Abstract
AIM
To evaluate the trending visual performance of different intraocular lenses (IOLs) over time after implantation.
METHODS
Ninety-one patients received cataract surgery with implantations of monofocal (Mon) IOLs, segmental refractive (SegRef) IOLs, diffractive (Dif) IOLs, and extended-depth-of-focus (EDoF) IOLs were included. The aberrations and optical quality collected with iTrace and OQAS within postoperative 6mo were followed and compared.
RESULTS
Most of the visual parameters improved over the postoperative 6mo. The postoperative visual acuity (POVA) of the Mon IOL, SegRef IOL, and EDoF IOL groups achieved relative stability in earlier states compared with the Dif IOL group. Nevertheless, the overall visual performance of the 3 IOLs continued to upturn in small extents within the postoperative 6mo. The optical quality initially improved in the EDoF IOL group, then in the Mon IOL, SegRef IOL, and Dif IOL groups. POVA and objective visual performance of the Mon IOL and EDoF IOL groups, as well as POVA and visual quality of the Dif IOL group, improved in the postoperative 1mo and stabilized. Within the postoperative 6mo, gradual improvements were observed in the visual acuity and objective visual performance of the SegRef IOL group, as well as in the postoperative optical quality of the Dif IOL group.
CONCLUSION
The visual performance is different among eyes implanted with different IOLs. The findings of the current study provide a potential reference for ophthalmologists to choose suitable IOLs for cataract patients in a personalized solution.
Keywords: intraocular lenses, visual performance, changing characteristics, high-order wavefront aberration
INTRODUCTION
With the improving technology and requirements for living quality, cataract treatment has advanced into the refractive era. Although traditional monofocal (Mon) intraocular lens (IOLs) provide enough distance vision, the lack of accommodation results in a dependence on glasses for close work and daily life. Owing to optical advancement, multifocal intraocular lenses (MIOLs) including segmental refractive (SegRef) IOLs, diffractive (Dif) IOLs, and extended-depth-of-focus (EDoF) IOLs bring about significant improvement of the intermediate and near vision of patients after implantation[1]–[3].
Previous studies showed that Mon and MIOLs have different characteristics of visual performance, and the structural differences among SegRef, Dif, and EDoF IOLs lead to various optical quality and visual experience[4]–[6]. Ocular higher-order aberrations (HOA) and optical quality can be recorded by digitally simulating the visual performance of the whole eye and separating the aberrations of ocular surface, intraocular, and whole eye. Therefore, researchers can further determine the visual status, identify the possible visual problems, and provide more accurate evaluation for IOLs implantations[7]–[8]. Previous studies have evaluated the visual performance of Mon and MIOLs after implantation, respectively[9]–[11]. However, no cohort study had been conducted to comprehensively compare the sustaining changes of visual performance including visual acuity, total high-order wavefront aberration (THOA), and optical quality after the implantations of the present IOLs.
In this study, we continuously investigated the visual acuity, THOA, and optical quality for 6mo after implantation of Mon IOLs, SegRef IOLs, Dif IOLs, and EDoF IOLs, while analyzing the change rates of visual performance. The visual and optical characteristics of the IOLs was comprehensively evaluated. Therefore, we aimed to provide a scientific reference for the individualized selection of IOLs in future clinical practices.
SUBJECTS AND METHODS
Ethical Approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University with IRB approval (Medical Research Review No. 50, 2022), and all procedures adhered to the principles of the Declaration of Helsinki. All individuals who participated in the study understood the protocols and filled out the informed consent forms.
Study Design and Patients
This is a single-centre retrospective cohort study. The study subjects included 91 patients (91 eyes) with age-related cataract who treated in the First Affiliated Hospital of Guangzhou Medical University from November 2020 to August 2021, based on the inclusion criteria and exclusive criteria. They underwent cataract surgery and unilateral implantation of 4 different types of IOLs, including: Mon IOL group, 21 eyes of 21 patients. SegRef IOL group, 19 eyes of 19 patients. Dif IOL group, 29 eyes of 29 patients. EDoF IOL group, 22 eyes of 22 patients.
Preoperative Examination
All patients underwent a comprehensive preoperative ophthalmic examination, including measurement of uncorrected visual acuity (UCVA) by using a logMAR distance visual acuity chart at 5 m, intraocular pressure measurement (NIDEK, Gamagori, Japan), slit lamp microscope evaluation, optical biometer IOL Master (Carl Zeiss, Jena, Germany), corneal topography imaged by Pentacam comprehensive eye scanner (Oculus Optikgeraete, Wetzlar, Germany), noncontact specular microscope (Topcon, Tokyo, Japan), Tracey-iTrace visual function analyzer (iTrace, Texas, USA), optical quality analysis system OQAS™II (Visiometrics, Castelldefels, Spain) and fundoscopy.
Surgery
All patients underwent standard cataract surgery by the same experienced surgeon (Cheng H). The procedure started with a 2.8-mm primary incision and a 0.8-mm lateral incision using a keratome. Continuous curvilla-capsulorhexis of approximately 5.0-mm diameter, water stratification, phacoemulsification (Alcon, Fort Worth, USA), and polishing were then performed. Finally, the IOL was implanted into the phacocyst with a syringe and the position of the IOL was adjusted with the help of a Verion image-guided system (Alcon, Fort Worth, USA), followed by a watertight incision. The procedure was consistent for all patients. The patients were treated according to the standard nursing care after ophthalmic surgery. Postoperative topical therapy included levofloxacin 5 mg/mL (Santen, Japan) and pranoprofen 1 mg/mL (Senju Pharmaceutical, Japan) for 4wk, tobramycin and dexamethasone 5 mg/mL (Alcon, USA) for 2wk.
