Abstract
Objective
Self‐monitored point‐of‐care urate‐measuring devices are an underexplored strategy to improve adherence to urate‐lowering therapy and clinical outcomes in gout. This study observed patient‐led urate self‐monitoring practice and assessed its influence on allopurinol adherence, urate control, and health‐related quality of life.
Methods
People with gout (n = 31) and prescribed allopurinol self‐monitored their urate concentrations (HumaSens2.0plus) at baseline and thereafter monthly for 12 months (3 months per quarter). Adherence to allopurinol was measured using medication event monitoring technology (Medication Event Monitoring System cap). Time spent below the target urate concentration (<0.36 mmol/L) was determined. Health‐related quality of life was measured using a survey (EuroQoL EQ‐5D‐5L). Gout flares were recorded. Two‐tailed Spearman correlation and the Wilcoxon matched‐pairs signed‐rank test (P < 0.05) were used for statistical comparisons.
Results
Most participants were male (94%) and had urate concentrations below the target (74%) at baseline. Overall, seven participants demonstrated repeated periods of “missed doses” (two or fewer allopurinol doses missed consecutively) and “drug holidays” (three or more missed doses). Most participants (94%) persisted with allopurinol. Time spent within the target urate concentration increased 1.3‐fold (from 79% to 100%; P = 0.346), and the incidence of gout flares decreased 1.6‐fold (from 8 to 5; P = 0.25) in the final quarter compared to that in the first quarter of the study. Health‐related quality of life was reduced for participants reporting at least one gout flare (median utility values 0.9309 vs 0.9563, P = 0.04).
Conclusion
Patient‐led urate self‐monitoring may support the maintenance of allopurinol adherence and improve urate control, thus reducing the incidence of gout flares. Further research on patient‐led urate self‐monitoring in a randomized controlled study is warranted.
INTRODUCTION
Gout is a chronic inflammatory arthritis characterized by acute episodes of painful joints known as “gout flares.” The acute inflammation is triggered by monosodium urate crystals in joint synovial fluid, often associated with chronically elevated urate concentrations. 1 Despite effective urate‐lowering therapy (ULT) (eg, allopurinol) for long‐term management, up to 50% of people with gout discontinue therapy within the first six months. 2 Patient‐reported factors that influence ULT adherence behavior include the understanding of ULT and its importance for preventing gout flares and experiences of health care professionals providing gout management advice. 3 An intervention that addresses these factors should improve ULT adherence and support the attainment of target urate concentration (<0.36 mmol/L), 4 thereby decreasing the incidence of gout flares. Because gout flares are associated with poor quality of life (eg, decreased work productivity and self‐care 5 ), such an intervention may also improve social outcomes.
SIGNIFICANCE & INNOVATIONS.
Point‐of‐care urate level testing may improve gout management by supporting medication adherence and attaining clinical targets.
Time within the target urate concentration may be increased the longer people with gout self‐monitor urate concentrations.
The incidence of gout flares may be decreased the longer people with gout self‐monitor urate concentrations.
Previous interventions to improve gout management that account for ULT adherence have been pharmacist led, nurse led, shared care (reviewed in the study by Sinnappah et al 6 ), and patient centered. 7 Although the impact of these interventions on ULT adherence appear positive, the reporting of adherence measures is poor and must be interpreted carefully. 6 Further, only a nurse‐led intervention improved ULT adherence while also accounting for persistence. 8 , 9 , 10 The high rate of discontinuation and reinitiation of ULT 11 and the lack of appropriate measurement of adherence in gout, particularly for persistence, may in part explain the limited effect of these interventions on urate concentration control.
Researchers designing interventions to improve gout patient adherence may benefit from reflecting on other chronic conditions in which patient‐led self‐monitoring services improve medication adherence 12 and clinical outcomes, such as blood pressure control 13 in hypertension. The benefits of self‐monitoring using a biomarker is recognized globally, 14 with the World Health Organization stating the approach fosters active patient participation in their health care. 15 Consequently, point‐of‐care (POC) devices for blood glucose are subsidized in many countries (eg, the National Diabetes Services Scheme in Australia 16 ). However, despite having effective medications, a measurable biomarker, and POC devices available, self‐monitoring remains underexplored in gout. 7 Given that understanding of ULT and gout flares among people with gout impacts their persistence, 3 urate concentration self‐monitoring may support and develop patient understanding of how ULT impacts gout, thereby facilitating adherence. This observational proof‐of‐concept feasibility study aimed to examine patient‐led urate concentration self‐monitoring by assessing the impact of this practice on their adherence to allopurinol, urate concentration control, incidences of gout flares, health‐related quality of life, and medical resource use.
PATIENTS AND METHODS
Study design and participants
This observational proof‐of‐concept feasibility study (Australian and New Zealand Clinical Trial Registry identifier: ACTRN12621001730897) was conducted from June 2021 to April 2023 (see Supplementary Data S1 for additional details). Ethical approval was obtained from the University of Sydney Human Research Ethics Committee (2021/216). Participants across Australia were recruited from a database of people with gout interested in participating in research (created by the Gout Self‐Management App study, 17 which included some gout education) and through advertisements on social media. Recruitment was stratified (1:1 rural to urban), with rurality assessed using the postcode remoteness area rating. 18
Eligible participants were people with gout who were prescribed allopurinol at study enrollment (but may not have initiated therapy), at least 18 years old, and proficient in the English language. People who were assisted in taking their medication were ineligible to participate. Written informed consent for participation was obtained from eligible individuals.
