Abstract
Objectives
This study aimed to explore the potential causal associations between serum uric acid (SUA) and the risk of colorectal cancer, colon cancer and rectal cancer.
Methods
Twenty-six SUA-related single nucleotide polymorphisms which were identified by a large meta-analysis of genome-wide association studies (GWASs) were used as instrumental variables in the two-sample Mendelian randomization (MR) study. Meta-analyses were used to synthesize the results of multiple GWASs which were extracted from the MRC Integrative Epidemiology Unit GWAS database for each type of cancer. The inverse variance weighted (IVW) method was used as the primary MR method to analyze the association between SUA and colorectal cancer risk. Several sensitivity analyses were performed to test the robustness of results.
Results
The IVW method showed that there were no causal relationships between SUA and the risk of colorectal cancer [odds ratio (OR): 1.0015; 95% confidence interval (CI): 0.9975–1.0056] and colon cancer (OR: 1.0015; 95% CI: 0.9974–1.0055). The SUA levels were negative correlated with rectal cancer risk (OR: 0.9984; 95% CI: 0.9971–0.9998). The similar results were observed in both males (OR: 0.9987; 95% CI: 0.9975–0.9998) and females (OR: 0.9985; 95% CI: 0.9971–0.9999). The sensitivity analyses suggested no evidence of heterogeneity or horizontal pleiotropy. The leave-one-out analyses showed that one SNP (rs1471633) significantly drove the causal effect of SUA on rectal cancer risk. The MR-Egger regression and weighted median both showed that there were no causal relationships between SUA and the risk of colorectal cancer and its subtypes.
Conclusion
Overall, there was no linear causal association between SUA and the risk of colorectal cancer. However, further research is needed to investigate the role of higher SUA levels such as hyperuricemia or gout in the occurrence of colorectal cancer.
Keywords: uric acid, colorectal cancer, Mendelian randomization, causal association, meta-analysis
Introduction
Colorectal cancer is the world’s second deadly cancer with 1.93 million new cases and 903,859 deaths in 2022. The incidence and mortality of colorectal cancer increase with age and are higher in males than females (1). The identification of pathogenic factors is very important for the prevention and control of colorectal cancer, but the factors driving the increasing incidence and mortality are still largely unknown. Established nonmodifiable risk factors of colorectal cancer are age, male sex and family history, and the modifiable risk factors are smoking, alcohol use, obesity and red meat (2). The nonmodifiable risk factors are generally immutable, so it is necessary to explore whether there are potentially modifiable risk factors for colorectal cancer.
Serum uric acid (SUA) is the final oxidation product of purine nucleotide degradation. Some physiological or pathological reactions will cause the imbalance of SUA metabolism, resulting in hyperuricemia (3, 4). Hyperuricemia is a chronic progressive disease without obvious clinical symptoms and is associated with gout, cardiovascular diseases, metabolic syndrome and kidney diseases (5). Many studies have also explored the associations between elevated SUA levels and cancer risk, but the conclusions were inconsistent. SUA as an antioxidant with beneficial effects has been reported a protective role against cancer (6, 7). A systematic review of prospective studies reported that elevated SUA levels had no statistically significant association with overall cancer risk and results for specific cancers were limited and mainly negative (8). Mounting evidence indicated that high SUA levels represent a risk factor in various cancers through generating inflammatory reactions and oxidative stress (9, 10). For colorectal cancer, a population-based prospective cohort study found no significant association between SUA and colorectal cancer risk (11). A large Swedish cohort study reported that elevated SUA levels were positively associated with the risk of colorectal cancer in males (12). The similar results were also found in Chinese males (13). Another cohort study using the UK Biobank database found that SUA showed a U-shaped association with colon cancer risk in females and males with SUA ≤ 3.5 mg/dL and females with SUA > 8.4 mg/dL had a higher risk of rectal cancer than those with SUA between 4.4 and 5.4 mg/dL (14). The above controversial conclusions might be attributed to potential confounding factors and reverse causation, which were the limitations of observational studies.
Mendelian randomization (MR) is a method that uses genetic variants as instrumental variables to analyze the unbiased causal effects of exposures and outcomes. Since the distribution of genetic variants is random and genotype is not influenced by diseases, it can avoid some limitations of observational studies (15). Kobylecki et al. used urate solute carrier family 2 member 9 rs7442295 genotype as an instrumental variable and found that high plasma urate levels were genetically associated with high cancer incidence and high all-cause mortality (16). There were potential causal associations between higher SUA levels and prostate cancer risk in East Asian population (17). However, the results of a one-sample MR study from the UK Biobank database did not support a causal relationship between SUA and lung cancer (18). Jiang et al. also did MR analysis to identify the causal effect of SUA on eight site-specific cancers risk and a total of six MR methods all showed no significant causality (19). However, it was unknown whether SUA was causally associated with colorectal cancer risk.