Outcome and Assessment
All patients (91 eyes) were reviewed at 1wk, 1, 3, and 6mo after surgery according to the routine clinical policy for cataract patients. The following examinations should be completed at each review, including: 1) Measurement of best corrected visual acuity (BCVA). In the Mon IOL group, best corrected distance visual acuity (BCDVA) was measured only at distance (5 m) by using logMAR distance visual acuity chart. BCDVA, best corrected intermediate visual acuity (BCIVA) and best corrected near visual acuity (BCNVA) were measured at distance (5 m), intermediate (80 cm), and near (40 cm) distances by using logMAR visual acuity charts in the other groups. 2) Diopter examination. The diopter of spherical, cylindrical and spherical equivalent were recorded. 3) Wavefront aberration measurement. Wavefront aberrations were measured by using Tracey-iTrace visual function analyzer in the dark room. The intraocular THOA and encircled energy function (EEF) were obtained by software Tracey (version 6.1.0). 4) Optical quality assessment. OQAS™ II was used to objectively evaluate the optical quality of the operated eyes in the same dark room condition. The spherical and cylindrical lenses were completely corrected with the low-order aberration correction system. If the astigmatism was greater than 0.50 D, an additional cylindrical lens was placed to correct the astigmatism. The objective scattering index (OSI), modulation transfer function cutoff frequency (MTF cutoff), strehl ratio (SR), and predicted visual acuity (PVA) 100%, 20%, 9% were obtained by the instrument. All the above examinations were performed by the same experienced ophthalmic technician, and were performed on the same day for each subject.
Statistical Analysis
In this study, SPSS 26.0 software was used for statistical processing. Count data, measurement data with normal distribution and measurement data with skewed distribution were described by mean (standard deviation) and median (interquartile range), respectively. The main effects, interaction effects and simple effects of different IOLs and postoperative (PO) time on each index were analyzed by repeated measures analysis of variance. Rank correlation analysis was used to analyze the correlation between the two skewed measurement data. Chi-square test was used for the comparison of multiple groups of count data, and analysis of variance and Kruskal-Wallis H test were used for the comparison of multiple groups of measurement data according to the data distribution, and further pairwise comparisons were performed if the difference was statistically significant. P<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
According to the inclusion and exclusion criteria, 91 eyes of 91 subjects were included in this study, which were divided into 4 different groups (Mon IOL group, SegRef IOL group, Dif IOL group, EDoF IOL group). The baseline and preoperative parameters of the included subjects were analyzed and integrated. There were no significant differences among the 4 groups in terms of gender, age, UCVA, axial length, mean keratometry (Km), corneal astigmatism, corneal endothelial cell counts, and IOL power (Table 1).
Table 1. Baseline of preoperative patient demographics.
| Parameters | Mon IOLs (21 patients) | SegRef IOLs (19 patients) | Dif IOLs (29 patients) | EDoF IOLs (22 patients) | χ²/H/F value | P |
| Sex (male)a | 8 (38.1%) | 10 (52.6%) | 10 (34.5%) | 8 (36.4%) | 1.783 | 0.619 |
| Age (y)b | 65 (14) | 68 (8) | 69 (8) | 67.5 (10) | 3.167 | 0.367 |
| UCVA (logMAR)b | 0.6 (0.5) | 0.5 (0.4) | 0.5 (0.3) | 0.45 (0.6) | 0.480 | 0.923 |
| Axial length (mm)c | 23.50 (0.69) | 23.40 (0.72) | 23.72 (0.53) | 23.57 (0.76) | 0.974 | 0.409 |
| Km (D)b | 43.99 (0.94) | 43.81 (1.82) | 44.12 (0.87) | 44.05 (1.58) | 1.461 | 0.691 |
| Corneal astigmatism (D)b | -0.53 (0.20) | -0.52 (0.30) | -0.46 (0.28) | -0.60 (0.32) | 2.290 | 0.514 |
| Corneal endothelial cell counts (/mm2)c | 2567 (263) | 2589 (282) | 2573 (275) | 2581 (319) | 0.022 | 0.996 |
| IOL power (D)c | 22.02 (2.06) | 20.83 (1.87) | 20.65 (1.97) | 21.18 (2.47) | 1.892 | 0.137 |
aCount data were described as absolute numbers (percentages), and comparison between groups was analyzed using the Chi-square test; bThe measurement data that did not meet the normal distribution were described by the median (interquartile range), and the Kruskal-Wallis H test was used for comparison between groups; cThe measurement data that meet the normal distribution were described by mean (SD), and comparison between groups was performed by completely randomized analysis of variance. IOLs: Intraocular lens; logMAR: Logarithm of the minimum angle of resolution; UCVA: Uncorrected distance visual acuity; Km: Mean keratometry; Mon IOLs: Monofocal intraocular lens; SegRef IOLs: Segmental refractive intraocular lens; Dif IOLs: Diffractive intraocular lens; EDoF IOLs: Extended-depth-of-focus intraocular lens.
Trends of Parameters Over Time
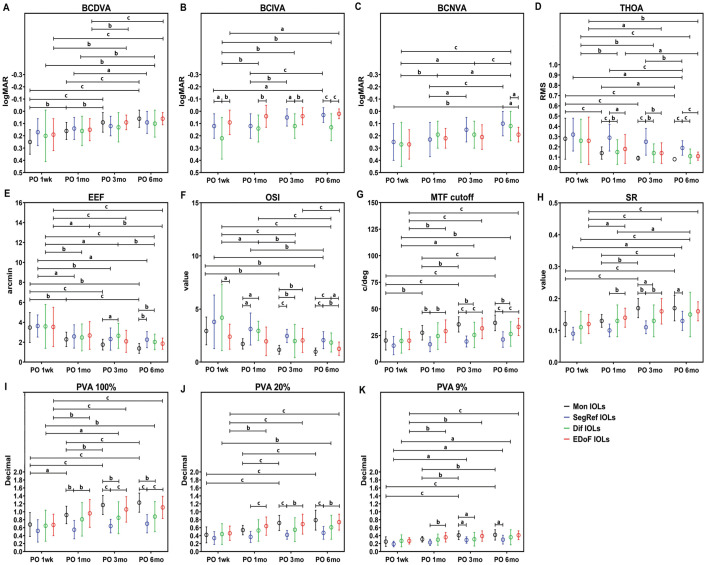
This study statistically compared the VA, aberration, and visual quality of patients in each group at 4 follow-up time points (Figure 1).