Study procedures
Demographic data were collected at study enrollment (Supplementary Data S1). All study procedures were conducted via telehealth, and equipment was mailed to participants. Participants received a POC device with associated consumables (HumaSens2.0plus Multiparameter System; HUMAN Diagnostics Worldwide), which determines urate concentration using a capillary blood sample (ie, finger prick), and were trained on using the device by JSC using video conferencing. Accuracy of the device is comparable to pathology testing. 19 , 20 Participants also received their allopurinol every three months, as prescribed by their health care professional. A Medication Event Monitoring System (MEMS) cap (Aardex) recorded the date and time of bottle opening as a proxy for medication taking.
Participants manually recorded (eg, a written record or a text sent to a study investigator (TJFM or JSC)) urate concentrations using the POC device at least once a month for 12 months. Consumables (25 testing strips every three months) were provided to test more frequently, as dictated by the participant. Urate concentration data were collected during monthly telehealth visits (approximately five minutes) with a study investigator (TJFM or JSC). Data from the MEMS cap were uploaded by participants through a mobile phone application during each telehealth visit. In addition, urate concentration data and information on any gout flares and/or adverse events experienced were reported. Participants were informed that staying below the target urate concentration (0.36 mmol/L) reduces their risk of gout flares. After each visit, participants were provided a graphical representation of their urate concentrations, including the urate target concentration. Participants were aware that their medication‐taking behavior was monitored, but they were not provided these data. At the study conclusion, the POC device and MEMS cap were returned to study investigators.
Based on MEMS, gout flare, and urate concentration data, the study clinician (ROD, rheumatologist) could recommend a change in allopurinol dosage to the participants’ prescriber. Dosage adjustments remained at the discretion of the prescriber and were communicated to study investigators by participants. Gout flares were defined 21 and reported to study investigators immediately or during telehealth visits.
Operational definitions of adherence
We used the proposed timelines, events, objectives, and sources framework 22 , 23 and the International Society for Medication Adherence Medication Adherence Reporting Guideline 24 to ensure suitable operationalization and quantification and consistent adherence reporting (Supplementary Data S1). Adherence phases were defined as the following:
Initiation: a delay of more than seven days between the prescription of allopurinol and the first dose recorded by the MEMS cap was considered noninitiation of the study drug. Participants already taking allopurinol were dispensed a new supply.
Implementation: variability in the implementation of allopurinol was defined using an adaption of Urquhart's “Rule of Sixes.” 25 Observed patterns of suboptimal implementation were defined as “missed doses” and “drug holidays,” categorized by the number of adherent days after the period of missed doses (Figure 1). Each participant was assigned an “implementation type” based on their most common pattern observed.
Persistence: participants who did not open the pill bottle for ≥30 consecutive days were assumed to have stopped taking allopurinol.
Figure 1.
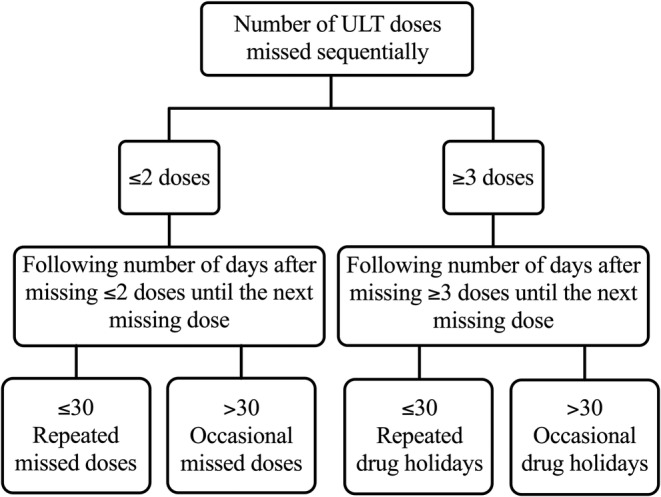
Flowchart of ULT implementation terminology. ULT, urate‐lowering therapy.
Health‐related quality of life, health care use, and costs
Participants completed the EuroQoL EQ‐5D‐5L questionnaire 26 at baseline; at 3‐, 6‐, 9‐, and 12‐month follow‐ups; and when they experienced a gout flare to determine their health‐related quality of life. A health utility score (ranging from −0.301 to 1) was generated at each time point using an Australian value set. 27 Medical resource usage was collected at baseline and at 1‐, 2‐, 4‐, 6‐, 9‐, and 12‐month follow‐ups using a questionnaire 28 adapted for gout (Supplementary Data S1). Data are reported as counts of items of resource use. A comparison of the expected direct costs of a self‐monitoring service to the medical costs of a gout flare was undertaken.
Statistical analysis
Demographics were analyzed using descriptive statistics. The period urate concentrations were <0.36 mmol/L and were determined using a linear interpolation method. 29 When applicable, data were described in monthly increments or as study quarters (three months per quarter). The relationship between self‐monitoring events and measured outcomes was assessed with the two‐tailed nonparametric Spearman correlation or the Wilcoxon matched‐pairs sign ranked test (P < 0.05) using GraphPad PRISM (version 9.5.0) and R Studio (version 2022.12.0+353). For participants lost to follow‐up, MEMS and urate data were analyzed up to the last data point collected. For adherence analysis, data before dosage escalation were excluded when applicable.
Summary utilities were determined for each participant by calculating the area under the curve for the utility scores using a trapezoidal method. Trends in the utility scores over time were assessed using linear mixed modeling. Utility and EuroQoL visual analog scale (EQ‐VAS) scores were compared at baseline, at month 12, and in the presence and absence of gout flares using Stata (version 17.0; StataCorp) (Supplementary Data S1). The EQ‐VAS score (0–100) represents the participant's self‐assessment of their health (0 being the worst health imaginable and 100 being the best).