In this study, a two-sample MR study was conducted to explore the potential causal relationships between SUA and colorectal cancer risk and its subtypes with 26 single nucleotide polymorphisms (SNPs) as instrumental variables. The findings would provide additional evidences for the causal effect of SUA on the colorectal cancer risk.
Methods
Study design
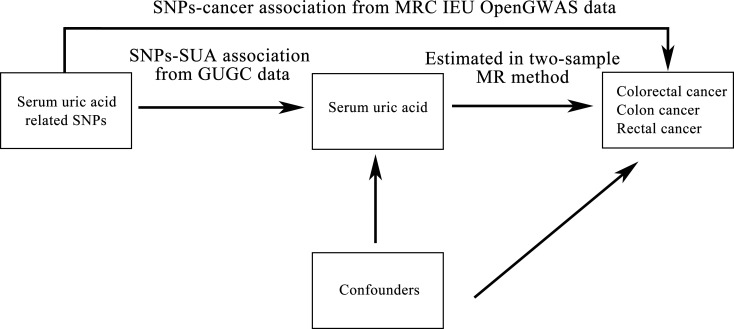
The causal relationships between SUA and the risk of colorectal cancer, colon cancer and rectal cancer were inferred using two-sample MR study with SUA associated SNPs as instrumental variables. Figure 1 shows the diagram of two-sample MR analysis of SUA and cancer risk. Three core assumptions should be satisfied to guarantee MR effect estimates without bias. First, the SNPs should have a strong association with SUA. Second, the SNPs should have been independent of confounding. Third, the SNPs should have affected cancer risk only via SUA and could not have a direct association.
Figure 1.
Diagram of two-sample Mendelian randomization analysis of serum uric acid levels and cancer risk. SNP: single nucleotide polymorphism; SUA: serum uric acid; GUGC: Global Urate Genetics Consortium; MR: Mendelian randomization; IEU: Integrative Epidemiology Unit; GWAS: genome-wide association study.
Genetic instruments for SUA
The SNPs-SUA sample was extracted from a large meta-analysis of 48 genome-wide association studies (GWASs) of 110,347 European individuals based on Global Urate Genetics Consortium (GUGC) data. In each GWAS, genotype imputation was carried out using the HapMap 2 data as the reference (20). The mean (standard deviation) of SUA levels in these studies ranged from 3.9 to 6.1 mg/dL (0.92 to 1.68 mg/dL) ( Supplementary Figure S1 ). To satisfy the MR assumptions, we restricted the instrumental SNPs to those highly associated with SUA levels (P<5.00E-8) and used a conservative clumping threshold with r2<0.001 to ensure linkage equilibrium. In addition, F-statistic value was used to assess the strength of each SNP. The SNP with F-statistic value larger than 10 was considered to have a strong potential to predict SUA levels. Finally, a total of 26 SNPs which explained 7.0% of the variance in SUA levels were screened to perform the MR analysis. The overall population and gender-specific effects for SNPs-SUA associations are displayed in Supplementary Tables S1 and S2 , respectively.
Genetic association with outcomes
The summary results of colorectal cancer and its subtypes were extracted from the latest MRC Integrative Epidemiology Unit (IEU) (University of Bristol) GWAS database (https://gwas.mrcieu.ac.uk/, Accessed 05 August 2023). According to the phenotypes of GWASs, they were classified as colorectal cancer, colon cancer, and rectal cancer-associated GWASs. Because there were several GWASs for each cancer, meta-analyses with random effect models were applied to synthesize the results of these GWASs.
Statistical analysis
The fixed-effect inverse variance weighted (IVW) method was used as the primary MR method to derive an overall weighted estimate of the potential causal effect of SUA levels on cancer risk. This method assumed that all SNPs were valid instruments and combined individual MR estimates across SNPs. The same analyses were conducted in overall population, males and females, respectively. In order to test the robustness of results, several sensitivity analyses were conducted. First, the significant heterogeneity indicates the presence of horizontal pleiotropy that instrumental SNPs affect the outcome not through the exposure of interest. Therefore, Cochran’s Q test was used to assess the heterogeneity among individual SNP estimates and horizontal pleiotropy was further examined using MR-Egger regression. Second, the leave-one-out analysis was conducted to investigate the possibility that the causal association was driven by a single SNP. In addition, the MR-Egger regression and weighted median method were implemented as complements to the IVW method. The MR-Egger regression estimated the causal effects in the presence of pleiotropic effects and the weighted median method could robustly assess the causal effects even if over half of the weight came from invalid instruments.
