Prevention of malaria requires a combination of laboratory and operational research and political will to provide affordable effective drugs
Summary points
Malaria kills millions of people every year
Basic scientific research is advancing rapidly in many disciplines; research with immediate practical applications in endemic countries is moving more slowly
Novel ways of deploying insecticides and impregnated nets are effective, but new insecticides are needed
Vaccine research is moving fast, but a vaccine for deploying in endemic areas is some way off
Increasing drug resistance in South East Asia has been countered with drugs containing artemisins
As resistance spreads in Africa the key challenge is to find affordable drugs
Introduction
Malaria is one of the leading causes of morbidity and death worldwide, with over 100 million cases and at least a million deaths a year. Most of these deaths are in the poorest regions of the world. Because malaria is a highly complex disease, the diversity of research to prevent and treat it is probably greater than for any other disease. It ranges from modelling climate change and satellite data to predict epidemics through to elucidating the genome sequence of the malaria parasite Plasmodium falciparum and the mosquito vector Anopheles gambiae.1–5 The cultural diversity and poverty of seriously affected populations in Africa, Asia, and South America present particular challenges. Here social science, especially anthropology and economic research, have as important a role as traditional laboratory work. Malaria research is exciting because it is rapidly moving and cross disciplinary. It is also depressing, as the spread of drug resistant parasites, extreme poverty, and the collapse of health services under the impact of HIV or war undermine much of this progress.
Sources and selection criteria
We focus on three areas of malaria research that are developing rapidly and are likely to have an impact in endemic areas in the foreseeable future: prevention of infection by using measures to control vectors, prevention of disease by vaccination, and drug treatment. We supplemented information from published and unpublished data using PubMed and WHO search strategies, directed especially to the identification of good recent reviews.
Preventing transmission by mosquitoes
Basic research has developed several techniques to reduce transmission of malaria by mosquitoes, with theoretical promise long term. Genetic manipulation enables genes for refractoriness to malaria to be inserted into Anopheles mosquitoes, although how these genes would be driven through the mosquito population of Africa remains unclear.6 The genome of A gambiae, the principle vector in Africa, has recently been published.4 This will open up the range of possible targets and may guide the development of alternative insecticides.3 In reality, however, it is in the imaginative use of new ways of deploying two old tools, bed nets and insecticides (especially when they are combined) that advances likely to have an impact in the medium term are occurring.
Insecticide treated nets constitute one of the cheapest, simplest, and most effective methods of preventing morbidity and mortality from malaria in Africa and Asia.7,8 The greatest challenges over the next 10 years will be to expand coverage of affordable nets through public and private sectors to all who need them. This is a big task requiring a diverse alliance between groups unfamiliar with each other: textile industry, pesticide manufacturers, health departments, government regulators, United Nations agencies, non-governmental organisations, distributors, retailers, and community organisations. Special approaches are being developed to reach disadvantaged and poor people, who often need the nets the most. Each cultural and economic setting needs a different approach, and combined entomological and socioeconomic research is needed in each setting.
New insecticides are needed for nets
Despite the hurdles, progress is being made, and coverage of bed nets is increasing, even in the most unlikely countries—Afghanistan for example.9 There are, however, risks in relying on a single control tool—particularly one that depends on insecticide for its effect. The evolution of resistance to the insecticide dicophane (DDT) during the 1960s contributed to the demise of the Malaria Eradication Programme. The eradication campaign eliminated malaria from Europe and North America by using DDT sprayed on walls and led to major reductions in malaria (subsequently partially reversed) in Asia and southern America. However, behavioural and physiological resistance by the mosquito led to the collapse of the campaign (assisted by pressure from environmental activists and political complications).
