Abstract
Diabetes mellitus and its complications are a known public health problem nowadays. Diabetic nephropathy is one of the main complications and the result of multiple mechanisms, including: activation of the renin-angiotensin-aldosterone system, formation of advanced glycation end products and chronic inflammation that led to glomerular and tubulo-interstitial damage producing mesangial expansion and glomerulosclerosis, which finally results in chronic kidney disease. Early detection of diabetic nephropathy is essential for adequate intervention to stop, or at least slow down its progression. Multiple markers have been described, not only the classic ones such as serum creatinine, urea, and albuminuria, but at this point also novel biomarkers such as neutrophil gelatinase-associated lipocalin, tumor necrosis factor 1 receptor and monocyte chemoattractant protein-1, among others. The aim of this article was to provide an update review of the role of biomarkers in the diagnosis of diabetic nephropathy.
Key Words: Biomarkers, Diabetic nephropathy, Diabetic kidney disease, Diagnosis, Quality of life
Diabetes mellitus (DM) has been a chronic disease with an increasing prevalence in recent years (1). Currently, according to the International Diabetes Federation, over 537 million people have DM (1, 2). It is considered a serious public health problem because of its impact on quality of life and associated health costs (3). Among its chronic complications, one of the most frequent is diabetic nephropathy (DN), which is associated with significant morbidity and mortality, and is the leading cause of end-stage renal disease (ESRD) (4, 5). DN is characterized by abnormal albumin excretion in the urine with or without a reduced glomerular filtration rate (GFR) (6). This occurs in 20-40% of patients with DM (7). Between 23 and 36% of type 1 DM (T1D) patients will develop albuminuria, a situation that also occurs in around 38% of those with type 2 DM (T2D) (8).
The main modifiable risk factors are increased urine albumin excretion, (6) hyperglycemia, high blood pressure, dyslipidemia, obesity, and smoking (6, 9). The non-modifiable risk factors are advanced age, female gender, and the duration of DM (9).
DN in its early diagnosis and timely intervention in diabetic patients are important to slow down the progression of renal function decline and prevent ESRD (10).
The aim of this narrative review was to update the usefulness of serum and urinary biomarkers in the diagnosis of DN, and its clinical relevance is to improve the management of DN and to limit its impact on the morbimortality which will result in maintaining a better quality of life for the patient and lower health system costs for this chronic disease.
Methods
Search strategy: MEDLINE and EMBASES electronic databases were searched for completed studies of any design except case reports, case series, letters to the editor and conference proceedings, from database inception between 2005 and 2022. The Medical Subject Heading (MeSH) used were "diabetic kidney disease" or “diabetic nephropathy”, and “biomarkers”.
Inclusion and exclusion criteria: Inclusion criteria were studies published in English involving patients of any age. Systematic reviews, clinical trials, prospective cohort studies, cross-sectional and retrospective studies, and narrative reviews related to the objective of this manuscript were included. The investigation was limited to articles related to human beings, so exclusion criteria was non-human studies.
Screening: A total of 6660 citations were identified; 658 duplicates were removed; 6002 titles and abstracts were screened against eligibility criteria. No information produced outside of traditional publishing and literature distribution channels was included. 5890 titles were excluded at the title and abstract screen, 98 eligible full-text papers met the inclusion criteria. This process is summarized in figure 1.
Figure 1.
Flowchart of narrative review process
Data extraction and synthesis: Results from the included papers were extracted to a table about pathophysiology, diagnosis, traditional and novel biomarkers.
Quality assessment: The quality of this narrative review was evaluated using the SANRA scale, obtaining 12 points (11).
Results
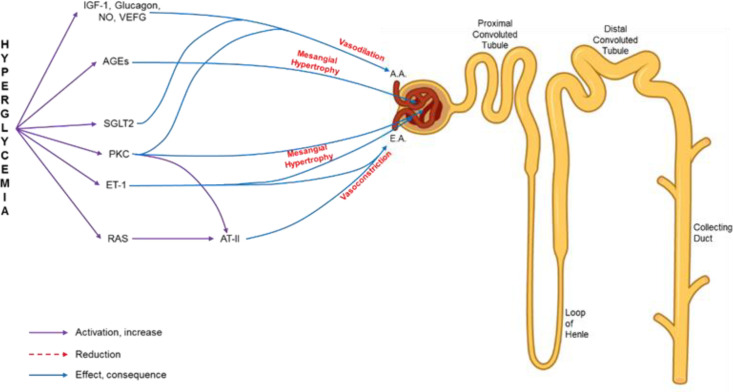
Pathophysiology of diabetic nephropathy: Hyperglycemia is currently recognized as the initial mediator of metabolic and hemodynamic alterations in the kidney, associated with other pathophysiological events, (12, 13) summarized in figure 2, and as follows:
Figure 2.
Representative diagram of the nephron and the alterations that contribute to diabetic nephropathy. A.A.: afferent arteriole; E.A.: efferent arteriole; ET-1: endothelin 1; RAS: renin angiotensin system; AT-II: angiotensin II; IGF-1: insulin-like growth factor 1; NO: nitric oxide; VEFG: vascular endothelial growth factor; AGEs: advanced glycation end products; PKC: protein kinase C; SGLT-2: type 2 sodium-glucose co-transporter.
Hemodynamic alterations: Activation of the renin-angiotensin system (RAS) increases the level of angiotensin II, produces contraction of the efferent arteriole, and increases intraglomerular pressure, resulting in hyperfiltration and damage to the glomerular basement membrane (GBM) (14-18).