All statistical analyses were performed using R software (version 4.1.2) with “meta” package (version 6.10) for meta-analyses, “ggplot2” package (version 3.4.1) for forest plots and “TwoSampleMR” package (version 0.5.6) for MR analyses. Two-tail P<0.05 was considered statistically significant.
Results
Association between SNP and cancer risk
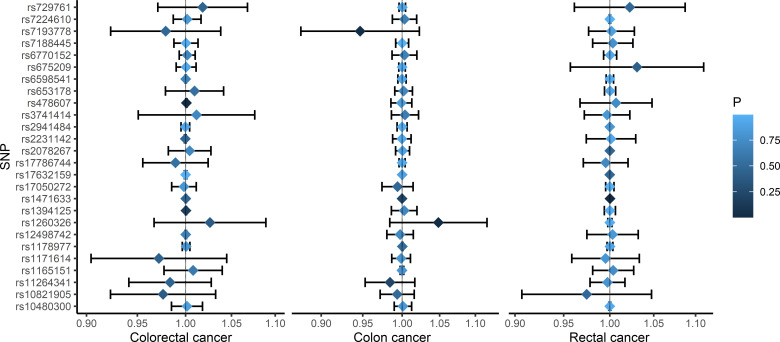
This study included ten cancer-related GWASs containing two colorectal cancer studies (8,679 cases and 543,000 controls), four colon cancer studies (7,862 cases and 1,0657,37 controls) and four rectal cancer studies (4,803 cases and 1,457,495 controls). Details of the GWASs used in this study are shown in Table 1 . Meta-analyses showed that none of the 26 SUA associated SNPs was significantly related to the risk of colorectal cancer, colon cancer and rectal cancer ( Supplementary Figures S2 – S4 ). The association between each SNP and cancer risk from the meta-analyses was integrated in Figure 2 . All the 26 SNPs were used as the instrumental variables in the MR analyses for SUA-colorectal cancer and its subtypes.
Table 1.
Characteristics of exposure dataset and outcome datasets.
| Phenotype | Consortium | Cases | Controls | Sample size | Population |
|---|---|---|---|---|---|
| Serum uric acid | GUGC | 110347 | European | ||
| Colorectal cancer | 8679 | 543000 | 551679 | European | |
| Ieu-b-4965 | UK Biobank | 5657 | 372016 | 377673 | European |
| Finn-b-C3_COLORECTAL_EXALLC | FinnGen | 3022 | 170984 | 174006 | European |
| Colon cancer | 7862 | 1065737 | 1073599 | European | |
| Ukb-d-C3_COLON | Neale lab | 2437 | 358757 | 361194 | European |
| Ukb-d-C18 | Neale lab | 2226 | 358968 | 361194 | European |
| Finn-b-C3_COLON_EXALLC | FinnGen | 1803 | 174006 | 175809 | European |
| Finn-b-C3_COLON_ADENO_EXALLC | FinnGen | 1396 | 174006 | 175402 | European |
| Rectal cancer | 4803 | 1457495 | 1461128 | European | |
| Ukb-b-19425 | MRC-IEU | 1085 | 461925 | 463010 | European |
| Ukb-b-1251 | MRC-IEU | 1470 | 461540 | 463010 | European |
| Ukb-d-C_RECTUM | Neale lab | 1170 | 360024 | 360024 | European |
| Finn-b-C3_RECTUM_EXALLC | FinnGen | 1078 | 174,006 | 175084 | European |
GUGC, Global Urate Genetics Consortium; MRC-IEU, MRC Integrative Epidemiology Unit.
Figure 2.
Forest plots of the associations between SNPs and the risk of colorectal cancer, colon cancer, and rectal cancer. SNP: single nucleotide polymorphism.
Association between SUA and cancer risk
There was no association between SUA and colorectal cancer risk [odds ratio (OR): 1.0015; 95% confidence interval (CI): 0.9975, 1.0056]. The similar result was also found in males (OR:0.9989; 95%CI: 0.9968, 1.0009) and females (OR:0.9992; 95%CI: 0.9976, 1.0009). Similarly, the SUA levels were not significantly associated with colon cancer in overall population [(OR (95% CI): 1.0015 (0.9974, 1.0055)], males [(OR (95% CI): 1.0013 (0.9978, 1.0048)] and females [(OR (95% CI): 1.0016 (0.9973, 1.0059)]. However, the IVW method indicated that there was causal relationship between SUA and rectal cancer risk. The elevated SUA levels could significantly reduce the risk of rectal cancer (OR: 0.9984; 95% CI: 0.9971, 0.9998). The similar result was also found in males (OR: 0.9987; 95% CI: 0.9975, 0.9998) and females (OR: 0.9985; 95% CI: 0.9971, 0.9999) ( Table 2 ).