The recent evolution and spread of mosquitoes resistant to the successor pyrethroid insecticides is threatening to reduce the potency of nets and to undermine present gains.10,11 A gambiae resistant to all known pyrethroids is now found in several southern and west African countries, forcing control programmes in southern Africa to revert to spraying houses with DDT to contain the problem of pyrethroid resistant Anopheles funestus.12 As net usage increases in Africa, further selection of pyrethroid resistance seems inevitable. To preserve the impregnated nets a new type of insecticide must be developed within this decade to replace or supplement the pyrethroids, otherwise the current WHO campaign, Roll Back Malaria, risks going the same way as the Malaria Eradication Programme. New classes of insecticide are not easy to find; no new class has been registered for vector control in over two decades.13 Industry is always searching for novel chemistry for crop protection, whereas malaria vector control constitutes less than 3% of the global pesticide market, so any new insecticide will be a byproduct of the agrochemical sector. If nothing suitable can be found, older classes of insecticides such as organophosphates and carbamates not previously used on nets but with a safe toxicology profile may have to be revived. Such insecticides are still widely used in agriculture, and field tests show they can kill pyrethroid resistant mosquitoes when used on nets.14
Treated nets have operational limitations
Nets need to be retreated annually to maintain their efficacy, and this has been one of the major limitations on their use. Few operational systems for retreatment exist in Africa. The most promising solution is the novel “long lasting net,” where the insecticide is able to withstand repeated washing up to the net's durability of 5 years. The insecticide is incorporated within polyethylene fibre or within a resin that coats the surface of polyester fibres.15 Bioavailability is achieved by diffusion of insecticide to the surface after washing. Several companies are active in this area, and rapid advances are anticipated. How the logistical issues around delivery and financing of the nets are to be overcome in practice remains to be seen.
Impregnated nets are not appropriate to every situation. Some of the gravest malaria burdens occur in countries undergoing conflict or among refugee populations.9 New ways of protecting individuals using materials that are already widely used have been successful in this setting. During emergencies safe pyrethroid insecticides (for example, permethrin) may be applied to a variety of surfaces, such as tents and blankets, or incorporated into the fibres of the plastic tarpaulins used as shelter material (fig 1).16,17 Such measures have good short term effect and are easy to implement. Pakistan's solution was to dip cattle in pyrethroid, although this is mainly used in Asia where Anopheles mosquitoes feed more readily on cattle than on humans.18
Figure 1.
KARSTEN THIELKER/AP PHOTO
During emergencies, insecticides can be incorporated into plastic tarpaulins
Vaccines
Much movement, some progress
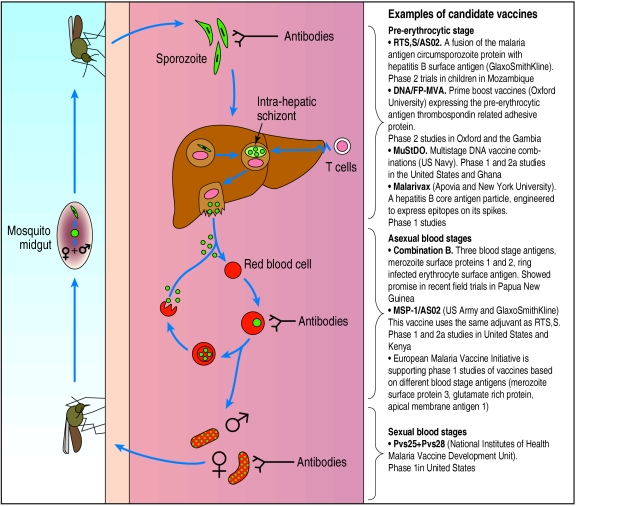
In the 1970s the passive transfer of purified immunoglobulin and administration of irradiated malaria sporozoites were both shown to protect against malaria. Neither solution is practical (the irradiated sporozoite “vaccine” requires bites from over 1000 infected mosquitoes over several months to confer protection). They did, however, show that it is possible to generate effective immunity against malaria. The challenge is to mimic these protective responses with a vaccine directed at antigens expressed in one of the three life cycle stages in humans.19 Figure 2 illustrates this cycle and outlines some of the (multiple) other candidate vaccines entering phase 1 or 2 trials.
Figure 2.