Metabolic alterations: Hyperglycemia leads to increased glycolysis by stimulating the polyol and hexosamine pathways, formation of advanced glycation end products (AGEs), and activation of protein kinase C (PKC) (13, 15).
AGEs cause cell damage by altering the function of laminin and type IV collagen, increasing the permeability of the GBM (6, 19-21). Additionally, they bind to proinflammatory receptors, which activate the production of interleukin (IL) 1 and 6, tumor necrosis factor alpha (TNF-α), transforming growth factor β1 (TGF-β1), vascular endothelial growth factor (VEGF), and reactive oxygen species (19, 22, 23).
On the other hand, PKC contributes to DN by increasing levels of prostaglandin E2 and nitric oxide, leading to vasodilation of the afferent arteriole and enhances the action of angiotensin II in the efferent arteriole, favoring the development of glomerular hyperfiltration (24-26). Also, this stimulates the production of fibronectin and type IV collagen, resulting in thickening of the GBM (27). Hyperglycemia itself causes dilation of the afferent arteriole through the release of vasoactive mediators such as insulin-like growth factor 1, glucagon, VEGF and prostaglandins (28).
Inflammatory changes: To sum up contributions to the development of DN, diabetic patients suffer chronic activation of innate immunity and a proinflammatory state (29). The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) regulates the expression of genes related to inflammation and apoptosis (30). Hyperglycemia increases the NF-κB expression through activation of Toll-like receptors (17, 31-33). In DN, NF-κB activation is associated with proteinuria and interstitial cell infiltrate (30, 34, 35).
Inflammatory cytokines such as TNF-α and IL 1, 6 and 18 are expressed in higher amounts in kidneys of patients with DM. These cytokines correlate directly with the degree of albuminuria and possibly increase vascular endothelial cell permeability, contributing to glomerular hypercellularity and thickening of the GBM (36-39).
Hyperglycemia increases the expression of the sodium-glucose cotransporter 2 (SGLT-2), which, in contexts of DM, is counterproductive (40, 41). This causes hemodynamic and metabolic alterations, increasing sodium and glucose reabsorption, stimulating the macula densa of the distal tubule, causing dilation of the afferent arteriole by tubulo-glomerular feedback, and increasing further intraglomerular pressure (42-46).
Diagnosis:
Traditional biomarkers:
Creatinine and estimated GFR: Serum creatinine is the most widely used biomarker to assess kidney function; it corresponds to the end-product of the creatine and creatine phosphate metabolism. Anyhow, creatinine measurement is prone to interferences, and inaccuracies due to physiological and technological aspects.
Its association with muscle mass, tubular secretion, diet, and comorbidities such as advanced liver disease, cause its clinical limitations. So does extrarenal clearance of serum creatinine possibly due to intestinal bacteria, relevant in advanced CKD (47) The serum creatinine concentration starts to increase when approximately 40–50% of the kidney parenchyma is damaged (48, 49). This limits its use as a marker for the early diagnosis of CKD (50). Different formulas used to estimate the GFR based on serum creatinine level are widely used, but they lack of accuracy since they do not measure direct renal tissue injury and have a low sensitivity to small changes in renal function (51-53). Additionally, there are differences in the estimation formulas of the GFR based on serum creatinine (eGFRcr), which limits, even more, its utility (54).
Albuminuria: Albuminuria is a traditional biomarker for DN. Albumin is almost completely reabsorbed in the renal tubules. The urinary albumin-creatinine ratio (UACR) is the method of choice for detecting albuminuria using a simple urine sample. Albuminuria, defined as a urine albumin excretion equal to or greater than 30 mg/day, is considered a sign of glomerulo-tubulopathy, which correlates with renal structural changes (47). It is the strongest predictor of kidney disease and cardiovascular morbimortality (55, 56).
However, prognostic by albuminuria is not specific for DN. Approximately 30% of patients with DN do not have albuminuria and therefore, eGFR is a better biomarker in the prediction of DN development and its progression (57-59). Studies show that patients with T2D without albuminuria have a high risk of renal dysfunction progression due to inflammatory mechanisms and lesions at the level of the proximal renal tubule, through alteration of tubuloglomerular feedback (60). The characterization of non-albuminuric forms of DN reinforces the importance of adding the estimated GFR to the measurement of albuminuria (59). GFR and albuminuria are independent predictors of the course of kidney disease and risk of mortality, and therefore both should be evaluated in screening for DN (61).
Cystatin C: Cystatin C is a low molecular weight protein, which concentration correlates with GFR. Its superiority over other renal function markers is due to its ability to remain free of binding proteins to be filtered in the glomeruli and to be almost completely reabsorbed in the proximal convoluted tubule. It has less interindividual variation, as it is independent of muscle mass, gender, age, and inflammatory conditions (62).
The estimation GFR based on serum Cystatin C (eGFRcys) has been suggested to show better clinical utility for detecting nephropathy in patients with normoalbuminuria, as well as predicting the progression of nephropathy in patients with albuminuria (63). Studies suggest that serum cystatin C could increase before creatinine in the presence or progression of DN, but its significance in DN is still on debate since there is no high-grade evidence to recommend it as a screening method for DN (64-68).
Additionally, urinary excretion of Cystatin C is elevated early in diabetes and prediabetic nephropathy and it suggests tubular injury (55).