Table 2.
Mendelian randomization estimates of the causal relationships between serum uric acid levels and cancer risk using inverse variance weighted method.
| Population | nsnp | Colorectal cancer | Colon cancer | Rectal Cancer | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Overall | 26 | 1.0015 (0.9975, 1.0056) | 0.459 | 1.0015 (0.9974, 1.0055) | 0.459 | 0.9984 (0.9971, 0.9998) | 0.028 |
| Male | 26 | 0.9989 (0.9968, 1.0009) | 0.285 | 1.0013 (0.9978, 1.0048) | 0.459 | 0.9987 (0.9975, 0.9998) | 0.025 |
| Female | 26 | 0.9992 (0.9976, 1.0009) | 0.364 | 1.0016 (0.9973, 1.0059) | 0.467 | 0.9985 (0.9971, 0.9999) | 0.037 |
OR, odds ratio; CI, confidence interval.
Sensitivity analysis
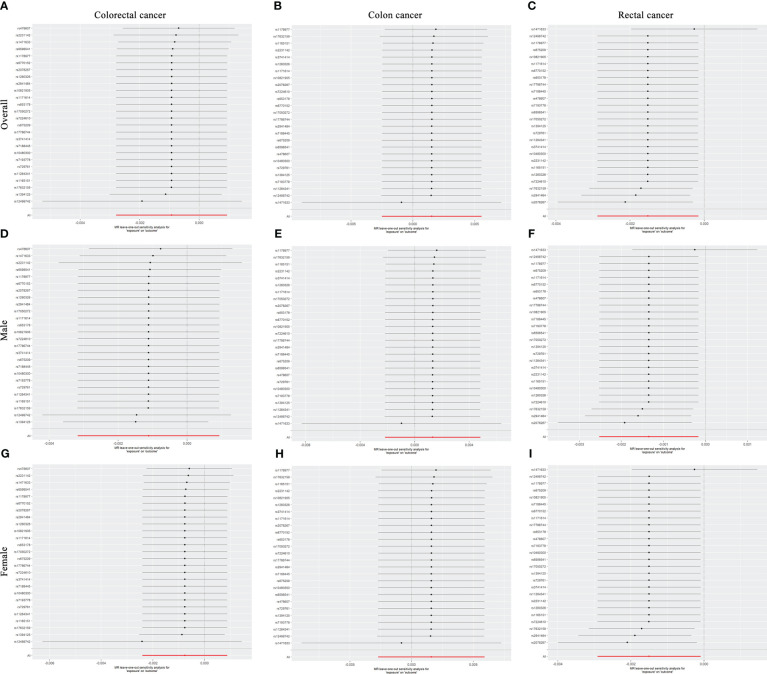
The Cochran’s Q tests showed that there was no heterogeneity across SNPs. The P values of the MR-Egger regressions were all greater than 0.05, suggesting that there was no horizontal pleiotropy ( Table 3 ). The leave-one-out analyses found that rs1471633 in the PDZK1 gene had great possibility to drive the causal association between SUA and rectal cancer risk in overall population, males and females ( Figures 3A–H ). The causal association became not statistically significant if rs1471633 was excluded in the MR analysis ( Figures 3C, F, I ). The MR-Egger regression and weighted median method both showed that there were no causal relationships between SUA and the risk of colorectal cancer, colon cancer and rectal cancer ( Table 4 ).
Table 3.
Heterogeneity and MR-Egger pleiotropy test for the causal relationships between serum uric acid levels and cancer risk.
| Phenotype | Cochran’s Q test | P | MR-Egger | SE | P |
|---|---|---|---|---|---|
| Q | Intercept | ||||
| Overall | |||||
| Colorectal cancer |
12.64 | 0.981 | -1.09E04 | 1.82E-04 | 0.554 |
| Colon cancer | 7.31 | 0.999 | -1.68E04 | 5.39E-04 | 0.758 |
| Rectal cancer | 9.15 | 0.998 | 1.55E-04 | 1.88E-04 | 0.420 |
| Male | |||||
| Colorectal cancer |
12.46 | 0.982 | -9.24E05 | 2.04E04 | 0.655 |
| Colon cancer | 7.31 | 0.999 | -1.56E04 | 5.22E04 | 0.767 |
| Rectal cancer | 8.96 | 0.998 | 1.51E04 | 1.72E04 | 0.389 |
| Female | |||||
| Colorectal cancer |
12.78 | 0.979 | -1.17E04 | 1.70E04 | 0.496 |
| Colon cancer | 7.33 | 0.999 | -1.58E04 | 5.76E04 | 0.786 |
| Rectal cancer | 9.60 | 0.998 | 7.62E05 | 1.99E04 | 0.705 |
SE, standard error.
