Abstract
Purpose
Estradiol valerate (Progynova®) is used as hormone therapy to supplement estrogen deficiency. This study aimed to assess the bioequivalence of an estradiol valerate tablet and its generic form, under fasting and fed conditions.
Methods
A randomized, open-label, single-dose, 2-period crossover study was conducted on healthy postmenopausal Chinese female volunteers under fasting and fed conditions. For each period, the subjects received either a 1 mg tablet of estradiol valerate or its generic. Blood samples were collected before dosing and up to 72 hours after administration. Plasma levels of total estrone, estradiol, and unconjugated estrone were quantified using a validated liquid chromatography-tandem mass spectrometry method.
Results
A total of 54 volunteers were enrolled in this study. The primary pharmacokinetic parameters, including Cmax, AUC0-t, and AUC0-∞, were similar for the two drugs under both fasting and fed conditions, with 90% confidence intervals for the geometric mean ratios of these parameters, all meeting the bioequivalence criterion of 80–125%. A total of 48 adverse events (AEs) were reported in the fed study compared with 24 AEs in the fasting study.
Conclusion
Estradiol valerate and its generic form were bioequivalent and well tolerated under both fasting and fed conditions.
Keywords: bioequivalence, estradiol valerate, postmenopausal, pharmacokinetics, safety
Introduction
Estradiol is a crucial natural estrogen in females that plays a significant role in maintaining secondary sexual characteristics, fertility, and various physiological systems. Insufficient estradiol levels can lead to menstrual irregularities, infertility,1 and various symptoms during perimenopause including hot flashes, vulvovaginal atrophy, dyspareunia, urinary incontinence, and sleep disorders.2 Hormone therapy (HT) is commonly used to alleviate these symptoms and to prevent related diseases.3 Estradiol valerate, an oral long-acting estrogen supplement, is important for HT.4 It has also been approved for the prevention of pregnancy and the treatment of heavy menstrual bleeding.5,6
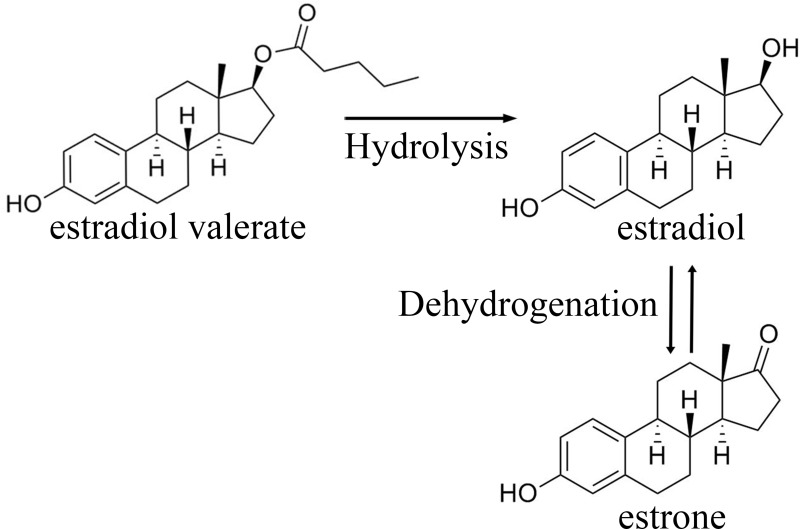
After oral administration of estradiol valerate, cleavage of 17β-estradiol and valeric occurs during absorption in the intestine or first liver passage. This process results in the production of estradiol and its metabolites including estrone (Figure 1). Ninety-five percent of the administered dose is metabolized by cytochrome P450 3A enzyme, which then enters the systemic circulation thereafter.7 A maximum serum estradiol concentration of 73.3 pg/mL was achieved approximately 6 h after a single administration of a tablet containing 3 mg of estradiol valerate under fasting conditions.8 For a tablet containing 2 mg estradiol valerate under fasting conditions, maximum serum estradiol concentrations of 30.79 pg/mL are achieved at approximately 8 hours.9 Estradiol binds extensively (98%) to serum proteins, primarily albumin (61%) and sex hormone-binding globulin (37%).10,11 Estradiol and its metabolites are mainly excreted in urine, with a terminal half-life of approximately 14–17 hours.8,12
Figure 1.
Estradiol valerate and its metabolites.
Estradiol valerate tablets, which were initially developed in Germany, have been approved for multiple indications. Despite these applications, generic versions of estradiol valerate tablets have not been successfully developed. Generic drugs offer significant economic and societal benefits including reduced healthcare costs and improved medication accessibility. This study, designed according to FDA guidelines, aimed to assess the bioequivalence (BE) and safety of estradiol valerate tablets and its generic in healthy Chinese postmenopausal female volunteers under fasting and fed conditions.
Materials and Methods
Study Drugs
An estradiol valerate tablet (strength: 1 mg, batch number: 200101, expiry date: 2024/12) was produced by Zhejiang Xianju Pharmaceutical Co., Ltd. The reference preparation (strength: 1 mg; batch number: 371A; expiry date: 2022/4) was produced by DELPHARM Lille S.A.S.
Subjects and Ethics
Eligible participants were healthy postmenopausal females aged 45–65 years with a body mass index within the range of 18–28 kg/m2. They weighed more than 45 kg and had been in the postmenopausal period for more than 12 months. Other eligibility criteria included endometrial thickness <5 mm, follicle-stimulating hormone (FSH) above 40 IU/l, and estradiol level <110 pmol/l. The health status of the subjects was evaluated based on their medical history, physical examination, 12-lead electrocardiography, low-dose chest CT, and laboratory tests.
The exclusion criteria were a history of significant illness or allergy, abuse of drugs or alcohol (2 units/day), excessive smoking (5 cigarettes/day), regular intake of caffeine or tea (8 cups/day), use of any drug within the past 14 days, use of any other investigational drugs within the past 90 days, and a positive result in the alcohol breath test or positive screening for drug abuse and nicotine. Subjects were also excluded if they had a history of breast cancer, endometrial cancer, or other estrogen-dependent tumors; a history of deep vein thrombosis or other thrombosis; gynecological transvaginal color Doppler ultrasound indicating uterine fibroids or submucosal fibroids larger than 2 cm, adenomyosis, endometrial polyps, or ovarian tumors; use of estrogen or progestogen in the past 6 months; received thyroid hormone replacement therapy; received a vaccine in the past 7 days; underwent gynecological or surgical procedures in the past 6 months; donated blood or experienced significant bleeding (450 mL) in the past 2 months; consumed grapefruit in the past 3 days; used drugs inhibiting or inducing hepatic drug-metabolizing enzymes in the past 30 days; or had an intolerance to venipuncture or a history of fainting or needle phobia.