New biomarkers: Current research is aiming to find more sensitive, specific, and precise biomarkers that allow the early detection of kidney damage, even before the appearance of microalbuminuria or a GFR decrease. New biomarkers initially found in acute kidney injury, are being studied to determine their value in the evaluation of CKD (51). According to the classification of sample origin, biomarkers are urinary or serum (table 1); meanwhile according to the site of injury, there are glomerular and tubular biomarkers (table 2). Pathophysiological mechanism classification considers renal dysfunction, inflammation, and oxidative stress biomarkers (55).
Table 1.
Classification of new biomarkers according to sample origin
| Urinary biomarkers | Serum biomarkers |
|---|---|
| Alpha-1 microglobulin Ceruloplasmin Cyclophilin A IgG KIM-1 MCP-1 Megalin NGAL 8-oxo-7,8-dihydro-2-deoxyguanosine TNF-alpha Transferrin Type IV collagen VDBP |
NGAL NT-proBNP TNFR1 and TNFR2 Uric acid |
KIM-1: Kidney injury molecule 1; MCP-1: Monocyte chemoattractant protein-1; NGAL: neutrophil gelatinase-associated lipocalin; NT-proBNP: N-Terminal B-type natriuretic peptide prohormone; TNF-alpha: Tumor necrosis factor ALPHA; TNFR1: Tumor necrosis factor 1 receptor; TNFR2: Tumor necrosis factor 2 receptor; VDBP: vitamin D binding protein.
Table 2.
Classification of new biomarkers according to place of lesion
| Glomerular biomarkers | Tubular biomarkers |
|---|---|
| Ceruloplasmin IgG Transferrin Type IV collagen TNFR1 and TNFR2 |
KIM-1 NGAL Megalin Alpha-1 microglobulin VDBP |
Tubular biomarkers:
Neutrophil gelatinase-associated lipocalin (NGAL): NGAL is a low molecular weight glycoprotein, a constituent of neutrophil granules that also is expressed in the kidney. This protein is freely filtered by the glomerulus and reabsorbed in the proximal tubules. When epithelial tubular cells of the kidneys are acutely injured, there is a marked increase in urinary NGAL concentrations and might go with an increase in de novo synthesis of NGAL within the tubules (62, 69).
Although an increase in urinary as well as serum NGAL has shown to be a reliable marker for the early diagnosis of acute kidney injury, its association with CKD progression remains controversial. It correlates inversely with GFR, but does not correlate with HbA1c. At the same time, T1D and T2D patients with normoalbuminuria can present elevated values of urinary NGAL, predicting albuminuria (65, 69). Its sensibility and specificity were reported to be 94% and 90%, respectively (70).
Kidney injury molecule 1 (KIM-1): KIM-1 is a glycoprotein found in the proximal tubules and is a sensitive marker of proximal tubular injury. Increased levels of KIM-1 have been found in DM patients, independently of albuminuria. KIM-1 has been associated with DN progression (71). With a cut-off point of 32.00 ng/g and sensitivity 93.8% it’s reported sensibility and specificity are 93.8% and 88.5%, respectively (72).
Vitamin D binding protein (VDBP): VDBP is the main plasmatic transporter of vitamin D. It is vital in the biosynthesis of 1,25 dihydroxy-vitamin D within the renal proximal tubules, binding 25-hydroxy-vitamin D to VDBP and actively recovering the complex by endocytosis from the glomerular filtrate. Higher expression of VDBP was found in patients with DN, although the reasons remain unclear. It is suggested that high concentrations are associated with renal tubular damage as well low serum levels of vitamin D, which has also been found a factor associated with DN. This marker has a sensitivity of 98.8% and a specificity of 80%, for a cut-off point of 216 ng/mg (73).
Megalin: Megalin is a multiligand receptor expressed in proximal tubular cells that reabsorbs filtered albumin and correlates cross-sectional with albuminuria (74). There are 2 types of assays: A-megalin, which is elevated in patients with early DN and microalbuminuria (75), and C-megalin, which is associated with persistent microalbuminuria and useful specially in patients with low-normal UACR levels (74).
Alpha-1 microglobulin: Urinary alpha-1 microglobulin is reabsorbed in the proximal tubule. It is an early biomarker of tubular injury that predicts microalbuminuria in T2D patients (76).
Glomerular biomarkers:
Transferrin: Transferrin is an iron-binding protein with low molecular weight and low ionic load that easily crosses the glomerular barrier (77). Urinary transferrin appears elevated prior to the development of microalbuminuria in patients with DM, suggesting that it may be an early biomarker of glomerular damage and could predict microalbuminuria (78, 79).
Ceruloplasmin: Ceruloplasmin is a copper-carrying protein that crosses the glomerular barrier with difficulty due to its negative load. Elevated urine ceruloplasmin levels usually predict microalbuminuria in T2D patients with normoalbuminuria (78-80).
IgG: Urinary IgG is a biomarker of glomerular damage that usually appears elevated along with transferrin and ceruloplasmin and predicts microalbuminuria in patients with DM (78, 79).
Type IV Collagen: Type IV collagen is the principal component of the glomerular basement membrane and mesangial matrix and is present in podocytes and the proximal tubule. Hyperglycemia stimulates the synthesis of type IV collagen. Urinary type IV collagen is a structural damage biomarker and its level increases as DN progresses (81, 82).
