Abstract
Objective
Emerging research suggests that rheumatoid arthritis (RA) is associated with intestinal dysbiosis. This prospective pilot study evaluates changes in intestinal microbial composition in patients with RA initiating treatment with either methotrexate (MTX) or a tumor necrosis factor inhibitor (TNFi).
Methods
Consecutive patients, fulfilling the 2010 American College of Rheumatology/EULAR classification criteria for RA, who started treatment with either MTX or TNFi delivered a stool sample upon initiation of immunosuppression and 3 months later. A 16S ribosomal RNA gene‐based validated microbiota test (GA‐map Dysbiosis Index Score [DIS], Genetic Analysis, Oslo, Norway) was used to evaluate for the presence and degree of dysbiosis. Fecal levels of Prevotella copri (P. copri) were analyzed by custom‐made quantitative polymerase chain reaction. Changes in microbial composition were analyzed in relation to changes in disease activity, as measured by the disease activity score based on 28‐joint counts, using C‐reactive protein.
Results
At baseline, dysbiosis was present in 33 of 50 (66%) participants and more common in participants with more than 2 years of disease duration (P = 0.019). At the 3‐month follow‐up, 27 of 50 (54%) were good treatment responders and the DIS had improved in 14 of 50 (28%). Participants initiating TNFi more often exhibited improvement in the DIS compared with those initiating MTX (P = 0.031). P. copri was identified in 32 of 50 (64%) at baseline. An improvement in disease activity score based on 28‐joint counts, using C‐reactive protein was associated with a simultaneous decrease in P. copri abundance (rs = 0.30, P = 0.036).
Conclusion
This study affirms that dysbiosis is a feature of RA. Although patients were not randomized to MTX or TNFi, the findings suggest that specific therapies may differentially modulate the gastrointestinal microbiota in RA. The association between P. copri and treatment response requires further study.
INTRODUCTION
The etiology of rheumatoid arthritis (RA) is multifactorial. Although host genetics may contribute to disease susceptibility, environmental factors, such as smoking, may increase the likelihood of disease development. 1 Our current understanding of the key environmental factors associated with RA pathogenesis is limited, but recent studies suggest that alterations in the gastrointestinal (GI) microbiota are present early in the disease course. 2
Prior studies have demonstrated an association between immune response to microbes in the intestine and synovial inflammation in patients with RA. 3 Cross‐sectional studies have consistently reported the presence of intestinal dysbiosis in both early and established RA. 4 , 5 , 6 Furthermore, Prevotella copri (P. copri) has been associated with RA and RA development based on both epidemiologic studies and a well‐defined molecular mimicry model. 2 , 3 , 4 , 5
To date, few studies have explored the intestinal microbiota in relation to usage of immunomodulatory treatments in RA. Small, prospective studies have suggested that successful methotrexate (MTX) therapy in patients with RA may partially normalize the intestinal microbiota and that specific microbial alterations may predict treatment response to MTX. 4 , 7 , 8 To our knowledge, no studies have prospectively evaluated the impact of tumor necrosis factor inhibitors (TNFi) on intestinal dysbiosis in RA. However, in inflammatory bowel disease (IBD), TNFi administration has been associated with a reduction of intestinal dysbiosis that parallels improvements in disease activity with reduced intestinal inflammation. 9
This prospective, observational pilot study investigated patients with RA initiating treatment with either MTX or TNFi at baseline and after 3 months. Our aims were to (1) examine the relationship between the baseline degree of intestinal dysbiosis and clinical characteristics of patients with RA and (2) evaluate changes in the degree of intestinal dysbiosis following treatment with MTX or TNFi. Finally (3), given the prior evidence linking P. copri to RA disease pathogenesis, we wanted to investigate P. copri abundance in relation to disease characteristics and treatment response. In an exploratory aim, this study also sought to explore the relationship between a candidate marker of intestinal inflammation (ie, fecal calprotectin) and intestinal dysbiosis in patients with RA.
MATERIALS AND METHODS
Patient eligibility
This study consecutively enrolled patients with RA fulfilling the 2010 American College of Rheumatology/EULAR classification criteria between February 2016 and March 2018. Eligible patients had to be initiated on therapy with either MTX or TNFi at the departments of Rheumatology in Lund, Sweden, and Karlskrona, Sweden. 10 Patients were included regardless of disease duration, and patients initiating TNFi were included irrespective of ongoing background disease‐modifying antirheumatic drug (DMARD) therapy as long as it had been prescribed at a stable dosage for 3 months before the study start and remained unaltered during the study period. Treatment selection was based on physician discretion and typically reflected the national guidelines on the management of RA from the Swedish Society for Rheumatology. 11 In general, during this time period, these guidelines suggested MTX as the first line DMARD treatment for RA and TNFi as the first‐line biologic DMARD therapy in refractory cases. Patients were excluded if they met any of the following exclusion criteria: a history of IBD, a history of diverticulitis, previous surgery of the GI tract (except appendectomies), a history of alcohol abuse, failure to comply with protocol, antibiotic use within the last 4 weeks, or ongoing biologic or targeted synthetic DMARD therapy. Patients were also excluded if the initiation of the study drug coincided with the initiation or cessation of another DMARD.