Figure 3.
Leave-one-out analyses for serum uric acid levels on cancer.
Table 4.
Mendelian randomization estimates of the causal relationships between serum uric acid levels and cancer risk using MR-Egger and Weighted median method.
| Population | nsnp | Colorectal cancer | Colon cancer | Rectal cancer | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| MR-Egger | |||||||
| Overall | 26 | 1.0046 (0.9851, 1.0245) | 0.652 | 1.0046 (0.9851, 1.0246) | 0.652 | 0.9959 (0.9896, 1.0022) | 0.216 |
| Male | 26 | 0.9994 (0.9963, 1.0025) | 0.721 | 1.0038 (0.9874, 1.0205) | 0.657 | 0.9965 (0.9915, 1.0015) | 0.181 |
| Female | 26 | 0.9997 (0.9976, 1.0018) | 0.773 | 1.0047 (0.9824, 1.0274) | 0.687 | 0.9972 (0.9903, 1.0041) | 0.433 |
| Weighted median | |||||||
| Overall | 26 | 1.0022 (0.9979, 1.0065) | 0.316 | 1.0022 (0.9979, 1.0065) | 0.316 | 0.9992 (0.9973, 1.0010) | 0.357 |
| Male | 26 | 0.9990 (0.9967, 1.0014) | 0.433 | 1.0020 (0.9982, 1.0057) | 0.301 | 0.9993 (0.9977, 1.0008) | 0.340 |
| Female | 26 | 0.9937 (0.9977, 1.0011) | 0.467 | 1.0023 (0.9976, 1.0070) | 0.339 | 0.9992 (0.9974, 1.0012) | 0.402 |
OR, odds ratio; CI, confidence interval.
Discussion
The uric acid is a very important metabolite in human body. It is the end product of the oxidation of xanthine and hypoxanthine catalyzed by xanthine oxidoreductase. This study conducted a two-sample MR study to explore the potential causal relationship between SUA and colorectal cancer risk with GWAS databases available online. The SUA was found causal effect on the risk of rectal cancer with IVW method, but the sensitivity analyses did not support the finding. The similar result was also observed in males and females, respectively.
More and more studies supported the carcinogenic effect of SUA. It was reported that the activation of xanthine oxidase during SUA production leads to the increase of reactive oxygen species (ROS) in vivo (21). ROS activated signaling pathways related to proliferation, survival, angiogenesis and metastasis, promote the occurrence, development and metastasis of tumors (22, 23). SUA could also cause the upregulation of mRNA levels of inflammasome‐related genes (e.g. IL‐1β and NLRP3) through monosodium urate crystals and soluble uric acid, giving rise to oncogenes potential (24, 25). Yiu et al. conducted a cohort study on 493,281 persons and found that altered SUA levels were associated with the risk of hepatobiliary, kidney, nonmelanoma skin and other cancers in males, head and neck and other cancers in females (12). An UK cohort study including 444,462 participants showed that high SUA levels were associated with an increased risk of pancreatic cancer and kidney cancer in females and gallbladder cancer in males (26, 27). SUA was also found positively associated with the risk of prostate, esophagus, stomach, liver, pancreatic, lung, ovarian, renal and bladder cancers in two prospective studies in Korean population (28, 29). A meta-analysis found that gout was a risk factor of urological cancers, digestive system cancers and lung cancer (30). Of the above reported cancers that were positively associated with SUA, only non-tobacco related cancer and prostate cancer were found causal relationships with SUA in published MR studies (16, 17). Similarly, several cohort studies reported that high SUA levels increased the risk of colorectal cancer in Swedish (12) and cancer in Chinese males (13). However, this MR study did not find positive causal associations between SUA and the risk of colorectal cancer, colon cancer and rectal cancer. It implied that the results of previous cohort studies may be affected by unobservable confounding factors.