Tumor necrosis factor 1 receptor (TNFR1) and Tumor necrosis factor 2 receptor (TNFR2): Elevated serum TNFR1 and TNFR2 concentrations are strong independent predictors of renal function decline leading to ESRD in patients with T2D. Elevated serum concentrations of TNFR1 or TNFR2 in T2D are associated with early glomerular structural lesions. These receptors showed the strongest associations with a reduced percentage of fenestrated endothelium (83).
Inflammation biomarkers:
Monocyte chemoattractant protein-1 (MCP-1): MCP-1 is a proinflammatory cytokine that plays a role in the recruitment of macrophages and monocytes, being detectable in urine of patients with different kidney diseases. A significant increase in MCP-1 levels has been found in urine of T2D patients with macroalbuminuria. Its measurement would especially be useful to determine the prognosis of DN (84, 85).
Tumor Necrosis Factor Alpha (TNF-α): TNF-α is a cytokine involved in systemic inflammation. It is produced primarily by activated macrophages. Urinary excretion of TNF-α is increased in T2D patients with microalbuminuria and macroalbuminuria compared to those without albuminuria (86).
Biomarkers of oxidative stress:
Urinary 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oHdG): Oxidative DNA damage causes the production of 8-oHdG, which is eliminated in the urine and so considered a biomarker of oxidative stress. Higher levels of 8-oHdG in urine indicate significant progression of DN (82). Eventually, this biomarker might predict long-term mortality in DM (87).
Other biomarkers:
B-type natriuretic peptide prohormone (NT-proBNP): The BEAt-DKD consortium, which assessed biomarkers to predict the rate of estimated GFR reduction in the early stages of CKD in patients with DM, showed that NT-proBNP predicts reduction of estimated GFR (88).
Periostin: Periostin is a urinary biomarker with high diagnostic precision in DN. Periostin reaches a sensitivity of 98% and a specificity of 80%. It is useful for early detection of DN (89, 90).
Cyclophilin A: Cyclophilin A is used as a urinary biomarker for early detection of DN. It has a high diagnostic precision in DN and registers a sensitivity of 84% and a specificity of 86% (89, 90).
Uric acid: Serum uric acid is associated with insulin resistance and endothelial dysfunction. Uric acid increases in chronic kidney disease and is associated with GFR reduction.
Also, it predicts microalbuminuria in T1D patients, being an early biomarker of DN (91, 92).
Proteomic: Proteomics is the large-scale study of proteins, which allows the identification and quantification of hundreds of proteins or peptides, and their variation under conditions of health or illness (93). In recent years, proteomics has become a promising method for identifying a wide variety of kidney disease biomarkers in urine, which is easily accessible, a very stable medium for proteins, and non-invasive for the patient (94).
The PRIORITY trial (Proteomic Prediction and Renin Angiotensin Aldosterone System Inhibition Prevention of Early Diabetic Nephropathy in Type 2 Diabetic Patients with Normoalbuminuria) confirmed that the test assessing a panel of 273 proteins and peptides using mass spectrometry, known as CKD273 (CKD classifier 273), is useful in the determination of the risk for DN progression (95).
MicroRNA: MicroRNAs are relatively stable particles and can be measured in serum, plasma, urine, and saliva by polymerase chain reaction, microarrays, RNA sequencing, and in situ hybridization. The microRNAs miR-192-5p and miR-130b have been identified and could be useful in the early detection, monitoring, and treatment efficacy of CKD (96).
Lipidomics analysis: Lipidomics is the large-scale study of pathways and networks of lipids. Alterations in lysophosphatidylethanolamine, phosphatidylethanolamine and triacylglycerol may be associated with alterations in the lipid metabolism in DN and the severity of the development of nephropathy, being able to identify early from advanced damage (97).
Conclusion: DN results in multiple alterations at glomerular and tubulo-interstitial level caused by hyperglycemia, hemodynamic factors, oxidative stress, and underlying chronic inflammation. These alterations can be detected earlier by the novel biomarkers, allowing timely diagnosis and treatment, to stop or slow down glomerular and tubular damage, thus reducing morbidity and mortality and so, maintaining a better quality of life for the patient, and reducing health system costs.
Acknowledgments
None stated by the authors.
Funding:
None stated by the authors.
Conflict of Interests:
None stated by the authors.
Authors’ contribution:
Marcio Concepción: conceptualization, methodology, investigation, writing – review & editing, project administration. Juan Quiroz: investigation, writing – original draft. Jacsel Suárez: investigation, writing – original draft. José Paz: investigation, writing – review & editing. Pela Roseboom: investigation, writing – original draft. Sofía Ildefonso: investigation, writing – original draft. Denis Cribillero: investigation, writing – review & editing. Francisca Zavaleta: investigation, writing – review & editing. Julia Coronado: investigation, writing – review & editing. Luis Concepción: investigation, writing – review & editing.