Figure 1. Outcomes of visual performance index of the different IOL groups at PO 1wk, 1, 3, and 6mo.
aP<0.05; bP<0.01; cP<0.001. PO: Postoperative; BCDVA: Best corrected distance visual acuity; BCIVA: Best corrected intermediate visual acuity; BCNVA: Best corrected near visual acuity; THOA: Total high-order wavefront aberration; EEF: Encircled energy function; OSI: Objective scattering index; MTF cutoff: Modulation transfer function cutoff frequency; SR: Strehl ratio; PVA: Predicted visual acuity; logMAR: Logarithm of the minimum angle of resolution; IOLs: Intraocular lens; Mon IOLs: Monofocal intraocular lens; SegRef IOLs: Segmental refractive intraocular lens; Dif IOLs: Diffractive intraocular lens; EDoF IOLs: Extended-depth-of-focus intraocular lens.
With the extending time postoperatively, the THOA, EEF, and OSI of the Mon IOL group showed a trend of decrease, while the BCDVA, MTF cutoff, SR, PVA100%, PVA20%, and PVA9% showed an increasing trend. Among them, the BCDVA, OSI, MTF cutoff, SR, PVA100%, PVA20%, and PVA9% had no significant variations of trending after PO 3mo (Figure 1).
The THOA, EEF, and OSI of the SegRef IOL group showed a decreasing trend, while the BCDVA, BCIVA, BCNVA, SR, PVA9% was in an increase. Among them, the EEF had no significant change in trend after PO 1mo, while the BCIVA, BCNVA and PVA9% exhibited no significant change of trending after PO 3mo (Figure 1).
The THOA, EEF, and OSI of the Dif IOL group showed a decreasing trend, while the BCDVA, BCIVA, BCNVA, MTF cutoff, SR, PVA100%, PVA20%, and PVA9% showed an increasing trend. Among these, the trends of the BCIVA and THOA had no significant change after PO 1mo, while there were no significantly changing trend found in the OSI, MTF cutoff, and PVA100% after PO 3mo (Figure 1).
The THOA, EEF, and OSI of the EDoF IOL group showed a decreasing trend, while the BCDVA, BCIVA, MTF cutoff, SR, PVA100%, PVA20%, and PVA9% showed an increasing trend. Among them, the MTF cutoff, SR, PVA100%, PVA20%, and PVA9% had no significant change in trending after PO 1mo, while the BCDVA didn't trend significantly after PO 3mo (Figure 1).
Comparison Between Groups at Each Time Point
Subsequently, we further compared the indicators of the visual acuity, aberration, and visual quality of patients among groups at each follow-up time point (Figure 1).
At each time point, there was no statistical difference in the BCDVA between the 4 groups (Figure 1A). On the other hand, except for PO 1mo, the BCIVA in the Dif IOL group was generally lower than that in the SegRef IOL and EDoF IOL groups (Figure 1B). In terms of the BCNVA, the EDoF IOL group was significantly lower than the SegRef IOL and Dif IOL groups at the PO 6mo (Figure 1C).
The THOA of the SegRef IOL group was significantly higher than the other groups after PO 1mo (Figure 1D). On the other hand, the EEF of the Dif IOL group was significantly higher than that of the Mon IOL group at PO 3mo and PO 6mo. Moreover, the EEF of the SegRef IOL group was significantly higher than that of the Mon IOL group at PO 6mo (Figure 1E).
In terms of the OSI, the EDoF IOL group was significantly lower than the Dif IOL group at PO 1wk (PO 1wk); the Mon IOL group was significantly lower than the SegRef IOL and Dif IOL groups at PO 1mo; the Mon IOL group was significantly lower than the other 3 groups at PO 3mo; the Mon IOL and EDoF IOL group were significantly lower than the SegRef IOL and Dif IOL groups at PO 6mo (Figure 1F). In terms of the MTF cutoff and PVA100%, the SegRef IOL group was significantly lower than the Mon IOL and EDoF IOL groups after PO 1mo, and the Dif IOL group was significantly lower than the Mon IOL group at PO 3mo and PO 6mo (Figure 1G, 1I). In terms of the SR, the SegRef IOL group was significantly lower than the EDoF IOL group at PO 1mo and PO 3mo, and was significantly lower than the Mon IOL group at PO 3mo and PO 6mo (Figure 1H). In terms of the PVA20%, the SegRef IOL group was significantly lower than the EDoF IOL group after PO 1mo and was significantly lower than the Mon IOL group at PO 3mo and PO 6mo (Figure 1J). In terms of the PVA9%, the SegRef IOL group was significantly lower than the EDoF IOL group at PO 1mo, and the SegRef IOL and Dif IOL groups was significantly lower than the Mon IOL group at PO 3mo, while the SegRef IOL group was significantly lower than the Mon IOL group at PO 6mo (Figure 1K).
Comparison of Change Rates Between Each Time Point
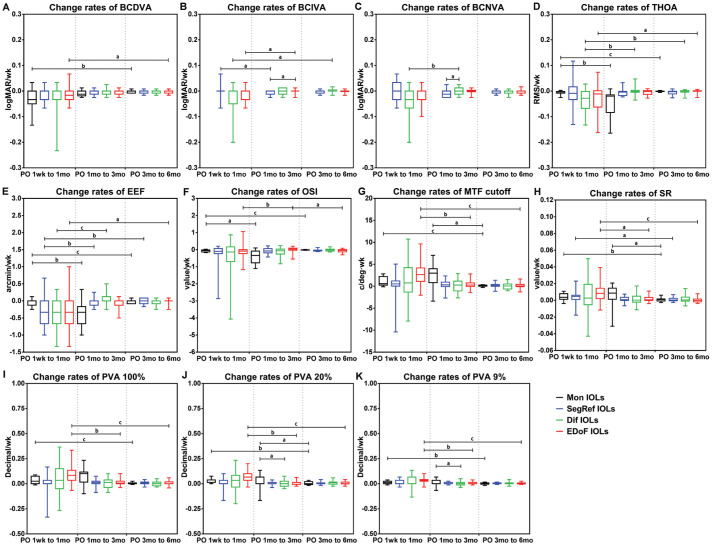
The change rates of the visual acuity, aberration and visual quality indicators of each group within different periods (PO 1wk to 1mo, PO 1 to 3mo, PO 3 to 6mo) were analyzed and compared (Figure 2).