RESULTS
Participant characteristics
In total, 32 people with gout were enrolled, with one participant withdrawing (Figure 2). Participants were predominantly male (94%) and more than 50 years old (74% [range 34–86 years old]) (Table 1). Most participants were diagnosed with gout more than 10 years ago (89% [range 5–45 years]) and had been prescribed allopurinol for at least 5 years (71% [range 0–40 years]). At baseline, the most common dosing regimen of allopurinol was 300 mg once daily (55% [range 100–600 mg]; one participant took 300 mg every second day). The average urate concentration at baseline was 0.33 mmol/L (range 0.20–0.57 mmol/L). Most participants (63%) had not received specialist care for their gout. Information on participant care at baseline is provided (Table 1).
Figure 2.
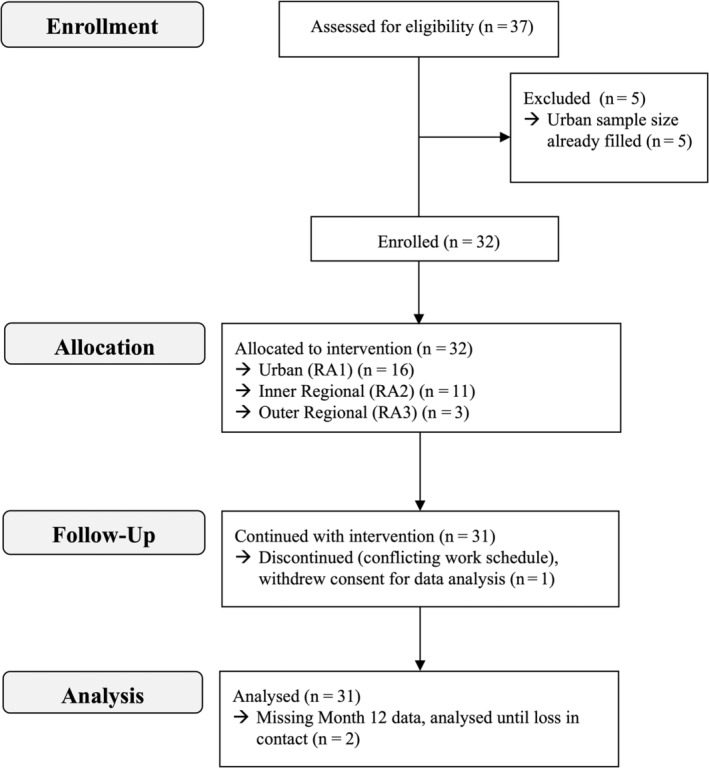
Consolidated Standards of Reporting Trials diagram. Urban location was based on an RA category of 0 from the Australian Statistical Geography Standard (inner regional = 1, outer regional = 2). RA, remoteness area.
Table 1.
Baseline characteristics of people with gout self‐monitoring their urate concentrations*
| Baseline characteristic | Participants (N = 31) |
|---|---|
| Age, y | 60 (34–86) |
| Male, n (%) | 29 (94) |
| Living in an urban a area, n (%) | 17 (55) |
| Allopurinol dosage, mode (range), mg/day | 300 (100–600) |
| Urate concentration, mmol/L | 0.33 (0.20–0.57) |
| Years since gout diagnosis | 21 (5–45) b |
| Years since first allopurinol prescription | 11 (0–40) b |
| Years between gout diagnosis and first allopurinol prescription | 9 (0–37) b |
| Seen a rheumatologist and/or a specialist for their gout, n (%) | 11 (37) c |
| Have urate concentration pathology tests every 12 mo, n (%) | 10 (33) c |
| Received an explanation of how allopurinol works by their health care professional, n (%) | 16 (53) c |
Data reported as mean (range), unless stated otherwise.
Australian Statistical Geography Standard remoteness area category 0.
Of 28 participants.
Of 30 participants.
Self‐management and urate control
Participants self‐monitored reliably, with lancing technique and device battery changes being the only issues reported. Collectively, participants recorded their urate concentration 831 times (Figure 3). One participant did not report a 12‐month urate concentration (loss of contact). Each participant recorded their urate concentration, on average, 18 times (median 1.5 times per month [range 1.1–6.5 times per month]) and measured consistently throughout the 12 months (quarter 1, median 5 readings per participant [range 3–22]; quarter 4, median 4 readings per participant [range 3–26]; P = 0.17).
Figure 3.
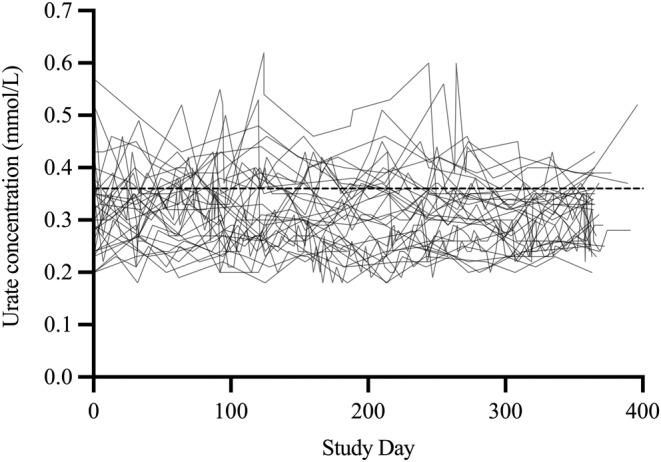
Urate concentrations self‐measured by participants (N = 31) using a point‐of‐care device (HumaSens2.0plus Multiparameter System). The dashed line represents the recommended target urate concentration range (<0.36 mmol/L). The final study visits for 11 participants exceeded 365 days (range 305–396 days), reflecting the availability of participants.