References
- 1.Faselis C, Katsimardou A, Imprialos K, et al. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18:117–24. doi: 10.2174/1570161117666190502103733. [DOI] [PubMed] [Google Scholar]
- 2.Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: A global perspective. Endocrinol Metab Clin North Am. 2021;50:337–55. doi: 10.1016/j.ecl.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Khan MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107–11. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugahara M, Pak WLW, Tanaka T, Tang SCW, Nangaku M. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology (Carlton) 2021;26:491–500. doi: 10.1111/nep.13860. [DOI] [PubMed] [Google Scholar]
- 5.Samsu N. Diabetic nephropathy: Challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. doi: 10.1155/2021/1497449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain S, Jamali MC, Habib A, et al. Diabetic kidney disease: An overview of prevalence, risk factors, and biomarkers. Clin Epidemiol Glob Health. 2021;9:2–6. [Google Scholar]
- 7.American Diabetes Association Professional Practice Committee. Chronic kidney disease and risk management: Standards of medical care in diabetes—2022. Diabetes Care. 2022;45: S175–184. doi: 10.2337/dc22-S011. [DOI] [PubMed] [Google Scholar]
- 8.Retnakaran R, Cull CA, Thorne KI, Adler AI. Risk factors for renal dysfunction in type 2 diabetes: U K Prospective Diabetes Study 74. Diabetes. 2006;55:1832–9. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 9.Tziomalos K, Athyros VG. Diabetic Nephropathy: New risk factors and improvements in diagnosis. Rev Diabet Stud. 2015;12:110–8. doi: 10.1900/RDS.2015.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tye S, Denig P, Heerspink H. Precision medicine approaches for diabetic kidney disease: opportunities and challenges. Nephrology Dialysis Transplantation. 2021;36:3–9. doi: 10.1093/ndt/gfab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4 doi: 10.1186/s41073-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meza Letelier CE, San Martín Ojeda CA, Ruiz Provoste JJ, Frugone Zaror CJ. Pathophysiology of diabetic nephropathy: a literature review. Medwave. 2017;17:e6839. doi: 10.5867/medwave.2017.01.6839. [DOI] [PubMed] [Google Scholar]
- 13.Toth-Manikowski S, Atta MG. Diabetic Kidney Disease: Pathophysiology and Therapeutic Targets. J Diabetes Res. 2015;2015:697010. doi: 10.1155/2015/697010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonner R, Albajrami O, Hudspeth J, Upadhyay A. Diabetic kidney disease. Prim Care. 2020;47:645–659. doi: 10.1016/j.pop.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Dagar N, Das P, Bisht P, et al. Diabetic nephropathy: A twisted thread to unravel. Life Sci. 2021;278:119635. doi: 10.1016/j.lfs.2021.119635. [DOI] [PubMed] [Google Scholar]
- 16.Rudberg S, Rasmussen LM, Bangstad HJ, Osterby R. Influence of insertion/deletion polymorphism in the ACE-I gene on the progression of diabetic glomerulopathy in type 1 diabetic patients with microalbuminuria. Diabetes Care. 2000;23:544–8. doi: 10.2337/diacare.23.4.544. [DOI] [PubMed] [Google Scholar]
- 17.Malek V, Suryavanshi SV, Sharma N, Kulkarni YA, et al. Potential of renin-angiotensin-aldosterone system modulations in diabetic kidney disease: Old players to new hope! Rev Physiol Biochem Pharmacol. 2021;179:31–71. doi: 10.1007/112_2020_50. [DOI] [PubMed] [Google Scholar]
- 18.Warren AM, Knudsen ST, Cooper ME. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin Ther Targets. 2019;23:579–91. doi: 10.1080/14728222.2019.1624721. [DOI] [PubMed] [Google Scholar]
- 19.Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end products in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S254–8. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 20.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–88. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 21.Walton HA, Byrne J, Robinson GB. Studies of the permeation properties of glomerular basement membrane: cross-linking renders glomerular basement membrane permeable to protein. Biochim Biophys Acta. 1992;1138:173–83. doi: 10.1016/0925-4439(92)90035-l. [DOI] [PubMed] [Google Scholar]
- 22.Alicic RZ, Tuttle KR. Novel therapies for diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:121–33. doi: 10.1053/j.ackd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Fukami K, Yamagishi SI, Ueda S, Okuda S. Role of AGEs in diabetic nephropathy. Curr Pharm Des. 2008;14:946–52. doi: 10.2174/138161208784139710. [DOI] [PubMed] [Google Scholar]
- 24.Bank N, Aynedjian HS. Role of EDRF (nitric oxide) in diabetic renal hyperfiltration. Kidney Int. 1993;43:1306–12. doi: 10.1038/ki.1993.183. [DOI] [PubMed] [Google Scholar]
- 25.Ruan X, Arendshorst WJ. Role of protein kinase C in angiotensin II-induced renal vasoconstriction in genetically hypertensive rats. Am J Physiol. 1996;270:F945–52. doi: 10.1152/ajprenal.1996.270.6.F945. [DOI] [PubMed] [Google Scholar]
- 26.Williams B, Schrier RW. Glucose-induced protein kinase C activity regulates arachidonic acid release and eicosanoid production by cultured glomerular mesangial cells. J Clin Invest. 1993;92:2889–96. doi: 10.1172/JCI116911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noh H, King GL. The role of protein kinase C activation in diabetic nephropathy. Kidney Int Suppl. 2007:S49–53. doi: 10.1038/sj.ki.5002386. [DOI] [PubMed] [Google Scholar]