Ames et al. firstly proposed that SUA as an antioxidant and scavenger of free radicals was working on singlet oxygen which can reduce the risk of various cancer types (31). The urate could activate potent immune stimulants and trigger anticancer immune response by reversing immunosuppression or as an adjuvant (32). In this MR study, it was found that SUA was a protective agent against rectal cancer and the protective effect of SUA on rectal cancer risk was influenced by rs1471633. The rs1471633 was reported to correlate with the decreasing trend of PDZK1 mRNA, whose protein had been shown to be a key regulatory scaffolding protein in tethering urate transportsome complex (33, 34). The previous studies reported inconsistent expression levels of PDZK1 across different cancers. The low-level PDZK1 expression had been reported in renal cell carcinoma (35) and gastric cancer (36), and exhibited tumor suppressive effects. In contrast, the PDZK1 expression was upregulated in breast cancer (37), thyroid cancer (38) and hepatocellular carcinoma (39). Therefore, further research is needed to explore the effect of PDZK1 expression on rectal cancer risk. However, the causal association between SUA and rectal cancer risk was not found in the MR-Egger regression and weighted median method. Given the robustness of results, the SUA levels were considered no causal relationship with rectal cancer risk.
Mi et al. reported that lowest uric acid groups (≤3.5 mg/dL) and highest uric acid groups (>8.4 mg/dL) both had higher risk of colorectal cancer compared with the reference groups (4.4~5.4 mg/dl), and concluded the U-shaped association between SUA and colorectal cancer risk (14). It was demonstrated that SUA was a powerful antioxidant at physiological concentrations, while it was a pro-oxidant molecule at high intracellular concentrations (40). The SUA levels as continuous variable in this MR study were relatively in the normal range which may limit the correlation analyses between SUA and colorectal cancer risk. Besides, the results of correlation analyses may vary when SUA had different types of variables in the models. Therefore, further research is needed to investigate the role of higher SUA levels such as hyperuricemia or gout in the occurrence of colorectal cancer.
This study had some strengths. First, MR analysis was used to analyze the causal effects of SUA on colorectal cancer risk to avoid confounding and reverse causality biases. The sensitivity analyses were applied to increase the robustness of the obtained results. Second, the data of colon cancer and rectal cancer from more variety of databases were abstracted to assess the causal effects of SUA on colorectal cancer risk. Moreover, meta-analyses with random effect models incorporated all available GWASs summary data to analyze the genetic association with colorectal cancer.
Several issues should be considered in this study. First, the causal effect obtained in MR study did not indicate an effect on the risk of colorectal cancer after changing SUA levels. Second, the causality between SUA and colorectal cancer risk may also vary by within or out of the normal range for SUA levels. The SUA levels may not be a simple linear association but a U-shaped or J-shaped association with cancer risk. This study was limited to the absence of information about SNPs-SUA and SNPs-cancer stratified by SUA levels in the available GWAS datasets. Third, the results may only be extrapolated to the European population. Future studies could consider other ethnicities and appropriate stratification analyses.
Conclusions
Overall, there was no linear causal association between SUA and the risk of colorectal cancer. However, further research is needed to investigate the role of higher SUA levels such as hyperuricemia or gout in the occurrence of colorectal cancer.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The GWASs used in this study can be available from the latest MRC Integrative Epidemiology Unit GWAS database at https://gwas.mrcieu.ac.uk.
Author contributions
JSZ: Formal Analysis, Writing – original draft, Writing – review & editing. RF: Conceptualization, Writing – original draft, Writing – review & editing. JWZ: Validation, Writing – original draft. SHZ: Validation, Writing – original draft. ZFL: Validation, Writing – original draft. ZL: Project administration, Writing – review & editing. XL: Data curation, Writing – original draft. XLX: Data curation, Writing – original draft. YLC: Data curation, Writing – original draft. ZJH: Conceptualization, Supervision, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Joint Fund project for Science and Technology Innovation of Fujian Province (Grant number: 2020Y9018) and Natural Science Foundation of Fujian Province (Grant number: 2023J01317). The funding sources have no role in study design, data collection, analysis, interpretation and the writing of the manuscript.