Figure 2. Change rates of visual performance index of the different IOL groups at PO 1wk to 1mo, PO 1 to 3mo, and PO 3 to 6mo.
aP<0.05; bP<0.01; cP<0.001. PO: Postoperative; BCDVA: Best corrected distance visual acuity; BCIVA: Best corrected intermediate visual acuity; BCNVA: Best corrected near visual acuity; THOA: Total high-order wavefront aberration; EEF: Encircled energy function; OSI: Objective scattering index; MTF cutoff: Modulation transfer function cutoff frequency; SR: Strehl ratio; PVA: Predicted visual acuity; logMAR: Logarithm of the minimum angle of resolution; IOLs: Intraocular lens; Mon IOLs: Monofocal intraocular lens; SegRef IOLs: Segmental refractive intraocular lens; Dif IOLs: Diffractive intraocular lens; EDoF IOLs: Extended-depth-of-focus intraocular lens.
In the Mon IOL group, the declining rate of the THOA, EEF, and OSI in the period PO 1wk to 1mo was significantly faster than that in the other periods, whilst the rising rate of the BCDVA, PVA100%, and PVA9% was significantly faster than that in the period PO 3 to 6mo (Figure 2A, 2D-2F, 2I, 2K). The increasing rate of the MTF cutoff, SR, and PVA20% in the periods of the PO 1wk to 1mo and PO 1 to 3mo were significantly higher compared with that in the period PO 3 to 6mo (Figure 2G-2H, 2J).
In the SegRef IOL group, the declining rate of the EEF during the period PO 1wk to 1mo was significantly faster than that in the other periods, while the increasing rate of the BCIVA was significantly faster than that in the period PO 1mo to 3mo. Moreover, the increasing rate of the SR was significantly faster than that in the period PO 3 to 6mo (Figure 2B, 2E, 2H).
In the Dif IOL group, the declining rate of the THOA during the period PO 1wk to 1mo was significantly faster than that in the other periods, while the change rate of the EEF and BCNVA was significantly larger than that in the period PO 1 to 3mo. Moreover, the increasing rate of the BCIVA was significantly faster than that the period PO 3 to 6mo (Figure 2B-2E).
In the period PO 1wk to 1mo, the MTF cutoff, SR, PVA100%, PVA20%, and PVA9% of the EDoF IOL group increased in a fastest speed (Figure 2G-2K), while the increasing rate of the BCIVA was significantly faster than that in the period PO 1 to 3mo. What's more, the change rate of the BCDVA, THOA, and EEF was significantly larger than that in the period PO 3 to 6mo in the EDoF IOL group (Figure 2A, 2B, 2D, 2E). In the periods of PO 1wk to 1mo and PO 3 to 6mo, the OSI of the EDoF IOL group declined faster than that in the period PO 1mo to 3mo (Figure 2F).
On the other hand, during the PO 1 to 3mo, the increasing rate of the BCIVA and BCNVA of the SegRef IOL group was significantly slower than that of the EDoF IOL and Dif IOL groups respectively (Figure 2B, 2C). Meanwhile, the PVA20% and PVA9% of the Mon IOL group was significantly changing faster than that of the Dif IOL group (Figure 2J, 2K).
Correlation Analysis
Finally, we conducted a graded correlation analysis on the BCDVA, aberration index, and visual quality index of the 4 groups at PO 6mo (Table 2).
Table 2. Correlation analysis between BCDVA and each index in IOL groups at PO 6mo.
| BCDVA | Mon IOLs |
SegRef IOLs |
Dif IOLs |
EDoF IOLs |
||||
| CC (r) | P | CC (r) | P | CC (r) | P | CC (r) | P | |
| THOA | 0.114 | 0.622 | 0.199 | 0.415 | -0.254 | 0.184 | 0.080 | 0.723 |
| EEF | 0.018 | 0.939 | 0.138 | 0.573 | -0.178 | 0.354 | -0.036 | 0.875 |
| OSI | -0.048 | 0.837 | 0.127 | 0.605 | 0.690 | <0.001 | 0.549 | 0.008 |
| MTF cutoff | -0.347 | 0.123 | 0.008 | 0.976 | -0.759 | <0.001 | -0.182 | 0.417 |
| SR | -0.007 | 0.975 | 0.035 | 0.888 | -0.733 | <0.001 | -0.146 | 0.517 |
| PVA100% | -0.346 | 0.125 | -0.039 | 0.876 | -0.782 | <0.001 | -0.184 | 0.413 |
| PVA20% | -0.069 | 0.768 | 0.018 | 0.943 | -0.773 | <0.001 | -0.265 | 0.233 |
| PVA9% | -0.057 | 0.807 | 0.081 | 0.742 | -0.687 | <0.001 | -0.234 | 0.294 |
CC: Correlation coefficient; BCDVA: Best corrected distance visual acuity; THOA: Total high-order wavefront aberration; EEF: Encircled energy function; OSI: Objective scattering index; MTF cutoff: Modulation transfer function cutoff frequency; SR: Strehl ratio; PVA: Predicted visual acuity; IOLs: Intraocular lens; Mon IOLs: Monofocal intraocular lens; SegRef IOLs: Segmental refractive intraocular lens; Dif IOLs: Diffractive intraocular lens; EDoF IOLs: Extended-depth-of-focus intraocular lens.
At PO 6mo, the MTF cutoff, SR, PVA100%, PVA20%, and PVA9% in the Dif IOL group were positively correlated with the BCDVA (P<0.05), whilst the OSI was negatively correlated with the BCDVA in the Dif IOL and EDoF IOL groups (P<0.05).