- 28.Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc Taiwan Yi Zhi. 2018;117:662–75. doi: 10.1016/j.jfma.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 29.García-García PM, Getino-Melián MA, Domínguez-Pimentel V, Navarro-González JF. Inflammation in diabetic kidney disease. World J Diabetes. 2014;5:431–43. doi: 10.4239/wjd.v5.i4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanz AB, Sanchez-Niño MD, Ramos AM, et al. NF-kappaB in renal inflammation. J Am Soc Nephrol. 2010;21:1254–62. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 31.Suryavanshi SV, Kulkarni YA. NF-κβ: A potential target in the management of vascular complications of diabetes. Front Pharmacol. 2017;8:798. doi: 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vianna HR, Soares CMBM, Tavares MS, Teixeira MM, Silva AC. Inflammation in chronic kidney disease: the role of cytokines. Bras J Nefrol. 2011;33:351–64. doi: 10.1590/s0101-28002011000300012. [DOI] [PubMed] [Google Scholar]
- 33.Wan S, Wan S, Jiao X, et al. Advances in understanding the innate immune-associated diabetic kidney disease. FASEB J. 2021;35:e21367. doi: 10.1096/fj.202002334R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mezzano S, Aros C, Droguett A, et al. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19:2505–12. doi: 10.1093/ndt/gfh207. [DOI] [PubMed] [Google Scholar]
- 35.Sakai N, Wada T, Furuichi K, et al. p38 MAPK phosphorylation and NF-kappa B act ivation in human crescentic glomerulonephritis. Nephrol Dial Transplant. 2002;17:998–1004. doi: 10.1093/ndt/17.6.998. [DOI] [PubMed] [Google Scholar]
- 36.DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol. 2003;284:F113–21. doi: 10.1152/ajprenal.00026.2002. [DOI] [PubMed] [Google Scholar]
- 37.Mariño E, Cardier JE. Differential effect of IL-18 on endothelial cell apoptosis mediated by TNF-alpha and Fas (CD95) Cytokine. 2003;22:142–8. doi: 10.1016/s1043-4666(03)00150-9. [DOI] [PubMed] [Google Scholar]
- 38.Moriwaki Y, Yamamoto T, Shibutani Y, et al. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism. 2003;52:605–8. doi: 10.1053/meta.2003.50096. [DOI] [PubMed] [Google Scholar]
- 39.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–42. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 40.Hu R, Layton A. A computational model of kidney function in a patient with diabetes. Int J Mol Sci. 2021;22:5819. doi: 10.3390/ijms22115819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlotides G, Mertens PR. Sodium-glucose cotransport inhibitors: mechanisms, metabolic effects and implications for the treatment of diabetic patients with chronic kidney disease. Nephrol Dial Transplant. 2015;30:1272–6. doi: 10.1093/ndt/gfu299. [DOI] [PubMed] [Google Scholar]
- 42.Chen SJ, Lv LL, Liu BC, Tang RN. Crosstalk between tubular epithelial cells and glomerular endothelial cells in diabetic kidney disease. Cell Prolif. 2020;53:e12763. doi: 10.1111/cpr.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahtal N, Lenoir O, Tharaux PL. Glomerular endothelial cell crosstalk with podocytes in diabetic kidney disease. Front Med. 2021;8:659013. doi: 10.3389/fmed.2021.659013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prattichizzo F, de Candia P, Ceriello A. Diabetes and kidney disease: emphasis on treatment with SGLT-2 inhibitors and GLP-1 receptor agonists. Metabolism. 2021;120:154799. doi: 10.1016/j.metabol.2021.154799. [DOI] [PubMed] [Google Scholar]
- 45.Tuttle KR. Back to the future: Glomerular hyperfiltration and the diabetic kidney. Diabetes. 2016;66:14–6. doi: 10.2337/dbi16-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeni L, Norden AGW, Cancarini G, Unwin RJ. A more tubulocentric view of diabetic kidney disease. J Nephrol. 2017;30:701–17. doi: 10.1007/s40620-017-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delanaye P, Cavalier E, Pottel H. Serum creatinine: Not so simple! Nephron. 2017;136:302–8. doi: 10.1159/000469669. [DOI] [PubMed] [Google Scholar]
- 48.Pasala S, Carmody JB. How to use… serum creatinine, cystatin C and GFR. Arch Dis Child Educ Pract Ed. 2017;102:37–43. doi: 10.1136/archdischild-2016-311062. [DOI] [PubMed] [Google Scholar]
- 49.Liu KZ, Tian G, Ko AC, et al. Detection of renal biomarkers in chronic kidney disease using microfluidics: progress, challenges and opportunities. Biomed Microdevices. 2020;22:29. doi: 10.1007/s10544-020-00484-6. [DOI] [PubMed] [Google Scholar]
- 50.Richard M, Erosha Premaratne JG. Estimating glomerular filtration rate in diabetes using serum cystatin C. Clin Biochem Rev. 2011;32:61–7. [PMC free article] [PubMed] [Google Scholar]
- 51.Satirapoj B. Tubulointerstitial biomarkers for diabetic nephropathy. J Diabetes Res. 2018;2018:2852398. doi: 10.1155/2018/2852398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kashani K, Rosner MH, Ostermann M. Creatinine: From physiology to clinical application. Eur J Intern Med. 2020;72:9–14. doi: 10.1016/j.ejim.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 53.Allison SJ. The CKD–EPI equation—accurately stratifying risk in CKD. Nat Rev Nephrol. 2012;8:371. doi: 10.1038/nrneph.2012.94. [DOI] [PubMed] [Google Scholar]