Disease activity and treatment response
Disease activity, measured as disease activity score based on 28‐joint counts, using CRP (DAS28‐CRP), was assessed at baseline and at 3 months follow‐up, and the change in disease activity (ΔDAS28‐CRP) between these two time points was used as a continuous measure of treatment response. Furthermore, participants were categorized as showing good, moderate, or no treatment response in accordance with EULAR response criteria. 12
Patient reported measures
At baseline, GI symptoms were assessed using the validated Gastrointestinal Symptom Rating Scale (GSRS). 13 This Swedish rating scale is a self‐administered questionnaire that includes 15 items rated on a 7‐grade Likert scale. It encompasses the following five dimensions: reflux, abdominal pain, constipation, indigestion, and diarrhea. Usage of antibiotics, coffee, proton pump inhibitors, and smoking were assessed by a local questionnaire and alcohol consumption by the Alcohol Use Disorders Identification Test questionnaire. 14
GI microbiota analysis
At baseline and at 3 months follow‐up, participants delivered stool samples, collected at home, which were briefly stored in a refrigerator, and transported to a −80°C freezer within 12 hours of collection. The Dysbiosis Index Score (DIS) was measured with the GA‐map Dysbiosis Test (Genetic Analysis), which quantifies intestinal microbial balance on a 5‐grade ordinal scale, in which values of three or more denotes dysbiosis. This test analyzes the 16S ribosomal RNA (rRNA) sequence (V3‐V9) by using 54 labeling probes aimed at 368 strains spread across 36 genera, 10 bacterial classes, and 6 phyla (Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, Tenericutes, and Verrucomicrobia). 15 The GA‐map Dysbiosis Test results demonstrate good agreement with the results of MiSeq Illumina high‐throughput 16S rRNA amplicon sequencing in identifying intestinal dysbiosis. 15 The test has also been validated in relation to Scandinavian cohorts of healthy controls. 15 , 16 , 17 All samples in the current study were analyzed simultaneously to minimize batch effects.
Fecal levels of P. copri were analyzed in triplicates with a custom‐made quantitative polymerase chain reaction together with Baseclear at baseline and at 3 months (Leiden, The Netherlands) (Supplementary methods). The abundance of P. copri bacteria per gram of feces was calculated, and log 2 values (2logPcopri) were used for analysis. A two‐fold change in P. copri levels was regarded as a significant increase or decrease of P. copri abundance.
Intestinal inflammation was studied by measurement of fecal calprotectin (F‐calprotectin), which was analyzed with a polyclonal Elisa (Calpro AS). Levels of F‐calprotectin greater than or equal to 50 μg/g were considered elevated. 18
Statistical analysis
To evaluate the relationships between the DIS or P. copri levels with clinical features, nonparametric testing, including the Mann‐Whitney U‐test and Spearman's rank correlation test, were used. The same nonparametric tests were used to evaluate how changes in the DIS and P. copri levels relate to therapy and treatment response. A P value less than 0.05 was considered statistically significant. Given the pilot nature of the study, a power calculation was not performed, and there were no corrections made for multiple hypothesis testing.
RESULTS
Patient characteristics
Sixty‐four patients fulfilled the study inclusion criteria, of which 50 participants completed the study according to protocol. Out of those not completing the study (n = 14), a majority (n = 9) failed to deliver both stool samples, and another subgroup (n = 3) failed to complete 3 months of prescribed therapy.
Patient characteristics are shown in Table 1. At baseline, 29 of the 32 patients who initiated TNFi therapy were already prescribed conventional synthetic DMARD therapy (n = 29 of 32), in most cases MTX (n = 24 of 32). Among the included participants, there was a female predominance, and on average, participants had moderate RA disease activity at baseline. Disease duration was, as expected from the clinical treatment algorithm previously described, lower in participants starting MTX compared with TNFi (median 0.4 vs 6.4 years, respectively). The presence of anticitrullinated peptide antibodies and rheumatoid factor (RF) were more common in participants initiating TNFi than MTX.
Table 1.
