated instruments gas chromatography and FTIR were used to the qualitative evaluation of the degraded hydrocarbons of diesel. Present study also suggested that strains A. pittii ED1 and P. aeruginosa BN have potential for bioremediation of diesel oil polluted environments.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
There is no conflict of interest, whatsoever.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sonam Dohare and Hemant Kumar Rawat have contributed equally to this work.
References
- 1.Kumar R, De M. Enhanced degradation of petroleum hydrocarbons by Klebsiella michiganensis RK and Acinetobacter baumannii IITG19 isolated from local soil sources. Int J Environ Sci Technol. 2023;20:13387–13398. doi: 10.1007/s13762-023-04790-3. [DOI] [Google Scholar]
- 2.Yang H, Kim G, Cho KS. Bioaugmentation of diesel-contaminated soil with Pseudomonas sp. DTF1. Int J Environ Sci Technol. 2023;20:12499–12510. doi: 10.1007/s13762-023-04846-4. [DOI] [Google Scholar]
- 3.Xue J, Wu Y, Liu Z, et al. Characteristic assessment of diesel-degrading bacteria immobilized on natural organic carriers in marine environment: the degradation activity and nutrient. Sci Rep. 2017;7:8635. doi: 10.1038/s41598-017-08832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosal D, Ghosh S, Dutta TK, Ahn Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): a review. Front Microbiol. 2016 doi: 10.3389/fmicb.2016.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imam A, Kanaujia PK, Ray A, Suman SK. Removal of petroleum contaminants through bioremediation with integrated concepts of resource recovery: a review. Indian J Microbiol. 2021;61:250–261. doi: 10.1007/s12088-021-00928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huettel M. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the deepwater horizon oil spill. Appl Environ Microbiol. 2011;77:7962–7974. doi: 10.1128/AEM.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novas-Anguita Z, García-Gusano D, Iribarren D. A review of techno-economic data for road transportation fuels. Renew Sustain Energy Rev. 2019;112:11–26. doi: 10.1016/j.rser.2019.05.041. [DOI] [Google Scholar]
- 8.Farber R, Rosenberg A, Rozenfeld S, Benet G, Cahan R. Bioremediation of artificial diesel-contaminated soil using bacterial consortium immobilized to plasma-pretreated wood waste. Microorganisms. 2019;7:1–17. doi: 10.3390/microorganisms7110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patowary K, Patowary R, Kalita MC, Deka S. Development of an efficient bacterial consortium for the potential remediation of hydrocarbons from contaminated sites. Front Microbiol. 2016;7:1–14. doi: 10.3389/fmicb.2016.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaczorek E, Cieślak K, Bielicka-Daszkiewicz K, et al. The Influence of rhamnolipids on aliphatic fractions of diesel oil biodegradation by microorganism combinations. Indian J Microbiol. 2013;53:84–91. doi: 10.1007/s12088-012-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patowary K, Patowary R, Kalita MC, Deka S. Characterization of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Front Microbiol. 2017;8:1049. doi: 10.3389/fmicb.2017.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bento FM, Camargo FAO, Okeke BC, Frankenberger WT. Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour Technol. 2005;96:1049–1055. doi: 10.1016/j.biortech.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hawash AB, Alkooranee JT, Abbood HA, Zhang J, Sun J, Zhang X, Ma F. Isolation and characterization of two crude oil-degrading fungi strains from Rumaila oil field, Iraq. Biotechnol Rep. 2018;17:104–109. doi: 10.1016/j.btre.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi XQ, Zhang JJ, Zhao S, Zhou NY. Bioaugmentation with a consortium of bacterial nitrophenol-degraders for remediation of soil contaminated with three nitrophenol isomers. Environ Pollut. 2013;172:33–41. doi: 10.1016/j.envpol.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Mujahid TY, Wahab A, Padhiar SH, Subhan SA, Baloch MN, Pirzada ZA. Isolation and characterization of hydrocarbon degrading bacteria from petroleum contaminated sites. Asian J Microbiol Biotechnol Environ Sci. 2018;20:1255–1259. doi: 10.6000/1927-5129.2015.11.32. [DOI] [Google Scholar]