This study was approved by the Independent Ethics Committee of Clinical Trials in Deyang People’s Hospital. This study was conducted in accordance with the principles of the Declaration of Helsinki and the requirements for Good Clinical Practice. All participants provided written informed consent prior to participating in the study. This trial was registered at the Chinese Clinical Trial Center (ChiCTR2300072157). The execution dates of the study were from April 7, 2022, to July 2, 2022.
Study Design
This study was conducted at the Phase 1 Clinical Trial Center of the Deyang People’s Hospital. It consisted of two separate parts: fasting and fed. Each part was a randomized, open-label, single-dose, 2-period crossover study.
Based on the results of a previous clinical study,13 the intra-individual coefficient of variation (CV) of estradiol was approximately 15% and the geometric mean ratio (GMR) was about 0.95 under fasting condition. To achieve an equivalence interval of 80–125% with 90% power and a bilateral significance level of 0.05, the calculated sample size was 16. Considering potential dropouts and visit losses, the final sample size for the fasting study was set at 24. For the fed study, the inter-individual CV was approximately 15%, and the GMR was approximately 0.90. The final sample size of this study was 30.
All participants were randomly assigned to the sequence reference test (RT) or the test reference (TR) at the beginning of the study. Sequence TR received the 1 mg test tablet of estradiol valerate in the first period and the reference tablet in the second period. Sequence RT had the opposite administration sequence. The two dosing periods were separated using a 7-day washout period.
The subjects were admitted to the study center one or 2 days before drug administration for further evaluation of their eligibility through inquiries and examinations. In the fasting study, the test or reference product was administered with 240 mL of warm water after an overnight fast of at least 10 h. In the fed study, subjects fasted for at least 10 h and then consumed a high-fat, high-calorie breakfast (800–1000 kcal) within 30 min before drug administration. Standard diets were provided at 4 and 10 h after dosing. Water consumption was prohibited within 1 h before and 2 h after dosing, under both fasting and fed conditions. The intake of alcohol, food, or beverages containing caffeine or xanthine (such as coffee, tea, chocolate, and cola) was not permitted from 3 days before administration until the end of the study. Additionally, the participants were not allowed to leave the center freely and bring their own food, smoke, or drink.
Blood Sample
Sample collection should encompass the absorption, distribution, and elimination of drugs. The sampling time should not be less than 3 times the terminal elimination half-life. Given that the terminal elimination half-life of estradiol is approximately 14–17 hours,12 sampling time was set at 72 h. In the fasting study, 24 blood samples were collected at 1 h, 30 min, and 0 h before dosing; at 20 and 40 min; and at 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 24, 48, and 72 h after dosing. Food intake can decrease the time to peak of estradiol and increase its maximum concentration, but it does not alter estradiol exposure.8,9,14 Therefore, in the fed study, 25 blood samples were collected at 1 h, 30 min, and 0 h before dosing, and at 15, 30, 45 min, 1, 1.25, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 9, 10, 12, 14, 24, 48, and 72 h after dosing. Approximately 6 mL of venous blood was collected and transferred to anti-coagulation tubes containing ethylene diamine tetraacetic acid-K2. The samples were centrifuged at 1200 × g for 10 minutes at 2–8°C within 60 min of collection. The plasma was then separated and placed into three labeled cryotubes, which were temporarily stored at −20°C within 90 min and subsequently transferred to a temperature not higher than −60°C within 24 h.
Sample Analysis
The plasma concentrations of total estrone, estradiol, and unconjugated estrone were determined using a validated liquid chromatography tandem mass spectrometer (Hangzhou Leading Pharmatech Co., Ltd, Hangzhou, China). Plasma concentrations after administration were corrected by subtracting the baseline concentration, which was calculated as the mean concentration 1, 0.5, and 0 h before dosing. Negative concentrations were adjusted to zero. The linear quantitation ranges were as follows: 0.10–50.00 ng/mL for the total estrone, 1.00–100.00 pg/mL for the estradiol, and 5.00–500.00 pg/mL for unconjugated estrone. Lower limits of quantitation (LLOQ) were 0.10 ng/mL for total estrone, 1.00 pg/mL for estradiol, and 5.00 pg/mL for unconjugated estrone, respectively. The maximum precisions (CV%) of total estrone, estradiol, and unconjugated estrone were 4.6%, 14.4%, and 9.0%, respectively. The accuracies of the total estrone, estradiol, and unconjugated estrone across the assay range were all within −1.3%–10.7%. The analytes in whole blood remained stable for 2 h in an ice bath. Similarly, the analytes in the plasma maintained their stability after storage at room temperature for 46 h. The treated plasma samples remained stable even after storage at 4°C for 140 hours. Furthermore, the analytes in the plasma remained stable even after storage at −20°C and −80°C for 42 days. The analytes in the plasma remained stable after five freeze–thaw cycles.
Safety Analysis
The safety analysis included all subjects who were administered either a test or a reference estradiol valerate tablet. Safety assessment included vital signs (blood pressure, pulse, and temperature), physical examination, laboratory tests (hematology, biochemistry, urinalysis, coagulation function, and thyroid function), electrocardiography, and observation of adverse events (AEs). Vital signs were monitored before dosing and at 2, 6, 12, 24, 48, and 72 h after drug administration. Other examinations were performed at the beginning and end of each period. All recorded AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE 5.0). Follow-up was conducted for all AEs until resolution or stabilization of the events.