- 54.Moazzeni SS, Arani RH, Hasheminia M, Tohidi M, Azizi F, Hadaegh F. High incidence of chronic kidney disease among iranian diabetic adults: using CKD-EPI and MDRD equations for estimated glomerular filtration rate. Diabetes Metab J. 2021;45:684–97. doi: 10.4093/dmj.2020.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uwaezuoke SN. The role of novel biomarkers in predicting diabetic nephropathy: a review. Int J Nephrol Renovasc Dis. 2017;10:221–31. doi: 10.2147/IJNRD.S143186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barzilay JI, Buzkova P, Shlipak MG, et al. Hospitalization rates in older adults with albuminuria: the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2020;75:2426–33. doi: 10.1093/gerona/glaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52:1036–40. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 58.An JH, Cho YM, Yu HG, et al. The clinical characteristics of normoalbuminuric renal insufficiency in Korean type 2 diabetic patients: a possible early-stage renal complication. J Korean Med Sci. 2009;24:S75–81. doi: 10.3346/jkms.2009.24.S1.S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27:195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- 60.Porrini E, Ruggenenti P, Mogensen CE, et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3:382–91. doi: 10.1016/S2213-8587(15)00094-7. [DOI] [PubMed] [Google Scholar]
- 61.Jones RH, Hayakawa H, Mackay JD, Parsons V, Watkins PJ. Progression of diabetic nephropathy. Lancet. 1979;1:1105–6. doi: 10.1016/s0140-6736(79)91788-4. [DOI] [PubMed] [Google Scholar]
- 62.Zhang D, Ye S, Pan T. The role of serum and urinary biomarkers in the diagnosis of early diabetic nephropathy in patients with type 2 diabetes. PeerJ. 2019;2019:1–14. doi: 10.7717/peerj.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yarkova NА, Borovkov NN, Zanozina ОV, Nosov VP. Cystatin C in the diagnosis of chronic kidney disease in patients with type 2 diabetes mellitus. Clinical Med. 2013;5:89–92. [Google Scholar]
- 64.Lee BW, Ihm SH, Choi MG, Yoo HJ. The comparison of cystatin C and creatinine as an accurate serum marker in the prediction of type 2 diabetic nephropathy. Diabetes Res ClinPract. 2007;78:428–34. doi: 10.1016/j.diabres.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 65.Lacquaniti A, Donato V, Pintaudi B, et al. “Normoalbuminuric” diabetic nephropathy: tubular damage and NGAL. Acta Diabetol. 2013;50:935–42. doi: 10.1007/s00592-013-0485-7. [DOI] [PubMed] [Google Scholar]
- 66.Iliadis F, Didangelos T, Ntemka A, et al. Glomerular filtration rate estimation in patients with type 2 diabetes: Creatinine- or cystatin C-based equations? Diabetologia. 2011;54:2987–94. doi: 10.1007/s00125-011-2307-1. [DOI] [PubMed] [Google Scholar]
- 67.Oddoze C, Morange S, Portugal H, Berland Y, Dussol B. Cystatin C is not more sensitive than creatinine for detecting early renal impairment in patients with diabetes. Am J Kidney Dis. 2001;38:310–6. doi: 10.1053/ajkd.2001.26096. [DOI] [PubMed] [Google Scholar]
- 68.Dedual MA, Wueest S, Challa TD, et al. Obesity-induced increase in cystatin C alleviates tissue inflammation. Diabetes. 2020;69:1927–35. doi: 10.2337/db19-1206. [DOI] [PubMed] [Google Scholar]
- 69.De Carvalho JA, Tatsch E, Hausen BS, et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin Biochem. 2016;49:232–6. doi: 10.1016/j.clinbiochem.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 70.He P, Bai M, Hu JP, et al. Significance of neutrophil gelatinase-associated lipocalin as a biomarker for the diagnosis of diabetic kidney disease: A systematic review and meta-analysis. Kidney Blood Press Res. 2020;45:497–509. doi: 10.1159/000507858. [DOI] [PubMed] [Google Scholar]
- 71.Bonventre JV. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc. 2014;125:293–9. [PMC free article] [PubMed] [Google Scholar]
- 72.Abdelraheem S, Ahmed N, Zahran FE, Mohammed G, Ibrahim ESI. Diagnostic performance of kidney injury molecule-1 for detection of abnormal urinary albumin-to-creatinine ratio in type 2 diabetes mellitus. J Immunoassay Immunochem. 2021;42:1954947. doi: 10.1080/15321819.2021.1954947. [DOI] [PubMed] [Google Scholar]
- 73.Fawzy MS, Abu AlSel BT. Assessment of vitamin D-binding protein and early prediction of nephropathy in type 2 Saudi diabetic patients. J Diabetes Res. 2018;2018:8517929. doi: 10.1155/2018/8517929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishiwaka H, Niihata K, Kinoshita M, et al. Urinary C-megalin as a novel biomarker of progression to microalbuminuria: A cohort study based on the diabetes Distress and Care Registry at Tenri (DDCRT 22) Diabetes Res Clin Pract. 2022;186:109810. doi: 10.1016/j.diabres.2022.109810. [DOI] [PubMed] [Google Scholar]
- 75.Ogasawara S, Hosojima M, Kaseda R, et al. Significance of urinary full-length and ectodomain forms of megalin in patients with type 2 diabetes. Diabetes Care. 2012;35:1112–8. doi: 10.2337/dc11-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shore N, Khurshid R, Saleem M. Alpha-1microglobulin: a marker for early detection of tubular disorders in diabetic nephropathy. J Ayub Med Coll Abbottabad. 2010;22:53–55. [PubMed] [Google Scholar]