Vaccines entering clinical trial target distinct life cycle stages of parasite and are designed to elicit different types of immune response
Before vaccine has left liver and invaded red blood cells
Much of the energy and investment in malaria vaccines has targeted the pre-erythrocytic stage of the parasite. A vaccine aimed at this stage is commercially attractive as it would protect travellers as well as those in endemic areas. RTS,S/AS02 (GlaxoSmithKline) is the first malaria vaccine to offer convincing protection in a clinical trial. In laboratory challenges about 40% of vaccinated people were protected against malaria, and in field trials in the Gambia the vaccine had a 71% efficacy at 2 months. Protection was, however, short lived: in the last 6 weeks of the 15 week follow up, efficacy fell to zero, yielding an overall efficacy of 34%.20
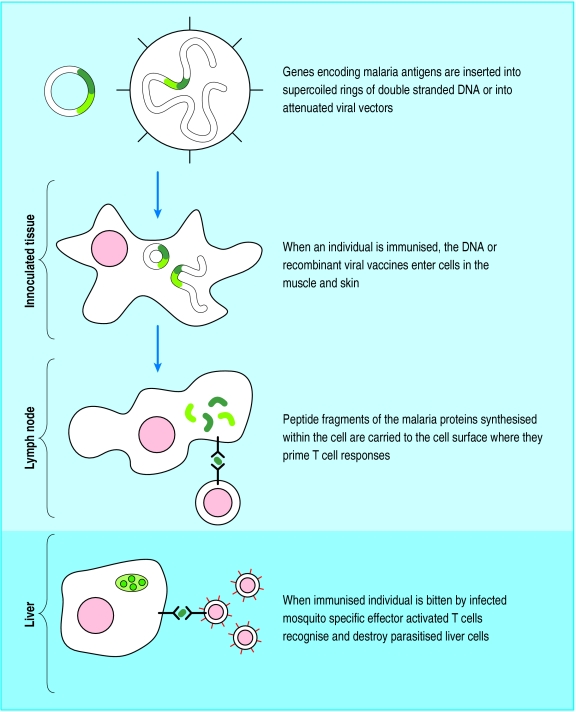
Although RTS,S was designed to elicit antibody responses capable of neutralising circulating parasites, antibody levels correlated only weakly with protection. Evidence shows that T cells are central to protection elicited by vaccines used in the pre-erythrocytic stage. Recently, DNA (as opposed to protein) based vaccines have proved excellent at stimulating the cellular immune response, at least in animal models (fig 3). Immunisation with a DNA plasmid followed by recombinant modified vaccinia Ankara, both encoding a rodent malaria protein, induced complete protection from challenge with sporozoites in mice.21 With these encouraging results, P falciparum vaccines based on the same “heterologous prime boost” schedule entered clinical trials in Oxford in 1999. A series of studies on sporozoite challenge are in progress with this immunisation regimen and also with a potentially more immunogenic regimen employing two recombinant poxviruses (AVS Hill, personal communication, 2002). Phase 1 field trials of both the DNA modified vaccinia Ankara and the novel recombinant fowlpox modified vaccinia Ankara prime boost immunisation strategy have already recently been undertaken in the Gambia, with promising results (V Moorthy, personal communication, 2002).
Figure 3.
Immunogenicity of DNA based vaccines is constantly being improved. Approaches include replacing the native DNA sequence of the pathogen with a DNA sequence that uses the mammalian genetic code to encode same protein (“mammalian codon bias”); adding sequences that increase antigen uptake into Langerhans' cells, injection together with DNA encoding cytokines and follow up immunisation with either recombinant protein antigen or same antigen encoded by another DNA vector
Once vaccine has invaded red blood cells
Clinical assessment of potential vaccines to be used in the blood stage have lagged behind those targeting the pre-erythrocytic stages. This is partly because they carry little commercial interest (residents of endemic countries rather than travellers would be the target population) and partly because there is no safe malaria blood stage challenge in humans. This may change however, with the development of polymerase chain reaction techniques to monitor low level parasitaemia. Researchers in Australia have challenged volunteers with low infective doses. Using polymerase chain reaction they successfully monitored the increase of parasitaemia until it was detectable by standard thick film microscopy. The rate of increase of parasitaemia over this period should correlate with the degree of immunity in the blood stage and therefore serve as a relatively safe phase 2 test of a malaria vaccine to be used in the blood stage.19 Several candidate vaccines targeting the blood stage are available (fig 2), all using traditional recombinant protein but with novel adjuvants.
Vaccines against the mosquito stage of the malaria life cycle
During the blood stages of malaria infection some parasites differentiate to produce male and female gametocytes; it is these that infect the mosquito. A novel group of transmission blocking vaccines works on the principle that antibodies generated in the human host and directed at gametocyte or ookinete antigens can block parasite development in the mosquito vector when ingested together with parasites in the blood meal. Transmission blocking vaccines can be developed more rapidly than the disease modifying vaccines, as no parasite challenge of volunteers is required. Anopheles mosquitoes are fed on infected erythrocytes that have been mixed with serum from immunised individuals. Infection is monitored by dissection of mosquitoes. The first transmission blocking vaccine will shortly be entering clinical trials. It is based on surface proteins of the P vivax ookinete and is highly immunogenic in animal studies. The homologous P falciparum vaccine should follow soon. The main drawback of transmission blocking vaccines is that benefit would be conferred on the community rather than the vaccinated individual. It is unlikely that these vaccines would ever be used alone but rather as a component of vaccines that target other stages of the life cycle.