Abbreviations
SUA, serum uric acid; MR, Mendelian randomization; SNP, single nucleotide polymorphism; GWAS, genome-wide association study; GUGC, Global Urate Genetics Consortium; IEU, Integrative Epidemiology Unit; IVW, inverse variance weighted; OR, odds ratio; CI, confidence interval; ROS, reactive oxygen species.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1394320/full#supplementary-material
References
- 1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 2. Sninsky JA, Shore BM, Lupu GV, Crockett SD. Risk factors for colorectal polyps and cancer. Gastrointest Endosc Clin N Am. (2022) 32:195–213. doi: 10.1016/j.giec.2021.12.008 [DOI] [PubMed] [Google Scholar]
- 3. Li L, Zhang Y, Zeng C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am J Transl Res. (2020) 12:3167–81. [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang S, Wang Y, Cheng J, Huangfu N, Zhao R, Xu Z, et al. Hyperuricemia and cardiovascular disease. Curr Pharm Des. (2019) 25:700–9. doi: 10.2174/1381612825666190408122557 [DOI] [PubMed] [Google Scholar]
- 5. Gill D, Cameron AC, Burgess S, Li X, Doherty DJ, Karhunen V, et al. Urate, blood pressure, and cardiovascular disease: evidence from mendelian randomization and meta-analysis of clinical trials. Hypertension. (2021) 77:383–92. doi: 10.1161/HYPERTENSIONAHA.120.16547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuo C-F, See L-C, Yu K-H, Chou I-J, Chiou M-J, Luo S-F. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatol (Oxford). (2013) 52:127–34. doi: 10.1093/rheumatology/kes223 [DOI] [PubMed] [Google Scholar]
- 7. Taghizadeh N, Vonk JM, Boezen HM. Serum uric acid levels and cancer mortality risk among males in a large general population-based cohort study. Cancer Causes Control. (2014) 25:1075–80. doi: 10.1007/s10552-014-0408-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yan S, Zhang P, Xu W, Liu Y, Wang B, Jiang T, et al. Serum uric acid increases risk of cancer incidence and mortality: A systematic review and meta-analysis. Mediators Inflammation. (2015) 2015:764250. doi: 10.1155/2015/764250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strasak AM, Rapp K, Hilbe W, Oberaigner W, Ruttmann E, Concin H, et al. The role of serum uric acid as an antioxidant protecting against cancer: prospective study in more than 28 000 older Austrian women. Ann Oncol. (2007) 18:1893–7. doi: 10.1093/annonc/mdm338 [DOI] [PubMed] [Google Scholar]
- 10. Mi S, Gong L, Sui Z. Friend or foe? An unrecognized role of uric acid in cancer development and the potential anticancer effects of uric acid-lowering drugs. J Cancer. (2020) 11:5236–44. doi: 10.7150/jca.46200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kühn T, Sookthai D, Graf ME, Schübel R, Freisling H, Johnson T, et al. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer. (2017) 117:1572–9. doi: 10.1038/bjc.2017.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yiu A, van Hemelrijck M, Garmo H, Holmberg L, Malmström H, Lambe M, et al. Circulating uric acid levels and subsequent development of cancer in 493,281 individuals: findings from the AMORIS Study. Oncotarget. (2017) 8:42332–42. doi: 10.18632/oncotarget.16198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li W, Liu T, Siyin ST, Zhang Q, Wang Y, Cao L, et al. The relationship between serum uric acid and colorectal cancer: a prospective cohort study. Sci Rep. (2022) 12:16677. doi: 10.1038/s41598-022-20357-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mi N, Huang J, Huang C, Lin Y, He Q, Wang H, et al. High serum uric acid may associate with the increased risk of colorectal cancer in females: A prospective cohort study. Int J Cancer. (2022) 150:263–72. doi: 10.1002/ijc.33807 [DOI] [PubMed] [Google Scholar]
- 15. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 16. Kobylecki CJ, Afzal S, Nordestgaard BG. Plasma urate, cancer incidence, and all-cause mortality: A mendelian randomization study. Clin Chem. (2017) 63:1151–60. doi: 10.1373/clinchem.2016.268185 [DOI] [PubMed] [Google Scholar]
- 17. Deng Y, Huang J, Wong MC. Association between serum uric acid and prostate cancer risk in East Asian populations: a Mendelian randomization study. Eur J Nutr. (2023) 62:1323–9. doi: 10.1007/s00394-022-03076-7 [DOI] [PubMed] [Google Scholar]