DISCUSSION
Different studies evaluating the visual performance of IOLs after implantation have been previously reported. Pedrotti et al[9] compared the BCDVA, BCIVA, and BCNVA after implantation of Mon and EDoF IOLs, and compared the MTF cutoff and SR by using OQAS at PO 3mo. They found the intermediate and near visual acuity was better after EDOF IOLs than after aspheric Mon IOLs implantation while maintaining similar levels of visual quality, except for halo perception. Alió et al[10] and Ruiz-Mesa et al[11] used Itrace and OQAS to analyze the visual acuity and visual performance of SegRef IOLs and EDoF IOLs at 1wk, 1mo and PO 3mo, respectively. They found SegRef IOLs and EDoF IOLs restored distance, intermediate, and near visual function after cataract surgery, and provided good optical and visual quality with high level of patient satisfaction. However, the trending performance of visual outcomes after implanting different IOLs with different optical properties over a period of time has not been reported before. The outcomes and change rates of visual acuity, visual quality, and optical quality were evaluated and compared between 4 different types of IOLs in the present study, by using the visual acuity chart, Itrace, and OQAS. Therefore, this study provides a potential reference of comprehensive for the individualized selection of IOLs in clinical practices.
Visual acuity is the most straightforward indicator of the effectiveness of cataract surgery. In this study, the BCDVA of all the IOL groups reached a higher level (≤0.19±0.06 logMAR) at PO 6mo, but the BCIVA of the Dif IOL group was generally worse than that of the SegRef IOL and EDoF IOL groups. With the extension of time postoperatively, the BCNVA of the EDoF IOL group was also significantly worse than that of the SegRef IOL and Dif IOL groups, which was in consistent with the findings of Walkow et al[12] and Cochener et al[13]. We consider that this is related to the design of IOLs itself. Dif IOLs allows the light entering the human eye form double focal points of far and near, so the intermediate visual acuity is relatively poorer. Due to the reason that EDoF IOLs lack fixed near-focus optical design, the extended focal segment makes it difficult to maintain consistent image quality in the near vision, thus the near visual acuity is relatively poorer[14].
With the prolongation of the time after implantation, except for that the BCNVA of the EDoF IOL group did not significantly change within the PO 1wk to 6mo, the BCDVA, BCIVA, BCNVA of the other groups (Mon IOL group only included BCDVA) showed an increasing trend. This was identical to the conclusion of Ganesh et al[15]. This indicated that the near vision after implanting EDoF IOLs had a smaller range of variation within the PO 1wk to 6mo and tended to be stable in the early period PO. It is considered to be related with the poorer BCNVA. We also found that the PO visual acuity of the Mon IOL, SegRef IOL, and EDoF IOL groups tended to be stable at PO 3mo, which was earlier than that of the Dif IOL group. This could be resulted from the fact that Dif IOLs distribute light energy at different focal points in the distance and near through diffraction. Therefore, the optical splitting and defocusing factors lead to the decreasing contrast of retinal imaging. The brain needs to gradually adapt to the image falling on the retina, resulting in a relatively late stabilization time[16].
We also found that the visual acuity of Mon IOL, Dif IOL, and EDoF IOL groups showed the most obvious improvement in the period PO 1wk to 1mo, while the visual acuity of the SegRef IOL group showed a steady trend within PO 6mo. This may be due to the strong anti-inflammatory measures applied to eyes in the early PO period, and the intraocular environment recovered well in the early period postoperatively. Therefore, the visual acuity of the eyes with Mon, Dif and EDoF IOLs significantly improved in a fast speed. However, the PO visual acuity and objective visual performance of the eyes with SegRef IOLs are more sensitive to eccentricity and tilt[17]–[19], which hinders the early lens capsule stability recovery and partially offsets the rapid improvement in visual performance. This is the possible reason why the visual performance showed significant improvements at PO 6mo when the lens capsule gains further stability.
In this study, both iTrace and OQAS were used to examine the objective visual performance after surgery. The repeatability of these two instruments has been validated in previous works[20]–[21].
The iTrace quantitatively measures the wavefront aberrations in eyes. Wavefront aberration is one of the indicators to evaluate the imaging effect of optical systems, which is usually expressed and calculated by root mean square (RMS)[22]. Among wavefront aberrations, the aberration that cannot be corrected by refraction is called THOA, while its numerical magnitude and the combination of different fractional order aberrations can still have a great adverse impact on visual performance even under good refractive conditions[23]–[24]. EEF is the point spread function (PSF) calculated by iTrace based on wavefront aberration through data integration. The visual angle of the human eye at 50%, which forms the best visual quality, is then simulated according to PSF. With smaller value, the indicator represents higher objective visual acuity and better visual quality.
We found that the THOA of the SegRef IOL group was higher than that of the other 3 groups in the period PO 1wk to PO 6mo, which was the same as the results of former studies[25]–[27]. Most researchers considered that this was due to the design of the optical part in SegRef IOLs itself, resulting in a large coma in the eye[28]. The coma is generally thought to result in reduced objective visual quality[29]. The EEF of the SegRef IOL and Dif IOL groups was also higher than that of the Mon IOL group with time extension, which could also be related to the optical structure design of the IOLs.
With the passage of time after IOLs implantation, the THOA and EEF of all groups showed a downward trend. In addition to the THOA of the Dif IOL group and the EEF of the SegRef IOL group, which tended to be stable after PO 1mo, the THOA and EEF of the other groups showed partial improvement. This was also different from the changing visual acuity after surgery. It was showed that the overall visual quality of the eyes with Mon IOLs, SegRef IOLs, and EDoF IOLs was not stable within the following PO 6mo, which was slower than the stabilization of visual acuity. A previous literature indicated that the incidence of non-adhesion between the posterior capsule of lens and the optical part of IOLs decreased significantly from PO 1wk to 2mo, which was strongly correlated with visual quality[30]. Meanwhile, the complete stability of PO optical quality was reported to took a long time, different from the visual acuity.
Though the THOA of the SegRef IOL group showed no significant change from PO 1wk to 6mo, it decreased obviously from PO 1wk to 1mo and reached a low level in the other groups. This was similar to the changes in the PO visual acuity. It could also be owing to the sensitivity of SegRef IOLs to eccentricity and tilt. The improvement, however, relied on the increasing intraocular environmental stability. In addition, the EEF of all groups showed greatest declines in the period PO 1wk to 1mo. This illustrated that the objective visual acuity improved in rapid speed, which verified the early benefits of suppressing intraocular inflammation on the structural optical properties after IOLs implantation.