Pharmacokinetic and Statistical Analysis
This study employed non-compartmental analysis using Phoenix WinNonlin 7.0 software (Certara Inc., Princeton, NJ, USA) and SAS 9.4 (SAS Institute, Inc., Cary, NC, United States) to calculate pharmacokinetic (PK) parameters, evaluate bioequivalence, and conduct statistical analysis on other data. The primary PK parameters included the maximum drug concentration in plasma (Cmax), area under the plasma concentration-time curve from time 0 to the last time point (AUC0-t), and area under the curve from 0 to infinity (AUC0-∞). The secondary PK parameters included the time to reach the peak concentration (Tmax), the elimination rate constant (λz), and observed elimination terminal half-life (t1/2). PK parameters were presented as the arithmetic mean value (mean) and standard deviation (SD). The value was treated as 0 when the plasma concentration was below the limit of quantification. A statistical analysis of the differences in PK parameters (Cmax, AUC0-t, AUC0-∞, t1/2) for total estrone, estradiol, and unconjugated estrone between the test formulation (Group T) and the reference formulation (Group R), both under fasting and fed conditions, was conducted using paired t-tests. The differences in Tmax for the same products between the two groups under both conditions were analyzed using the paired rank sum test. Additionally, we used the independent group t-test to analyze the demographic characteristics of the TR sequence and RT sequence groups under fasting and fed conditions, while the chi-square test was used to analyze the occurrence of adverse events. A p-value of less than 0.05 was considered statistically significant.
After a natural logarithmic transformation, GMRs (test/reference) and its 90% confidence intervals (CIs) were calculated. The BE between the two formulations was considered acceptable if the 90% CIs for the GMR of Cmax, AUC0–t, and AUC0-∞ were within the range of 80–125%. The BE of the two formulations was based on the PK parameters of the total estrone, while the PK parameters of the estradiol and unbound estrone are only used as reference and not as criteria for determining BE.
Results
Subjects
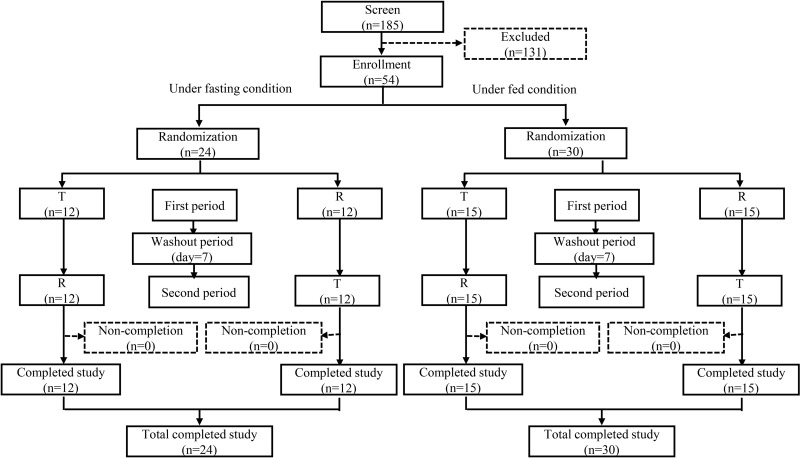
A total of 185 postmenopausal Chinese females were screened, and 54 eligible participants were enrolled in the study. All the enrolled participants were of Han ethnicity. The demographic characteristics of the subjects in the fasting and fed studies are presented in Table 1. The age, height, weight, and BMI of the participants were comparable across the treatment groups, both under fasting and fed conditions (p > 0.05). All participants, comprising 24 individuals in the fasting study and 30 individuals in the fed study, were randomized and successfully completed the trials (Figure 2).
Table 1.
Demographic Characteristics of Subjects in the Fasting and Fed Studies
| Characteristics | Fasting | Fed | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RT (N =12) | TR (N =12) | Total (N=24) | P | RT (N=15) | TR (N=15) | Total (N=30) | P | ||
| Age (years) | Mean ± SD | 53.75 ± 3.08 | 54.33 ± 4.48 | 54.04 ± 3.77 | 0.714 | 54.67 ± 3.56 | 54.00 ± 3.12 | 54.33 ± 3.30 | 0.590 |
| Median | 53.00 | 54.00 | 53.00 | 54.00 | 53.00 | 53.50 | |||
| Min, Max | 49.00, 59.00 | 46.00, 61.00 | 46.00, 61.00 | 50.00, 62.00 | 49.00, 59.00 | 49.00, 62.00 | |||
| Height (cm) | Mean ± SD | 153.61 ± 6.30 | 153.43 ± 4.06 | 153.52 ± 5.18 | 0.936 | 153.65 ± 3.79 | 152.91 ± 4.43 | 153.28 ± 4.07 | 0.624 |
| Median | 156.50 | 154.15 | 154.80 | 153.50 | 152.60 | 153.05 | |||
| Min, Max | 142.60, 161.50 | 146.80, 161.20 | 142.60, 161.50 | 147.90, 161.50 | 144.80, 162.20 | 144.80, 162.20 | |||
| Weight (kg) | Mean ± SD | 56.68 ± 7.02 | 57.35 ± 5.22 | 57.01 ± 6.06 | 0.792 | 56.95 ± 6.56 | 56.11 ± 5.97 | 56.53 ± 6.18 | 0.717 |
| Median | 55.10 | 57.20 | 56.55 | 55.40 | 55.10 | 55.25 | |||
| Min, Max | 45.20, 67.10 | 50.10, 68.40 | 45.20, 68.40 | 47.00, 70.00 | 48.70, 68.50 | 47.00, 70.00 | |||
| BMI (kg/m2) | Mean ± SD | 23.93 ± 2.04 | 24.33 ± 2.26 | 24.13 ± 2.12 | 0.654 | 24.05 ± 2.18 | 23.93 ± 2.12 | 23.99 ± 2.11 | 0.886 |
| Median | 24.20 | 23.40 | 23.65 | 23.50 | 24.10 | 23.70 | |||
| Min, Max | 20.30, 26.30 | 20.90, 27.40 | 20.30, 27.40 | 20.50, 27.80 | 20.90, 28.00 | 20.50, 28.00 | |||
Notes: The statistical method employed in this table is the independent group t-test.
Abbreviations: BMI,body mass index; SD, standard deviation; P, p-value.
Figure 2.
Study design and disposition of subjects.
Abbreviations: T, test drug; R, reference drug; n, number of subjects.