Baseline patient characteristics*
| All patients (N = 50) | MTX‐start (n = 18) | TNFi‐start (n = 32) | |
|---|---|---|---|
| Age, y (SD) | 58 (11) | 59 (11) | 58 (11) |
| Female, n (%) | 39 (78) | 14 (78) | 25 (78) |
| ACPA positive, n (%) | 38 (76) | 11 (61) | 27 (84) |
| RF positive, n (%) | 34 (68) | 11 (61) | 23 (72) |
| HAQ, mean (SD) a | 0.85 (0.47) | 0.76 (0.51) | 0.90 (0.45) |
| Disease duration, y, median, (IQR) | 4.4 (0.7–12) | 0.4 (0.3–1.1) | 6.4 (3.1–17.3) |
| DAS28‐CRP, mean (SD) | 3.9 (1.2) | 3.9 (0.8) | 3.9 (1.4) |
| Daily coffee consumption, cups (SD) | 4.3 (4.3) | 3.9 (1.9) | 4.5 (5.2) |
| Smoking, ever, n (%) a | 34 (68) | 10 (56) | 24 (75) |
| Smoking, package y, mean (SD) a | 13 (16) | 12 (16) | 14 (16) |
| Current smoker, n (%) | 8 (16) | 3 (17) | 5 (13) |
| PPI usage, n (%) | |||
| Daily | 11 (22) | 3 (17) | 8 (25) |
| Sporadic | 8 (16) | 2 (11) | 6 (19) |
| None | 31 (62) | 13 (72) | 18 (56) |
| NSAID, n (%) | |||
| Daily | 12 (24) | 4 (22) | 8 (25) |
| Sporadic | 10 (20) | 4 (22) | 6 (19) |
| None | 28 (56) | 10 (59) | 18 (56) |
| Study drug | |||
| MTX po, n (%) | 16 (89) | N/A | |
| MTX sc, n (%) | 2 (11) | N/A | |
| MTX weekly dose mg, mean (SD) | 19 (2.7) | N/A | |
| Etanercept | N/A | 31 (97) | |
| Infliximab | N/A | 1 (3) | |
| Ongoing treatment | |||
| MTX, po, n (%) | N/A | 16 (50) | |
| MTX, sc, n (%) | N/A | 8 (25) | |
| MTX weekly dose mg, mean (SD) | N/A | 14 (8) | |
| Sulfasalazine, n (%) | 0 (0) | 6 (19) | |
| Prednisolone, n (%) | |||
| 0 mg | 27 (54) | 11 (61) | 16 (50) |
| 1–5 mg | 13 (26) | 4 (22) | 9 (28) |
| 6–10 mg | 7 (14) | 3 (17) | 7 (22) |
| >10 mg | 0 (0) | 0 (0) | 0 (0) |
| BMI, mean (SD) b | 26 (4.4) | 27 (2.7) | 26 (4.7) |
| F‐calprotectin μg/g, median | 25 (12–51) | 27 (17–90) | 22 (11–41) |
| Elevated (≥50 ug/g), n (%) | 12 (24) | 5 (28) | 7 (22) |
ACPA, anticitrullinated peptide antibodies; BMI, body mass index; DAS28‐CRP, disease activity score based on 28‐joint counts; HAQ, health assessment questionnaire; IQR, interquartile range; MTX, methotrexate, N/A, not applicable; NSAID, nonsteroidal antiinflammatory drug; po, per os; PPI, proton pump inhibitor; RF, rheumatoid factor; sc, subcutaneous.
Data missing = 3.
Data missing = 6.
Intestinal dysbiosis in RA
At baseline, a DIS of greater than or equal to 3, indicating the presence of intestinal dysbiosis, was present in 33 out of 50 participants (Figure 1), with similar proportions in participants initiating MTX (11 of 18 [61%]) and TNFi (22 of 32 [69%]). The baseline DIS did not correlate with disease activity or disease duration (Table 2). However, when comparing participants with established (>2 years, n = 29) and early disease (≤2 years from first RA symptoms, n = 21), the DIS was higher in participants with established disease (mean 3.0 vs 2.3; P = 0.019) (Figure 1A).
Figure 1.