- 16.Onur G, Yilma ZF, Icgen B. Diesel oil degradation potential of a bacterium inhabiting petroleum hydrocarbon contaminated surface waters and characterization of its emulsification ability. J Surfactants Deterg. 2015;18:707–717. doi: 10.1007/s11743-015-1697-3. [DOI] [Google Scholar]
- 17.Sadare OO, Daramola MO. Bio-catalytic degradation of dibenzothiophene (DBT) in petroleum distillate (diesel) by Pseudomonas spp. Sci Rep. 2023;13:6020. doi: 10.1038/s41598-023-31951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastida F, Eldridge DJ, García C, et al. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISMEJ. 2021;15:2081–2091. doi: 10.1038/s41396-021-00906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mara K, Decorosi F, Viti C, Giovannetti L, Cristiana M, Maida I, Perrin E, Fondi M, Vaneechoutte M, Nemec A, Van Den Barselaar M, Dijkshoorn L, Fani R. Molecular and phenotypic characterization of Acinetobacter strains able to degrade diesel fuel. Res Microbiol. 2012;163:161–172. doi: 10.1016/j.resmic.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Koolivand A, Abtahi H, Parhamfar M, Saeedi R, Coulon F, Kumar V, Villaseñor J, Sartaj M, Najarian N, Shahsavari M, Seyedmoradi P, Rahimi L, Bagheri F. The effect of petroleum hydrocarbons concentration on competition between oil-degrading bacteria and indigenous compost microorganisms in petroleum sludge bioremediation. Environ Technol Innov. 2022 doi: 10.1016/j.eti.2022.102319. [DOI] [Google Scholar]
- 21.Assil Z, Esegbue O, Mašek O, Gutierrez T, Free A. Specific enrichment of hydrocarbon clastic bacteria from diesel-amended soil on biochar particles. Sci Total Environ. 2021;762:143084. doi: 10.1016/j.scitotenv.2020.143084. [DOI] [PubMed] [Google Scholar]
- 22.Salam LB, Obayori OS, Hawa O. Hydrocarbon degradation and biosurfactant production by an acenaphthene-degrading Pseudomonas species. Soil Sediment Contam. 2016;25:837–856. doi: 10.1080/15320383.2016.1217826. [DOI] [Google Scholar]
- 23.Yan S, Wang Q. Characterization of oil-degrading bacteria from oil-contaminated soil and activity of their enzymes. Biotechnol Biotechnol Equip. 2013;27:3932–3938. doi: 10.5504/BBEQ.2013.0050. [DOI] [Google Scholar]
- 24.Gao J, Ming J, Xu M, et al. Isolation and characterization of a high-efficiency marine diesel oil-degrading bacterium. Pet Sci. 2021;18:641–653. doi: 10.1007/s12182-020-00540-z. [DOI] [Google Scholar]
- 25.Varjani SJ, Rana DP, Jain AK, Bateja S, Upasani VN. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int Biodeterior Biodeg. 2015;103:116–124. doi: 10.1016/j.ibiod.2015.03.030. [DOI] [Google Scholar]
- 26.Srimathi M, Suganthi M, Sugitha S, et al. Characterization of crude oil degrading marine bacterium Bacillus licheniformis. Indian J Microbiol. 2024 doi: 10.1007/s12088-024-01222-9. [DOI] [Google Scholar]
- 27.Palanisamy N, Ramya J, Kumar S, Vasanthi NS, Chandran P. Diesel biodegradation capacities of indigenous bacterial species isolated from diesel contaminated soil. J Environ Heal Eng. 2014 doi: 10.1186/s40201-014-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulrich AC, Guigar SE, Foght JM, Semple KM, Pooley K, Armstrong JE, Biggar KW. Effect of salt on aerobic biodegradation of petroleum hydrocarbons in contaminated groundwater. Biodegradation. 2009;20:27–38. doi: 10.1007/s10532-008-9196-0. [DOI] [PubMed] [Google Scholar]
- 29.Hong JH, Kim J, Choi OK, Cho KS, Ryu HW. Characterization of a diesel-degrading bacterium, Pseudomonas aeruginosa IU5, isolated from oil-contaminated soil in Korea. World J Microbiol Biotechnol. 2005;21:381–384. doi: 10.1007/s11274-004-3630-1. [DOI] [Google Scholar]
- 30.You Y, Shim J, Cho CH, Ryu MH, Shea PJ, Kamala-Kannan S, Chae JC, Oh BT. Biodegradation of BTEX mixture by Pseudomonas putida YNS1 isolated from oil-contaminated soil. J Basic Microbiol. 2013;53:469–475. doi: 10.1002/jobm.201200067. [DOI] [PubMed] [Google Scholar]
- 31.Luo Q, Zhang JG, Shen XR, Sui X, Fan ZQ. Characterization of a novel diesel oil-degrading Pseudomonas sp. strain F4. Fresenius Environ Bull. 2013;22:689–697. [Google Scholar]
- 32.Shukor MY, Dahalan FA, Jusoh AZ, Muse R, Shamaan NA, Syed MA. Characterization of a diesel-degrading strain isolated from a hydrocarbon-contaminated site. J Environ Biol. 2009;30:145–150. doi: 10.54987/jebat.v1i1.24. [DOI] [PubMed] [Google Scholar]
- 33.Lee M, Woo SG, Ten LN. Characterization of novel diesel-degrading strains Acinetobacter haemolyticus MJ01 and Acinetobacter johnsonii MJ4 isolated from oil-contaminated soil. World J Microbiol Biotechnol. 2012;28:2057–2067. doi: 10.1007/s11274-012-1008-3. [DOI] [PubMed] [Google Scholar]