PK Results and BE Results
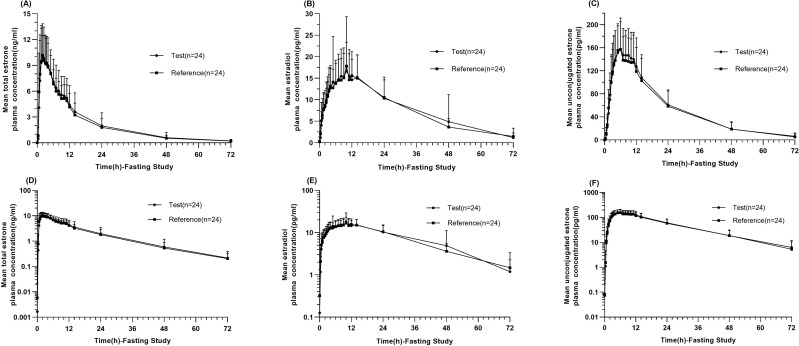
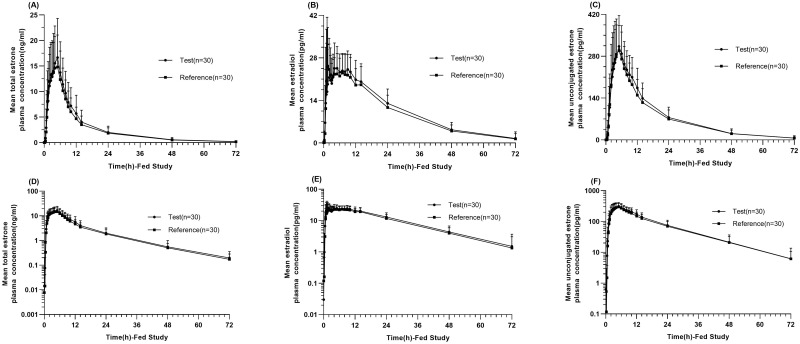
Linear and semi-logarithmic of the mean plasma concentration-time profiles of the total estrone, estradiol, and unconjugated estrone showed similarities between the reference and test estradiol valerate formulations under both fasting (Figure 3A–F) and fed conditions (Figure 4A–F). Blood samples from all 54 subjects were analyzed, and the major PK parameters of estrone, estradiol, and unconjugated estrone were calculated and summarized in Table 2 for both fasting and fed conditions. According to the BE set, all 90% CIs for the GMRs of Cmax, AUC0-t, and AUC0-∞ between the test and reference products were within the range of 80–125% under both fasting and fed conditions (Table 3).
Figure 3.
Pharmacokinetic analysis in the fasting study. Linear and Semi-logarithmic of mean plasma concentration-time profiles of total estrone (A and D), estradiol (B and E), and unconjugated estrone (C and F) after single oral administration of test and reference estradiol valerate tablet in the fasting study. Data represent the mean value, and error bars represent the standard deviation.
Figure 4.
Pharmacokinetic analysis in the fed study. Linear and Semi-logarithmic of mean plasma concentration-time profiles of total estrone (A and D), estradiol (B and E), and unconjugated estrone (C and F) after single oral administration of test and reference estradiol valerate tablet in the fed study. Data represent the mean value, and error bars represent the standard deviation.
Table 2.
Pharmacokinetic Parameters of Total Estrone, Estradiol, and Unconjugated Estrone After Single Oral Administration of Test and Reference Estradiol Valerate Tablet Under Fasting and Fed Conditions
| Pharmacokinetic Parameters | Mean ± SD (CV%) | Median (Min, Max) | |||||
|---|---|---|---|---|---|---|---|
| Cmax (ng/mL) | AUC0-t (h*ng/mL) | AUC0-∞ (h*ng/mL) | t1/2 (h) | Tmax (h) | |||
| Fasting | Total estrone | T (n=24) | 11.77 ± 4.47 (38.03) | 156.73 ± 74.64 (47.62) | 161.49 ± 77.53 (48.01) | 14.07 ± 3.84 (27.31) | 2.00 (1.00, 5.00) |
| R (n=24) | 11.79 ± 2.94 (24.94) | 148.52 ± 52.68 (35.47) | 152.75 ± 54.53 (35.70) | 13.71 ± 3.35 (24.44) | 2.00 (0.67, 6.00) | ||
| P | 0.965 | 0.260 | 0.253 | 0.628 | 0.662 | ||
| Estradiol | T (n=24) | 18.90 ± 7.24 (38.31) | 559.04 ± 233.87 (41.83) | 563.02 ± 215.64 (38.30) | 14.36 ± 4.77 (33.19) | 10.00 (4.00, 48.00) | |
| R (n=24) | 20.47 ± 13.42 (65.56) | 543.52 ± 191.37 (35.21) | 575.99 ± 215.47 (37.41) | 14.48 ± 4.17 (28.83) | 10.00 (0.67, 14.00) | ||
| P | 0.525 | 0.710 | 0.645 | 0.966 | 0.628 | ||
| Unconjugated estrone | T (n=24) | 174.38 ± 50.63 (29.03) | 3755.65 ± 1276.88 (34.00) | 3903.86 ± 1382.49 (35.41) | 13.05 ± 3.99 (30.61) | 6.00 (2.50, 12.00) | |
| R (n=24) | 169.02 ± 53.45 (31.62) | 3660.96 ± 1336.78 (36.51) | 3779.91 ± 1440.75 (38.12) | 12.92 ± 3.26 (25.22) | 5.00 (3.50, 11.00) | ||
| P | 0.467 | 0.527 | 0.454 | 0.849 | 0.703 | ||
| Fed | Total estrone | T (n=30) | 19.07 ± 7.33 (38.44) | 198.25 ± 84.34 (42.54) | 202.06 ± 86.50 (42.81) | 12.40 ± 3.53 (28.50) | 4.50 (1.50, 7.00) |
| R (n=30) | 16.92 ± 6.61 (39.05) | 180.30 ± 75.82 (42.05) | 184.20 ± 75.96 (41.24) | 12.59 ± 3.58 (28.41) | 4.00 (1.50, 9.00) | ||
| P | 0.018 | 0.006 | 0.008 | 0.719 | 0.688 | ||
| Estradiol | T (n=30) | 34.54 ± 13.14 (38.04) | 738.08 ± 215.49 (29.20) | 814.33 ± 378.55 (46.49) | 16.00 ± 14.16 (88.52) | 2.25 (0.75, 14.00) | |
| R (n=30) | 33.98 ± 11.97 (29.64) | 692.17 ± 203.79 (36.97) | 739.87 ± 266.44 (38.71) | 14.41 ± 8.84 (22.04) | 2.00 (0.75, 12.00) | ||
| P | 0.756 | 0.029 | 0.039 | 0.177 | 0.171 | ||
| Unconjugated estrone | T (n=30) | 348.71 ± 103.36 (22.04) | 5376.32 ± 1987.37 (36.97) | 5521.38 ± 2137.10 (38.71) | 12.06 ± 2.66 (22.04) | 5.00 (2.00, 9.00) | |
| R (n=30) | 315.17 ± 94.15 (29.87) | 5048.42 ± 1777.71 (35.21) | 5191.23 ± 1824.84 (35.15) | 12.39 ± 2.88 (23.22) | 5.00 (2.00, 10.00) | ||
| P | 0.005 | 0.048 | 0.071 | 0.569 | 0.271 | ||
Notes: The statistical methods employed in this table is paired t-tests and paired rank sum test. The CV% represents the inter-individual variation in the arithmetic mean of pharmacokinetic parameters.