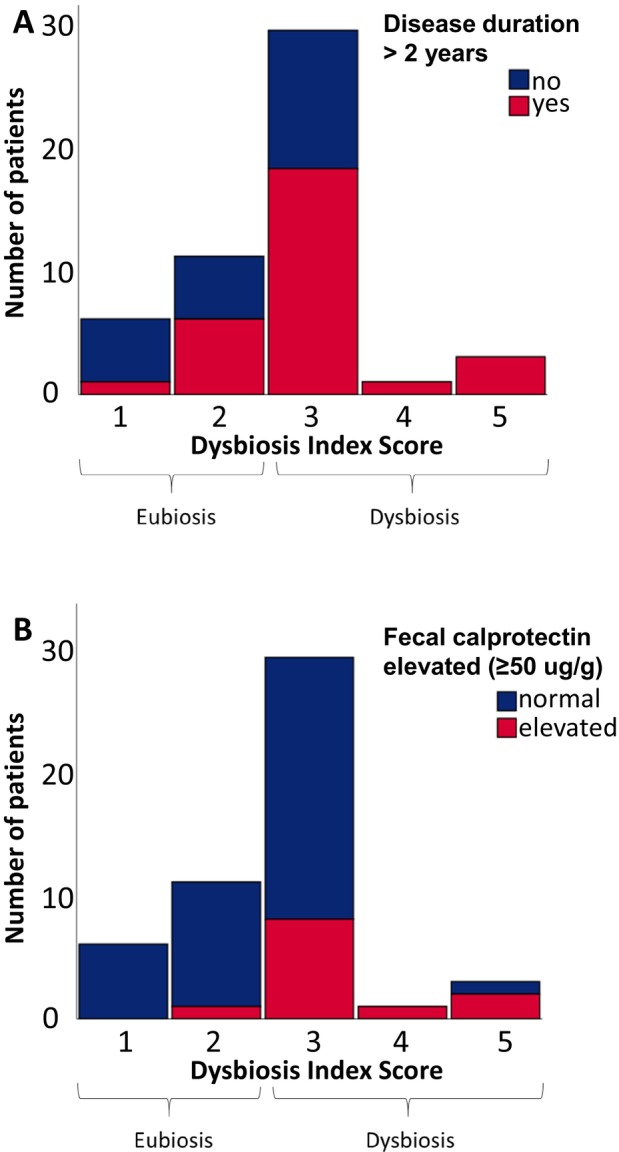
Histogram of the Dysbiosis Index Score at baseline. Each bar represents number of patients. A Dysbiosis Index Score of 3–5 represents intestinal dysbiosis and 1–2 eubiosis. In (A), patients with rheumatoid arthritis disease with a duration of more than 2 years are marked in red. In (B), patients with elevated fecal calprotectin, indicating intestinal inflammation, are marked in red.
Table 2.
The Dysbiosis Index Score (a five‐step semiquantitive measure of intestinal dysbiosis) and P. copri levels in relation to disease activity, disease duration, fecal calprotectin, and serology at baseline*
| Correlation with Dysbiosis Index Score, rs | Correlation with P. copri, rs | |
|---|---|---|
| DAS28‐CRP | 0.11, P = 0.479 | −0.04, P = 0.780 |
| Disease duration | 0.22, P = 0.118 | 0.11, P = 0.445 |
| F‐calprotectin | 0.33, P = 0.018 | −0.06, P = 0.677 |
| Dysbiosis Index Score | N/A | −0.11, P = 0.430 |
| Median levels, (yes vs no) | ||
|---|---|---|
| Dysbiosis Index Score | P. copri, ng per g feces | |
| RF‐positivity | 3.0 vs 3.0, P = 0.304 | 1.2e‐3 vs 0.0, P = 0.034 |
| ACPA‐positivity | 3.0 vs 3.0, P = 0.789 | 9.6e‐5 vs 4.5e‐5, P = 0.340 |
ACPA, anticitrullinated peptide antibodies; DAS28‐CRP, disease activity score based on 28‐joint counts; N/A, not applicable; P. copri, Prevotella copri; RF, rheumatoid factor.
Twelve patients (24%) exhibited elevated levels of F‐calprotectin suggestive of intestinal inflammation. Increased F‐calprotectin levels were associated with higher (worse) DIS (rs = 0.33 P = 0.018), and the DIS was higher in participants with elevated F‐calprotectin (mean 3.3 vs 2.5, P = 0.006) (Figure 1B).
GI symptoms, as assessed by the GSRS, were uncommon in this cohort (Supplementary Table 1). The DIS was not associated with the GSRS, body mass index, concomitant medications (nonsteroidal antiinflammatory drugs/prednisolone/proton pump inhibitor), serological profile, smoking, or alcohol habits (Table 2 and Supplementary Table 2).
Intestinal dysbiosis and inflammation at follow‐up
At the 3‐month follow‐up, 54% of the study participants exhibited good EULAR‐defined treatment response, whereas no treatment response occurred in 26% of the participants. Numerically, more participants who initiated MTX exhibited a good treatment response compared with TNFi (12 of 18 [67%] vs 15 of 32 [47%]).