OQAS based on the dual-channel technology quantitatively measures the diffraction and scattering data of eyes[21],[31]. The most important parameters detected by OQAS include: 1) OSI that reflects the forward scattering of the refractive medium, and its lower value proves better optical quality. 2) MTF cutoff that represents the spatial frequency corresponding to the minimum resolution of the human eye in the modulation transfer function curve. The higher the value of MTF cutoff, the better the optical quality. 3) SR that represents the ratio of light intensity between the actual optical system of the human eye and the optical system without aberrations. The larger the value of SR, the better the optical quality. 4) Based on the modulation transfer function curve, PVA100%, PVA20% and PVA9% can simulate the contrast visual acuity of human eyes under 100%, 20% and 9% light intensities.
We found that the optical quality of the Mon IOL and EDoF IOL groups was better than that of the SegRef IOL and Dif IOL groups at PO 1mo. With further extension of PO time, the optical quality of the Mon IOL group reached a better state than that of the SegRef IOL and Dif IOL groups. This was similar to the conclusion of a previous studie[32]. This is considered to be related to the optical features of IOLs: Mon IOLs carriy no diffractive structures. In this case, the light energy dispersion and light wave interference generated by passing through the IOLs are lower, resulting in better optical quality. Besides, the Echelette diffraction grating and achromatic design of EDoF IOLs assist to correct the external focus caused by the wavelength change. They also offset the occurrence of glare and halo, and improve the contrast sensitivity of eyes. Therefore, the PO optical quality of the eyes with EDoF IOLs was almost equally good as those with Mon IOLs.
As time went on after surgery, except for the MTF cutoff, PVA100%, and PVA20% of the SegRef IOL group, the other optical quality indices of other groups showed significant improvement from PO 1wk to 6mo. This result indicates that most optical quality indices had a process of improvement with the extension of PO time, which is in consistent with the reports from some researchers[11],[33]. It was also found that the optical quality indices of the EDoF IOL group mainly stabilized at PO 1mo, and that of the Mon IOL group mainly stabilized at PO 3mo. However, the PVA20% and PVA9% of the Dif IOL were not stable in PO 6mo, as well as the OSI and SR of the SegRef IOL group. This is because of that the diffraction of light will produce greater mutual interference by Dif IOLs under lower contrast conditions[34]. As it is mentioned previously, after the implantation of SegRef IOLs, the stability of optical quality could be related to the recovery in lens capsule's stability, eccentricity, and tilt[35]–[36].
In this study, it was also found that the optical quality indices of the Mon IOL and EDoF IOL groups had the fastest improvement from PO 1wk to 1mo. In the SegRef IOL and Dif IOL groups, the change rates of most optical quality indices basically remained consistent within PO 6mo. It indicates that the PO optical quality of the eyes with Mon and EDoF IOLs significantly advanced at the early stage after implantation, in consistent with the visual acuity and aberration indices. The PO optical quality of the SegRef IOL and Dif IOL groups improved gently in the early and middle period after surgery. The gradual increase in the optical quality of the eyes with SegRef IOLs could to be related to the unstable state of IOLs in the pouch during an early PO phase, and again the sensitivity to eccentricity and tilt. Nevertheless, the slow improvement of the PO optical quality of the Dif IOL group might be resulted from a poorer neural adaptability. Due to the diffraction and defocus caused by Dif IOLs, the visual center needs to undergo complex processing and image suppression[37]. However, the reasons for which the inconsistency between the change rates of the PO optical quality and visual acuity/aberration in the eyes with Dif IOLs need to be further researched in the future.
We also found that the MTF cutoff, SR, PVA100%, PVA20%, and PVA9% were positively correlated with THE BCDVA in the Dif IOL group at PO 6mo, while the OSI was negatively correlated with the BCDVA in the Dif IOL and EDoF IOL groups at PO 6mo. These results fully reflected the correlation between subjective and objective visual performance in patients with IOL implants. It brings new guiding significance for mutual prediction and evaluation between subjective and objective examinations.
Generally, Dif IOLs provided lower PO intermediate visual acuity and EDoF IOLs offered lower PO near visual acuity compared with other types of presbyopic correction IOLs. Mon and EDoF IOLs had significantly better PO optical and visual quality than SegRef and Dif IOLs. The stability of PO visual acuity was achieved earlier in the eyes with Mon, SegRef, and EDoF IOLs. However, the overall visual quality of these 3 IOLs was not stable within PO 6mo. The optical quality of the eyes with EDoF IOLs improved first, followed by Mon IOLs, then SegRef and Dif IOLs. The PO visual acuity and objective visual performance of the eyes with Mon and EDoF IOLs, as well as the PO visual acuity and visual quality of the eyes implanted Dif IOLs, showed significant improvement from PO 1wk to 1mo, and then gradually stabilized. Within the PO 6mo, there was a gradual improvement in the visual acuity and objective visual performance in the eyes with SegRef IOL implants, as well as an improvement in the optical quality of those with Dif IOLs. Based on these findings, we can tailor PO diagnosis and treatment plans to meet the diverse needs of patients' lifestyles. The results of the current study may enable the ophthalmologists to select the most appropriate IOL for each patient and make more accurate prediction about the PO outcomes, thereby improving the overall quality of clinical care.
Footnotes
Authors' contributions: Study concept and design (Huang H, Cheng H, Gao L); collection, management, analysis and interpretation of data (Li BW, Huang MS, Guo SL, Zeng YY, Cheng L, Yao SY, Lin JQ, Liu L, Yang Y, Lu XM); preparation, review, or approval of the manuscript (Huang H, Li BW). All authors read and approved the final manuscript.