Treatment
Drug resistance and affordability are key issues
Although chloroquine still works well for non-falciparum malaria, chloroquine resistant falciparum malaria is now nearly universal, and multidrug resistant malaria is spreading. In South East Asia multidrug resistant malaria is now so widespread that virtually no antimalarial can be used alone reliably. New rapid methods of drug screening hold out the promise of much faster identification of novel antimalarials in the longer term.20 It is mainly the investigation of older drugs used in novel combinations, however, that has produced a solution in this region. The artemisin group of drugs, derived from a traditional Chinese herbal drug, combined with a variety of partner drugs, some well known (for example, mefloquine) and some novel (for example, lumefantrine) have proved highly effective even in multidrug resistant malaria.21 Artemisin combinations may delay and possibly reverse the development of drug resistance through a variety of mechanisms.22 A rolling programme of efficacy and operational trials in Thailand and Vietnam mean that clinical research has kept pace with the advance of drug resistance in this region.
Additional educational resources
plasmodb.org/—the Plasmodium falciparum genome
www.ensembl.org/Anopheles_gambiae/—the Anopheles genome project
rbm.who.int/—WHO Roll Back Malaria website (contains many useful links)
www.malariavaccine.org—the Malaria Vaccine Initiative
www.mmv.org—Medicines for Malaria Venture
Yamey G. Global campaign to eradicate malaria. BMJ 2001;322:1191-2.
Multidrug resistant malaria is not yet a major problem in east and central Africa. The current target of research is finding an affordable drug that works. Because of economic constraints the successor drug to chloroquine has been sulfadoxine-pyrimethamine (Fansidar; Roche). Several countries have recently changed to this as first line treatment. Even before they did, however, parasitological resistance of over 20% to sulfadoxine-pyrimethamine in east Africa was common, and over 40% has been reported in some areas.23 The situation is likely to be critical within a couple of years.24 Doubtless, artemisin containing combinations would work as well as they do in South East Asia in case management, but cost is a problem. Chloroquine and sulfadoxine-pyrimethamine cost around 15 cents (10p) for a treatment course, and artemisin combinations cost over $1 (64p). Since the average family gets many episodes of malaria a year, and household incomes of less than $10 (£6.40) a month are not uncommon, this raises serious problems. Scientific research is contributing in two different ways. One is in bringing forward novel but affordable drugs—a pioneering partnership between GlaxoSmithKline, WHO, and academic institutions has fast tracked a relatively cheap new antimalarial, dapsone-chlorproguanil (Lapdap), based on combining two older drugs.25 The other is in operational research, applied anthropology, and economics in trying to determine the best way to deliver an effective existing drug at a cost and in a way most can afford. It is likely, however, that long term subsidy will be essential to provide effective, affordable antimalarial drug combinations for all. This is a political rather than a scientific problem, and until it is solved, making the best use of monotherapy against steadily increasing resistance remains an important practical scientific goal.
Acknowledgments
MR also works for HealthNet International, Pakistan.
Footnotes
Competing interest: None declared.