At follow‐up, 14 participants showed a decrease (improvement) of their DIS, whereas 11 exhibited an increase (worsening) of their DIS. For those initiating MTX, six (33%) experienced a worsening of their DIS at follow‐up, and two (11%) experienced an improvement in their DIS. For participants initiating TNFi, 5 (15%) experienced worsening of their DIS, whereas 12 (38%) experienced an improvement in their DIS. There was a significant difference between the mean change in DIS between participants initiating MTX and TNFi (0.28 vs −0.39, P = 0.031) at follow‐up (Figure 2).
Figure 2.
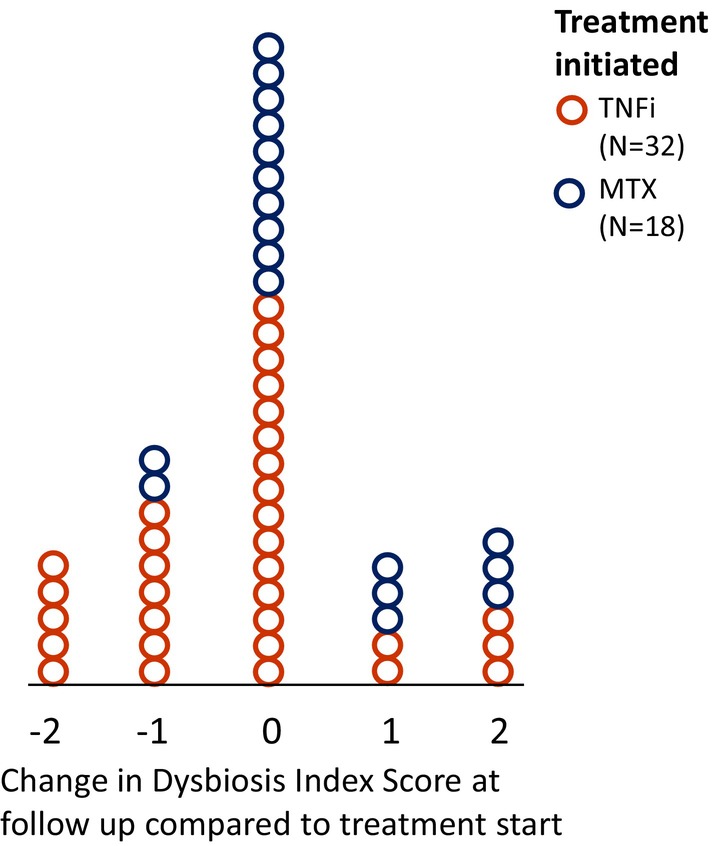
Change in intestinal microbial balance after initiation of TNFi or MTX. Dot‐plot showing change in the Dysbiosis Index Score in patients starting treatment with either TNFi or MTX at 3 months follow‐up. An improvement of the Dysbiosis Index Score was more common in patients starting TNFi than MTX. MTX, methotrexate; TNFi, tumor necrosis factor inhibitor.
Changes in intestinal dysbiosis in relation to treatment response
Among participants achieving good EULAR‐defined treatment response, seven exhibited a worsening of the DIS and five exhibited an improvement in the DIS. For those achieving no treatment response, three exhibited a worsening of the DIS and five exhibited an improvement (Figure 3).
Figure 3.
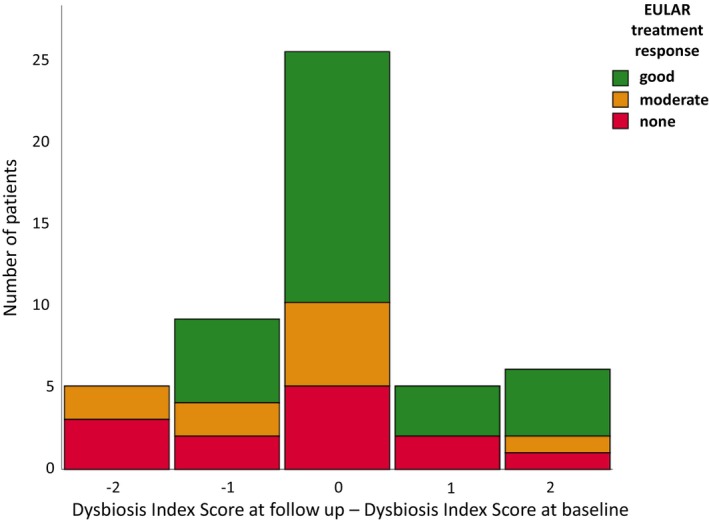
Histogram of the change in Dysbiosis Index Score at follow‐up. Each bar represents number of patients. Good treatment response could be seen both in patients exhibiting an improvement (negative value on x‐axis) and a worsening (positive value on x‐axis) of the Dysbiosis Index Score at follow‐up.