Abbreviations: SD, standard deviation; CV, coefficient of variation; T, test drug; R, reference drug; Cmax, maximum observed drug concentration in the plasma; Tmax, time from administration to maximum observed plasma concentration; AUC 0-t, area under the curve from time zero to the last quantifiable time point; AUC0-∞, area under the curve from zero to infinity; t1/2, terminal half-life; P, p-value.
Table 3.
Geometric Mean and the Corresponding 90% Confidence Intervals for the Primary Pharmacokinetic Parameters of Total Estrone, Estradiol, and Unconjugated Estrone After Single Oral Administration of Test and Reference Estradiol Valerate Tablet Under Fasting and Fed Conditions
| Pharmacokinetic Parameters(Unit) | Geometric mean | CV (%) | 90% Confidence Interval (%) | Power (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Test | Reference | T/R ratio (%) | ||||||
| Fasting (n=24) |
Total estrone | AUC0-t (h*ng/mL) | 145.39 | 140.91 | 103.18 | 11.81 | 97.33–109.37 | 99.99 |
| AUC0-∞ (h*ng/mL) | 149.74 | 144.87 | 103.36 | 11.83 | 97.49–109.58 | 99.99 | ||
| Cmax (ng/mL) | 11.02 | 11.43 | 96.38 | 17.68 | 88.35–105.14 | 97.19 | ||
| Estradiol | AUC0-t (h*pg/mL) | 519.37 | 516.32 | 100.59 | 23.82 | 89.53–113.02 | 87.55 | |
| AUC0-∞ (h*pg/mL) | 521.09 | 533.92 | 97.60 | 14.83 | 90.40–105.36 | 99.62 | ||
| Cmax (pg/mL) | 17.81 | 18.13 | 98.22 | 29.90 | 84.96–113.55 | 62.93 | ||
| Unconjugated estrone | AUC0-t (h*pg/mL) | 3560.13 | 3462.61 | 102.82 | 13.44 | 96.22–109.87 | 99.94 | |
| AUC0-∞ (h*pg/mL) | 3682.68 | 3559.67 | 103.46 | 13.78 | 96.65–110.74 | 99.85 | ||
| Cmax (pg/mL) | 168.12 | 161.60 | 104.04 | 14.26 | 96.97–111.62 | 99.65 | ||
| Fed (n=30) |
Total estrone | AUC0-t (h*ng/mL) | 182.74 | 164.81 | 110.88 | 13.53 | 104.51–117.64 | 95.69 |
| AUC0-∞ (h*ng/mL) | 186.16 | 169.20 | 110.03 | 13.36 | 103.78–116.64 | 97.61 | ||
| Cmax (ng/mL) | 17.83 | 15.68 | 113.68 | 18.71 | 104.78–123.32 | 61.40 | ||
| Estradiol | AUC0-t (h*pg/mL) | 708.49 | 658.47 | 107.60 | 12.07 | 102.06–113.43 | 99.89 | |
| AUC0-∞ (h*pg/mL) | 756.36 | 693.09 | 109.13 | 13.04 | 103.08–115.53 | 98.94 | ||
| Cmax (pg/mL) | 32.82 | 32.08 | 102.31 | 19.88 | 93.83–111.55 | 98.50 | ||
| Unconjugated estrone | AUC0-t (h*pg/mL) | 5076.92 | 4734.95 | 107.22 | 11.76 | 101.84–112.89 | 99.95 | |
| AUC0-∞ (h*pg/mL) | 5198.40 | 4870.12 | 106.74 | 12.28 | 101.16–112.63 | 99.94 | ||
| Cmax (pg/mL) | 335.05 | 302.27 | 110.84 | 13.45 | 104.52–117.56 | 95.97 | ||
Notes: The CV% represents the intra-individual variation in the geometric mean of pharmacokinetic parameters.
Abbreviations: CV, coefficient of variation; n, number of subjects; AUC 0-t, area under the curve from time zero to the last quantifiable time point; AUC0-∞, area under the curve from zero to infinity; Cmax, maximum observed drug concentration in plasma.
Safety Results
Thirteen participants experienced treatment emergent adverse events (TEAEs) under fasting condition. Among these, 13 TEAEs were reported in 8 subjects who received the test drug, and 11 TEAEs were recorded in 8 subjects after administration of the reference drug. All TEAEs were of mild intensity and resolved without intervention. In the fed study, 21 participants experienced TEAEs. Among them, 28 TEAEs were reported in 13 subjects who received the test drug and 20 TEAEs were observed in 11 subjects who received the reference drug. All TEAEs were mild, except for one case of increased triglyceride levels after administration of the test drug, which was classified as grade 2 (Table 4). No serious adverse events (SAE) were observed during the fasting and fed studies.
Table 4.