- 18. Horsfall LJ, Hall IP, Nazareth I. Serum urate and lung cancer: a cohort study and Mendelian randomization using UK Biobank. Respir Res. (2021) 22:179. doi: 10.1186/s12931-021-01768-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang M, Ren L, Chen S, Li G. Serum uric acid levels and risk of eight site-specific cancers: A mendelian randomization study. Front Genet. (2021) 12:608311. doi: 10.3389/fgene.2021.608311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Köttgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. (2013) 45:145–54. doi: 10.1038/ng.2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Battelli MG, Bortolotti M, Polito L, Bolognesi A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:2557–65. doi: 10.1016/j.bbadis.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 22. Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, et al. ROS generation and antioxidant defense systems in normal and Malignant cells. Oxid Med Cell Longev. (2019) 2019:6175804. doi: 10.1155/2019/6175804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. (2014) 63:976–81. doi: 10.2337/db13-1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cabău G, Crișan TO, Klück V, Popp RA, Joosten LA. Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol Rev. (2020) 294:92–105. doi: 10.1111/imr.12833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oğuz N, Kırça M, Çetin A, Yeşilkaya A. Effect of uric acid on inflammatory COX-2 and ROS pathways in vascular smooth muscle cells. J Recept Signal Transduct Res. (2017) 37:500–5. doi: 10.1080/10799893.2017.1360350 [DOI] [PubMed] [Google Scholar]
- 26. Huang C-F, Huang J-J, Mi N-N, Lin Y-Y, He Q-S, Lu Y-W, et al. Associations between serum uric acid and hepatobiliary-pancreatic cancer: A cohort study. WJG. (2020) 26:7061–75. doi: 10.3748/wjg.v26.i44.7061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dai X-Y, He Q-S, Jing Z, Yuan J-Q. Serum uric acid levels and risk of kidney cancer incidence and mortality: A prospective cohort study. Cancer Med. (2020) 9:5655–61. doi: 10.1002/cam4.3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim YR, Choi CK, Lee YH, Choi SW, Kim HY, Shin MH, et al. Association between albumin, total bilirubin, and uric acid serum levels and the risk of cancer: A prospective study in a korean population. Yonsei Med J. (2021) 62:792–8. doi: 10.3349/ymj.2021.62.9.792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oh Y-J, Lee YJ, Lee E, Park B, Kwon J-W, Heo J, et al. Cancer risk in Korean patients with gout. Korean J Intern Med. (2022) 37:460–7. doi: 10.3904/kjim.2020.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang W, Xu D, Wang B, Yan S, Wang X, Yin Y, et al. Increased risk of cancer in relation to gout: A review of three prospective cohort studies with 50,358 subjects. Mediators Inflammation. (2015) 2015:680853. doi: 10.1155/2015/680853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U.S.A. (1981) 78:6858–62. doi: 10.1073/pnas.78.11.6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Ma X, Su C, Peng B, Du J, Jia H, et al. Uric acid enhances the antitumor immunity of dendritic cell-based vaccine. Sci Rep. (2015) 5:16427. doi: 10.1038/srep16427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferreira C, Prestin K, Hussner J, Zimmermann U, Meyer Zu Schwabedissen HE. PDZ domain containing protein 1 (PDZK1), a modulator of membrane proteins, is regulated by the nuclear receptor THRβ. Mol Cell Endocrinol. (2018) 461:215–25. doi: 10.1016/j.mce.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 34. Anzai N, Miyazaki H, Noshiro R, Khamdang S, Chairoungdua A, Shin H-J, et al. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem. (2004) 279:45942–50. doi: 10.1074/jbc.M406724200 [DOI] [PubMed] [Google Scholar]
- 35. Tao T, Yang X, Zheng J, Feng D, Qin Q, Shi X, et al. PDZK1 inhibits the development and progression of renal cell carcinoma by suppression of SHP-1 phosphorylation. Oncogene. (2017) 36:6119–31. doi: 10.1038/onc.2017.199 [DOI] [PubMed] [Google Scholar]
- 36. Zhao C, Tao T, Yang L, Qin Q, Wang Y, Liu H, et al. Loss of PDZK1 expression activates PI3K/AKT signaling via PTEN phosphorylation in gastric cancer. Cancer Lett. (2019) 453:107–21. doi: 10.1016/j.canlet.2019.03.043 [DOI] [PubMed] [Google Scholar]
- 37. Kim H, Abd Elmageed ZY, Davis C, El-Bahrawy AH, Naura AS, Ekaidi I, et al. Correlation between PDZK1, Cdc37, Akt and breast cancer Malignancy: the role of PDZK1 in cell growth through Akt stabilization by increasing and interacting with Cdc37. Mol Med. (2014) 20:270–9. doi: 10.2119/molmed.2013.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Di Maro G, Orlandella FM, Bencivenga TC, Salerno P, Ugolini C, Basolo F, et al. Identification of targets of Twist1 transcription factor in thyroid cancer cells. J Clin Endocrinol Metab. (2014) 99:E1617–26. doi: 10.1210/jc.2013-3799 [DOI] [PubMed] [Google Scholar]
- 39. Chen X, Wang X, Zhu F, Qian C, Xu F, Huang X, et al. HBV infection-related PDZK1 plays an oncogenic role by regulating the PI3K-akt pathway and fatty acid metabolism and enhances immunosuppression. J Immunol Res. (2022) 2022:8785567. doi: 10.1155/2022/8785567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Allegrini S, Garcia-Gil M, Pesi R, Camici M, Tozzi MG. The Good, the Bad and the New about Uric Acid in Cancer. Cancers (Basel). (2022) 14:4959. doi: 10.3390/cancers14194959 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: The GWASs used in this study can be available from the latest MRC Integrative Epidemiology Unit GWAS database at https://gwas.mrcieu.ac.uk.