P. copri in relation to disease characteristics and treatment response
At baseline, P. copri was identified in measurable levels (above 1.0e‐6 ng/g faces) in 32 of 50 (64%) participants, with similar proportions in participants initiating MTX (11 of 18 [61%]) and TNFi (21 of 32 [66%]), respectively. P. copri baseline levels were not significantly associated with disease activity, disease duration or early disease (as defined previously), F‐calprotectin, or the DIS. P. copri was more prevalent among participants who were RF positive (Table 2).
After 3 months, a decrease in P. copri was observed in 19 participants, whereas 18 exhibited an increase in P. copri. Changes in P. copri levels were evenly distributed among MTX‐starters and TNFi‐starters, with 7 of 18 [39%] and 12 of 32 [38%] exhibiting a decrease in P. copri levels, respectively.
A decline in P. copri levels between treatment start and the 3‐month follow‐up was associated with an improvement in the EULAR‐defined treatment response (rs = 0.30, P = 0.034). This association with treatment response was evident in patients who were RF positive (Figure 4).
Figure 4.
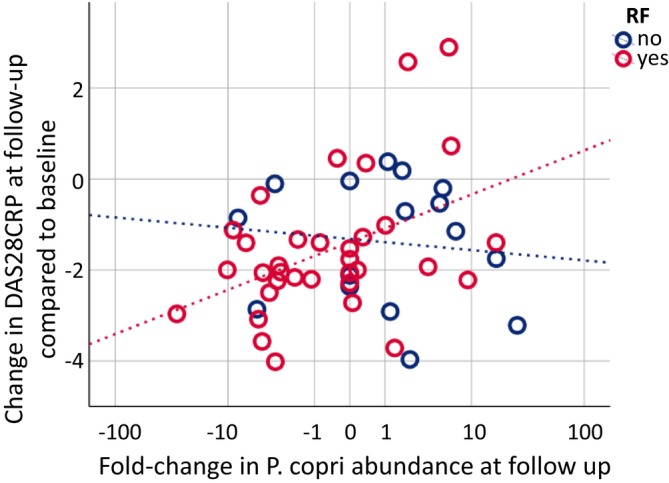
Change in disease activity in relation to change in P. copri abundance. At follow‐up, 3 months after treatment start, change in disease activity is associated with change in P. copri abundance in patients with positive RF (rs = 0.344, P = 0.046, n = 34) but not patients with negative RF (rs = −0.17, P = 0.541, n = 16). The majority of patients with positive RF who experienced a positive response to their immunosuppressive therapy also exhibited a decline in P. copri levels (lower left quartile). DAS28‐CRP, disease activity score based on 28‐joint counts; P. copri, Prevotella copri; RF, rheumatoid factor.
DISCUSSION
The present study affirmed that intestinal dysbiosis is present in a prospective cohort of consecutive patients with RA initiating therapy with either MTX or TNFi. This pilot study also found that participants initiating therapy with TNFi were more likely to have an improvement in their extent of intestinal dysbiosis compared with those initiating therapy with MTX. Although the impact of MTX on GI microbiota has been studied, to our knowledge, this is the first study to report longitudinal changes in the intestinal microbiota among patients with RA receiving TNFi.
The finding that changes in intestinal microbiota were observed in patients with RA who commenced on TNFi therapy is consistent with the results of studies in other patient populations, such as IBD and spondyloarthritis. 19 Although GI inflammation and dysbiosis are recognized disease features of IBD and spondyloarthritis, our understanding of GI‐tract involvement in RA is still evolving. One cross‐sectional study in RA suggested the possibility of normalization of the microbiota after starting treatment with TNFi, 20 but that study did not evaluate changes in intestinal microbiota over time. In our study, 31 out of 32 patients treated with TNFi received etanercept, which is a soluble TNF‐receptor. In contrast to monoclonal antibodies, this TNFi has failed to show efficacy in IBD. Further studies are needed to elucidate how this TNFi may interact with the GI immune system and microbiota and if this differs from TNFi of monoclonal antibody type.