Summary of Adverse Events Adverse Drug Reaction in Fasting and Fed Studies
| Fasting | Fed | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (n=24) | R (n=24) | Total, n (%) | P | T (n=30) | R (n=30) | Total, n (%) | P | |||||
| Instance | n (%) | Instance | n (%) | Instance | n (%) | Instance | n (%) | |||||
| Total AEs | 13 | 8 (33.33) | 11 | 8 (33.33) | 13 (54.17) | 1.000 | 28 | 13 (43.33) | 20 | 11 (36.67) | 21 (70.00) | 0.598 |
| TEAE | 13 | 8 (33.33) | 11 | 8 (33.33) | 13 (54.17) | 1.000 | 28 | 13 (43.33) | 20 | 11 (36.67) | 21 (70.00) | 0.598 |
| ADR | 2 | 2 (8.33) | 0 | 0 (0.00) | 2 (8.33) | 0.489 | 6 | 6 (20.00) | 3 | 3 (10.00) | 9 (30.00) | 0.472 |
| AE of grade 1 | 13 | 8 (33.33) | 11 | 8 (33.33) | 13 (54.17) | 1.000 | 27 | 13 (43.33) | 20 | 11 (36.67) | 21 (70.00) | 0.598 |
| AE of grade 2 | 0 | 0 | 0 | 0 | 0 | / | 1a | 1 (3.33) | 0 | 0 | 1 (3.33) | 1.000 |
| AE of grade 3 and above | 0 | 0 | 0 | 0 | 0 | / | 0 | 0 | 0 | 0 | 0 | / |
| SAE | 0 | 0 | 0 | 0 | 0 | / | 0 | 0 | 0 | 0 | 0 | / |
| SADR | 0 | 0 | 0 | 0 | 0 | / | 0 | 0 | 0 | 0 | 0 | / |
Notes: The statistical method employed in this table is the chi-square test. Values are presented as number of AEs (incidence rate, n%). aIncreased triglyceride.
Abbreviations: T, test drug; R, reference drug; n, number of subjects; Aes, adverse events; TEAE, treatment-emergent adverse event; SAE, serious adverse event; ADR, adverse drug reaction; SADR, serious adverse drug reaction; P, p-value.
The most frequent TEAEs in the fasting study were increased uric acid (25.0%), while increased thyroid-stimulating hormone (30.0%) was most commonly observed in the fed study (Table 5). Except for one case (anxiety state) that was monitored by the physician and stabilized, 84.7% of the TEAEs (61/72) recovered, and an additional 11 TEAEs were classified as being in remission. Aside from the subjects who received combination medication after completing the PK sample collection owing to anxiety, no other subjects used combination medication during the fasting and fed studies.
Table 5.
The Number of Treatment-Emergent Adverse Events and Incidence Rate According to the System Organ Class in Fasting and Fed Study
| System Organ Class | Preferred Terms | Fasting | Fed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test (n=24) | Reference (n=24) | Total (n=24) | P | Test (n=30) | Reference (n=30) | Total (n=30) | P | ||
| Infections and infestations | Upper respiratory tract infection | 0 | 0 | 0 | / | 0 | 1 (3.33) | 1 (3.33) | 1.000 |
| Respiratory, thoracic and mediastinal disorders | Irritable cough | 0 | 0 | 0 | / | 0 | 1 (3.33) | 1 (3.33) | 1.000 |
| Investigations | Increased thyroid stimulating hormone | 2 (8.33) | 0 | 2 (8.33) | 0.489 | 6 (20.00) | 3 (10.00) | 9 (30.00) | 0.472 |
| Increased uric acid | 2 (8.33) | 4 (16.67) | 6 (25.00) | 0.666 | 3 (10.00) | 4 (13.33) | 7 (23.33) | 1.000 | |
| Increased triglyceride | 1 (4.17) | 1 (4.17) | 2 (8.33) | 1.000 | 4 (13.33) | 0 | 4 (13.33) | 0.112 | |
| Increased LDL cholesterol | 3 (12.50) | 0 | 3 (12.50) | 0.234 | 1 (3.33) | 0 | 1 (3.33) | 1.000 | |
| Increased cholesterol | 1 (4.17) | 0 | 1 (4.17) | 1.000 | 1 (3.33) | 0 | 1 (3.33) | 1.000 | |
| Increased glucose | 0 | 0 | 0 | / | 1 (3.33) | 0 | 1 (3.33) | 1.000 | |
| Increased γ- glutamyl transpeptidase | 0 | 0 | 0 | / | 2 (6.67) | 0 | 2 (6.67) | 0.492 | |
| Increased Alanine aminotransferase | 0 | 0 | 0 | / | 1 (3.33) | 1 (3.33) | 2 (6.67) | 1.000 | |
| Increased Aspartate aminotransferase | 0 | 0 | 0 | / | 0 | 1 (3.33) | 1 (3.33) | 1.000 | |
| Urine white blood cell positive | 0 | 1 (4.17) | 1 (4.17) | 1.000 | 3 (10.00) | 2 (6.67) | 5 (16.67) | 1.000 | |
| Urine red blood cell positive | 0 | 2 (8.33) | 2 (8.33) | 0.489 | 0 | 0 | 0 | / | |
| Decreased White blood | 0 | 1 (4.17) | 1 (4.17) | 1.000 | 0 | 0 | 0 | / | |
| Decreased Neutrophil | 0 | 0 | 0 | / | 0 | 1 (3.33) | 1 (3.33) | 1.000 | |
| Increased eosinophil | 0 | 0 | 0 | / | 0 | 1 (3.33) | 1 (3.33) | 1.000 | |
| Increased pulse | 0 | 0 | 0 | / | 2 (6.67) | 1 (3.33) | 2 (6.67) | 1.000 | |
| Increased blood pressure | 2 (8.33) | 0 | 2 (8.33) | 0.489 | 2 (6.67) | 2 (6.67) | 2 (6.67) | 1.000 | |
| Decreased blood pressure | 1 (4.17) | 1 (4.17) | 2 (8.33) | 1.000 | 0 | 1 (3.33) | 1 (3.33) | 1.000 | |
| Psychiatric disorders | Anxiety state | 0 | 0 | 0 | / | 0 | 1 (3.33) | 1 (3.33) | 1.000 |
| Neurological disorders | Tongue numbness | 0 | 0 | 0 | / | 1 (3.33) | 0 (0.00) | 1 (3.33) | 1.000 |
| Headache | 1 (4.17) | 0 | 1 (4.17) | 1.000 | 0 | 0 | 0 | / | |
| Skin and subcutaneous tissue disorders | Rash | 0 | 1 (4.17) | 1 (4.17) | 1.000 | 0 | 0 | 0 | / |
| Gastrointestinal disorders | Abdominal pain | 0 | 0 | 0 | / | 1 (3.33) | 0 | 1 (3.33) | 1.000 |
Notes: The statistical method employed in this table is the chi-square test. Values are presented as number of AEs (incidence rate, n%).