Nine participants were within the target urate concentration range and one participant was above the target urate concentration range for the entire study. For each participant, urate concentrations fluctuated, on average, by 0.19 mmol/L (range 0.08–0.40 mmol/L) throughout the study. The proportion of time a participant's urate concentration was within the target range increased by 1.3‐fold (study quarter 1, 79%; study quarter 4, 100%; P = 0.35) (Figure 4A).
Figure 4.
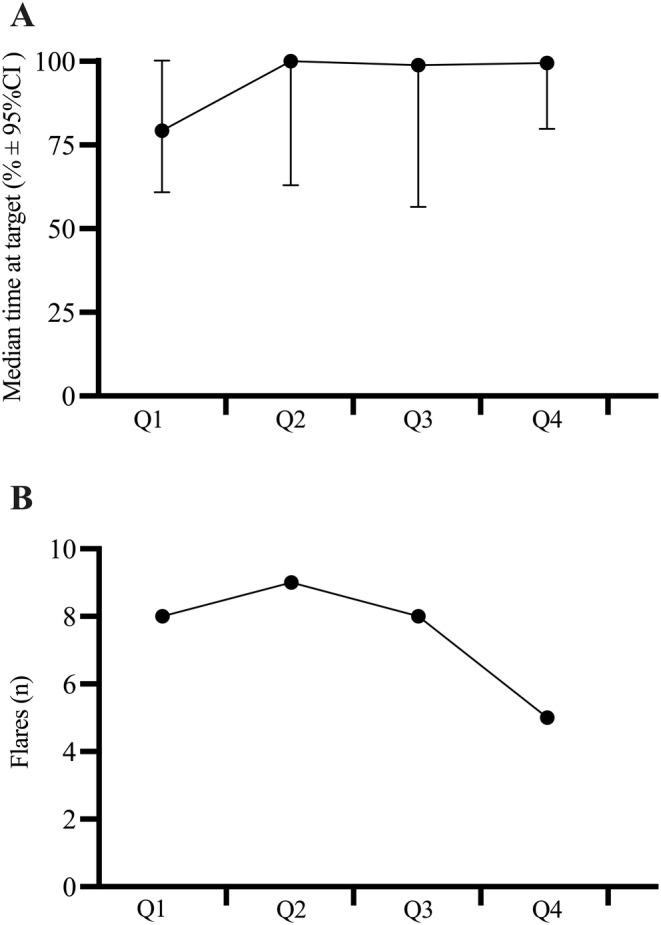
Change in clinical outcomes with urate self‐monitoring. The (A) proportion of time (median time percentage ± 95% CI) spent within the urate concentration target range and the (B) number of gout flares per quarter for 31 people with gout self‐monitoring urate concentrations over 12 months. Each study quarter lasted 3 months. CI, confidence interval; Q, quarter.
Six participants up‐titrated their dosage of allopurinol (eg, Figure 5A), and one participant switched to febuxostat (Figure 5B) after recording urate concentrations above the target. Although allopurinol dosing recommendation letters were provided to the participants’ general practitioner, participants told study investigators that they were the ones who instigated the conversation with their general practitioners about their dosage during their standard consultations. Almost half of the participants (48%) reported a gout flare. Of these, seven reported experiencing more than one gout flare. The incidence of gout flares decreased 1.6‐fold (quarter 1, eight gout flares; quarter 4, five gout flares; P = 0.25) (Figure 4B).
Figure 5.
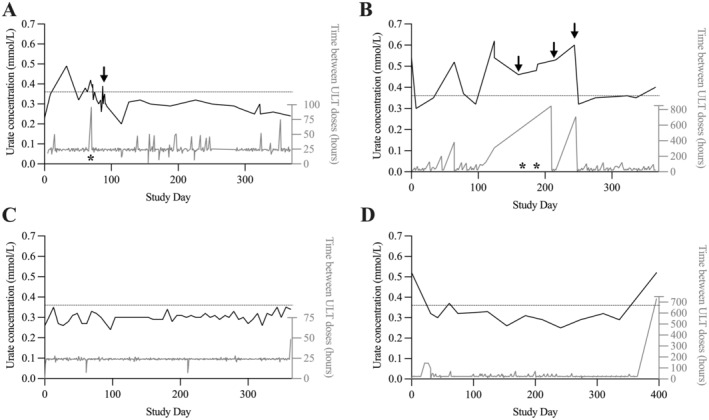
Examples of participant experiences urate self‐monitoring and their corresponding adherence behavior. Urate concentration: black line, left axis. ULT adherence: gray line, right axis, measured using MEMS. Asterisks denotes a gout flare. Arrow represents change in ULT therapy (eg, dosage alteration). Dashed line: target urate concentration (<0.36 mmol/L). (A) Participant up‐titrated allopurinol dosage after recording elevated urate concentration, and subsequent concentrations were within the target concentration. (B) Participant discontinued allopurinol, continued to record elevated urate concentration, and returned to therapy. Subsequent concentrations were close to or within the target range. (C) Participant was persistent, and urate concentrations were within the target range. (D) Participant was persistent, and recorded urate concentrations were within the target range. Then participant ceased taking allopurinol and recorded a concentration above the target range. MEMS, Medication Event Monitoring System; ULT, urate‐lowering therapy.