- 34.Chettri B, Singha NA, Mukherjee A, Rai AN, Chattopadhyay D, Singh AK. Hydrocarbon degradation potential and competitive persistence of hydrocarbon oclastic bacterium Acinetobacter pittii strain ABC. Arch Microbiol. 2019;201:1129–1140. doi: 10.1007/s00203-019-01687-z. [DOI] [PubMed] [Google Scholar]
- 35.Ho MT, Li MSM, McDowell T, MacDonald J, Yuan ZC. Characterization and genomic analysis of a diesel-degrading bacterium, Acinetobacter calcoaceticus CA16, isolated from Canadian soil. BMC Biotechnol. 2020 doi: 10.1186/s12896-020-00632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jerin I, Rahi MS, Sultan T, Islam MS, Sajib SA, Hoque KMF, Reza MA. Diesel degradation efficiency of Enterobacter sp., Acinetobacter sp. and Cedecea sp. isolated from petroleum waste dumping site: a bioremediation view point. Archives Microbiol. 2021;203:5075–5084. doi: 10.1007/s00203-021-02469-2. [DOI] [PubMed] [Google Scholar]
- 37.Jiang S, Fan Q, Zhang Z, Deng Y, Wang L, Dai Q, Wang J, Lin M, Zhou J, Long Z, He G, Zhou Z. Biodegradation of oil by a newly isolated strain Acinetobacter junii wco-9 and its comparative pan-genome analysis. Microorganisms. 2023;11:407. doi: 10.3390/microorganisms11020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goveas LC, Selvaraj R, Vinayagam R, Alsaiari AA, Alharthi NS, Sajankila SP. Nitrogen dependence of rhamnolipid mediated degradation of petroleum crude oil by indigenous Pseudomonas sp. WD23 in seawater. Chemosphere. 2022;304:135235. doi: 10.1016/j.chemosphere.2022.135235. [DOI] [PubMed] [Google Scholar]
- 39.Chettri B, Singha NA, Singh AK. Efficiency and kinetics of Assam crude oil degradation by Pseudomonas aeruginosa and Bacillus sp. Archives Microbiol. 2021;203:5793–5803. doi: 10.1007/s00203-021-02567-1. [DOI] [PubMed] [Google Scholar]
- 40.Yan ZF, Xu KW, Wu J. Synergism between multi-Pseudomonas and cutinase for biodegradation of crude oil-based derivatives. Curr Microbiol. 2022;80:30. doi: 10.1007/s00284-022-03139-2. [DOI] [PubMed] [Google Scholar]
- 41.Rehman R, Ali MI, Ali N, Badshah M, Iqbal M, Jamal A, Huang Z. Crude oil biodegradation potential of biosurfactant-producing Pseudomonas aeruginosa and Meyerozyma sp. J Haz Mat. 2021;418:126276. doi: 10.1016/j.jhazmat.2021.126276. [DOI] [PubMed] [Google Scholar]
- 42.Muthukumar B, Al Salhi MS, Narenkumar J, Devanesan S, Rao TN, Kim W, Rajasekar A. Characterization of two novel strains of Pseudomonas aeruginosa on biodegradation of crude oil and its enzyme activities. Environ Pollut Barking Essex 1987. 2022;304:119223. doi: 10.1016/j.envpol.2022.119223. [DOI] [PubMed] [Google Scholar]
- 43.Ljesevic M, Gojgic-Cvijovic G, Teruyo I, HashimotoS NT, Bulatovic S, Ilic M, Beskoski V. Biodegradation of the aromatic fraction from petroleum diesel fuel by Oerskovia sp. followed by comprehensive GC×GC-TOF MS. J Hazar Mate. 2019;363:227–232. doi: 10.1016/j.jhazmat.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Zdarta A, Smułek W, Pacholak A, Dudzińska-Bajorek B, Kaczorek E. Surfactant addition in diesel oil degradation—how can it help the microbes? J Environ Health Sci Eng. 2020;18:677–686. doi: 10.1007/s40201-020-00494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sowani H, KulkarniM ZS. Uptake and detoxification of diesel oil by a tropical soil actinomycete Gordonia amicalis HS-11: cellular responses and degradation perspectives. Environ Pollut. 2020;263:114538. doi: 10.1016/j.envpol.2020.114538. [DOI] [PubMed] [Google Scholar]
- 46.Vaishnavi J, Devanesan S, Al Salhi MS, RajasekarA SelviA, Srinivasan P, Govarthanan M. Biosurfactant mediated bioelectrokinetic remediation of diesel contaminated environment. Chemosphere. 2021;264:128377. doi: 10.1016/j.chemosphere.2020.128377. [DOI] [PubMed] [Google Scholar]
- 47.Revathy T, Jayasri MA, Suthindhiran K. Biodegradation of PAHs by Burkholderia sp. VITRSB1 isolated from marine sediments. Scientifica. 2015;1:1–9. doi: 10.1155/2015/867586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, YuB LJ, Naidu R, Chen Z. Simultaneous adsorption and biodegradation (SAB) of diesel oil using immobilized Acinetobacter venetianus on porous material. Chem Eng J. 2016;289:463–470. doi: 10.1016/j.cej.2016.01.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.