It is unclear whether the observed changes in intestinal microbiota in patients treated with TNFi therapy contribute to the pathobiology of RA and/or treatment response. However, the present study followed a significant relationship between reduction in P. copri abundance and response to therapy in both treatment groups. Accumulating evidence suggests that P. copri is directly involved in autoimmune reactions, leading to articular inflammation in RA. 21 A previous cross‐sectional study reported a greater abundance of P. copri in untreated patients with RA compared with patients with RA receiving immunomodulatory treatment. 2 The interplay between intestinal microbes and the immune system in relation to systemic autoimmunity is an active area of research, 21 , 22 and future clinical trials in RA may consider evaluating the abundance of P. copri as a candidate biomarker of treatment response.
Although this study was not adequately powered to analyze treatment response in relation to dysbiosis as measured by the DIS, many participants who exhibited good treatment response did so without an improvement in the DIS (Figure 3). Although the reasons for this discrepancy are unknown, it is possible that further measurement of specific bacteria species could provide greater insight into how different therapies alter the intestinal microbiota in RA. 21 , 23 Furthermore, external factors (eg, age, sex) beyond the intestinal microbiota may modulate treatment response in RA and should be considered in future studies.
This study also demonstrated that P. copri was more prevalent in participants with positive RF, compared with participants with negative RF, which is a finding consistent with recent reports. 21 , 24 Future studies of larger magnitude are needed to evaluate whether P. copri can serve as a biomarker for RA treatment response in both patients with positive and negative RF.
The observed relationship between intestinal dysbiosis and elevated levels of F‐calprotectin was intriguing and may reflect the dynamic relationship between dysbiosis and inflammation in the intestinal mucosa. Similar results have previously been reported in arthritis animal models. 25 Endoscopic studies involving biopsies may help to further elucidate the relationship between the intestinal immune system and microbiota in relation to RA development.
We were unable to confirm the findings of a previous study, which demonstrated partial resolution of intestinal dysbiosis following oral MTX therapy. 4 The present study population differed from that of the previous report in respect to geographic origin, disease activity, age, and disease duration. Specifically, the prior study included a majority of patients with high disease activity.
This study has notable limitations. First, although the microbial analyses of fecal samples were based on the Dysbiosis Index Score, which has shown comparable performance in identifying intestinal dysbiosis in relation to MiSeq Illumina high‐throughput 16S rRNA amplicon sequencing, the DIS has limited power to identify novel bacterial strains and to provide comprehensive insight into all bacterial strains present in a sample. Based on the promising results of this pilot study, future analyses using high‐throughput sequencing of the full metagenome are warranted.
Further limitations of this observational study include the fact that patients were not randomized to treatment, and therefore, confounding factors may contribute to the observed treatment arm differences. Twenty‐four out of 32 patients initiating TNFi were on a stable dose of MTX at baseline and continued using this drug in unaltered dosage throughout the study period, which may have influenced group comparisons. Also, the number of included patients is limited, restricting further subgroup analyses. The strengths of this study include its prospective design, prespecified objectives and outcomes, as well as the inclusion of consecutive patients receiving two different therapeutic approaches.
In summary, the present study demonstrated alterations of the intestinal microbiota between treatment start and 3‐month follow‐up in patients with RA initiating MTX or TNFi. Reduction of P. copri, a bacterial species previously suggested to be of pathogenic importance in RA, paralleled observed improvements in RA disease activity. Future prospective studies in larger cohorts, encompassing analysis of the complete intestinal microbiome, are needed to validate these results and further explore the relevance of microbial changes induced by immunosuppression in RA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr Andréasson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Andréasson, Olofsson, Alrawi, Wallman, Volkmann.
Acquisition of data
Andréasson, Olofsson, Alrawi, Klaassens, Holster, Wallman.
Analysis and interpretation of data
Andréasson, Olofsson, Lagishetty, Klaassens, Holster, Hesselstrand, Jacobs, Wallman, Volkmann.
Supporting information
Disclosure Form
Appendix S1: Supplemental methods
ACKNOWLEDGMENTS
We are indebted to our study nurse Elna Haglund for skillful assistance in organizing the study. We appreciate the advice and inspiration from Professor Meliha Kapetanovic in the early phases of this study.
Supported by the NIH National Heart, Lung, and Blood Institute (grant K23‐HL‐150237‐01), the Anna‐Greta Crafoord Foundation, the Swedish Medical Society, the Swedish Rheumatism Association, and Stiftelsen Ulla och Roland Gustafssons Donationsfond.
Additional supplementary information cited in this article can be found online in the Supporting Information section (https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/acr2.11673).
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11673.