Self‐management and adherence
The impact of self‐monitoring urate concentrations on attainment of the target urate concentration, adherence to allopurinol, and optimization of the allopurinol dose is illustrated with representative case examples (Figure 5). For four participants, there was a notable decrease in urate concentrations (P < 0.02). For some participants, self‐monitoring did not impact their adherence behavior or their urate concentrations (eg, Figure 5C).
Twenty‐nine participants had complete adherence data over a median period of 364 days. Two participants had incomplete adherence data, recording up to day 305 (the last two months were missing) and day 368 (the last nine days were missing). There were 1,315 missed doses of ULT during the study, with a median of 32 per participant (range 1–180 missed doses). Further, 91.2% (range 37.3%–99.7%) of doses were taken as prescribed.
Initiation and implementation
One participant commenced allopurinol during the study because they had received a prescription for allopurinol just before enrollment. The remaining participants had already initiated ULT. Overall, 773 events indicative of suboptimal implementation were identified, including 58 occasional missed doses, 619 repeated missed doses, 92 repeated drug holidays, and 4 occasional drug holidays. One participant recorded “perfect” implementation, with consistent alignment between the prescribed and actual dose taking for the entire study period. For the remaining participants, five different “implementation types” were identified (Supplementary Table S1): (1) no missed doses (n = 2), (2) predominantly occasional missed doses (n = 4), (3) predominantly repeated missed doses (n = 8), (4) both repeated missed doses and repeated drug holidays (n = 7), and (5) a mix of occasional missed doses and repeated missed doses (n = 10). No obvious trends in implementation types over time were observed (Supplementary Table S2).
Persistence
Overall, 29 participants persisted with allopurinol (most consecutive missed doses, median 4 days [range 2–35 days]). For the two participants who discontinued therapy, one had recorded urate concentrations of more than 0.36 mmol/L and ceased taking allopurinol for 35 days. The participant then switched to febuxostat (treatment duration two months) but consequently ceased taking febuxostat and reinitiated allopurinol 26 days later (Figure 5B). The other participant had self‐recorded urate concentrations below 0.36 mmol/L. At study completion, 30 days after discontinuing allopurinol, their urate concentration was 0.52 mmol/L (Figure 5D).
Health‐related quality of life, health care use, and costs
Individual utility and EQ‐VAS scores are presented in Supplementary Figures S1 and S2 and Supplementary Tables S3 and S4. There was no trend in the utilities and EQ‐VAS scores over time, so linear mixed modeling was not conducted. The median utility score was 0.96. Utilities were >0.9 throughout the study for 16 participants, regardless of gout flare occurrence. The median EQ‐VAS score was 80.00.
The median utility score (0.96 and 0.96, P = 0.59) and EQ‐VAS score (80 and 80, P = 0.63) at baseline and month 12, respectively, did not change. The median utility and EQ‐VAS scores during a gout flare were lower than at other times (0.92 vs 0.96, P = 0.0056, and 70 vs 80, P = 0.0042, respectively). For participants who experienced at least one gout flare, the median utility and EQ‐VAS scores were lower compared to those who did not experience a gout flare (0.93 vs 0.96, P = 0.039 and 80 vs 84, P = 0.0027, respectively).
Self‐reported medical resource use is summarized in Supplementary Table S5. The comparison of the expected costs of a self‐monitoring service and the reported medical costs of a gout flare (eg, medications, clinician time) is presented in Supplementary Table S6. The self‐monitoring service is expected to cost about A$285 per year per person, whereas the reported cost of gout flares (adjusted for inflation) ranges from about A$390 to >A$4,000 per flare.
DISCUSSION
Self‐monitoring urate concentration may support people with gout to adhere to their ULT and attain and maintain target urate concentration, thus reducing the incidences of gout flares. Incidentally, this may provide a rationale for dosage up‐titration. Our study is the first to determine the impact of urate self‐monitoring on urate concentration control and ULT adherence behavior while considering important clinical variables, such as urate concentration, gout flare frequency, health‐related quality of life, and medical resource use. As a proof‐of‐concept feasibility study, our findings support large‐scale evaluation of patient‐led self‐monitoring of urate concentration in people with gout.
The increased time spent within the target urate concentration range suggests that urate self‐monitoring fosters practices that improve urate control, such as adherence to ULT. Our findings are consistent with a patient‐centered study in which urate self‐monitoring facilitated the attainment of target urate concentration in 80% of people with gout. 7 Many variables impact urate concentration beyond adherence to ULT, such as hydration, weight, diet, time of the day, and taking other medications. 30 Regular self‐monitoring of urate enables people with gout to self‐assess how their behavior influences their urate control, which can motivate people to modify their behavior to achieve optimal urate control. This goal setting requires the patient to know the target urate concentration. The behavioral impact of urate self‐monitoring aligns with the COM‐B framework, 31 in which the ability to use a urate device (Capacity), have access to a urate device (Opportunity), and have an understanding of the target urate concentration (Motivation) encourages “Behavioral change,” such as improved ULT adherence. This is analogous to people with diabetes, self‐monitoring glucose 32 concentration and modifying their behavior to achieve optimal glucose control. 33
Urate concentrations within an individual varied (even with the same allopurinol dosage), irrespective of their adherence and time spent within the target urate concentration. This biologic variation should be considered when interpreting a urate concentration in isolation. Additionally, although the trigger of a gout flare remains unclear, there is evidence that fluctuations in urate concentrations may increase the risk of a gout flare. 34 Trends in urate concentration over time may better reflect an individual's urate control to inform clinical decision‐making, especially periods of urate fluctuation.