Foundations: Supported by the “Municipal School (College) Joint Funding (Zhongnanshan Medical Foundation of Guangdong Province) Project” of Guangzhou Municipal Science and Technology Bureau (No.202201020458); the “Guangzhou Health Science and Technology General Guidance Project (Western Medicine Project)” of Guangzhou Municipal Health Commission (No.20231A011083).
Conflicts of Interest: Li BW, None; Huang H, None; Huang MS, None; Guo SL, None; Gao L, None; Zeng YY, None; Cheng L, None; Yao SY, None; Lin JQ, None; Liu L, None; Yang Y, None; Lu XM, None; Cheng H, None.
REFERENCES
- 1.Cho JY, Won YK, Park J, Nam JH, Hong JY, Min S, Kim N, Chung TY, Lee EK, Kwon SH, Lim DH. Visual outcomes and optical quality of accommodative, multifocal, extended depth-of-focus, and monofocal intraocular lenses in presbyopia-correcting cataract surgery: a systematic review and Bayesian network meta-analysis. JAMA Ophthalmol. 2022;140(11):1045–1053. doi: 10.1001/jamaophthalmol.2022.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanclerz P, Toto F, Grzybowski A, Alio JL. Extended depth-of-field intraocular lenses: an update. Asia Pac J Ophthalmol (Phila) 2020;9(3):194–202. doi: 10.1097/APO.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Lan Q, Sun T, Tang C, Yang T, Duan H, Liu R, Qi H. Binocular visual function after unilateral versus bilateral implantation of segmented refractive multifocal intraocular lenses: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2022;260(4):1205–1213. doi: 10.1007/s00417-021-05496-3. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Zheng TY, Lu Y. Comparative analysis of visual performance and astigmatism tolerance with monofocal, bifocal, and extended depth-of-focus intraocular lenses targeting slight myopia. J Ophthalmol. 2020;2020:9283021. doi: 10.1155/2020/9283021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochener B, Lafuma A, Khoshnood B, Courouve L, Berdeaux G. Comparison of outcomes with multifocal intraocular lenses: a meta-analysis. Clin Ophthalmol. 2011;5:45–56. doi: 10.2147/OPTH.S14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altemir-Gomez I, Millan MS, Vega F, Bartol-Puyal F, Gimenez-Calvo G, Larrosa JM, Polo V, Pablo LE, Garcia-Martin E. Comparison of visual and optical quality of monofocal versus multifocal intraocular lenses. Eur J Ophthalmol. 2020;30(2):299–306. doi: 10.1177/1120672119827858. [DOI] [PubMed] [Google Scholar]
- 7.Baur ID, Yan WJ, Auffarth GU, Khoramnia R, Łabuz G. Optical quality and higher order aberrations of refractive extended depth of focus intraocular lenses. J Refract Surg. 2023;39(10):668–674. doi: 10.3928/1081597X-20230831-01. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Li FF, Xia HJ, Zhou J. Visual quality after implantation of trifocal intraocular lenses in highly myopic eyes with different axial lengths. Int J Ophthalmol. 2021;14(3):371–377. doi: 10.18240/ijo.2021.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedrotti E, Chierego C, Talli PM, Selvi F, Galzignato A, Neri E, Barosco G, Montresor A, Rodella A, Marchini G. Extended depth of focus versus monofocal IOLs: objective and subjective visual outcomes. J Refract Surg. 2020;36(4):214–222. doi: 10.3928/1081597X-20200212-01. [DOI] [PubMed] [Google Scholar]
- 10.Alió JL, Piñero DP, Plaza-Puche AB, Chan MJ. Visual outcomes and optical performance of a monofocal intraocular lens and a new-generation multifocal intraocular lens. J Cataract Refract Surg. 2011;37(2):241–250. doi: 10.1016/j.jcrs.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Mesa R, Blanch-Ruiz J, Ruiz-Santos M, Montés-Micó R. Optical and visual quality assessment of an extended depth-of-focus intraocular lens based on spherical aberration of different sign. Int Ophthalmol. 2021;41(3):1019–1032. doi: 10.1007/s10792-020-01659-z. [DOI] [PubMed] [Google Scholar]
- 12.Walkow T, Liekfeld A, Anders N, Pham DT, Hartmann C, Wollensak J. A prospective evaluation of a diffractive versus a refractive designed multifocal intraocular lens. Ophthalmology. 1997;104(9):1380–1386. doi: 10.1016/s0161-6420(97)30127-4. [DOI] [PubMed] [Google Scholar]
- 13.Cochener B, Concerto Study Group Clinical outcomes of a new extended range of vision intraocular lens: International Multicenter Concerto Study. J Cataract Refract Surg. 2016;42(9):1268–1275. doi: 10.1016/j.jcrs.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Böhm M, Petermann K, Hemkeppler E, Kohnen T. Defocus curves of 4 presbyopia-correcting IOL designs: Diffractive panfocal, diffractive trifocal, segmental refractive, and extended-depth-of-focus. J Cataract Refract Surg. 2019;45(11):1625–1636. doi: 10.1016/j.jcrs.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Ganesh S, Brar S, Rp N, Rathod D. Clinical outcomes, contrast sensitivity, reading performance and patient satisfaction following bilateral implantation of AT LARA 829MP EDoF IOLs. Clin Ophthalmol. 2021;15:4247–4257. doi: 10.2147/OPTH.S331860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palomino Bautista C, Carmona González D, Castillo Gómez A, Bescos JA. Evolution of visual performance in 250 eyes implanted with the Tecnis ZM900 multifocal IOL. Eur J Ophthalmol. 2009;19(5):762–768. doi: 10.1177/112067210901900513. [DOI] [PubMed] [Google Scholar]