- 77.Papadopoulou-Marketou N, Kanaka-Gantenbein C, Marketos N, Chrousos GP, Papassotiriou I. Biomarkers of diabetic nephropathy: A 2017 update. Crit Rev Clin Lab Sci. 2017;54:326–42. doi: 10.1080/10408363.2017.1377682. [DOI] [PubMed] [Google Scholar]
- 78.Narita T, Hosoba M, Kakei M, Ito S. Increased urinary excretions of immunoglobulin G, ceruloplasmin, and transferrin predict development of microalbuminuria in patients with type 2 diabetes. Diabetes Care. 2006;29:142–4. doi: 10.2337/diacare.29.1.142. [DOI] [PubMed] [Google Scholar]
- 79.Narita T, Sasaki H, Hosoba M, et al. Parallel increase in urinary excretion rates of immunoglobulin G, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patients. Diabetes Care. 2004;27:1176–81. doi: 10.2337/diacare.27.5.1176. [DOI] [PubMed] [Google Scholar]
- 80.Lee MJ, Jung CH, Kang YM, et al. Serum ceruloplasmin level as a predictor for the progression of diabetic nephropathy in Korean men with type 2 dtiabetes mellitus. Diabetes Metab J. 2015;39:230–9. doi: 10.4093/dmj.2015.39.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kotajima N, Kimura T, Kanda T, et al. Type IV collagen as an early marker for diabetic nephropathy in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 2000;14:13–7. doi: 10.1016/s1056-8727(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 82.Tomino Y, Suzuki S, Azushima C, et al. Asian multicenter trials on urinary type IV collagen in patients with diabetic nephropathy. J Clin Lab Anal. 2001;15:188–92. doi: 10.1002/jcla.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pavkov ME, Weil EJ, Fufaa GD, et al. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int. 2016;89:226–34. doi: 10.1038/ki.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colhoun HM, Marcovecchio ML. Biomarcadores de enfermedad renal diabética. Diabetología. 2018;61:996–1011. doi: 10.1007/s00125-018-4567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kajitani N, Shikata K, Nakamura A, Nakatou T, Hiramatsu M, Makino H. Microinflammation is a common risk factor for progression of nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;88:171–6. doi: 10.1016/j.diabres.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 86.Navarro JF, Mora C, Gomez M, et al. Influence of renal involvement on peripheral blood mononuclear cell expression behaviour of tumour necrosis factor-a and interleukin-6 in type 2 diabetic patients. Nephrol Dial Transplant. 2008;23:919–26. doi: 10.1093/ndt/gfm674. [DOI] [PubMed] [Google Scholar]
- 87.Broedbaek K, Weimann A, Stovgaard ES, et al. Urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine as a biomarker in type 2 diabetes. Free Radic Biol Med. 2011;51:1473–9. doi: 10.1016/j.freeradbiomed.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 88.Kammer M, Heinzel A, Willency JA, et al. BEAt-DKD Consortium Integrative analysis of prognostic biomarkers derived from multiomics panels helps discrimination of chronic kidney disease trajectories in people with type 2 diabetes. Kidney Int. 2019;96:1381–8. doi: 10.1016/j.kint.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 89.Abdel Ghafar MT, Hamed Shalaby K, Ibrahim Okda H, et al. Assessment of two novel renal tubular proteins in type 2 diabetic patients with nephropathy. J Investig Med. 2020;68:748–55. doi: 10.1136/jim-2019-001135. [DOI] [PubMed] [Google Scholar]
- 90.Salem NA, El Helaly RM, Ali IM, et al. Urinary Cyclophilin A and serum Cystatin C as biomarkers for diabetic nephropathy in children with type 1 diabetes. Pediatr Diabetes. 2020;21:846–55. doi: 10.1111/pedi.13019. [DOI] [PubMed] [Google Scholar]
- 91.Rosolowsky ET, Ficociello LH, Maselli NJ, et al. Highnormal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3:706–13. doi: 10.2215/CJN.04271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jalal DI, Maahs DM, Hovind P, et al. Uric acid as a mediator of diabetic nephropathy. Semin Nephrol. 2011;31:459–65. doi: 10.1016/j.semnephrol.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neuhoff N, Kaiser T, Wittke S, et al. Mass spectrometry for the detection of differentially expressed proteins: a comparison of surface-enhanced laser desorption/ionization and capillary electrophoresis/mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:149–56. doi: 10.1002/rcm.1294. [DOI] [PubMed] [Google Scholar]
- 94.Wu J, Chen YD, Gu W. Urinary proteomics as a novel tool for biomarker discovery in kidney diseases. J Zhejiang Univ Sci B. 2010;11:227–37. doi: 10.1631/jzus.B0900327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tofte N, Lindhardt M, Adamova K, et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8:301–12. doi: 10.1016/S2213-8587(20)30026-7. [DOI] [PubMed] [Google Scholar]
- 96.Peters LJF, Floege J, Biessen EAL, Jankowski J, van der Vorst EPC. MicroRNAs in Chronic Kidney Disease: Four Candidates for Clinical Application. Int J Mol Sci. 2020;21:6547. doi: 10.3390/ijms21186547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu T, Xu X, Zhang L, et al. Lipidomics reveals serum specific lipid alterations in diabetic nephropathy. Front Endocrinol (Lausanne) 2021;12:781417. doi: 10.3389/fendo.2021.781417. [DOI] [PMC free article] [PubMed] [Google Scholar]