The frequency of gout flares decreased in the last six months of self‐monitoring, which might reflect improvements in urate control. Although this could reflect regression toward the mean, 35 people may also have identified dietary triggers for gout, allowing them to subsequently avoid certain foods. Additionally, access to a POC urate device enabled participants to identify elevations in urate concentration, thereby identifying a greater risk of painful gout flares and consequently adjusting behavior to lower their urate concentration. This behavior is consistent with the fear of hypoglycemia in people with diabetes, encouraging glucose self‐monitoring to ensure adequate glucose control. 36
Our participants were persistent in taking allopurinol, with only two participants discontinuing therapy over 12 months. This may reflect the real‐time feedback on their urate control provided by self‐monitoring urate, as participants could contextualize their behavior (including medication taking), while informing medication decisions and gout understanding. This is consistent with the effect of self‐monitoring glucose in people with diabetes on adherence to antiglycemic medication. 33 This ability to reflect on real‐time feedback on urate control may also allow for people who struggle to initiate ULT to engage more with their new medication. Our participants were also motivated; most had been prescribed allopurinol for more than five years (so they had overcome initiation barriers 3 ). Given that most people with gout discontinue ULT within six months of their first prescription, 2 the evaluation of urate self‐monitoring in people with gout who are initiating or reinitiating ULT would be of interest because real‐time feedback on urate control may assist in encouraging people with gout to persist with ULT, particularly when the risk of gout flares is high.
Importantly, awareness of adherence monitoring does not impact participant adherence behavior. 37 Despite being persistent, participants demonstrated variation in their allopurinol adherence behavior, with repeated and patterned missed doses. Forgetfulness, continuing to experience gout flares while on therapy (ie, belief medication is not working), or experiencing less gout flares while on therapy (ie, belief medication is no longer necessary) are possible reasons for irregular allopurinol dosing. 3 However, allopurinol appears to be a forgiving medication, 38 with participants’ urate control being adequate despite these missed doses.
For our two discontinuers, urate self‐monitoring influenced their decisions to cease therapy. One discontinued ULT in response to poorly controlled gout (ie, elevated urate concentrations, experiencing gout flares regularly), whereas the other had well‐controlled gout (ie, achieved target urate concentrations, absence of gout flares). A perception that ULT is ineffective or unnecessary is a common reason for discontinuing ULT, 39 particularly in the absence of feedback on urate control. Interestingly, both discontinuers reinitiated allopurinol after reassurance that ULT was effective and necessary from self‐monitoring their urate during their period of nonpersistence. This highlights the ability of self‐monitoring interventions to provide opportunities for shared decision‐making with relevant health care professionals informed by patient‐recorded evidence.
Urate self‐monitoring provides real‐time data to inform patient‐led shared decision‐making with clinicians (eg, aid with ULT prescribing, as experienced by some of our participants) and/or pharmacists (eg, supporting people when dispensing ULT). Further, many people with gout express the desire to suspend their ULT. 39 Urate self‐monitoring may help to inform the optimal duration of these drug holidays 40 in consultation with their health care professional. By empowering people with gout to contribute to, and to take charge of, their gout management, this approach may evolve from self‐management toward self‐efficacy. 41
This study provides preliminary evidence to support further research on patient‐led urate self‐monitoring through a randomized controlled trial. Our data suggest that a urate self‐monitoring service has the potential of being cost neutral if one gout flare per year is avoided. Further, delivery of the service was equitable because all study procedures were delivered by telehealth, enabling people with gout in rural regions who face barriers to accessing health care 42 to participate. Additionally, our participants valued the opportunity to self‐monitor urate, as evident by our low withdrawal rate and minimal missing data. Further, the brief interactions with study investigators allowed participants to integrate urate concentration self‐monitoring into their routine. Engaging stakeholders to identify the barriers and facilitators to implementation of urate concentration self‐monitoring is essential. Future research should consider including people with gout at different stages of adherence, particularly those initiating ULT in whom persistence is often poor. Engaging people with gout whose urate concentration is consistently above the target concentration or who have gout flares despite persistence in taking allopurinol beyond initiation is also required. Further examination of the cost‐effectiveness of long‐term self‐monitoring of urate on a larger scale is also required to understand the health care benefits of this intervention.
A limitation of our study was restricting recruitment to people with gout who were already prescribed ULT, and thus we are unable to assess the impact of urate self‐monitoring on the desire to initiate ULT. Additionally, participants with a target urate concentration at baseline were recruited. Therefore, the findings from this study are potentially conservative. Despite this, they still experienced gout flares and were able to derive benefit from self‐monitoring urate concentration. We also recruited from a database of people with gout interested in research, some of whom had participated in previous gout management studies and as such are likely motivated to manage their gout. Supplying allopurinol to participants may have improved adherence to ULT because this removed the inconvenience of collecting a new prescription, a known barrier to medication adherence. 43
Patient‐led urate self‐monitoring by people with gout using a POC device may support their adherence to ULT. When people with gout could self‐monitor urate concentration, they maintained a target urate concentration and experienced a reduced incidence of gout flares. This approach to gout management has the potential to shift clinical practice toward empowering people to be invested in their gout management. Through research on intervention implementation, there is potential to establish a subsidy program analogous to those in place for other conditions that encourage self‐monitoring. Further, a patient‐led approach considers accessibility of individuals for whom specialist care is unaffordable and/or inaccessible. By allowing people to generate their own record of urate concentrations, people with gout will play a fundamental role in conversations with their health care professional and foster condition self‐ownership.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article and revising it critically for important intellectual content, and all authors approved the final version to be published. Dr Stocker had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Michael, Chan, Coleshill, Hughes, Day, Stocker.
Acquisition of data
Michael, Chan.