Abbreviations: T, test drug; R, reference drug; n, number of subjects; P, p-value.
No significant differences were found in the overall incidence of TEAEs, AEs across various organ systems, or the severity levels of AEs between the test and reference formulations, whether administered under fasting or fed conditions (p > 0.05).
Discussion
This study aimed to compare the PK profiles and assess the BE of estradiol valerate tablet and its generic formulation. A total of 54 volunteers completed the study, with BE determined based on the primary PK parameters for total estrone, estradiol, and unconjugated estrone in fasting and fed studies. Both drugs were well tolerated and no SAE was reported. This study was conducted on healthy postmenopausal females, comprising fasting and fed conditions. The PK parameters of the total estrone served as the primary indicators for equivalence assessment, whereas the PK parameters of the estradiol and unconjugated estrone provided supplementary data. These procedures were aligned with the US Food and Drug Administration-recommended BE guidelines for estradiol valerate.15
There is indeed a scarcity of prior PK studies on the single-dose administration of 1 mg estradiol valerate tablets. Only one BE study has been published on the estradiol valerate tablets monopreparation.12 The study involved 32 postmenopausal Caucasian females and compared two 2 mg estradiol valerate tablets solely under fasting conditions. Blood samples were collected for up to 48 h after dosing, revealing bioequivalence between both formulations. Compared with the PK parameters of a single dose of the compound preparation containing 2 mg estradiol valerate administered under fasting conditions, this study found a longer tmax and a shorter t1/2. This suggests that the monopreparation of estradiol valerate in the 1 mg dosage form is absorbed more slowly and eliminated more rapidly. Our findings revealed that in vivo exposure to estradiol and estrogen was higher in the fed study than in the fasting study, consistent with prior research outcomes.8 Estrogen and estradiol possess notable lipid solubility, indicating that a high-fat diet can augment body exposure.
The generic development of estradiol valerate presents a significant challenge, owing to the endogenous nature of estradiol. The levels of estradiol in the human body are regulated through metabolism and physiological processes, which can be influenced by estrogen-containing food intake. When estradiol valerate tablets are administered to the body, it becomes complex and difficult to determine whether the detected estradiol is absorbed from the drug or already present in the body. To address this, we conducted baseline measurements of serum estradiol and FSH levels before administration to exclude any interference from endogenous estrogen. Low baseline concentrations of estradiol, total estrone, and unconjugated estrone were observed. Furthermore, a washout period of 7 days was implemented, exceeding the seven elimination half-lives of estradiol valerate, to minimize any potential influence from the administration of the first period.
The safety profile of the tested formulation was comparable to that of a reference formulation. Both formulations were well tolerated, with no reports of SAE. AEs were predominantly related to laboratory examinations. The AEs observed in this study were generally consistent with those listed on the estradiol valerate label. The most frequently reported AEs included increased serum uric acid, thyroid-stimulating hormone, and triglyceride levels. Studies have indicated that estrogen stimulation can lead to thyroid cell proliferation and subsequent elevation of thyroid hormones,16–18 which was more commonly observed in our study. Therefore, the AEs were determined to be related to the study drugs. Estradiol has been shown to upregulate the intestinal ATP-binding cassette subfamily G member 2 expression via the phosphatidylinositol 3-kinase/protein kinase B pathway, promoting the excretion of urate and consequently reducing serum urate levels.19 Studies have also demonstrated the ability of estrogen to reduce serum uric acid levels in postmenopausal women.20 Thus, the elevated levels of uric acid detected in this study were deemed unrelated to the administered drug. In recent years, population-based observational studies have found that estrogen therapy is associated with a reduction in the incidence and mortality of cardiovascular diseases.21–23 Estradiol has been found to downregulate serum total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels while increasing high-density lipoprotein cholesterol levels in menopausal and postmenopausal women.24–26 This may be accomplished by modulating the expression of the hepatic apolipoprotein A5, thereby reducing triglyceride levels.27 However, the lipid-related AEs observed in this study included increased serum triglyceride, cholesterol, and low-density lipoprotein cholesterol levels. The incidence of lipid-related AEs was higher in the test preparation than in the reference preparation. Additionally, the fed study demonstrated a higher incidence compared to the fasting study. This difference could be attributed to the metabolic and dietary status of postmenopausal subjects, and individual variations among subjects. There have been rarely reported cases where estrogen therapy has resulted in a significant increase in plasma triglycerides in patients with pancreatitis. However, no statistically significant difference in incidence was found between the test and reference preparation in our study. This suggests the need for further research with more evidence.
Conclusions
This was a phase 1 clinical trial of estradiol valerate tablet and its generic conducted in healthy postmenopausal female subjects. The results of this study confirmed the BE of the estradiol valerate tablet and its generic in this population. In addition, the estradiol valerate tablet demonstrated a safety profile similar to that of the generic version.
Acknowledgments
Li Zhang and Mupeng Li are co-first authors of this study. We thank all enrolled participants, investigators, and individuals who contributed to this study.
Funding Statement
This study was supported by Zhejiang XianJu Pharmaceutical Co., Ltd., the Science and Technology Research Project of Sichuan Provincial Administration of Traditional Chinese Medicine (2021MS198), and the Incubation Project of Deyang People’s Hospital (FHS202204). The funder was not involved in the study design, data collection, analysis, interpretation, article writing, or the decision to submit the manuscript for publication.
Data Sharing Statement
Original data supporting the Conclusions of this study will be provided by the author (Li Zhang) and should be retained.
Ethics Approval and Informed Consent
This study was reviewed and approved by the Clinical Trial Ethics Committee of Deyang People’s Hospital. All participants provided written informed consent to participate in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The trial staff interpreted all data for this study independently from the sponsor. Li Zhang, Mupeng Li, Lianlian Fan, Fangfang Liu, Peiwen Zhang, Qian Huang, and Gang Mai are employees of the Deyang People’s Hospital, and Jianzhong Shentu is an employee of the First Affiliated Hospital, Zhejiang University School of Medicine. The authors have no other relevant affiliations, financial involvement, or interests in any organization or entity related to the subject matter or materials discussed in this manuscript.