- 17.Tanabe H, Shojo T, Yamauchi T, Takase K, Akada M, Tabuchi H. Comparative visual performance of diffractive bifocal and rotationally asymmetric refractive intraocular lenses. Sci Rep. 2022;12(1):19394. doi: 10.1038/s41598-022-24123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XM, Xie LX, Huang YS. Effects of decentration and tilt at different orientations on the optical performance of a rotationally asymmetric multifocal intraocular lens. J Cataract Refract Surg. 2019;45(4):507–514. doi: 10.1016/j.jcrs.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 19.Cheng KW, Meng JQ, Wei L, Hu XX, Lu Y, Zhu XJ. Comparison of tolerance to decentration and tilt in the early postoperative period between the continuous range and bifocal intraocular lens implantation in myopic eyes. Zhonghua Yan Ke Za Zhi. 2022;58(7):513–520. doi: 10.3760/cma.j.cn112142-20220403-00154. [DOI] [PubMed] [Google Scholar]
- 20.Ju RH, Qu HK, Wu ZM, Chen Y, Wu LN, Long Y, Wang Z. Comparison of visual performance with iTrace analyzer following femtosecond laser-assisted cataract surgery with bilateral implantation of two different trifocal intraocular lenses. Int J Ophthalmol. 2023;16(11):1773–1781. doi: 10.18240/ijo.2023.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin YW, Lu Y, Xiang AQ, Fu YY, Zhao Y, Li YJ, Hu T, Du KX, Hu SF, Fu QM, Wu XY, Wen D. Comparison of the optical quality after SMILE and FS-LASIK for high myopia by OQAS and iTrace analyzer: a one-year retrospective study. BMC Ophthalmol. 2021;21(1):292. doi: 10.1186/s12886-021-02048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng X, Thibos LN, Bradley A. Estimating visual quality from wavefront aberration measurements. J Refract Surg. 2003;19(5):S579–S584. doi: 10.3928/1081-597X-20030901-14. [DOI] [PubMed] [Google Scholar]
- 23.Applegate RA, Marsack JD, Ramos R, Sarver EJ. Interaction between aberrations to improve or reduce visual performance. J Cataract Refract Surg. 2003;29(8):1487–1495. doi: 10.1016/s0886-3350(03)00334-1. [DOI] [PubMed] [Google Scholar]
- 24.Applegate RA, Ballentine C, Gross H, Sarver EJ, Sarver CA. Visual acuity as a function of Zernike mode and level of root mean square error. Optom Vis Sci. 2003;80(2):97–105. doi: 10.1097/00006324-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Lian HF, Ma WS, Wei QH, Yuan XY. A comparative study on early vision quality after implantation of refractive segmental and diffractive multifocal intraocular lens. Pak J Med Sci. 2020;36(7):1607–1612. doi: 10.12669/pjms.36.7.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatinel D, Loicq J. Clinically relevant optical properties of bifocal, trifocal, and extended depth of focus intraocular lenses. J Refract Surg. 2016;32(4):273–280. doi: 10.3928/1081597X-20160121-07. [DOI] [PubMed] [Google Scholar]
- 27.Song XH, Liu X, Wang W, Zhu YN, Qin ZW, Lyu DN, Shentu XC, Xv W, Chen PQ, Ke Y. Visual outcome and optical quality after implantation of zonal refractive multifocal and extended-range-of-vision IOLs: a prospective comparison. J Cataract Refract Surg. 2020;46(4):540–548. doi: 10.1097/j.jcrs.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 28.Ramón ML, Piñero DP, Pérez-Cambrodí RJ. Correlation of visual performance with quality of life and intraocular aberrometric profile in patients implanted with rotationally asymmetric multifocal IOLs. J Refract Surg. 2012;28(2):93–99. doi: 10.3928/1081597X-20111213-02. [DOI] [PubMed] [Google Scholar]
- 29.Hu C, Ravikumar A, Hastings GD, Marsack JD. Visual interaction of 2nd to 5th order Zernike aberration terms with vertical coma. Ophthalmic Physiol Opt. 2020;40(5):669–679. doi: 10.1111/opo.12718. [DOI] [PubMed] [Google Scholar]
- 30.Zhu XJ, He WW, Yang J, Hooi M, Dai JH, Lu Y. Adhesion of the posterior capsule to different intraocular lenses following cataract surgery. Acta Ophthalmol. 2016;94(1):e16–e25. doi: 10.1111/aos.12739. [DOI] [PubMed] [Google Scholar]
- 31.Liao X, Li JY, Tan QQ, Tian J, Lin J, Lan CJ. Comparison of visual quality after implantation of A1-UV and SN60WF aspheric intraocular lens. Int J Ophthalmol. 2020;13(11):1727–1732. doi: 10.18240/ijo.2020.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedrotti E, Bruni E, Bonacci E, Badalamenti R, Mastropasqua R, Marchini G. Comparative analysis of the clinical outcomes with a monofocal and an extended range of vision intraocular lens. J Refract Surg. 2016;32(7):436–442. doi: 10.3928/1081597X-20160428-06. [DOI] [PubMed] [Google Scholar]
- 33.Shen JY, Zhang LM, Ni S, Cai L, Guo HK, Yang J. Comparison of visual outcomes and quality of life in patients with high myopic cataract after implantation of AT LISA tri 839MP and LS-313 MF30 intraocular lenses. J Ophthalmol. 2022;2022:5645752. doi: 10.1155/2022/5645752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gil MÁ, Varón C, Cardona G, Buil JA. Far and near contrast sensitivity and quality of vision with six presbyopia correcting intraocular lenses. J Clin Med. 2022;11(14):4150. doi: 10.3390/jcm11144150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X, Liu XM, Li DF, Wang XY, Huang YS. Therapeutic effect of a new implantation method of rotationally asymmetric multifocal intraocular lenses on visual quality. Int Ophthalmol. 2023;43(12):4621–4629. doi: 10.1007/s10792-023-02862-4. [DOI] [PubMed] [Google Scholar]
- 36.Gallenga PE, Mastropasqua L, Lobefalo L. Vertical imbalance in pseudophakia. Eur J Implant Refract Surg. 1991;3(2):131–133. [Google Scholar]
- 37.Rosa AM, Miranda ÂC, Patrício M, McAlinden C, Silva FL, Murta JN, Castelo-Branco M. Functional magnetic resonance imaging to assess the neurobehavioral impact of Dysphotopsia with multifocal intraocular lenses. Ophthalmology. 2017;124(9):1280–1289. doi: 10.1016/j.ophtha.2017.03.033. [DOI] [PubMed] [Google Scholar]