Analysis and interpretation of data
Michael, Wright, Aslani, Hughes, Stocker.
Supporting information
Disclosure Form
Supplementary Data S1: Extended Methodology
Supplementary Figure S1: Individual Utility Plots with gout flares marked by red arrows.
Supplementary Figure S2: Individual EQ‐VAS Scores with gout flares marked by red arrows.
Supplementary Table S1: The number of adherence events recorded for each participant and proposed implementation ‘types’
Supplementary Table S2: Observed implementation patterns at each quarter of the study period (each quarter is approximately 91 days)
Supplementary Table S3: Summary of utility scores from the EQ‐5D‐5L survey.
Supplementary Table S4: Summary of Individual EQ‐VAS scores.
Supplementary Table S5: Gout related healthcare resources use.
Supplementary Table S6: Cost comparison of a self‐monitoring service and reported costs of a gout flare in the literature.
ACKNOWLEDGMENTS
We thank Aardex, Belgium, for their technological support with the MEMS equipment and EBOS International, New Zealand, for coordinating the acquirement of HumaSens2.0plus devices. We thank St. Vincent's Hospital Clinical Pharmacy in Darlinghurst, New South Wales, Australia, for dispensing allopurinol for our study participants. We also thank our participants for the time and effort dedicated by them to this study. Without them, this research would not be possible. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Australian New Zealand Clinical Trials Registry identifier: ACTRN12621001730897.
Supported by Arthritis Australia (National Research Program grant 2021).
Additional supplementary information cited in this article can be found online in the Supporting Information section (http://onlinelibrary.wiley.com/doi/10.1002/arc2.11666).
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11666.
REFERENCES
- 1. Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet 2016;388:2039–2052. [DOI] [PubMed] [Google Scholar]
- 2. Coleshill MJ, Day RO, Tam K, et al. Persistence with urate‐lowering therapy in Australia: a longitudinal analysis of allopurinol prescriptions. Br J Clin Pharmacol 2022;88:4894–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spragg JCJ, Michael TJF, Aslani P, et al. Optimizing adherence to allopurinol for gout: patients' perspectives. Br J Clin Pharmacol 2023;89:1978–1991. [DOI] [PubMed] [Google Scholar]
- 4. Robinson PC, Stamp LK. The management of gout: much has changed. Aust Fam Physician 2016;45:299–302. [PubMed] [Google Scholar]
- 5. Edwards NL, Sundy JS, Forsythe A, et al. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Econ 2011;14:10–15. [DOI] [PubMed] [Google Scholar]
- 6. Sinnappah KA, Stocker SL, Chan JS, et al. Clinical interventions to improve adherence to urate‐lowering therapy in patients with gout: a systematic review. Int J Pharm Pract 2022;30:215–225. [DOI] [PubMed] [Google Scholar]
- 7. Riches PL, Alexander D, Hauser B, et al. Evaluation of supported self‐management in gout (GoutSMART): a randomised controlled feasibility trial. Lancet Rheumatol 2022;4:e320–e328. [DOI] [PubMed] [Google Scholar]
- 8. Doherty M, Jenkins W, Richardson H, et al. Efficacy and cost‐effectiveness of nurse‐led care involving education and engagement of patients and a treat‐to‐target urate‐lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018;392:1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abhishek A, Jenkins W, La‐Crette J, et al. Long‐term persistence and adherence on urate‐lowering treatment can be maintained in primary care‐5‐year follow‐up of a proof‐of‐concept study. Rheumatology (Oxford) 2017;56:529–533. [DOI] [PubMed] [Google Scholar]
- 10. Fuller A, Jenkins W, Doherty M, et al. Nurse‐led care is preferred over GP‐led care of gout and improves gout outcomes: results of Nottingham Gout Treatment Trial follow‐up study. Rheumatology (Oxford) 2020;59:575–579. [DOI] [PubMed] [Google Scholar]
- 11. Harrold LR, Andrade SE, Briesacher B, et al. The dynamics of chronic gout treatment: medication gaps and return to therapy. Am J Med 2010;123:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fletcher BR, Hartmann‐Boyce J, Hinton L, et al. The effect of self‐monitoring of blood pressure on medication adherence and lifestyle factors: a systematic review and meta‐analysis. Am J Hypertens 2015;28:1209–1221. [DOI] [PubMed] [Google Scholar]
- 13. Bray EP, Holder R, Mant J, et al. Does self‐monitoring reduce blood pressure? Meta‐analysis with meta‐regression of randomized controlled trials. Ann Med 2010;42:371–386. [DOI] [PubMed] [Google Scholar]
- 14. McBain H, Shipley M, Newman S. The impact of self‐monitoring in chronic illness on healthcare utilisation: a systematic review of reviews. BMC Health Serv Res 2015;15:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization . WHO Guideline on Self‐Care Interventions for Health and Well‐Being, 2022 Revision. Geneva: World Health Organization; 2022. https://www.who.int/publications/i/item/9789240052192 [Google Scholar]
- 16. National Diabetes Services Scheme . Accessed June 2, 2023. https://www.ndss.com.au/products/
- 17. Day RO, Frensham LJ, Nguyen AD, et al. Effectiveness of an electronic patient‐centred self‐management tool for gout sufferers: a cluster randomised controlled trial protocol. BMJ Open 2017;7:e017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Australian Bureau of Statistics . 1270.0.55.005 ‐ Australian Statistical Geography Standard (ASGS): volume 5 ‐ remoteness structure, July 2016. Accessed 2023. https://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/1270.0.55.005Main+Features1July%202016?OpenDocument