References
- 1.Dieni CV, Contemori S, Biscarini A, Panichi R. De novo synthesized estradiol: a role in modulating the cerebellar function. Int J Mol Sci. 2020;21(9):3316. doi: 10.3390/ijms21093316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duralde ER, Sobel TH, Manson JE. Management of perimenopausal and menopausal symptoms. BMJ. 2023;382:e072612. doi: 10.1136/bmj-2022-072612 [DOI] [PubMed] [Google Scholar]
- 3.McNeil M. Menopausal hormone therapy: understanding long-term risks and benefits. JAMA. 2017;318(10):911–913. doi: 10.1001/jama.2017.11462 [DOI] [PubMed] [Google Scholar]
- 4.The Hormone Therapy Position Statement of The North American Menopause Society” Advisory P. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29(7):767–794. doi: 10.1097/GME.0000000000002028 [DOI] [PubMed] [Google Scholar]
- 5.Kiley JW, Shulman LP. Estradiol valerate and dienogest: a new approach to oral contraception. Int J Womens Health. 2011;3:281–286. doi: 10.2147/IJWH.S22645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30(1):1–13. doi: 10.1007/s12325-012-0071-3 [DOI] [PubMed] [Google Scholar]
- 7.Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16[alpha]-hydroxylation of 17[beta]-estradiol. Metabolism. 2001;50(9):1001–1003. doi: 10.1053/meta.2001.25592 [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. FDA labelling information-NATAZIA (estradiol valerate and estradiol valerate/dienogest) tablets, for oral use.04/29/2022; 2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/022252s007lbl.pdf. Accessed July 5, 2024.
- 9.Zimmerman H, Thebault JJ, Duvauchelle T, Mignot A, Renoux A, Gualano V. Pharmacokinetics of estradiol valerate 2mg + dienogest 2mg (climodien(R) 2/2) after single and repeated oral administration in healthy postmenopausal women. Clin Drug Investig. 2000;20(2):123–134. doi: 10.2165/00044011-200020020-00007 [DOI] [PubMed] [Google Scholar]
- 10.Hoy SM, Scott LJ. Estradiol valerate/dienogest: in oral contraception. Drugs. 2009;69(12):1635–1646. doi: 10.2165/11202820-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 11.Zeun S, Lu M, Uddin A, Zeiler B, Morrison D, Blode H. Pharmacokinetics of an oral contraceptive containing oestradiol valerate and dienogest. Eur J Contracept Reprod Health Care. 2009;14(3):221–232. doi: 10.1080/13625180902850039 [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann H, Koytchev R, Mayer O, Borner A, Mellinger U, Breitbarth H. Pharmacokinetics of orally administered estradiol valerate. Results of a single-dose cross-over bioequivalence study in postmenopausal women. Arzneimittelforschung. 1998;48(9):941–947. [PubMed] [Google Scholar]
- 13.Timmer CJ, Geurts TB. Bioequivalence assessment of three different estradiol formulations in postmenopausal women in an open, randomized, single-dose, 3-way cross-over study. Eur J Drug Metab Pharmacokinet. 1999;24(1):47–53. doi: 10.1007/BF03190010 [DOI] [PubMed] [Google Scholar]
- 14.Whalen KL, Rose R. Estradiol valerate/dienogest: a novel oral contraceptive. Ann Pharmacother. 2011;45(10):1256–1261. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration. Draft Guidance on Estradiol; 2010. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Estradiol_tabs_84500_84499_81295_RC_12-10.pdf. Accessed July 5, 2024.
- 16.Kaminski J, Junior CM, Pavesi H, Drobrzenski B, Amaral GMD. Effects of oral versus transdermal estradiol plus micronized progesterone on thyroid hormones, hepatic proteins, lipids, and quality of life in menopausal women with hypothyroidism: a clinical trial. Menopause. 2021;28(9):1044–1052. doi: 10.1097/GME.0000000000001811 [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Xu T, Ma L, Chang W. Signal pathway of estrogen and estrogen receptor in the development of thyroid cancer. Front Oncol. 2021;11:593479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Z, Yang S, Wei M, et al. The isoforms of estrogen receptor alpha and beta in thyroid cancer. Front Oncol. 2022;12:916804. doi: 10.3389/fonc.2022.916804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Zhao T, Shan L, Cao L, Zhu X, Xue Y. Estradiol regulates intestinal ABCG2 to promote urate excretion via the PI3K/Akt pathway. Nutr Metab. 2021;18(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akin F, Bastemir M, Alkis E, Kaptanoglu B. SHBG levels correlate with insulin resistance in postmenopausal women. Eur J Intern Med. 2009;20(2):162–167. [DOI] [PubMed] [Google Scholar]
- 21.Bush TL, Barrett-Connor E, Cowan LD, et al. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the lipid research clinics program follow-up study. Circulation. 1987;75(6):1102–1109. doi: 10.1161/01.CIR.75.6.1102 [DOI] [PubMed] [Google Scholar]
- 22.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991;20(1):47–63. [DOI] [PubMed] [Google Scholar]
- 23.Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325(11):756–762. doi: 10.1056/NEJM199109123251102 [DOI] [PubMed] [Google Scholar]
- 24.Whitcroft SI, Crook D, Marsh MS, Ellerington MC, Whitehead MI, Stevenson JC. Long-term effects of oral and transdermal hormone replacement therapies on serum lipid and lipoprotein concentrations. Obstet Gynecol. 1994;84(2):222–226. [PubMed] [Google Scholar]
- 25.Bhathena RK, Anklesaria BS, Ganatra AM, Pinto R. The influence of transdermal oestradiol replacement therapy and medroxyprogesterone acetate on serum lipids and lipoproteins. Br J Clin Pharmacol. 1998;45(2):170–172. doi: 10.1046/j.1365-2125.1998.00658.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325(17):1196–1204. doi: 10.1056/NEJM199110243251702 [DOI] [PubMed] [Google Scholar]
- 27.Luo F, Guo Y, Ruan GY, Peng R, Li XP. Estrogen lowers triglyceride via regulating hepatic APOA5 expression. Lipids Health Dis. 2017;16(1):72. doi: 10.1186/s12944-017-0463-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data supporting the Conclusions of this study will be provided by the author (Li Zhang) and should be retained.