REFERENCES
- 1. Klareskog L, Rönnelid J, Saevarsdottir S, et. al. The importance of differences; On environment and its interactions with genes and immunity in the causation of rheumatoid arthritis. J Intern Med 2020;287:514–533. [DOI] [PubMed] [Google Scholar]
- 2. Scher JU, Sczesnak A, Longman RS, et. al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013;2:e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pianta A, Arvikar SL, Strle K, et. al. Two rheumatoid arthritis‐specific autoantigens correlate microbial immunity with autoimmune responses in joints. J Clin Invest 2017;127:2946–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang X, Zhang D, Jia H, et. al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med 2015;21:895–905. [DOI] [PubMed] [Google Scholar]
- 5. Wang H, Ong E, Kao JY, et. al. Reverse microbiomics: a new reverse dysbiosis analysis strategy and its usage in prediction of autoantigens and virulent factors in dysbiotic gut microbiomes from rheumatoid arthritis patients. Front Microbiol 2021;12:633732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Wright K, Davis JM, et. al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med 2016;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Artacho A, Isaac S, Nayak R, et. al. The pretreatment gut microbiome is associated with lack of response to methotrexate in new‐onset rheumatoid arthritis. Arthritis Rheumatol 2021;73:931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scher JU, Nayak RR, Ubeda C, et. al. Pharmacomicrobiomics in inflammatory arthritis: gut microbiome as modulator of therapeutic response. Nat Rev Rheumatol 2020;16:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magnusson MK, Strid H, Sapnara M, et. al. Anti‐TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J Crohns Colitis 2016;10:943–952. [DOI] [PubMed] [Google Scholar]
- 10. Aletaha D, Neogi T, Silman AJ, et. al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–2581. [DOI] [PubMed] [Google Scholar]
- 11. Swedish Society for Rheumatology . Guidelines for the treatment of rheumatoid arthritis. Accessed April 18, 2024. https://riktlinjer.svenskreumatologi.se/riktlinjer‐och‐rekommendationer/riktlinjer‐for‐lakemedelsbehandling‐vid‐reumatoid‐artrit/
- 12. Wells G, Becker JC, Teng J, et. al. Validation of the 28‐joint disease activity score (DAS28) and European League Against Rheumatism response criteria based on C‐reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Svedlund J, Sjödin I, Dotevall G. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 1988;33:129–134. [DOI] [PubMed] [Google Scholar]
- 14. Babor T, Higgins‐Biddle JC, Aaunders JB, et. al. Audit. The alcohol disorders identification test. Guidelines for use in primary care. World Health Organization 2001. Accessed April 18, 2024. https://www.paho.org/sites/default/files/Auditmanual_ENG.pdf [Google Scholar]
- 15. Casén C, Vebø HC, Sekelja M, et. al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther 2015;42:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei S, Bahl MI, Baunwall SMD, et. al. Determing gut microbial dysbiosis: a review of applied indexes for assessment of intestinal microbiota imbalances. Appl Environ Microbiol 2021;87:e00395–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandl T, Marsal J, Olsson P, et. al. Severe intestinal dysbiosis is prevalent in primary Sjögren's syndrome and is associated with systemic disease activity. Arthritis Res Ther 2017;19:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waugh N, Cummins E, Royle P, et. al. Faecal calprotectin testing for differentiating amongst inflammatory and non‐inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess 2013;17:xv–xix, 1–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dai Q, Xia X, He C, et. al. Association of anti‐TNF‐a treatment with gut microbiota of patients with ankylosing spondylitits. Pharmacogenet Genomics 2002;32:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Picchianti‐Diamanti A, Panebianco C, Salemi S, et. al. Analysis of gut microbiota in rheumatoid arthritis patients: disease‐related dysbiosis and modifications induced by etanercept. Int J Mol Sci 2018;19:2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seifert JA, Bemis EA, Ramsden K, et. al. Association of antibodies to Prevotella copri in anti‐CCP‐positive individuals at‐risk for developing rheumatoid arthritis and in those with early or established rheumatoid arthritis. Arthritis Rheumatol 2023;75:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao T, Wei Y, Zhu Y, et al. Gut microbiota and rheumatoid arthritis: from pathogenesis to novel therapeutic opportunities. Front Immunol 2022;13:1007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chriswell ME, Lefferts AR, Clay MR. Clonal IgA and IgG autoantibodies from individuals at risk for rheumatoid arthritis identify and arthritogenic strain of Subdoligranulum. Sci Transl Med 2022;14:eabn5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alpizar‐Rodriguez D, Lesker TR, Gronow A, et. al. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann Rheum Dis 2019;78:590–593. [DOI] [PubMed] [Google Scholar]
- 25. Kim M‐J, Kim J‐Y, Kang M, et. al. Reduced fecal calprotectin and inflammation in a murine model of atopic dermatitis following probiotic treatment. Int J Mol Sci 2020;21:3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1: Supplemental methods