- 19. Paraskos J, Berke Z, Cook J, et al. An analytical comparison between point‐of‐care uric acid testing meters. Expert Rev Mol Diagn 2016;16:373–382. [DOI] [PubMed] [Google Scholar]
- 20. Fabre S, Clerson P, Launay J‐M, et al. Accuracy of the HumaSensplus point‐of‐care uric acid meter using capillary blood obtained by fingertip puncture. Arthritis Res Ther 2018;20:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaffo AL, Dalbeth N, Saag KG, et al. Validation of a definition of flare in patients with established gout. Arthritis Rheumatol 2018;70:462–467. [DOI] [PubMed] [Google Scholar]
- 22. Dima AL, Allemann SS, Dunbar‐Jacob J, et al. TEOS: a framework for constructing operational definitions of medication adherence based on timelines–events–objectives–sources. Br J Clin Pharmacol 2021;87:2521–2533. [DOI] [PubMed] [Google Scholar]
- 23. Dima AL, Allemann SS, Dunbar‐Jacob J, et al. Methodological considerations on estimating medication adherence from self‐report, electronic monitoring and electronic healthcare databases using the TEOS framework. Br J Clin Pharmacol 2023;89(7):1918–1927. [DOI] [PubMed] [Google Scholar]
- 24. De Geest S, Zullig LL, Dunbar‐Jacob J, et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med 2018;169:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Urquhart J. Pharmacodynamics of variable patient compliance: implications for pharmaceutical value. Adv Drug Deliv Rev 1998;33:207–219. [DOI] [PubMed] [Google Scholar]
- 26. Buchholz I, Janssen MF, Kohlmann T, et al. A systematic review of studies comparing the measurement properties of the three‐level and five‐level versions of the EQ‐5D. Pharmacoeconomics 2018;36:645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Norman R, Mulhern B, Lancsar E, et al. The use of a discrete choice experiment including both duration and dead for the development of an EQ‐5D‐5L value set for Australia. Pharmacoeconomics 2023;41:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brogan PA, Arch B, Hickey H, et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in childhood polyarteritis nodosa: an open‐label, randomized, bayesian noninferiority trial. Arthritis Rheumatol 2021;73:1673–1682. [DOI] [PubMed] [Google Scholar]
- 29. Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993;69:236–239. [PubMed] [Google Scholar]
- 30. Shaffer A, Rahn E, Saag K, et al. Variation in serum urate levels in the absence of gout and urate lowering therapy. BMC Rheumatol 2021;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dailey G. Assessing glycemic control with self‐monitoring of blood glucose and hemoglobin A(1c) measurements. Mayo Clin Proc 2007;82:229–236. [DOI] [PubMed] [Google Scholar]
- 33. Gopalan A, Kellom K, McDonough K, et al. Exploring how patients understand and assess their diabetes control. BMC Endocr Disord 2018;18:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uhlig T, Karoliussen LF, Sexton J, et al. Fluctuation and change of serum urate levels and flares in gout: results from the NOR‐Gout study. Clin Rheumatol 2022;41:3817–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol 2005;34:215–220. [DOI] [PubMed] [Google Scholar]
- 36. Snoek FJ, Malanda UL, de Wit M. Self‐monitoring of blood glucose: psychological barriers and benefits. European Diabetes Nursing 2008;5:112–115. [Google Scholar]
- 37. Sutton S, Kinmonth AL, Hardeman W, et al. Does electronic monitoring influence adherence to medication? Randomized controlled trial of measurement reactivity. Ann Behav Med 2014;48:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hill‐McManus D, Marshall S, Soto E, et al. Integration of pharmacometrics and pharmacoeconomics to quantify the value of improved forgiveness to nonadherence: a case study of novel xanthine oxidase inhibitors for gout. Clin Pharmacol Ther 2019;106:652–660. [DOI] [PubMed] [Google Scholar]
- 39. Emad Y, Dalbeth N, Weinman J, et al. Why do patients with gout not take allopurinol? J Rheumatol 2022;49:622–626. [DOI] [PubMed] [Google Scholar]
- 40. Perez‐Ruiz F, Herrero‐Beites AM, Carmona L. A two‐stage approach to the treatment of hyperuricemia in gout: the “dirty dish” hypothesis. Arthritis Rheum 2011;63:4002–4006. [DOI] [PubMed] [Google Scholar]
- 41. Bodenheimer T, Lorig K, Holman H, et al. Patient self‐management of chronic disease in primary care. JAMA 2002;288:2469–2475. [DOI] [PubMed] [Google Scholar]
- 42. Street TD, Somoray K, Richards GC, et al. Continuity of care for patients with chronic conditions from rural or remote Australia: a systematic review. Aust J Rural Health 2019;27:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mondesir FL, Levitan EB, Malla G, et al. Patient perspectives on factors influencing medication adherence among people with coronary heart disease (CHD) and CHD risk factors. Patient Prefer Adherence 2019;13:2017–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary Data S1: Extended Methodology
Supplementary Figure S1: Individual Utility Plots with gout flares marked by red arrows.
Supplementary Figure S2: Individual EQ‐VAS Scores with gout flares marked by red arrows.
Supplementary Table S1: The number of adherence events recorded for each participant and proposed implementation ‘types’
Supplementary Table S2: Observed implementation patterns at each quarter of the study period (each quarter is approximately 91 days)
Supplementary Table S3: Summary of utility scores from the EQ‐5D‐5L survey.
Supplementary Table S4: Summary of Individual EQ‐VAS scores.
Supplementary Table S5: Gout related healthcare resources use.
Supplementary Table S6: Cost comparison of a self‐monitoring service and reported costs of a gout flare in the literature.