References
- 1.Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers DJ, Randolph SE, Snow RW, Hay SI. Satellite imagery in the study and forecast of malaria. Nature. 2002;415:710–715. doi: 10.1038/415710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman SL, Subramanian GM, Collins FH, Venter JC. Plasmodium, human, and Anopheles genomics and malaria. Nature. 2002;415:702–709. doi: 10.1038/415702a. [DOI] [PubMed] [Google Scholar]
- 4.Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 5.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lycett GJ, Kafatos FC. Antimalarial mosquitoes? Nature. 2002;417:387–388. doi: 10.1038/417387a. [DOI] [PubMed] [Google Scholar]
- 7.Lengeler C. Cochrane Library. Issue 2. Oxford: Update Software; 2002. Insecticide-treated bednets and curtains for preventing malaria. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong-Schellenberg JRM, Abdulla S, Nathan R, Mukasa O, Lengeler C. Effect of large-scale social marketing of insecticide-treated nets on child survival in rural Tanzania. Lancet. 2001;357:1241–1247. doi: 10.1016/S0140-6736(00)04404-4. [DOI] [PubMed] [Google Scholar]
- 9.Rowland M, Nosten F. Malaria epidemiology and control in refugee camps and complex emergencies. Ann Trop Med Parasitol. 2001;95:741–754. doi: 10.1080/00034980120103405. [DOI] [PubMed] [Google Scholar]
- 10.Chandre F, Darriet F, Manga L, Akogbeto M, Faye O, Mouchet J, et al. Status of pyrethroid resistance in Anopheles gambiae. Bull WHO. 1999;77:230–234. [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis CF, Miller JE, Hassan Hodjati M, Kolaczinski JH, Kasumba I. Can anything be done to maintain the effectiveness of pyrethroid-impregnated bednets against malaria vectors? Phil Trans R Soc Lond. 1998;353:1769–1775. doi: 10.1098/rstb.1998.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 13.Zaim M, Guillet P. Alternative insecticides: an urgent need. Trends Parasitol. 2002;18:161–163. doi: 10.1016/s1471-4922(01)02220-6. [DOI] [PubMed] [Google Scholar]
- 14.Kolaczinski JH, Fanello C, Herve J-P, Conway DJ, Carnevale P, Curtis CF. Experimental and molecular genetic analysis of pyrethroid and non-pyrethroid insecticide impregnated bednets in an area of pyrethroid resistance. Bull Entomol Res. 2000;90:125–132. doi: 10.1017/s0007485300000237. [DOI] [PubMed] [Google Scholar]
- 15.N'Guessan R, Darriet F, Doannio JMC, Chandre F, Carnevale P. Olyset Net efficacy against pyrethroid- resistant Anopheles and Culex after 3 years' field use in Cote d'Ivoire. Med Vet Entomol. 2001;15:97–104. doi: 10.1046/j.1365-2915.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt S, Rowland M, Nasir M, Kamal M, Kemp E. Pyrethroid sprayed tents for malaria control: an entomological evaluation in Pakistan's North West Frontier Province. Med Vet Entomol. 1995;9:344–352. doi: 10.1111/j.1365-2915.1995.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 17.Rowland M, Durrani N, Hewitt S, Mohammed N, Bouma M, Carneiro I, et al. Permethrin treated chaddars and top-sheets: appropriate technology for protection against malaria in Afghanistan and other complex emergencies. Trans R Soc Trop Med Hyg. 1999;93:465–472. doi: 10.1016/s0035-9203(99)90341-3. [DOI] [PubMed] [Google Scholar]
- 18.Rowland M, Durrani N, Kenwood M, Urahman H, Mohammad N, Hewitt S. Control of malaria in Pakistan by applying deltamethrin insecticide to cattle: a community-randomised trial. Lancet. 2001;357:1837–1841. doi: 10.1016/S0140-6736(00)04955-2. [DOI] [PubMed] [Google Scholar]
- 19.Good MF. Towards a blood-stage vaccine for malaria: are we following all the leads? Nature Rev Immunol. 2001;1:117–125. doi: 10.1038/35100540. [DOI] [PubMed] [Google Scholar]
- 20.Ridley RG. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature. 2002;415:686–693. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- 21.Van Vugt M, Looareesuwan S, Wilairatana P, McGready R, Villegas L, Gathmann I, et al. Artemether-lumefantrine for the treatment of multidrug-resistant falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:545–548. doi: 10.1016/s0035-9203(00)90082-8. [DOI] [PubMed] [Google Scholar]
- 22.Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Brockman A, et al. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. doi: 10.1016/s0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- 23.Mutabingwa TK, Maxwell CA, Sia IG, Msuya FH, Mkongewa S, Vannithone S, et al. A trial of proguanil-dapsone in comparison with sulfadoxine-pyrimethamine for the clearance of Plasmodium falciparum infections in Tanzania. Trans R Soc Trop Med Hyg. 2001;95:433–438. doi: 10.1016/s0035-9203(01)90207-x. [DOI] [PubMed] [Google Scholar]
- 24.White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, et al. Averting a malaria disaster. Lancet. 1999;353:1965–1967. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]
- 25.Mutabingwa T, Nzila A, Mberu E, Nduati E, Winstanley P, Hills E, et al. Chlorproguanil-dapsone for treatment of drug-resistant falciparum malaria in Tanzania. Lancet. 2001;358:1218–1223. doi: 10.1016/S0140-6736(01)06344-9. [DOI] [PubMed] [Google Scholar]